1. Introduction
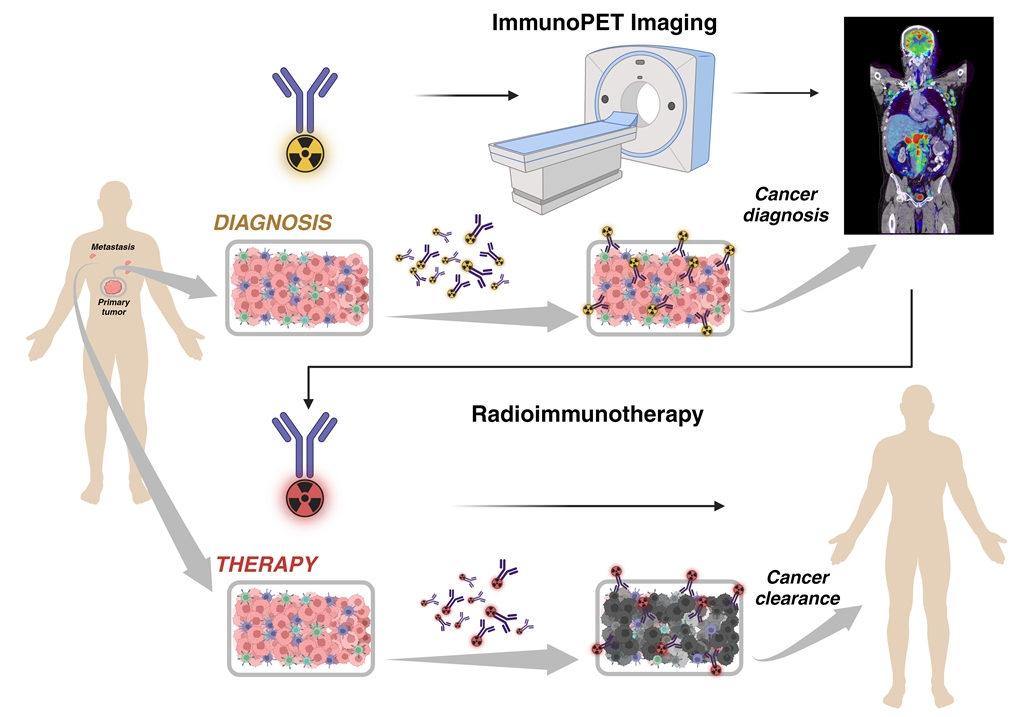
The potential use of a single molecular targeting agent for both diagnostic and therapeutic purposes, known as theragnostic drugs, represents an opportunity to improve the management of cancer patients. In Nuclear Medicine, radioimmunotheragnostics drugs involve the coordinated use of a “matched pair” radiopharmaceutical for diagnostic imaging and targeted therapy using β or α-emitting isotopes [
1,
2]. This integrated approach is the epitome of the personalized medicine. By combining a specific tumor biomarker, quantitatively imaged with high sensitivity by positron emission tomography (PET), with a theragnostic β or α -emitting radionuclide pair, linked to the same targeted molecule, it becomes possible to regulate tumor development in individual patients. Traditionally, antibodies directed against exposed antigens that are overexpressed in tumors but not in healthy tissues, have been the preferred targeting molecules due to their high specificity and selectivity. However, full-length antibodies used in immunoPET imaging have several drawbacks such as slow pharmacokinetics and prolonged circulating half-life due to their high molecular weight. As a result, PET imaging is typically delayed for several days after injection, resulting in increased background radiation and degraded image quality. In addition, when coupled with a therapeutic radionuclide, the circulating activity poses a risk of potentially damaging healthy tissues such as bone marrow, kidney, and liver [
3].
In today’s landscape of cutting-edge molecular targeting therapy and cancer immunotherapy, there’s an urgent need to advance the clinical care of cancer patients. This requires the seamless integration of novel molecular imaging techniques and innovative radioisotope-based targeted therapies. One such pioneering approach is radioimmunotheragnosis a fusion of immunoPET and radioimmunotherapy (RIT) techniques. In this comprehensive review, we discuss the latest studies and advancements in this revolutionary technology, which is poised to redefine the landscape of cancer management [
4,
5,
6].
For therapy, RIT uses monoclonal antibodies (mAbs) labeled with α-particle, β, or Auger electron (AE) emitting radionuclides to selectively deliver radiation to cancer cells. The mAbs specifically bind to cancer cell surface-expressed antigens, delivering lethal doses of radiation directly to the tumor while sparing healthy tissue [
7]. Commonly used isotopes for antibody labeling include iodine-131, yttrium-90, and lutetium-177, among others. These isotopes emit different types of radiation with different ranges and energies, allowing for tailored treatment depending on the characteristics of the tumor [
6]. Compared to traditional external beam radiation therapy, RIT offers the advantage of targeted delivery, potentially reducing systemic toxicity and sparing healthy tissues. Some issues with RIT include optimizing dosimetry, managing hematologic toxicities such as myelosuppression, and addressing the development of resistance.
For imaging, immunoPET combines the specificity of mAbs with the sensitivity and resolution of PET imaging. Antibodies are labeled with positron-emitting radionuclides, allowing for non-invasive imaging of antigen expression in vivo [
1,
8]. ImmunoPET has multiple clinical applications, including cancer diagnosis, staging, treatment response assessment, and patient stratification. It provides valuable information about tumor characteristics, such as antigen expression levels and heterogeneity, which helps to plan a personalized treatment. This technique offers high sensitivity and quantitation capacity, as well as high specificity due to the targeting capability of the antibodies. A major challenge is to achieve a good match between the isotope and the size of the antibody. The combination of immunoPET and radioimmunotherapy offers several advantages. ImmunoPET provides non-invasive imaging of antigen expression, which can help select patients most likely to benefit from radioimmunotherapy. In addition, can be used to assess tumor response to therapy and guide treatment modifications if necessary. Furthermore, immunoPET can provide valuable pharmacokinetic data, such as antibody biodistribution and clearance rates, which can be used to calculate dosimetry and optimize treatment protocols [
9].
Antibodies in ImmunoPET and RIT
As a primary function, antibodies and antibody-like molecules designed and used for oncology treatment, should recognize their target antigen with high specificity and binding affinity. On the other hand, antigens should have high expression in tumors and in the surrounding microenviroment [
10], but low expression in normal tissues to minimize background noise in imaging. In addition, the epitopes of the antigen recognized by the antibody must be present on the tumor cell surface to facilitate the interaction and further accessibility.
In this regard, there are many tumor-antigens that are under investigation as targets for various molecules designed to specifically detect them. In this review we will discuss most of these types of antibodies, focusing on their characteristics, advantages and limitations.
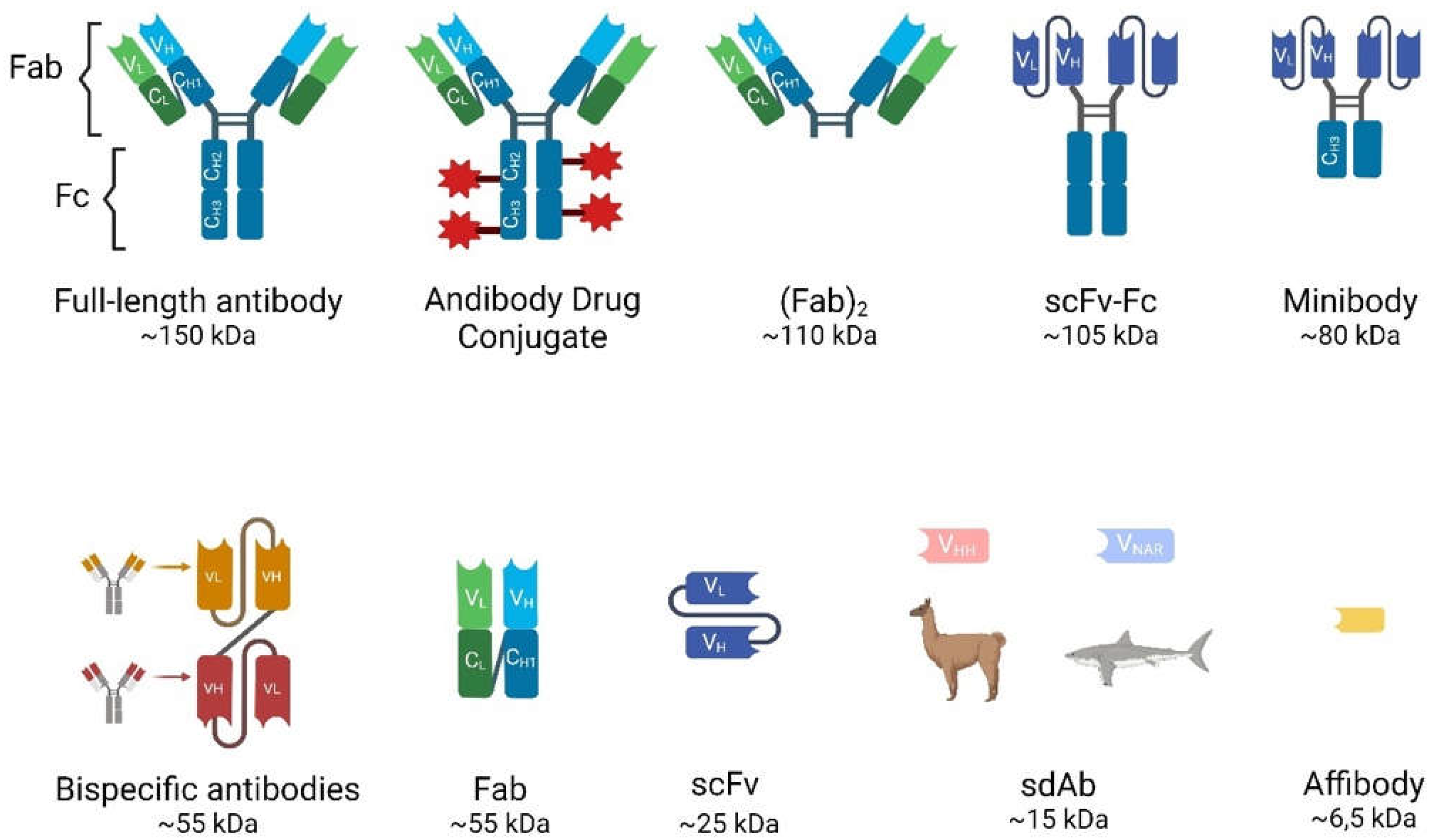
Antibodies can be classified into different types based on their size, structures and mechanisms of function (
Figure 1)
Full-Length Antibodies (Abs)
These glycoproteins, also known as immunoglobulins (Ig), are essential components of the immune system that recognize and neutralize pathogens such as bacteria and viruses. They have been the focus of research and medical applications for decades, including diagnostics, therapeutics and immunoassays. Different types of antibodies have unique structures, functions, and applications. In humans, antibodies are divided into five classes or isotypes: IgA, IgD, IgE, IgM and IgG, the latter being the most common.
