1. Introduction
Diseases of the vitreoretinal interface result in a three-dimensional (3D) remodelling of retinal tissue and there has been recently a growing interest in the potential role of the retinal volumetric analysis in the diagnosis, treatment planning and monitoring of these pathological entities (1–8). Indeed, it has been suggested that retinal volumetric analysis would better reflect the in vivo alterations of retinal tissue caused by specific pathologies (1). The clinical relevance of specific retinal volumes as biomarkers has been already demonstrated in several medical retinal diseases, such as diabetic retinopathy (DR), age-related macular degeneration (AMD) or glaucoma, ultimately contributing to improved patient outcomes and vision preservation (3,5,7,9–11). In the vitreoretinal field, it was shown that the volume of specific entities, such as the foveal cavity (FC) in lamellar macular holes (LMHs), were associated with visual acuity at baseline and with the natural history of the disease during the follow-up period (1). Moreover, in eyes with epiretinal membrane (ERM), higher pre-operative macular volumes showed association with worse visual outcome after surgery (12).
Optical-coherence-tomography (OCT) uses light waves in order to acquire a reflectivity profile of the retina, showing cross-sectional images of in vivo 3D tissue structures (13). Thanks to its fast acquisition, high-resolution and non-invasive properties, OCT is an essential tool in ophthalmology for the diagnosis and monitoring of pathologies, as well as for research purposes (14). Several innovations to improve its quality have been recently made, including the development of new automated analysis algorithms to detect retinal surfaces or layers allowing volumetric analysis (15,16). Nevertheless, automated volumetric calculations are still limited to the total retinal volume and the volumes of single retinal layers; whereas, volumes of specific entities, such as FC or epiretinal proliferation (EPR) in eyes with LMHs, cannot be automatically extracted by manufacturer’s software. The need for complex processes to calculate volumes, requiring the use of deep learning machines or external software, limits significantly the use of volumetric analysis in the clinical, surgical practice and research field (1,6,17,18). Indeed, although some machine learning-based tools have been introduced in the healthcare setting, prudence and vigilance are still recommended due to the persistence of various challenges (19). Additionally, the use of an external software requires extra procedures for installation, export and upload of OCT scans in the system for manual processing(1).
The aim of this study is to describe and validate a novel and easy OCT-based method, without the need for deep learning machines or external software, to manually calculate volumes of specific entities in retinal pathologies, which cannot be automatically detected, segmented or analysed by current OCT software.
2. Materials and Methods
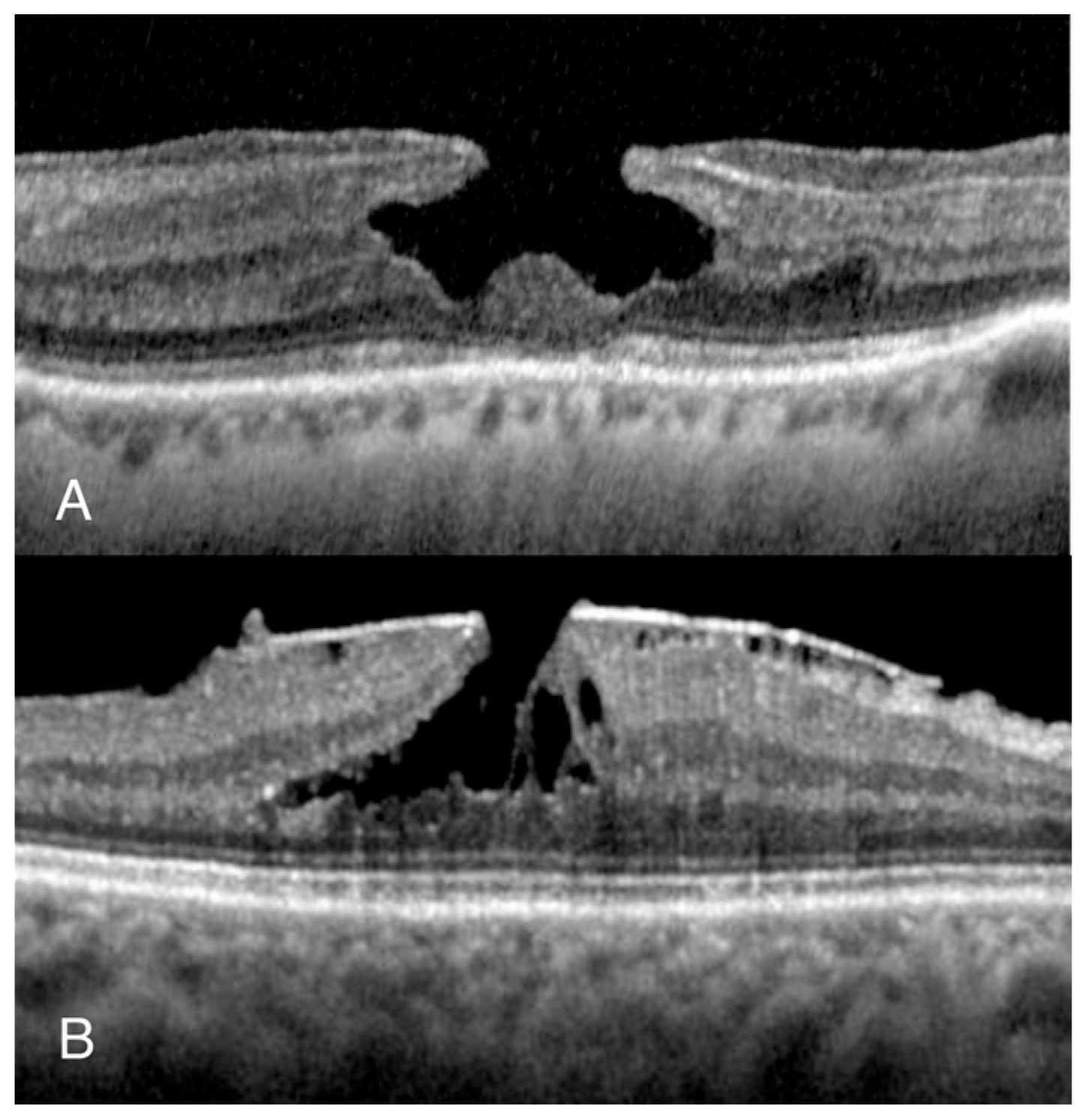
We conducted a retrospective, non-interventional observational study including eyes with normal foveal profile (NFP) and LMH. Patients with NFP were identified through retrospectively evaluating the OCT scans of patients who attended Manchester Royal Eye Hospital for myodesopsias. Patients with LMHs were identified from the electronic surgical database of the vitreoretinal unit, searching for those awaiting surgery for from January 2020 to January 2023. We included both degenerative LMH (D-LMH) and ERM foveoschisis (ERM-FS), as defined by the OCT-based consensus definition for LMH (20) (
Figure 1).
