Submitted:
12 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Plant Material
2.2. Morphological Features with Industrial Value
2.3. Preparation and Extraction of Low Molecular Weight Antioxidants
2.4. Total Phenolic Compounds (TPC)
2.5. Free Radical Scavenging Activity (RSA)
2.6. Antioxidant Capacity (AC)
2.7. Lipid Peroxidation (LP)
2.8. Experimental Design and Statistical Analysis
3. Results
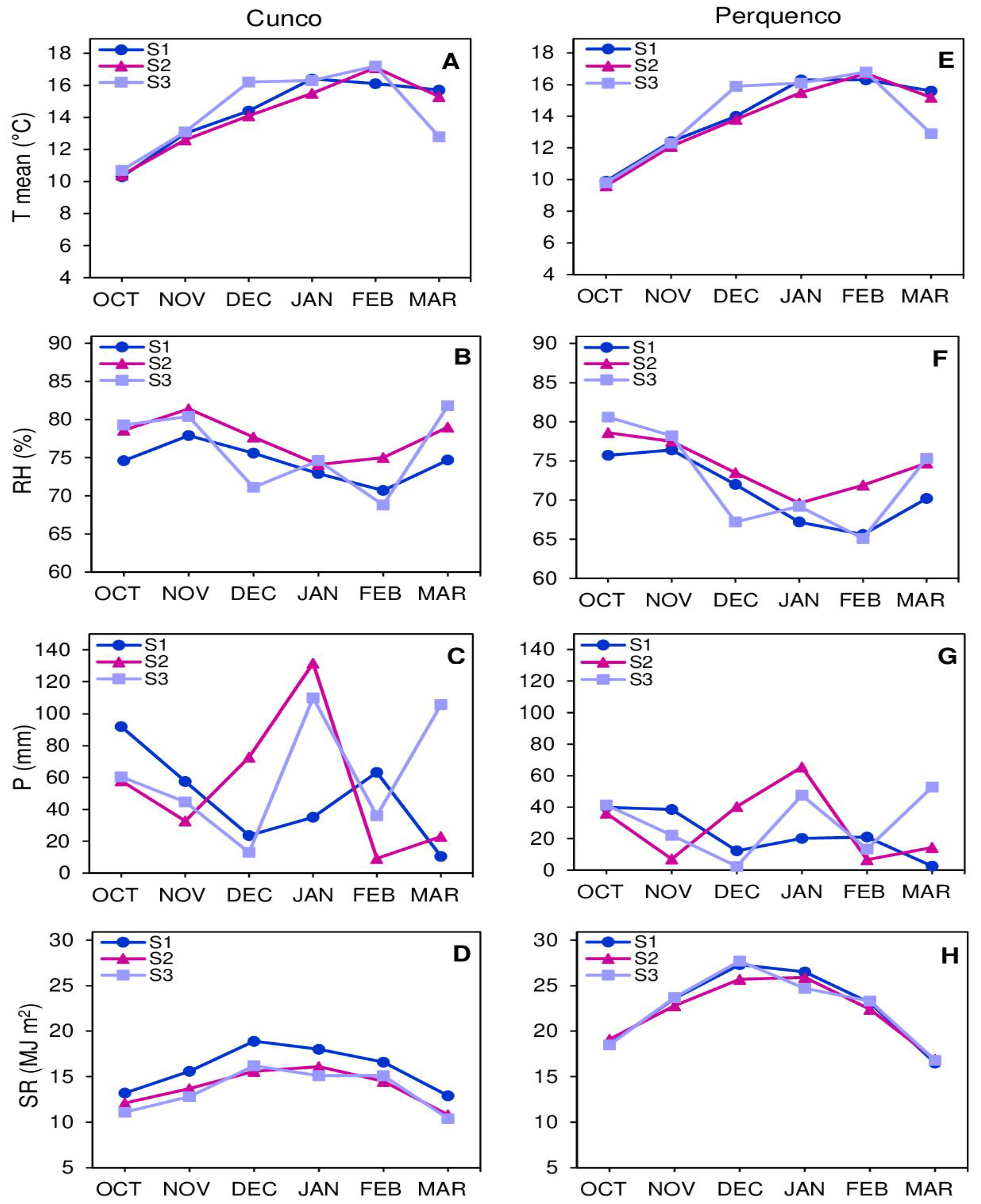
3.1. Experimental Sites and Weather Conditions
3.2. Morphological Features in Hazelnut
3.3. Antioxidant Properties in Hazelnut Kernels and Shells