IgG is composed of four polypeptide chains; two heavy chains (containing one variable domain VH and three to four constant domains CH1, CH2, CH3) and two identical light chains (containing one variable domain VL and one constant domain CL) linked by disulfide bonds (see
Figure 1). In the antibody structure can also be distinguished as an antigen-binding fragment (Fab) and a crystallizable fragment (Fc), together forming a Y-shape that allows the variable part to be exposed and able to recognize its corresponding antigen [
11]. The heterotetramer has a molecular weight of 150 kDa and a half-life in the bloodstream of approximately 21 days before being eliminated mainly by intracellular enzymatic degradation. They cannot be eliminated by the kidneys or the liver due to their large size.
Antibody binding to the cancer cells can lead to cancer cell death by a variety of mechanisms, such as by exerting neutralizing or apoptotic effects and promoting innate immune responses (antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC)). In addition, these antibodies can be engineered for various functions, such as drug conjugation, radioimmunotherapy and immunoPET imaging.
Although these Abs have been used for most medical treatments and research, the full-length antibodies have some limitations, such as slow blood clearance and low target/background ratio (especially important to achieve good quality for PET imaging).
Antibody Fragments
Various antibody fragments can be prepared by enzymatic digestion with papain or pepsin or by genetic engineering to overcome the drawbacks of full-length antibodies. Compared to full-length Abs, they exhibit shorter serum half-lives (4-20 h), faster elimination from the bloodstream, lower immunogenicity, since most of them lack the Fc region, and the ability to homogeneously penetrate tissues, including solid tumors, allowing for better target/background ratios for imaging [
1]. The most frequently used antibody fragments in clinical developments are (
Figure 1):
Fragment Antigen-Binding (Fab and (Fab’)2): Fab is a 55-kDa monovalent fragment consisting of a complete light chain and the heavy chain variable (VH) domain and the first constant (CH1) region. It can be produced by papain digestion of an intact antibody or recombinantly in several high-yield expression systems. This fragment retains antigen-binding specificity and the smaller size improves tissue penetration and reduces non-specific binding. Useful for certain diagnostic and therapeutic applications, although lower molecular stability may be a limitation compared to full-length antibodies. On the other hand, (Fab’)2 is another antibody derivative produced by pepsin digestion of IgG. It retains the bivalent binding capacity of IgG immunoglobulins but lacks the constant region (Fc) and is consequently smaller in size (~110kDa).
Single-chain variable fragments (scFvs): These fragments are engineered antibodies consisting of the variable regions of the heavy and light chains connected by a variable peptide linker to form a single-chain molecule. With a molecular weight of only 25-30 kDa, scFvs have even better tissue penetration and rapid clearance from the bloodstream through the urinary system. They can be produced in bacterial systems, which is cost-effective, but their lower stability and functional affinity (avidity) compared to full-length antibodies and rapid renal clearance have limited their use in the clinic. Multivalent scFvs, constructed by connecting VH and VL fragments with short “GGGGS” peptide linkers, include diabodies (~60 kDa), triabodies (~90 kDa) and tetrabodies (~110 kDa). These can serve as advantageous vectors for radioimmunoimaging because their increased size enhances affinity and prolongs blood circulation [
12,
13].
Minibodies: These constructions are also engineered Ab fragments. They are produced by combining scFv molecules with human IgG1 constant heavy chain-3 (CH3). These fragments have accelerated blood clearance compared to full-sized antibodies, making them particularly useful for in vivo imaging as accumulation in the kidney can be avoided [
13]
Bispecific Antibodies: Unlike monospecific antibodies, bispecific antibodies have two different antigen-binding sites, allowing them to bind to two different antigens simultaneously. The specific antigens/epitopes can be localized either on the same cell or on different cells. They are emerging as potent therapeutics agents for cancer immunotherapy. The disadvantages are the complex engineering and consistent production to overcome the molecule instability. The bispecific antibodies targeting two different cells are mostly T cell engagers (BiTE, Bispecific T cell engagers) that link a cancer cell to an effector T cell. A cancer-specific scFv is joined with a T cell-binding scFv (anti-CD3) via a glycine-serine peptide linker [
14,
15]. Upon crosslinking, the T cell is activated to kill the bound target cancer cell by secreting perforin and other granzymes. These cytolytic proteins can form pores on the cancer cell membrane, resulting in cancer cell lysis. The BiTE format has emerged as a potent antibody-based therapeutic since the regulatory approval of blinatumomab (anti-CD19xanti-CD3) in B-cell malignancies [
16].
Single domain antibodies (sdAb): Heavy chain antibodies are a class of antibodies found in camelids and sharks that are characterized by the absence of the light chain and the CH1 domain. The variable domain of their heavy chain can be efficiently cloned, expressed and purified in a heterologous system such as
E. coli to yield a single domain antibody of approximately 15 kDa, also known as VHH from camelids, commercially called nanobody (Nb), and VNAR, natural heavy-cahin only antibody derived from sharks. These sdAbs have unique biochemical and biophysical properties such as their stability, high solubility, thermal and proteolytic resistance [
17]. Single domain antibodies are the smallest but functional natural antibody-derived fragments that retain high specificity and good affinity binding properties, also exhibiting faster pharmacokinetics than the original structure. Their small molecular weight allows for much deeper tissue penetration, more homogeneous distribution within the tumor microenvironment and a faster blood clearance through the renal excretion route, increasing the tumor-to-background ratio and image quality in PET experiments. In addition, nanobodies may be superior for targeting brain tumors or brain metastases from systemic tumors due to their ability to cross the brain-blood-barrier, attributed to their smaller size and different physicochemical properties compared to conventional antibodies [
18]. Moreover, their shorter half-life can be matched with rapidly decaying radionuclides for diagnosis and tumor treatment [
15,
19,
20]. (
Figure 2). The problem of rapid renal clearance, especially if they are developed as therapeutic tools, can be solved by PEGylation or fusion with albumin-binding proteins [
21]. Other favourable feature for clinical use is its low immunogenicity since VHH structure is highly similar to human VH structure and the small size of VHH implies a low number of potentially immunogenic epitopes [
22]. Nevertheless, humanization of VHH is possible to further reduce its immunogenicity [
23].
Affibody molecules (AB): they are even smaller engineered proteins (6.5 kDa) that bind with high affinity to a variety of target molecules, mimicking monoclonal antibodies [
24]. Affibodies can be used for protein purification, enzyme inhibition, diagnostic imaging and ultimately targeted therapy. The robust structure of affibody molecules, composed of alpha helices and lacking disulfide bridges, allows them to be conjugated to radionuclides without compromising their binding capacity. Their small size also helps achieve high-contrast images [
25,
26]. For example, affibody “ABY-002” has been developed for HER2 imaging using SPECT (radiolabeled with
111In) and for PET (radiolabeled with
68Ga) [
27].
Antibody–drug conjugates (ADCs): They consist of a small molecule drug (payload) covalently attached to a monoclonal antibody via a chemical linker to target tumor-expressing antigens and destroy cancer cells. The toxic payloads most commonly used in approved and clinical-stage ADCs are microtubule disruptors or DNA-damaging agents [
28]. PET imaging of radiolabeled ADCs has been used to determine the pharmacokinetics, biodistribution and tissue uptakes of ADCs [
29] as radioisotopes such as
64Cu,
86Y,
89Zr, and
124I can be conjugated to ADC without affecting the drug properties [
30].