We first manually acquired volumes of the foveal area (defined as the retinal tissue within a circle of 500-microns radius centred on the foveal centre) in patients with NFP and compared them with the automated measurements provided by the OCT software; then we measured specific volumes, that cannot be automated by current OCT software, in eyes with LMH. For both groups, only eyes with “dense macular volume” preset of Heidelberg Spectralis (Engineering GmbH, Heidelberg, Germany) were included. This involves a macular volume scan acquisition with a 49-line horizontal raster and foveal centration, covering an area of 30° by 30°. Two observers (KS, NA) independently acquired the required measurements, according to the method described below, and the inter-personal variability of data acquisition was evaluated. In addition, to evaluate the intra-observer variability, the same two observers independently re-acquired the same measurements six weeks later. In the event of outliers, a third observer (G.M.) was in charge of the review and check of the measurements acquired.
Accordingly to the United Kingdom (UK) guidance, retrospective data collection is regarded as audit for service evaluation, and therefore ethical approval was not required.
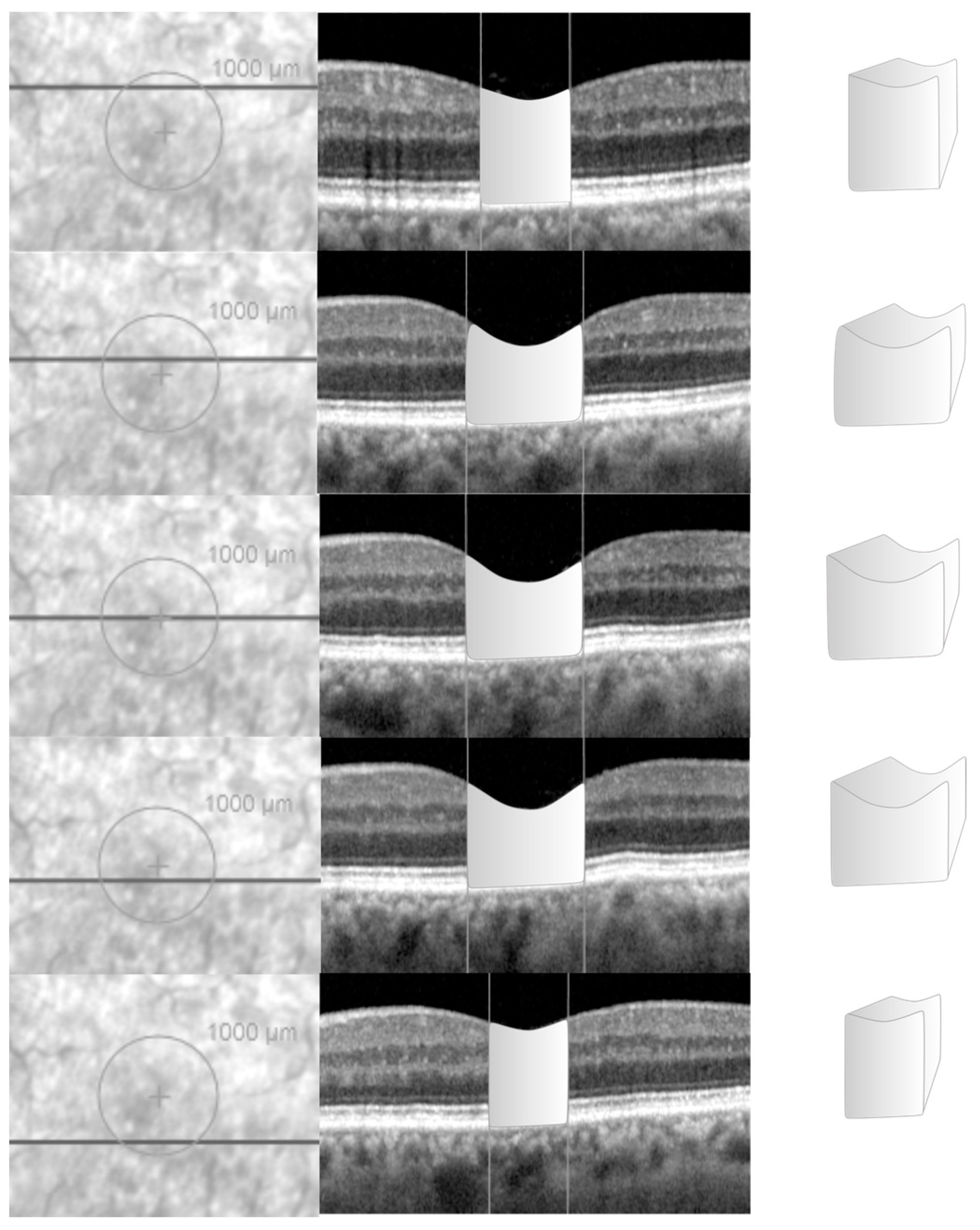
3.1. Data Acquisition in the NFP Group
For each eye with NFP, the horizontal b-scan intersecting the centre of the fovea was identified and the vertical green reference line was adjusted to the foveal centre. This enabled the foveal centre to be highlighted on the infrared imaging (
Figure 2).
