Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
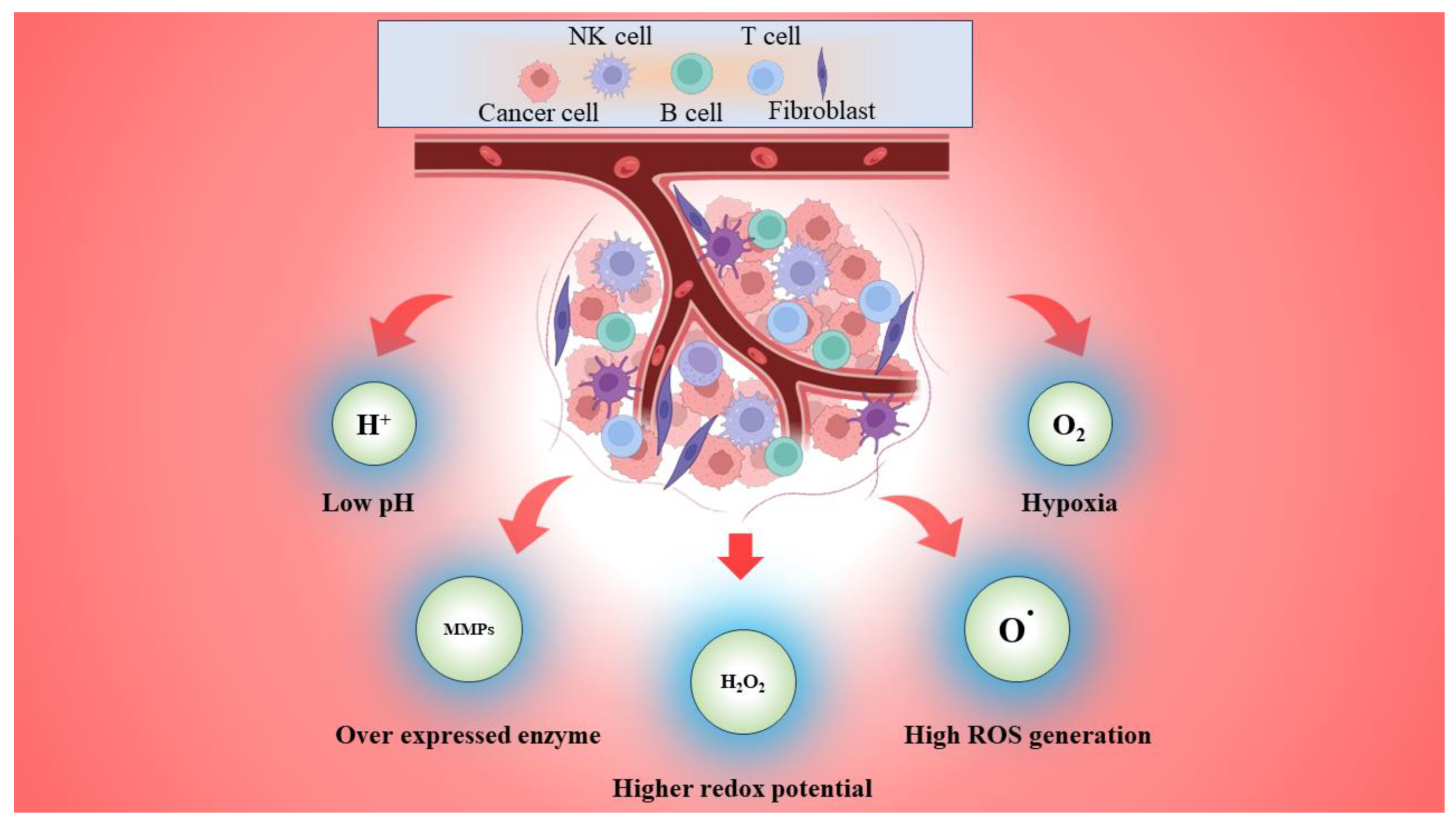
2. Tumor Microenvironment (TME)
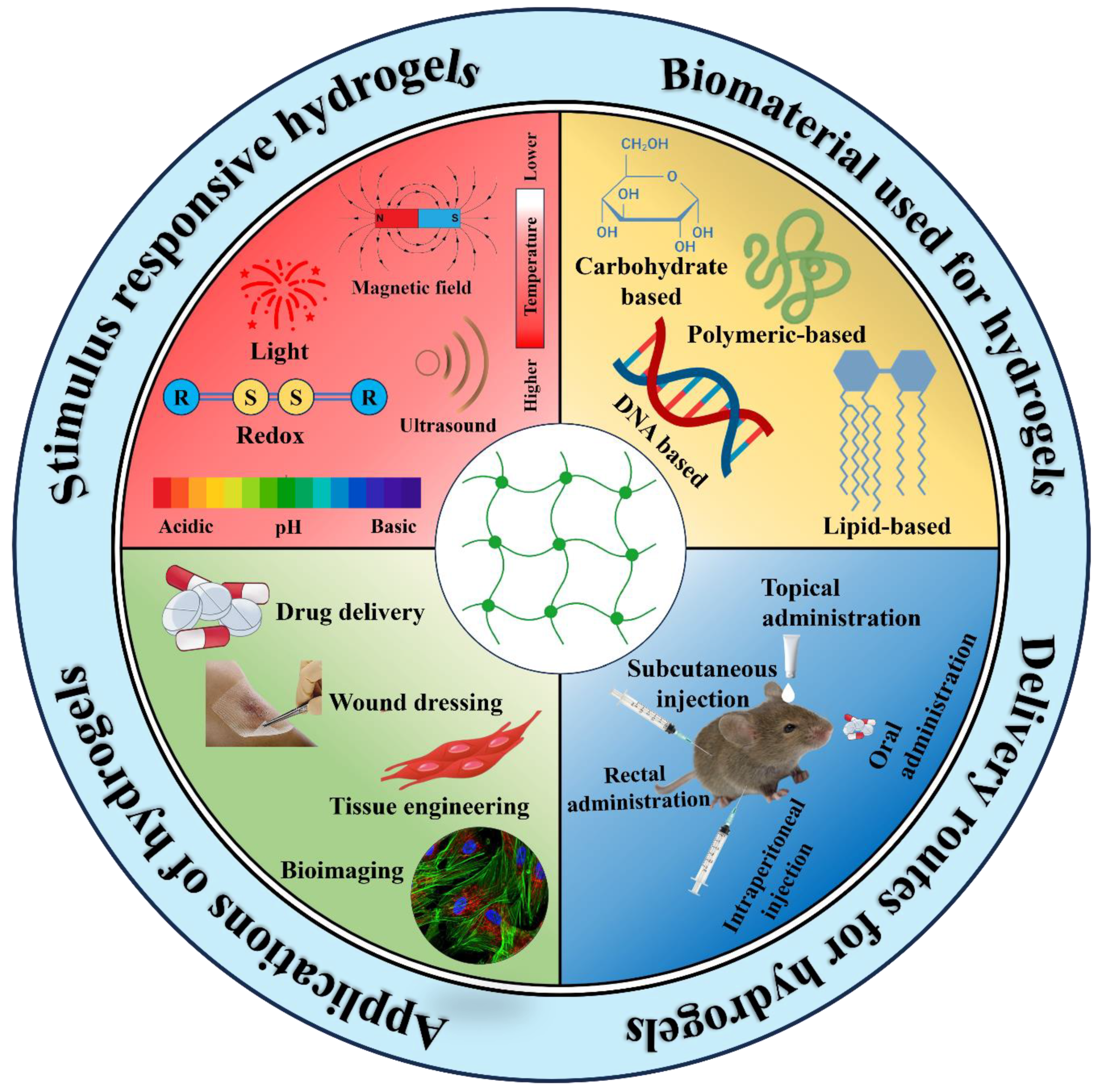
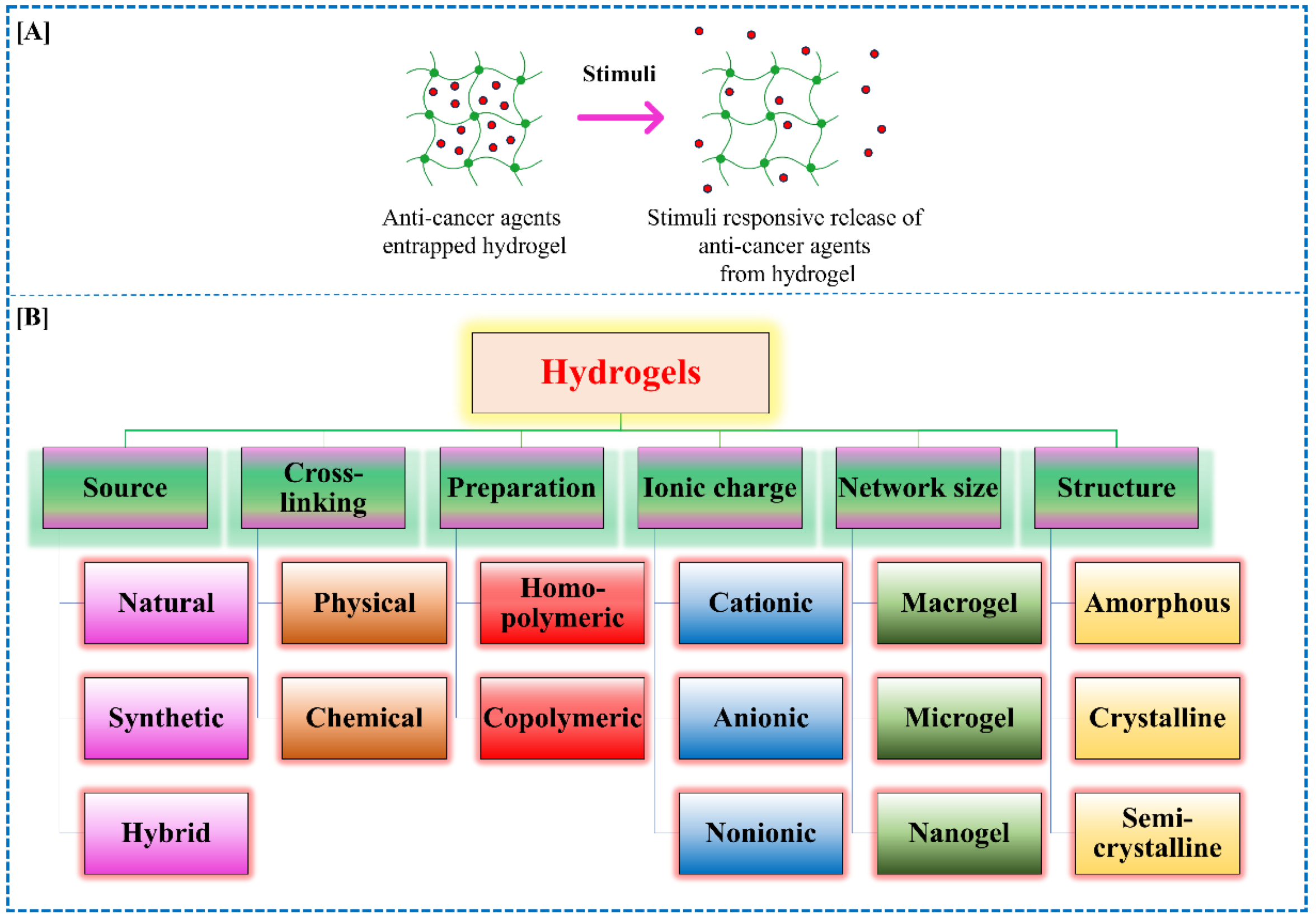
3. Hydrogels: An Overview
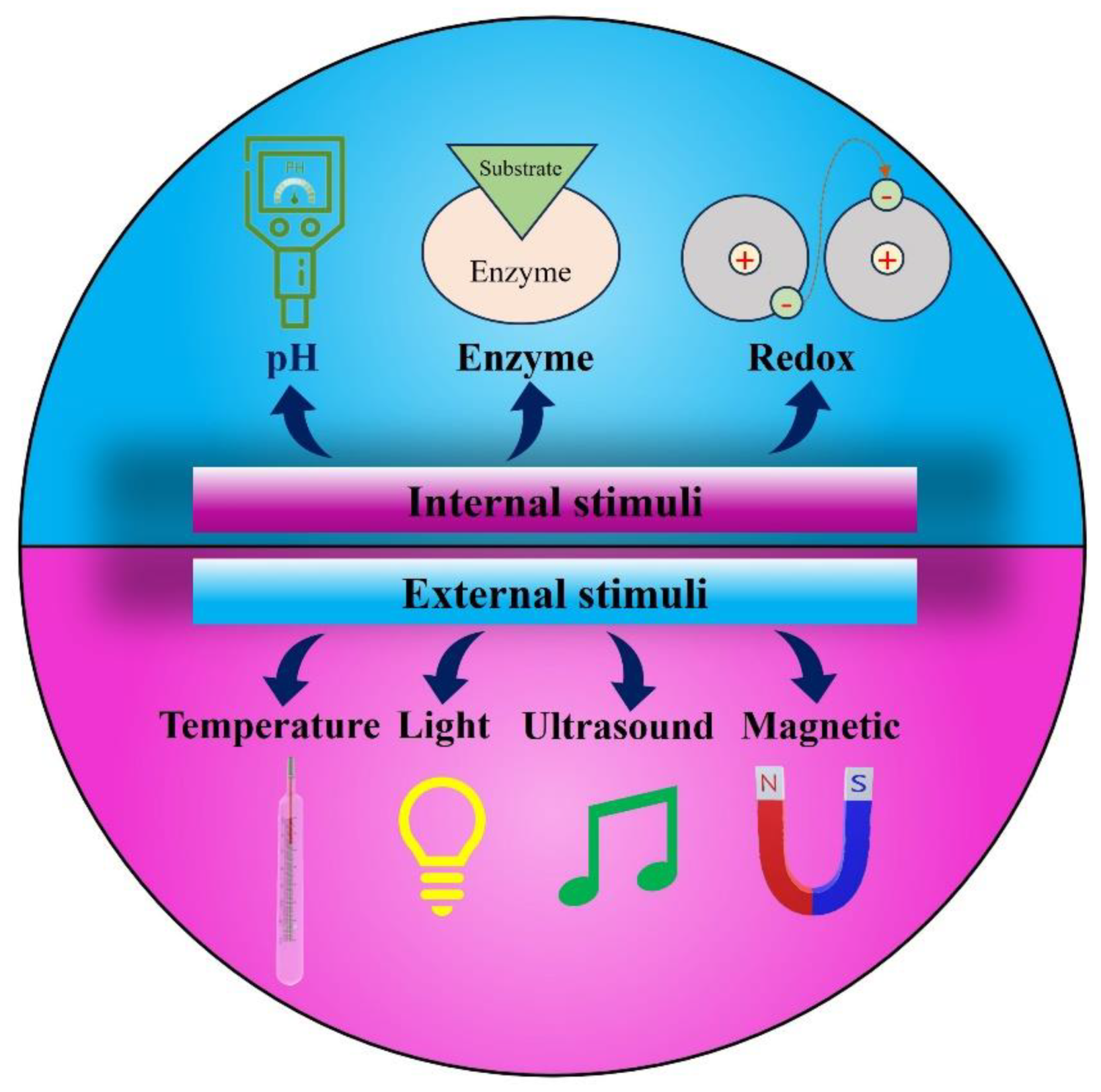
4. Stimulus Responsive Hydrogels for Cancer Therapy
4.1. Chemical Stimuli Responsive Hydrogels
4.1.1. pH Responsive Hydrogels
4.1.2. Redox Responsive Hydrogels
| Stimuli | Polymers | Drug/dye/ Ligand |
Cross-linking agent | Preparation method | Cancer/model/Route | Brief finding | References |
|---|---|---|---|---|---|---|---|
| pH | CS and PEO | Amoxicillin, Metronidazole | Glyoxal | Cross-linking | Peptic ulcer | In the acidic environment of the stomach fluid, prepared hydrogels may be helpful for the localized administration of antibiotics. | [34] |
| PAA: PEO | SAM, NAM, CHC, PDN | TDIC | Cross-linking | GI tract | The pH-dependent swelling of IPN granules in the matrix significantly determines drug release across all studied types. | [39] | |
| Gelatin: PEO | Riboflavin | Glyoxal | Cross-linking | For oral delivery | Gelatin and gelatin-PEO hydrogels swell based on pH and high molecular weight PEO. | [42] | |
| PEG and L-Lactide | DOX and TET | MA–PLLA–PEG–PLLA–MA | Cross-linking | GI tract | pH mediated drug release observed (slow release of DOX in acidic buffers as well as fast release of TET) | [71] | |
| PEG-6000 and MAA | - | MBA | Radical polymerization reaction | Male albino rabbits | The nanogels were well-tolerated with no toxic effects in animals. | [45] | |
| Gelatin and Pluronic F127 | CUR | FCHO | Schiff base cross-linking | Diabetes mellitus | Displayed antibacterial and antioxidative activity and biocompatibility, facilitated wound closure and enhancing tissue regeneration | [72] | |
| Lap®/CS/PVA | CUR | Lap®CUR | Cross-linking | Breast cancer cells (MDA-MB 231) and bacteria S. aureus, E. coli and H. pylori | Had good blood compatibility, excellent antioxidant properties, and antibacterial activity. | [51] | |
| PEI-Co-MAA | Mesalazine | MBA | Free radical polymerization | Colorectal diseases | Hydrophilic drugs may be delivered at colon site via hydrogels. | [52] | |
| Black seed extract and β-CD, MAA | Perindopril Erbumine | MBA | Free radical polymerization | - | At alkaline pH, hydrogels demonstrated more swelling and in vitro drug release compared to acidic pH, with no adverse effects observed in animals. | [53] | |
| CMTKG/ PVP/PAM |
DS | MBA | Free radical polymerization | - | The higher drug released was observed at physiological pH (pH 7.4) then acidic (pH 1.2) from hydrogel. | [73] | |
| PU−PEI and PU−CA | Ciprofloxacin, Bromophenol blue and Pyronin Y |
- | Aminolysis and Stiglich esterification mechanism, Physical cross-linking | Chronic Wounds | PU-PEI films exhibited significantly higher antibacterial activity than PU-CA films, and they discharged more cargo at an acidic pH than PU-CA films did at an alkaline pH. | [56] | |
| CMA and CS | DS | - | Ionic complexation | Dermal drug delivery, HaCat cells | The viability of HaCat cells was nearly 100% in the presence of hydrogels and DS, indicating the potential of CMA/CS PECs for pH-responsive dermal drug delivery. | [32] | |
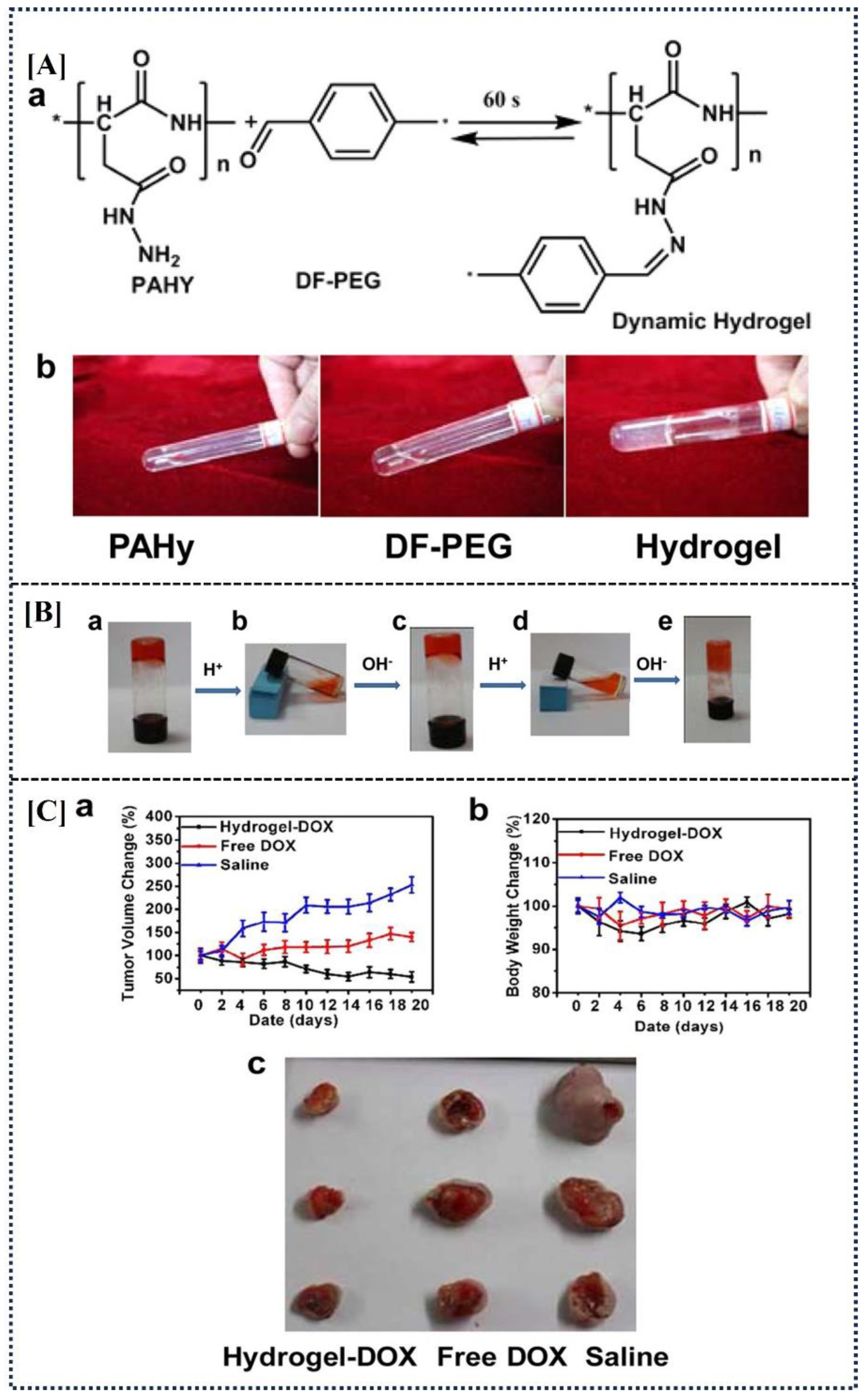
| DF-PEG, PAHy | DOX | - | Chemical cross-linking | Human fibrosarcoma | The DOX-loaded hydrogel exhibited enhanced efficacy, achieving approximately 80% tumor inhibition by day 20, suggesting its potential as a highly effective treatment for human fibrosarcoma | [33] |
|
| Redox | PEG | PDS, BSA | - | - | L929 mouse fibroblast | When hydrogels are treated with thiol-containing reducing agents, they break down quickly, facilitating the release of the encapsulated payload (such as BSA) more quickly. | [21] |
