1. Introduction
After the pioneering work of Fujishima and Honda (1972) [
1] on the photocatalytic properties of TiO
2 numerous studies carried out to elucidate the principles, mechanisms and mode of action of TiO
2 for environmental remediation purposes. The development of innovative coatings containing TiO
2 as a photo-catalyst was in the foreground of research activities with the aim to be used as building and construction materials mainly outdoors, e.g. on building facades in high traffic roads for the degradation of priority air pollutants (NOx and volatile organic compounds) in the urban atmosphere.
Research over the past decades has well documented that heterogeneous photocatalysis leads to the degradation of inorganic and organic pollutants under various environmental conditions. Photo-induced reactions in the heterogeneous phase proceed differently from those in the homogeneous phase. Due to the interaction between the functional groups of the adsorbed chemical compounds and the adsorbent surfaces, bond lengths and bond angles between individual atoms of the adsorbed compounds are altered. This leads to changes in the light absorption behaviour of the compounds. Changes in the absorption behaviour are generally of a bathochromic nature (red-shift). In addition changes in the relative intensities of the individual bands (hyper-chromic effects) might also be very important. As a consequence, compounds which practically do not absorb tropospheric light (λ > 290 nm), once they are adsorbed on various surfaces e.g. soil, might be transformed or degraded under atmospheric conditions due to changes in the absorption behaviour and the reaction with reactive species formed on the surface.
Numerous studies in the literature reported photo-induced degradation of compounds of different chemical classes, e.g., aromatics, insecticides, and herbicides adsorbed onto silica gel or soil after irradiation with UV light (λ> 290 nm) of the solar spectrum, indicating a total degradation of the organic compounds (photo-mineralization). In some of these studies an attempt was made to correlate the rates of the heterogeneous photo-oxidation (photo-mineralization, % of CO
2 production) of chemicals adsorbed onto e.g. silica gel with their ionization potential [
2,
3,
4,
5]. This correlation suggests that the photochemical oxidative degradation of environmental chemicals can be estimated merely by measuring their ionization potentials.
Unlike the heterogeneous photo-catalysis with solar-or UV-light and the degradation of substances adsorbed onto soil, particulate matter or SiO
2 surfaces, the photo-induced degradation of chemicals with semiconductors e.g. TiO
2 proceeds via another way. It is an energetically interesting process because it operates near ambient temperature using (basically) solar energy/UV to initiate photo-catalytic reactions. The impact of solar or UV-light on TiO
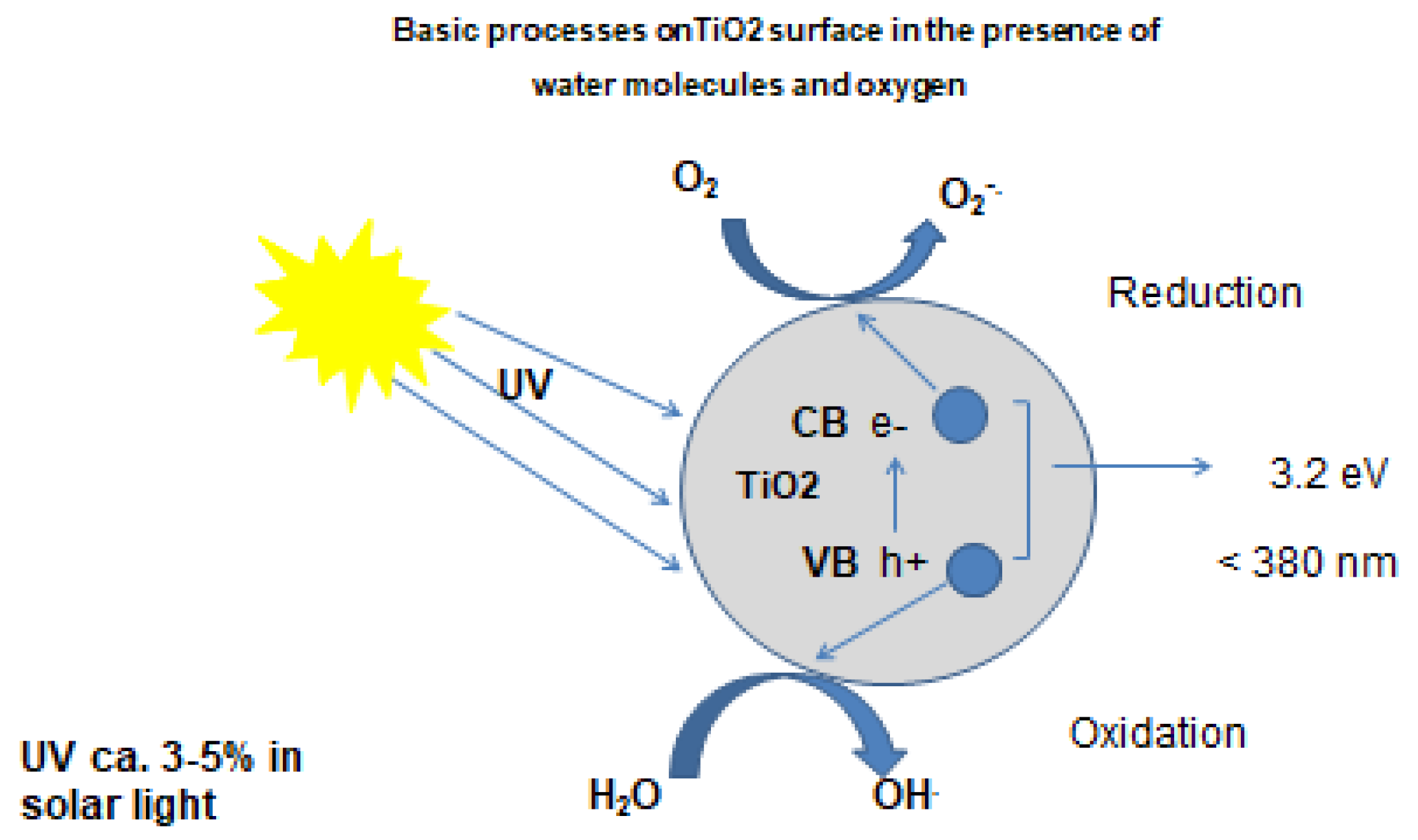
2 leads to the separation of charges through the movement of an electron from the valence band (VB) of TiO
2 to the conduction band (CB) leaving a positive hole behind (
Figure 1) [
6]. TiO
2 is considered to be the most used photo-catalyst for the removal of pollutants due to its highly strong oxidative ability even at low UV irradiation. The best photo-catalytic performance with the maximum quantum yield, which represents the number of reactions per absorbed photon, is achieved by TiO
2. Moreover, TiO
2 is a chemically stable material (no photo-corrosion), very common and relative cheap.
