Submitted:
15 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Selection
- No medical contraindications for surgical endodontic therapy (ASA-PS class I or II).
- Diagnosis of periapical periodontitis by preoperative cone-beam computed tomography (CBCT).
- Root resection and retrograde root canal filling were conducted with the aid of a surgical microscope and with specialized instruments and treatment protocols optimized for EMS.
- The patient provided informed consent for the treatment.
2.3. Surgical Treatment Procedures and Follow-up
2.4. Data Collection and Outcome Assessment
2.5. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Arx, T. Failed root canals: the case for apicoectomy (periradicular surgery). J Oral Maxillofac Surg 2005, 63, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Akerblom, A.; Hasselgren, G. The prognosis for endodontic treatment of obliterated root canals. J Endod 1988, 14, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Salehrabi, R.; Rotstein, I. Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. J Endod 2004, 30, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Gorni, F.G.; Gagliani, M.M. The outcome of endodontic retreatment: a 2-yr follow-up. J Endod 2004, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Rahbaran, S.; Lewsey, J.; Gulabivala, K. Outcome of primary root canal treatment: systematic review of the literature - part 1. Effects of study characteristics on probability of success. Int Endod J 2007, 40, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Rahbaran, S.; Lewsey, J.; Gulabivala, K. Outcome of primary root canal treatment: systematic review of the literature -- Part 2. Influence of clinical factors. Int Endod J 2008, 41, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Karabucak, B.; Setzer, F. Criteria for the ideal treatment option for failed endodontics: surgical or nonsurgical? Compend Contin Educ Dent 2007, 28, 391–397, quiz 398, 407. [Google Scholar] [PubMed]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: a review. J Endod 2006, 32, 601–623. [Google Scholar] [CrossRef]
- Setzer, F.C.; Shah, S.B.; Kohli, M.R.; Karabucak, B.; Kim, S. Outcome of endodontic surgery: a meta-analysis of the literature--part 1: Comparison of traditional root-end surgery and endodontic microsurgery. J Endod 2010, 36, 1757–1765. [Google Scholar] [CrossRef]
