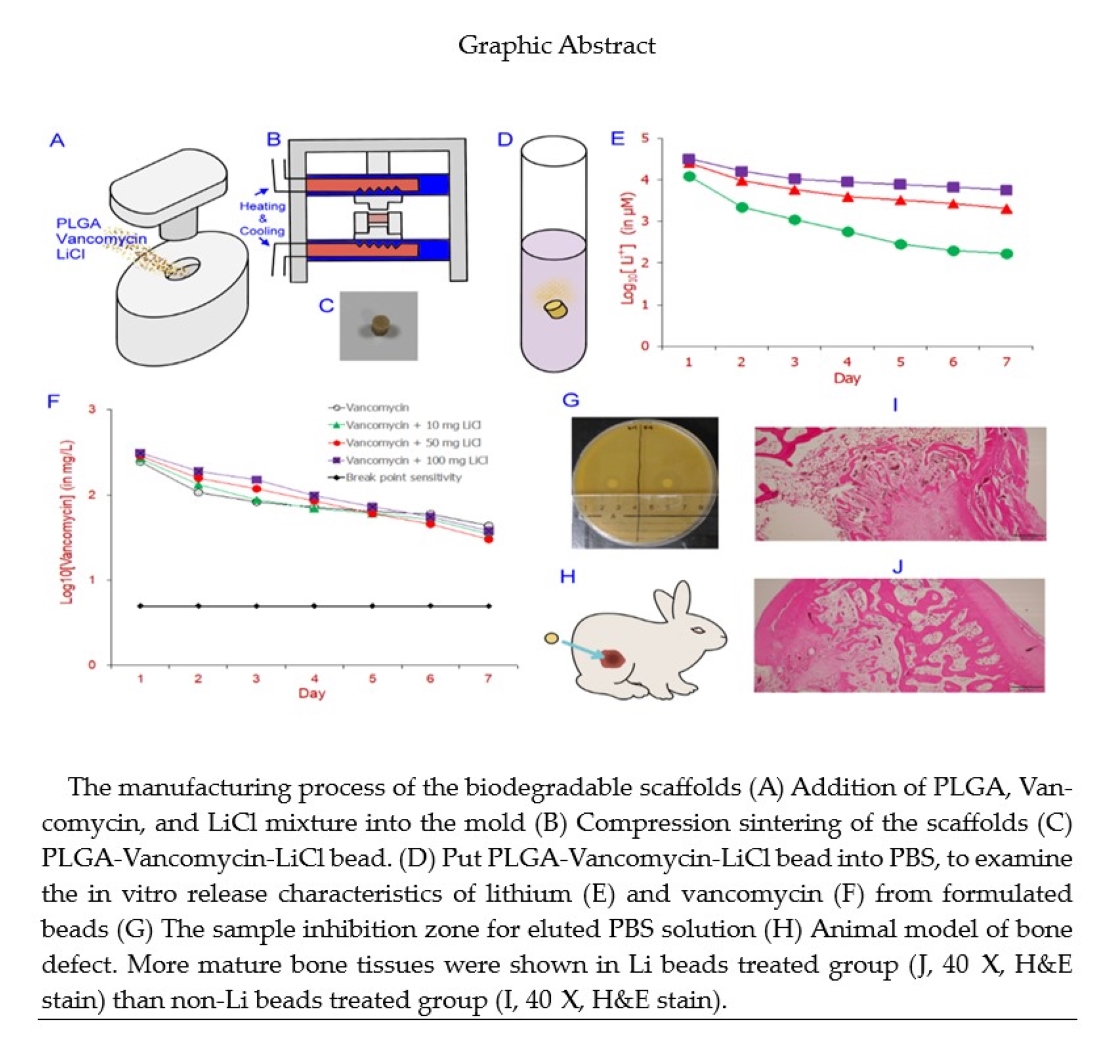
1. Introduction
Despite the advances in surgical techniques and the availability of newly developed antibiotics, bone infections after surgical procedures continue to be a difficult problem for surgeons [
1]. The combination of surgical intervention and an effective antibiotic remains the mainstay of treatment in the present time [
2]. Systemic and topical antibiotic therapies are usually used [
3,
4]. The most common of these is the incorporation of antibiotics into polymethylmethacrylate (PMMA) [
5,
6], a notion that was originally used by Buchholz et al [
2]. The antibiotic impregnated PMMA beads provide a high concentration of local antibiotics and have the advantage over intravenous injection in having low systemic levels, thereby producing minimal allergic reactions and systemic complications. Antibiotic-impregnated PMMA beads provide a high concentration of topical antibiotics and have the advantage of low systemic levels over intravenous administration, resulting in minimal allergic reactions and systemic complications. PMMA beads may require a second operation for removal after prolonged implantation [
1,
3].
A biodegradable carrier as a slow-release bead for delivering antibiotics may be better than PMMA beads and intravenous antibiotics. Biodegradable beads provide a long-term bactericidal concentration of antibiotics, and the biodegradability is alterable from weeks to months to treat many types of infections. Since the biodegradable beads dissolve gradually and the soft tissue or bone defect slowly fills with tissue, it is thus unnecessary for bone/tissue reconstruction [
7]. A large number of biocompatible and biodegradable substrates, such as bioceramics, polymers, bioglasses, and composite materials, have been tried as possible methods for topical antibiotics to treat osteomyelitis in vitro and in vivo [
8]. Poly (lactide-
co-glycolide) (PLGA) copolymer is one of the favorable biodegradable materials. It induces a minimal inflammatory response, is nontoxic, and can be absorbed with no accumulation in the vital organs. PLGA has been used as carrier materials for antibiotics [
9]. In 1999, the authors used a hot compressing molded method to fabricate PLGA antibiotic beads and achieved in vitro antibiotic release lasting more than 32 days. [
8,
9]. Additionally, the authors reported antibiotic release for 56 days from the PLGA vancomycin beads with a rabbit model in 2002 [
10]. In 2007, the authors have adopted compression sintering and ultrasonic welding techniques to produce biodegradable polymer capsules that can simultaneously release vancomycin and rhBMP-2 [
24]. In 2016, the authors used the PLGA antibiotic beads to eradicate S. aureus infection in damaged bone [
14].
Lithium ion (Li
+) is a well-known essential trace element and is a mood stabilizer found in cereals, vegetables and replenished in drinking water [
15]. Lithium has been used to treat bipolar disorder, and numerous studies have shown that lithium can prevent apoptosis, prolong lifespan, and is widely used as an anticancer drug in combination therapy [
16]. In addition, lithium affects the proliferation of hematopoietic stem cells (HSCs) and its protective effect against cadmium, another toxic metal, has been reported [
17]. Recent studies on this theme have shown that Li upregulates proliferation of mesenchymal stem cells (MSCs), stimulates osteogenic and inhibits adipogenic differentiation of MSCs by initiating the Wnt/β-catenin pathway [
18,
19]. To provide a solution for infected bone defects, Li modified PLGA antibiotic beads could be used for bone restoration and defect augmentation. The PLGA antibiotic beads used for bone repair were biodegradable, bioactive and biocompatible. Lithium detoxification is at least partly mediated by Li
+ efflux via a Na
+/H
+ antiporter in
E.
coli [
13]. Previous studies have shown that the size of the inhibition zone caused by vancomycin can determine the ability of vancomycin to inhibit bacterial growth. However, the combined effects of lithium and vancomycin on inhibition zone and relative activity of vancomycin against
Staphylococcus aureus are not clear. The possible molecular mechanism is worth exploring.