Positive hole (h+) and the free electron (e-) could recombine very fast (in femtoseconds) if no other molecules are present in the photo-chemical system. Recombination is inhibited when water molecules, oxygen or other compounds are present. In this case, positive holes in the valence band and the electrons in the conduction band may react at the air/catalyst interface. The formation of reactive oxygen species and co-occurring redox-reactions are reported. The photocatalytic effectiveness of a semiconductor surface is measured by its ability to generate positive holes in the valence and promoting electrons to the conduction band initiating redox reactions of charge carriers with other molecules present in the photochemical system [
7,
8].
Building materials modified/enriched with TiO
2 gained an increasing importance in the last years given the full range of possible applications. TiO
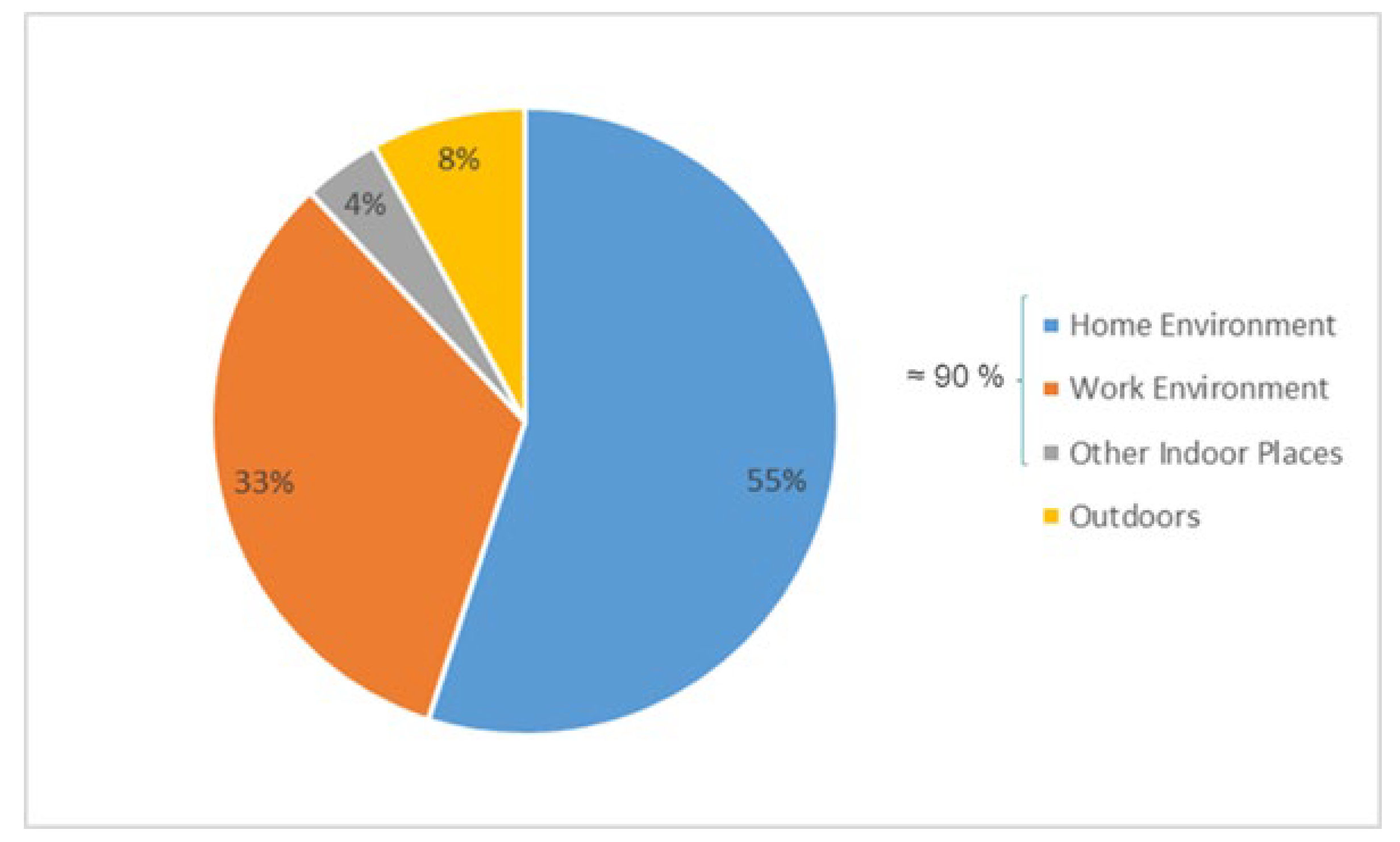
2-based photocatalytic materials (e.g. paints) are initially developed and used as outer coatings in the facades of buildings to clean the polluted air using the solar energy only. Since we spend ca.85–90% of our time indoors (
Figure 2) substantial efforts were made to investigate further the photo-catalytic activity of materials containing TiO
2 or modified (doped) TiO
2 towards priority air pollutants e.g. NO, NO
2, and VOCs frequently accumulated in indoor environments
A number of transition metals, such as V, Cr, Fe, Mn, Ni, Co, Cu, and Zn as well as non-metals (N), have been explored to reduce the energy gap facilitating the movement of electrons and, thus, expanding the absorption spectral range of modified TiO2 towards the visible light.
Experiments done with manganese (Mn) doped TiO
2 indicate the total degradation of NO at indoor-like irradiation conditions, too [
10].
In the present short review the focus is on the heterogeneous photo-catalysis using TiO2 for the degradation of NO/NO2, benzene and toluene (at typical indoor/outdoor concentration levels), once in contact with the semiconductor surface. The surface chemistry at the air/catalyst interface and the formation of reactive oxygen species with co-occurring redox reactions are investigated. Factors affecting the application of TiO2 for the degradation of air contaminants as individual compounds and mixtures are discussed. The photocatalytic activity of Mn doped TiO2 on the degradation of NO under indoor-like illumination was evaluated. Moreover, the formation of by-products, particularly carbonyls, by the irradiation of TiO2 enriched building materials is investigated.
2. Adsoption of Volatile Organic Compounds on the TiO2 Surface
Adsorption of pollutants e.g. benzene, toluene, aldehydes onto a photo-catalyst surface plays a great role in the efficacy of the photocatalytic oxidation (PCO) technology for air purification applications. TiO2 has three main allotropic forms. Rutile, anatase and brookite. The principal forms employed as photocatalysts are anatase and rutile. The use of brookite is limited due to its photoelectric properties e.g. higher band gap. In addition, pure brookite without rutile or anatase is rather difficult to be prepared so that, until recently, its photocatalytic properties have not been extensively studied.
Generally, the affinity for the adsorption of organic compounds on anatase is higher than on rutile and the adsorption capacity of O
2 in rutile is lower than on αnatase. This also leads to an increased recombination rate of e- and h+. In a study on the adsorption performance of P25 (70% anatase and 30% rutile), it was showed that alcohols possess higher adsorption efficiency compared to ketones, aromatics, and alkanes. Boulamanti et al. examined the adsorption of aromatic VOCs on P25 and reported that the adsorption constants calculated from a Langmuir-Hinshelwood model follow the order: ethyl-benzene < benzene < o-xylene < p-xylene ∼ m-xylene ∼ toluene. The low adsorption of ethyl-benzene and o-xylene was partly ascribed to their molecular structures, which give rise to bigger stereo-chemical hindrance during adsorption [
11].
