Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
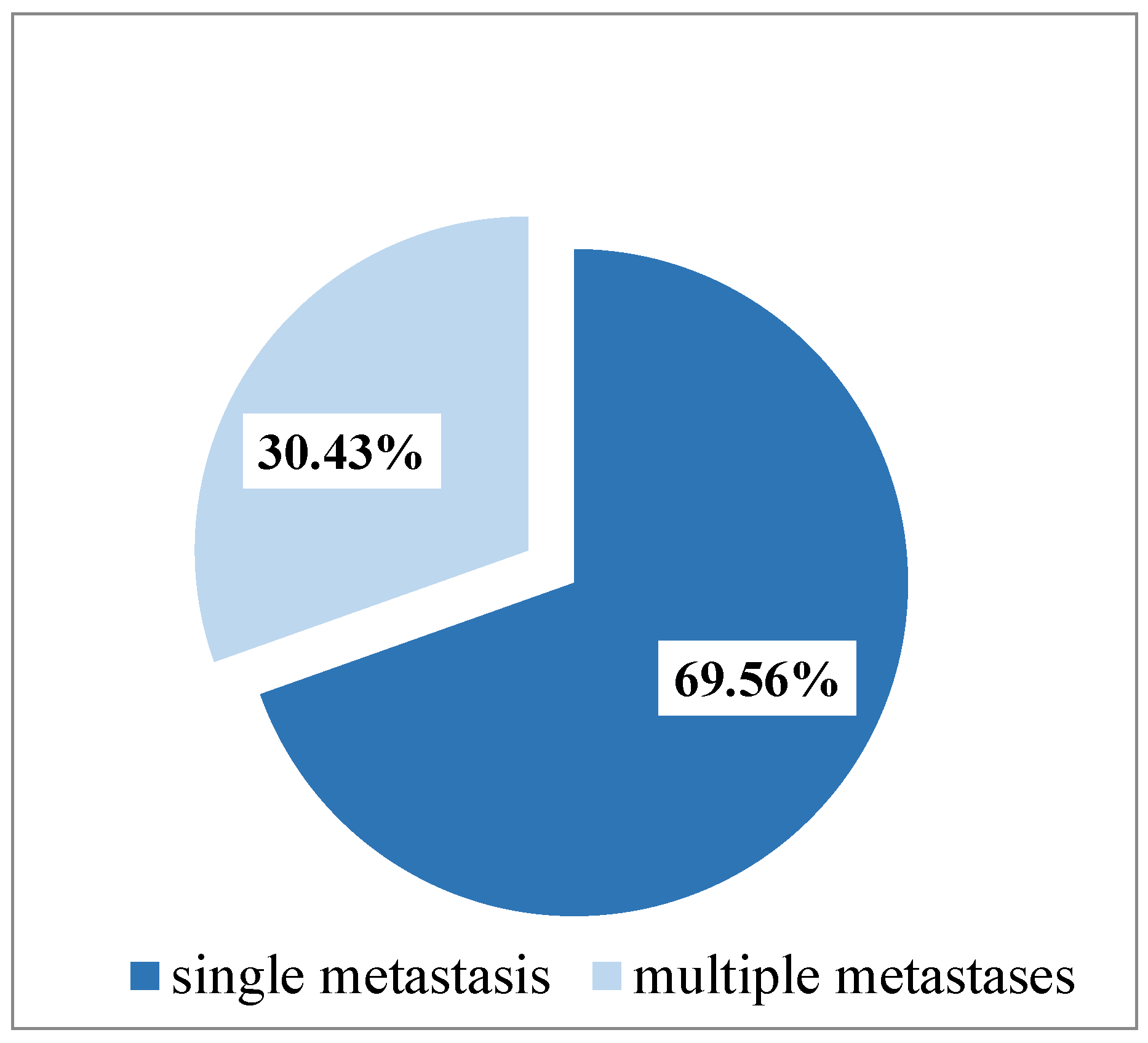
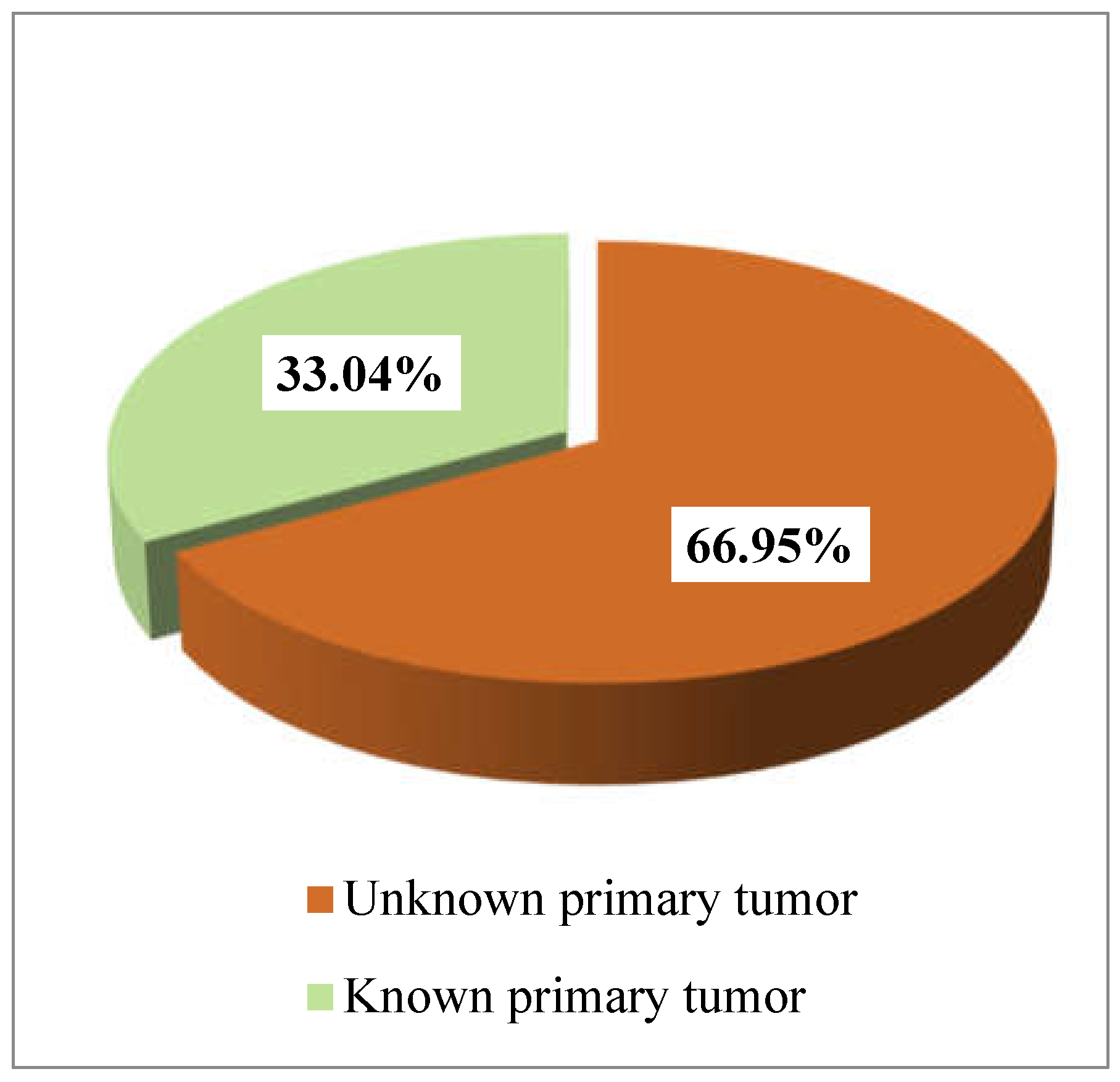
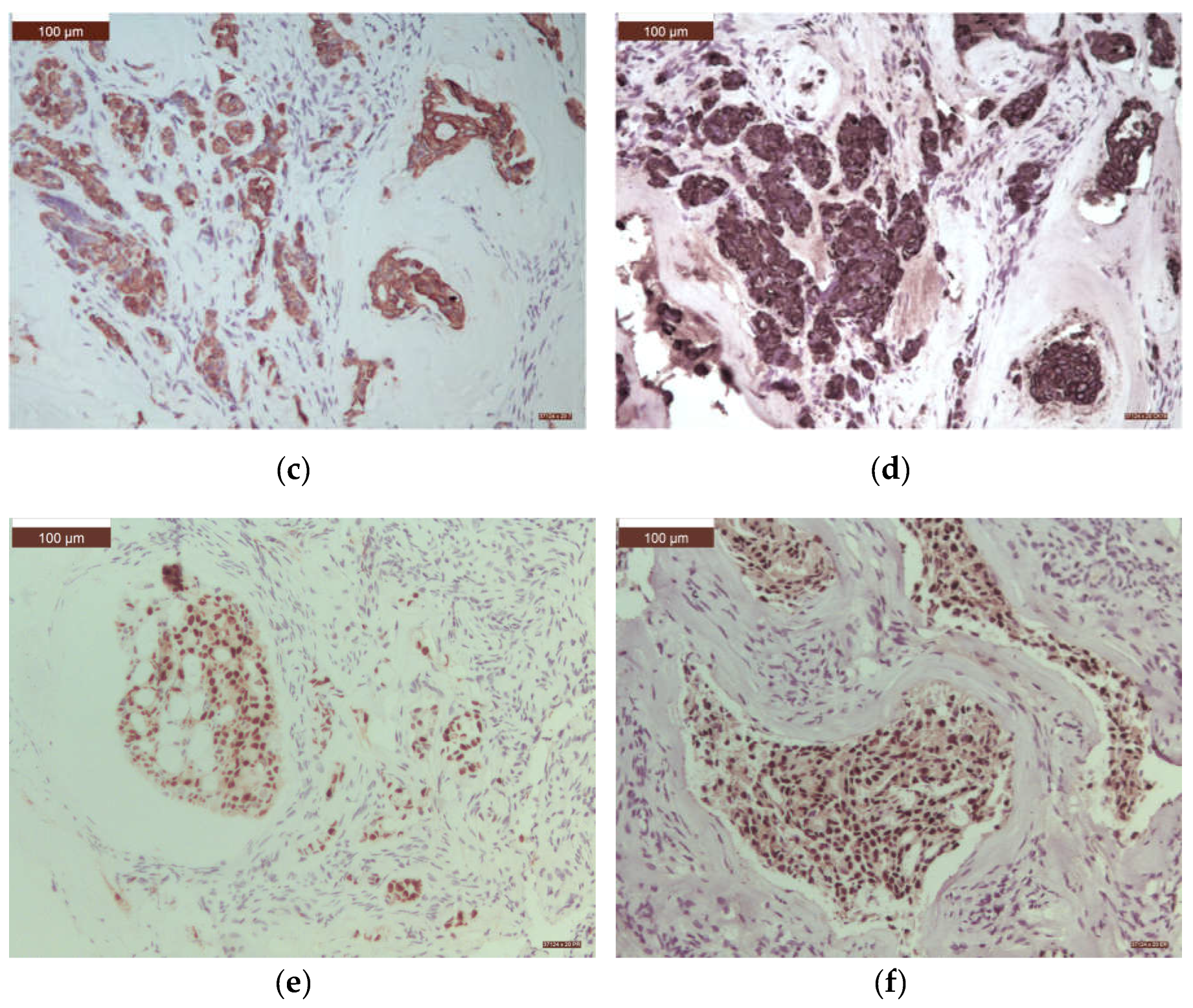
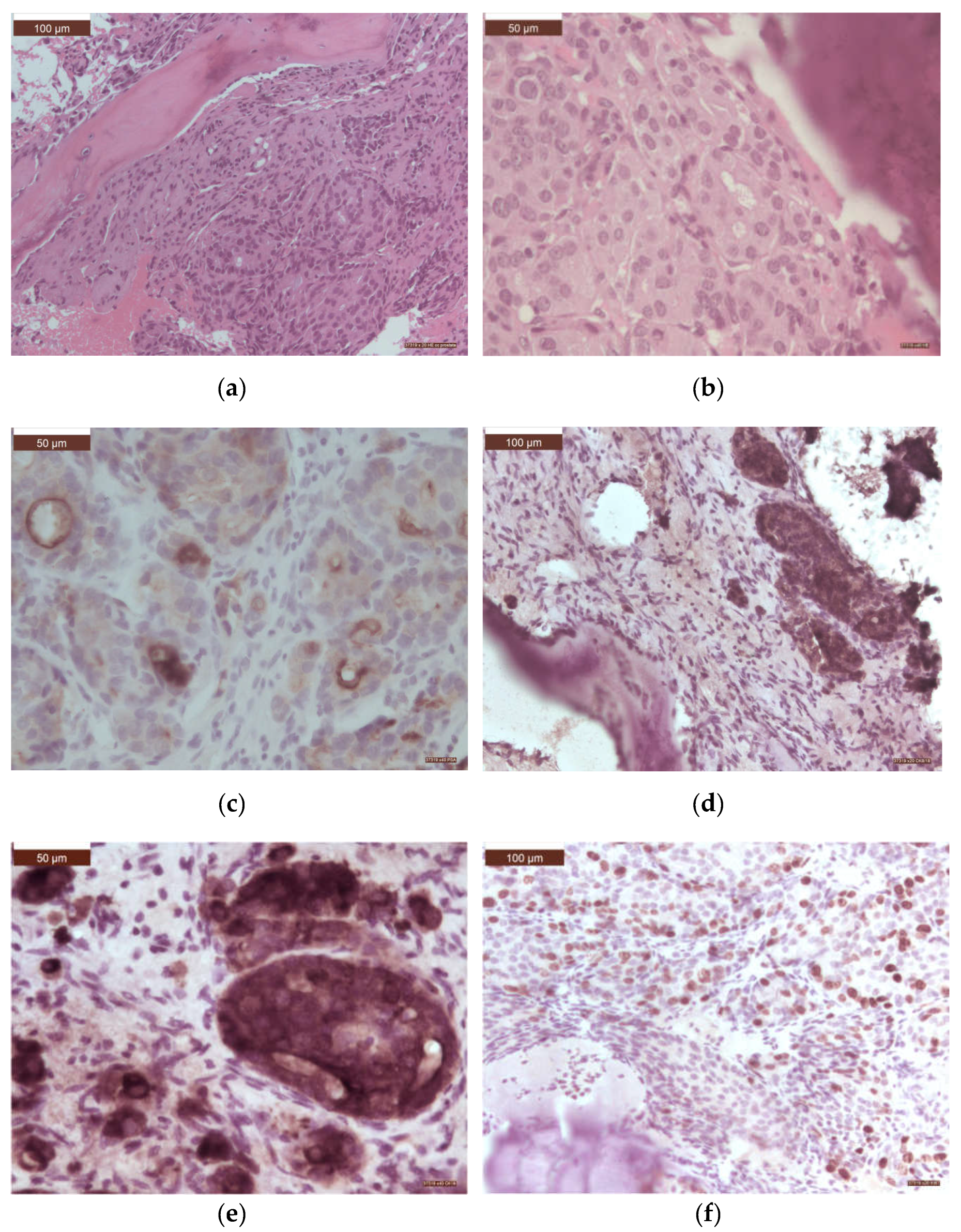
3. Results