A circle overlay, with a diameter of 1000 micron and centred on the fovea, was added to the infra-red image in Spectralis. The visualization mode was changed from the default vertical scaling (1:1 pixel) to 1:1 μm before acquisition of data. For each horizontal b-scan confined within the circle, the vertical green reference line was adjusted on the OCT scan in order to intersect the borders of the overlay, a vertical line using the software tool was drawn on the OCT scan to highlight those borders as reference parameters. Using the freehand integrated caliper tool, the perimeter of the retina, included between the two borders of the circle drawn, was drawn to acquire its desired cross-sectional area (
Figure 2). The same process was repeated for all the scans within the circle. The distance between each linear scan was found through the “Information” option on the OCT software. The volume of the retinal tissue, included within a diametre of 1000 microns across the fovea, was calculated using the formula: Volume (mm³) = ∑area [mm²] x OCT-scan distance [mm].
This manual volumetric volume was compared to the retinal volume within the central 1000 microns from the fovea calculated in an automated modality by Spectralis and extracted from the “Thickness Map” tab as the volume of the retina within the central circle of the 1, 3, 6 ETDRS circle diameters.
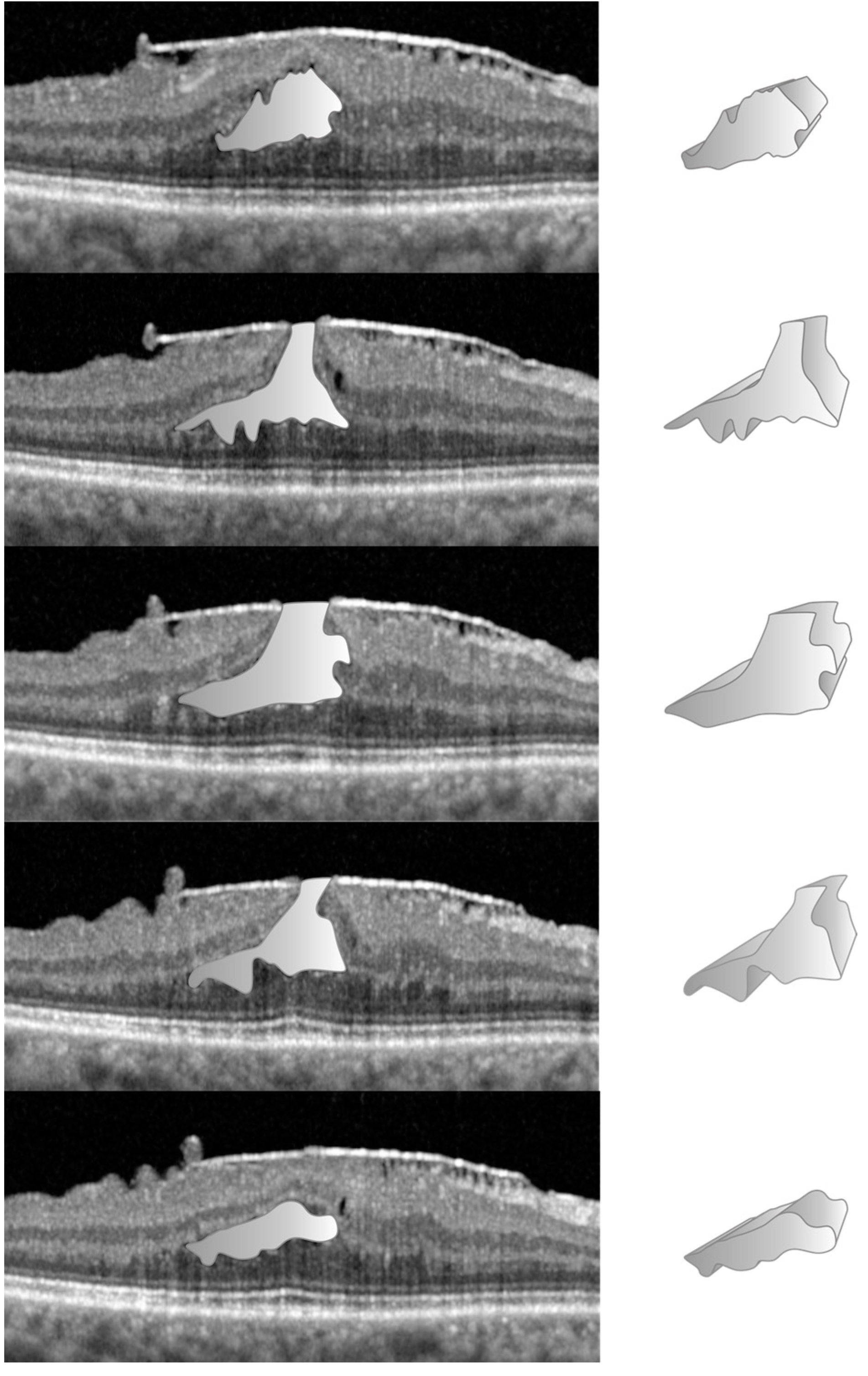
3.2. Data Acquisition in the LMH Group
The manual technique, to establish the volumes of specific entities like the FC in D-LMH or FS in ERM-FS, is more straight forward than NFP as no reference circle is required. From the “Display” tab, we used the afore-mentioned freehand caliper tool, present in the Spectralis system, to calculate the cross-sectional area of the FC or FS on a single horizontal B-Scan (
Figure 3).