3. Formation of Singlet Oxygen (1 O2) during the Photocatalytic Processes
The formation of singlet oxygen during the photocatalytic processes with various oxides e.g. TiO
2, vanadium (V
2O
5) and molybdenum oxides (M
OO
3) was reported [
12]. Singlet oxygen could react and degrade organic compounds leading to the production of CO
2 and water. Singlet oxygen is formed through the reaction of superoxide anion with positive holes
(O2•− + h+ → 1O2 or O2).
According to M.V. Vishnetskaya and I.S. Tomskiy the reaction between singlet oxygen and toluene leads to various intermediates (maleic acid and maleic anhydride) that
finally degrade to the end products CO2 and H2O [
12]
. The photocatalysis in relation to organic compounds is mainly an oxidative process; usually reduced is only the molecular oxygen. It is possible that a molecule initially oxidized by positive holes and then it is reduced again by conduction band electrons. This can be seen as a special case of recombination, that is possibly leading to the formation of singlet oxygen (
1O
2).
4. Degradation of Benzene and Toluene and NO as Model Compounds on TiO2 Enriched Materials
In numerous studies the photocatalytic behaviour of TiO
2 enriched materials and the removal of benzene and toluene was studied. The vast majority of experiments for the heterogeneous photocatalysis of pollutants carried out at ppmv concentration levels, much higher of those typically found in the real environment [
13,
14,
15,
16,
17].
In our studies two types of experiments carried out:
-
a)
Experiments carried out in a 30 m3 environmental chamber (INDOORTRON facility at the EC-Joint research Centre, Ispra, Italy) to estimate the degradation of NO and NO2 on panels covered with a mineral silicate paint enriched with 10% and 5% of TiO2, respectively (table 1). The initial concentration of NO was 220 ppbv, the temperature was set at 23 0C and the humidity level at 50%. After six hours of irradiation with UV-light (S1), NO was degraded to more than 80% while the degradation of NO2 reaches values up to 60%. The paint materials (without UV) had an insignificant effect on NO removal (5.9%), while the corresponding value for NO2 was 26.2 %.
Table 1. Composition of the investigated mineral silicate paint.
Component Content (percentage by weight)
Cement (containing TiO2) 43
Fine sand 52.6
Cohesion agents (Methyl-hydroxy-ethylcellulose) 0.65
Super-plasticizer (sulphonated melamin) 1.4
Defoaming agent (fatty alcohols, polyacrylate) 0.47
Re-dispersible resin (Vinyl copolymer) 1.72
Higher NO
2 than NO adsorption phenomena on the surface of the sample (mineral silicate paint) was the main reason of the higher amount of NO
2 removal on the blank sample. An experiment with the same type of mineral silicate paint containing 5% of TiO
2 (S3) resulted to 29, 6 % removal of NO, significantly less than with the same paint containing 10% of TiO
2. Reduction of NO
2 was about 33, 6 %. Also by this experiment higher NO
2 than NO adsorption on the surface of the sample was the main reason of the NO
2 removal (Table 2) [
18].
Table 2.
Photo-catalytic degradation of NOx on mineral silicate paints with different content of TiO2 (220 ppbv NO) [modified].
Table 2.
Photo-catalytic degradation of NOx on mineral silicate paints with different content of TiO2 (220 ppbv NO) [modified].
| %NO reduction |
%NO reduc- tion due to TiO2 |
% NO2 reduction |
%NO2 re-
duction due to TiO2 |
Sample
Name |
With UV |
Without UV |
With UV |
Without UV |
| S1 |
82.4 |
5.9 |
73.9 |
60.5 |
26.2 |
27.6 |
| S2 |
8.5 |
5.0 |
0 |
32.9 |
37.9 |
0 |
| S3 |
29.6 |
4.5 |
21.1 |
33.6 |
27.4 |
0.7 |
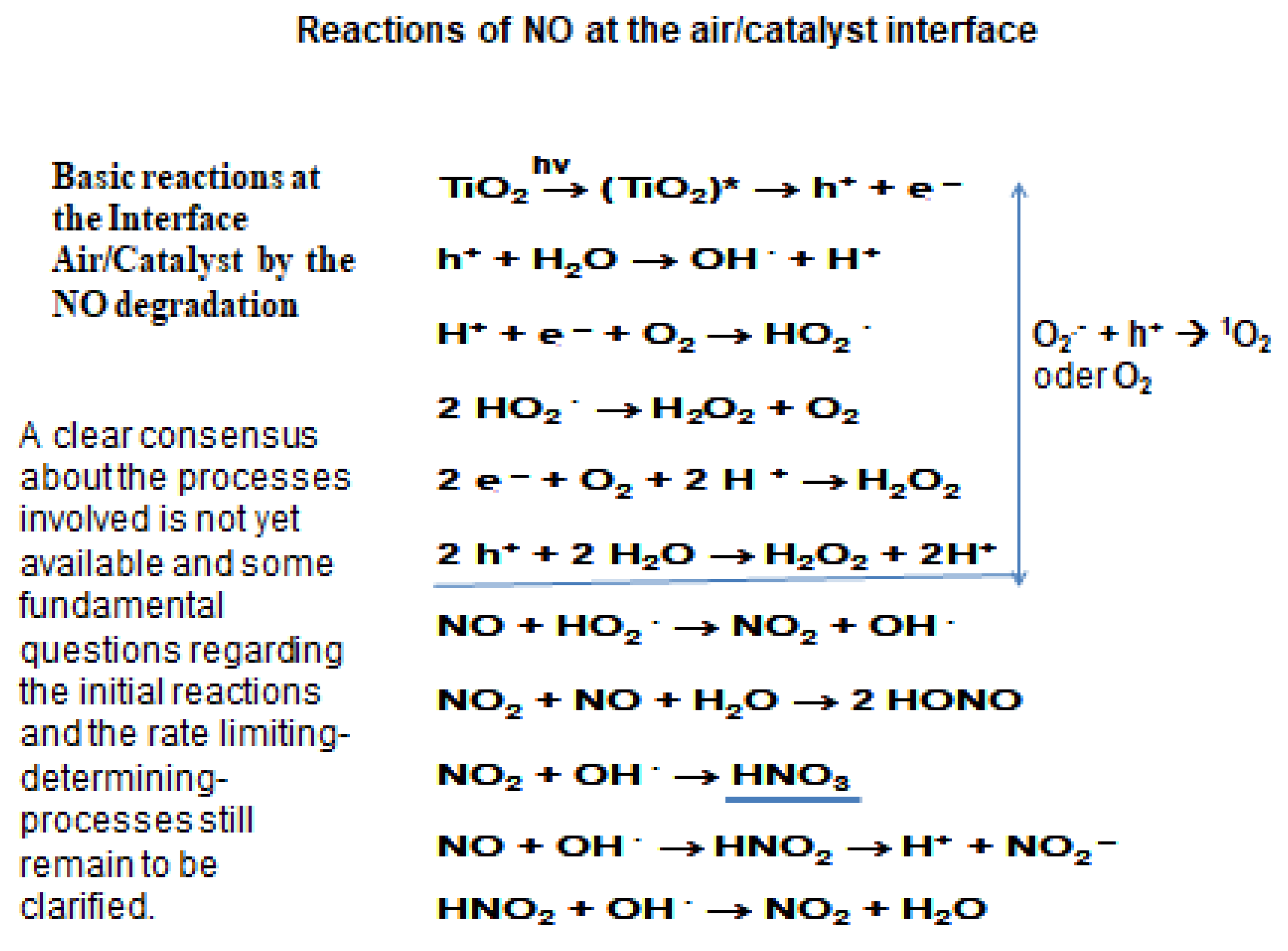
As an intermediate by the NO degradation, NO2 is formed that further oxidized to nitrate, the end-product of the NO degradation (scheme 1). The presence of nitrites and nitrates after the photocatalytic reactions has been qualitatively documented on the surface of the TiO2-based materials through TOF-SIMS (Time of Flight-Secondary Ion Mass Spectrometry)(Figure 3). It should also be mentioned that the degradation of NO proceeds not only heterogeneously on the TiO2 surface, but also in the gas phase via the oxidation with oxygen (2NO +O2 -> 2NO2) to form NO2. Elimination of NO2 from the gas phase through adsorption on the TiO2-containing materials is the speed-determining step for the NO oxidation to NO2.