Isotopes
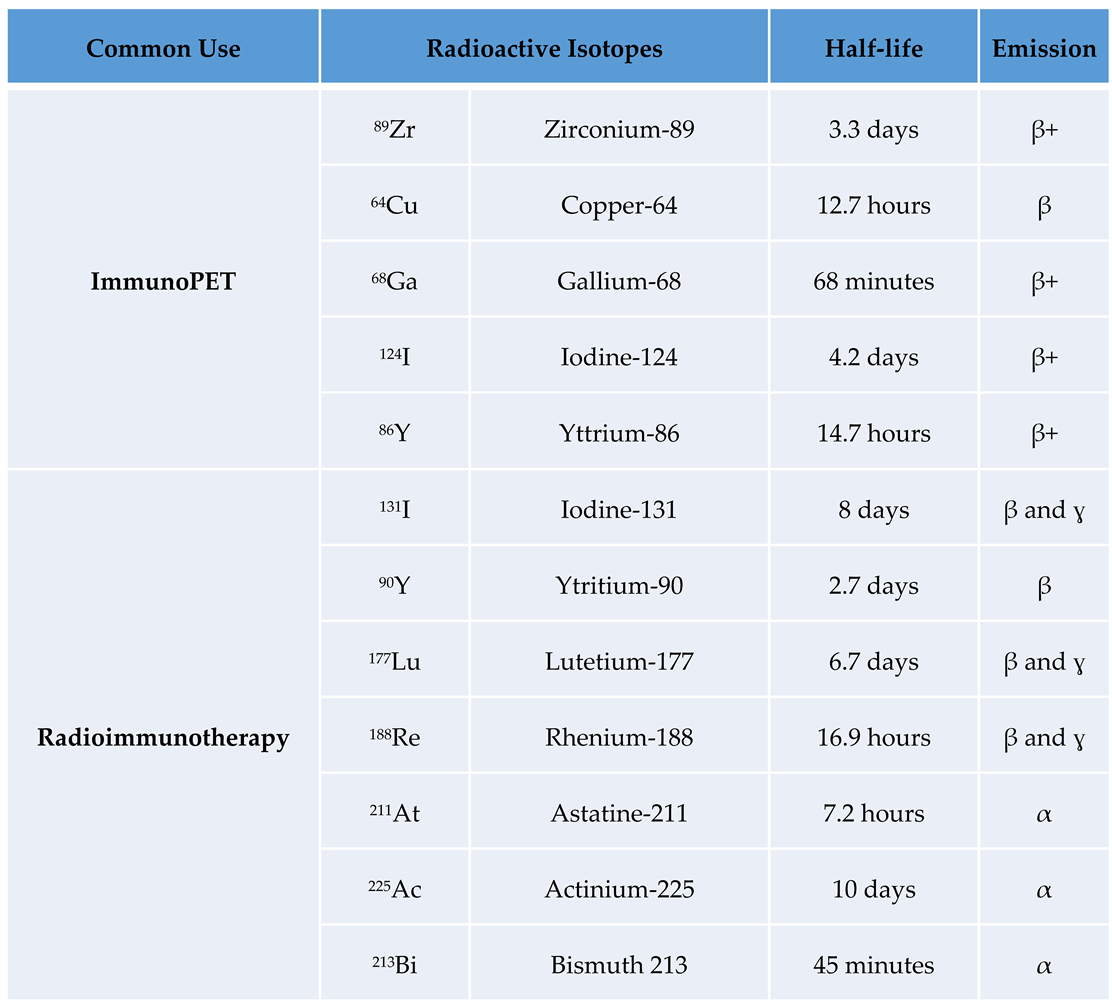
There are a number of isotopes used in nuclear medicine as theragnostic agents. The choice of radioactive isotopes is critical for the efficacy and safety of immunoPET and RIT. Here’s an overview of some of the most common used radioactive isotopes, focusing on their properties, advantages, and limitations (
Table 1):
Commonly Used Radioactive Isotopes in ImmunoPET
- -
89Zr (Zirconium-89)
The relatively long half-life of
89Zr (half-life: 78.4 hours) matches well with the pharmacokinetics of intact antibodies, allowing for sufficient time for the antibody to target the tumor and for imaging to be performed at later time points. This is particularly useful for imaging slow-accumulating targets. Importantly, the long half-life also means higher radiation dose to the patient. In addition,
89Zr has a high energy positron emission, β+ (23%; 396 keV) and β+ (6%; 824 keV).
89Zr-immuno-PET tracers have been used clinically to image HER2 [
31,
32] receptors and VEGF-A [
33]. In addition
89Zr PD-1/PD-L1 has been used in different clinical trials [
32,
34].
- -
64Cu (Copper-64)
The intermediate half-life of
64Cu (half-life: 12.7 hours) is suitable for imaging with smaller antibody fragments, such as Fab fragments and single-chain variable fragments (scFv). It provides a balance between adequate imaging time and lower radiation dose. However, the shorter half-life compared to
89Zr may not be ideal for imaging with full antibodies.
64Cu has been evaluated in preclinical studies labeling full Abs as durvalumab [
35] and (Fab’)
2 for
64Cu-labeled CD4+ T cell targeting (38) and also in patients for trastuzumab PET imaging [
36].
- -
68Ga (Gallium-68)
The short half-life (68 minutes) is ideal for imaging with very small antibody fragments, peptides, or nanobodies.
68Ga is readily available from a
68Ge/
68Ga generator, making it convenient for clinical use. It has been used extensively in preclinical studies and the HER2-nanobody has been used successfully in patients with breast cancer. The short imaging window limits the flexibility of imaging schedules and may not be suitable for intact antibodies or other large biomolecules that require more time to accumulate at the target site [
37,
38].
- -
124I (Iodine-124)
The long half-life (100.3 hours) is excellent for imaging with full antibodies and allows delayed imaging, which can improve contrast by allowing non-specific background signal to clear. Antibody radiolabeling with
124I for immuno-PET imaging has successfully produced radiotracers for tumor detection in colorectal cancer [
39], thyroid cancer [
40] and gastric cancer [
41].
124I has high energy positron emissions β+ (12%; 687 keV) and β+ (11%; 975 keV), which may result in poorer image resolution. There is also a risk of dehalogenation, where iodine is released from the antibody and can accumulate in non-target tissues, such as the thyroid.
- -
86Y (Yttrium-86)
With a half-life of 14.7 hours,
86Y is suitable for medium-sized antibody fragments and provides good image quality due to its appropriate positron energy β+ (32%) decay; 394–1437 keV. However, it has less favorable properties than
89Zr due to the high energy γ-rays produced by the isotope.
86Y is less widely used and less readily available than other isotopes. It also has complex decay schemes that can complicate image quantification. This radiometal has been studied in various preclinical models for labeling F(ab’)2 fragments and mAbs [
42,
43].
Commonly Used Radioactive Isotopes in Radioimmunotherapy
- -
131I (Iodine-131)
It was the first theranostic isotope used.
131I is β and ɣ emitter that has been extensively used in RIT because of its well-established chemistry and ability to be easily conjugated to antibodies. Its beta particles provide effective cytotoxicity, and the gamma emissions allow for imaging and dosimetry. Rituximab [
44], and tositumomab [
45] labeled with
131I have been tested in patients and the latter has been approved for radiopharmaceutical therapy in USA and EU. Gamma emissions require special handling and patient isolation to avoid radiation exposure to others. Dehalogenation may lead to accumulation of free iodine in the thyroid, requiring thyroid protection.
- -
90Y (Yttrium-90)
The
90Y pure beta emitter produces high-energy particles that are highly effective at killing cancer cells. The lack of gamma emission reduces radiation exposure to medical personnel and others. On the other hand, it cannot be used for imaging for the same reason. The high-energy beta particles can damage surrounding healthy tissue if the tumor is small or near sensitive structures.
90Y-ibritumomab tiuxetan has been used as a first-line treatment for follicular lymphoma [
46].
- -
177Lu (Lutetium-177)
177Lu has favorable radiation properties, with beta particles for therapeutic effects and gamma emissions for imaging and dosimetry. Its intermediate beta energy and relatively short path length make it suitable for treating smaller tumors and minimizing damage to surrounding tissues. It has been successfully used in preclinical studies and several clinical trials in non-Hodgkin lymphoma, ovarian cancer, prostate cancer and neuroendocrine tumors. [
177Lu]Lu-PSMA-617 is approved for resistant prostate cancer in the US since 2022 and [
177Lu]Lu-DOTA-TATE is approved for gastroenteropancreatic neuroendocrine tumours since 2018 in the UE and USA [
47,
48,
49].
- -
188Re (Rhenium-188)
188Re emits both beta particles for therapy and gamma rays for imaging. It is available from a
188W/
188Re generator, providing a convenient and continuous supply. Its shorter half-life (16,9 h) is advantageous for reducing long-term radiation exposure. On the other hand, this short half-life requires rapid targeting and treatment protocols.
188Re has been used in many clinical trials to label various compounds such as liposomes or hydroxyethylidine diphosphonate [
50,
51].
- -
211At (Astatine-211)
It is an alpha emitter with a half life of 7.2 hours. Alpha particles have a high linear energy transfer (LET) and are extremely effective at killing cancer cells with minimal damage to surrounding tissue. This makes
211At particularly effective for targeting micrometastases and isolated tumor cells. The short half-life requires rapid targeting, and the production and handling of
211At can be challenging. Its alpha emissions limit the penetration depth, making it less suitable for large tumors. Several
211At based radionuclide therapies are under investigation in various clinical trials [
52].
- -
225Ac (Actinium-225)
This is another alpha emitter (10 days half-life). It has a median lethal dose several orders of magnitude greater than
213Bi because of its longer half-life and subsequent alpha emissions from its decay products. Each decay of
225Ac to
209Bi produces four high-energy alpha particles, greatly increasing its potency. The main limitation is that its alpha emissions limit the depth of penetration, making it less suitable for large tumors. It has been tested for targeted alpha particle therapy in prostate cancer patients [
53].
- -
213Bi (Bismuth 213)
Another emerging alpha emitter isotope with a half-life of 45 minutes. It can be produced using a
225Ac/
213Bi generator. The short half-life requires rapid targeting. Production and handling of
211At can be challenging. The anti-leukemic effects of lintuzumab labeled with
225Ac and
213Bi have been studied in patients with acute myeloid leukemia [
54].
Factors Influencing the Choice of Isotope
Several factors must be considered when selecting isotopes for targeted molecular imaging and therapy to optimize efficacy and safety. The half-life of the isotope should match the biological half-life of the targeting molecule to maximize tumor localization while minimizing off-target radiation exposure. Full antibodies generally require isotopes with longer half-lives, while smaller fragments or peptides can use isotopes with shorter half-lives. In imaging, lower positron energy is preferred because it improves image resolution; high-energy positrons travel further before annihilation, reducing spatial resolution. The type of radiation emitted by the isotope also plays a critical role in therapeutic efficacy and potential collateral damage; alpha particles are highly effective but have limited penetration, while beta particles can treat larger volumes but may affect surrounding tissues. In addition, the availability and production of isotopes such as
68Ga or
188Re, which can be obtained from generators, make them convenient for clinical use. Finally, dosimetry and safety considerations are paramount; radiation dose to the patient should be minimized to ensure effective imaging, and it should be noted that isotopes with longer half-lives typically deliver higher radiation doses [
8].