| PEG-SH and Fe-EDTA | Dextran | DVS | One-pot cross-linking | NIH/3T3 mouse fibroblasts | These gels offer a potentially useful platform for separating the behaviour of degradation in response to reduction stimuli from the initial mechanical properties. | [58] | |
| Resilin | RGD | DTSSP | - | NIH/3T3 fibroblasts | Demonstrated the degradation and cytocompatibility of DTSSP-crosslinked RZ10-RGD, showcasing their potential for biomedical appications | [67] |
1.2. Biological Stimuli Responsive Hydrogels
4.2.1. Enzyme Responsive Hydrogels
4.2.2. Glucose Responsive Hydrogels
| Stimuli | Polymers | Drug/dye/ ligand |
Cross-linking agent | Preparation method | Cancer/model/Route | Brief finding | References |
|---|---|---|---|---|---|---|---|
| Enzyme (HRP) | Alginate and tyramine | Phenol | HRP/H2O2 | Enzymatic cross-linking | - | Findings demonstrate the viability of a unique synthesised alginate with phenols as an alternate material to typical unmodified alginates. | [80] |
| Enzyme (GOx) | N-hydroxyimide–heparin | DOX | EDC/NHS, GOx |
Radical polymerization reaction | HeLa, HepG2, and NIH-3T3 cells | Drug is released from hydrogel in the enzyme responsive manner | [81] |
| Enzyme (MMP) | Tm | TMZ, BG | - | - | C6 glioma cells, BALB/c nude male mice, orthotropic glioma model | Hydrogels reduced MGMT expression in vivo, rendering TMZ-resistant glioma cells more responsive to TMZ treatment. Additionally, post-surgery, these hydrogels significantly enhanced TMZ efficacy in glioma growth inhibition and reduced recurrence of TMZ-resistant gliomas | [82] |
| Enzyme (β-gal) | PLGA−PEG−PLGA | 5-FU, LAPONITE | 2-ethyl-hexanoate as a catalyst | Bulk ring-opening co-polymerization | MCF-7, female nude mice (ICR-nu/nu), PC-3, | Prodrug 5-FU−β-gal and nanocomposite gels were injected locally once, and the combination had long-lasting anticancer activity in vivo with no side effects. | [99] |
| Enzyme (MMP) | dPG | DOX | peptide | Nano-precipitation | HeLa cells, MCTS, primary fibroblasts | The digested multistage pNGs demonstrated enhanced diffusive transport through a dense gel matrix, pNGs facilitates the infiltration of functional chemotherapeutic medication into deeper tissue regions in tumor-like MCTS | [88] |
| Enzyme (MMP) | PEG | DOX, MIONPs, RGDS | - | Surface-initiated photopolymerization | HeLa cells, Mouse fibroblast | Targeted nanocarriers highly internalized and efficiently carry and release DOX into the nucleus of HeLa cells within 2 hours. | [87] |
| Enzyme (dextranases) | Dextran | - | HDI or DDI | - | SD rats, human colonic ferme-ntation model | Dextran hydrogels were degraded in vitro by a model dextranase, as well as in vivo in rats and a human colonic fermentation model. | [89] |
| Glucose | PBA and glucose | PBA | AIBN as an initiator | Radical polymerization | Insulin delivery | Mechanism was not studied but could be used for insulin delivery | [94] |
| Glucose | IA-0 peptide | Gox, Catal-ase, Insulin | - | Solid phase method | STZ-induced diabetic mice | In vitro and in vivo studies demonstrated that the developed hydrogels regulate blood glucose levels. | [98] |
4.3. Physical Stimuli Responsive Hydrogels
4.3.1. Thermo-Responsive Hydrogels
| Stimuli | Polymers | Drug/dye/ Ligand |
Cross-linking agent | Preparation method | Cancer/model/Route | Brief finding | References |
|---|---|---|---|---|---|---|---|
| Thermo | Chitosan | DSF | - | Physical cross-linking | SMMC-7721 cells | High biocompatibility hydrogels that quickly gelled at body temperature and showed dose-dependently greater cytotoxicity compared to the free DSF solution may be given at room temperature. | [113] |