Scheme 1.
Figure 3. Material (silicate paint) + TiO
2+ UV +NO/Air/20% Rel.humidity, after the analysis with TOF-SIMS (Time of Flight MS). The sample clearly shows peaks assigned to NO
2 (mass 46) and NO
3 (mass 62) in mass/unit [
18].
Scheme 1.
Figure 3. Material (silicate paint) + TiO
2+ UV +NO/Air/20% Rel.humidity, after the analysis with TOF-SIMS (Time of Flight MS). The sample clearly shows peaks assigned to NO
2 (mass 46) and NO
3 (mass 62) in mass/unit [
18].
-
b)
In addition, experiments conducted with low concentrations of benzene/toluene (7-8 ppbv) and NO (40 ppbv) in environmental (glass) chambers of a volume of 0.45 m
3. At low concentration levels the degradation of benzene and toluene at 23
0 C and at humidity levels of 60 and 20%, reaches up levels for benzene between 25-77%, and for toluene under the same conditions between 75 and 90%, after six hours of irradiation, respectively. The photocatalytic degradation of NO on TiO
2 enriched surfaces (gypsum board) is not influenced at any humidity level under the conditions in our study. After three hours of irradiation NO is almost quantitatively degraded. According to the data obtained, oxidation of NO to NO2 and the subsequent conversion to nitrate (scheme 1), seems to be also under these conditions the key process leading to the elimination of NO at both humidity levels (20 and 60%). The addition of toluene or benzene to NO does not have a measurable impact on NO degradation at 20% or 60% relative humidity. The photo-induced degradation of NO on the plaster surface strongly differs from that reported with other photocatalytic materials where changes in relative humidity result to changes in the photo-degradation of NO [
13,
14,
18].
Most studies with priority pollutants carried out with single compounds, either with toluene, benzene or NO present in the photochemical system. In a few studies the compounds are subjected as a mixture to irradiation with UV in the presence of TiO2. This was done to simulate and evaluate the photo-induced degradation of mixtures of these
chemicals on TiO
2 enriched surfaces because in the real environment, indoors and outdoors, toluene, benzene and NO/NO
2 are mostly present as mixtures. Experiments carried out with distinct mixtures of volatile organic compounds at low concentrations (7 to 8 ppbv for benzene/toluene, and 40 ppbv for NO) and at humidity levels of 20 and 60% under the same experimental conditions as by the degradation of the individual compounds [
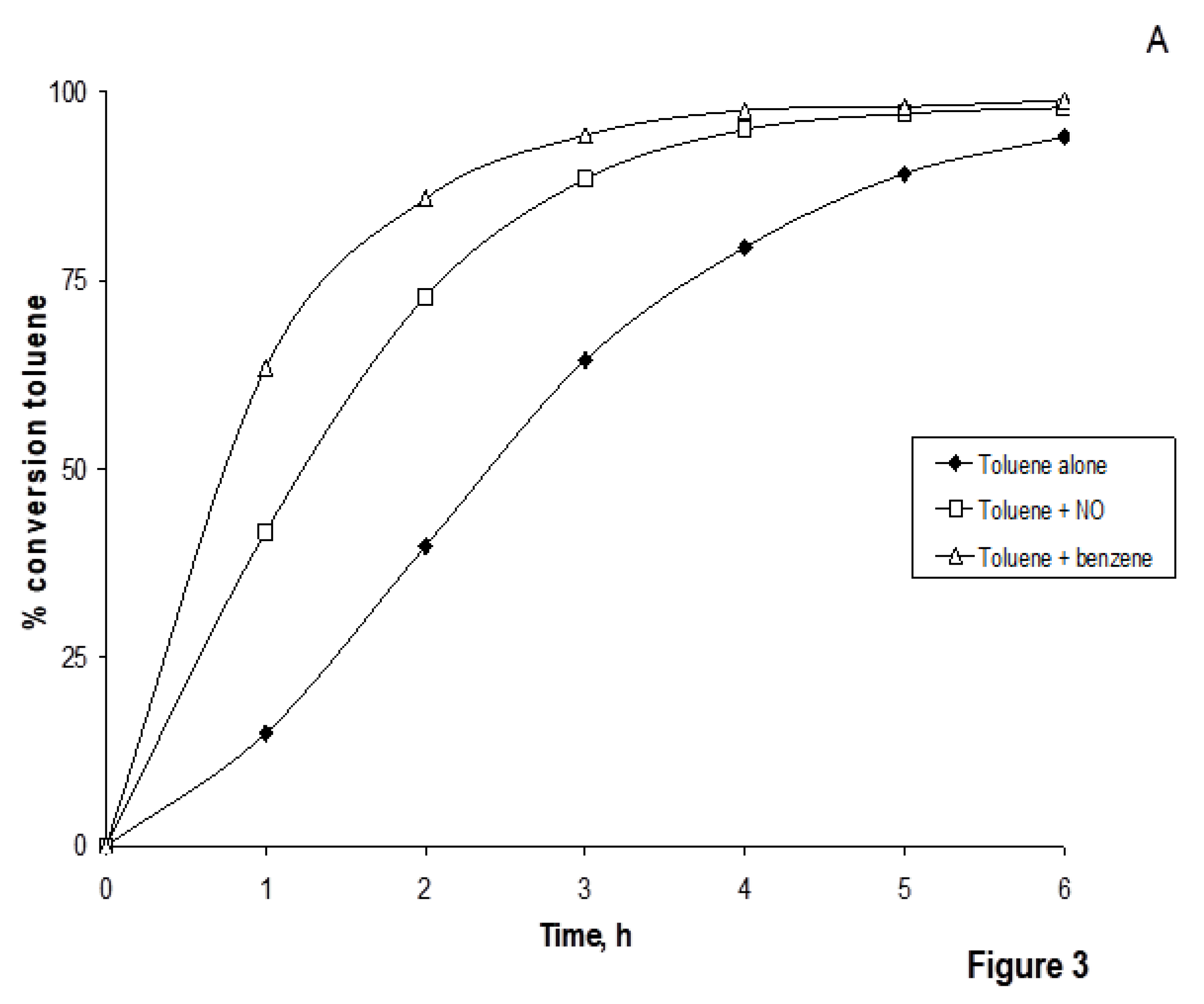
19]. The photocatalytic degradation of toluene (in mixtures with NO or benzene) was strongly influenced by the presence of benzene and/or nitrogen oxide at 20% relative humidity. After four hours of
UV-irradiation, toluene as individual compound was degraded to 77%, while in mixture with NO or benzene the photo-induced degradation of toluene after four hours was 92-93% (
Figure 4). After six hours of UV-irradiation, toluene as individual compound was degraded to 91%, while in mixture with NO or benzene the photo-induced degradation of toluene in the same time was 100% (Figure. 3). At humidity levels of 60% the degradation of toluene in mixture with NO and benzene reached up levels between 70-80%. Hence, the impact of nitrogen oxide and benzene on the degradation of toluene at the 60% humidity level was found to be negligible.
