Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
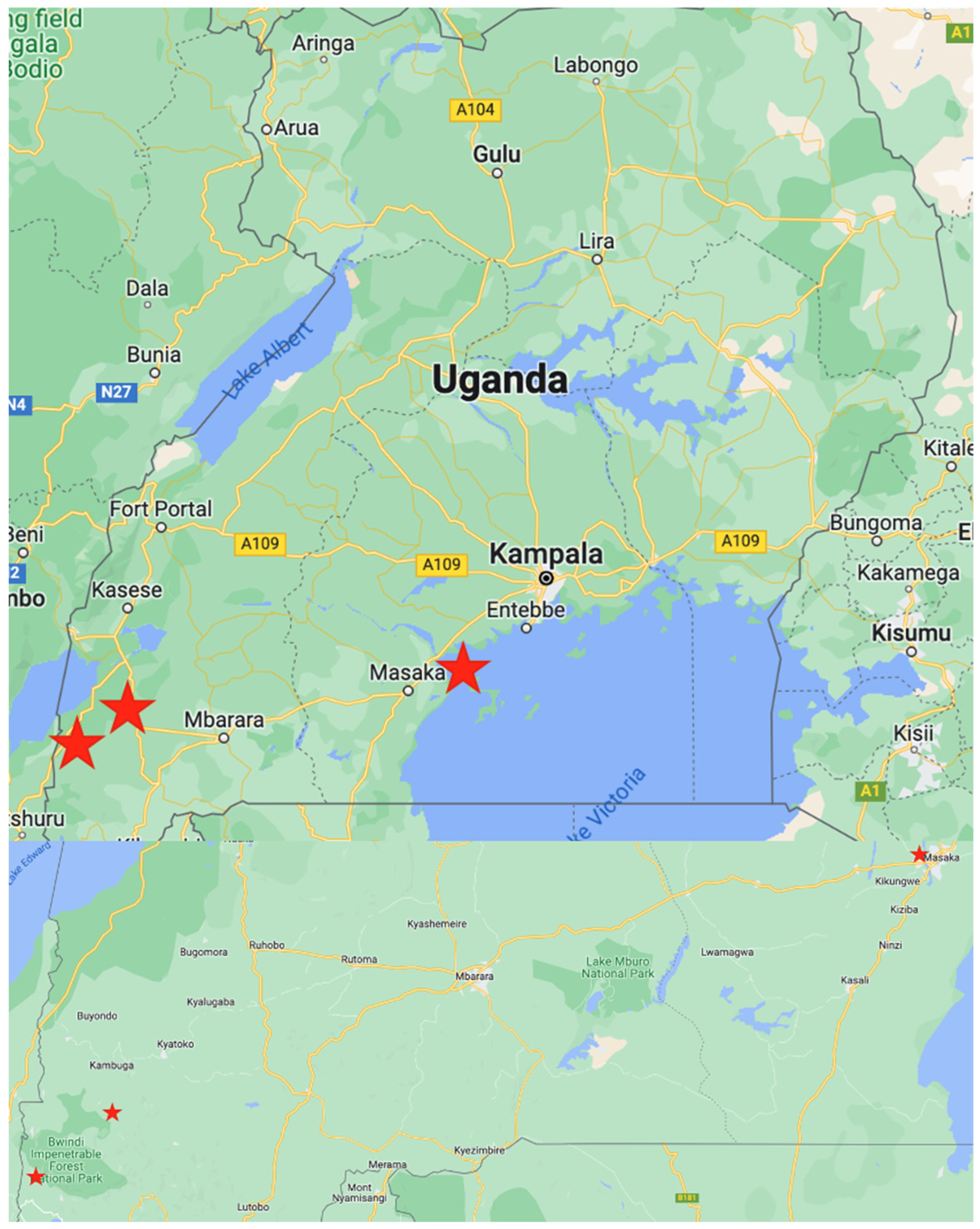
2.1. Sample Collection
2.2. pH Determination
2.3. Free Acidity Determination
2.4. Metals Content Determination
2.5. Fourier Transform Infrared Spectroscopy Analysis
2.6. Moisture Content Determination
3. Results
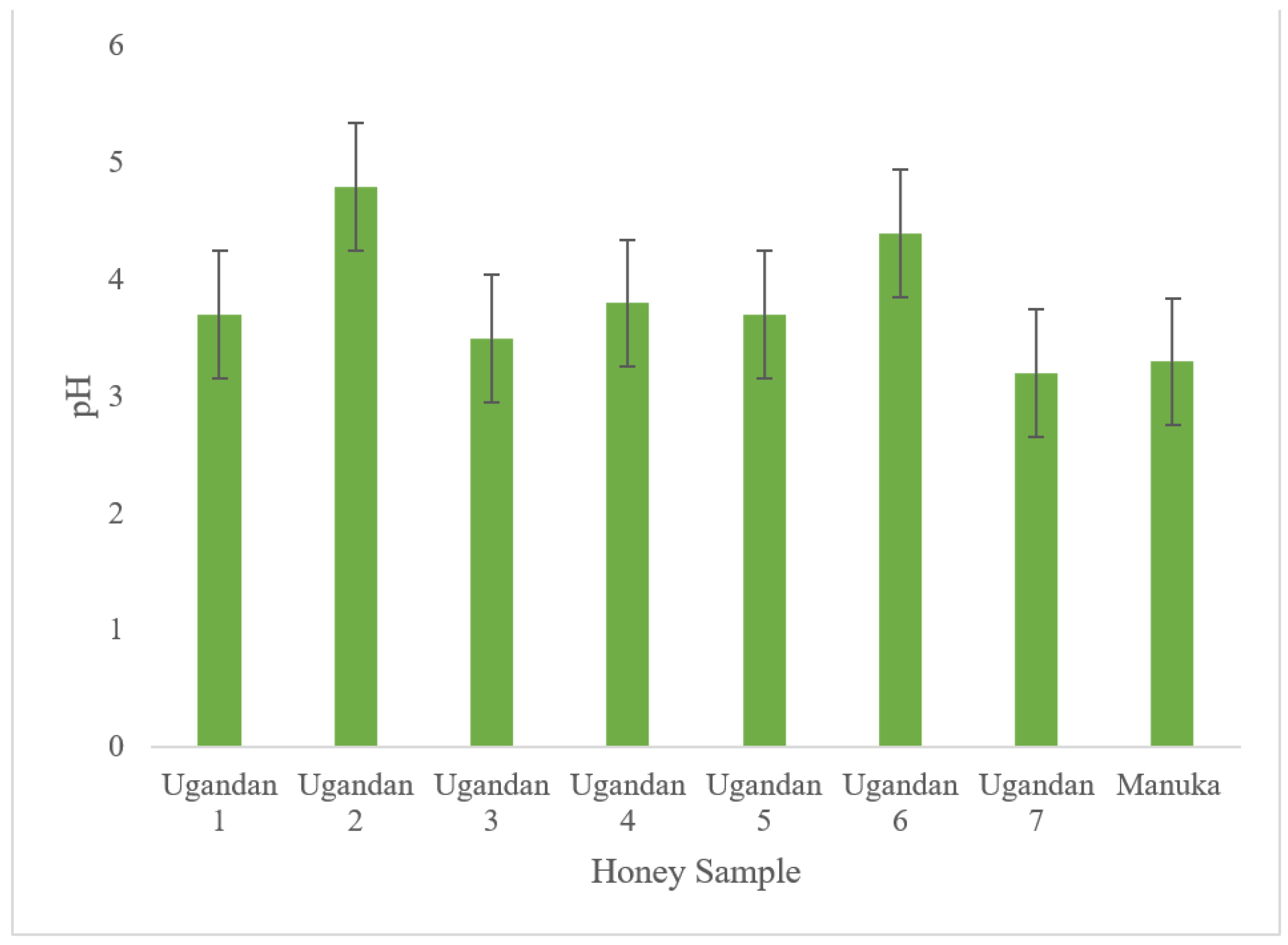
3.1. pH
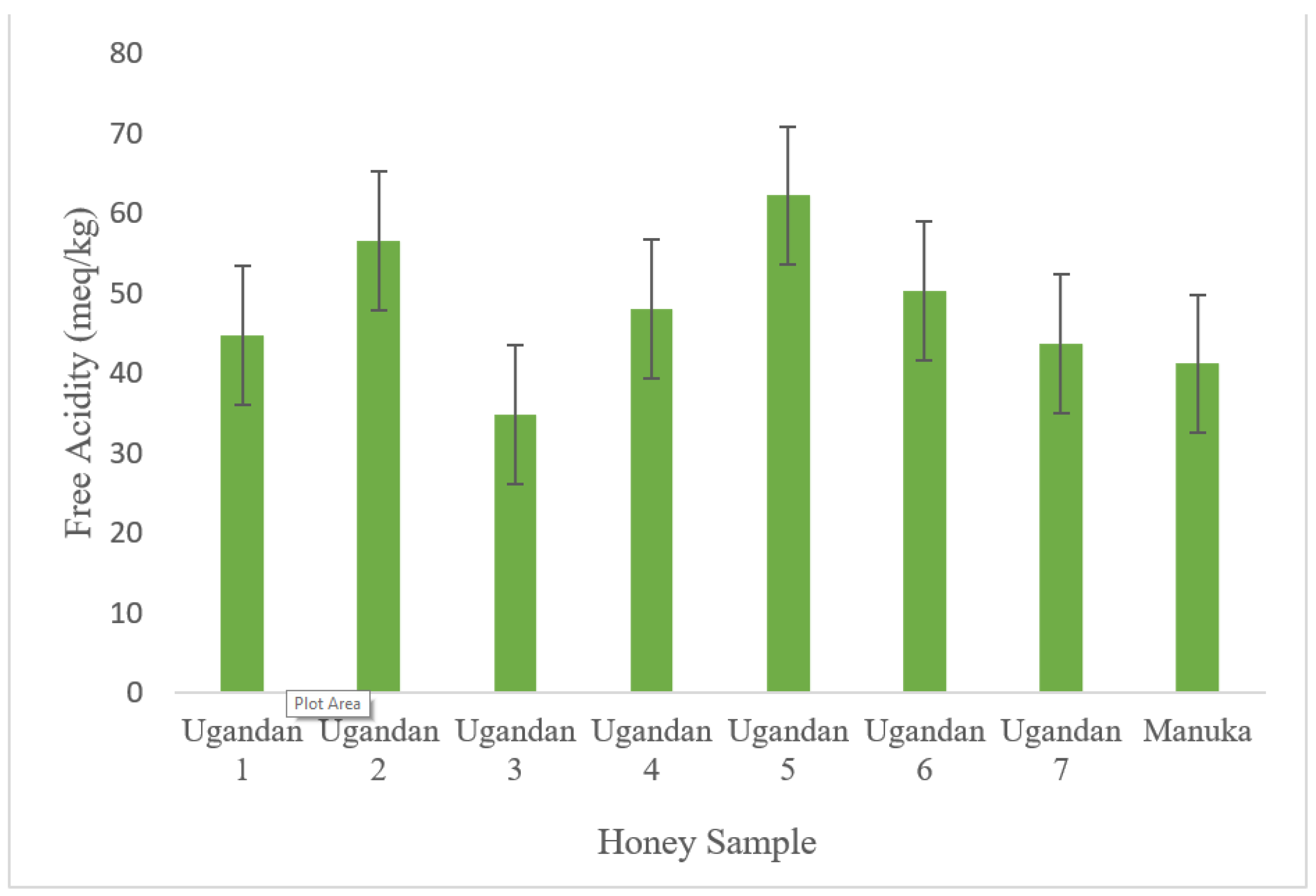