In this study, we developed PLGA-vancomycin-LiCl delivery beads for the treatment of bone infection. The objective of the present study was (I) to prepare sustained release Li and antibiotic beads using matrix materials including PLGA, LiCl, and vancomycin (II) to examine the in vitro release characteristics of lithium and vancomycin from formulated beads (III) to investigate the effects of the beads elution on osteogenesis of MSCs (IV) to investigate the effects of the beads elution on bioassay of antibacterial activity (V) to examine the in vivo release characteristics of Li and vancomycin from formulated beads in a rabbit model (VI) to examine the repaired tissues of the rabbits following beads treatment.
2. Materials and Methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Chang Gung Memorial Hospital (No: 2017120803, The approval date of our animal experiments was 20180528). PLGA was supplied by Boehringer Ingelheim, Germany. The poly(lactide-co-glycolide acid) polymer Resomer RG503 (lactide:glycolide, 50:50) (molecular weight: 33,000 Da) was purchased from Boehringer Ingelheim (Ingelheim am Rhein, Germany). Powdered vancomycin and lithium chloride (LiCl) were purchased from Sigma. A Millipore Ultrapure Water System (Watford, UK) was used to obtain HPLC-grade water. Acetonitrile purchased from Mallinckrodt Baker. Heptane sulfonic acid was purchased from Fisher Ltd. Commercially available human mesenchymal stem cells (MSCs) cell line and MesenPRO RS™ medium were purchased from Invitrogen.
2.1. Fabrication of a Biodegradable PLGA Drug Delivery System for Antibiotic and Lithium Delivery
PLGA (125 mg), vancomycin (25 mg), LiCl (10, 50, or 100 mg) were loaded into molds, then with hot compression molded at 55° C (
Figure 1). Each of the antibiotic beads was incubated in 5 mL of PBS (pH 7.4) at 37° C for 24 hours to assay the elution rate of vancomycin and lithium from PBS in vitro. At each 24-hour interval, the PBS was drawn, and the beads and were re-submerged in fresh buffer. The removed PBS with vancomycin and lithium was frozen at -70° C until study.
2.2. In Vitro Analysis of Vancomycin and Lithium Release
The concentrations of vancomycin in the collected fluid were determined by a high-performance liquid chromatography (HPLC) assay method. The separating column was a C8 Symmetry HPLC column (Waters Asia Ltd). The mobile phase contained 0.01 M heptane sulfonic acid (Fisher, Loughborough, UK) and acetonitrile (Mallinckrodt Baker, KY; 85/15, v/v). Absorbance was monitored at 280 nm under a flow rate of 1.4 mL/min. The concentrations of lithium in the collected fluid was determined by Dimension® EXL™ 200 Integrated Chemistry System.
2.3. In Vitro Analysis of Antibacterial Activity in the Collected PBS with Vancomycin and Lithium
Staphylococcus aureus (ATCC 259523) was cultured in static at 37°C for 24 h in 12 mL of broth (LB broth, GIBCO, Thermo Fisher Scientific). Minimal inhibitory concentration (MIC) of vancomycin with or without Li in the collected fluid against S. aureus was measured with an antibiotic tube dilution method in Cation-Supplemented Mueller- Hinton Broth (Difco Laboratories, Detroit, MI). Medium was diluted serially twice in tubes containing 0.5 mL Broth. The S. aureus inocula for each series of tubes were 0.5 mL of an overnight culture containing 5 × 105 colony forming units/mL. MIC was defined as the lowest antibiotic concentration preventing turbidity after 24 hours of incubation at 37°C.
Relative activity of vancomycin with or without Li against S. aureus was determined by an antibiotic disk diffusion method applied to the nutrient broth. Each sample was diluted or concentrated to 50 mg/mL. Solution (8 mL) from each buffer sample was harvested daily and pipetted onto 7-mm disks, which was placed on the nutrient agar plates (Difco Laboratories) seeded with a lawn of S. aureus. Zones of inhibition were measured with a micrometer after 16 hours to 18 hours of incubation at 35°C. The equation for relative activity was relative activity (%) = (diameter of sample inhibition zone- disk diameter)/(diameter of maximum inhibition zone- disk diameter).
2.4. Effects of the Collected PBS with Vancomycin and Lithium on Osteogenesis of MSCs
StemPro® BM MSCs were purchased from Gibco (Life Technologies Corporation). Expand primary MSCs with 2% FBS MesenPRO RS™ medium (Gibco) containing 2 mM L-glutamine and 5 μg/mL gentamicin in T-75 flasks and maintained in an incubator at 37 °C /5% CO2. The media was changed every three days, and the cells were split at 80–90% confluence. The cells were used at early passage (< 5 passages) for all experiments.
Approximately 2.5×105 MSCs were seeded onto a 100 mm cell culture dish. Transfer 100 μL of the collected liquid with or without vancomycin and lithium to the medium daily. After culturing for 14 d, the cultured cells were rinsed with PBS. Total RNA was extracted using a Qiagen RT kit (Qiagen, USA). To detect the Runx 2, osteopontin (OPN), osteocalcin (OCN), and GAPDH RNA transcripts, cDNA was analyzed using an ABI PRISM 7900 sequence detection system and TaqMan PCR Master Mix (Applied Biosystems, CA). Total protein was extracted using M-PER protein extraction reagent (Thermo, USA). Protein sample quantitation with a protein assay kit (Pierce Biotechnology, IL), separated with 7.5% SDS-PAGE, and protein expression was detected for phosphor-GSK-3β (Ser9) (Abcam), GSK-3β (Cell Signaling), and Runx 2 (Millipore).
2.5. Fabrication of a Biodegradable PLGA Drug Delivery System for Antibiotic and Lithium Delivery
PLGA (125 mg), vancomycin (25 mg, Sigma), and LiCl (100 mg, Sigma) were loaded into molds, then with hot compression molded at 55° C to form biodegradable vancomycin and Li delivery systems for in vivo study.
2.6. Animal Model of Bone Defect
Eight adult male New Zealand white rabbits weighing 2.9–3.5 kg was used. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Chang Gung Memorial Hospital (No: 2017120803). The methods were carried out in accordance with the approved guidelines. At first surgery, a cylindrical cavity (15 mm X 12 mm X 10 mm) was made at the side of the right femur distal end and obliterated with a poly (methyl-methacrylate) (PMMA) spacer. The wound was closed with 3-0 nylon sutures. After 2 weeks, the PMMA spacer was removed, and one PLGA drug delivery bead was inserted into the cavity and then the wound was closed. After the implantation of the PLGA drug delivery system, fluid content was aspirated from each femoral cavity on days 7, 14, 28, and 42 after implantation. Minimal inhibitory concentration (MIC) of vancomycin in the collected fluid against Staphylococcus aureus (ATCC 259523) was measured. Four rabbits in each group will killed at 8 weeks for histologic observation.
2.6.1. Li Detection
The concentrations of Li in the collected fluid was determined by Dimension® EXL™ 200 Integrated Chemistry System.
2.6.2. Vancomycin Detection by HPLC
The concentrations of vancomycin in the collected fluid from femur were determined from a HPLC assay method as previous description.
2.7. Immunohistochemical Evaluation
After decalcification, the specimens harvested from femoral cavities were embedded in paraffin, cut into 5-μ m thick sections, and processed for H&E staining.
2.8. Statistical Analysis
Student-test was used to analyze the LiCl treated group/Non LiCl treated group ratio in this study. Data were represented as mean ± standard division (SD). The p-values for the Student's t-test were performed using the SPSS software package (Version 12.0, Chicago: SPSS Inc.). A p-value of < 0.05 was considered statistically significant.