Therefore, the choice of radioactive isotope for immunoPET depends on a balance of factors including the half-life of the isotope, the type of targeting molecule, the required image resolution, and the clinical context.
89Zr and
124I are preferred for full antibodies due to their long half-lives, while
64Cu and
68Ga are suitable for smaller fragments and peptides due to their shorter half-lives and rapid imaging capabilities [
6]. Understanding these properties helps to optimize immunoPET for accurate and effective molecular imaging. The selection of radioactive isotopes for radioimmunotherapy depends on balancing therapeutic efficacy, safety, and practical considerations.
131I and
90Y are well-established in clinical practice, with distinct advantages for different tumor types and sizes.
177Lu offers a versatile option with both therapeutic and imaging capabilities. Emerging isotopes such as
213Bi,
225Ac and
211At offer promising alternatives for specific clinical scenarios, particularly for targeting micrometastases. Understanding the characteristics and clinical implications of each isotope helps to tailor radioimmunotherapy to achieve optimal patient outcomes [
36].
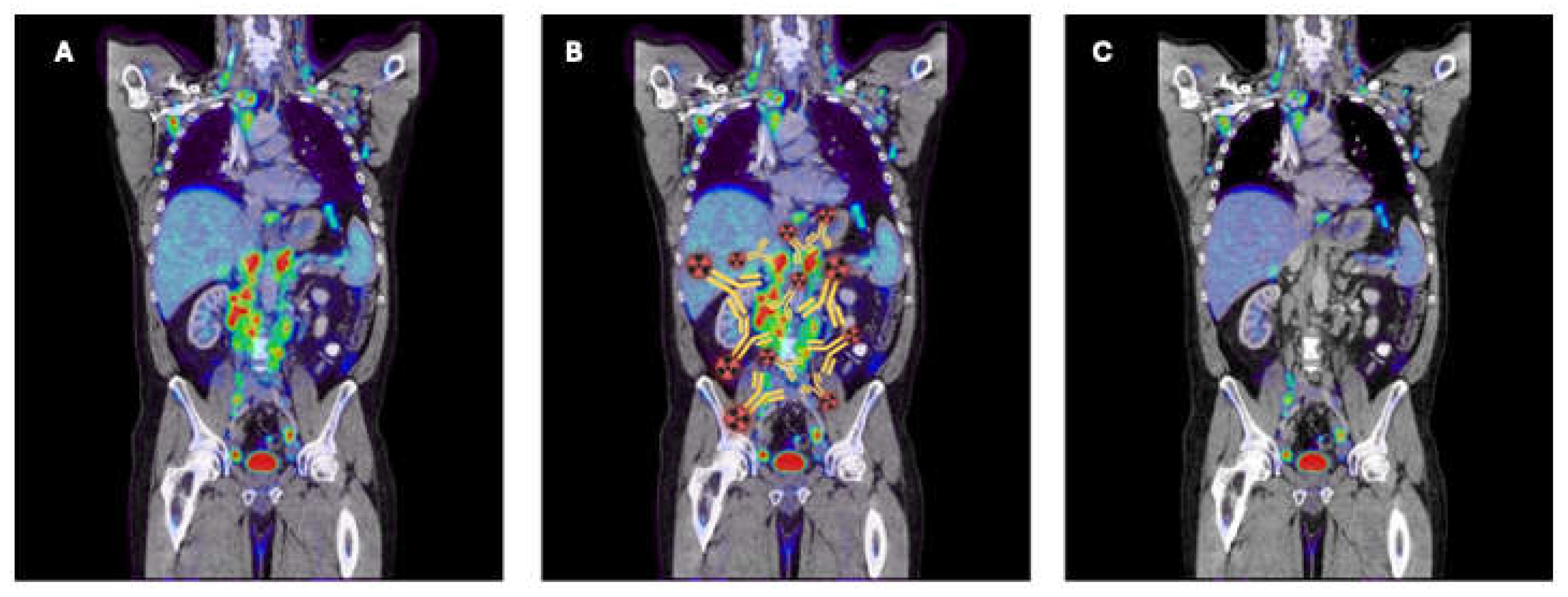
Pretargeted ImmunoPET and Radioimmunotherapy (PRIT): General Strategy
Pretargeting methodologies hold promise for enhancing antibody binding to tumor cells. A key example involves functionalizing antibodies or their fragments with biotin, avidin, and specific oligonucleotides such as phosphorodiamidate morpholinos or peptide nucleic acids. These molecules are then engineered to be bispecific. Subsequently, the modified monoclonal antibodies are typically administered intravenously. After a waiting period of 24–72 hours to allow for clearance of unbound conjugates, radionuclides tagged with a complementary counterpart capable of recognizing only the conjugated mAbs are administered intravenously, intraperitoneally, or locally [
29]. The small size of the radioligand ensures rapid biodistribution and clearance, typically within a few hours, thereby minimizing off-target irradiation of healthy tissues.
Unlike traditional radioimmunoconjugates, pretargeted approaches decouple the steps of tumor-targeting and payload delivery, enhancing tumor uptake while minimizing exposure to normal tissues. Critical considerations include antibody immunogenicity and specificity, radioisotope availability, and clinical feasibility. Each step can be optimized independently, making pretargeting systems adaptable to different tumor targets, types, and radioisotopes. However, despite its versatility, pretargeting presents complexities and unique challenges for clinical translation and optimal patient use [
41].
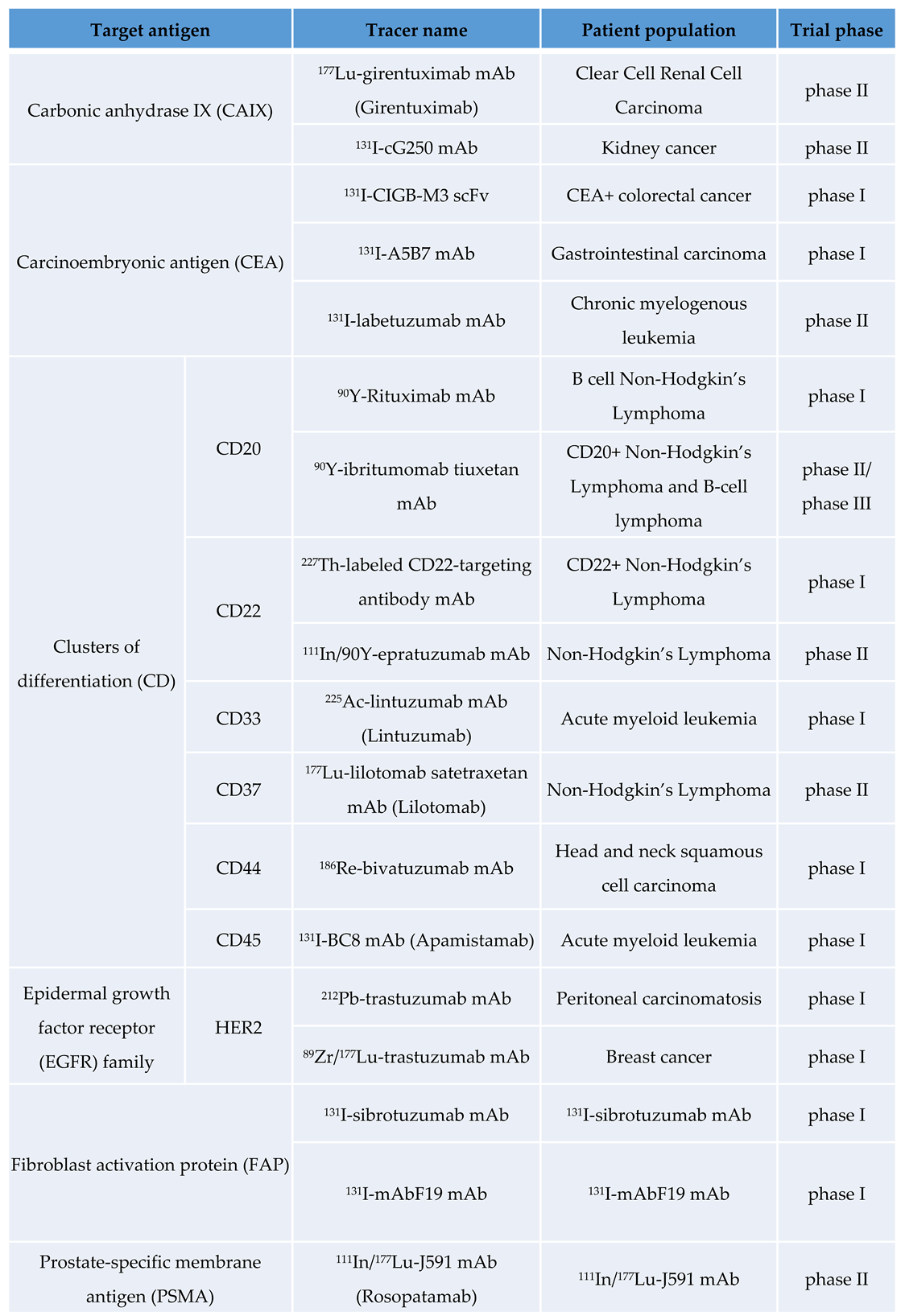
Radioimmunotheragnosis Applications in Cancer
Radioimmunotheranostics has a long history dating back to the 1930s when Hertz et al. introduced the concept [
55]. If the current potential of radioimmunotheranostics is realized, it will validate the substantial investments in research and development and the clinical translation of new agents to improve patient outcomes. The most significant advances are expected in cancers with the highest incidence and mortality rates, such as breast cancer. Additionally, significant improvements may occur in cancers with lower incidence but very high mortality rates, including pancreatic, ovarian, small-cell lung, and hepatobiliary cancers. However, expanding the use of radiotheranostics presents numerous challenges [
2,
45].
We will review some of the most common applications in preclinical and clinical cancer research (
Table 2).