3.2. Free Acidity
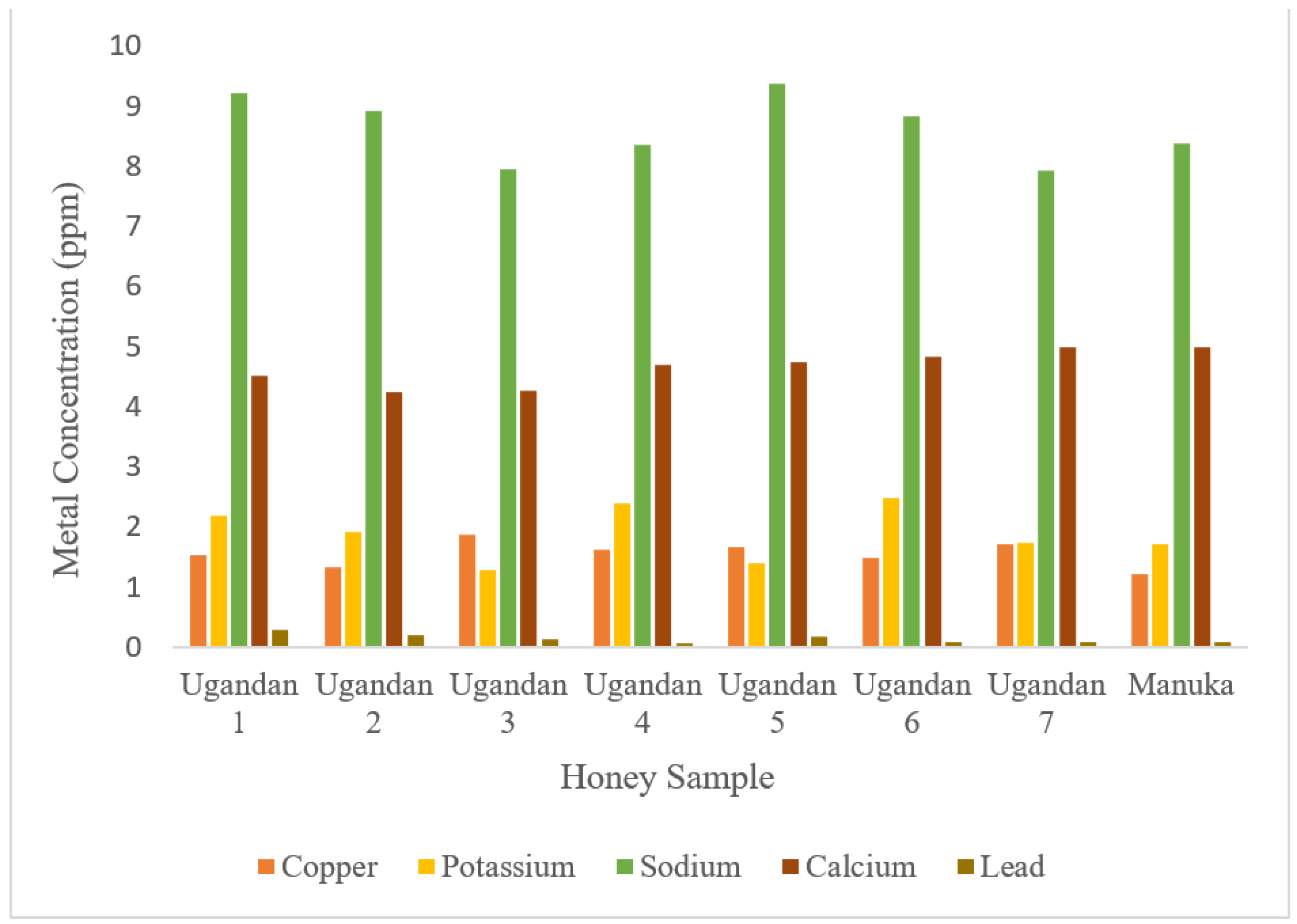
3.3. Determination of Metals
3.4. Moisture Content
3.5. Fourier Transform Infrared Spectroscopy Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez García, J.C.; Barciela García, J.; Herrero Latorre, C.; García Martín, S.; Peña Crecente, R.M. Direct and Combined Methods for the Determination of Chromium, Copper, and Nickel in Honey by Electrothermal Atomic Absorption Spectroscopy. J. Agric. Food Chem. 2005, 53, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Costa, P. Physical Properties of Honeys Produced in the Northeast of Brazil. Int. J. Food Stud. 2013, 2, 118–125. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single Food Stuff Comprises Many Drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Afik, O.; Hallel, T.; Dag, A.; Shafir, S. The Components That Determine Honeybee (Apis Mellifera) Preference between Israeli Unifloral Honeys and the Implications for Nectar Attractiveness. Isr. J. Plant Sci. 2009, 57, 253–261. [Google Scholar] [CrossRef]
- Honey Market Size, Share & Trends Analysis Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/honey-market (accessed on 20 October 2023).