| PLA-PEG-PLA | CTX and CpG-ODN | - | - | CT26 cells, CT26 bearing mice | The outcomes demonstrate that this combined approach decreases CTX toxicity while generating a cytotoxic T cell response that efficiently suppresses tumour growth, extends survival, and significantly increases the tumour cure rate. | [118] | |
| L-lysine and L-alanine- based diblock copolypeptide |
PTX | - | - | Glioblastoma (HK308 cells) | Hydrogel loaded with paclitaxel caused less cellular inflammation, tissue damage, and reactive astrocytes than either hydrogel or cremaphor-taxol (the usual taxol-carrier), In-vivo studies suggest local tumor control and improved survival. | [115] | |
| HPCS and F127-CHO | ICG and BSA, CaO2 NPs, Bi2S3 | - | Schiff-base linking | L929 cells, 4 T1 cells, BALB/c nude mice | ICG@CaO2-BSA nanoparticles' CaO2 breaks down in the TME to produce Ca2+ and H2O2. In addition, ICG produces ROS when exposed to NIR radiation. Furthermore, when Bi2S3 nanorods and ICG are exposed to near-infrared radiation, they produce a photothermal effect that raises the temperature of tumour tissues, which helps to precisely destroy tumour cells. | [119] | |
| MXene nanosheets | DOX, FeCl2 solution, Gellan gum | - | Physical cross linking | A549 and L-929 cells |
MXene@GG demonstrates superior photothermal properties and precise drug release control. Additionally, cell studies confirm MXene@GG's high biocompatibility and the sustained anticancer efficacy of DOX. | [114] | |
| CS-g-PNIPAM | GO-CET/CPT11 and shRNA | NIPAM and MAA | Free radical polymerization | U87 cells (Glioblastoma), 3T3 fibroblasts, BALB/c nude mice |
In vitro studies suggested cell apoptosis, reduced SLP2 protein expression and inhibited cell migration. In vivo studies confirmed 40% tumor size compared with the untreated control group after 12 days | [116] | |
| PDLLA-PEG-PDLLA | BVZ and DOX | Stannous octoate as catalyst | Ring-opening polymerization | HaCaT and HeLa cells, Hela xeno-graft nude | In vitro studies showed negligible cytotoxicity on HeLa and HaCaT, In vivo studies suggest that hydrogel co-loaded with BVZ and DOX effectively suppressed tumors for 36 days post single intratumoral injection, with no harm to vital organs. | [120] | |
| Alginate grafted PNIPAM | DOX | EDC, NHS, MES buffer | ATRP | AT3B-1 cells | DOX gradually release from hydrogel, enhanced cellular uptake, good biocompatibility and increased efficacy in cancer cell death. | [117] | |
| Light | Azobenzene and α-CD functionalized HA | MSNs-AuNBs, DOX | NIR radiation | In situ self-assembly | HaCaT and SCC, MCS | Upregulation of hyaluronidase (HAase) near the tumour tissue will cause hydrogel HA degradation and the release the drug from hydrogel, which could take up by tumour cells and deliver drug to cell nuclei. | [121] |
| CSMA | Gnp substrate, LAP | 405 nm laser | - | MCF-7, HepG2, Hela, healthy and cancer patient’s blood | Study indicates that the isolation platform had acceptable biocompatibility and had isolated the selected cells successfully. This light-responsive hydrogel has better potential for use in clinical applications. | [122] | |
| Ti3C2 MXene/ Cellulose | DOX | ECH | Chemical cross-linking | HepA1-6, SMMC-7721, HepG2, U-118MG and U-251 MG cells, BALB/c or C57BL/6 mice | The results showed the promise of the nanoplatform for use in cancer therapy by demonstrating the combination of PTT and adjuvant chemotherapy delivered via this nanoplatform destroyed tumours instantly and prevented tumour relapse. Notably, DOX released from hydrogel and have excellent photothermal action. | [123] | |
