Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Definitions of Persistent Bacteremia
3. Definitions and Consequences of Persistent and Uncontrolled Infection
4. What Is the Role of Repeating Blood Cultures to Identify Those at Risk for Persistent Infection ?
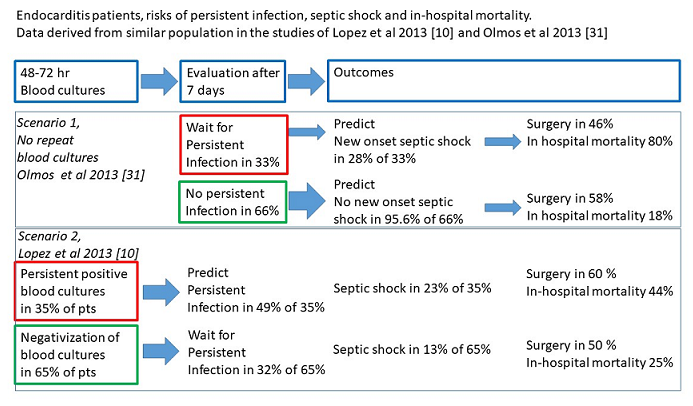
5. The Incidence, Risks and Associations of Persistent Infection
6. Why Do We Need Negative Blood Cultures as the Starting Point of Antibiotic Therapy Duration ?
7. What Is the Time Needed to Clear Bacteria from Affected Valves in Endocarditis ?
8. Conclusions
References
- Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015 Oct 13;132(15):1435-86. Epub 2015 Sep 15. Erratum in: Circulation. 2015 Oct 27;132(17):e215. Erratum in: Circulation. 2016 Aug 23;134(8):e113. Erratum in: Circulation. 2018 Jul 31;138(5):e78-e79. PMID: 26373316. [CrossRef]
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015 Nov 21;36(44):3075-3128. Epub 2015 Aug 29. PMID: 26320109. [CrossRef]
- Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, Caselli S, Doenst T, Ederhy S, Erba PA, Foldager D, Fosbøl EL, Kovac J, Mestres CA, Miller OI, Miro JM, Pazdernik M, Pizzi MN, Quintana E, Rasmussen TB, Ristić AD, Rodés-Cabau J, Sionis A, Zühlke LJ, Borger MA; ESC Scientific Document Group. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023 Oct 14;44(39):3948-4042. Erratum in: Eur Heart J. 2023 Sep 20;: Erratum in: Eur Heart J. 2024 Jan 1;45(1):56. PMID: 37622656. [CrossRef]
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016 Jun 13;16:286. PMID: 27296858; PMCID: PMC4906775. [CrossRef]
- Kuehl R, Morata L, Boeing C, Subirana I, Seifert H, Rieg S, Kern WV, Kim HB, Kim ES, Liao CH, Tilley R, Lopez-Cortés LE, Llewelyn MJ, Fowler VG, Thwaites G, Cisneros JM, Scarborough M, Nsutebu E, Gurgui Ferrer M, Pérez JL, Barlow G, Hopkins S, Ternavasio-de la Vega HG, Török ME, Wilson P, Kaasch AJ, Soriano A; International Staphylococcus aureus collaboration study group and the ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis. 2020 Dec;20(12):1409-1417. Epub 2020 Aug 4. PMID: 32763194. [CrossRef]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994 Mar;96(3):200-9. [CrossRef]
- Gutschik E. Microbiological recommendations for the diagnosis and follow-up of infective endocarditis. Clin Microbiol Infect. 1998;4 Suppl 3:S10-S16. [CrossRef]
- Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS; ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005 Jun 22;293(24):3012-21. [CrossRef]
- Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Paré C, Almirante B, Muñoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton-Suty C, Jones SB, Casabé J, Morris A, Corey GR, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007 Mar 28;297(12):1354-61. PMID: 17392239. [CrossRef]
- López J, Sevilla T, Vilacosta I, Sarriá C, Revilla A, Ortiz C, Ferrera C, Olmos C, Gómez I, San Román JA. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J. 2013 Jun;34(23):1749-54. [CrossRef]
- Blumberg EA, Robbins N, Adimora A, Lowy FD. Persistent fever in association with infective endocarditis. Clin Infect Dis. 1992 Dec;15(6):983-90. PMID: 1457671. [CrossRef]
- Olaison L, Hogevik H, Alestig K. Fever, C-reactive protein, and other acute-phase reactants during treatment of infective endocarditis. Arch Intern Med. 1997 Apr 28;157(8):885-92. PMID: 9129548. [CrossRef]
- 24. Revilla A, López J, Vilacosta I, Villacorta E, Rollán MJ, Echevarría JR, Carrascal Y, Di Stefano S, Fulquet E, Rodríguez E, Fiz L, San Román JA. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J. 2007 Jan;28(1):65-71. Epub 2006 Oct 10. PMID: 17032690. [CrossRef]
- Graupner C, Vilacosta I, SanRomán J, Ronderos R, Sarriá C, Fernández C, Mújica R, Sanz O, Sanmartín JV, Pinto AG. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002 Apr 3;39(7):1204-11. PMID: 11923047. [CrossRef]