4. Discussion
PLGA is one of the most suitable biodegradable polymer materials for the synthesis of tissue engineering drug delivery devices [
19,
20,
21]. The material is biodegradable and biocompatible, exhibits a large range of erosion times, and is mechanically adjustable. Drug release kinetics can be controlled by the polymerization rate of lactide and glycolide as well as molecular weight of PLGA [
11,
22]. The degradation of PLGA produces lactic acid and glycolic acid, which are finally degraded to CO
2 and H
2O [
41]. PLGA is an FDA-approved polymer that is widely used for the control of small molecule drugs, proteins, and other large molecules. Therefore, we selected PLGA as the carrier material for vancomycin-containing beads.
Bacterial infections during surgery can be destructive and are often associated with considerable morbidity and poor functional outcomes. Treatment of postoperative infection requires surgical debridement, removal of implants and all necrotic tissue, and the administration of systemic antibiotics. Delivering local antibiotics by using antibiotic-impregnated biodegradable beads have been proposed to provide a sustained release of antibiotics to infected areas, replacing intravenous antibiotics infusion. In this study, we developed PLGA-LiCl-vancomycin beads for the treatment of bone infection. PLGA has been demonstrated to be an excellent material for various healthcare applications, including tissue engineering, regenerative medicine, the fabrication of cardiovascular stents, and orthopedic interventions [
12,
24,
25,
26], owing greatly to the polymer’s favorable biocompatibility and to its safe degradation products [
26]. PLGA is a degradable polymeric material with superior properties that has been extensively researched. The material has a glass transition temperature of 40-60 °C and a melting temperature of 262 °C which is suit for our fabrication process with hot compression molded at 55° C (
Figure 1). Furthermore, because of the absence of organic solvents during bead preparation, these vancomycin/PLGA beads reduces the problems caused by organic solvents which destroying drugs or residues in the body thus applicable for clinical use for the treatment of infections in bone tissue.
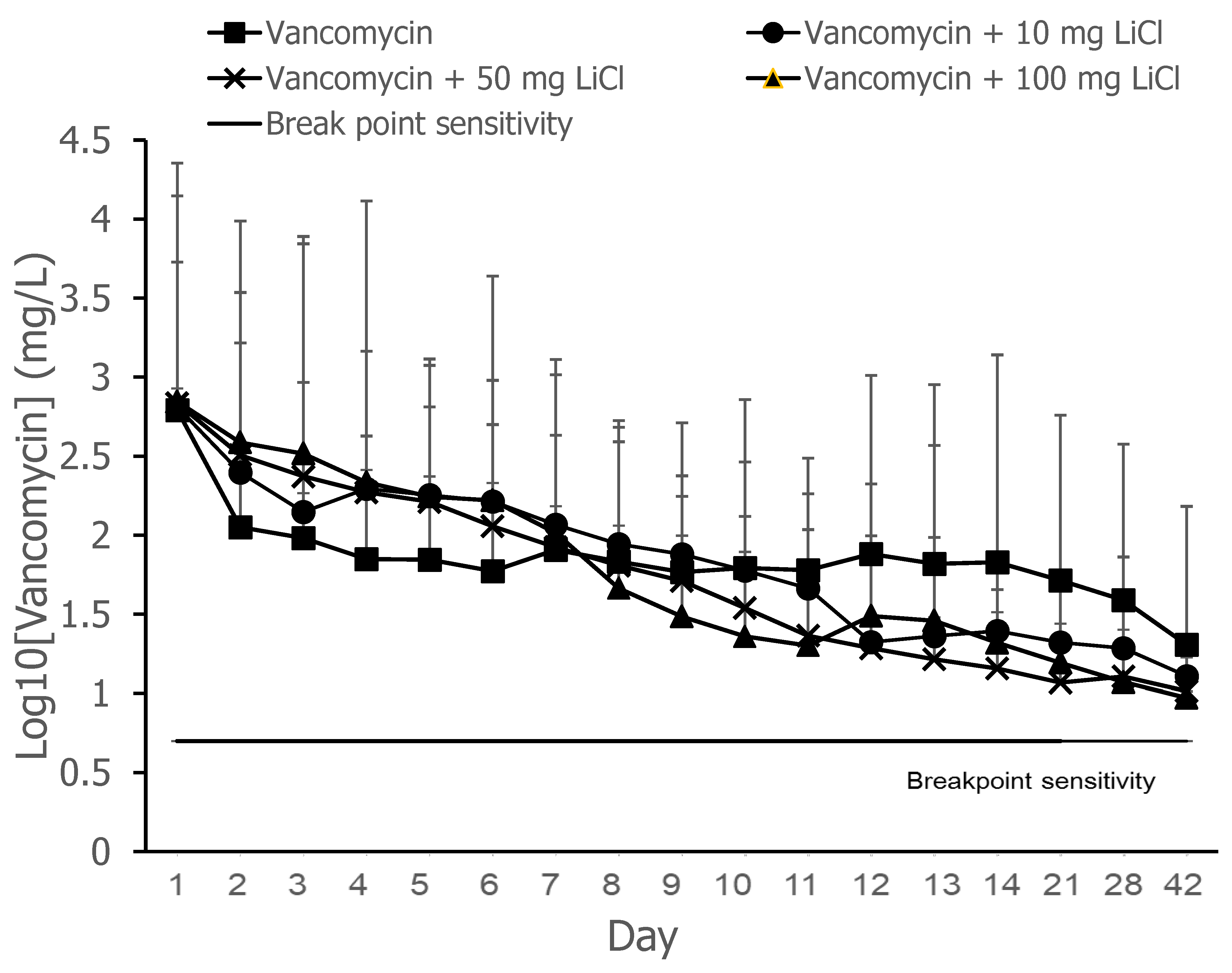
The release curves of vancomycin from the PLGA-vancomycin-LiCl delivery beads are shown in
Figure 2. Gradual release of vancomycin was observed from the biodegradable drug delivery beads over the entire duration, remaining above the breakpoint sensitivity level through day 42 in vitro. Vancomycin release was the most obvious during the first 48 h. The initial burst release of vancomycin was justified by the fast release of vancomycin located on the surfaces of PLGA drug delivery beads [
19]. This burst release of vancomycin molecules at the surgical site could successfully eradicate bacteria causing infection, followed by 4 to 6 weeks of a constant release above the breakpoint sensitivity level [
9,
10]. The slower following release could give continuous dosing of vancomycin as a long-term therapy. The continuous dosing of drug molecules was attributed to the slow diffusion of vancomycin molecules held inside their pores [
16,
19].
Antibiotic concentrations associated with antibiotic bone cements may cause skeletal cell toxicity and prevent fracture healing [
27]. Previous studies suggested dose-dependent effects of vancomycin on cells. Local administration of vancomycin at high levels may have cytotoxic effects. However, at lower vancomycin doses (4-400 μg/mL), vancomycin did not significantly impair osteogenic proliferation or function [
28,
29]. Local levels of vancomycin of 1000 μg/mL and less have little or no effect on osteoblast replication, and concentrations of 10,000 μg/mL cause cell death. Vancomycin is less toxic than cefazolin to osteoblasts at higher concentrations and may be a better antibiotic for local administration in the treatment of similarly sensitive bacterial infections [
30]. Vancomycin and tobramycin at doses greater than 2000 μg/mL severely decreased chondrocytes proliferation. The balance between the targeted microbicidal effects and host cellular toxicity is critical for skeletal cell survival and function [
27]. Regarding the clinical indication, the microbicidal effect is often investigated, but toxicity to osteoblasts has rarely been examined. In the present study, the authors developed biodegradable drug delivery beads for the treatment of bacterial infections. The highest vancomycin concentrations eluted from the PLGA-vancomycin-LiCl delivery beads (type IV) were 700.3 μg/mL in vitro (
Figure 2) which lower than the inhibitory concentration of vancomycin on osteoblast replication (1000 μg/mL) [
30]. Our data suggested that PLGA-vancomycin-LiCl delivery beads may be a good choice for local administration in the treatment of similarly sensitive bacterial infections.