The FC/FS surface area was acquired for each linear OCT scan in which FC/FS was present. The volume of the FC or FS was calculated multiplying the sum of the FC/FS surface areas for the distance between the OCT scans, using the same formula. Due to the limitation of the machine to detect, and consequently to calculate those volumes, comparison with automated software measurements was not possible.
3.3. Statistical Analysis
All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 29.0.2 (IBM Corp, Armonk NY). Statistical significance was defined as p<0.05. Inter- and intra-observer variation and difference with automated OCT machine measurements were assessed with Bland-Altman Plots (21). The bias (mean of the differences in measurement) was presented with 95% confidence intervals (CI) (±1.96 times the standard error [SE] of the differences). The coefficient of repeatability (CR) was measured as the Standard Deviation [SD] of differences * 1.96. The 95% lower and upper limits of agreement (LOA) were calculated as Bias ± CR. The 95% CIs of each respective upper and lower LOA were calculated as ±1.96 times the SE of the respective limit and approximated as per Bland et al. [SE=√((3s^2)/n), in the formula “s” was the SD of the differences and “n” the sample size].
Power calculations for each comparison with Bland Altman plots was performed as per Lu et al. (22). The power calculation for a specified sample size was conducted using the following parameters: Bias, SD of the bias, the targeted clinical agreement limit, available sample size, targeted CI of the upper and lower (95% CI) for the LOAs and targeted confidence level of LOA (95%). Clinical agreement limit was determined as the maximum allowable difference that would be clinically significant, conservatively set to 0.01mm3. Due to a sparsity in the literature regarding comparable measurements the NFP (volume measurements in the central 1mm) and LMH group, we conducted a power calculation following collecting 30 NFP and 32 LMH pairs, respectively. With our sample size, we achieved over 90% power in both groups.
3. Results
We report on 62 eyes, of which 30 eyes with NFP and 32 with LMH. The former group was used to validate the proposed measurement method through the comparison of human to OCT automated measurements. In both groups, we assessed inter- and intra-observer variability.
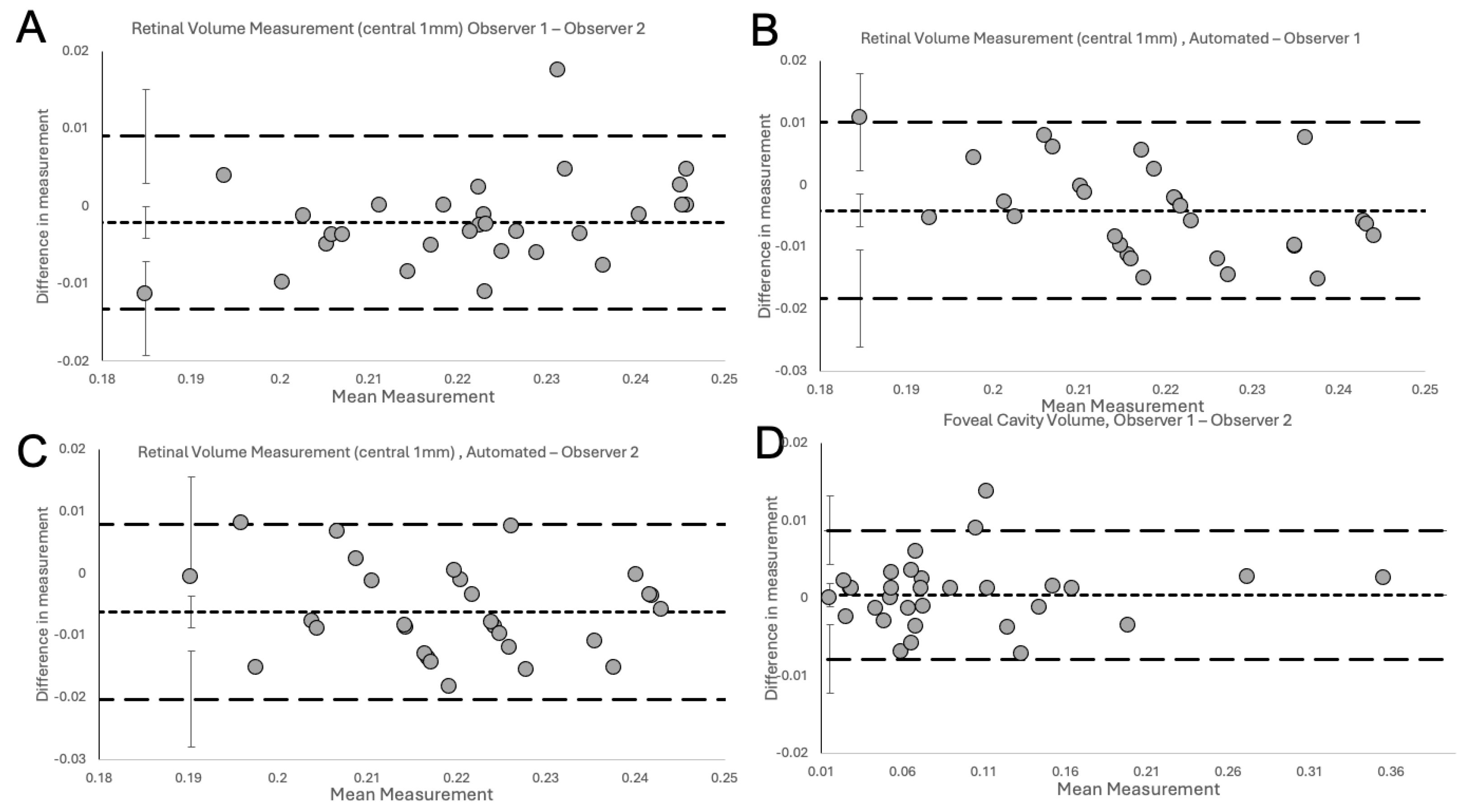
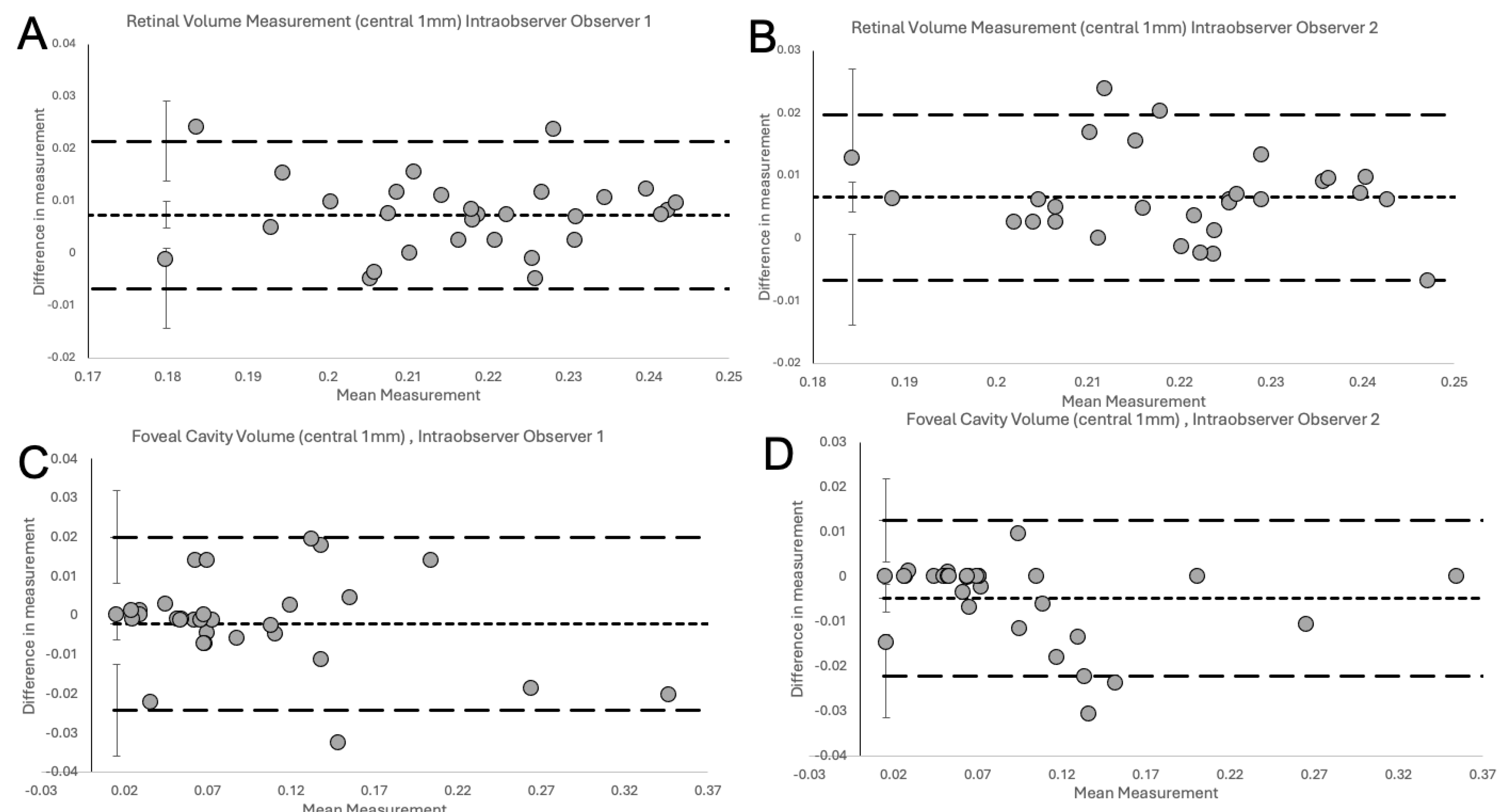
The Bland-Altman Plots (
Figure 4) show the degree of excellent agreement for interobserver variability between the two observers (
Figure 4A), observer 1 and Spectralis (
Figure 4B), observer 2 and Spectralis (
Figure 4C) for measurements of RV in NFP eyes and between the two observers for measurements of FC/FS volume in LMHs (
Figure 4D).