- Ramos-Martínez A, Calderón-Parra J, Miró JM, Muñoz P, Rodríguez-Abella H, Valerio M, de Alarcón A, Luque R, Ambrosioni J, Fariñas MC, Goenaga MÁ, Oteo JA, Martínez Marcos FJ, Vinuesa D, Domínguez F; Spanish Collaboration on Endocarditis — Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES) (see Appendix). Effect of the type of surgical indication on mortality in patients with infective endocarditis who are rejected for surgical intervention. Int J Cardiol. 2019 May 1;282:24-30. Epub 2019 Jan 10. PMID: 30718134. [CrossRef]
- Garcia Granja PE, Lopez J, Vilacosta I, Saéz C, Cabezón G, Olmos C, Jerónimo A, Pérez JB, De Stefano S, Maroto L, Carnero M, Monguio E, Pulido P, de Miguel M, Gomez Salvador I, Carrasco-Moraleja M, San Román JA. Prognostic impact of cardiac surgery in left-sided infective endocarditis according to risk profile. Heart. 2021 Dec;107(24):1987-1994. Epub 2021 Sep 11. PMID: 34509995. [CrossRef]
- de Miguel M, López J, Vilacosta I, Olmos C, Sáez C, Cabezón G, Zulet P, Jerónimo A, Gómez D, Pulido P, Lozano A, Oña A, Gómez-Salvador I, San Román JA. Clinical Profile and Prognosis of Patients with Left-Sided Infective Endocarditis with Surgical Indication Who Are Not Operated. Microorganisms. 2024 Mar 19;12(3):607. PMID: 38543658; PMCID: PMC10975654. [CrossRef]
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: A prospective study. Ann Intern Med. 1982 Oct;97(4):496-503. PMID: 6751182 . [CrossRef]
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991 Nov 1;115(9):674-80. PMID: 1929035. [CrossRef]
- Chang FY, MacDonald BB, Peacock JE Jr, Musher DM, Triplett P, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore). 2003 Sep;82(5):322-32. PMID: 14530781. [CrossRef]
- Yoon HJ, Choi JY, Kim CO, Kim JM, Song YG. A comparison of clinical features and mortality among methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus endocarditis. Yonsei Med J. 2005 Aug 31;46(4):496-502. PMID: 16127774; PMCID: PMC2815834. [CrossRef]
- Riedel DJ, Weekes E, Forrest GN. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob Agents Chemother. 2008 Jul;52(7):2463-7. Epub 2008 May 12. PMID: 18474578; PMCID: PMC2443910. [CrossRef]
- Bae IG, Federspiel JJ, Miró JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG Jr; International Collaboration on Endocarditis-Microbiology Investigator. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009 Nov 1;200(9):1355-66. PMID: 19811099; PMCID: PMC3600359. [CrossRef]
- Casapao AM, Davis SL, McRoberts JP, Lagnf AM, Patel S, Kullar R, Levine DP, Rybak MJ. Evaluation of vancomycin population susceptibility analysis profile as a predictor of outcomes for patients with infective endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2014 Aug;58(8):4636-41. Epub 2014 Jun 2. PMID: 24890596; PMCID: PMC4136033. [CrossRef]
- Herrera-Hidalgo L, Fernández-Rubio B, Luque-Márquez R, López-Cortés LE, Gil-Navarro MV, de Alarcón A. Treatment of Enterococcus faecalis Infective Endocarditis: A Continuing Challenge. Antibiotics (Basel). 2023 Apr 4;12(4):704. PMID: 37107066; PMCID: PMC10135260 . [CrossRef]
- Oldberg K, Rasmussen M. Enterococcus faecalis in blood cultures-a prospective study on the role of persistent bacteremia. Diagn Microbiol Infect Dis. 2021 Sep;101(1):115433. Epub 2021 May 18. PMID: 34139401. [CrossRef]
- Olmos C, Vilacosta I, Sarriá C, López J, Ferrera C, Sáez C, Vivas D, Hernández M, Sánchez-Enrique C, García-Granja PE, Pérez-Cecilia E, Maroto L, San Román JA. Streptococcus bovis endocarditis: Update from a multicenter registry. Am Heart J. 2016 Jan;171(1):7-13. Epub 2015 Oct 21. PMID: 26699595. [CrossRef]