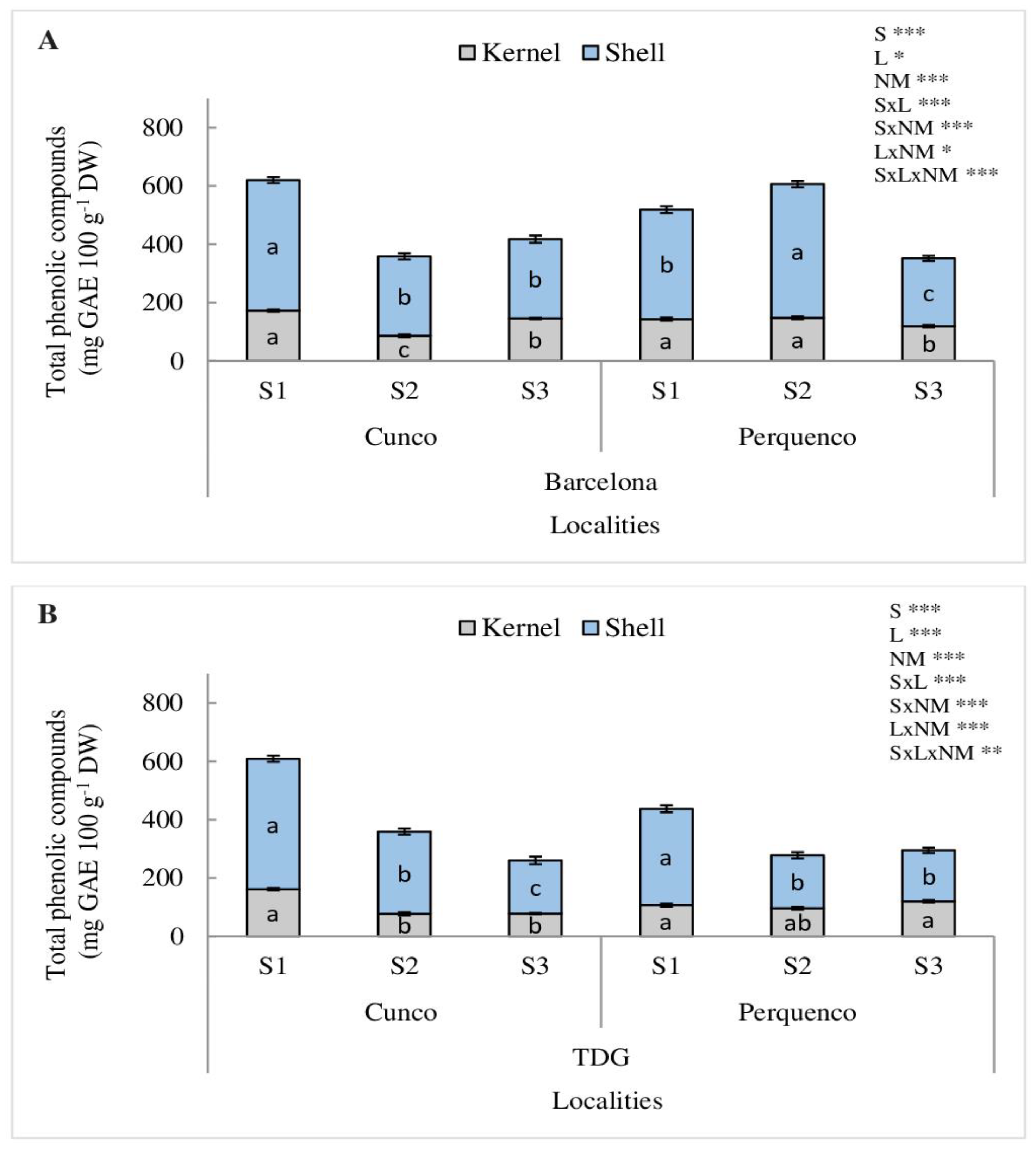
3.3.1. Total Phenolic Compounds
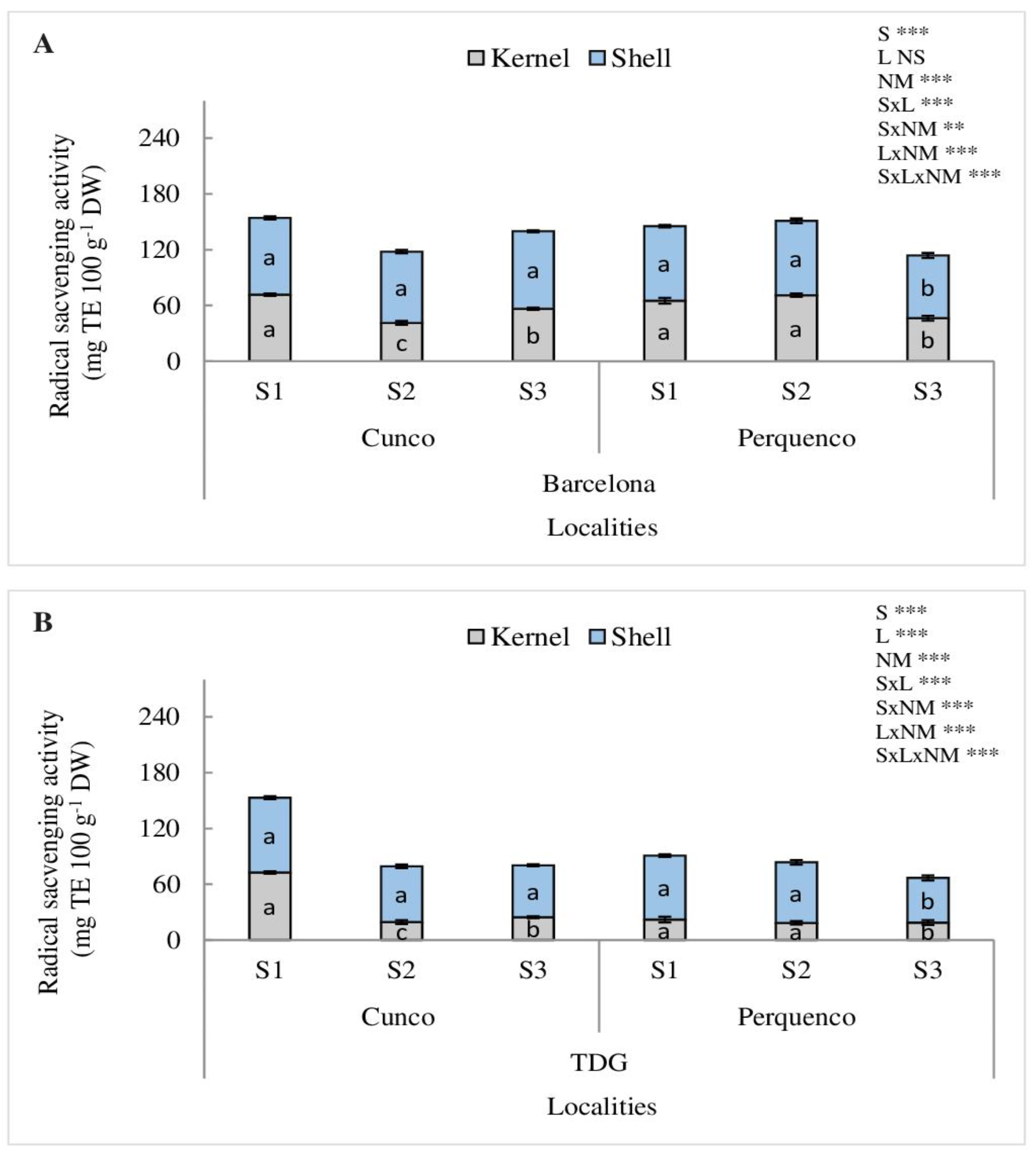
3.3.2. Free Radical Scavenging Activity
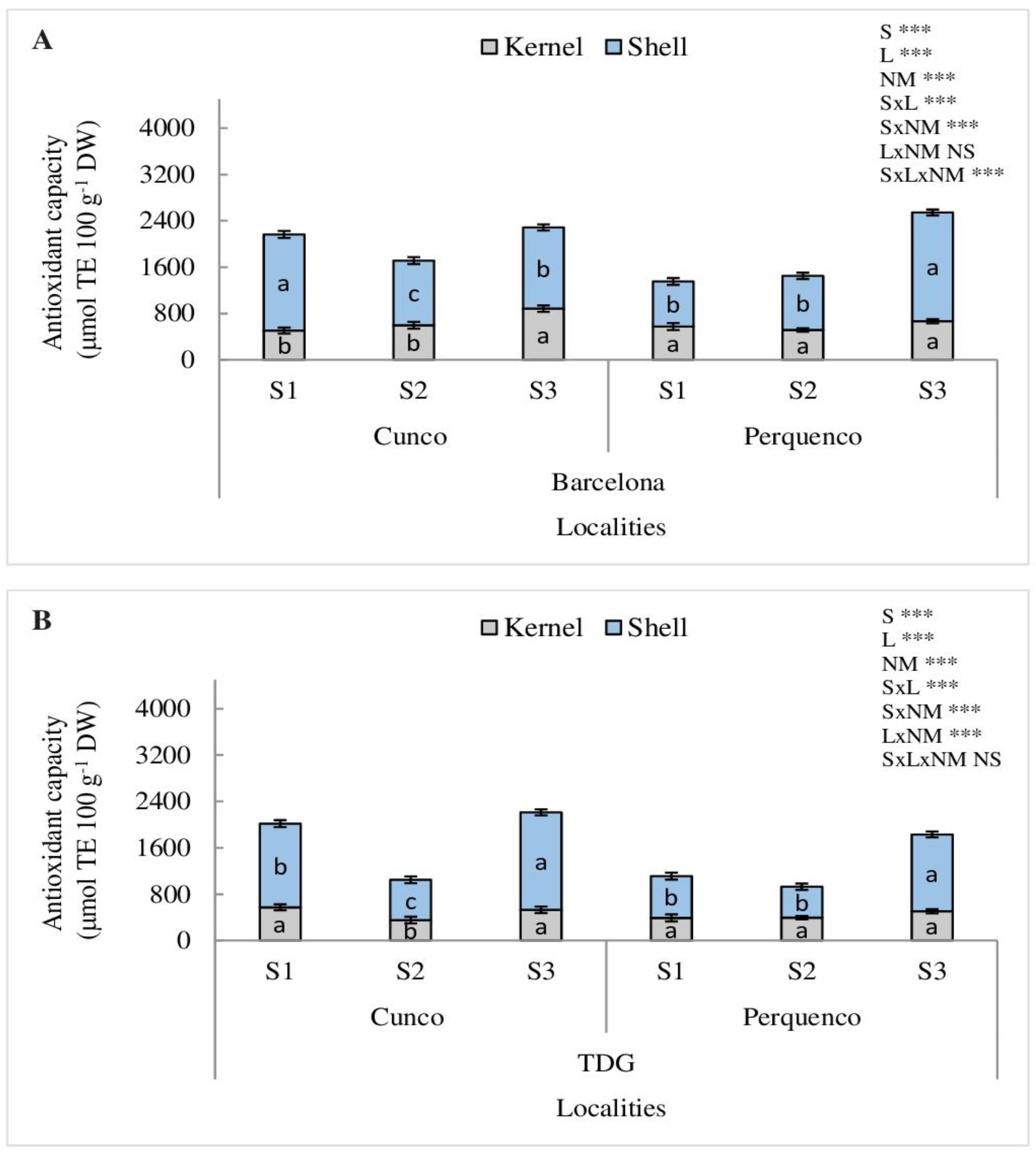
3.3.3. Antioxidant Capacity
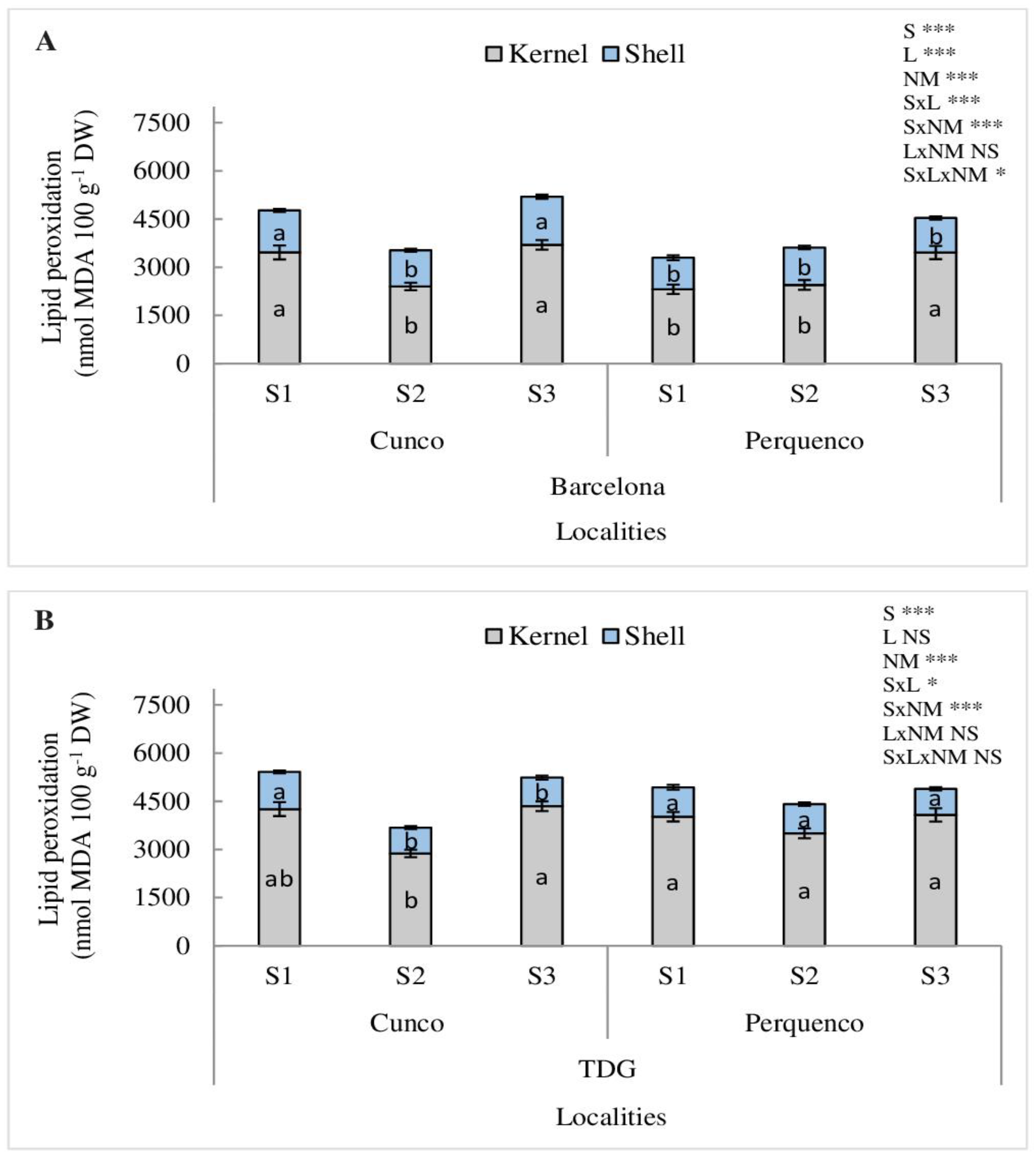
3.3.4. Lipid Peroxidation