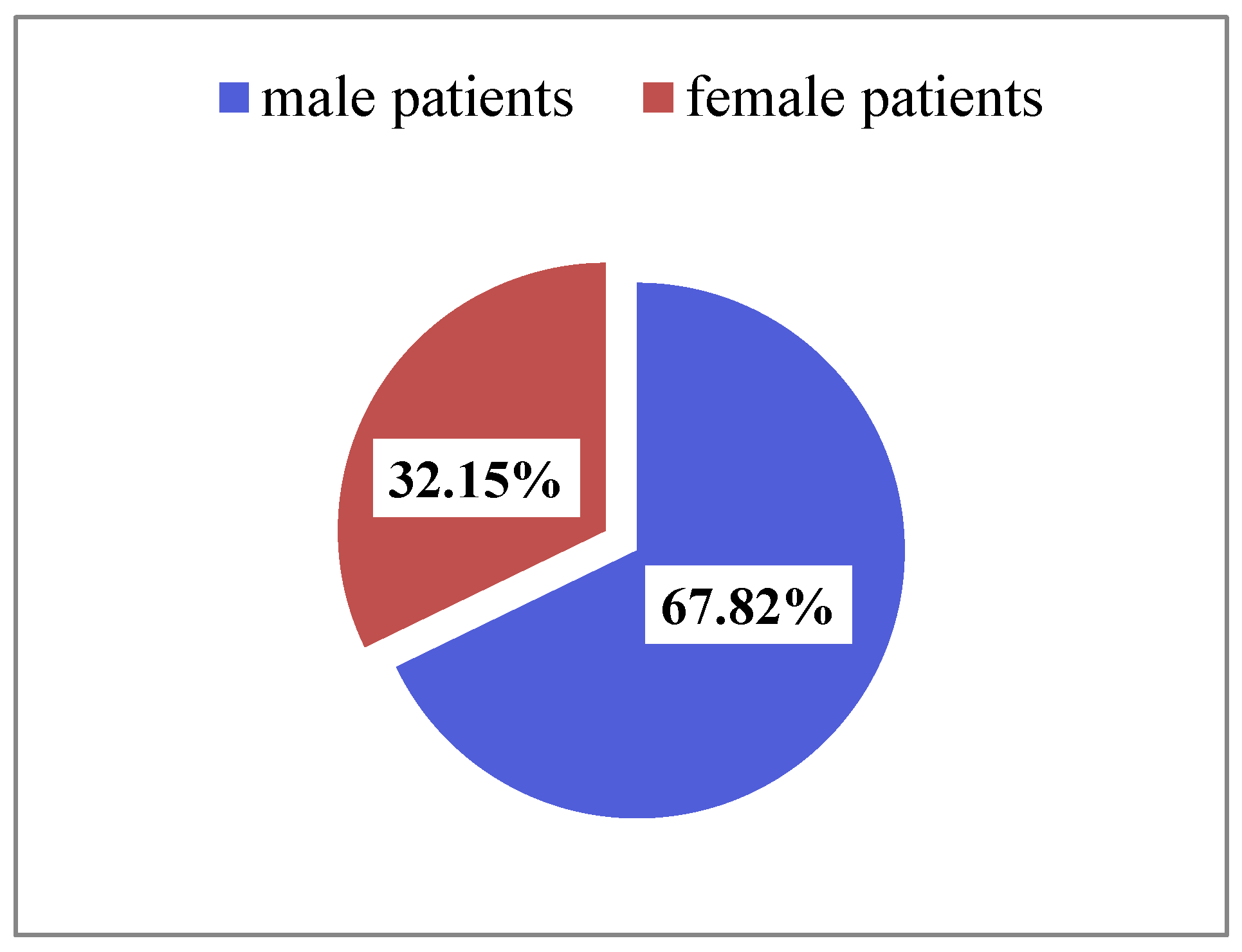
3.1. Sociodemographic Characteristics of Patients
3.2. Topography of Spinal Metastasis
3.3. Formatting of Mathematical Components
3.3. Time Interval between the Diagnosis of the Primary Tumor and the Appearance of the Primary Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziu, E.; Viswanathan, V.K.; Mesfin, F.B. Spinal Metastasis. In: StatPearls [Internet]. StatPearls Publishing: Treasure Island (FL), 2024.