- El Rafei A, DeSimone DC, Narichania AD, Sohail MR, Vikram HR, Li Z, Steckelberg JM, Wilson WR, Baddour LM. Comparison of Dual β-Lactam therapy to penicillin-aminoglycoside combination in treatment of Enterococcus faecalis infective endocarditis. J Infect. 2018 Nov;77(5):398-404. Epub 2018 Jun 30. PMID: 29969596 . [CrossRef]
- Ramos-Martı´nez A, Pericàs JM, Ferna´ndez-Cruz A, Muñoz P, Valerio M, Kestler M, et al. (2020) Four weeks versus six weeks of ampicillin plus ceftriaxone in Enterococcus faecalis native valve endocarditis: A prospective cohort study. PLoS ONE 15(8): e0237011. [CrossRef]
- van der Vaart TW, Stuifzand M, Boekholdt SM, Cramer MJ, Bonten MJM, Prins JM, van der Meer JTM. The prevalence of persistent bacteraemia in patients with a non-staphylococcal infective endocarditis, a retrospective cohort study. Int J Cardiol. 2022 Nov 15;367:49-54. Epub 2022 Aug 21. PMID: 36002040. [CrossRef]
- Olmos C, Vilacosta I, Fernández C, López J, Sarriá C, Ferrera C, Revilla A, Silva J, Vivas D, González I, San Román JA. Contemporary epidemiology and prognosis of septic shock in infective endocarditis. Eur Heart J. 2013 Jul;34(26):1999-2006. Epub 2012 Oct 11. PMID: 23060453. [CrossRef]
- Morris AJ, Drinkovic D, Pottumarthy S, Strickett MG, MacCulloch D, Lambie N, Kerr AR. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis. Clin Infect Dis. 2003 Mar 15;36(6):697-704. Epub 2003 Mar 4. PMID: 12627353. [CrossRef]
- Upton A, Drinkovic D, Pottumarthy S, West T, Morris AJ. Culture results of heart valves resected because of streptococcal endocarditis: insights into duration of treatment to achieve valve sterilization. J Antimicrob Chemother. 2005 Feb;55(2):234-9. Epub 2005 Jan 13. PMID: 15649988. [CrossRef]
- Mekontso Dessap A, Zahar JR, Voiriot G, Ali F, Aissa N, Kirsch M, Brun-Buisson C. Influence of preoperative antibiotherapy on valve culture results and outcome of endocarditis requiring surgery. J Infect. 2009 Jul;59(1):42-8. Epub 2009 May 5. PMID: 19481815. [CrossRef]
- Voldstedlund M, Fuursted K, Bruun NE, Arpi M. Comparison of heart valve culture between two Danish endocarditis centres. Scand J Infect Dis. 2012 Jun;44(6):405-13. Epub 2012 Jan 31. PMID: 22292569. [CrossRef]
- Halavaara M, Martelius T, Järvinen A, Antikainen J, Kuusela P, Salminen US, Anttila VJ. Impact of pre-operative antimicrobial treatment on microbiological findings from endocardial specimens in infective endocarditis. Eur J Clin Microbiol Infect Dis. 2019 Mar;38(3):497-503. Epub 2019 Jan 24. PMID: 30680557; PMCID: PMC6394703. [CrossRef]
- Gisler V, Dürr S, Irincheeva I, Limacher A, Droz S, Carrel T, Englberger L, Sendi P. Duration of Pre-Operative Antibiotic Treatment and Culture Results in Patients With Infective Endocarditis. J Am Coll Cardiol. 2020 Jul 7;76(1):31-40. PMID: 32616160. [CrossRef]
- Fillâtre P, Gacouin A, Revest M, Maamar A, Patrat-Delon S, Flécher E, Fouquet O, Lerolle N, Verhoye JP, Le Tulzo Y, Tattevin P, Tadié JM. Determinants and consequences of positive valve culture when cardiac surgery is performed during the acute phase of infective endocarditis. Eur J Clin Microbiol Infect Dis. 2020 Apr;39(4):629-635. Epub 2019 Nov 26. PMID: 31773364. [CrossRef]
- Johansson G, Sunnerhagen T, Gilje P, Ragnarsson S, Rasmussen M. Risk factors for and consequences of positive valve cultures in patients who undergo cardiac surgery while receiving antimicrobial treatment for infective endocarditis. Infect Dis (Lond). 2024 Mar;56(3):244-254. Epub 2023 Dec 15. PMID: 38100548. [CrossRef]
| AHA guideline 2015 | ESC guideline 2015/ 2023 | |
| Persistent infection |
Persistent bacteremia or fever lasting >5–7 days despite appropriate antibiotic therapy
|
Persisting fever and positive blood culture ( > 7–10 days) despite an appropriate antibiotic regimen |
| Action required |
Excluding other sites of infection and fever.