3.4. Principal Components Analysis
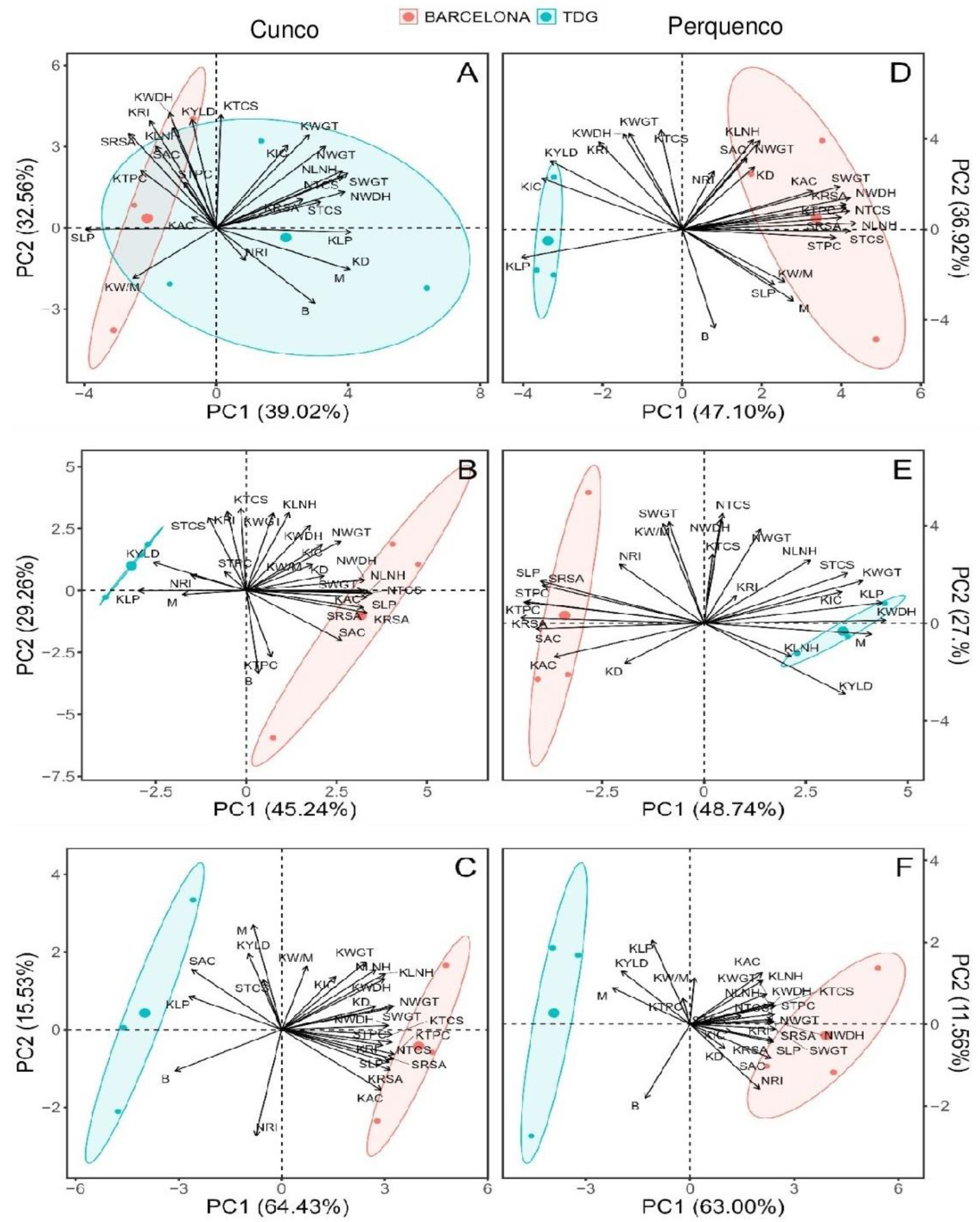
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuts Dried Fruits Statistical Yearbook. International Nut and Dried Fruit Council. Available online: https://inc.nutfruit.org/technical-projects/ (accessed on 25 April 2023).