Bone healing is a complex physiological process that is initiated and controlled by growth factors, such as bone morphogenetic protein-2 (BMP-2). Because the sintering temperature for hot compression molded (55° C) is not suite for rhBMP-2 protein, we have adopted three steps of compression sintering and ultrasonic welding techniques to manufacture rhBMP-2 containing polymer capsules in previous study [
24]. Since rhBMP-2 or Wnt 3a protein are costly and rapidly loses its activity in culture, we investigated the possibility of replacing it with inexpensive commercially available Wnt agonists, specifically lithium chloride (LiCl) for certain applications of MSCs. Wnt signaling was shown to promote the osteogenesis of MSCs. LiCl up-regulated Wnt signaling thus increased the osteogenic capacity of MSCs [
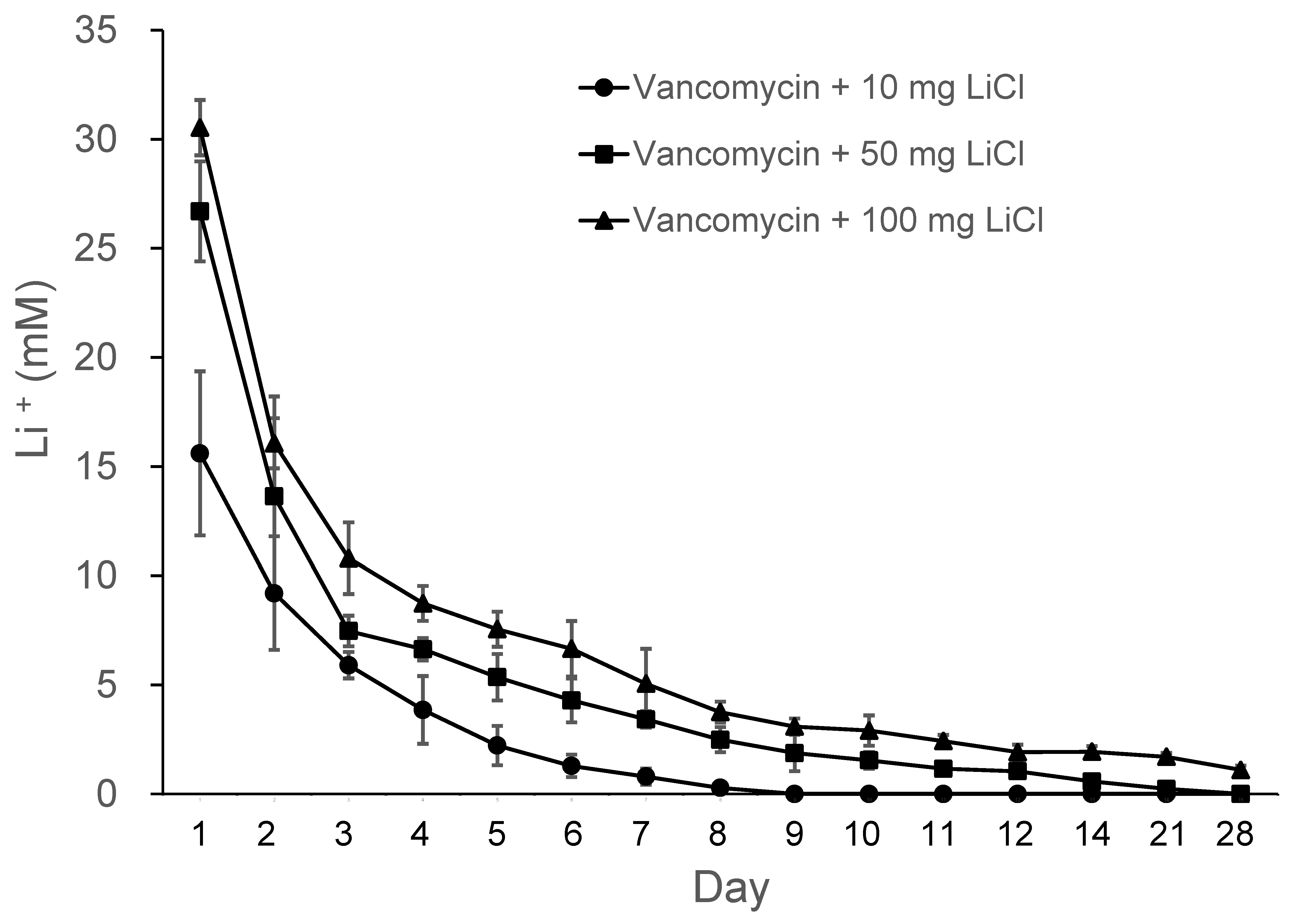
31]. In the present study, we used LiCl as an osteogenic enhancer of MSCs and adopted compression sintering techniques to manufacture LiCl containing polymer beads. The release curves of Li from the PLGA-vancomycin-LiCl delivery beads are shown in
Figure 3. Gradual release of Li was observed from the Type II beads for 10 days, Type III beads for 14 days, and Type IV beads for 28 days. Lithium release was the most obvious during the first 48 h. The melting points of LiCl and vancomycin were 614°C and 175°C, respectively. The sintering temperature was set at 55°C, which was higher than polymers’ glass transition temperature (
40-60 °C), but low enough to avoid destroying the vancomycin and LiCl.