- Cerqueira, B.P.; dos Santos do Nascimento Carvalho, M.; de Barros Pontes, F.G. Epidemiological Characterization of Patients with Spinal Tumors in Alagoas, Brazil. J. Bras. Neurocirur. 2023, 34, 291–296. [Google Scholar] [CrossRef]
- Shakil, H.; Malhotra, A.K.; Badhiwala, J.H.; Karthikeyan, V.; Essa, A.; He, Y.; Fehlings, M.G.; Sahgal, A.; Dea, N.; Kiss, A.; Witiw, C.D.; Redelmeier, D.A.; Wilson, JR. Contemporary trends in the incidence and timing of spinal metastases: A population-based study. Neuro-Oncol. Adv. 2024, 6, vdae051. [Google Scholar] [CrossRef]
- World Health Organization. Who Report on Cancer: Setting Priorities, Investing Wesely and Providing Care for All. Geneva: World Health Organization, 2020.
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS One. 2020, 15, e0234927. [Google Scholar] [CrossRef]
- Cortez, P.R. Spinal metastasis: diagnosis, treatment and prognosis - Integrative review from 2012 to 2017. Coluna/Columna 2020, 19, 58–66. [Google Scholar] [CrossRef]
- Esperança-Martins, M.; Roque, D.; Barroso, T.; Abrunhosa-Branquinho, A.; Belo, D.; Simas, N.; Costa, L. Multidisciplinary Approach to Spinal Metastases and Metastatic Spinal Cord Compression—A New Integrative Flowchart for Patient Management. Cancers (Basel). 2023, 15, 1796. [Google Scholar] [CrossRef]
- Fomchenko, E.I.; Bayley, J.C.; Alvarez-Breckenridge, C.; Rhines, L.D.; Tatsui, C.E. Spinal Metastases and the Evolving Role of Molecular Targeted Therapy, Chemotherapy, and Immunotherapy. Neurospine 2022, 19, 978–993. [Google Scholar] [CrossRef]
- Truong, V.T.; Al-Shakfa, F.; Phan, P.; Newman, N.; Boubez, G.; Shedid, D.; Yuh, S.J.; Wang, Z. Does the Region of the Spine Involved with Metastatic Tumor Affect Outcomes of Surgical Treatments? World Neurosurg. 2021, 156, e139–e151. [Google Scholar] [CrossRef]
- Botelho, R.V.; de Oliveira, M.F.; Rotta, J.M. Quantification of Vertebral Involvement in Metastatic Spinal Disease. Open Orthop J. 2013, 7, 286–291. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yang, L.; Yang, X.; Zhang, H.; Li, J.; Qiao, R.; Hu, Y. Epidemiological Characteristics of 1196 Patients with Spinal Metastases: A Retrospective Study. Orthop. Surg. 2019, 11, 1048–1053. [Google Scholar] [CrossRef]
- Lu, C.; Gonzalez, R.G.; Jolesz, F.A; Wen, P.Y.; Talcott, J.A. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J. Support. Oncol. 2005, 3, 305–312. [Google Scholar] [CrossRef]