- Agrichile Ferrero Hazelnut Company. Available online: https://agrichile.cl/noticias/mas-de-550-productoresparticiparon-de-las-dos-jornadas-del-meeting-anual-del-avellano-2023/ (accessed on 25 April 2023).
- Meriño-Gergichevich, C.; Luengo-Escobar, A.; Alarcón, D.; Reyes-Díaz, M.; Ondrasek, G.; Morina, F.; Ogass, K. Combined spraying of boron and zinc during fruit set and premature stage improves yield and fruit quality of European hazelnut cv. Tonda di Giffoni. Front. Plant Sci. 2021, 12, 984. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. Morphological Traits, Kernel Composition and Sensory Evaluation of Hazelnut (Corylus avellana L.) Cultivars Grown in Poland. Agronomy 2019, 9, 703. [Google Scholar] [CrossRef]
- Manterola-Barroso, C.; Godoy, K.; Alarcón, D.; Padilla, D.; Meriño-Gergichevich, C. Antioxidants in Shell and Nut Yield Components after Ca, Mg and K Preharvest Spraying on Hazelnut Plantations in Southern Chile. Plants 2022, 11, 3536. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, E.D.; Türkhan, A.; Bulut, M.; Koyuncu, M.; Güler, E.; Sayın, F.; Muradoğlu, F. The Effect of Altitude on Phenolic, Antioxidant and Fatty Acid Compositions of Some Turkish Hazelnut (Coryllus avellana L.) Cultivars. Molecules 2023, 28, 5067. [Google Scholar] [CrossRef]
- Solar, A.; Medic, A.; Slatnar, A.; Mikulic-Petkovsek, M.; Botta, R.; Rovira, M.; Sarraquigne, J.P.; Silva, A.; Veberic, R.; Stampar, F.; et al. The Effects of the cultivar and environment on the phenolic contents of hazelnut kernels. Plants 2022, 11, 3051. [Google Scholar] [CrossRef]
- Agrometeorología Red Agrometeorológica INIA. Available online: https://agrometeorologia.cl/ (accessed on 7 May 2023).
- Ferrão, A.C.; Guiné, R.P.F.; Ramalhosa, E.; Lopes, A.; Rodrigues, C.; Martins, H.; Gonçalves, R.; Correia, P.M.R. Chemical and physical properties of some hazelnut varieties grown in Portugal. Agronomy 2021, 11, 1476. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Simsek, A.; Aykut, O. Evaluation of the microelement profile of Turkish hazelnut (Corylus avellana L.) varieties for human nutrition and health. Int. J. Food Sci. Nutr. 2007, 58, 677–688. [Google Scholar] [CrossRef]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management and nuts storage for enhancing product quality and safety: An overview. J. Sci. Food Agric. 2020, 101, 27–43. [Google Scholar] [CrossRef]
- Özdemir, F.; Akinci, I. Physical and nutritional properties of four major commercial Turkish hazelnut varieties. J. Food Eng. 2004, 63, 341–347. [Google Scholar] [CrossRef]
- Ercisli, S.; Ozturk, I.; Kara, M.; Kalkan, F.; Seker, H.; Duyar, O.; Erturk, Y. Physical properties of hazelnuts. Int. Agrophys. 2011, 25, 115–121. [Google Scholar]
- Petriccione, M.; Ciarmiello, L.F.; Boccacci, P.; De Luca, A.; Piccirillo, P. Evaluation of ‘Tonda di Giffoni’ hazelnut (Corylus avellana L.) clones. Sci. Hortic. 2010, 124, 153–158. [Google Scholar] [CrossRef]
- Delprete, C.; Sesana, R. Mechanical characterization of kernel and shell of hazelnuts: Proposal of an experimental procedure. J. Food Eng. 2014, 124, 28–34. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Determination of size and shape features of hazelnuts using multivariate analysis. Acta Sci. Pol. Hortorum Cultus. 2017, 16, 49–61. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A.; Smith, D.C.; McCluskey, R.L. ‘Sacajawea’ hazelnut. Hortic. Sci. 2008, 43, 255–257. [Google Scholar] [CrossRef]
- Solar, A.; Stampar, F. Characterisation of selected hazelnut cultivars: Phenology, growing and yielding capacity, market quality and nutraceutical value. J. Sci. Food Agric. 2011, 91, 1205–1212. [Google Scholar] [CrossRef]
- Mohammadzedeh, M.; Fattahi, R.; Zamani, Z.; Khadivi-Khub, A. Genetic Identity and Relationships of Hazelnut (Corylus avellana L.) Landraces as Revealed by Morphological Characteristics and Molecular Markers. Sci. Hortic. 2014, 167, 17–26. [Google Scholar] [CrossRef]
- Cristofori, V.; Bertazza, G.; Bignami, C. Changes in kernel chemical composition during nut development of three Italian hazelnut cultivars. Fruits 2015, 70, 311–322. [Google Scholar] [CrossRef]
- Ilić, P.; Mićić, N.; Đurić, G.; Tojnko, S.; Solares, A.; Bosančić, B. Pomological identification of hazelnut cultivars (Corylus avellana L.) in plantations Bosnia and Herzegovina. Agric. Conspec. Sci. 2017, 84, 389–395. [Google Scholar]
- Correia, P.; Rodrigues, C.; Filipe, A.; Guiné, R. Evaluation of the Biometric Characteristics of Hazelnuts. In Proceedings of the FABE 2019: Food and Biosystems Engineering Conference, Crete Island, Greece, 30 May–2 June 2019. [Google Scholar]
- Özdemir, M.; Açkurt, F.; Kaplan, M.; Yıldız, M.; Löker, M.; Gürcan, T.; Biringen, G.; Okay, A.; Seyhan, F.G. Evaluation of new Turkish hybrid hazelnut (Corylus avellana L.) varieties: Fatty acid composition, a-tocopherol content, mineral composition and stability. Food Chem. 2001, 73, 411–415. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Hazelnuts (Corylus). Genet. Resour. Temp. Fruit Nut Crops. 1991, 290, 791–838. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A.; Smith, D.C.; Brenner, L.K. Variance components and heritability of nut and kernel defects in hazelnut. Plant Breed. 1993, 110, 144–152. [Google Scholar] [CrossRef]