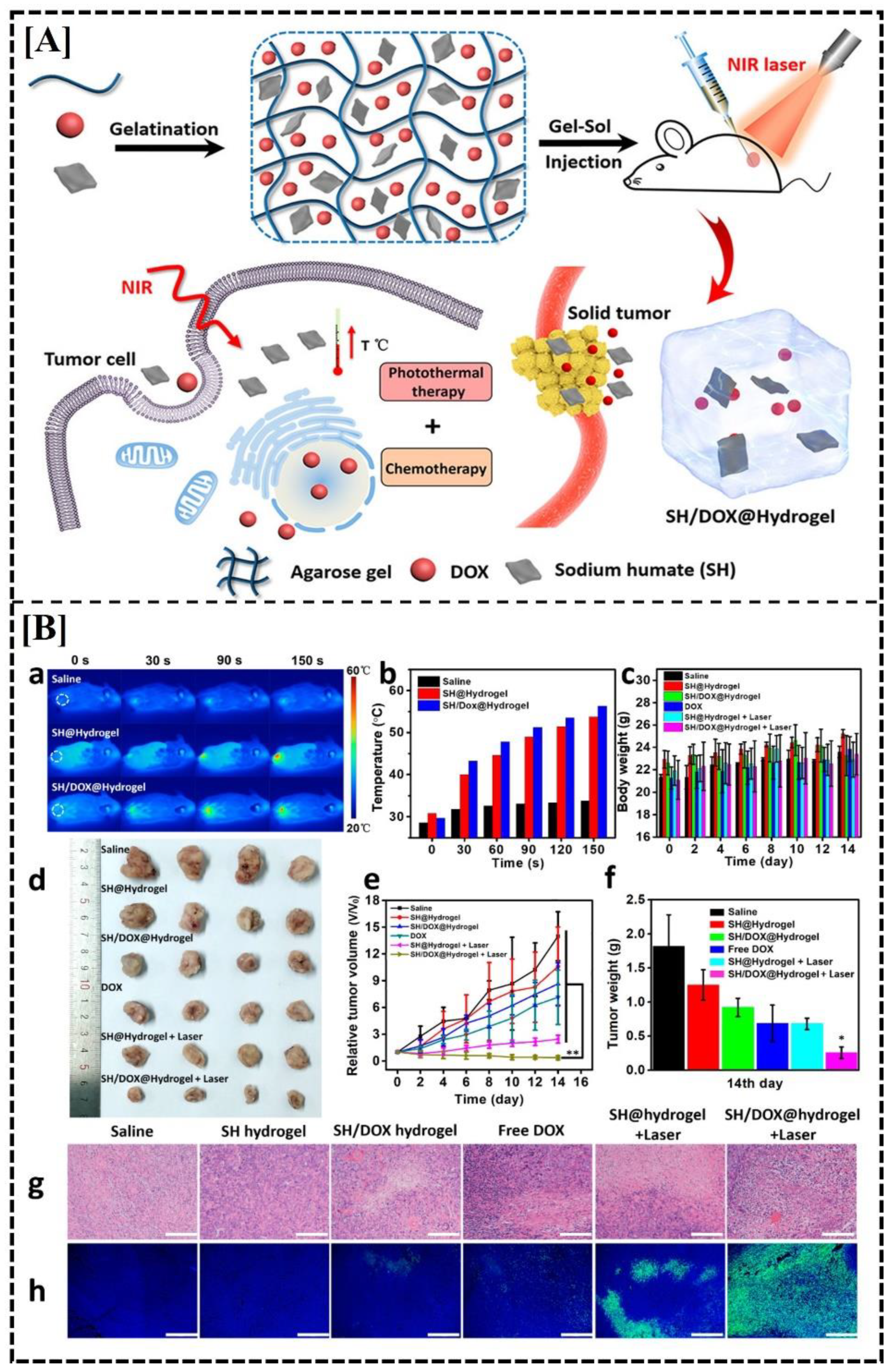
| Humic acid/ Agarose |
SH and DOX | - | Physical cross-linking | 4T1 cells, 4T1 tumor-bearing BALB/c mice | In vivo studies suggest improved antineoplastic efficacy of free drugs in tumoral tissues as compared to the local distribution of free drugs | [124] | |
| MC | MSNs, DOX | - | - | 3T3 mouse fibroblast and Cal27 human OSCC, female BALB/c mice | Chemotherapy and phototherapy together produced a less toxic, long-lasting synergistic anti-tumor impact both in vitro and in vivo. | [125] |
4.3.2. Light Responsive Hydrogels
4.4. Multi-Responsive Hydrogels
| Stimuli | Polymers | Drug/dye/ Ligand |
Cross-linking agent | Preparation method | Cancer/disease model/Route | Brief finding | References |
|---|---|---|---|---|---|---|---|
| Thermo-and pH-sensitive | PNVCL, Vim, PVP | 5-FU | MBA | Free radical polymerization | Neoplastic cells | Hydrogels of P(NVCL-co-VIm)/PVP across varied pH and temperature conditions offer promise for targeted drug delivery applications. | [142] |
| Thermo-and pH-sensitive | PNIAAm-co-IA and CS | DOX | GP | Free radical polymerization | Breast cancer (MCF-7 cells) | Lower concentrations in an acidic environment (37°C) demonstrated faster DOX release than a neutral pH and 40°C. The hydrogels are cytocompatible and have negligible or no cytotoxicity, according to the cytotoxicity analysis. | [143] |
| Thermo- and pH-responsive | PGA and PNH | Lysozyme | Carbodiimide | Radical polymerization, Ring-opening polymerization |
- | The hydrogel's potential as a smart drug carrier is highlighted by the quicker rates of lysozyme release at pH 7.4 along with decreased crosslinking density and PNH content. At pH 4.0, the release of lysozyme is slowed down due to protonation of the PGA portion. | [144] |
| pH and glucose | PEGS-PBA-BA and CS-DA-LAG | rGO@PDA and Metformin | - | Double dynamic bond of Schiff-base and phenylboronate ester | Type II diabetic foot wounds | With their increased adhesion, stimuli-responsive metformin release, and self-healing properties, PC hydrogels have shown effective in helping chronic athletic diabetic foot wounds recover. | [95] |
| pH and ROS responsive | POD, CE | DS and MF | Groups from POD and CE | Schiff base linkages and boronic ester bonds | Chronic diabetic wound | Results both in vitro and in vivo showed anti-infection, anti-oxidation, and anti-inflammatory effects at first, which were followed by enhanced angiogenesis and faster wound healing. | [138] |
| Thermo- and pH-responsive | PCLA | DOX-pH-GA, BA |
- | Covalent cross-linking | Hepatocellular carcinoma | The in vivo investigation demonstrated the efficacious inhibition of tumour growth by the DOX-releasing hydrogel depot. These results demonstrate the pH-responsive hydrogel's intriguing potential for localised anticancer therapy. | [145] |
| pH and Enzyme | HEMA and MAA | 5-FU | OLZ-AC | Radical copolymerization | HCT116 colon cells, rat colonic fluid | Local 5-FU release occurs at the colon location, and high 5-FU concentrations overcome cancer therapy resistance by promoting necroptosis in colon cancer cells. | [146] |
| Thermo and Enzyme | PEG, MMP peptide | DOX, TSLs, | Michael-type reaction responsible for cross linking | Thiol-maleimide reaction, chemical crosslinking | AoAF and NIH3T3 cells | Investigations into in-situ drug delivery and degradation demonstrate that TSL-gel reacts to local environmental factors such as temperature and enzymatic stimulation. | [147] |
5. Limitations/Disadvantages of Hydrogels
6. Conclusion and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global Cancer Statistics 2022 GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J C.