- Selaru, S.; Sava, A.; Scripcariu, D.V.; Costea, C.F.; Dumitrescu, A.M.; Costăchescu, B.; Dumitrescu, G.F.; Ciupilan, C.; Vatavu, R.; Haba, R.M.; Poroch, V.; Dima-Cozma, L.C.; Vornicu, V.; Stan, C.I. Epidemiological and pathological characteristics of spinal metastases from gastrointestinal cancers – a series of 40 cases. Rom. J. Morphol. Embryol. 2023, 64, 225–234. [Google Scholar] [CrossRef]
- Marchi Candido, P.B.; Pinheiro, R.P.; Peria, F.M.; Nogueira Toledo, V.; Tavares Costa, H.R.; Aparecido Defino, H.L. Unknown primary tumor sites in spinal metastasis. Coluna/Columna 2021, 20, 64–67. [Google Scholar] [CrossRef]
- Enkaoua, E.A.; Doursounian, L.; Chatellier, G.; Mabesoone, F.; Aimard, T.; Saillant, G. Vertebral Metastases. A Critical Appreciation of the Preoperative Prognostic Tokuhashi Score in a Series of 71 Cases. Spine. 1997, 22, 2293–2298. [Google Scholar] [CrossRef]
- Helweg-Larsen, S.; Sorensen, P.S.; Kreiner, S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1163–1169. [Google Scholar] [CrossRef]
- Husband, D.J.; Grant, K.A.; Romaniuk, C.S. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br. J. Radiol. 2001, 74, 15–23. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Pendleton, C.; Wolinsky, J.P.; Gokaslan, Z.L.; Sciubba, D.M. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurg. 2009, 65, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, F.; Liu, L.; Liu, X.D. Pathological Investigation of Vertebral Tumor Metastasis from Unknown Primaries - a Systematic Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 1047–1049. [Google Scholar] [CrossRef]
- Wright, E.; Ricciardi, F.; Arts, M.; Buchowski, J.M.; Chung, C.K.; Coppes, M.; Crockard, A.; Depreitere, B.; Fehlings, M.; Kawahara, N.; Lee, C.S.; Leung, Y.; Martin-Benlloch, A.; Massicotte, E.; Mazel, C.; Oner, C.; Peul, W.; Quraishi, N.; Tokuhashi, Y.; Tomita, K.; Ulbricht, C.; Verlaan, J.J.; Wang, M.; Choi, D. Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg. 2018, 114, e809–17. [Google Scholar] [CrossRef]
- Başdelioğlu, K. Features of Spinal Metastases: A Retrospective View. Int. J. Spine Surg. 2021, 15, 119–129. [Google Scholar] [CrossRef]
- Bratu, A.M.; Raica, V.P.; Sălcianu, I.A.; Zaharia, C.; Popa, V.B.; Lupu, A.R.; Ştefănescu, V.; Dobrea, C-M.; Iana, G.; Marinescu, A.N. MRI differential diagnosis: bone metastases versus bone lesions due to malignant hemopathies. Rom. J. Morphol. Embryol. 2017, 58, 1217–1228. [Google Scholar]