- Guerrero Contreras, J.; Galdames Gutierrez, R.; Ogass Contreras, K.; Pérez Fuentealba, S. First report of Diaporthe foeniculina causing black tip and necrotic spot on hazelnut kernel in Chile. Plant Dis. 2020, 104, 975. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S. Hazelnut kernel defects and associated fungi in three provinces in Turkey. In Proceedings of the VII International Scientific Agriculture Symposium Agrosym 2016, Jahorina, Bosnia and Herzegovina, 6–9 October 2016; pp. 1312–1318. [Google Scholar]
- Battilani, P.; Chiusa, G.; Arciuolo, R.; Somenzi, M.; Fontana, M.; Castello, G.; Spigolon, N. Diaporthe as the main cause of hazelnut defects in the Caucasus region. Phytopathol. Mediterr. 2018, 57, 320–333. [Google Scholar]
- Pscheidt, J.W.; Heckert, S.; Wiseman, M.; Jones, L. Fungi associated with and influence of moisture on development of kernel mold of hazelnut. Plant Dis. 2019, 103, 922–928. [Google Scholar] [CrossRef]
- Wiman, N.G.; Webber III, J.B.; Wiseman, M.; Merlet, L. Identity and pathogenicity of some fungi associated with hazelnut (Corylus avellana L.) trunk cankers in Oregon. PLoS ONE 2019, 14, e0223500. [Google Scholar] [CrossRef]
- Beyhan, N.; Marangoz, D. An investigation of the relationship between reproductive growth and yield loss in hazelnut. Sci. Horticulturae. 2007, 113, 208–215. [Google Scholar] [CrossRef]
- Thompson, M.M.; Lagerstedt, H.B.; Mehlenbacher, S.A. Hazelnuts 125 184 Janick J. & Moore JN. Fruit Breed. 1996, 3, 1. [Google Scholar]
- McCluskey, R.L.; Mehlenbacher, S.A.; Azarenko, A.N.; Smith, D.C. “Santiam” Hazelnuts (OSU509.064); Extension Service of the Oregon State University: Corvallis, United States of America, 2005; 3p. [Google Scholar]
- McCluskey, R.; Mehlenbacher, S.; Smith, D. “Yamhill” Hazelnuts (OSU542.102); Extension Service of the Oregon State University: Corvallis, OR, USA, 2009; p. 3. [Google Scholar]
- Guerrero, C.J.; Merino-Gergichevich, C.; Ogass, C.K.; Alvarado, N.C.; Sobarzo, M.V. Quality and condition features of hazelnut (Corylus avellana L.) cv. Barcelona grown in South-Central of Chile. Rev. Fac. Cienc. Agrar. 2015, 47, 1–14. [Google Scholar]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and kernel traits and chemical composition of hazelnut (Corylus avellana L.) cultivars. J. Sci. Food Agric. 2008, 88, 1091–1098. [Google Scholar] [CrossRef]
- Jakopic, J.; Petkovsek, M.M.; Likozar, A.; Solar, A.; Stampar, F.; Veberic, R. HPLC-MS identification of phenols in hazelnut (Corylus avellana L.) kernels. Food Chem. 2011, 124, 1100–1106. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Sürek, E.; Büyükkileci, A.O. Extraction of antioxidant compounds from hazelnut wastes using subcritical water. GIDA J. Food 2018, 43, 211–221. [Google Scholar]
- Yilmaz, M.; Karakaya, O.; Balta, M.F.; Balta, F.; Yaman, İ. Changes in biochemical properties in Çakıldak hazelnut cultivar depending on the inner fruit size. Akademik. Ziraat. Dergisi. 2019, 8, 61–70. [Google Scholar] [CrossRef]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential Valorization of Edible Nuts By-Products: Exploring the Immune-Modulatory and Antioxidants Effects of Selected Nut Shells Extracts in Relation to Their Metabolic Profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. Investigation of changes in total phenol, flavonoid, antioxidant activity, fatty acids, polyphenol and mineral profiles of hazelnut kernels dried in air, oven and microwave. JSFA Reports 2023, 3, 72–81. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Islam, A.; Küçük, M. Fat and fatty acid composition of hazelnut kernels in vacuum packages during storage. Grasas Y Aceites 2005, 56, 263–266. [Google Scholar] [CrossRef]
- Mercanlıgil, S.M.; Arslan, P.; Alasalvar, C.; Okut, E.; Akgül, E.; Pınar, A.; Geyik, P.; Tokgözoğlu, L.; Shahidi, F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur. J. Clin. Nutr. 2007, 61, 212–220. [Google Scholar] [CrossRef]
- Alasalvar, C.; Pelvan, E.; Topal, B. Effects of roasting on oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.). Int. J. Food Sci. Nutr. 2010, 61, 630–642. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Stefanucci, A.; Ferrante, C.; Menghini, L.; Orlando, G.; Brunetti, L.; Locatelli, M.; Dimmito, M.; Novellinoc, E.; et al. Nutraceutical potential of Corylus avellana daily supplements for obesity and related dysmetabolism. J. Funct. Foods 2018, 47, 562–574. [Google Scholar] [CrossRef]
- Pelvan, E.; Öktem Olgun, E.; Karadağ, A.; Alasalvar, C. Phenolic profiles and antioxidant activity of Turkish Tombul hazelnut samples (natural, roasted, and roasted hazelnut skin). Food Chem. 2018, 244, 102–108. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat. J. Food Compos. Anal. 2009, 22, 184–188. [Google Scholar] [CrossRef]
- Li, H.; Parry, J.W. Phytochemical compositions, antioxidant properties, and colon cancer antiproliferation effects of Turkish and Oregon hazelnut. Food Sci. Nutr. 2011, 2, 1142–1149. [Google Scholar] [CrossRef]
- 16PTECFS-66647; Sustainability and Efficient Use of Resources in the Production of European Hazelnut (Corylus avellana L.) in South-Central Chile. CORFO: Santiago, Chile, 2017.