- Zhu, Y.; Jia, H.; Duan, Q.; Wu, F. Nanomedicines for Combating Multidrug Resistance of Cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2021, 13, e1715. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; ur Rehman, A.; ud Din, F. Nanotechnology: From in Vivo Imaging System to Controlled Drug Delivery. Nanoscale Res Lett 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Shankar, A.; Modi, U.; Patel, S. New Insights from Nanotechnology in SARS-CoV-2 Detection, Treatment Strategy and Prevention. Mater Today Chem 2023, 101478. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Jodha, B.; Prabina, K.E.; Aggarwal, N.; Patel, S. Recent Advances in Phytochemical Based Nano-Drug Delivery Systems to Combat Breast Cancer: A Review. J Drug Deliv Sci Technol 2022, 103832. [Google Scholar] [CrossRef]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive Nanotherapeutics for Precision Drug Delivery and Cancer Therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2019, 11, e1527. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, A.; Kataria, B.; Gupta, D. Nanoparticle-Based Methodologies for Targeted Drug Delivery—an Insight. Journal of Nanoparticle Research 2021, 23, 87. [Google Scholar] [CrossRef]
- Niazi, M.; Alizadeh, E.; Zarebkohan, A.; Seidi, K.; Ayoubi-Joshaghani, M.H.; Azizi, M.; Dadashi, H.; Mahmudi, H.; Javaheri, T.; Jaymand, M. Advanced Bioresponsive Multitasking Hydrogels in the New Era of Biomedicine. Adv Funct Mater 2021, 31, 2104123. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, S. Protein Nanocarriers for the Delivery of Phytoconstituents. In Nanotechnology Based Delivery of Phytoconstituents and Cosmeceuticals; Pooja, D., Kulhari, H., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 229–264. ISBN 978-981-99-5314-1. [Google Scholar]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Current Biology 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Truffi, M.; Sorrentino, L.; Corsi, F. Fibroblasts in the Tumor Microenvironment. Tumor Microenvironment: Non-Hematopoietic Cells 2020, 15–29. [Google Scholar]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Tumor Microenvironment-enabled Nanotherapy. Adv Healthc Mater 2018, 7, 1701156. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor Microenvironment-Responsive Intelligent Nanoplatforms for Cancer Theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Advanced materials 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv Drug Deliv Rev 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New Progress and Prospects: The Application of Nanogel in Drug Delivery. Materials Science and Engineering: C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Kilic Boz, R.; Aydin, D.; Kocak, S.; Golba, B.; Sanyal, R.; Sanyal, A. Redox-Responsive Hydrogels for Tunable and “On-Demand” Release of Biomacromolecules. Bioconjug Chem 2022, 33, 839–847. [Google Scholar] [CrossRef]
- Municoy, S.; Alvarez Echazu, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M. V; Alvarez, G.S.; Hardy, J.G. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int J Mol Sci 2020, 21, 4724. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv Drug Deliv Rev 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Redox-Responsive Nano-Carriers as Tumor-Targeted Drug Delivery Systems. Eur J Med Chem 2018, 157, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers (Basel) 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and Application of PH-Responsive Drug Delivery Systems. Journal of Controlled Release 2022, 348, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Khan, R.A.; Choonara, Y.E. Design, Preparation, and Functionalization of Nanobiomaterials for Enhanced Efficacy in Current and Future Biomedical Applications. Nanotechnol Rev 2022, 11, 1802–1826. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Li, C.; Sang, S.; Chen, L.; Long, J.; Qiu, C.; Jin, Z. Targeted Delivery of Hydrogels in Human Gastrointestinal Tract: A Review. Food Hydrocoll 2023, 134, 108013. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv Drug Deliv Rev 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Jin, S.; Chen, Y. Synthesis and Swelling Properties of PH-sensitive Hydrogels Based on Chitosan and Poly (Methacrylic Acid) Semi-interpenetrating Polymer Network. J Appl Polym Sci 2005, 98, 1720–1726. [Google Scholar] [CrossRef]
- Solanki, R.; Rajput, P.K.; Jodha, B.; Yadav, U.C.S.; Patel, S. Enhancing Apoptosis-Mediated Anticancer Activity of Evodiamine through Protein-Based Nanoparticles in Breast Cancer Cells. Sci Rep 2024, 14, 2595. [Google Scholar] [CrossRef]
- Ortiz, J.A.; Sepúlveda, F.A.; Panadero-Medianero, C.; Murgas, P.; Ahumada, M.; Palza, H.; Matsuhiro, B.; Zapata, P.A. Cytocompatible Drug Delivery Hydrogels Based on Carboxymethylagarose/Chitosan PH-Responsive Polyelectrolyte Complexes. Int J Biol Macromol 2022, 199, 96–107. [Google Scholar] [CrossRef]
- Li, L.; Gu, J.; Zhang, J.; Xie, Z.; Lu, Y.; Shen, L.; Dong, Q.; Wang, Y. Injectable and Biodegradable PH-Responsive Hydrogels for Localized and Sustained Treatment of Human Fibrosarcoma. ACS Appl Mater Interfaces 2015, 7, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Amiji, M.M. Preparation and Characterization of Freeze-Dried Chitosan-Poly (Ethylene Oxide) Hydrogels for Site-Specific Antibiotic Delivery in the Stomach. Pharm Res 1996, 13, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wirsen, A.; Albertsson, A.-C. Novel PH-Sensitive Chitosan Hydrogels: Swelling Behavior and States of Water. Polymer (Guildf) 2000, 41, 4589–4598. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. PH-Responsive Self-Healing Injectable Hydrogel Based on N-Carboxyethyl Chitosan for Hepatocellular Carcinoma Therapy. Acta Biomater 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.Y.; Abdel-Razik, E.A.; Abdel-Bary, E.M.; El-Sherbiny, I.M. Chitosan-based Interpolymeric PH-responsive Hydrogels for in Vitro Drug Release. J Appl Polym Sci 2007, 103, 2864–2874. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Heras, A.; Aranaz, I.; Galed, G.; Fernández-Valle, M.E.; Argüelles-Monal, W. Effect of Chemical Crosslinking on the Swelling and Shrinking Properties of Thermal and PH-responsive Chitosan Hydrogels. Macromol Biosci 2003, 3, 612–619. [Google Scholar] [CrossRef]
- Bilia, A.; Carelli, V.; Di Colo, G.; Nannipieri, E. In Vitro Evaluation of a PH-Sensitive Hydrogel for Control of GI Drug Delivery from Silicone-Based Matrices. Int J Pharm 1996, 130, 83–92. [Google Scholar] [CrossRef]
- Monir, T.S.B.; Afroz, S.; Khan, R.A.; Miah, M.Y.; Takafuji, M.; Alam, M.A. PH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic Acid by Gamma Radiation. Journal of Composites Science 2019, 3, 58. [Google Scholar] [CrossRef]
- Nho, Y.C.; Lim, Y.M.; Lee, Y.M. Preparation, Properties and Biological Application of PH-Sensitive Poly (Ethylene Oxide)(PEO) Hydrogels Grafted with Acrylic Acid (AAc) Using Gamma-Ray Irradiation. Radiation Physics and Chemistry 2004, 71, 239–242. [Google Scholar] [CrossRef]