- Shoemaker, S. 7 Unique Health Benefits of Honey. Available online: https://www.healthline.com/nutrition/benefits-of-honey (accessed on 20 October 2023).
- Ssali, M. Bees Will Give You More. Available online: https://www.monitor.co.ug/uganda/magazines/farming/bees-will-give-you-more-4016822 (accessed on 20 October 2023).
- Nakalya, E. Uganda Produces 4,000 Metric Tonnes of Honey per Annum - Report | Monitor. Available online: https://www.monitor.co.ug/uganda/news/national/uganda-produces-4-000-metric-tonnes-of-honey-per-annum-report-4349326 (accessed on 20 October 2023).
- Oromokoma, C.; Kasangaki, P.; Akite, P.; Mugume, R.; Kajobe, R.; Mangusho, G.; Matovu, M.; Chemurot, M. First Physicochemical Analysis of Stingless Bee Honey from Uganda. J. Apic. Res. 2023, 0, 1–10. [Google Scholar] [CrossRef]
- Gela, A.; Hora, Z.A.; Kebebe, D.; Gebresilassie, A. Physico-Chemical Characteristics of Honey Produced by Stingless Bees (Meliponula Beccarii) from West Showa Zone of Oromia Region, Ethiopia. Heliyon 2021, 7, e05875. [Google Scholar] [CrossRef] [PubMed]
- Abeshu, M.; Geleta, B. Medicinal Uses of Honey. Biol. Med. 2016, 8, 279. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial Activity of Manuka Honey and Its Components: An Overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial Effect of Different Types of Honey on Staphylococcus Aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.L.; Brown, H.L.; Jenkins, R.E. On the Antibacterial Effects of Manuka Honey: Mechanistic Insights. Res. Rep. Biol. 2015, 6, 215–224. [Google Scholar] [CrossRef]
- Girma, A.; Seo, W.; She, R.C. Antibacterial Activity of Varying UMF-Graded Manuka Honeys. PLOS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and Quantification of Methylglyoxal as the Dominant Antibacterial Constituent of Manuka (Leptospermum Scoparium) Honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Aal, A.M.; El-Hadidy, M.R.; El-Mashad, N.B.; El-Sebaie, A.H. Antimicrobial Effect of Bee Honey in Comparison to Antibiotics on Organisms Isolated From Infected Burns. Ann. Burns Fire Disasters 2007, 20, 83–88. [Google Scholar] [PubMed]
- Fan, F.; Roos, Y.H. Physicochemical Properties, Structural Transformation, and Relaxation Time in Strength Analysis for Honey Powder Models. Food Res. Int. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Google Maps. Available online: https://www.google.com/maps/place/Uganda/@0.8737302,31.0000435,8.19z/data=!4m6!3m5!1s0x1771a69f6499f945:0x874155ce43014549!8m2!3d1.373333!4d32.290275!16zL20vMDd0cDI?hl=en&entry=ttu (accessed on 1 January 2024).
- Honey. In Official Methods of Analysis of AOAC INTERNATIONAL; Godshall, M.A., Latimer, G.W., Jr., Eds.; Oxford University Press, 2023; p. 0 ISBN 978-0-19-761013-8.
- Afik, O.; Hallel, T.; Dag, A.; Shafir, S. The Components That Determine Honey Bee (Apis Mellifera) Preference between Israeli Unifloral Honeys and the Implications for Nectar Attractiveness. Isr. J. Plant Sci. 2009, 57, 253–261. [Google Scholar] [CrossRef]
- Price, R. The Analysis of Trace Elements in Honey by Flame and Graphite Furnace Atomic Absorption Spectrometry.