Haematological Malignacies
Long time ago researchers have investigated two different methods using radioactively labeled mouse monoclonal antibodies that target the CD20 antigen on B cells. In 2002, the FDA approved
90Y-ibritumomab tiuxetan, which became a breakthrough treatment for patients with B cell non-Hodgkin lymphomas (NHL) [
56]. In 2003 another anti-CD20-binding antibody,
131I-tositumomab, also received received FDA approval [
45,
57]. Despite their excellent clinical performance and limited toxicity in several trials combining conventional radioimmunotherapy with high-dose myeloablative conditioning chemotherapy, both drugs faced commercial failure. This led to the discontinuation of
131I-tositumomab in 2014.
This first-in-class monoclonal antibody was a commercial failure despite no significant toxicity. This failure was attributed in part to occasional communication gaps between oncologists and nuclear medicine physicians, as nuclear medicine was often viewed primarily as a diagnostic discipline with limited therapeutic applications, with a few exceptions. This market rejection has somewhat stalled the research and development of new antibodies for radioimmunotheragnosis [
3].
To overcome the challenges of poor tumor penetration and unfavorable pharmacokinetics associated with full-length antibodies, a series of hCD20-targeting single-domain antibodies (sdAbs) were developed. One of these sdAbs was radiolabeled with
68Ga for immunoPET imaging and with
177Lu for targeted therapy of lymphoma. [
58].
Building on the success of imaging, extensive preclinical evidence has shown that CD38-targeted RIT can effectively eradicate disseminated multiple myeloma (MM) [
59,
60,
61]. A novel two-step pretargeted RIT (pRIT) strategy, using a less immunogenic bispecific protein (028-Fc-C825) targeting both the CD38 antigen and the
90Y -DOTA ligand, together with
90Y-DOTA-biotin, significantly reduced tumor growth and prolonged survival in both MM and non-Hodgkin lymphoma (NHL) models [
59]. These findings highlight the superiority of CD38 for both immunoPET imaging and pRIT. The clinical translation of these CD38-targeted theranostic agents promises to improve the management of patients with MM (NCT03665155) [
60,
62,
63].
Breast Cancer
Over the past two decades, the human epidermal growth factor receptor 2 (HER2/ErbB2) has received considerable attention as a molecular imaging target. In addition to the clinical approval of HER2-targeted antibody therapeutics such as trastuzumab, trastuzumab emtansine (T-DM1), and pertuzumab, numerous antibody-based radiotracers have been developed to visualize HER2 expression [
64]. Among these, two HER2-specific VHHs, namely 2Rs15d and 5F7, have been radiolabeled with [
18F]TFPFN. ImmunoPET imaging with these probes effectively identified tumors with significant uptake while significantly reducing renal uptake. In addition, [
18F]AlF-NOTA-Tz-TCO-GK-2Rs15d has been developed and validated as a viable vehicle for radioimmunotherapy (RIT) after labeling with
177Lu or
131I [
48,
65,
66]. These advances offer valuable therapeutic options to complement the aforementioned immunoPET imaging. There are also a number of clinical trials using RIT in breast cancer, one of them is evaluating the safety and distribution of
131I-SGMIB-2Rs15d which has already completed patient enrollment (NCT02683083). pRIT also plays a role in breast cancer; a recent study reported that pRIT with anti-HER2-DOTA-pRIT +
177Lu-DOTA-Bn inhibited HER-2 positive breast cancer and significantly improved survival without inducing toxicity in normal tissues [
41]. Our group has also contributed to develop a theragnostic pair for immunoPET and RIT in triple negative breast cancer (TNBC) mouse model targeting MT1-MMP metalloprotease with promising results using 3 doses of [
177Lu]Lu-DOTA-LEM2/15 [
67].
In the phase II segment, pre/peri-menopausal women and eligible men, will receive [
177Lu]Lu-NeoB in addition to capecitabine, and in women, a gonadotropin-releasing hormone agonist (GnRHa) according to local clinical protocols. The trial is designed to evaluate the efficacy of this combination therapy in adult patients with ER+/HER2-, gastrin-releasing peptide receptor-positive (GRPR+) metastatic breast cancer (mBC) after progression on CDK4/6 inhibitor-based treatment. The study seeks to explore the preliminary efficacy of two different dose levels and administration regimens [
68,
69].
The treatment regimen consists of [
177Lu]Lu-NeoB and capecitabine, with [
68Ga]Ga-NeoB as the imaging agent. Participants will receive [
177Lu]Lu-NeoB in addition to capecitabine, with GnRHa administered according to local practice guidelines for eligible phase II women and men. As a PET imaging agent, [
68Ga]Ga-NeoB is being investigated as a tool to select patients for [
177Lu]Lu-NeoB therapy, particularly those with GRPR-overexpressing tumors, including mBC. Preclinical and clinical studies have demonstrated the favorable technical and diagnostic performance of [
68Ga]Ga-NeoB, producing high-quality images that allow for accurate interpretation [
70,
71] NCT06247995.
Prostate Cancer
Several mAbs have been developed that target both intracellular and extracellular epitopes of Prostate Specific Membrane Antigen (PSMA) [
72,
73,
74]. Among these, J591, a humanized mAb, has emerged as a promising candidate for clinical investigation in both imaging and therapeutic applications. Studies by Fung et al. have shown comparable surface binding and internalization rates for radiolabeled variants of J591, suggesting the feasibility and safety of using
177Lu- and
124I-or
89Zr-labeled J591 for theranostic purposes, potentially offering improved targeting of bone lesions compared to traditional imaging methods [
41] NCT00538668. These advances will change the management strategies for patients with PSMA-positive prostate cancer. Meanwhile, capromab, another mAb, has been investigated for its utility as a SPECT imaging and therapeutic agent when labeled with
90Y. However, its binding to an intracellular domain of PSMA limits its efficacy in detecting soft-tissue and bone metastases compared to mAbs targeting extracellular domains [
75,
76]. Because capromab binds to an epitope on the intracellular domain of PSMA, tracers derived from this mAb are limited to detecting dead cells and do not have advantages in imaging soft-tissue and bone metastases of PCas compared to tracers that bind to the extracellular domain [
49].
A phase II trial is currently underway to evaluate the therapeutic efficacy of 177Lu-TLX591, a radiolabeled PSMA-targeting antibody, in combination with external beam radiation therapy (EBRT) in patients with biochemically recurrent, oligometastatic, PSMA-expressing prostate cancer. TLX591, a PSMA-targeting antibody designed to be radiolabeled with a therapeutic radioisotope, represents a potential breakthrough in the treatment of PSMA-expressing tumors. NCT05146973
Even thought there is no antibody, the strategy followed for the theragnosis in prostate cancer with PSMA could be suitable for the prostate cancer antibodies moving from
177Lu to an alpha emitter. The dosimetry of
213Bi-PSMA-617 is within the range typically considered appropriate for clinical use. However, compared to
225Ac-PSMA-617, it presents challenges such as increased off-target radiation influenced by perfusion and a prolonged biological half-life of PSMA-617 in organs where dose limits are critical, exceeding the physical half-life of
213Bi. As a result, while still viable,
213Bi is emerging as a secondary option for radiolabeling in targeted alpha therapy for prostate cancer [
77].
Hepatocelullar Carcinoma
There are several clinical trials involving anti-CEA therapies and other radiolabeled antibody fragments for hepatocellular carcinoma (HCC): Anti-CEA CIGB-M3 ScFv17II-131CRC (2011): This was a phase I clinical trial of the
131I-labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment [
78]. Other approach was CD147HAb18 metuximab F(ab′)
2 (Licartin
®®) (2010) NCT00829465, this trial investigated hepatic arterial injection of
131I-labeled metuximab combined with chemoembolization for unresectable hepatocellular carcinoma [
79]. An specific trial was performed to probe the clinical value of iodine [
131I] metuximab infusion combined with transcatheter arterial chemoembolization (TACE) for patients with post-intervention relapse of mid or advanced stage hepatocellular carcinoma. Adjuvant
131I-Metuximab for hepatocellular carcinoma after liver resection (2020, NCT00819650): This was a randomized, controlled, multicenter, open-label, phase II trial assessing the efficacy of adjuvant
131I-metuximab for hepatocellular carcinoma following liver resection (2020) NCT00819650 [
80,
81].
Colorrectal Cancer
In RIT clinical trials in colorectal cancer, there is one study describing the use of radiolabeled scFv. This was conducted in 2011 through a phase I using
131I-CIGB-M3 trivalent scFv specific for CEA targeting in patients with colorectal metastases [
2]. Feasibility was evaluated in a small cohort of 17 patients divided into two groups receiving either 0.3 mg or 1 mg of CIGB-M3 scFv for similar injected activities of about 185–259 MBq of
131I. Toxicity assessments demonstrated the low off-target toxicity of the scFv fragment in both groups and was associated with lower human anti-mouse antibody (HAMA) responses compared to patients receiving a single 1 mg dose of the parental CB-CEA-1 full antibody. Although the trivalent CIGB-M3 scFv showed interesting pharmacokinetic results and quite favorable dosimetry (0.07 ± 0.02 to 0.08 ± 0.02 mGy/MBq in the whole body, 1.1 ± 0.6 to 2.0 ± 1.3 mGy/MBq in the kidneys), it has not been further developed in clinical trials.
A new generation of bispecific Ab (bsAbs) formats was then evaluated to reduce the observed immunogenicity of the chimeric F(ab′)
2. The trispecific humanized TF2 Fab′ consists of an anti-histamine-succinyl-glutamine (HSG) fragment that is derived from the 679 anti-HSG IgG
1 and 2 fragments of the humanized anti-CEA derived from hMN-14 IgG
1 (labetuzumab) [
82]. The first-in-man study by Schoffelen et al. (NCT00860860), which was published in 2013, demonstrated the feasibility and safety of pRIT with TF2 and
177Lu-IMP-288 (a 1.5 kDa HSG peptide) in patients with metastatic CRC [
83]. In a previous imaging study with TF2/
111In-IMP-288, a 24-hour interval between the administration of the two treatments was found to be most appropriate. Patients received a mean injected activity of 5.6 GBq with no significant differences being observed between cohorts. However, the HAMA response to TF2 was measured to be quite high 1 week after administration, and this response gradually increased over the 8-week follow-up period, suggesting that TF2 was surprisingly immunogenic despite being humanized and lacking an Fc portion.