The plots did not demonstrate systemic disagreement in all comparisons. Regarding RV measurements, both human observers had excellent agreement between themselves (
Figure 4A,
Table 1), and between Spectralis (
Figure 4B-C,
Table 1), with small mean differences and narrow LOA. In details, the small mean differences between human observers and Spectralis were negative (-0.0041 for observer 1 and -0.0062 for observer 2), showing that human observers had slightly larger measurements than the OCT software. The human observers had a single outlier on both a measurement in
Figure 4A (RV) and
Figure 4D (FC/FS) which, on revisiting by the third observer, were human errors in measurement technique. With corrected measurements, these differences disappeared. However, we presented the original measured data to avoid bias. The outlier in
Figure 4A was a result of observer 1 slightly overestimating by error the area to be calculated, leading to larger than usual measurement. In contrast, observer 2, was slightly under measuring this scan. It should be highlighted that the quality of the considered scan was slightly reduced, possibly leading to error. Nonetheless, both these errors were small, and both human measurements were within the LOA compared to the automated measurement (
Figure 4B and C respectively). In
Figure 4D, observer 1 had not delineated an area of foveoschisis correctly and this led to overmeasurement to the volume of the schitic area.
After validation of RV measurements, we investigated the agreement between both human observers for calculation of specific volumes in pathological retinas, such FC or FS volume measurements in eyes with LMH. As Heidelberg Spectralis does not have inbuilt facility to automate the volume measurement of FC/FS, the evaluation of agreement between observers and Spectralis was not possible for those specific volumes. Nonetheless, we demonstrate high agreement between both human observers using the novel volume measurement technique on the Bland-Altman Plot (
Figure 5D,
Table 1). At 6-week measurement, we report excellent intra-observer agreement with low variability and small differences in measurements (
Figure 5). These were within the 95% CI for the LOA and no systematic bias was observed.
4. Discussion
In both medical and surgical retinal pathologies, volumetric analysis has been proposed as an important tool to identify biomarkers for diagnosis, understanding the disease mechanisms, clinical grading, monitoring, and response to interventions (1–8). For instance, in neovascular AMD, volumes of the intra- or sub-retinal fluid were described as reliable biomarkers for disease activity, as well as for visual function or outcome, thus playing an important role in planning customized treatment strategies (5,9–11). Similarly, in DR, evaluation of retinal or fluid cysts volume allows severity assessment or early detection of microvascular abnormalities, guiding interventions to prevent vision loss (3,6). In multiple sclerosis optic neuritis, increase of inner nuclear layer (INL) volume has shown association with occurrence of clinical relapses (7). In the glaucomatous field, 3D macular parameters (without necessary manual correction of artifacts in the clinical setting) has shown a diagnostic performance similar to or better than 2D measurements, allowing an early diagnosis of glaucoma and prompt start of treatment, as well as valuable insights into the extent of optic nerve damage (23). Recently, in the vitreoretinal field, Taşlıpınar Uzel et al (1) highlighted the importance of volumetric analysis in D-LMH reporting that FC volume was the only factor associated with baseline best corrected visual acuity (BCVA), whereas no correlation was detected between BCVA and linear OCT measurements, such as central retinal thickness (1). Moreover, FC and ERP volumes were the only parameters that showed a significant increment at last follow-up visit compared to baseline, detecting changes in the natural course of D-LMH earlier than the horizontal diameter measurements (1). In the light of their findings, Taşlıpınar Uzel et al (1) determined that tracking volumetric changes could offer deeper understanding of the dynamic transformations in retinal conditions like D-LMH, supporting physicians in making informed decisions regarding their treatment. Similarly, we demonstrated a highly predictive model for functional outcomes, following vitrectomy and internal limiting membrane (ILM) peeling in LMHs, utilizing specific OCT volumetric parameters, including FC or FS and ERP volumes (unpublished data). However, conversely to Taşlıpınar Uzel et al (1) approach, which necessitated use of an external software, our method did not require any external software to calculate the volume of specific entities, providing a simpler and quicker way to obtain volumes. Indeed, although ophthalmologists typically interpret retinal imaging using two-dimensional (2D) data subsets (2,24,25); due to the increase interest in volumetric analysis, several OCT devices have enabled the segmentation of retinal layers in eyes with NFP, allowing automated measuring of their volume in the macular area (15,16). However, these devices do not allow for automated calculation of specific entities in retinal pathologies. In order to overcome this problem, different strategies have been proposed in the scientific literature (1,6,17,18,26). Among the strategies proposed for measuring the volume of specific entities in retinal pathologies, the processing of OCT scans using external software like FIJI and ImageJ 2.0.0 was reported in the paper by Taşlıpınar Uzel et al (1). In this method, after exporting all linear OCT scans from the OCT software and uploading them into the external software, manual or semi-automated segmentation of the OCT images was required, using tools provided by the external platform, to trace the outlines of specific entities (1). Use of external software platforms to calculate volumes requires additional several steps, such as installation, compatibility issues with different operating systems, dependencies, or updates, additional costs, exportation of images. Moreover, they can have a steep learning curve for new users requiring significant time and effort in case of limited experience in image analysis or programming. Finally, manual intervention, such as manual segmentation or annotation of images, or human supervision in case of semi-automated measurements are required, while fully automated workflows can be challenging to implement, especially for complex image processing tasks or analyses requiring sophisticated algorithms. Beyond use of an external software, deep learning methods have emerged as powerful tools for calculation of volumes, typically involving semantic segmentation techniques that classify each pixel in an image into predefined categories, thereby delineating different regions or structures of interest (6,17,18). Starting from a large dataset of OCT images annotated with ground segmentations indicating the boundaries of the specific entities whose volumes need to be calculated, convolutional neural networks (CNNs), or other deep learning architectures such as U-Net, SegNet, DeepLab architectures, are chosen for semantic segmentation (27). While our method can potentially offer more efficient data for training supervised neural networks; several problematics have been reported for deep learning models in calculation of specific retinal entities such as incapacity of the model to segment small target regions (28), poor performance and challenges due to speckle noise and imaging artifacts (17), inaccurate measurements of fluid volume due to the sparse sampling density(18), ability to segment exclusively entities with high contrast with surrounding tissues on OCT scans (6), insensitivity to the location of the target (6).