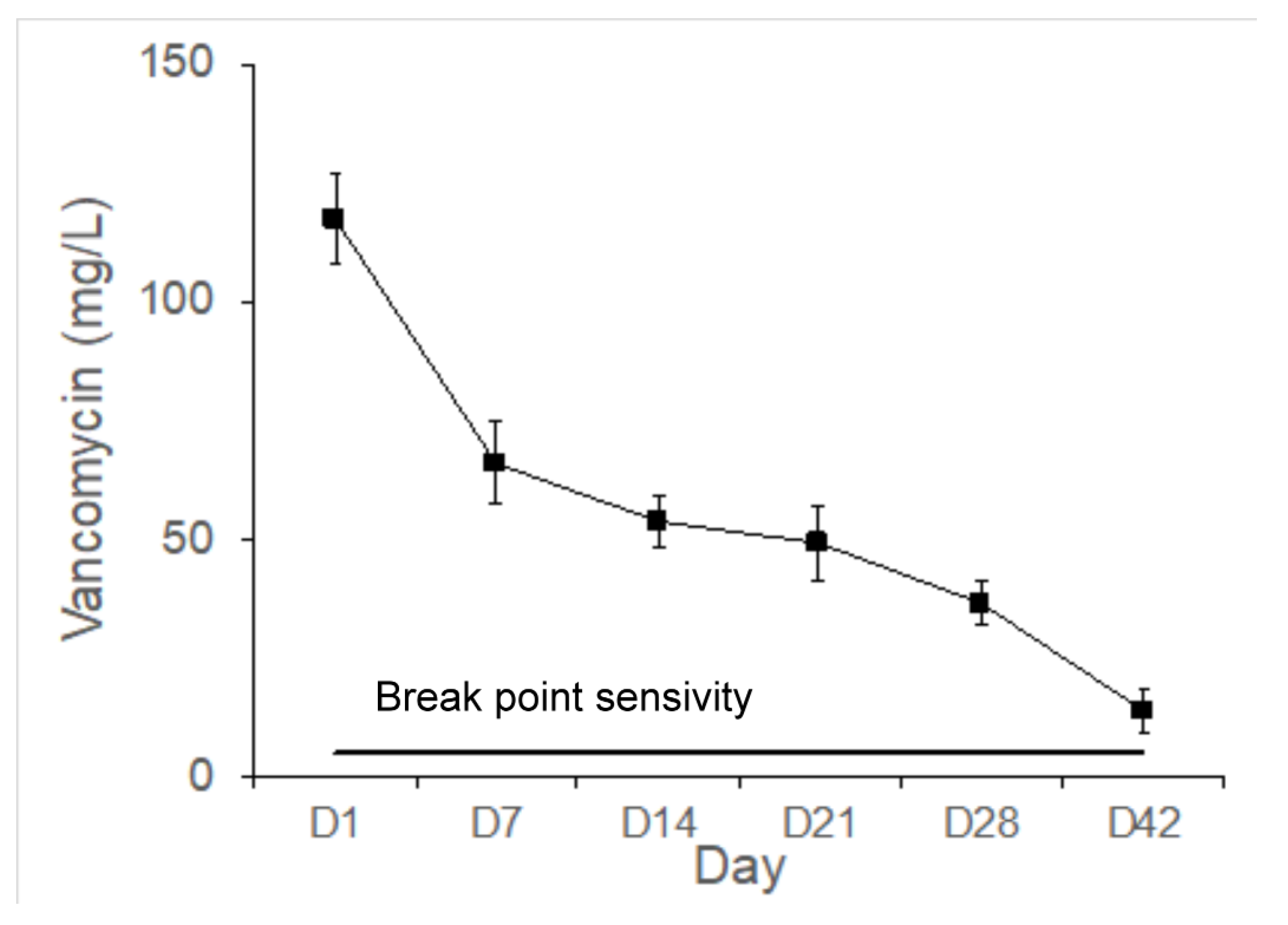
Staphylococcus aureus (S. aureus) is the most widespread etiology bacteria identified in traumatic and iatrogenic infections [
12,
32]. Breakpoint sensitivity of vancomycin in the collected fluid against S. aureus (ATCC 259523) was measured.
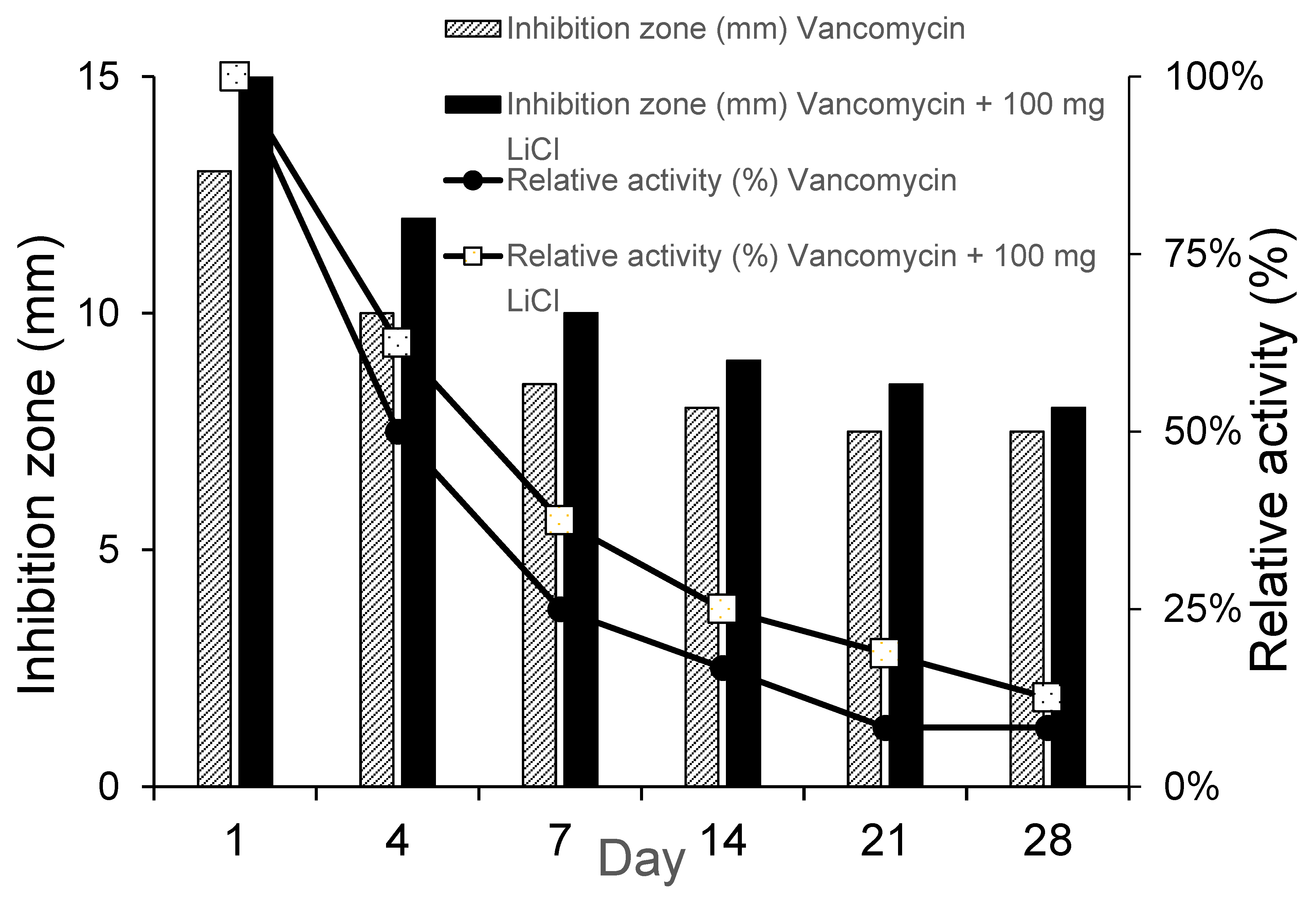
Figure 4 showed that the sample inhibition zone and relative activity were higher in Type IV beads than Type I beads in D1, D4, and D7 which suggested that the bactericidal effect of vancomycin was enhanced by Li co-treatment in Type IV beads as compare with Type I beads. Vancomycin is a type of glycopeptide antibiotic. The main bactericidal function of vancomycin is to inhibit bacterial cell wall synthesis and disturb the osmotic ability of the cell membrane in S. aureus [
33]. The permeability of the bacterial cell membrane and the synthesis of RNA can be modified [
34,
35]. LiCl increases the solubilization of the cell wall structure [
36], so it may increase the bactericidal effect of vancomycin.