The fact that benzene promotes the photocatalytic degradation of toluene (at RH 20%) in the photochemical system without the presence of NO is interesting. It cannot be explained through the generation of OH radicals following the interaction of water molecules with positive holes, only. Both compounds benzene and toluene react with the OH-radicals, while the rate constant of the reaction OH-toluene is greater (5.78 x10
-12 cm3/mol. s) than the rate constant of benzene (12.9x 10
-13 cm3/mol. s). Thus, when both compounds (benzene and toluene) are present in the photo-chemical system, the generated OH-radicals react (even with different rate constants) with both compounds; the OH radicals are not entirely available for one compound only. This means, that in the case of the irradiation of toluene as a single compound, the photocatalytic degradation would be faster than in the case of the irradiation of a mixture with benzene, because OH-radicals react with/ or are consumed by one compound, namely toluene, only. The substantial impact of benzene on the degradation of toluene is explained via a classical electrophilic substitution according to
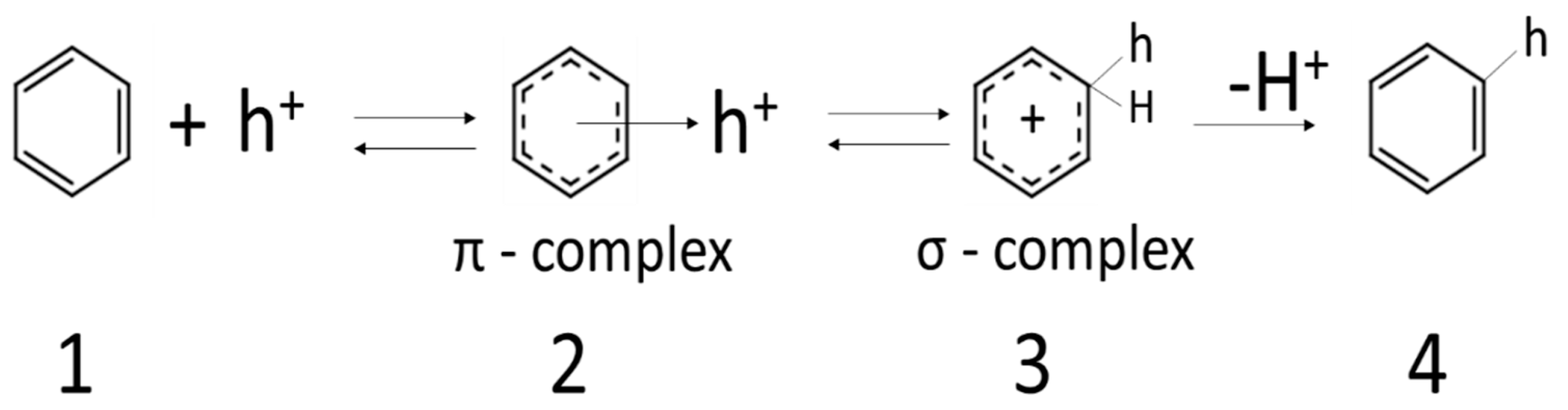
Figure 5 and Scheme 2. Positive holes react as electrophiles towards benzene.
Scheme 2.