- Amelot, A.; Terrier, L.M.; Cristini, J.; Buffenoir, K.; Pascal-Moussellard, H.; Carpentier, A.; Bonaccorsi, R.; Le Nail, L.R.; Mathon, B. Spinal metastases from lung cancer: Survival depends only on genotype, neurological and personal status, scarcely of surgical resection. Surg. Oncol. 2020, 34, 51–56. [Google Scholar] [CrossRef]
- Portales, F.; Thézenas, S.; Samalin, E.; Assenat, E.; Mazard, T.; Ychou, M. Bone metastases in gastrointestinal cancer. Clin. Exp. Metastasis 2015, 32, 7–14. [Google Scholar] [CrossRef]
- Ionescu, E.M.; Tieranu, C.G.; Maftei, D.; Grivei, A.; Olteanu, A.O.; Arbanas, T.; Calu, V.; Musat, S.; Mihaescu-Pintia, C.; Cucu, I.C. Colorectal Cancer Trends of 2018 in Romania-an Important Geographical Variation Between Northern and Southern Lands and High Mortality Versus European Averages. J. Gastrointest. Cancer. 2021, 52, 222–228. [Google Scholar] [CrossRef]
- Zhoobasarova, D.; Sadykova, A.; Muratov, Z.; Abdraeva, F.; Aitieva, A.; Aitieva, Z.; Zheenbekova1, D.; Ismailova, F.; Tazhibaeva, U.; Kyzy, A.M.; Abdullaeva, Z.; Kochkorbaeva, Z.; Maksatbek, T.; Keneshbaev, B.; Kadyrberdieva, M.; Sherieva, N. Optimization Diagnosis of Breast Cancer Vertebral Metastases. Advances in Breast Cancer Research 2021, 10, 156–164. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Crnalic, S.; Lofvenberg, R.; Bergh, A.; Widmark, A.; Hildingsson, C. Predicting survival for surgery of metastatic spinal cord compression in prostate cancer: a new score. Spine (Phila Pa 1976) 2012, 37, 2168–2176. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Karpathakis, A.; Kwan, A.; Mazhar, D.; Ansell, W.; Shamash, J.; Harper, P.; Rudman, S.; Powles, T.; Chowdhury, S. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int. 2013, 112, 176–181. [Google Scholar] [CrossRef]
- Zheng, D.X.; Soldozy, S.; Mulligan, K.M.; Levoska, M.A.; Cohn, E.F.; Finberg, A.; Alsaloum, P.; Cwalina, T.B.; Hanft, S.J.; Scott, J.F.; Rothermel, L.D.; Nambudirig, V.E. Epidemiology, management, and treatment outcomes of metastatic spinal melanoma. World Neurosurg. X. 2023, 18, 100–156. [Google Scholar] [CrossRef]
- dos Reis Oliveira, M.B.; Costa Souza, L.; Sampayo, E.J.G.; de Carvalho, G.S.; de Queiroz Mello, F.C.; Paschoal, M.E.M. The Impact of Lung Carcinoma Histology on the Frequency of Bone Metastases. Rev. Bras. Ortop. (Sao Paulo) 2019, 54, 524–530. [Google Scholar]