Lung Cancer
The investigators have shown that pRIT using a bispecific antibody (bsAb) can deliver a higher dose of radiation to a tumor than a directly radiolabeled anti-CEA antibody, and showed improved anti-tumor efficacy. This clinical trial is designed to evaluate pRIT using an all-new recombinant anti-CEA bsMAb and a 177Lu-labeled peptide for the treatment of CEA-expressing small cell lung cancer (SCLC) or CEA-expressing non small cell lung carcinoma (NSCLC) NCT01221675.
The L19SIP antibody is a fully human antibody, capable of preferentially localizing around tumor blood vessels while sparing normal tissue. The formation of new blood vessels is a rare event in adults (with the exception of the female reproductive tract), but is a characteristic pathological feature of most of aggressive cancers. The objective of this feasibility study is to evaluate the therapeutic potential of the L19SIP antibody, labeled with the radionuclide 131I in combination with radiochemotherapy for the treatment of patients with newly diagnosed, unresectable, locally-advanced NSCLC, following the promising results with this agent in previous clinical studies NCT01124812.
A phase I trial of radioimmunotherapy (Y90-Mx-DTPA-cT84.66) is being conducted following the completion of radiotherapy alone or in combination with systemic therapy in patients with unresectable or medically inoperable, non-metastatic, CEA-producing stage I-IIIB non-small cell lung cancer NCT00738452.
There is a recent clinical trial in 2024 where patients with small cell lung cancer, sarcoma and malignant melanoma will be treated with GD2-SADA:177Lu-DOTA complex; it is a two-step radioimmunotherapy, delivered as two separate products GD2-SADA and 177Lu-DOTA to assess safety and tolerability NCT05130255.
Others
Adults with leptomeningeal metastases from solid tumors are treated with 177Lu-DTPA-omburtamab, a radioactive labeling of a murine monoclonal antibody targeting B7-H3. Breast, NSCLC, malignant melanoma NCT04315246.
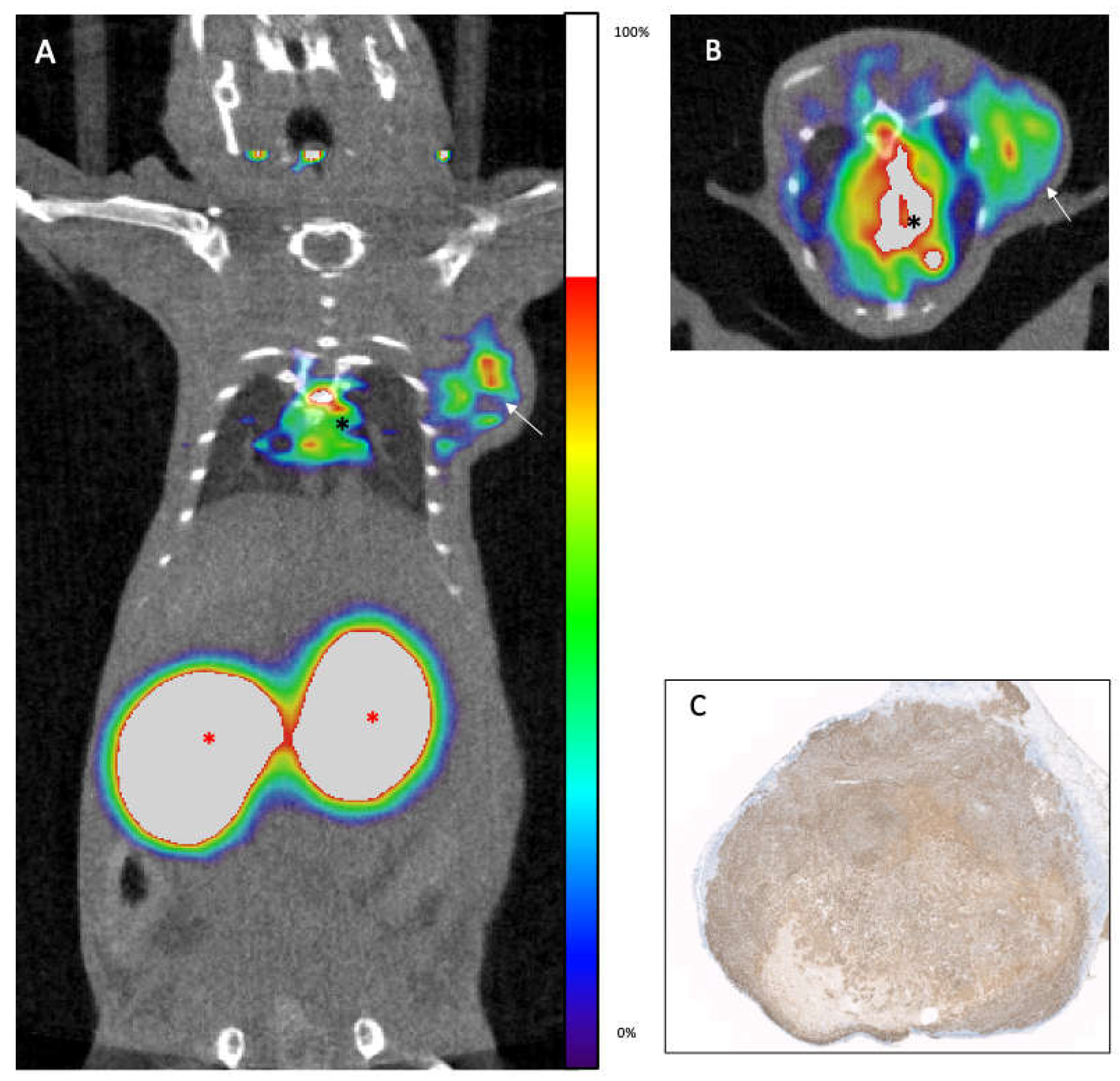
In (
Figure 3) we showed a figurative example of how an immunoPET image visualizes the target, in this case the paraaortic lymph nodes, and how they disappear after RIT.
Dosimetry
Radionuclide therapies deliver cytotoxic radiation to target tissues while minimizing damage to healthy tissues. The dose of radiation energy absorbed by a given tissue, measured in grays (Gy) is a critical parameter in determining the biological effects of radiation, such as cell death. Therefore, estimating absorbed radiation, or dosimetry, is essential to making radiation-based treatments less toxic and more effective.
In external-beam radiation therapy, dosimetry is a well-established practice in routine treatment planning. Parameters such as the duration and intensity of radiation, the region to be irradiated, and the target volume are relatively easy to manage. However, radionuclide treatments are more complex due to the physical and pharmacokinetic interactions of radiopharmaceuticals at the whole-body scale. This complexity makes it difficult to translate dosimetric measurements from research to routine clinical practice, where treatment planning is often based on the total administered radiation (activity) rather than site-specific absorbed doses [
84].
Traditionally, dosimetric techniques in nuclear medicine have focused on determining the maximum tolerated doses of radionuclides for individuals. Numerous studies have evaluated the pharmacokinetic properties and biodistribution of both classic and newly developed agents. While this approach is more common in research, it has led to simpler, widely adopted clinical activity-based protocols. These protocols help estimate the highest activities that can be administered to a patient without exceeding radiation limits for critical organs, such as approximately 40 Gy to the kidneys (in patients without risk factors for renal toxicity) and 2 Gy to the bone marrow. ImmunoPET imaging allows dosimetry calculations for these therapies and also predicts dose-limiting organs prior to the RIT [
85], optimizing the development and use of RIT agents.
Advances in imaging technology, particularly hybrid imaging, and improvements in dosimetry software, together with new diagnostic radiopharmaceuticals, have improved the definition of target volumes and their integration with pharmacokinetic and irradiation data. Further studies and some technical simplifications are needed for internal dosimetry to play a central role in routine theranostic planning [
59,
86,
87].
Future
The production, distribution, and storage of radiotheranostic agents face several challenges. There are significant differences within and between countries in the availability of medical cyclotrons, GMP-compliant production facilities, and dedicated treatment centers that meet radiation safety standards. These disparities result in significant cost differences between commercial suppliers and in-house manufacturers. Reliable distribution networks are critical to ensure the safe and timely delivery of these agents, especially given the rapid growth in demand. All radiotheranostic agents have a limited shelf life due to the radioactive half-life of the radionuclides (
Table 1). Unlike conventional cancer therapies, both manufacturing (central vs. local) and logistics (delivery, application, and waste management) must be adapted to compensate for these short shelf lives and the resulting limitations on the number of patients treated per production cycle [
3]. Challenges also include the need for nuclear medicine physicians who are fully trained in therapy planning, dosimetry, and response assessment, as well as facilities that comply with national radioactive waste management regulations. In addition, advances in PET technology and reconstruction algorithms are improving the spatial and temporal resolution of immunoPET images [
88]. Most immunoPET imaging is performed in patients with metastatic disease, who often lack histochemical confirmation of some tracer-avid lesions. However, the primary purpose of initial immunoPET imaging is to stratify patients by mapping the expression of specific biomarkers. A decrease in tracer-avid lesion uptake or size on post-treatment images suggests that these lesions are histopathologically positive.
It is difficult to design robust antibody fragments with high affinity to antigens, favorable pharmacokinetic profiles, low toxicity, and good stability over time. Fragments must also be able to undergo harsh radiochemical processes without inducing deleterious effects on the aforementioned properties. For small antibody fragments, such as scFv or single-domain antibodies (nanobodies), radiochemical processing is known to dramatically affect their affinity to antigens [
20,
89,
90]. The scarcity and heterogeneity of clinical studies also make it difficult to conclude on the efficacy of antibody fragments for RIT application. In fact, the small number of studies, the low number of patients enrolled, and the wide variety of fragment formats or targets is quite disappointing, resulting in little evidence representing the efficacy of RIT with fragments. However, such strategies remain of interest for RIT or nuclear imaging applications, but these strategies need to be optimized. Recent advances in phage-display technology, chemical and chemoenzymatic engineering, and radiolabeling protocols have provided new insights to circumvent most of these drawbacks and have shown promising results for antibody fragments in preclinical studies [
2,
91,
92].