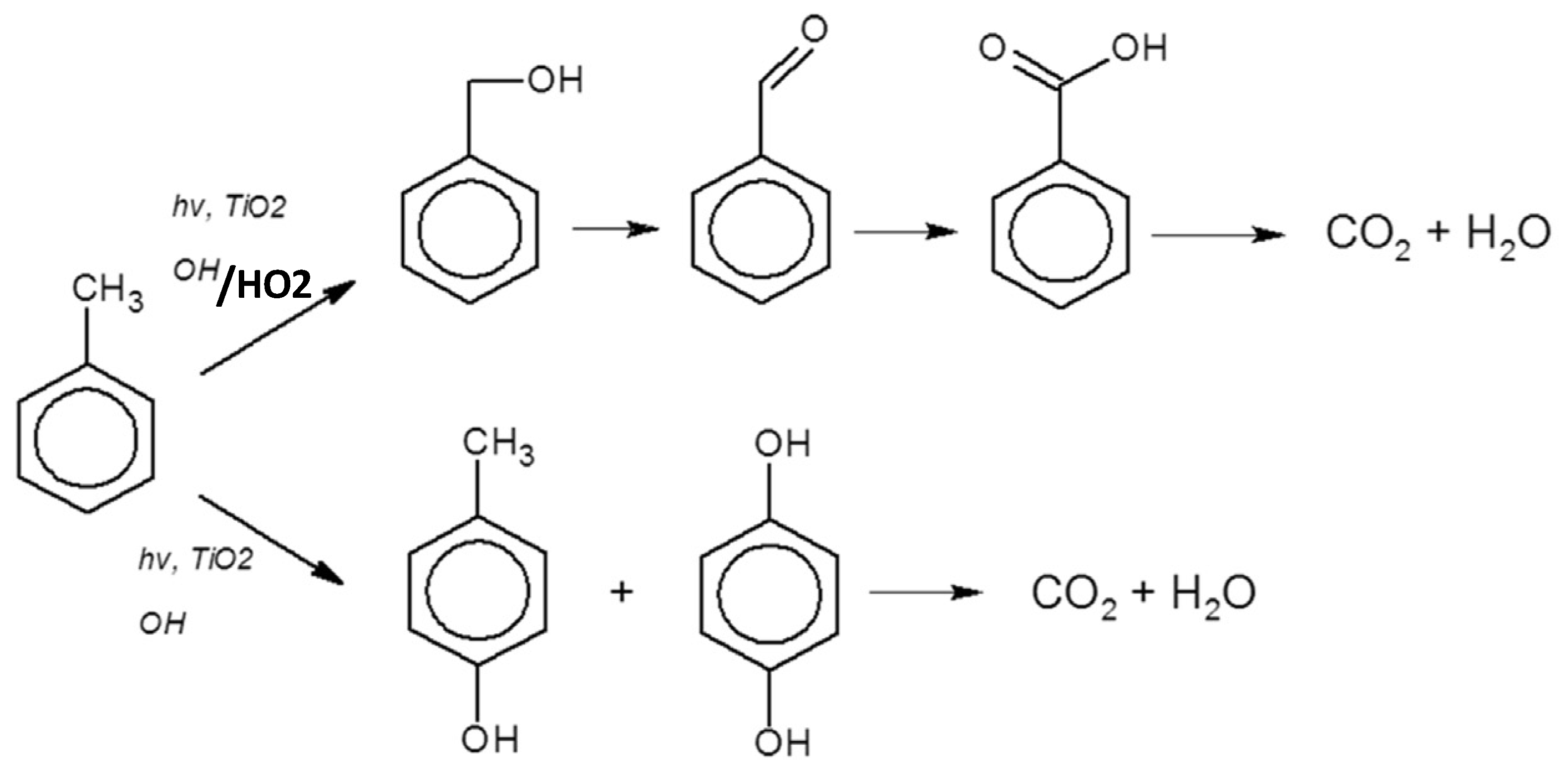
Through the reactions 1,2,3 (Scheme 2) the proton (H+) from the benzene ring initiates the formation of radicals (HO
2) that attacked toluene leading to products like benzyl alcohol, benzaldehyde, benzoic acid (
Figure 6).
5. Heterogeneous Photo-Catalysis of NO under Indoor-Like Illumination Conditions/Doping
The advantages connected with the application of TiO2 are limited due to its band gap of 3.2 eV. Hence, efforts made to increase the area of activity of TiO2 using visible light, which will extend its application to indoor environments. The activity of TiO2 depends on the lifetime of charge carriers - positive holes and electrons - produced on its surface. Recombination of positive holes and electrons occurs in an extremely short time before undergoing redox reactions.
Therefore, the primary challenge for an efficient photocatalytic process is to reduce or inhibit the recombination of charge carriers to maintain the photo-catalyst activity at a high level.
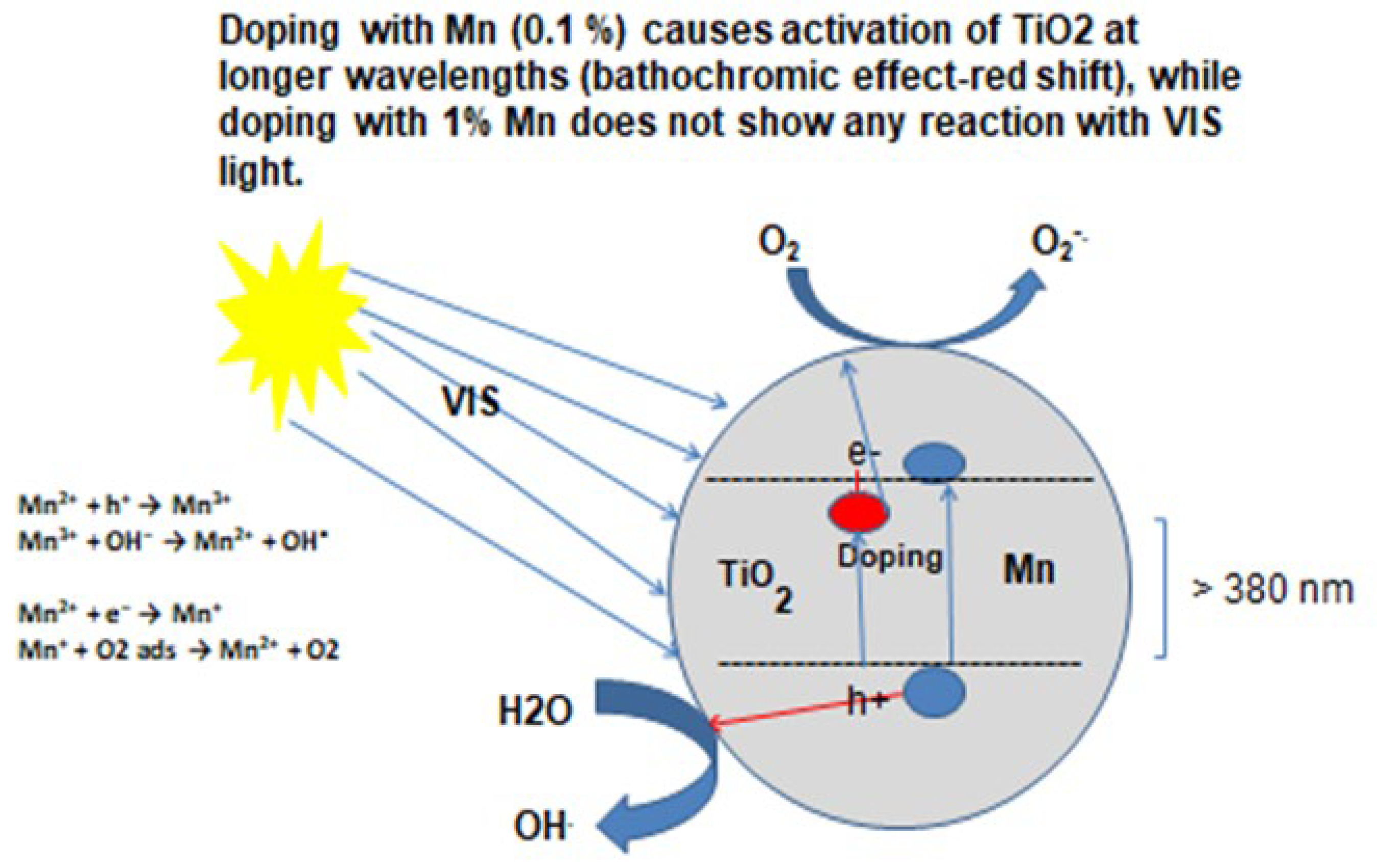
One way to reduce or inhibit recombination is to blend/dope TiO2 with transition metals, which create traps for electrons and block the charge carriers by reducing the recombination rate and facilitating the promotion of electrons to the conduction band. Doping (change/modification of the crystalline structure of TiO2) causes a bathochromic (red) shift, which results in a reduction in the energy gap leading to increased absorption in the visible light region.