Differently from other techniques, we proposed a novel measurement method applicable for any specific retinal entity of interest, using imaging protocols routinely present in a clinical setting. Indeed, in our method, the target areas for volumetric calculation are manually drawn using the inbuilt specific tool present in Heidelberg Spectralis from the display system. This method showed high degree of agreement for inter-observer variability for RV in NFP eyes and FC/FS volume in LMH ones, and between the single observers and the OCT software for RV in NFP eyes. Moreover, excellent intra-observer agreement, for both RV and FC/FS volumes, was described. Compared with approaches based on external software, this method does not require export of the OCT scans and upload in a different system resulting in a significantly easier and less time-consuming procedure.
Limitations of the study include its limited sample size. However, these data were sufficient to highlight excellent intra- and inter-observer agreement. Manual segmentation allows for detailed customization and fine-tuning of segmentation boundaries but can be time-consuming. Nonetheless, in small-sized studies with limited resources, manual acquisition may offer a more practical and cost-effective alternative, allowing researchers to maximize the utility of available resources without compromising on data quality or analysis.
In conclusion, we describe an easy, reliable and widely applicable method to calculate retinal volumes, showing high intra- and inter-observer agreement. In the light of the clinical relevance of specific entities in several medical and surgical retinal disease, we believe that this measurement method may be a valuable tool in both clinical and research settings.
Author Contributions
Conceptualization, M.L., M.F.,A.J., T.I. and G.M.; methodology, M.L., M.F.,A.J., T.I. and G.M.; software, G.M.; validation, K.S., N.A. and G.M..; formal analysis, G.M. investigation, M.L.,K.S.,N.A.,N.A.; resources, M.L.,K.S.,N.A.,N.A.; data curation, M.L.,K.S.,N.A.,N.A, G.M.; writing—original draft preparation, M.L.,M.F.,G.M.; writing—review and editing M.L., M.F.,A.J., T.I. and G.M.; visualization, K.S.,N.A.,N.A.; supervision M.F.,A.J., T.I. and G.M.; project administration M.L., M.F.,A.J., T.I. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is an anonymized non-interventional observational study and as per national guidelines from the National Code of Clinical Research, and the Health Research Authority (HRA), this study has ethical approval exemption, and no patient consent was required for participation. All procedures were completed prior to the design of this study. Patients were diagnosed and treated according to local guidelines and agreements and written consent from patients was acquired prior to all procedures as clinically indicated. This study does not report on the use of new or experimental protocols.
Informed Consent Statement
Patient consent was waived due to its retrospective nature.
Data Availability Statement
No new data were created for this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Taşlıpınar Uzel AG, Gelisken F, Kühlewein L, Neubauer J. VOLUMETRIC ANALYSIS OF LAMELLAR MACULAR HOLE: An Optical Coherence Tomography Study. Retina. 2023 Feb 1;43(2):209–14.
- Pi S, Hormel TT, Wang B, Bailey ST, Hwang TS, Huang D, et al. Volume-based, layer-independent, disease-agnostic detection of abnormal retinal reflectivity, nonperfusion, and neovascularization using structural and angiographic OCT. Biomed Opt Express, BOE. 2022 Sep 1;13(9):4889–906. [CrossRef]
- El Habib Daho M, Li Y, Zeghlache R, Boité HL, Deman P, Borderie L, et al. DISCOVER: 2-D multiview summarization of Optical Coherence Tomography Angiography for automatic diabetic retinopathy diagnosis. Artif Intell Med. 2024 Mar;149:102803.
- Nipp GE, Sarici K, Lee T, Hadziahmetovic M. Risk factors for worsening morphology and visual acuity in eyes with adult-onset foveomacular vitelliform dystrophy. Ophthalmol Retina. 2024 Mar 8;S2468-6530(24)00108-8. [CrossRef]
- von der Burchard C, Treumer F, Ehlken C, Koinzer S, Purtskhvanidze K, Tode J, et al. Retinal volume change is a reliable OCT biomarker for disease activity in neovascular AMD. Graefes Arch Clin Exp Ophthalmol. 2018 Sep;256(9):1623–9. [CrossRef]
- Guo Y, Hormel TT, Xiong H, Wang J, Hwang TS, Jia Y. Automated Segmentation of Retinal Fluid Volumes From Structural and Angiographic Optical Coherence Tomography Using Deep Learning. Transl Vis Sci Technol. 2020 Oct;9(2):54.