- Amiji, M.; Tailor, R.; Ly, M.-K.; Goreham, J. Gelatin-Poly (Ethylene Oxide) Semi-Interpenetrating Polymer Network with PH-Sensitive Swelling and Enzyme-Degradable Properties for Oral Drug Delivery. Drug Dev Ind Pharm 1997, 23, 575–582. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Guilherme, M.R.; Lima-Tenório, M.K.; Scariot, D.B.; Nakamura, C. V; Rubira, A.F.; Kunita, M.H. Synthesis and Characterization of a PH-Responsive Poly (Ethylene Glycol)-Based Hydrogel: Acid Degradation, Equilibrium Swelling, and Absorption Kinetic Characteristics. Colloid Polym Sci 2015, 293, 3611–3622. [Google Scholar] [CrossRef]
- Wang, S.; Attah, R.; Li, J.; Chen, Y.; Chen, R. A PH-Responsive Amphiphilic Hydrogel Based on Pseudopeptides and Poly (Ethylene Glycol) for Oral Delivery of Hydrophobic Drugs. ACS Biomater Sci Eng 2018, 4, 4236–4243. [Google Scholar] [CrossRef]
- Mahmood, T.; Sarfraz, R.M.; Mahmood, A.; Salem-Bekhit, M.M.; Ijaz, H.; Zaman, M.; Akram, M.R.; Taha, E.I.; Sahu, R.K.; Benguerba, Y. Preparation, In Vitro Characterization, and Evaluation of Polymeric PH-Responsive Hydrogels for Controlled Drug Release. ACS Omega 2024. [Google Scholar] [CrossRef] [PubMed]
- Halacheva, S.S.; Freemont, T.J.; Saunders, B.R. PH-Responsive Physical Gels from Poly (Meth) Acrylic Acid-Containing Crosslinked Particles: The Relationship between Structure and Mechanical Properties. J Mater Chem B 2013, 1, 4065–4078. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, N.B.; Milašinović, N.Z.; Popović, I.G.; Filipović, J.M.; Kalagasidis Krušić, M.T. Preparation and Characterization of PH-sensitive Hydrogels Based on Chitosan, Itaconic Acid and Methacrylic Acid. Polym Int 2011, 60, 443–452. [Google Scholar] [CrossRef]
- Frutos, G.; Prior-Cabanillas, A.; París, R.; Quijada-Garrido, I. A Novel Controlled Drug Delivery System Based on PH-Responsive Hydrogels Included in Soft Gelatin Capsules. Acta Biomater 2010, 6, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, B. Mucoadhesive and PH-Responsive Behavior of Gelatin Containing Hydrogels for Protein Drug Delivery Applications. Korea-Australia Rheology Journal 2020, 32, 41–46. [Google Scholar] [CrossRef]
- Hussain, K.; Aslam, Z.; Ullah, S.; Shah, M.R. Synthesis of PH Responsive, Photocrosslinked Gelatin-Based Hydrogel System for Control Release of Ceftriaxone. Chem Phys Lipids 2021, 238, 105101. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Namazi, H. PH-Sensitive Biosystem Based on Laponite RD/Chitosan/Polyvinyl Alcohol Hydrogels for Controlled Delivery of Curcumin to Breast Cancer Cells. Colloids Surf B Biointerfaces 2023, 231, 113585. [Google Scholar] [CrossRef]
- Asad, S.; Khan, S.A.; Ullah, K.; Mannan, A.; Murtaza, G. Synthesis, Characterization, and in-Vitro Evaluation of PH-Responsive PEI-MAA Polymeric Matrices Decorated with Mesalazine for Colonic Delivery. J Drug Deliv Sci Technol 2023, 88, 104926. [Google Scholar] [CrossRef]
- Noor, F.; Mahmood, A.; Zafar, N.; Sarfraz, R.M.; Rehman, U.; Ijaz, H.; Hussain, Z.; Ahmed, I.A.; Imam, M.T.; Al Abdulmonem, W. Fabrication of PH-Responsive Hydrogels of Perindopril Erbumine Using Black Seed Extract and β-Cyclodextrin Co-Polymerized with Methacrylic Acid and Methylene Bisacrylamide. J Drug Deliv Sci Technol 2023, 88, 104924. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Z.; Li, X.; Qiu, S.; Lei, J.; Li, D.; Mu, C.; Ge, L. Functionalization of an Injectable Self-Healing PH-Responsive Hydrogel by Incorporating a Curcumin/Polymerized β-Cyclodextrin Inclusion Complex for Selective Toxicity to Osteosarcoma. ACS Appl Polym Mater 2022, 4, 1243–1254. [Google Scholar] [CrossRef]
- Yang, X.; Kim, J. Β-Cyclodextrin Hydrogels Containing Naphthaleneacetic Acid for PH-sensitive Release. Biotechnol Bioeng 2010, 106, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Crago, M.; Schofield, T.; Zeng, H.; Vyas, H.K.N.; Müllner, M.; Mai-Prochnow, A.; Farajikhah, S.; Naficy, S.; Dehghani, F. Synthesis and Evaluation of Functionalized Polyurethanes for PH-Responsive Delivery of Compounds in Chronic Wounds. Gels 2023, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Fukino, T.; Yamagishi, H.; Aida, T. Redox-responsive Molecular Systems and Materials. Advanced Materials 2017, 29, 1603888. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Battistoni, C.M.; Liu, J.C. Redox-Responsive Hydrogels with Decoupled Initial Stiffness and Degradation. Biomacromolecules 2021, 22, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chemistry of Materials 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Poole, L.B. The Basics of Thiols and Cysteines in Redox Biology and Chemistry. Free Radic Biol Med 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Asai, D.; Xu, D.; Liu, W.; Quiroz, F.G.; Callahan, D.J.; Zalutsky, M.R.; Craig, S.L.; Chilkoti, A. Protein Polymer Hydrogels by in Situ, Rapid and Reversible Self-Gelation. Biomaterials 2012, 33, 5451–5458. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Cao, L.; Chen, R.; Tang, L.; Liu, C. Injectable and Redox-Responsive Hydrogel with Adaptive Degradation Rate for Bone Regeneration. J Mater Chem B 2014, 2, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Annis, I.; Barany, G. Disulfide Bond Formation in Peptides. Curr Protoc Protein Sci 2001, 23, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Sessler, J.L.; Kim, J.S. Disulfide-Based Multifunctional Conjugates for Targeted Theranostic Drug Delivery. Acc Chem Res 2015, 48, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.M.N.; Keshavarz, T. Bioinspired Polymeric Carriers for Drug Delivery Applications. In Stimuli responsive polymeric nanocarriers for drug delivery applications, volume 1; Elsevier, 2018; pp. 377–404. [Google Scholar]
- Su, R.S.; Galas Jr, R.J.; Lin, C.; Liu, J.C. Redox-responsive Resilin-like Hydrogels for Tissue Engineering and Drug Delivery Applications. Macromol Biosci 2019, 19, 1900122. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible Light-Induced Crosslinking and Physiological Stabilization of Diselenide-Rich Nanoparticles for Redox-Responsive Drug Release and Combination Chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lei, M.; Yan, L.; An, F. Diselenide-Crosslinked Zwitterionic Nanogels with Dual Redox-Labile Properties for Controlled Drug Release. Polym Chem 2020, 11, 2360–2369. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Lu, D.-Q.; Chen, L.; Yang, R.; Liu, D.; Zhang, B. Diselenide-Crosslinked Carboxymethyl Chitosan Nanoparticles for Doxorubicin Delivery: Preparation and in Vivo Evaluation. Carbohydr Polym 2022, 292, 119699. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable PH-Responsive Hydrogels for Controlled Dual-Drug Release. J Mater Chem B 2018, 6, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cheng, X.; Xiu, J.; Liu, M.; Liu, S.; Zhang, B.; Miao, Q.; Cun, D.; Yang, C.; Li, K. PH-Responsive Injectable Multifunctional Pluronic F127/Gelatin-Based Hydrogels with Hydrogen Production for Treating Diabetic Wounds. ACS Appl Mater Interfaces 2023, 15, 55392–55408. [Google Scholar] [CrossRef]