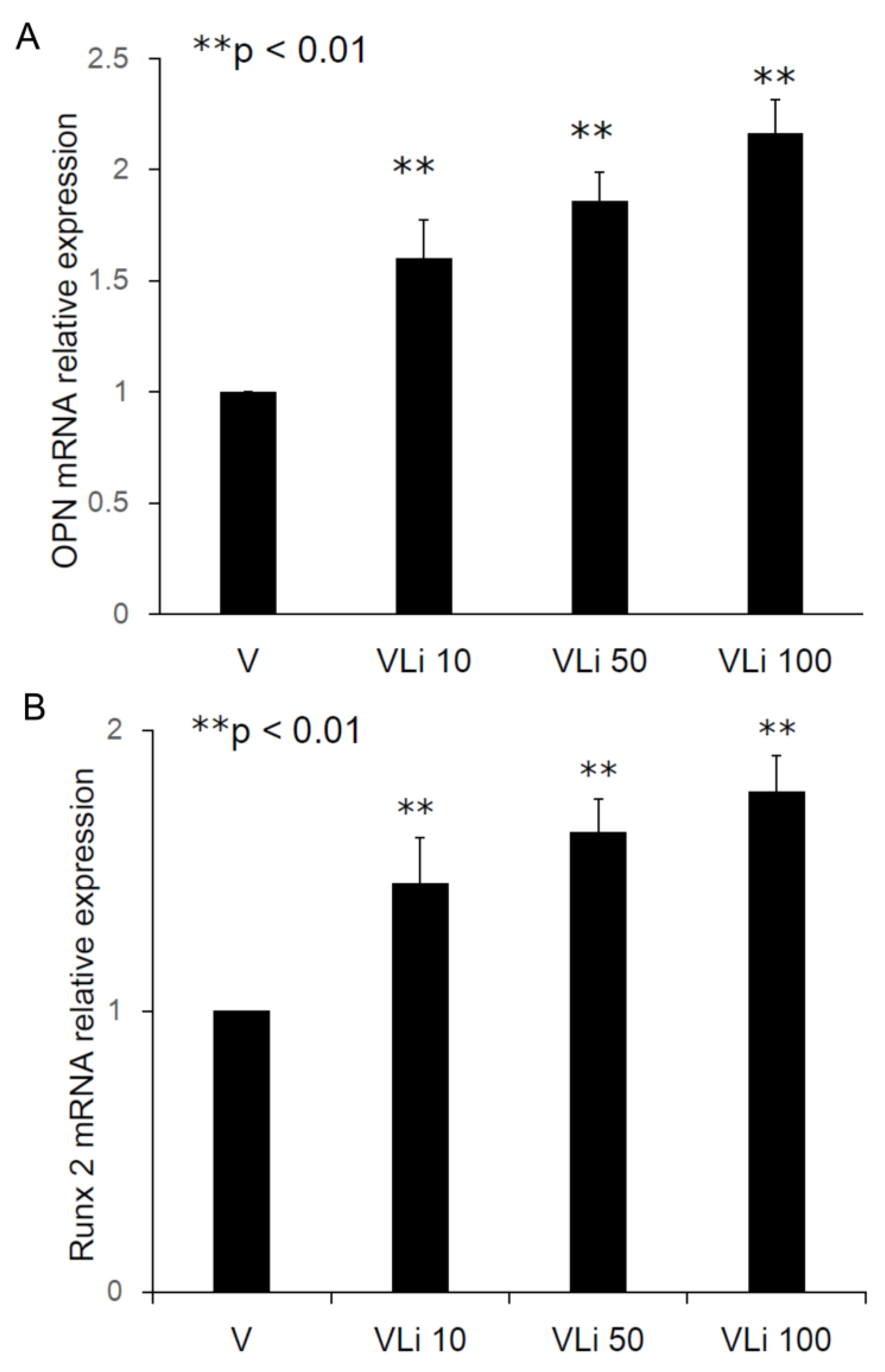
After treatment of MSCs by collected elution from PLGA-Vancomycin-LiCl beads, the dose dependent released Li was shown to promote the osteogenesis of MSCs by increasing in osteopontin (OPN) (
Figure 5a) and Runx2 mRNA (
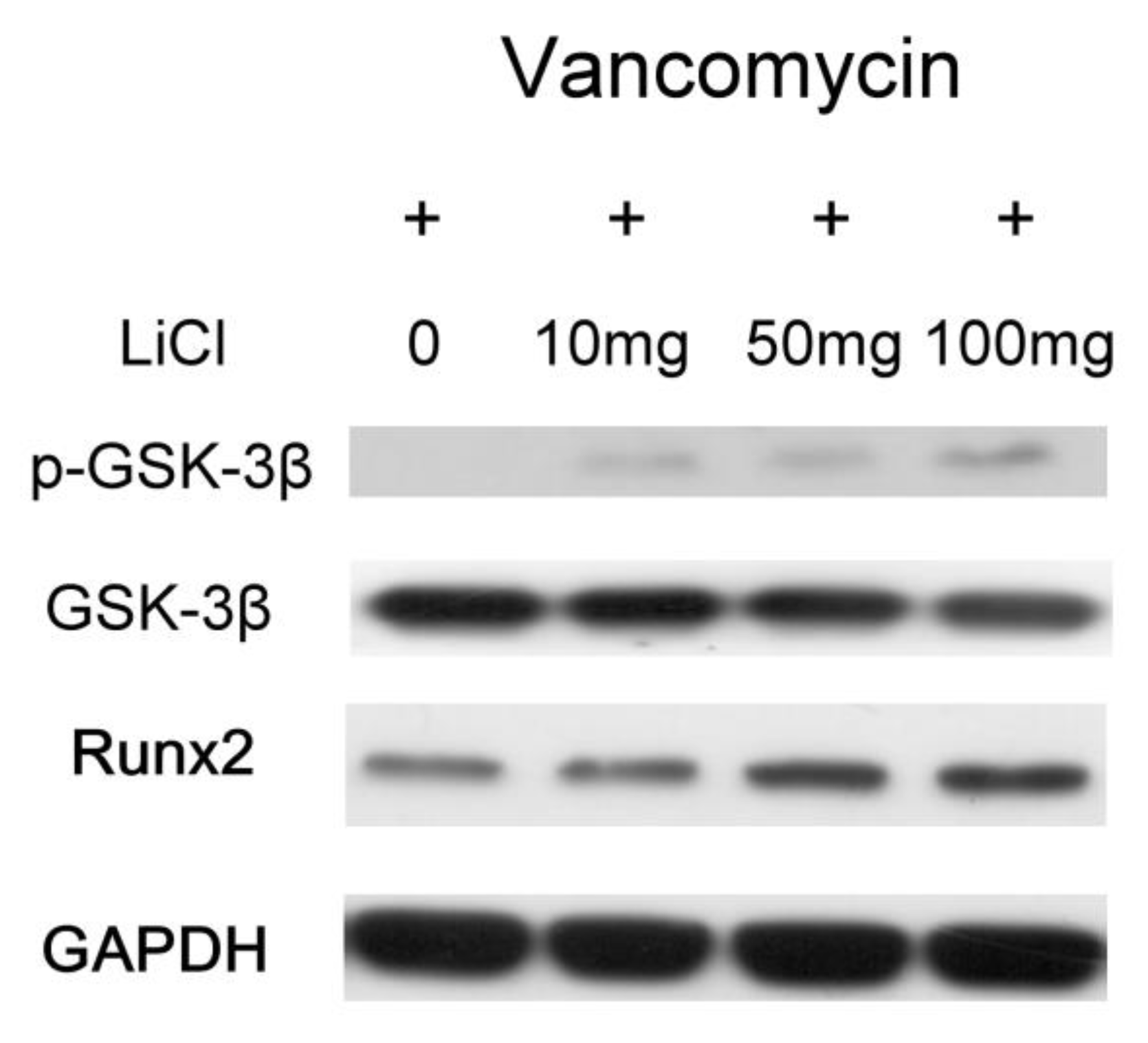
Figure 5b) expression. In addition, the released Li was shown to promote the osteogenesis of MSCs by increasing in phosphorylation of GSK-3β protein (
Figure 6). LiCl inhibits GSK3β activity and thereby stabilize free cytosolic β-catenin thus leading to intracellular accumulation of β-catenin. β-catenin subsequently translocated into the nucleus to promote the osteogenesis of MSCs by up-regulating Runx2 protein expression [
37,
38]. The authors have found that released Li stimulated MSC differentiation (
Figure 6), and previous studies have shown that Li is not carcinogenic [
39] or mutagenic [
40]. These attributes make Li a potential supplement for MSC in vitro culture medium optimization.
