1. Introduction
Gliomas are potentially lethal types of primary brain tumours that are characterized by aggressive capability and angiogenesis. [
1] Traditionally, they are classified Grade 1, 2,3,4 (also known as GBM, Glioblastoma multiforme).
Epidemiological data suggests that there are over 100, 000 cases of glioma diagnosed per year worldwide.
The incidence, predictive indicators and the prognosis of gliomas is different based on age, gender, ethnicity and genetic mutations. [
2]
Gliomas pose a medical and surgical challenge; they constitute 27% - 30% of all the primary CNS tumours and also account for 80% of all malignant primary CNS tumours. [
1,
2,
3,
4,
5,
6,
7]
The prognosis of diffuse gliomas in adults is poor based on a recent review on the therapeutic modalities utilized in the managing the disease. Therapy with curative intent in the management strategy is unlikely as gliomas tend to recur and have aggressive capabilities. [
5]
Even limiting the extent of resection (EOR) surgically, may lead to inadvertent residual tumour or resection of normal brain parenchyma which will either lead to early recurrence or immediate deterioration of quality-of-life (QoL).
Gliomas arise from glial cells which are non-neuronal, and are the supporting infrastructural components of the central nervous system (CNS) and the peripheral nervous system (PNS).
Glial cells which give rise to astrocytes, which are then responsible for maintenance of cellular homeostasis and production of astrocytes, thereby contributing to the blood-brain-barrier and protection of the neurons.
In the human and mammalian brain, glial cells constitute the microglia, ependymal cells, oligodendrocyte lineage and astrocytes. Some of these cells, in particular microglia, are the resident immune cells of the brain that survey their environment and respond to pathogens, toxins, and tumors. [
8,
9]
In the 2020 EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood, several important modifications were made i.e., glioblastoma (GBM) is now defined as a diffuse astrocytic glioma with no mutations in IDH genes nor histone H3 genes and is characterized by microvascular proliferation, necrosis and/or specific molecular features, including TERT promoter mutation, EGFR gene amplification and/or a +7/-10 cytogenetic signature, IDH-mutant glioblastoma is now referred to as IDH-mutant astrocytoma, WHO grade IV (4), homozygous H3.3 G34-mutant diffuse hemispheric gliomas constitute a novel glioma entity corresponding to WHO grade 4 (previously referred to as glioblastoma multiforme). [
10]
Notable hallmarks of gliomas as previously noted are access to vasculature and which correlates with distinctive patterns of transcription that potentiates glioma heterogeneity, angiogenesis, poor prognosis and treatment response, however as previously believed oncologic medical interventions particularly bevacizumab does not prolong progression-free-survival (PFS) nor overall survival (OS).
The current gold standard for the treatment of glioblastoma is care that includes surgery, adjuvant radiotherapy and temozolomide (TMZ) chemotherapy. Unfortunately, these treatment strategies are not effective in curing glioblastoma, hence the poor prognosis.
Novel agents such as nivolumab (an immune-therapeutic agent) are not superior to bevacizumab nor superior to temozolomide in patients diagnosed with glioblastoma without MGMT promoter methylation.
Invasive diagnosis of glioma diagnosis is on histopathology, however this is not always feasible based on location of the lesion or comorbidities. Therefore non-invasive techniques such as Magnetic Resonance Imaging ( MRI) are often utilized preoperatively, postoperatively and during follow-up where there is suspected tumour recurrence.
MRI imaging of gliomas depends on various sequences and the administration of contrast agents i.e. gadolinium-enhanced (GE) MRI with T1 and T2-weighted sequences. Christy and colleagues found the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of detecting intra-axial gliomas to be 93%, 77%, 80% and 90% respectively, this suggests MRI as a useful and powerful imaging modality. [
10,
11] However, infiltrative tumour growth within non-enhancing regions of FLAIR signal abnormality is not readily visualised with conventional MR sequences ( before and after therapy). [
12]
Other imaging techniques that are used for glioma management is ultrasonography with different modes e.g. elastography and DC. The drawback has been that it is user-depended and the advantage being that brain-shift can be accounted for when the craniotomy is performed. [
10,
12]
Molecular imaging may hold the key or be a potential adjunct in differentiating and characterising gliomas e.g. low-grade (LGG) vs. high grade (HGG), pseudoprogression and recurrence. These tools are in the mainstream of Nuclear Medicine diagnostic armamentarium, single-photon emission tomography (SPECT) or Positron emission tomography (PET).
Since angiogenesis is the hallmark of genetic aberration(s), in theory the potential use of PET 68[Ga]68Ga-labelled integrins (RGD) using integrins for the development of epigenetic-targeted therapy and theranostics.