- Meena, P.; Singh, P.; Warkar, S.G. Development and Assessment of Carboxymethyl Tamarind Kernel Gum-Based PH-Responsive Hydrogel for Release of Diclofenac Sodium. Eur Polym J 2023, 197, 112340. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T. Stimuli-Responsive Hydrogels for Manipulation of Cell Microenvironment: From Chemistry to Biofabrication Technology. Prog Polym Sci 2019, 98, 101147. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme Responsive Drug Delivery Systems in Cancer Treatment. Journal of controlled release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Anderson, D.G. Smart Approaches to Glucose-Responsive Drug Delivery. J Drug Target 2015, 23, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-F.; Chen, Y.; Liu, Y. Enzyme-Responsive Protein/Polysaccharide Supramolecular Nanoparticles. Soft Matter 2015, 11, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int J Mol Sci 2022, 23, 4421. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Reaume, M.; Hennenfent, M.; Lee, B.P.; Rajachar, R. Biomimetic Hydrogels with Spatial-and Temporal-Controlled Chemical Cues for Tissue Engineering. Biomater Sci 2020, 8, 3248–3269. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kawakami, K. Synthesis and Characterization of Both Ionically and Enzymatically Cross-Linkable Alginate. Acta Biomater 2007, 3, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Tang, Z.; He, H.; Li, W.; Wang, X.; Liao, C.; Sun, Y.; Wang, Q. Glucose Oxidase Triggers Gelation of N-Hydroxyimide–Heparin Conjugates to Form Enzyme-Responsive Hydrogels for Cell-Specific Drug Delivery. Chem Sci 2014, 5, 4204–4209. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable Postoperative Enzyme-Responsive Hydrogels for Reversing Temozolomide Resistance and Reducing Local Recurrence after Glioma Operation. Biomater Sci 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Sekhon, B.S. Matrix Metalloproteinases–an Overview. Res Rep Biol 2010, 1–20. [Google Scholar]
- Conlon, G.A.; Murray, G.I. Recent Advances in Understanding the Roles of Matrix Metalloproteinases in Tumour Invasion and Metastasis. J Pathol 2019, 247, 629–640. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’Drug Delivery and Tumor Targeting. Trends Pharmacol Sci 2018, 39, 766–781. [Google Scholar] [CrossRef]
- Chauhan, S.; Solanki, R.; Jangid, A.K.; Jain, P.; Pranjali, P.; Patel, S.; Guleria, A.; Pooja, D.; Kulhari, H. Manganese Nanocarrier for Matrix Metalloproteinase 9 Responsive Delivery of Irinotecan for Colon Cancer Treatment. Journal of Industrial and Engineering Chemistry 2023. [Google Scholar] [CrossRef]
- Nazli, C.; Demirer, G.S.; Yar, Y.; Acar, H.Y.; Kizilel, S. Targeted Delivery of Doxorubicin into Tumor Cells via MMP-Sensitive PEG Hydrogel-Coated Magnetic Iron Oxide Nanoparticles (MIONPs). Colloids Surf B Biointerfaces 2014, 122, 674–683. [Google Scholar] [CrossRef]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix Metalloproteinase-Sensitive Multistage Nanogels Promote Drug Transport in 3D Tumor Model. Theranostics 2020, 10, 91. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brøndsted, H. Dextran Hydrogels for Colon-Specific Drug Delivery. Journal of Controlled Release 1995, 36, 159–166. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Matranga, M.A.; Cortinas, A.B.; Delcassian, D.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-Responsive Nanoparticles for Rapid and Extended Self-Regulated Insulin Delivery. ACS Nano 2019, 14, 488–497. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-responsive Insulin and Delivery Systems: Innovation and Translation. Advanced Materials 2020, 32, 1902004. [Google Scholar] [CrossRef]
- Tanna, S.; Sahota, T.S.; Sawicka, K.; Taylor, M.J. The Effect of Degree of Acrylic Derivatisation on Dextran and Concanavalin A Glucose-Responsive Materials for Closed-Loop Insulin Delivery. Biomaterials 2006, 27, 4498–4507. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ikeda, S.; Harada, A.; Kataoka, K. Glucose-Responsive Polymer Bearing a Novel Phenylborate Derivative as a Glucose-Sensing Moiety Operating at Physiological PH Conditions. Biomacromolecules 2003, 4, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.-W.; Weir, G.C.; Langer, R. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid–Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. PH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cao, S.; Chen, X.; Liu, S.; Tan, H.; Wu, W.; Li, J. Super Long-Term Glycemic Control in Diabetic Rats by Glucose-Sensitive LbL Films Constructed of Supramolecular Insulin Assembly. Biomaterials 2012, 33, 8733–8742. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. PH-Sensitive Peptide Hydrogel for Glucose-Responsive Insulin Delivery. Acta Biomater 2017, 51, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hashimoto, H.; Katayama, T.; Ueda, N.; Nagahama, K. Injectable Biocatalytic Nanocomposite Hydrogel Factories for Focal Enzyme-Prodrug Cancer Therapy. Biomacromolecules 2021, 22, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem Soc Rev 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers (Basel) 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv Healthc Mater 2019, 8, 1801002. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A Review of Drug Release Mechanisms from Nanocarrier Systems. Materials Science and Engineering: C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
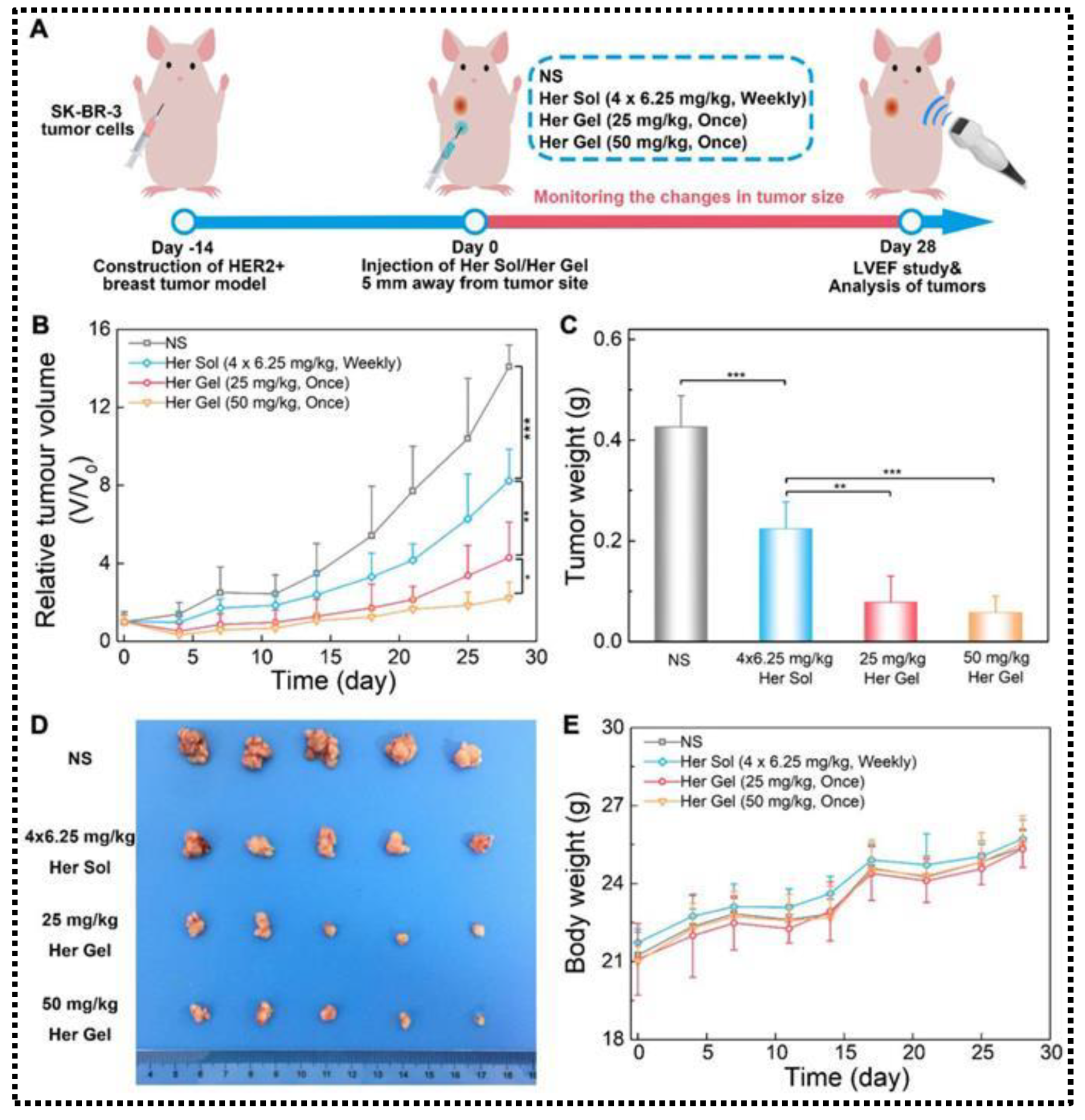
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable Hydrogels for the Sustained Delivery of a HER2-Targeted Antibody for Preventing Local Relapse of HER2+ Breast Cancer after Breast-Conserving Surgery. Theranostics 2019, 9, 6080. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive Sol–Gel Reversible Hydrogels. Adv Drug Deliv Rev 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. Journal of controlled release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv Funct Mater 2021, 31, 2008123. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly (N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable Thermosensitive Hydrogel-Based Drug Delivery System for Local Cancer Therapy. Colloids Surf B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Mondal, J.H.; Das, D. Peptide Hydrogels. RSC Adv 2013, 3, 9117–9149. [Google Scholar] [CrossRef]
- Nagahama, K.; Takahashi, A.; Ohya, Y. Biodegradable Polymers Exhibiting Temperature-Responsive Sol–Gel Transition as Injectable Biomedical Materials. React Funct Polym 2013, 73, 979–985. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater Sci Eng 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- He, J.; Zou, H.; Zhou, J.; Deng, C. Thermoresponsive MXene-Based Hydrogel for Controlled Anticancer Drug Release. J Drug Deliv Sci Technol 2024, 91, 105207. [Google Scholar] [CrossRef]