DOPING: Preparation of Two Admixtures Mn and TiO2- (0.1 and 1%- Mn-TiO2)
In our experiments, 0.1% (w/w) and 1% (w/w) Mn-TiO
2 admixtures were prepared and the ability of the modified photo-catalysts to degrade NO by both solar and indoor like-illumination was evaluated [
10]. In short, thirty grams of the photo-catalyst powder (0.1% and 1% doped Mn–TiO2) was spread homogeneously in a 0.1m radius Petri dish and placed in a 0.45 m
3 environmental test chamber in which a controlled atmosphere containing approximately 200 ppbv NO was created. Mn
2+ has the electronic configuration of
3d5.When it traps electrons the electronic configuration changes to
d6 and if it traps holes its electronic configuration to
d4, both highly unstable. To restore its stable electronic configuration, the trapped electron will be transferred to oxygen molecule and trapped hole to surface adsorbed water molecules to generate
superoxide (•O2-) radicals and
hydroxyl (OH•) radicals in line with the reactions shown in
Figure 7.
The results of our experiments clearly show that the photo-catalyst doped with 0.1% Mn was able to degrade NO by up to 95% in six hours under indoor-like illumination whereas TiO2 doped with 1% Mn did not have an impact on the degradation of NO under indoor illumination conditions. When considering these results, it was shown that adding a small amount of foreign ions (in our case, 0.1% manganese) in TiO2 extends its activity to visible light, while the addition of higher concentrations of manganese (1% Mn-TiO2) does not have an effect for the degradation of NO even under solar irradiation conditions.
The resulting optimal concentration used in our experiments was 0.1%. The doping atoms/cations represent catching points (traps) for electrons and/or holes. These catching points immobilize the charge carriers and reduce the recombination rate. Immobilized charge carriers can also recombine through tunnel processes in the crystal lattice when a dopant atom is in direct neighbourhood to another dopant atom it can be regarded as a recombination centre [
21]. Partial inactivation (around 10% per cycle) of the 0.1% with manganese doped photo-catalyst was observed after consecutive photocatalytic cycles. This can be attributed to the adsorption of HNO
3 formed during the photo-catalysis of NO onto the catalyst occupying active photo-catalyst centres [
10]. This effect had already been observed by Ohko et al. for photo-catalysis of NO and NO
2 using pure anatase TiO
2 [
22].
6. Byproducts: Emission of Low Molecular Weight Carbonyls by the Irradiation of TiO2-Enriched Paints
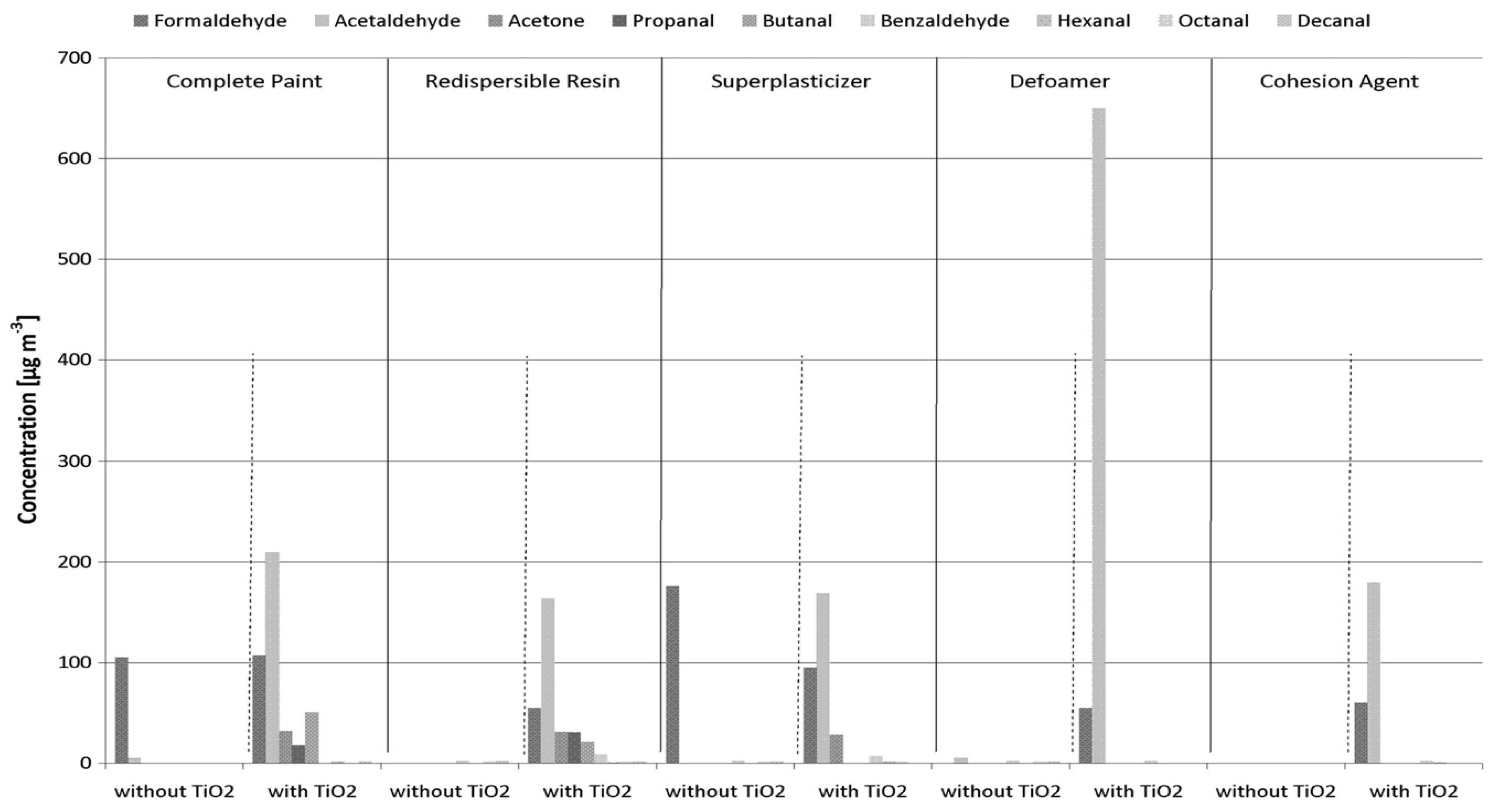
The development of TiO2-containing paints that are activated by UV and visible light opens up a wide range of air purifying applications in indoor environments.