- Lanjwani, M.F.; Channa, F.A. Minerals Content in Different Types of Local and Branded Honey in Sindh, Pakistan. Heliyon 2019, 5, e02042. [Google Scholar] [CrossRef]
- Sahlan, M.; Karwita, S.; Gozan, M.; Hermansyah, H.; Yohda, M.; Yoo, Y.J.; Pratami, D.K. Identification and Classification of Honey’s Authenticity by Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy and Chemometric Method. Vet. World 2019, 12, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, S.; Soceanu, A.; Birghila, S.; Birghila, C.; Matei, N.; Popescu, V.; Constanda, L.M. Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes 2022, 10, 2554. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An Overview of Physicochemical Characteristics and Health-Promoting Properties of Honeydew Honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Honey Quality and International Regulatory Standards: Review by the International Honey Commission. Available online: https://www.researchgate.net/publication/277618846_Honey_quality_and_international_regulatory_standards_review_by_the_International_Honey_Commission (accessed on 1 January 2024).
- Value-Added Products from Beekeeping. Codex Standard for Honey. Available online: https://www.fao.org/3/w0076e/w0076e30.htm (accessed on 21 March 2024).
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rysha, A.; Kastrati, G.; Biber, L.; Sadiku, V.; Rysha, A.; Zogaj, F.; Kabashi-Kastrati, E. Evaluating the Physicochemical Properties of Some Kosovo’s and Imported Honey Samples. Appl. Sci. 2022, 12, 629. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy Metals Determination in Honey Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Tutun, H.; Kahraman, H.A.; Aluc, Y.; Avci, T.; Ekici, H. Investigation of Some Metals in Honey Samples from West Mediterranean Region of Turkey. Vet. Res. Forum 2019, 10, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and Trace Elements in Different Honey Types Produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Honey Moisture Reduction and Its Quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis Cerana, Apis Dorsata and Apis Mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sanz, S.; Gradillas, G.; Jimeno, F.; Perez, C.; Juan, T. Fermentation Problem in Spanish North-Coast Honey. J. Food Prot. 1995, 58, 515–522. [Google Scholar] [CrossRef]
- Doner, L.W. The Sugars of Honey—A Review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Cavia, M.M.; Fernández-Muiño, M.A.; Gömez-Alonso, E.; Montes-Pérez, M.J.; Huidobro, J.F.; Sancho, M.T. Evolution of Fructose and Glucose in Honey over One Year: Influence of Induced Granulation. Food Chem. 2002, 78, 157–161. [Google Scholar] [CrossRef]
- Chirife, J.; Zamora, M.C.; Motto, A. The Correlation between Water Activity and % Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of Physicochemical and Antioxidant Properties of Sourwood and Other Malaysian Honeys: A Comparison with Manuka Honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Biliskov, N.; Bubalo, D.; Barišić, D. Application of Infrared Spectroscopy in Honey Analysis. Agric. Conspec. Sci. 2011, 76, 191–195. [Google Scholar]
- In Uganda: Low Access to Essential Goods, High Food Insecurity, with Slightly Improved Conditions among the Poorest. Available online: https://www.worldbank.org/en/programs/lsms/brief/in-uganda-low-access-to-essential-goods-high-food-insecurity-with-a-slight-improved-conditions-among-the-poorest (accessed on 22 October 2023).
- Uganda | Imports and Exports | World | Natural Honey | Value (US$) and Value Growth, YoY (%) | 2009 - 2020. Available online: https://trendeconomy.com/data/h2/Uganda/0409 (accessed on 26 November 2023).
- Bunce, B. Discover the 10 Countries That Produce the Most Honey. Available online: https://a-z-animals.com/blog/discover-the-countries-that-produce-the-most-honey/ (accessed on 7 December 2023).