For the development of antibody-based radiopharmaceuticals, we would like to emphasize the importance of multidisciplinary collaboration. Specifically, multiple approaches (e.g., genomic, serological, proteomic, biological, and bioinformatical approaches) should be leveraged to identify antigens highly or exclusively overexpressed on the surface of the tumor cells, tumor stromal cells, tumor vascular endothelial cells, immune cells, or beta cells.
Combination with established systemic therapies is an emerging, exciting approach with the potential to improve the outcomes of patients receiving radiotheranostic agents. Targeted radionuclide approaches currently being evaluated, in either preclinical studies or early-phase trials, include combinations with chemotherapy, radiosensitizers, EBRT and immunotherapies.
Novel targets and approaches for the development of radiotheranostics have focused primarily on targeting emitters of either α- or β-radiation to the surface of tumour cells followed by intracellular trafficking and retention, resulting in DNA damage. Novel and potentially clinically important radiotheranostic approaches are expanding the range of targets to include those present in the tumour microenvironment, such as blood vessels, cancer-associated fibroblasts (CAFs), the stromal matrix and immune cells [
20,
92,
93]. The stromal cells present in the tumour microenvironment are generally more genetically stable than tumor cells, which may downregulate or entirely lose expression of certain targets; stromal cells may also contribute to the development of an immunosuppressive microenvironment and to drug resistance [
94].
Conclusions
The careful deployment of radioimmunotheranostics in cancer patients has the potential to significantly improve treatment outcomes. However, several major challenges need to be addressed. There is an urgent need to generate evidence that will enable a wider range of radiotheranostic agents to gain regulatory approval and rapidly enter the market. In addition, strategies are needed to improve the global availability of radiotheranostics. The current success of radiotheranostics is likely to generate increased interest from both academia and industry in the identification and development of novel targeted agents. This is expected to lead to earlier and more accurate cancer detection, personalized treatments, and improved patient outcomes.
Author Contributions
Conceptualization, FM, JLMT; data curation, BB, GG, MRS, DSA; writing—original draft preparation, GG, GM, BB; writing—review and editing, FM; supervision, FM, JLMT. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NAME OF FUNDER, and the APC was funded by Comunidad de Madrid (S2022/BMD-7403 RENIM-CM) and by BBVA Foundation with the grant “Radioinmunotheragnostics for metastatic lung cancer with pretargeted clickable Ab Fragments (TherAbnostic)” Ref.: PR [19]_BIO_IMG_0096 (2020).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable
Acknowledgments
We extend our heartfelt gratitude to the staff of the Molecular Imaging Unit for their unwavering support in our daily work. Their encouragement and dedication provide us with the motivation and time to achieve excellence in our scientific endeavors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem Rev 2020, 120, 3787. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat Rev Clin Oncol 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Mulero, F. Editorial: ImmunoPET imaging in disease diagnosis and therapy assessment. Front Med 2023, 10, 1231525. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jiang, D.; Lee, H.J.; Li, M.; Kutyreff, C.J.; Engle, J.W.; et al. Development and Characterization of CD54-Targeted ImmunoPET Imaging in Solid Tumors. Eur J Nucl Med Mol Imaging 2020, 47, 2765. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.M.; Poty, S.; Sharma, S.K.; Lewis, J.S. Preclinical optimization of antibody-based radiopharmaceuticals for cancer imaging and radionuclide therapy-Model, vector, and radionuclide selection. J Labelled Comp Radiopharm 2018, 61, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br J Radiol 2018, 91, 20180440. [Google Scholar] [CrossRef] [PubMed]
- Mulero, F. ImmunoPET in oncology. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition). 2022, 41, 332–339. [Google Scholar] [PubMed]
- Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders-An Update. Cancers 2020, 12. [Google Scholar] [CrossRef]
- Harsini, S.; Alavi, A.; Rezaei, N. Introduction on Nuclear Medicine and Immunology. 2022, 1–13. [Google Scholar]
- Woof, J.M.; Burton, D.R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol 2004, 4, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Manafi-Farid, R.; Ataeinia, B.; Ranjbar, S.; Jamshidi Araghi, Z.; Moradi, M.M.; Pirich, C.; et al. ImmunoPET: Antibody-Based PET Imaging in Solid Tumors. Frontiers in Medicine 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Olafsen, T.; Wu, A. Antibody Vectors for Imaging. Seminars in Nuclear Medicine 2010, 40, 167–181. [Google Scholar] [CrossRef]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; et al. Single-Chain Variable Fragment-Based Bispecific Antibodies: Hitting Two Targets with One Sophisticated Arrow. Mol Ther Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: outstanding features for diagnostic and therapeutic applications. Anal Bioanal Chem 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; et al. Applications of nanobodies in brain diseases. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, A.O. Radiomics, aptamers and nanobodies: New insights in cancer diagnostics and imaging. Hum Antibodies 2021, 29, 1–15. [Google Scholar] [CrossRef]
- Mulero, F.; Oteo, M.; Garaulet, G.; Magro, N.; Rebollo, L.; Medrano, G.; et al. Development of anti-membrane type 1-matrix metalloproteinase nanobodies as immunoPET probes for triple negative breast cancer imaging. Frontiers in Medicine 2022, 9. [Google Scholar] [CrossRef]
- Wei, W.; Younis, M.H.; Lan, X.; Liu, J.; Cai, W. Single-Domain Antibody Theranostics on the Horizon. J Nucl Med 2022, 63, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Arbabi-Ghahroudi, M. Camelid Single-Domain Antibodies: Promises and Challenges as Lifesaving Treatments. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Sulea, T. Humanization of Camelid Single-Domain Antibodies. Methods Mol Biol 2022, 2446, 299–312. [Google Scholar] [PubMed]
- Gebauer, M.; Skerra, A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr Opin Chem Biol 2009, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Wållberg, H.; Orlova, A.; Rosestedt, M.; Hosseinimehr, S.J.; Tolmachev, V.; et al. Order of amino acids in C-terminal cysteine-containing peptide-based chelators influences cellular processing and biodistribution of 99mTc-labeled recombinant Affibody molecules. Amino Acids 2012, 42, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; et al. Site-Specific Radiometal Labeling and Improved Biodistribution Using ABY-027, A Novel HER2-Targeting Affibody Molecule–Albumin-Binding Domain Fusion Protein. Journal of Nuclear Medicine 2013, 54, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Carmon, K.S.; Azhdarinia, A. Application of Immuno-PET in Antibody–Drug Conjugate Development. Molecular Imaging 2018, 17, 1536012118801223. [Google Scholar] [CrossRef]
- Cahuzac, H.; Devel, L. Analytical Methods for the Detection and Quantification of ADCs in Biological Matrices. Pharmaceuticals 2020, 13. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med 2017, 42, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; et al. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- van Asselt, S.J.; Oosting, S.F.; Brouwers, A.H.; Bongaerts, A.H.; de Jong, J.R.; Lub-de Hooge, M.N.; et al. Everolimus Reduces (89)Zr-Bevacizumab Tumor Uptake in Patients with Neuroendocrine Tumors. J Nucl Med 2014, 55, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Malih, S.; Lin, W.; Tang, Z.; DeLuca, M.C.; Engle, J.W.; Alirezapour, B.; et al. Noninvasive PET imaging of tumor PD-L1 expression with (64)Cu-labeled Durvalumab. Am J Nucl Med Mol Imaging 2024, 14, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nature Reviews Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; et al. Synthesis, Preclinical Validation, Dosimetry, and Toxicity of 68Ga-NOTA-Anti-HER2 Nanobodies for IPET Imaging of HER2 Receptor Expression in Cancer. J Nucl Med 2013, 54, 776. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, A.G.; Schuhmacher, A.J.; Oteo, M.; Romero, E.; Camara, J.A.; de Martino, A.; et al. Targeting MT1-MMP as an ImmunoPET-Based Strategy for Imaging Gliomas. PLoS One 2016, 11, e0158634. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Pandit-Taskar, N.; O’Donoghue, J.A.; Humm, J.L.; Zanzonico, P.; Smith-Jones, P.M.; et al. (124)I-huA33 antibody PET of colorectal cancer. J Nucl Med 2011, 52, 1173–1180. [Google Scholar] [CrossRef]
- Samnick, S.; Al-Momani, E.; Schmid, J.S.; Mottok, A.; Buck, A.K.; Lapa, C. Initial Clinical Investigation of [18F]Tetrafluoroborate PET/CT in Comparison to [124I]Iodine PET/CT for Imaging Thyroid Cancer. Clin Nucl Med 2018, 43, 162–167. [Google Scholar] [CrossRef]
- Cheal, S.M.; Xu, H.; Guo, H.-F.; Patel, M.; Punzalan, B.; Fung, E.K.; et al. Theranostic pretargeted radioimmunotherapy of internalizing solid tumor antigens in human tumor xenografts in mice: Curative treatment of HER2-positive breast carcinoma. Theranostics 2018, 8, 5106. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. PET imaging of tumor angiogenesis in mice with VEGF-A-targeted (86)Y-CHX-A″-DTPA-bevacizumab. Int J Cancer 2011, 128, 920–926. [Google Scholar] [CrossRef]
- Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Garmestani, K.; Brechbiel, M.W.; Milenic, D.E. In Vitro and In Vivo Pre-Clinical Analysis of a F(ab’)(2) Fragment of Panitumumab for Molecular Imaging and Therapy of HER1 Positive Cancers. EJNMMI Res 2011, 1, 1. [Google Scholar] [CrossRef]