- Balk LJ, Coric D, Knier B, Zimmermann HG, Behbehani R, Alroughani R, et al. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Multiple Sclerosis Journal - Experimental, Translational and Clinical. 2019 Jul 1;5(3):2055217319871582.
- Nam KT, Yun C, Lee YJ, Choi M, Kang D, Oh J. Visual Outcome and Fluid Changes Between Eyes With Polypoidal Choroidal Vasculopathy Receiving Biosimilar CKD-701 or Reference Ranibizumab Therapy: A Post Hoc Analysis of a Phase 3 Randomized Clinical Trial. Curr Eye Res. 2024 Mar 7;1–8.
- Schmidt-Erfurth U, Mulyukov Z, Gerendas BSS, Margaron P, Lorand D, Bogunovic H, et al. A comparison of the therapeutic response between brolucizumab and aflibercept in the HAWK & HARRIER trials using deep learning-based OCT analysis. Investigative Ophthalmology & Visual Science. 2020 Jun 10;61(7):1159.
- Waldstein SM, Philip AM, Leitner R, Simader C, Langs G, Gerendas BS, et al. Correlation of 3-Dimensionally Quantified Intraretinal and Subretinal Fluid With Visual Acuity in Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2016 Feb;134(2):182–90. [CrossRef]
- Lee H, Jo A, Kim HC. Three-Dimensional Analysis of Morphologic Changes and Visual Outcomes in Neovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017 Feb 1;58(2):1337–45. [CrossRef]
- Rommel F, Brinkmann MP, Sochurek JAM, Prasuhn M, Grisanti S, Ranjbar M. Ocular Blood Flow Changes Impact Visual Acuity Gain after Surgical Treatment for Idiopathic Epiretinal Membrane. J Clin Med. 2020 Jun 7;9(6):1768. [CrossRef]
- Aumann S, Donner S, Fischer J, Müller F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In: Bille JF, editor. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]. Cham: Springer International Publishing; 2019 [cited 2024 Mar 22]. p. 59–85. Available from: . [CrossRef]
- Zeppieri M, Marsili S, Enaholo ES, Shuaibu AO, Uwagboe N, Salati C, et al. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina (Kaunas). 2023 Dec 3;59(12):2114.
- Tian J, Varga B, Tatrai E, Fanni P, Somfai GM, Smiddy WE, et al. Performance evaluation of automated segmentation software on optical coherence tomography volume data. J Biophotonics. 2016 May;9(5):478–89.
- Debuc D. A Review of Algorithms for Segmentation of Retinal Image Data Using Optical Coherence Tomography. In: Image Segmentation. 2011.
- Schlegl T, Waldstein SM, Bogunovic H, Endstraßer F, Sadeghipour A, Philip AM, et al. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology. 2018 Apr;125(4):549–58. [CrossRef]
- Li MX, Yu SQ, Zhang W, Zhou H, Xu X, Qian TW, et al. Segmentation of retinal fluid based on deep learning: application of three-dimensional fully convolutional neural networks in optical coherence tomography images. Int J Ophthalmol. 2019;12(6):1012–20. [CrossRef]
- Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2017 May 6;19(6):1236–46.
- Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020 Dec;104(12):1741–7.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–10.
- Mj L, Wh Z, Yx L, Hz M, Yc L, Mh J. Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. The international journal of biostatistics [Internet]. 2016 Jan 11 [cited 2024 May 25];12(2). Available from: https://pubmed.ncbi.nlm.nih.gov/27838682/.
- Verticchio Vercellin AC, Jassim F, Poon LYC, Tsikata E, Braaf B, Shah S, et al. Diagnostic Capability of Three-Dimensional Macular Parameters for Glaucoma Using Optical Coherence Tomography Volume Scans. Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):4998–5010.
- Kaiser PK, Wykoff CC, Singh RP, Khanani AM, Do DV, Patel H, et al. RETINAL FLUID AND THICKNESS AS MEASURES OF DISEASE ACTIVITY IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION. Retina. 2021 Aug;41(8):1579–86. [CrossRef]
- Schneider M, Bjerager J, Hodzic-Hadzibegovic D, Klefter ON, Subhi Y, Hajari J. Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2024 Feb 28; [CrossRef]
- Jiang X, Shen M, Wang L, de Sisternes L, Durbin MK, Feuer W, et al. Validation of a Novel Automated Algorithm to Measure Drusen Volume and Area Using Swept Source Optical Coherence Tomography Angiography. Transl Vis Sci Technol. 2021 Apr 14;10(4):11. [CrossRef]
- Hsiao CH, Sun TL, Lin PC, Peng TY, Chen YH, Cheng CY, et al. A deep learning-based precision volume calculation approach for kidney and tumor segmentation on computed tomography images. Computer Methods and Programs in Biomedicine. 2022 Jun 1;221:106861. [CrossRef]
- Bai F, Marques MJ, Gibson SJ. Cystoid macular edema segmentation of Optical Coherence Tomography images using fully convolutional neural networks and fully connected CRFs [Internet]. arXiv; 2017 [cited 2024 Jan 13]. Available from: http://arxiv.org/abs/1709.05324.
- Guo S, Tutela AC, Wagner R, Caputo AR. A comparison of the effectiveness of four biostains in enhancing visualization of the vitreous. J Pediatr Ophthalmol Strabismus. 2006 Oct;43(5):281–4. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).