- Setzer, F.C.; Kohli, M.R.; Shah, S.B.; Karabucak, B.; Kim, S. Outcome of endodontic surgery: a meta-analysis of the literature--Part 2: Comparison of endodontic microsurgical techniques with and without the use of higher magnification. J Endod 2012, 38, 1–10. [Google Scholar] [CrossRef]
- Kohli, M.R.; Berenji, H.; Setzer, F.C.; Lee, S.M.; Karabucak, B. Outcome of Endodontic Surgery: A Meta-analysis of the Literature-Part 3: Comparison of Endodontic Microsurgical Techniques with 2 Different Root-end Filling Materials. J Endod 2018, 44, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, M.J.; Friedman, S. Treatment outcome of surgical and non-surgical management of endodontic failures. J Can Dent Assoc 1997, 63, 364–371. [Google Scholar] [PubMed]
- Taschieri, S.; Machtou, P.; Rosano, G.; Weinstein, T.; Del Fabbro, M. The influence of previous non-surgical re-treatment on the outcome of endodontic surgery. Minerva Stomatol 2010, 59, 625–632. [Google Scholar] [PubMed]
- Kim, S.; Jung, H.; Kim, S.; Shin, S.J.; Kim, E. The Influence of an Isthmus on the Outcomes of Surgically Treated Molars: A Retrospective Study. J Endod 2016, 42, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yokoo, S.; Yamaguchi, T.; Suzuki, K.; Makiguchi, T. Factors Influencing Bone Healing after Extirpation with Endodontic Microsurgery-Microscopic Apicoectomy for Extensive Radicular Cysts. 北関東医学 2019, 69, 315–324. [Google Scholar] [CrossRef]
- Sukegawa, S.; Shimizu, R.; Sukegawa, Y.; Hasegawa, K.; Ono, S.; Fujimura, A.; Yamamoto, I.; Nakano, K.; Takabatake, K.; Kawai, H.; et al. Prognostic Factors in Endodontic Surgery Using an Endoscope: A 1 Year Retrospective Cohort Study. Materials (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Rud, J.; Andreasen, J.O.; Jensen, J.E. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg 1972, 1, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Molven, O.; Halse, A.; Grung, B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int J Oral Maxillofac Surg 1987, 16, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Rubinstein, R.A.; Kim, S. Short-term observation of the results of endodontic surgery with the use of a surgical operation microscope and super-EBA as root-end filling material. Journal of Endodontics 1999, 25, 43–48. [Google Scholar] [CrossRef]
- Chong, B.S.; Pitt Ford, T.R.; Hudson, M.B. A prospective clinical study of Mineral Trioxide Aggregate and IRM when used as root-end filling materials in endodontic surgery. 2003. Int Endod J 2009, 42, 414–420. [Google Scholar] [CrossRef]