It is to understand the pathophysiology, link it with the current known molecular markers and introduce integrins and how Nuclear Medicine can be utilized.
In a recent review by Bolcaen et al [
11] Novel Tyrosine Kinase Pathway inhibitors for targeted therapy of glioblastoma focussed on seven tyrosine kinase receptors, based on their role in GB.
2. Case Presentation
A 50-year-old male patient with no significant background history presented to the Accident and Emergency (A&E) unit complaining of a severe headache associated with recalcitrant nausea and vomiting. The headaches were progressive over several months. There was no other history of chronic medication/surgical operations or family history. Clinical tests that are performed are to assess orientation (GCS), mini-mental exam, eye reflexes to light stimuli. A lumbar puncture is not routinely done in the case of SOL (Space occupying lesions).
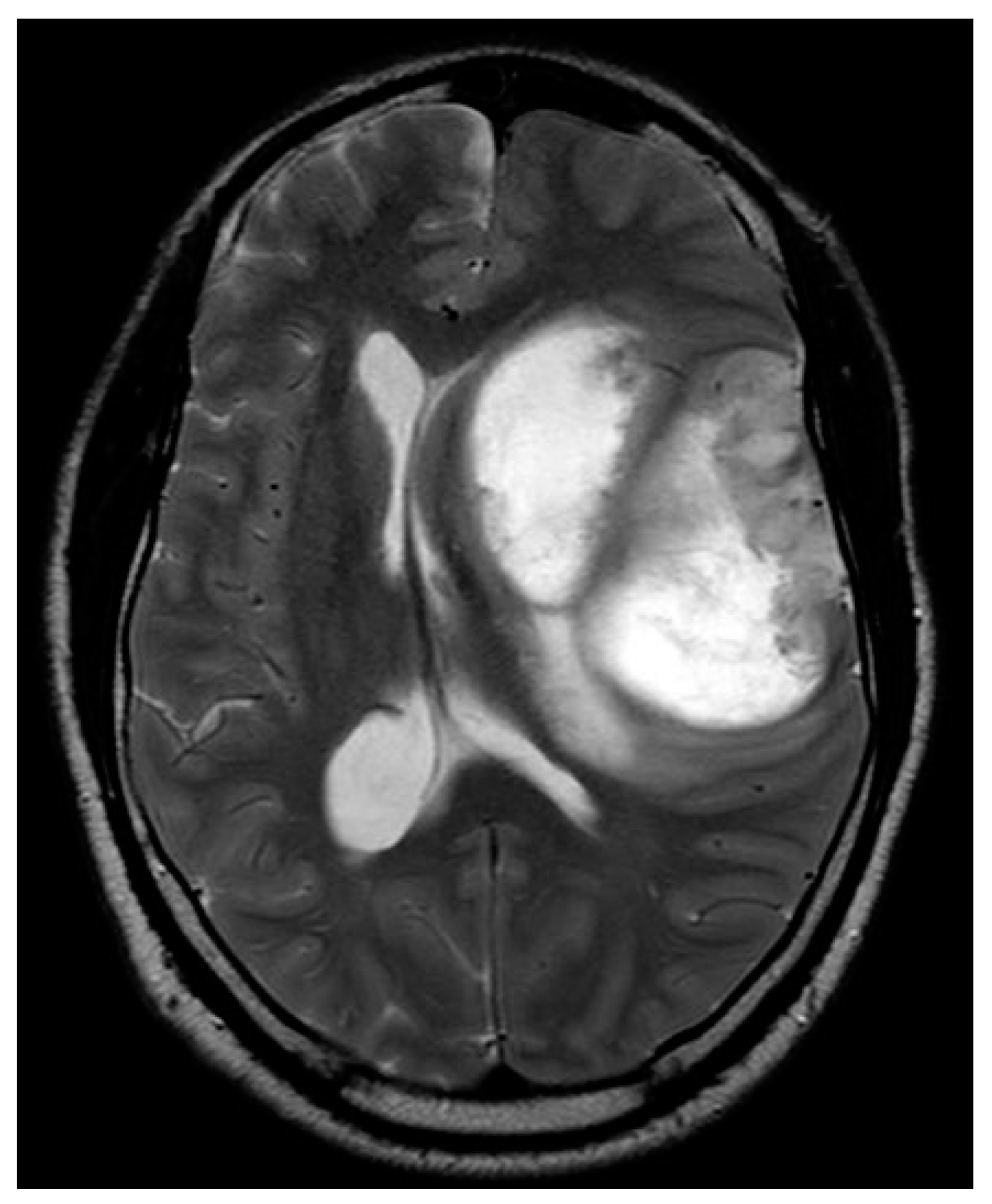
Figure A.