Lithium significantly enhances bone formation in rats [
24] and accelerates fracture healing clinically [
25]. In addition, Li is taken up by a variety of tissues, with bone and muscle containing the highest concentrations, making Li especially suitable for treating bone disorders [
17,
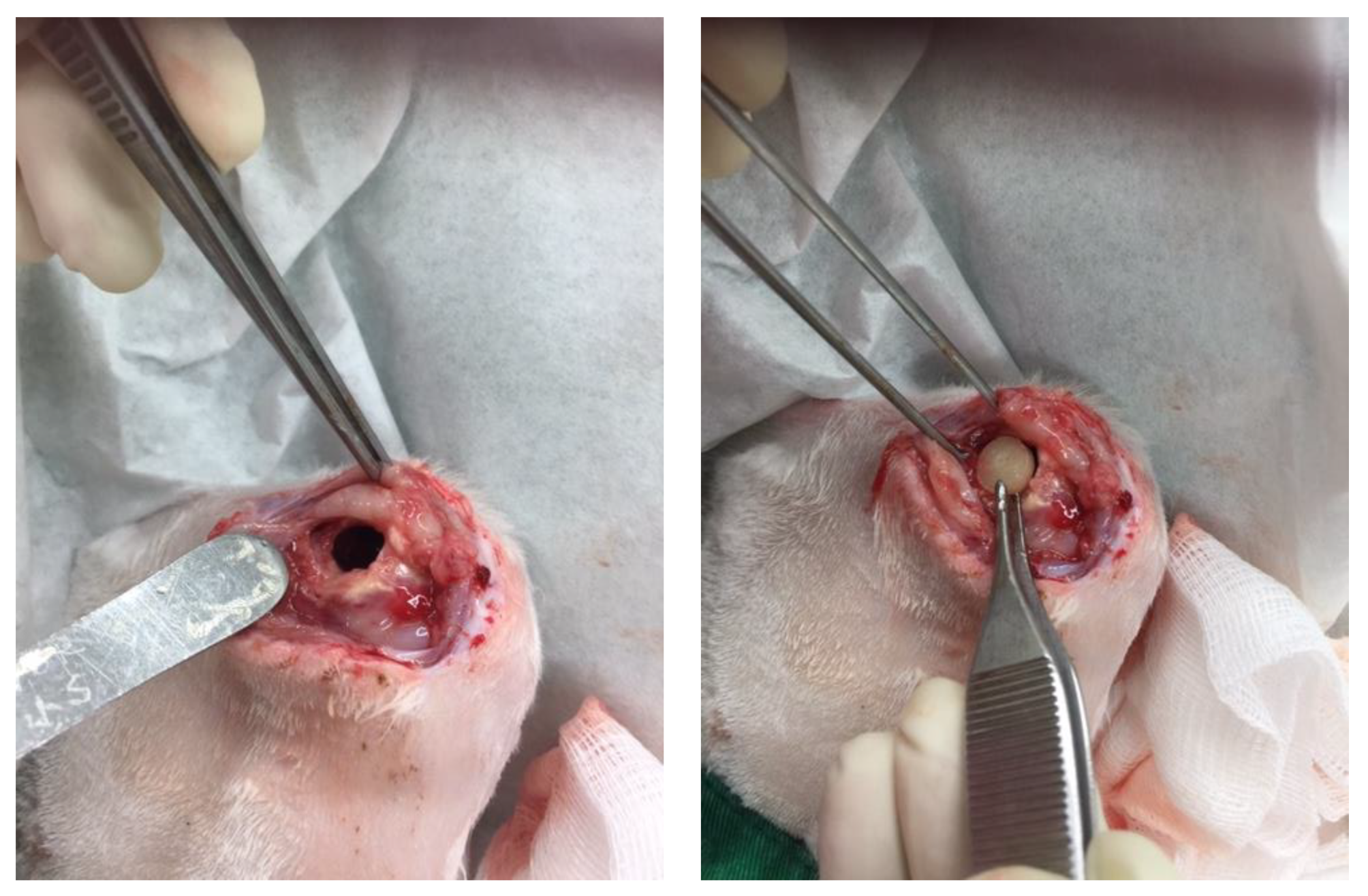
26]. To investigate the effects of released Li and vancomycin in vivo, we created a rabbit model. A cylindrical cavity was made at the side of the right femur distal end and one composite delivery bead was inserted into the cavity (
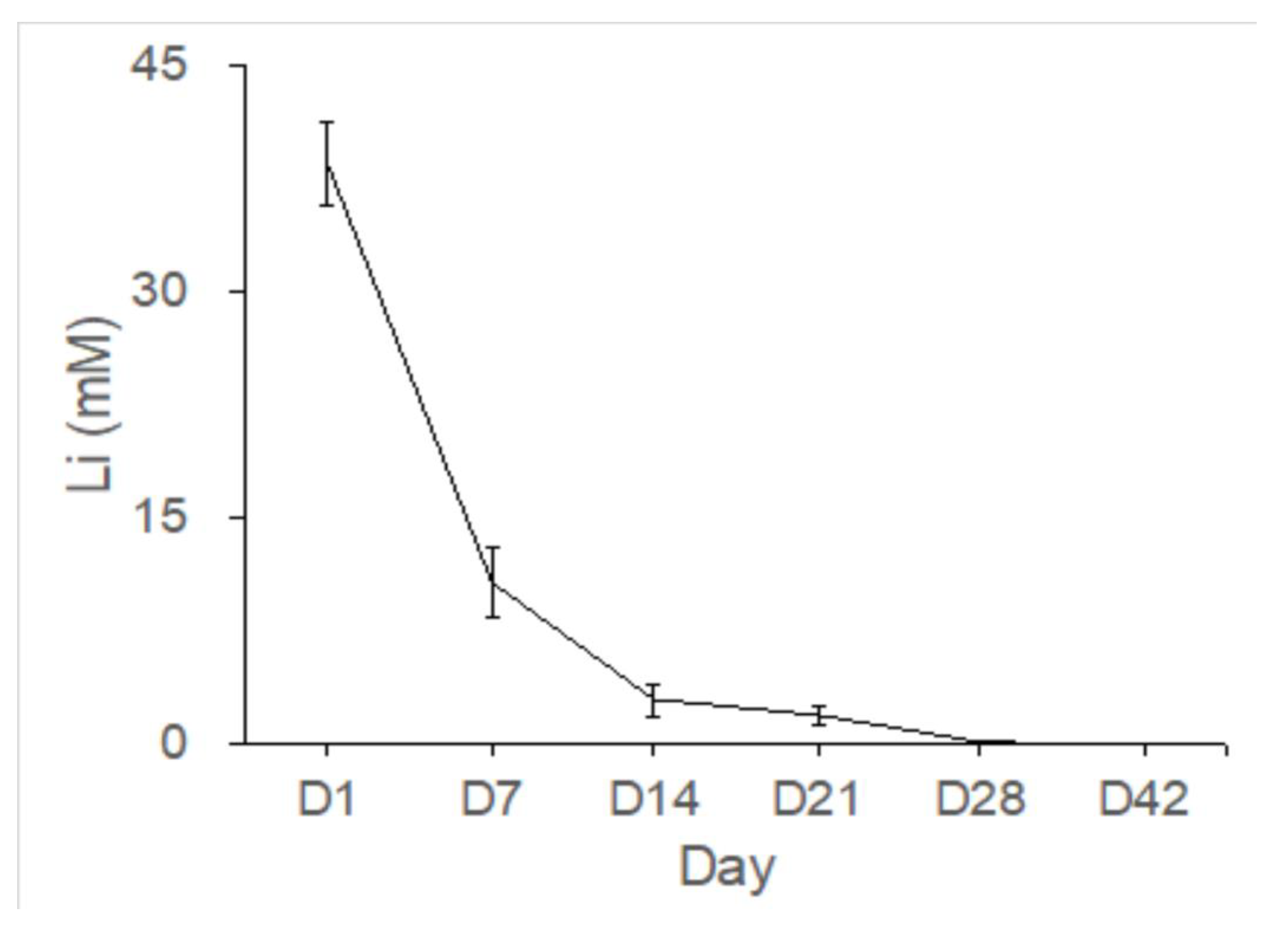
Figure 7). After implantation of the PLGA-Vancomycin-LiCl beads for 7, 14, 28, and 42 days, fluid content was aspirated from each femoral cavity and the concentration of Li (
Figure 8) and vancomycin (
Figure 9) were quantified.