- Ondrašek, G. Fondecyt 11160762; Quality Traits and Fruit Yield in Hazelnut (Corylus Avellana L.) Associated with Boron and Zinc Levels and Phenological Stage of Application in Plantations of Southern Chile. University of Zagreb Faculty of Agriculture: Zagreb, Croatia, 2016.
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F.; Liyanapathirana, C.M.; Ohshima, T. Turkish tombul hazelnut (Corylus avellana L.). Compositional characteristics. J. Agric. Food Chem. 2003, 51, 3790–3796. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, J.; Wang, H.; Zhang, X.; Zhang, Y.; He, C.; Ji, X.; Li, H. Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem. 2007, 103, 913–918. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Hoffmann, J.F.; Galli, V.; Zambiazi, R.C. Composition and impact of pre-and post-harvest treatments/factors in pecan nuts quality. Trends Food Sci Technol. 2023, 131, 46–60. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Mari, A.; de Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS Profiling Highlights Hazelnut (Nocciola Di Giffoni PGI) Shells as a Byproduct Rich in Antioxidant Phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef]

| Nut | Shell | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Thickness | Width | Weight | NRI | Thickness | Weight | |||
| Season | Locality | Cultivar | (mm) | (g) | (mm) | (g) | |||
| S1 | Cunco | Barcelona | 19.38 ± 0.61 a | 15.95 ± 0.46 a | 18.74 ± 0.65 a | 2.49 ± 0.28 a | 0.90 ± 0.09 a | 1.48 ± 0.05 a | 1.38 ± 0.16 a |
| S2 | 20.20 ± 0.29 a | 17.11 ± 0.15 a | 19.89 ± 0.24 a | 2.77 ± 0.14 a | 0.92 ± 0.01 a | 1.42 ± 0.04 a | 1.55 ± 0.05 a | ||
| S3 | 19.79 ± 0.26 a | 16.30 ± 0.05 a | 19.64 ± 0.27 a | 2.81 ± 0.06 a | 0.91 ± 0.89 a | 1.48 ± 0.03 a | 1.68 ± 0.06 a | ||
| Mean | 19.79 ± 0.39 | 16.45 ± 0.22 | 19.42 ± 0.39 | 2.69 ± 0.16 | 0.91 ± 0.99 | 1.46 ± 0.04 | 1.54 ± 0.09 | ||
| S1 | Perquenco | 20.41 ± 0.32 ab | 18.16 ± 0.13 ab | 21.09 ± 0.29 a | 2.92 ± 0.21 a | 0.96 ± 0.01 a | 1.59 ± 0.02 a | 1.78 ± 0.07 a | |
| S2 | 19.10 ± 0.39 b | 17.72 ± 0.55 b | 20.03 ± 0.66 a | 2.90 ± 0.14 a | 0.99 ± 0.01 a | 1.43 ± 0.05 a | 1.66 ± 0.10 a | ||
| S3 | 22.80 ± 1.49 a | 19.84 ± 1.37 a | 22.82 ± 1.41 a | 3.36 ± 0.04 a | 0.96 ± 0.01 a | 1.59 ± 0.02 a | 1.94 ± 0.04 a | ||
| Mean | 20.77 ± 0.73 | 18.57 ± 0.68 | 21.31 ± 0.79 | 3.06 ± 0.39 | 0.97 ± 0.01 | 1.54 ± 0.03 | 1.79 ± 0.07 | ||
| S1 | Cunco | TDG | 20.23 ± 0.60 a | 17.01 ± 0.31 a | 20.06 ± 0.52 a | 2.80 ± 0.24 a | 0.92 ± 0.01 a | 1.57 ± 0.04 a | 1.64 ± 0.18 a |
| S2 | 18.85 ± 0.14 a | 16.20 ± 0.06 a | 18.86 ± 0.24 a | 2.61 ± 0.05 a | 0.93 ± 0.01 a | 1.49 ± 0.03 a | 1.38 ± 0.02 a | ||
| S3 | 18.54 ± 0.54 a | 15.42 ± 0.04 a | 18.31 ± 0.09 a | 2.29 ± 0.10 a | 0.92 ± 0.01 a | 1.57 ± 0.04 a | 1.31 ± 0.06 a | ||
| Mean | 19.20 ± 0.43 | 16.21 ± 0.14 | 19.08 ± 0.28 | 2.57 ± 0.39 | 0.92 ± 0.01 | 1.54 ± 0.04 | 1.44 ± 0.09 | ||
| S1 | Perquenco | 18.70 ± 0.16 a | 16.53 ± 0.31 a | 19.03 ± 0.24 a | 2.58 ± 0.17 a | 0.95 ± 0.01 a | 1.48 ± 0.02 a | 1.39 ± 0.07 a | |
| S2 | 19.63 ± 0.37 a | 17.69 ± 0.17 a | 20.01 ± 0.08 a | 2.96 ± 0.10 a | 0.96 ± 0.02 a | 1.56 ± 0.06 a | 1.58 ± 0.05 a | ||
| S3 | 19.22 ± 0.22 a | 16.01 ± 0.07 a | 18.32 ± 0.13 a | 2.61 ± 0.10 a | 0.95 ± 0.01 a | 1.48 ± 0.02 a | 1.41 ± 0.03 a | ||
| Mean | 19.18 ± 0.25 | 19.74 ± 0.18 | 19.12 ± 0.15 | 2.71 ± 0.12 | 0.95 ± 0.01 | 1.51 ± 0.03 | 1.46 ± 0.05 | ||
| Significance | S | NS | NS | NS | NS | *** | ** | NS | |
| L | NS | *** | ** | ** | *** | * | * | ||