- Zhai, S.; Hu, P.; Liu, X.; Li, Z.; Wang, B.; Zhou, H.; Liu, Z.; Liu, X.; Li, Y.; Wei, F. Prognostic Analysis of Spinal Metastasis Secondary to Lung Cancer after Surgeries: A Unicentric, Large-Cohort, Retrospective Study. Orthop. Surg. 2022, 15, 70–78. [Google Scholar] [CrossRef]
- Ciuleanu, TE. Research and Standard of Care: Lung Cancer in Romania. Am. Soc. Clin. Oncol. Educ. Book. 2012, 437–41. [Google Scholar] [CrossRef]
- Aydinl, U.; Ozturk, C.; Bayram, S.; Sarihan, S.; Evrensel, T.; Yilmaz, H.S. Evaluation of lung cancer metastases to the spine. Acta Orthop. Belg. 2006, 72, 592–597. [Google Scholar]
- Groszman, L.; Hubermann, J.A.; Kooner, P.; Alamiri, N.; Bozzo, A.; Aoude, A. The Impact of Adjunct Medical Therapy on Survival after Spine Metastasis: A Systematic Review and Pooled Data Analysis. Cancers (Basel) 2024, 16, 1425. [Google Scholar] [CrossRef]
- Van den Brande, R.; Cornips, E.M.J.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef]
- Valesin Filho, E.S.; Tardini, R.; de Abreu, L.C.; Vieira Motter, B.; Adami, F.; Miller Reis Rodrigues, L. Epidemiological study of 55 patients with symptomatic metastatic spinal disease in Santo André - SP, Brazil. Coluna/Columna 2013, 12, 32–35. [Google Scholar]
- Hong, S.H.; Chang, B.S.; Kim, H.; Kang, D.H.; Chang, S.Y. An Updated Review on the Treatment Strategy for Spinal Metastasis from the Spine Surgeon’s Perspective. Asian Spine J. 2022, 16, 799–811. [Google Scholar] [CrossRef]
- Di Perna, G.; Cofano, F.; Mantovani, C.; Badellino, S.; Marengo, N.; Ajello, M.; Comite, L.M.; Palmieri, G.; Tartara, F.; Zenga, F.; Ricardi, U.; Garbossa, D. Separation surgery for metastatic epidural spinal cord compression: a qualitative review. J Bone Oncol. 2020, 25, 100320. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Murakami, H.; Kawahara, N.; Tomita, K.; Tsuchiya, H. Surgical metastasectomy in the spine: a review article. Oncologist. 2021, 26, e1833–e1843. [Google Scholar] [CrossRef]
- Al Farii, H.; Aoude, A.; Al Shammasi, A.; Reynolds, J.; Weber, M. Surgical Management of the Metastatic Spine Disease: A Review of the Literature and Proposed Algorithm. Global Spine J. 2023, 13, 486–498. [Google Scholar] [CrossRef]
- Nakanishi, K.; Uchino, K.; Watanabe, S.; Misaki, K.; Iba, H. Effect of Minimally Invasive Spine Stabilization in Metastatic Spinal Tumors. Medicina 2022, 58, 358. [Google Scholar] [CrossRef]