- von Arx, T.; Hanni, S.; Jensen, S.S. Clinical results with two different methods of root-end preparation and filling in apical surgery: mineral trioxide aggregate and adhesive resin composite. J Endod 2010, 36, 1122–1129. [Google Scholar] [CrossRef]
- Song, M.; Jung, I.Y.; Lee, S.J.; Lee, C.Y.; Kim, E. Prognostic factors for clinical outcomes in endodontic microsurgery: a retrospective study. J Endod 2011, 37, 927–933. [Google Scholar] [CrossRef]
- Barone, C.; Dao, T.T.; Basrani, B.B.; Wang, N.; Friedman, S. Treatment outcome in endodontics: the Toronto study--phases 3, 4, and 5: apical surgery. J Endod 2010, 36, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, M.K.; Tekin, U.; Kaval, M.E.; Solmaz, M.C. The outcome of apical microsurgery using MTA as the root-end filling material: 2- to 6-year follow-up study. Int Endod J 2016, 49, 245–254. [Google Scholar] [CrossRef]
- Tawil, P.Z.; Saraiya, V.M.; Galicia, J.C.; Duggan, D.J. Periapical microsurgery: the effect of root dentinal defects on short- and long-term outcome. J Endod 2015, 41, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Lee, Y.-L.; Tsai, Y.-L.; Lin, H.-J.; Chang, M.-C.; Chang, S.-F.; Chang, S.-H.; Jeng, J.-H. Outcome assessment of apical surgery: A study of 234 teeth. Journal of the Formosan Medical Association 2019, 118, 1055–1061. [Google Scholar] [CrossRef]
- Raedel, M.; Hartmann, A.; Bohm, S.; Walter, M.H. Three-year outcomes of apicectomy (apicoectomy): Mining an insurance database. J Dent 2015, 43, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Glickman, G.N.; Woodmansey, K.F.; He, J. Retrospective Analysis of Root-end Microsurgery Outcomes in a Postgraduate Program in Endodontics Using Calcium Silicate-based Cements as Root-end Filling Materials. J Endod 2020, 46, 345–351. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, Q.; Tan, X.; Song, D.; Zhang, L.; Huang, D. Comparison of Mineral Trioxide Aggregate and iRoot BP Plus Root Repair Material as Root-end Filling Materials in Endodontic Microsurgery: A Prospective Randomized Controlled Study. J Endod 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Pinto, D.; Marques, A.; Pereira, J.F.; Palma, P.J.; Santos, J.M. Long-Term Prognosis of Endodontic Microsurgery-A Systematic Review and Meta-Analysis. Medicina (Kaunas) 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, S.G.; Lee, S.J.; Kim, B.; Kim, E. Prognostic factors of clinical outcomes in endodontic microsurgery: a prospective study. J Endod 2013, 39, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.; Pitt Ford, T.R. Essential endodontology : prevention and treatment of apical periodontitis; Blackwell Science: Oxford, UK, 1998; pp. 368–369. [Google Scholar]