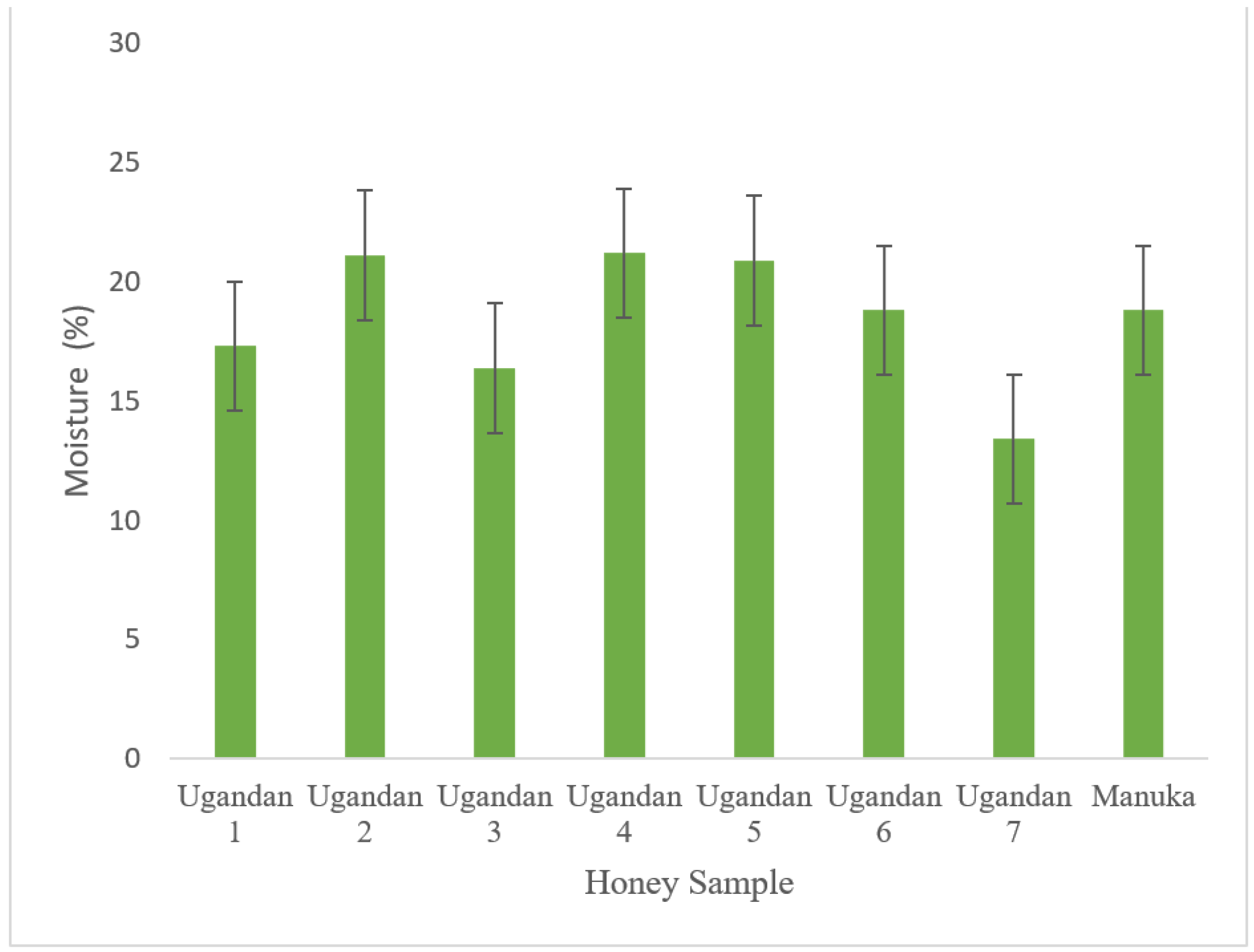
| Honey Sample | pH | Free Acidity (meq/kg) | Moisture (%) | Copper (ppm) | Potassium (ppm) | Sodium (ppm) | Calcium (ppm) | Lead (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.7 | 44.72 | 17.3 % | 1.54 | 2.18 | 9.21 | 4.52 | 0.28 |
| 2 | 4.8 | 56.52 | 21.1 % | 1.32 | 1.92 | 8.92 | 4.24 | 0.19 |
| 3 | 3.5 | 34.87 | 16.4 % | 1.87 | 1.29 | 7.94 | 4.27 | 0.13 |
| 4 | 3.8 | 48.06 | 21.2 % | 1.62 | 2.38 | 8.36 | 4.69 | 0.07 |
| 5 | 3.7 | 62.22 | 20.9 % | 1.67 | 1.39 | 9.38 | 4.75 | 0.18 |
| 6 | 4.4 | 50.34 | 18.8 % | 1.49 | 2.48 | 8.84 | 4.82 | 0.09 |
| 7 | 3.2 | 43.68 | 13.4 % | 1.71 | 1.73 | 7.93 | 4.99 | 0.09 |
| Manuka | 3.3 | 41.16 | 18.8 % | 1.22 | 1.72 | 8.37 | 4.98 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