- Knapp, B.; Govindan, A.; Patel, S.S.; Pepin, K.; Wu, N.; Devarakonda, S.; Buchowski, J.M. Outcomes in patients with spinal metastases managed with surgical intervention. Cancers (Basel). 2024, 16, 438. [Google Scholar] [CrossRef]
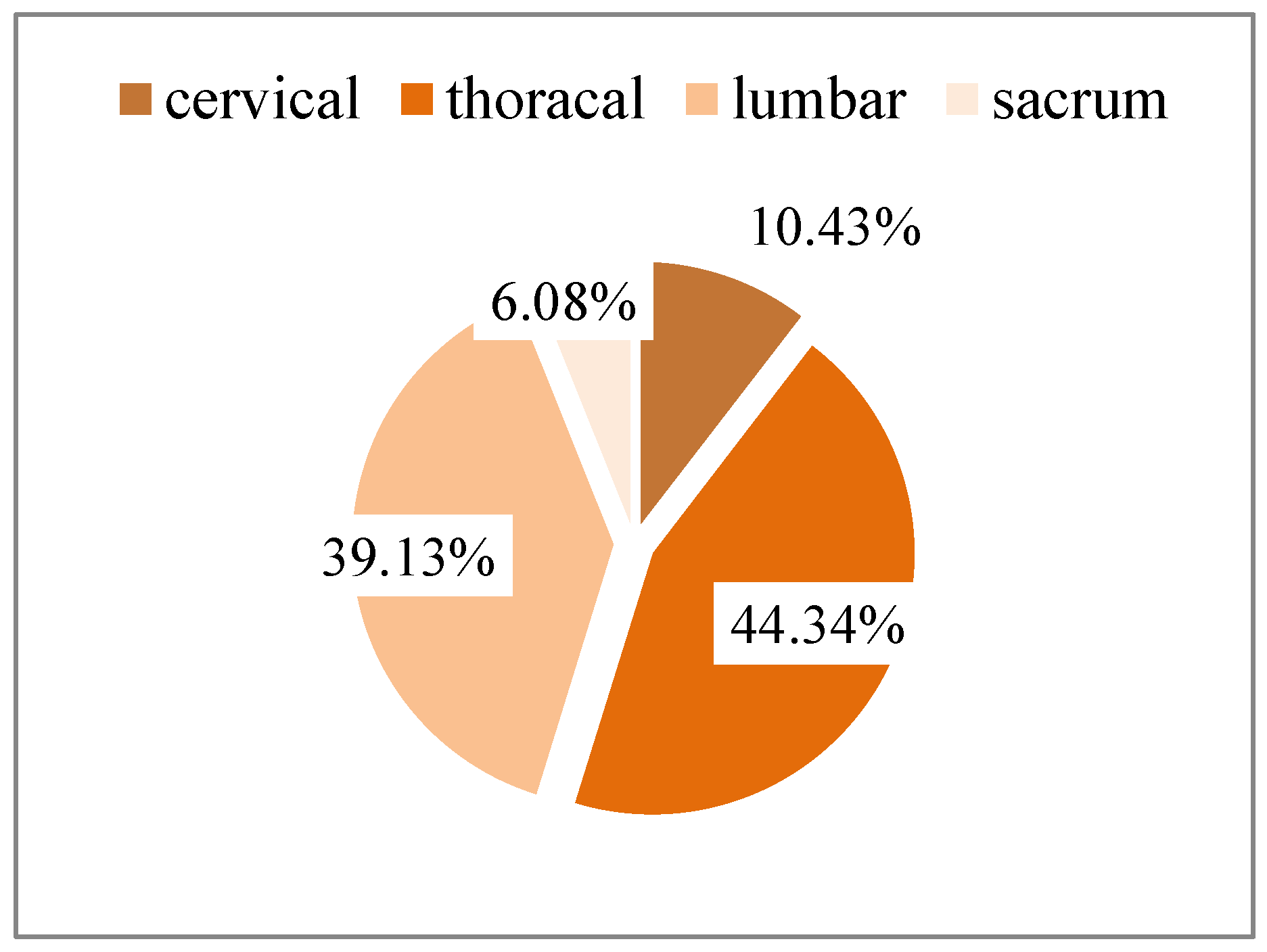
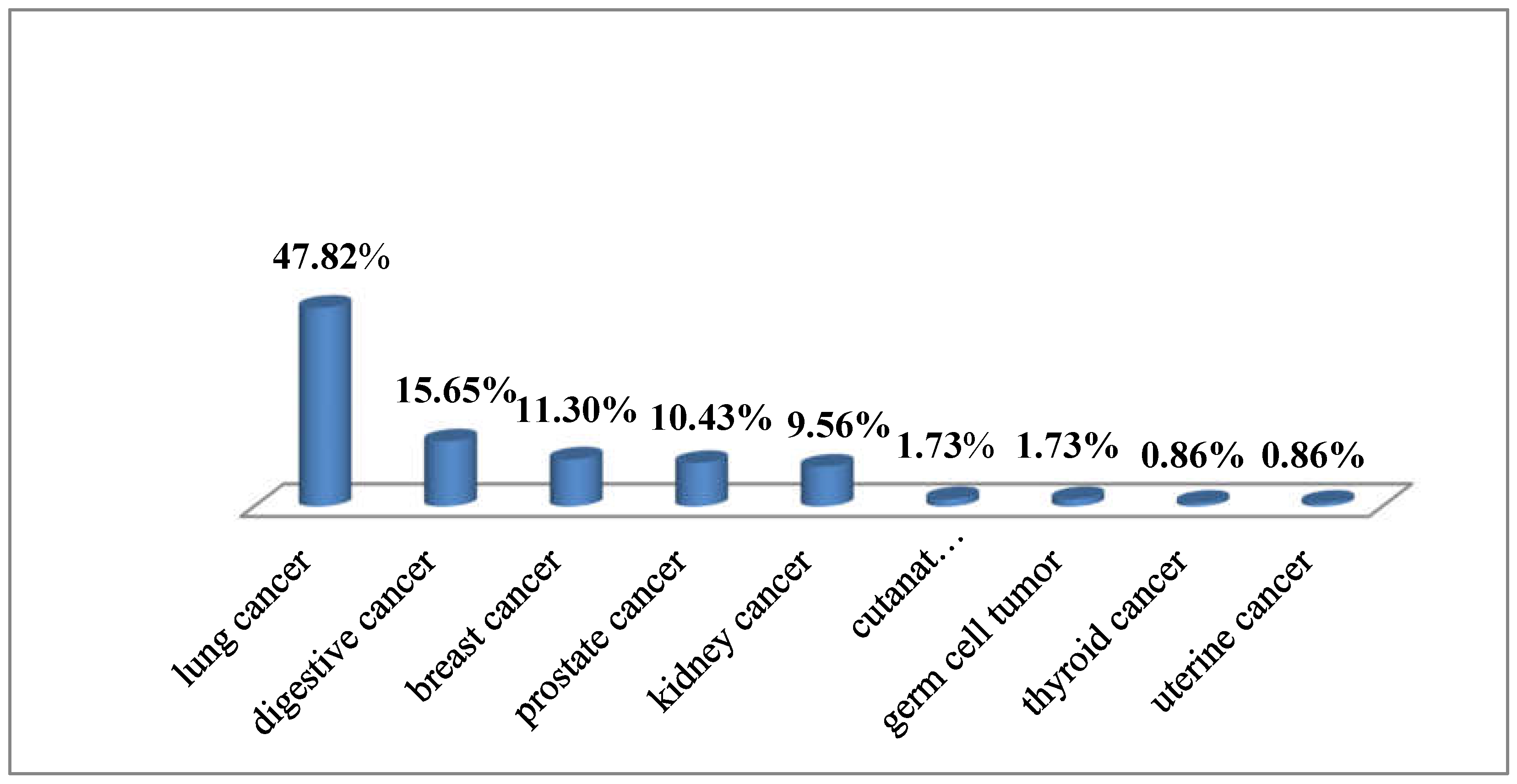

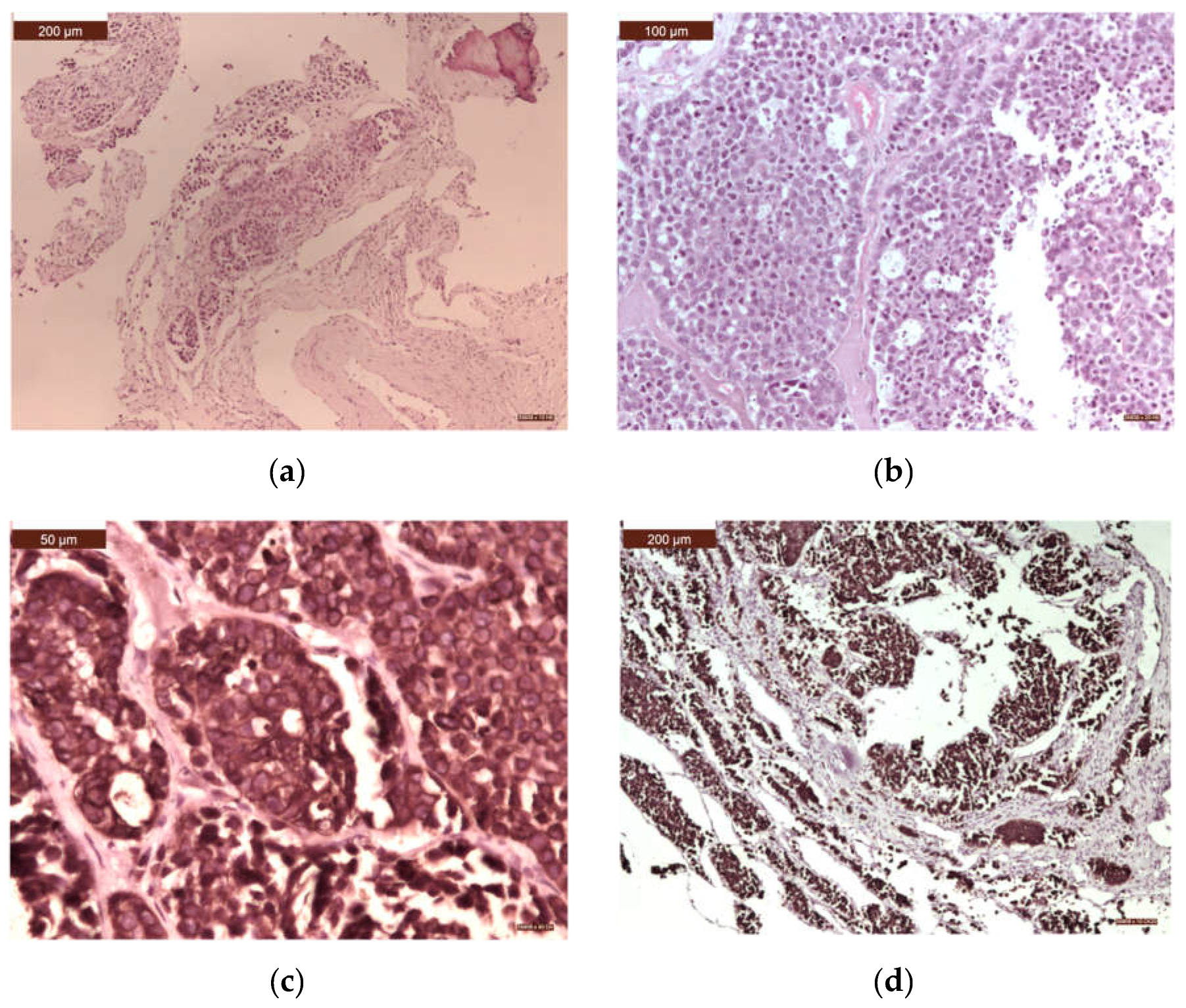
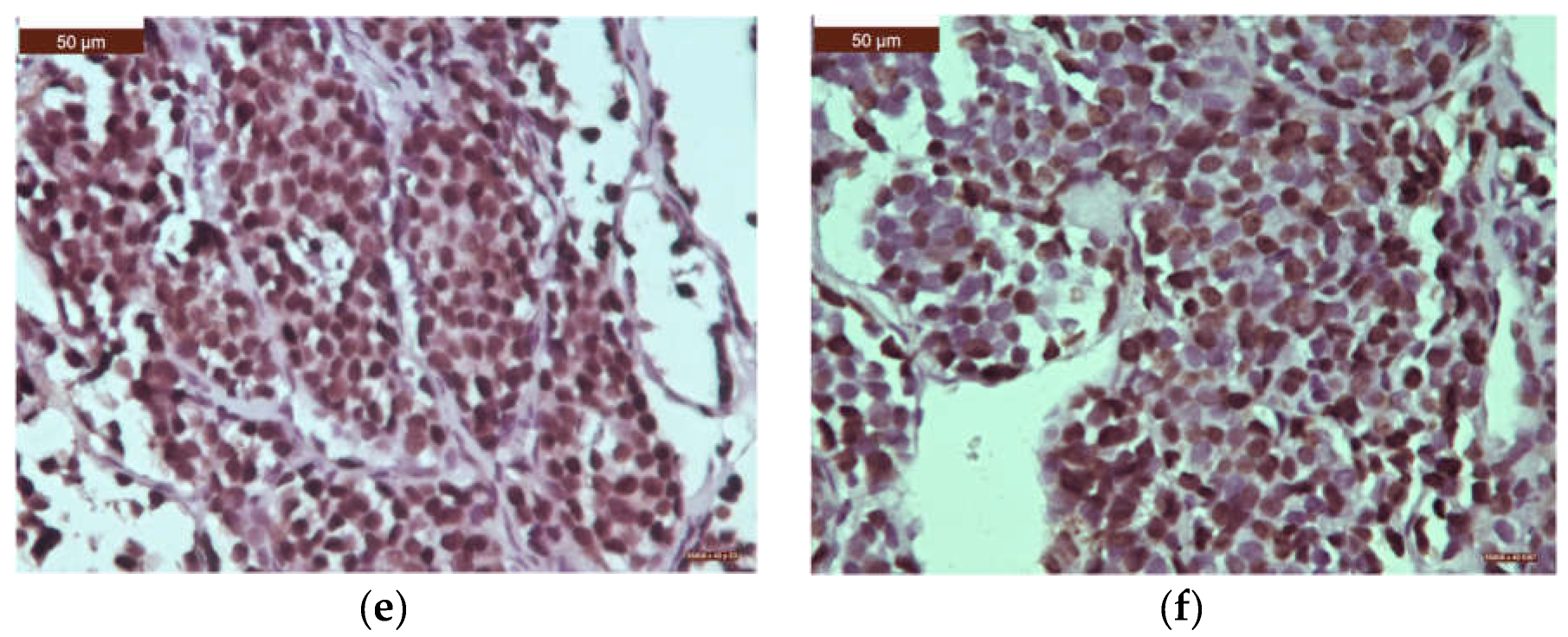
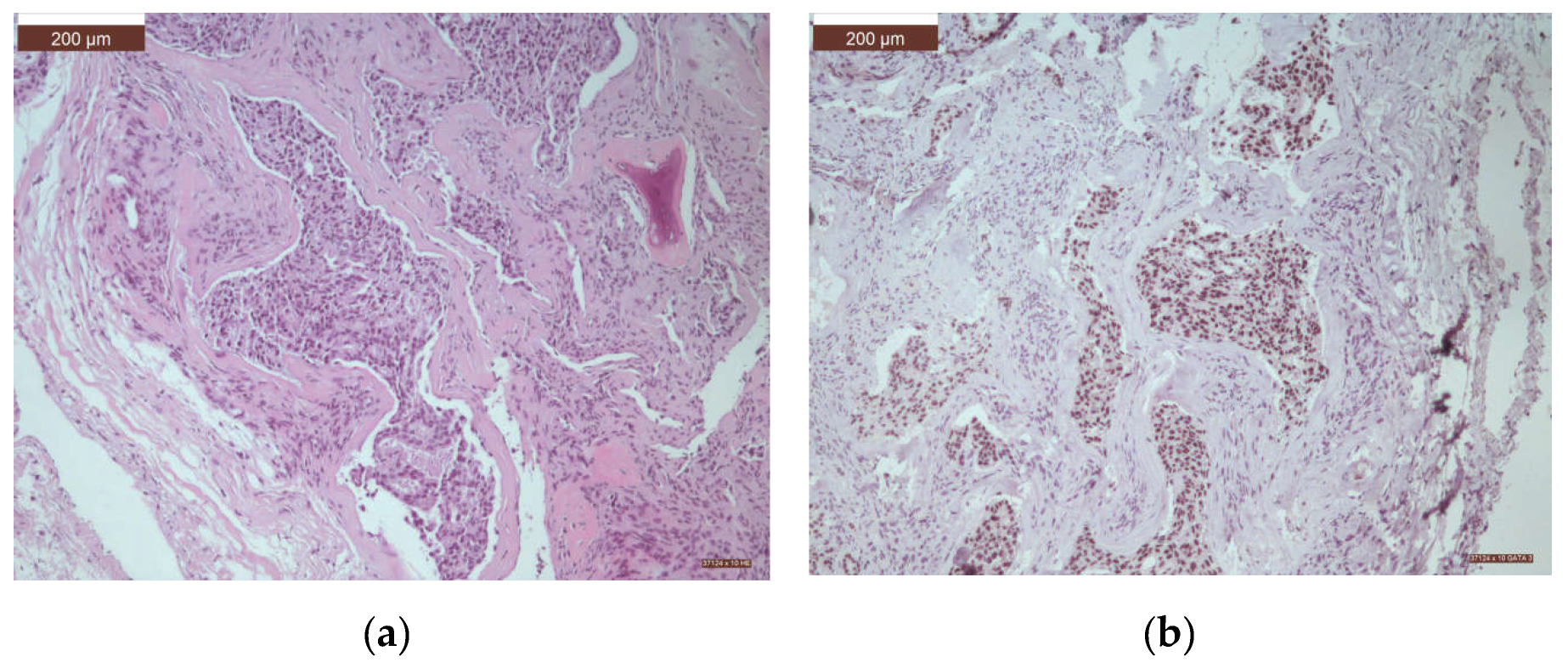
| Histological type | n | % |
|---|---|---|
| Adenocarcinoma | 20 | 17.39 |
| solid | 9 | 7.82 |
| acinar | 7 | 6.08 |
| papillary | 3 | 2.60 |
| colloid | 1 | 0.86 |
| Squamous cell carcinoma (moderate/poor differentiated) |
22 | 19.13 |
| Neuroendocrine tumors | 11 | 9.56 |
| Small cell carcinoma | 8 | 6.95 |
| Large cell neuroendocrine carcinoma | 3 | 2.60 |
| Pleomorphic carcinoma | 1 | 0.86 |
| Adenosquamous carcinoma | 1 | 0.86 |
| Total | 55 | 47.82 |
| Location | Histological type | n | % |
|---|---|---|---|
| Mucosa of the oral cavity | Squamous carcinoma (moderate/poor differentiated) |
2 | 1.73 |
| Stomach | Signet-ring cell carcinoma | 1 | 0.86 |
| Colorectal | Adenocarcinoma (moderate/poor differentiated) | 10 | 8.69 |
| Liver | Trabecular hepatocellular carcinoma (moderate differentiated) | 4 | 3.47 |
| Biliary tree | Cholangiocarcinoma | 1 | 0.86 |
| Total | 18 | 15.65 |
| Histological type | n | % |
|---|---|---|
| Infiltrating ductal carcinoma, NOS | 12 | 10.43 |
| Oncocytic carcinoma | 1 | 0.86 |
| Total | 13 | 11.30 |
| Location | Histological type | n | % |
|---|---|---|---|
| Prostate | Acinar adenocarcinoma | 12 | 10.43 |
| Kidney | Renal clear cell carcinoma | 11 | 9.56 |
| Skin cancer | Melanoma | 2 | 1.73 |
| Germ cell | Embryonar carcinoma | 1 | 0.86 |
| Seminoma | 1 | 0.86 | |
| Uterus | Endometrial endometrioid carcinoma | 1 | 0.86 |
| Thyroid | Follicular carcinoma | 1 | 0.86 |
| Total | 29 | 25.21 |
| Location of primary tumor | Time interval (months) |
|---|---|
| Lung | 17.1 |
| Gastrointestinal system | 19.56 |
| colon | 22.2 |
| liver/biliary tree | 38 |
| pancreas | unknown |
| the mucosa of the oral cavity | 8 |
| Breast | 23 |
| Prostate | 33 |
| Kidney | 20.75 |
| Cutaneous melanoma | 26 |
| Germ cell tumors | 1 |
| Thyroid | unknown |
| Uterus | unknown |
| Total | unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