- Grillo-López, A.J. Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert Rev Anticancer Ther 2002, 2, 485–493. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Fisher, R.I. Iodine-131 tositumomab (Bexxar): radioimmunoconjugate therapy for indolent and transformed B-cell non-Hodgkin’s lymphoma. Expert Rev Anticancer Ther 2004, 4, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.; De Filippi, R.; Lindén, O.; Viardot, A.; Hess, G.; Lerch, K.; et al. 90-yttrium-ibritumomab tiuxetan as first-line treatment for follicular lymphoma: updated efficacy and safety results at an extended median follow-up of 9.6 years. Ann Hematol 2022, 101, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.Y.; Kashani, R.; Zaorsky, N.G.; Spratt, D.E.; Kiess, A.P.; Michalski, J.M.; et al. Lutetium-177 DOTATATE: A Practical Review. Pract Radiat Oncol 2022, 12, 305–311. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; et al. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clinical Cancer Research 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chang, C.-H.; Chang, Y.-J.; Hsu, C.-W.; Chen, L.-C.; Chen, H.-L.; et al. Preliminary evaluation of acute toxicity of (188) Re-BMEDA-liposome in rats. J Appl Toxicol 2010, 30, 680–687. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Gui, J.; Liu, C.; Wang, Y.; Zhang, G.; et al. Efficacy and safety of (188)Re-HEDP in lung cancer patients with bone metastases: a randomized, multicenter, multiple-dose phase IIa study. Int J Clin Oncol 2021, 26, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; et al. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front Med 2022, 9, 1076210. [Google Scholar] [CrossRef]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 targeted alpha particle therapy for prostate cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr Radiopharm 2018, 11, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Hertz, S.; Roberts, A.; Evans, R.D. Radioactive Iodine as an Indicator in the Study of Thyroid Physiology. Proceedings of the Society for Experimental Biology and Medicine 1938, 38, 510–513. [Google Scholar] [CrossRef]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; et al. Randomized controlled trial of yttrium-90–labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. Journal of Clinical Oncology 2002, 20, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Leahy, M.F.; Seymour, J.F.; Hicks, R.J.; Turner, J.H. Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphoma. J Clin Oncol 2006, 24, 4418–4425. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Van Der Heyden, J.; et al. Theranostic Radiolabeled Anti-CD20 SdAb for Targeted Radionuclide Therapy of Non-Hodgkin Lymphoma. Mol Cancer Ther 2017, 16, 2828. [Google Scholar] [CrossRef] [PubMed]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; et al. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Green, D.J.; Orgun, N.N.; Jones, J.C.; Hylarides, M.D.; Pagel, J.M.; Hamlin, D.K.; et al. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Research 2014, 74, 1179–1189. [Google Scholar] [CrossRef]
- O’Steen, S.; Comstock, M.L.; Orozco, J.J.; Hamlin, D.K.; Wilbur, D.S.; Jones, J.C.; et al. The α-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood, The Journal of the American Society of Hematology 2019, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; O’Steen, S.; Lin, Y.; Comstock, M.L.; Kenoyer, A.L.; Hamlin, D.K.; et al. CD38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other B-cell malignancies. Blood, The Journal of the American Society of Hematology 2018, 131, 611–620. [Google Scholar] [CrossRef] [PubMed]
- bastienJamet, B.; Bailly, C.; Carlier, T.; Touzeau, C.; Nanni, C.; Zamagni, E.; et al. Interest of pet imaging in multiple myeloma. Frontiers in Medicine 2019, 6, 69. [Google Scholar]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Van Der Heyden, J.; et al. Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-Hodgkin lymphoma. Molecular Cancer Therapeutics 2017, 16, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.; Massa, S.; et al. 131I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clinical Cancer Research 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [PubMed]
- Magro, N.; Oteo, M.; Romero, E.; Ibáñez-Moragues, M.; Lujan, V.M.; Martínez, L.; et al. Target engagement of an anti-MT1-MMP antibody for triple-negative breast cancer PET imaging and beta therapy. Nuclear Medicine and Biology 2024, 108930. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Reffert, L.; Diehl, S.; Schönberg, S.O.; Wängler, C.; Hohenberger, P.; et al. GMP-compliant production of [(68)Ga]Ga-NeoB for positron emission tomography imaging of patients with gastrointestinal stromal tumor. EJNMMI Radiopharm Chem 2021, 6, 22. [Google Scholar] [CrossRef]
- Montemagno, C.; Raes, F.; Ahmadi, M.; Bacot, S.; Debiossat, M.; Leenhardt, J.; et al. In Vivo Biodistribution and Efficacy Evaluation of NeoB, a Radiotracer Targeted to GRPR, in Mice Bearing Gastrointestinal Stromal Tumor. Cancers 2021, 13. [Google Scholar] [CrossRef]
- Gruber, L.; Jiménez-Franco, L.D.; Decristoforo, C.; Uprimny, C.; Glatting, G.; Hohenberger, P.; et al. MITIGATE-NeoBOMB1, a Phase I/IIa Study to Evaluate Safety, Pharmacokinetics, and Preliminary Imaging of (68)Ga-NeoBOMB1, a Gastrin-Releasing Peptide Receptor Antagonist, in GIST Patients. J Nucl Med 2020, 61, 1749–1755. [Google Scholar] [CrossRef]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; et al. 68Ga/177Lu-NeoBOMB1, a Novel Radiolabeled GRPR Antagonist for Theranostic Use in Oncology. J Nucl Med 2017, 58, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bander, N.H. Technology insight: monoclonal antibody imaging of prostate cancer. Nature Clinical Practice Urology 2006, 3, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Research 1997, 57, 3629–3634. [Google Scholar] [PubMed]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J Nucl Med 2010, 51, 1293. [Google Scholar] [CrossRef]
- Petronis, J.D.; Regan, F.; Lin, K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clinical Nuclear Medicine 1998, 23, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Lütje, S.; Gerrits, D.; Molkenboer-Kuenen, J.D.; Herrmann, K.; Fracasso, G.; Colombatti, M.; et al. Characterization of Site-Specifically Conjugated Monomethyl Auristatin E–And Duocarmycin-Based Anti-PSMA Antibody–Drug Conjugates for Treatment of PSMA-Expressing Tumors. Journal of Nuclear Medicine 2018, 59, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; et al. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- González, G.P.; García, I.G.; González, J.G.; Sánchez, L.P.; Mirabal, M.V.; Marín, C.C.; et al. Phase I clinical trial of the 131I-Labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment. Cancer Biotherapy & Radiopharmaceuticals 2011, 26, 353–363. [Google Scholar]
- Wu, L.; Yang, Y.-F.; Ge, N.-J.; Shen, S.-Q.; Liang, J.; et al. Hepatic arterial Iodine-131–labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: interim safety and survival data from 110 patients. Cancer Biotherapy & Radiopharmaceuticals 2010, 25, 657–663. [Google Scholar]
- Wu, L.; Yang, Y.-F.; Ge, N.-J.; Shen, S.-Q.; Liang, J.; Wang, Y.; et al. Hepatic artery injection of 131 I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: a prospective nonrandomized study. European Journal of Nuclear Medicine and Molecular Imaging 2012, 39, 1306–1315. [Google Scholar] [CrossRef]
- Meyer, T.; Gaya, A.; Dancey, G.; Stratford, M.; Othman, S.; Sharma, S.; et al. A Phase I Trial of Radioimmunotherapy with I-131-A5B7 Anti-CEA Antibody in Combination with Combretastatin-A4-Phosphate in Advanced Gastrointestinal Carcinomas. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 2009, 15, 4484–4492. [Google Scholar] [CrossRef]
- Rossi, E.A.; Goldenberg, D.M.; Cardillo, T.M.; McBride, W.J.; Sharkey, R.M.; Chang, C.-H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proceedings of the National Academy of Sciences 2006, 103, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.; Franssen, G.M.; et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. British Journal of Cancer 2013, 109, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Lawhn-Heath, C.; Hope, T.A.; Martinez, J.; Fung, E.K.; Shin, J.; Seo, Y.; et al. Dosimetry in radionuclide therapy: the clinical role of measuring radiation dose. Lancet Oncol 2022, 23, e75–e87. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.N.; Visser, O.J.; Vosjan, M.J.; van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; et al. Biodistribution, radiation dosimetry and scouting of 90 Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89 Zr-ibritumomab tiuxetan and PET. European Journal of Nuclear Medicine and Molecular Imaging 2012, 39, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.G.; Nunes, R.; Coutinho, A.; Zaniboni, E.; Costa, L.; Barbosa, F.; Buchpiguel, C.A.; et al. Theranostics in Nuclear Medicine: Emerging and Re-Emerging Integrated Imaging and Therapies in the Era of Precision Oncology. 2020, 40. [Google Scholar] [CrossRef]
- Eberlein, U.; Cremonesi, M.; Lassmann, M. Individualized Dosimetry for Theranostics: Necessary, Nice to Have, or Counterproductive? J Nucl Med 2017, 58 (Suppl 2), 97s–103s. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, H.; Alavi, A.; El Naqa, I. (Eds.) Novel quantitative PET techniques for clinical decision support in oncology. Seminars in nuclear medicine; Elsevier, 2018. [Google Scholar]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nature Biotechnology 2009, 27, 331–337. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; Muyldermans, S.; Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opinion on Drug Delivery 2016, 13, 1149–1163. [Google Scholar] [CrossRef]
- Herrero Álvarez, N.; Bauer, D.; Hernández-Gil, J.; Lewis, J.S. Recent advances in radiometals for combined imaging and therapy in cancer. ChemMedChem 2021, 16, 2909–2941. [Google Scholar] [CrossRef]
- Lapi, S.E.; Scott, P.J.H.; Scott, A.M.; Windhorst, A.D.; Zeglis, B.M.; Abdel-Wahab, M.; et al. Recent advances and impending challenges for the radiopharmaceutical sciences in oncology. The Lancet Oncology 2024, 25, e236–e49. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nature Reviews Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nature Reviews Clinical oncology 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).