- Garrett, M.C.; O’Shea, T.M.; Wollenberg, A.L.; Bernstein, A.M.; Hung, D.; Staarman, B.; Soto, H.; Deming, T.J.; Sofroniew, M. V; Kornblum, H.I. Injectable Diblock Copolypeptide Hydrogel Provides Platform to Deliver Effective Concentrations of Paclitaxel to an Intracranial Xenograft Model of Glioblastoma. PLoS One 2020, 15, e0219632. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 ShRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int J Mol Sci 2020, 21, 7111. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly (N-Isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl Mater Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide Loaded Thermo-Responsive Hydrogel System Synergize with a Hydrogel Cancer Vaccine to Amplify Cancer Immunotherapy in a Prime-Boost Manner. Bioact Mater 2021, 6, 3036–3048. [Google Scholar] [CrossRef]
- Qian, Z.; Zhao, N.; Xu, S.; Yuan, W. In Situ Injectable Thermoresponsive Nanocomposite Hydrogel Based on Hydroxypropyl Chitosan for Precise Synergistic Calcium-Overload, Photodynamic and Photothermal Tumor Therapy. Carbohydr Polym 2024, 324, 121487. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Chou, H.-Y.; Hsu, W.-H.; Lai, J.-Y.; Lin, S.-Y.; Tsai, H.-C. Localized Controlled Release of Bevacizumab and Doxorubicin by Thermo-Sensitive Hydrogel for Normalization of Tumor Vasculature and to Enhance the Efficacy of Chemotherapy. Int J Pharm 2019, 572, 118799. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Parker, S.G.; Zhang, X.; Gooding, J.J.; Ru, Y.; Liu, Y.; Zhou, Y. Light-Induced Hydrogel Based on Tumor-Targeting Mesoporous Silica Nanoparticles as a Theranostic Platform for Sustained Cancer Treatment. ACS Appl Mater Interfaces 2016, 8, 15857–15863. [Google Scholar] [CrossRef]
- Chen, B.; Wang, G.; Huang, C.; Sun, Y.; Zhang, J.; Chai, Z.; Guo, S.-S.; Zhao, X.-Z.; Yuan, Y.; Liu, W. A Light-Induced Hydrogel Responsive Platform to Capture and Selectively Isolate Single Circulating Tumor Cells. Nanoscale 2022, 14, 3504–3512. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl Mater Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Yang, R.; Zhang, L.; Zhang, L.; Liu, G.; Xu, Z.; Kang, Y.; Xue, P. Injectable and Natural Humic Acid/Agarose Hybrid Hydrogel for Localized Light-Driven Photothermal Ablation and Chemotherapy of Cancer. ACS Biomater Sci Eng 2018, 4, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D. Near-Infrared Light-Responsive Hybrid Hydrogels for the Synergistic Chemo-Photothermal Therapy of Oral Cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Sessa, L.; Diana, R.; Piotto, S.; Concilio, S. Recent Progress in Photoresponsive Biomaterials. Molecules 2023, 28, 3712. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Advanced Materials 2019, 31, 1807333. [Google Scholar] [CrossRef]
- Peng, K.; Zheng, L.; Zhou, T.; Zhang, C.; Li, H. Light Manipulation for Fabrication of Hydrogels and Their Biological Applications. Acta Biomater 2022, 137, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Ahmed, R.; Marques, A.P.; Reis, R.L.; Demirci, U. Engineering Hydrogel-Based Biomedical Photonics: Design, Fabrication, and Applications. Advanced Materials 2021, 33, 2006582. [Google Scholar] [CrossRef]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J Am Chem Soc 2012, 134, 8848–8855. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; You, Y.; Wu, X.; Shao, S.; Gu, Q. Controlled Protein Delivery from Photosensitive Nanoparticles. J Biomed Mater Res A 2015, 103, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pianowski, Z.L.; Karcher, J.; Schneider, K. Photoresponsive Self-Healing Supramolecular Hydrogels for Light-Induced Release of DNA and Doxorubicin. Chemical communications 2016, 52, 3143–3146. [Google Scholar] [CrossRef]
- Jiang, J.; Qi, B.; Lepage, M.; Zhao, Y. Polymer Micelles Stabilization on Demand through Reversible Photo-Cross-Linking. Macromolecules 2007, 40, 790–792. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-Stimuli-Responsive Hydrogels and Their Medical Applications. New Journal of Chemistry 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive Nanocomposite Hydrogels for Biomedical Applications. Adv Funct Mater 2021, 31, 2005941. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A Spatiotemporal Release Platform Based on PH/ROS Stimuli-Responsive Hydrogel in Wound Repairing. Journal of Controlled Release 2022, 341, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, X. Multifunctional Smart Hydrogels: Potential in Tissue Engineering and Cancer Therapy. J Mater Chem B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Pu, K. Recent Advances in Dual-and Multi-Responsive Nanomedicines for Precision Cancer Therapy. Biomaterials 2022, 291, 121906. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-Stimuli Responsive Hydrogels Derived from Hyaluronic Acid for Cancer Therapy Application. Carbohydr Polym 2022, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- Güngör, A.; Özdemir, T.; Genç, R. Investigation of Use in 5-FU Release: Synthesis of Temperature and PH Responsive P (NVCL-Co-VIm)/PVP Hydrogels. Polymer Bulletin 2024, 81, 2091–2109. [Google Scholar] [CrossRef]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual Thermo-and PH-Sensitive Injectable Hydrogels of Chitosan/(Poly (N-Isopropylacrylamide-Co-Itaconic Acid)) for Doxorubicin Delivery in Breast Cancer. Int J Biol Macromol 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Zhao, C.; Zhuang, X.; He, P.; Xiao, C.; He, C.; Sun, J.; Chen, X.; Jing, X. Synthesis of Biodegradable Thermo-and PH-Responsive Hydrogels for Controlled Drug Release. Polymer (Guildf) 2009, 50, 4308–4316. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, S.H.; Phan, V.H.G.; Thambi, T.; Lee, D.S. Therapeutic Effects of Boronate Ester Cross-Linked Injectable Hydrogels for the Treatment of Hepatocellular Carcinoma. Biomater Sci 2021, 9, 7275–7286. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ma, R.; Wang, X.; Gao, J.; Zheng, Y.; Sun, Z. Enzyme and PH Responsive 5-Flurouracil (5-FU) Loaded Hydrogels Based on Olsalazine Derivatives for Colon-Specific Drug Delivery. Eur Polym J 2019, 118, 64–70. [Google Scholar] [CrossRef]
- Palmese, L.L.; Fan, M.; Scott, R.A.; Tan, H.; Kiick, K.L. Multi-Stimuli-Responsive, Liposome-Crosslinked Poly (Ethylene Glycol) Hydrogels for Drug Delivery. J Biomater Sci Polym Ed 2020, 32, 635–656. [Google Scholar] [CrossRef] [PubMed]
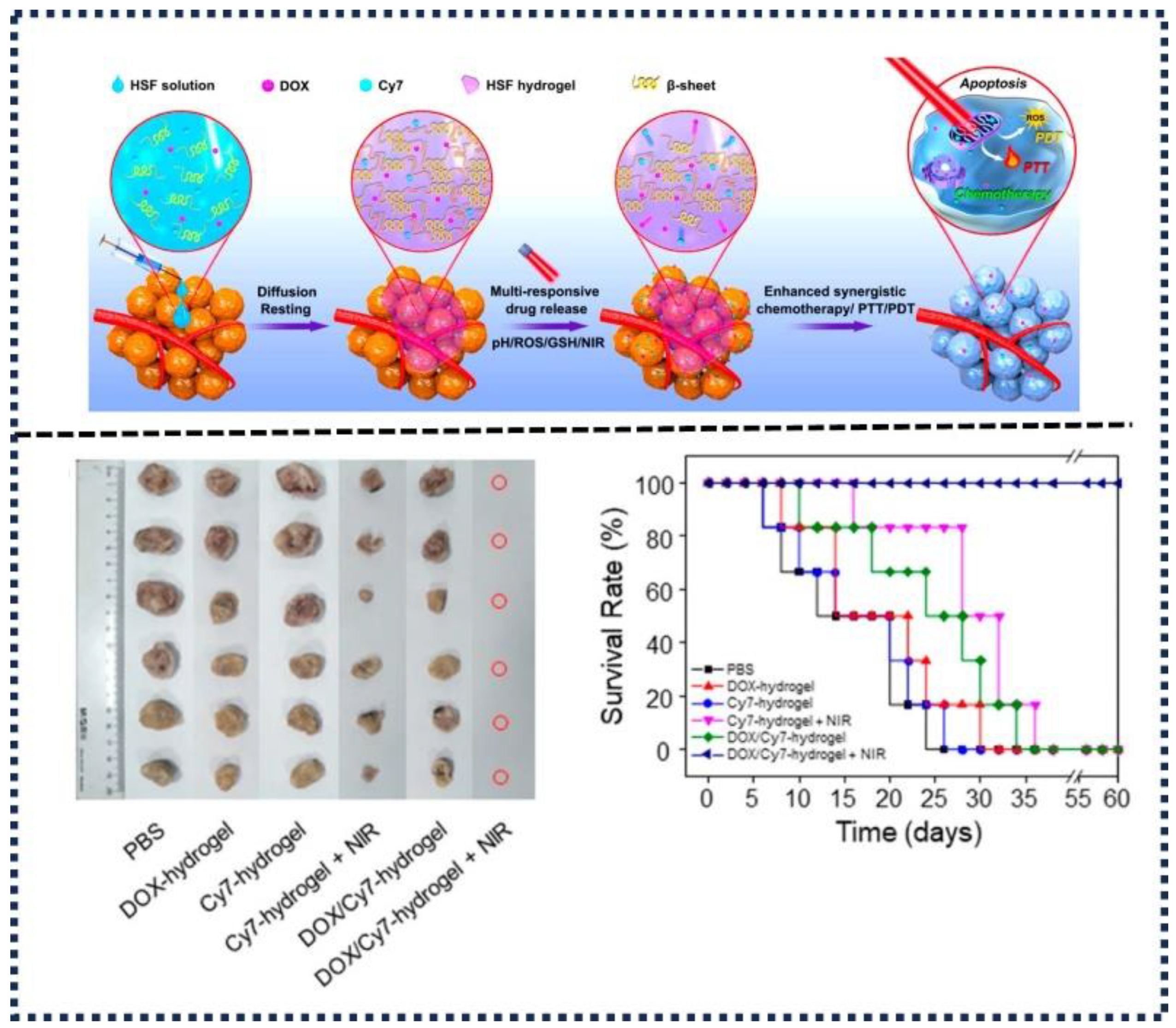
- Gou, S.; Xie, D.; Ma, Y.; Huang, Y.; Dai, F.; Wang, C.; Xiao, B. Injectable, Thixotropic, and Multiresponsive Silk Fibroin Hydrogel for Localized and Synergistic Tumor Therapy. ACS Biomater Sci Eng 2019, 6, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, J.; Sun, J.; Jiang, Y.; Zheng, W.; Hu, W.; Qian, H. Recent Advances on Thermosensitive Hydrogels-Mediated Precision Therapy. Asian J Pharm Sci 2024, 100911. [Google Scholar] [CrossRef]
- Cook, A.B.; Decuzzi, P. Harnessing Endogenous Stimuli for Responsive Materials in Theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive Nanocomposite Hydrogels for Biomedical Applications. Adv Funct Mater 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P. V Complicity of Degradable Polymers in Health-Care Applications. Mater Today Chem 2020, 16, 100236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