| C | ** | *** | *** | * | NS | NS | *** | ||
| SxL | * | NS | NS | NS | * | NS | NS | ||
| SxC | * | ** | ** | * | NS | * | ** | ||
| LxC | NS | ** | ** | NS | * | NS | * | ||
| SxLxC | * | * | * | NS | NS | NS | * | ||
| Kernel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Thickness | Width | Weight | KRI | IC | Kernel | |||
| Season | Locality | Cultivar | (mm) | (g) | (mm) | (%) | |||
| S1 | Cunco | Barcelona | 14.19 ± 0.92 a | 10.51 ± 1.00 a | 13.59 ± 0.94 a | 1.16 ± 0.16 a | 0.82 ± 0.04 a | 2.40 ± 0.32 b | 45.25 ± 2.56 a |
| S2 | 14.36 ± 0.70 a | 10.81 ± 0.75 a | 13.70 ± 0.67 a | 1.21 ± 0.07 a | 0.83 ± 0.04 a | 4.08 ± 0.33 a | 43.47 ± 1.50 a | ||
| S3 | 14.45 ± 0.62 a | 10.31 ± 0.38 a | 12.85 ± 0.45 a | 1.13 ± 0.03 a | 0.75 ± 0.03 a | 2.40 ± 0.32 b | 39.19 ± 1.72 b | ||
| Mean | 14.33 ± 0.75 | 10.54 ± 0.71 | 13.38 ± 2.06 | 1.16 ± 0.09 | 0.80 ± 0.04 | 2.96 ± 0.32 | 42.64 ± 1.93 | ||
| S1 | Perquenco | 13.66 ± 0.65 a | 10.89 ± 1.37 a | 13.10 ± 1.63 a | 1.13 ± 0.17 a | 0.83 ± 0.13 a | 1.71 ± 0.41 b | 37.35 ± 4.36 b | |
| S2 | 13.73 ± 0.30 a | 11.94 ± 0.14 a | 13.70 ± 0.13 a | 1.24 ± 0.04 a | 0.93 ± 0.02 a | 2.73 ± 0.43 a | 42.31 ± 1.50 a | ||
| S3 | 17.06 ± 1.54 a | 14.70 ± 1.20 a | 16.85 ± 1.35 a | 1.43 ± 0.01 a | 0.83 ± 0.13 a | 1.71 ± 0.41 b | 37.35 ± 4.36 b | ||
| Mean | 14.82 ± 2.49 | 12.51 ± 0.90 | 14.55 ± 1.04 | 1.35 ± 0.07 | 0.86 ± 0.09 | 2.05 ± 0.42 | 39.00 ± 3.41 | ||
| S1 | Cunco | TDG | 14.13 ± 0.78 a | 10.95 ± 0.78 ab | 12.59 ± 0.84 ab | 1.23 ± 0.11 a | 0.78 ± 0.06 a | 2.50 ± 0.38 b | 42.83 ± 0.78 b |
| S2 | 14.16 ± 0.06 a | 11.45 ± 0.04 a | 13.39 ± 0.08 a | 1.22 ± 0.02 a | 0.86 ± 0.09 a | 3.67 ± 0.39 a | 46.46 ± 0.05 a | ||
| S3 | 11.83 ± 0.85 a | 8.23 ± 0.22 b | 11.07 ± 0.56 b | 1.03 ± 0.06 a | 0.78 ± 0.06 a | 2.50 ± 0.38 b | 41.62 ± 1.78 b | ||
| Mean | 13.37 ± 0.56 | 10.21 ± 0.35 | 12.35 ± 0.49 | 1.16 ± 0.06 | 0.81 ± 0.07 | 2.89 ± 0.38 | 43.64 ± 0.87 | ||
| S1 | Perquenco | 12.65 ± 0.60 a | 10.72 ± 0.63 a | 13.65 ± 0.75 ab | 1.19 ± 0.09 a | 0.90 ± 0.04 a | 2.91 ± 0.41 b | 44.26 ± 1.49 b | |
| S2 | 14.39 ± 0.38 a | 11.92 ± 0.20 a | 14.66 ± 0.07 a | 1.38 ± 0.04 a | 0.94 ± 0.03 a | 4.13 ± 0.33 a | 46.93 ± 0.24 a | ||
| S3 | 14.08 ± 0.73 a | 10.37 ± 0.46 a | 12.72 ± 0.45 b | 1.21 ± 0.06 a | 0.90 ± 0.04 a | 2.91 ± 0.41 b | 44.26 ± 1.49 b | ||
| Mean | 13.71 ± 0.57 | 11.00 ± 0.43 | 13.68 ± 0.42 | 1.26 ± 0.06 | 0.91 ± 0.04 | 3.32 ± 0.38 | 45.15 ± 1.07 | ||
| Significance | S | NS | NS | NS | NS | * | *** | NS | |
| L | NS | ** | * | NS | *** | NS | NS | ||
| C | * | * | NS | NS | NS | NS | ** | ||
| SxL | ** | * | NS | NS | NS | NS | * | ||
| SxC | * | ** | * | NS | NS | NS | NS | ||
| LxC | NS | NS | NS | NS | NS | NS | NS | ||
| SxLxC | NS | NS | NS | NS | NS | NS | NS | ||
| Nut | ||||||
|---|---|---|---|---|---|---|
| Blank | Wrinkled/Misshapen Kernel | Double Kernel | Mold | |||
| Season | Locality | Cultivar | (%) | |||
| S1 | Cunco | Barcelona | 3 | 10 | 0 | 0 |
| S2 | 3 | 7 | 3 | 0 | ||
| S3 | 3 | 13 | 10 | 7 | ||
| Mean | 3 | 10 | 4.3 | 2.3 | ||
| S1 | Perquenco | 7 | 3 | 13 | 3 | |
| S2 | 0 | 13 | 3 | 0 | ||
| S3 | 0 | 13 | 10 | 0 | ||
| Mean | 2.3 | 9.6 | 8.6 | 0.3 | ||
| S1 | Cunco | TDG | 7 | 10 | 3 | 7 |
| S2 | 0 | 0 | 0 | 3 | ||
| S3 | 13 | 10 | 3 | 7 | ||
| Mean | 6.6 | 6.6 | 2 | 5.6 | ||
| S1 | Perquenco | 7 | 10 | 3 | 0 | |
| S2 | 0 | 7 | 0 | 17 | ||
| S3 | 3 | 10 | 3 | 10 | ||
| Mean | 3.3 | 9 | 2 | 9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