However, the degradation of
organic additives (Table 3) used in TiO
2-enriched paints, when irradiated with UV-light lead to the formation of carbonyl compounds like
formaldehyde, acetaldehyde, acetone and other low molecular weight carbonyls that are formed and emitted into the air (
Figure 8).
The formation and emissions of these compounds, particularly, into the indoor atmosphere, might substantially affect human health and wellbeing.
Table 3. Chemical substances as common additives in paints.
Cohesion agents (Methyl-hydroxy-ethylcellulose)
Super-plasticizer (Sulphonated melamine)
De-foaming agent (Fatty alcohols, polyacrylate)
Re-dispersible resin(Vinyl Copolymer)
Targeted studies are needed addressing the efficiency of photo-catalytic materials for the degradation of chemical and biological contaminants also considering the emission of eventually formed, toxicologically relevant by-products to evaluate the applicability of these materials and coatings in indoor environments.
7. Conclusions
The removal of NOx and VOCs at typical urban/indoor air levels using the photo-catalytic (TiO2- based) technology is feasible. Humidity affects VOC-photo-degradation. An increase in humidity inhibits photo-oxidation, due to the competition of water molecules with pollutant molecules for adsorption sites. The mixtures of VOCs and NO behave differently than the individual compounds after UV irradiation. This is an important fact for the evaluation of the efficiency of building materials (e.g. paints) for the removal of harmful air contaminants in ambient air and confined spaces. For the NO degradation at low concentrations humidity does not play a significant role.
The hypothesis on the role of aromatic compounds as possible inhibitors of the recombination process (h+ and e-) should be further tested to clarify, whether organic compounds with electrophilic/nucleophilic characteristics could act in analogy to water molecules and oxygen as a source for radicals and other oxidizing compounds.
The development of TiO2 photocatalytic paints to be applied indoors needs to be optimized to minimize the formation and emission of harmful substances.
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Parlar, H., Kotzias, D. Degradation of Environmental Chemicals in the Liquid and Adsorbed Phase. SCOPE 22 by John Wiley & Sons, (1985) Chapter 2.4, 81-105.
- Sheehan, P., Korte, F., Bourdeau, P. Appraisal of tests to predict the environmental behaviour of chemicals. SCOPE 22 by John Wiley&Son, (1985), 150-170.
- Gaeb, S., Schmitzer, J., Thamm, H.W., Parlar, H., Korte, F. Photo-Mineralization Rate of Organic-Compounds Adsorbed on Particulate Matter. Nature 1977, 5635, 331. [Google Scholar]
- Kotzias, D., Klein, W., Lotz, F., Nitz, S., Korte, F. Zur Photo-Induzierten Mineralisie- rung organischer Chemikalien. Chemosphere 1979, 5, 301–304. [Google Scholar]
- Hoffmann, M.R., Martin, S.T., Choi, W et al. Environmental Applications of Semiconductor Photocatalysis. Chem 1995, 95, 69–96. [Google Scholar]
- I.S. Mc Lintock, M. Ritchie. Reactions on titanium dioxide; photo-adsorption and oxidation of ethylene and propylene. Trans. Faraday Soc. 1965, 61, 1007–1016. [Google Scholar] [CrossRef]
- Chi Him A. Tsang, Kai Li, Yuxuan Zeng. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environment International 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Kotzias, D. Geiss, O, Tirendi, S. et al. The European indoor air monitoring and assessment Study (AIRMEX). Fresenius Environ. Bulletin 2009, 18, 670–681. [Google Scholar]
- Cacho, C., Geiss, O., Barrero-Moreno et al. Studies on photo-induced NO removal by Mn-doped TiO2 under indoor-like illumina- tion conditions. J. Photochem Photobiol A. Chem. 2011, 222, 304–6. [Google Scholar] [CrossRef]
- Boulamanti, A., Korologos C.A, Philippopoulos C. The rate of photocatalytic oxidation of aromatic volatile organic compounds in the gas-phase. Atmospheric Environment 2008, 42, 7844–7850. [Google Scholar] [CrossRef]
- Vishnetskaya, M.V, Tomskiy, I.S. Role of Singlet Oxygen in the Oxidation of Toluene on Vanadium,Molybdenum Catalytic Systems. Chemistry for Sustainable Development 2011, 19, 321–325. [Google Scholar]
- Ao, C.H., Lee, S.C. Mak,C. L. Photodegradation of volatile organic compounds (VOCs) and NO for indoor air purification using TiO2: promotion versus inhibition effect of NO. Appl. Catal. B 2003, 42, 119–129. [Google Scholar] [CrossRef]
- Devahasdin, S., Fan Jr. Ch., Li K. TiO2 photocatalytic oxidation of nitric oxide: transient behaviour and reaction kinetics. J. Photochem. Photobiol. A 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Sakamoto, K., Tonegawa, Y., Ishitani, O. Destruction of indoor air pollutants in TiO2- wall coated cylindrical flow reactor under 254 nm UV irradiation. J. Adv. Oxid. Technol. 1999, 4, 35–39. [Google Scholar]
- Obee,T. N., Brown, R.T. TiO2 photocatalysis for indoor air applications: effects of humidity and trace contaminant levels on the oxidations rates of formaldehyde, toluene and 1,3-butadiene. Environ. Sci. Technol. 1995, 29, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.H., Lee, S.C., Yu, J. C. Photocatalyst TiO2 supported on glass fiber for indoor air 242 purification: effect of NO on the photodegradation of CO and NO2. J. Photochem. Photobiol. A 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Barrero- Moreno, J. , Cacho, C., Geiss, O., Leva, P., Bellintani, A., Ceccone, G., Kotzias, D. Photocatalysis of Indoor/ Outdoor Pollutants by Consumer Friendly Titanium Dioxide Based Building Materials (Paints); (2006). Final report Contract N_ 22868-2005-09-T1CD ISP pp. 1– 72. European Commission-Joint Research Centre, Institute for Health and Consumer Protection: Ispra, Italy.
- Kotzias, D.; Binas, V.; Kiriakidis, G. Smart Surfaces: Photocatalytic Degradation of Priority Pollutants on TiO2-Based Coatings in Indoor and Outdoor Environments—Principles and Mechanisms. Materials 2022, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Presented at the World Chemistry Forum, May 22–24, 2019, Barcelona/Spain. Topics in Catalysis (2020) 63:875-881. [CrossRef]
- Bloh J.Z., PhD-Thesis (2012), Entwicklung von Zinkoxid-Photokatalysatoren für den Abbau von Luftschadstoffen, October 2012.
- Yoshihisa Ohko, Yuri Nakamura et al. (2009). Photocatalytic oxidation of nitrogen monoxide using TiO 2 thin films under continuous UV light illumination. Journal of Photochemistry and Photobiology A Chemistry 205(1):28-33. [CrossRef]
- Geiss O, Cacho C, et al. Photocatalytic degradation of organic paint consituents-formation of carbonyls. Building and Environment 2012, 48, 107–112. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).