Figure 8 showed the Li concentrations as measured in bone cavity tissue. Gradual release of Li
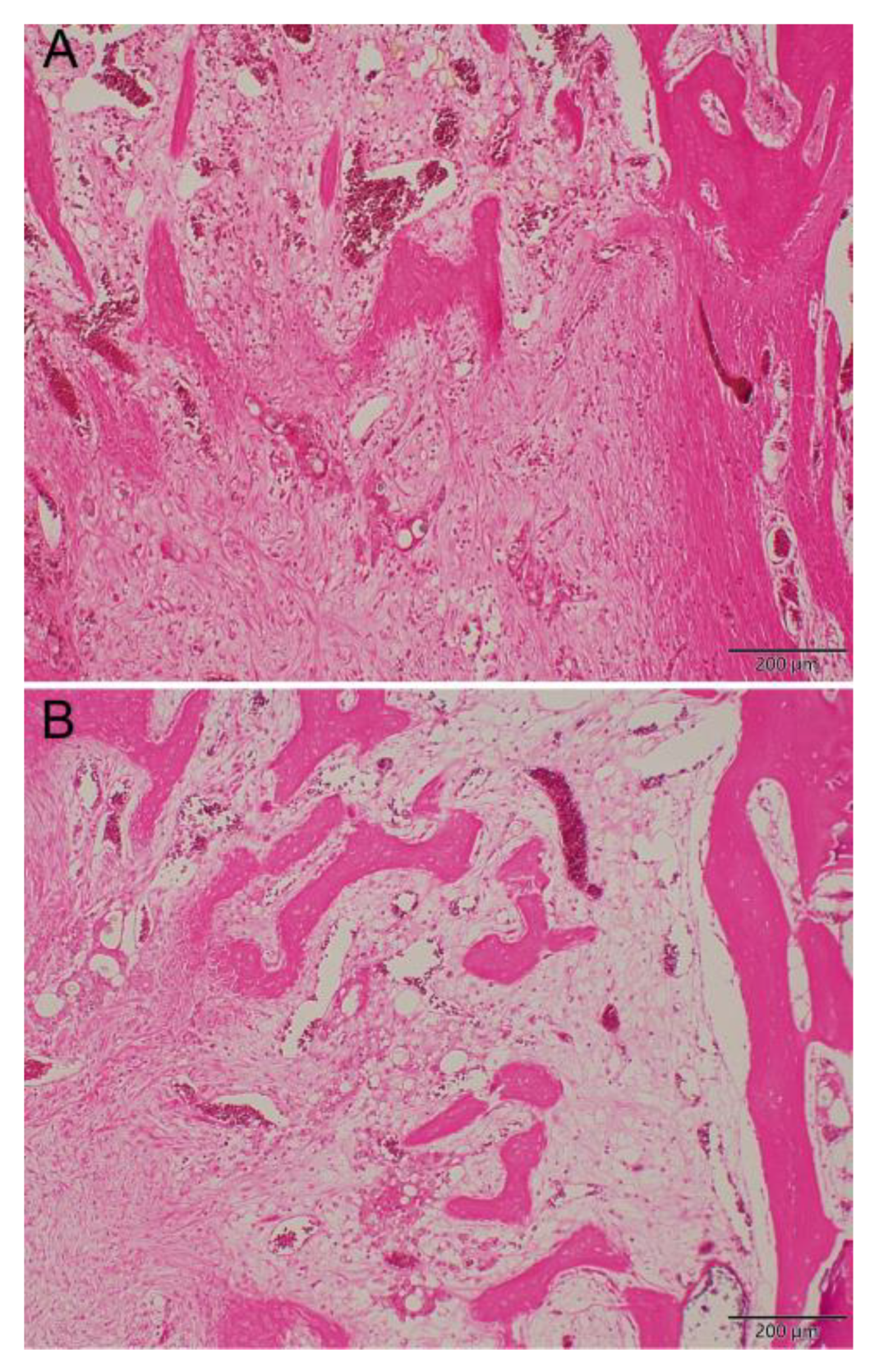
+ ion was observed from the Type IV beads for 42 days in vivo. Because Li molecular can be used as a Wnt signaling activator to promote the osteogenesis of MSCs, more mature bone tissues was shown in Li beads treated group (
Figure 10b) than non-Li beads treated group (
Figure 10a). The formerly mentioned delivery systems for vancomycin are very useful for bone reconstructive surgery [
5,
12,
27]. The localized release of a powerful antibiotic such as vancomycin at the early period following the surgery (1–6 days) can inhibit bacterial infection, thus avoiding severe complication and implant failure. However, for acute cases such as osteomyelitis; the drug delivery systems should be able to deliver therapeutic doses of antibiotic for at least 2 weeks. In the present study, long-term medication was provided by the PLGA-Li-vancomycin beads. They were able to deliver therapeutic doses of vancomycin up to six weeks in vivo (
Figure 9), thus controlling the bone infections.
In the present study, we offer a convenient method for multiple deliveries of Wnt agonists Li and antibiotics by biodegradable PLGA-Li-vancomycin beads to meet the specific antibiotic requirements for osteomyelitis patients. Although the current study has generated promising preliminary data, some limitations should be noted. We used a non-infected animal model, and therefore it is unclear whether the PLGA-Li-vancomycin beads might perform differently in infected tissue. Further evaluation of the PLGA-Li-vancomycin beads in an animal model of Staphylococcus aureus infection is necessary to address this limitation.
Author Contributions
Conceptualization, S.S.L, S.W.N. U, S.J.L; methodology, S.J.L, E.C.C, KYC; formal analysis, T.T.T, Y.S.C.,YLJ and CAC; investigation, L.J.Y, Y.S.C and CY.Y; data curation, S.S.L, CY.Y, and YJH; writing—original draft preparation, S.S.L, HYH ; writing—review and editing, S.W.N. U, S.J.L.; funding acquisition, SWN Ueng, C.C.N. All authors approved submission of the manuscript.