Surgery is indicated when persistent bacteremia or fever lasting >5–7 days despite appropriate antibiotic therapy and provided that other sites of infection and fever have been excluded |
Replacement of i.v. lines, repeat laboratory measurements, blood cultures, echocardiography, and the search for an intracardiac or extracardiac focus of infection. Surgery has been indicated when fever and positive blood cultures persist for several days (7–10 days) despite an appropriate antibiotic regimen and when extracardiac abscesses (splenic, vertebral, cerebral or renal) and other causes of fever have been excluded. However, the best timing for surgery in this difficult situation is unclear. Recently it has been demonstrated that persistent blood cultures 48–72 h after initiation of antibiotics are an independent risk factor for hospital mortality (Lopez 2013). These results suggest that surgery should be considered when blood cultures remain positive after 3 days of antibiotic therapy, after the exclusion of other causes of persistent positive blood cultures (adapted antibiotic regimen). |
| Uncontrolled infection |
AHA guidelines do not use the term uncontrolled infection and present individual reasons for surgery.
Persisting fever and positive blood cultures (>5-7 days), provided that other sites of infection and fever have been excluded, is one of them. |
Locally uncontrolled Infection (increasing vegetation size, abscess formation, false aneurysms, and the creation of fistulae) OR Persisting fever and positive blood culture (>7–10 days), OR infection due to fungi or multiresistant organisms or in the rare infections caused by Gram-negative bacteria. |
| Action required | Surgery is recommended as soon as possible. Rarely when there are no other reasons for surgery and fever is easily controlled with antibiotics, small abscesses or false aneurysms can be treated conservatively under close clinical and echocardiographic follow-up. |
| Study | Number of patients and % native valves | % of patients with positive valve culture | Influence of duration of antibiotic treatment on % positive valve culture |
| Morris 2003 [32] | 480 (62% native) | 30% | In terms of standard duration of antibiotic treatment completed: ≤50%: 116/214 (54.2%) >50%: 14/ 145 (9.7%) |
| Upton 2005 [33] | 131 (66% native), with streptococcal endocarditis | 19% | ≤14 days: 24/69 (34.8%) >14 days: 1/62 (1.6%) |
| Mekontso Dessap 2009 [34] | 90 (79% native) | 51% | ≤7 days: 35/45 (78%) >7 days: 11/45 (24%) |
| Voldstedlund 2012 [35] | 223 (85% native) | 26% | <14 days: 53/157 (33.8%) ≥14 days: 5/74 (6.7%) |
| Halavaara 2019 [36] | 87 (87% native) | 22% | <14 days: 19/53 (35.8%) ≥14 days: 0/34 (0%) |
| Gisler 2020 [37] | 231 (66% native) | 25% | ≤7 days: 31/60 (52%) >7 days: 27/171 (15.8%) ≤15 days: 47/125 (37.6%) >15 days: 11/96 (11.4%) ≤21 days: 53/169 (31.4%) >21 days: 5/62 (8.1%) In logistic regression analysis, contribution of antibiotic duration per 2 days on prediction of positive valve culture is absent after 21 days. Other strong predictors for positive valve cultures are Enterococcus spp and Staphylococcus spp. |
| Fillâtre 2020 [38] | 148 (81% native) | 31% | <14 days: 34/73 (46.6%) ≥14 days: 12/75 (16.0%) |
| Johansson 2023 [39] | 345 (73% native) | 23% | ≤11 days: 73/208 (35.1%) >11 days: 5/137 ( 3.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