- Hjorting-Hansen, E.; Andreasen, J.O. Incomplete bone healing of experimental cavities in dog mandibles. Br J Oral Surg 1971, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lustmann, J.; Friedman, S.; Shaharabany, V. Relation of pre- and intraoperative factors to prognosis of posterior apical surgery. J Endod 1991, 17, 239–241. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Jensen, S.S.; Hanni, S. Clinical and radiographic assessment of various predictors for healing outcome 1 year after periapical surgery. J Endod 2007, 33, 123–128. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Penarrocha, M.; Jensen, S. Prognostic factors in apical surgery with root-end filling: a meta-analysis. J Endod 2010, 36, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Pallares-Serrano, A.; Glera-Suarez, P.; Tarazona-Alvarez, B.; Penarrocha-Oltra, D.; Penarrocha-Diago, M.; Penarrocha-Diago, M. Healing of 295 Endodontic Microsurgery Cases After Long-Term (5-9 Years) Versus Middle-Term (1-4 Years) Follow-up. J Endod 2022, 48, 714–721. [Google Scholar] [CrossRef]
- Nair, P.N. New perspectives on radicular cysts: do they heal? Int Endod J 1998, 31, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Oginni, A.O.; Olusile, A.O. Follow-up study of apicectomised anterior teeth. SADJ 2002, 57, 136–140. [Google Scholar]
- Alantar, A.; Bloud, C.; Galéazzi, J.-M.; Baranes, M.; Lévy, G.; Chapireau, D.; Benlagha, N.; Maman, L. Success rate and recurrence frequency of periapical surgery: prospective study on 132 cases. Med Buccale Chir Buccale 2010, 16, 15–22. [Google Scholar] [CrossRef]
- Riis, A.; Taschieri, S.; Del Fabbro, M.; Kvist, T. Tooth Survival after Surgical or Nonsurgical Endodontic Retreatment: Long-term Follow-up of a Randomized Clinical Trial. J Endod 2018, 44, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Truschnegg, A.; Rugani, P.; Kirnbauer, B.; Kqiku, L.; Jakse, N.; Kirmeier, R. Long-term Follow-up for Apical Microsurgery of Teeth with Core and Post Restorations. J Endod 2020, 46, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Shin, S.J.; Kim, E. Outcomes of endodontic micro-resurgery: a prospective clinical study. J Endod 2011, 37, 316–320. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T. Frequency and type of canal isthmuses in first molars detected by endoscopic inspection during periradicular surgery. Int Endod J 2005, 38, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Yamada, M.; Kasahara, N.; Kasahara, M.; Odaka, K.; Fujii, R.; Miyayoshi, N.; Sekiya, S.; Sako, R.; Sugiuchi, A.; et al. Tooth Root Cross-section Variations of Significance for Endodontic Microsurgery and Predicted Risk of Concealed Canal Isthmus Based on Cross-sectional Morphology: Three-dimensional Morphological Analysis of Japanese Maxillary First Molars Using Micro-CT. Journal of Hard Tissue Biology 2019, 28, 153–158. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Taschieri, S.; Testori, T.; Francetti, L.; Weinstein, R.L. Surgical versus non-surgical endodontic re-treatment for periradicular lesions. Cochrane Database Syst Rev 2007, CD005511. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Hanni, S.; Jensen, S.S. 5-year results comparing mineral trioxide aggregate and adhesive resin composite for root-end sealing in apical surgery. J Endod 2014, 40, 1077–1081. [Google Scholar] [CrossRef]
- Caliskan, M.K.; Tekin, U.; Kaval, M.E.; Solmaz, M.C. The outcome of apical microsurgery using MTA as the root-end filling material: 2- to 6-year follow-up study. Int Endod J 2016, 49, 245–254. [Google Scholar] [CrossRef]
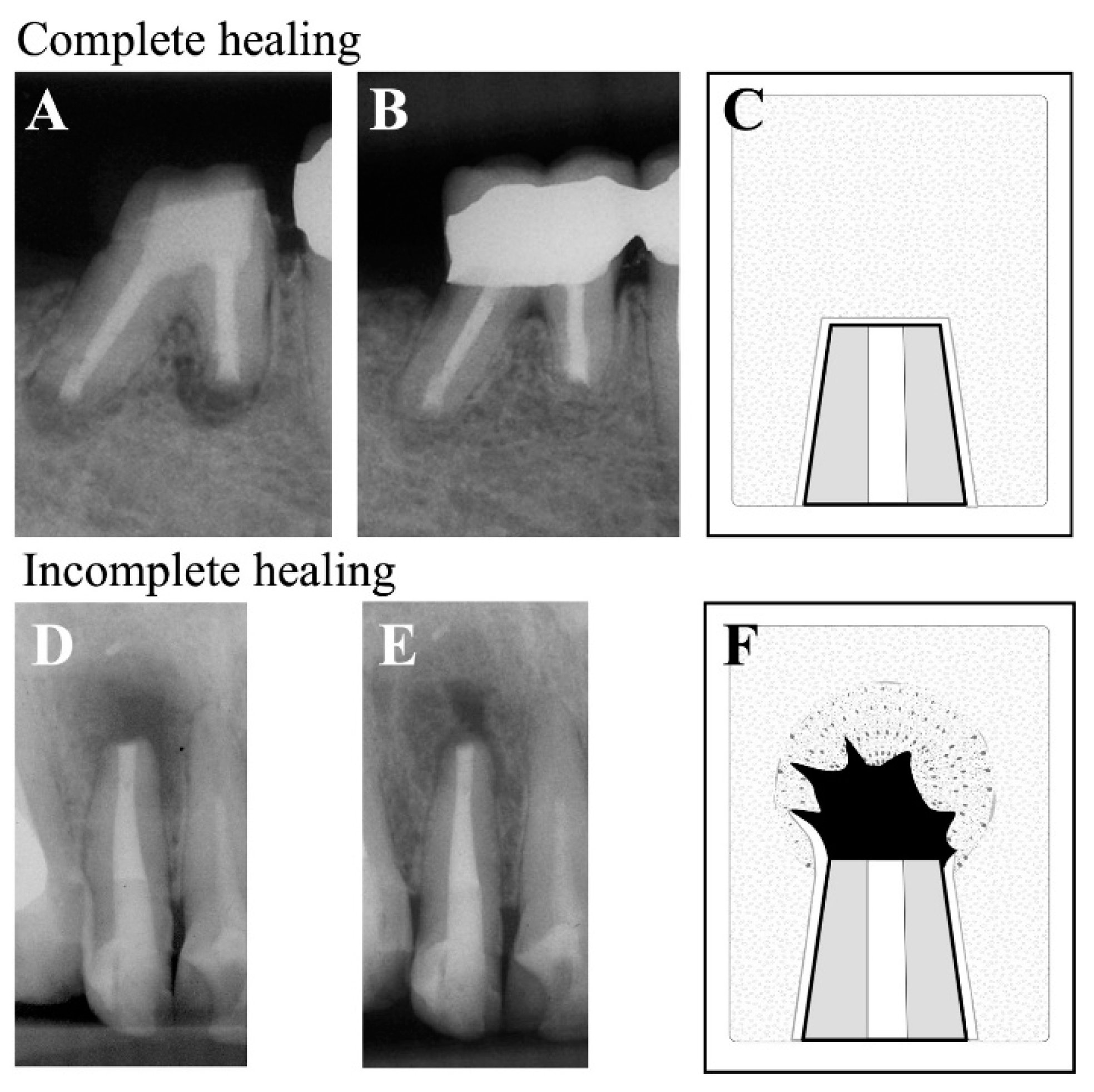
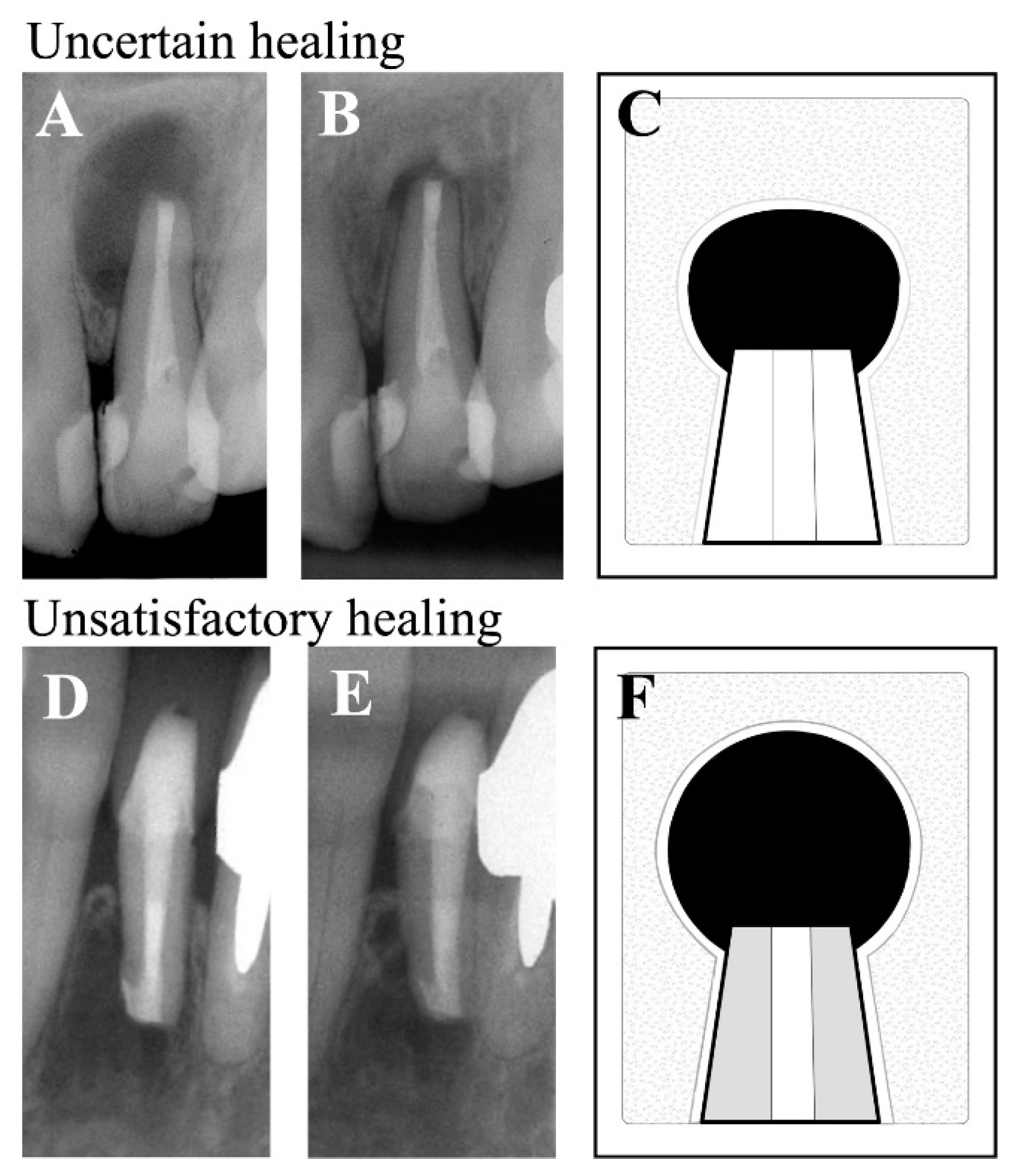
| Pathological diagnosis | n (%) |
|---|---|
| Radicular cyst | 26 (65) |
| Radicular granuloma | 8 (20) |
| Inflammatory granulation tissue | 6 (7.5) |
| Not collected | 6 (7.5) |
| Variable/Factor (n) | Clinical success (%) | Clinical failure (%) | OR (95%CI) | P value | |
|---|---|---|---|---|---|
| Sex (46) | Female | 31 (67.4) | 3 (6.5) | 0 (0-7.012) |
0.557 |
| Male | 12 (26.1) | 0 (0) | |||
| Age, y (46) | < 45 | 29 (63.1) | 2 (4.3) | 1.035 (0.02.-21.50) |
1 |
| ≥ 45 | 14 (30.4) | 1 (2.2) | |||
| Arch (46) | Maxillary | 39 (84.8) | 1 (2.2) | 17.08 (0.75-1165.82) |
0.041* |
| Mandibular | 4 (8.7) | 2 (4.3) | |||
| Lesion size, mm (46) | < 5 | 17 (37) | 0 (0) | Inf (0.11-Inf) |
0.524 |
| ≥ 5 | 27 (58.7) | 2 (4.3) | |||
| Lesion type (34) | Granuloma | 24 (70.6) | 2 (5.9) | 3.27 (0.04 -279.35) |
0.432 |
| Cyst | 7 (20.6) | 1 (2.9) | |||
| Pre-RCT (46) | Yes | 33 (71.8) | 0 (0) | Inf (1.14-Inf) |
0.019* |
| No | 10 (21.7) | 3 (6.5) | |||
| Post core (46) | Yes | 17 (37) | 2 (4.3) | 0.561 (0.01- 6.91) |
0.561 |
| No | 26 (56.5) | 1 (2.2) | |||
| Isthmus (46) | Yes | 18 (39.1) | 2 (4.3) | 0.368 (0.01- 7.58) |
0.572 |
| No | 25 (54.4) | 1 (2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