W image demonstrates a left frontotemporal-insular mass with peripheral irregular solid components and central necrotic components. There is subfalcine herniation.
Figure A.
W image demonstrates a left frontotemporal-insular mass with peripheral irregular solid components and central necrotic components. There is subfalcine herniation.
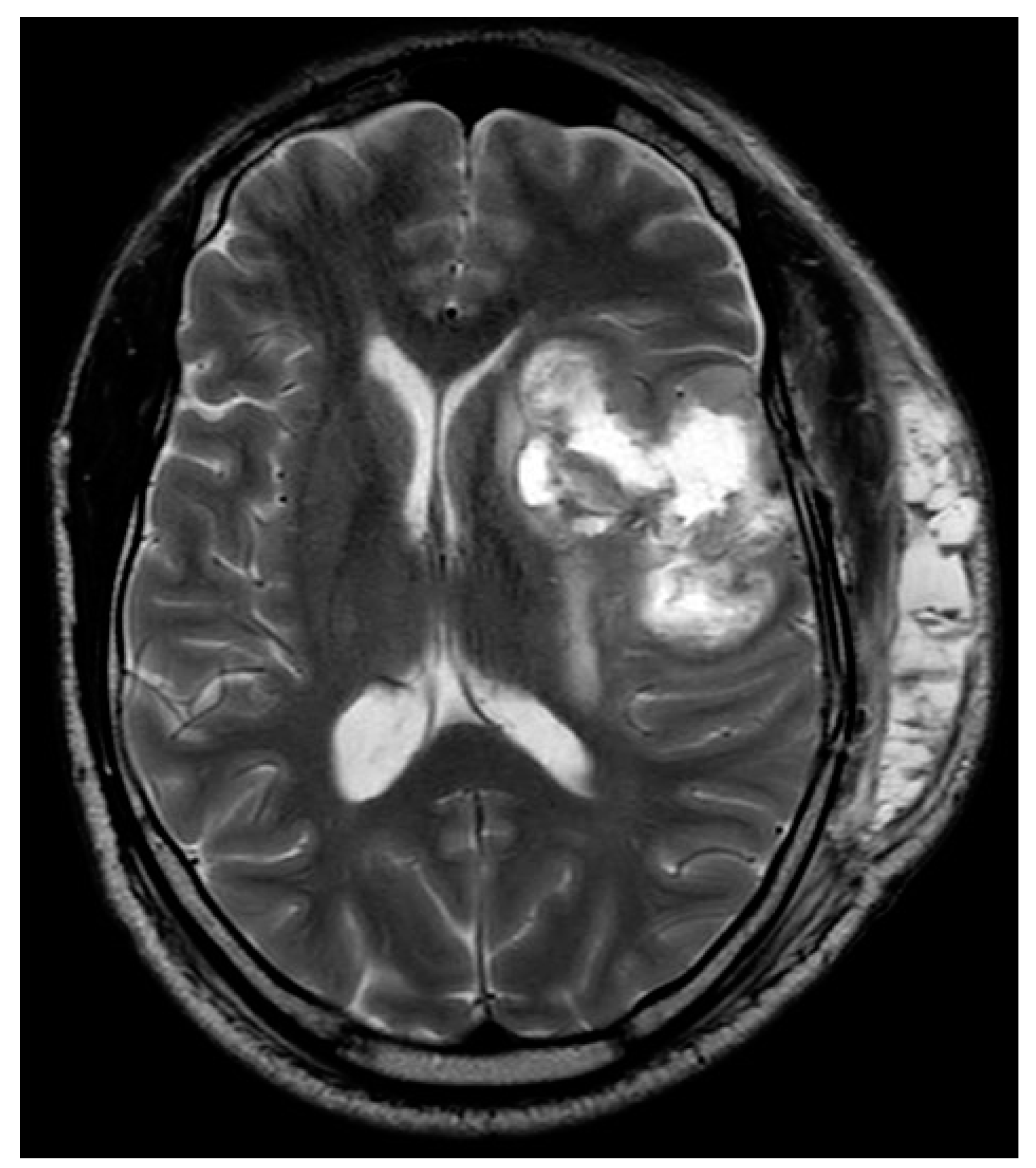
Figure B.
W image acquired 7 days postoperatively demonstrates subtotal resection with residual peripheral solid components. The central necrotic component is smaller, with reduction in mass effect.
Figure B.
W image acquired 7 days postoperatively demonstrates subtotal resection with residual peripheral solid components. The central necrotic component is smaller, with reduction in mass effect.
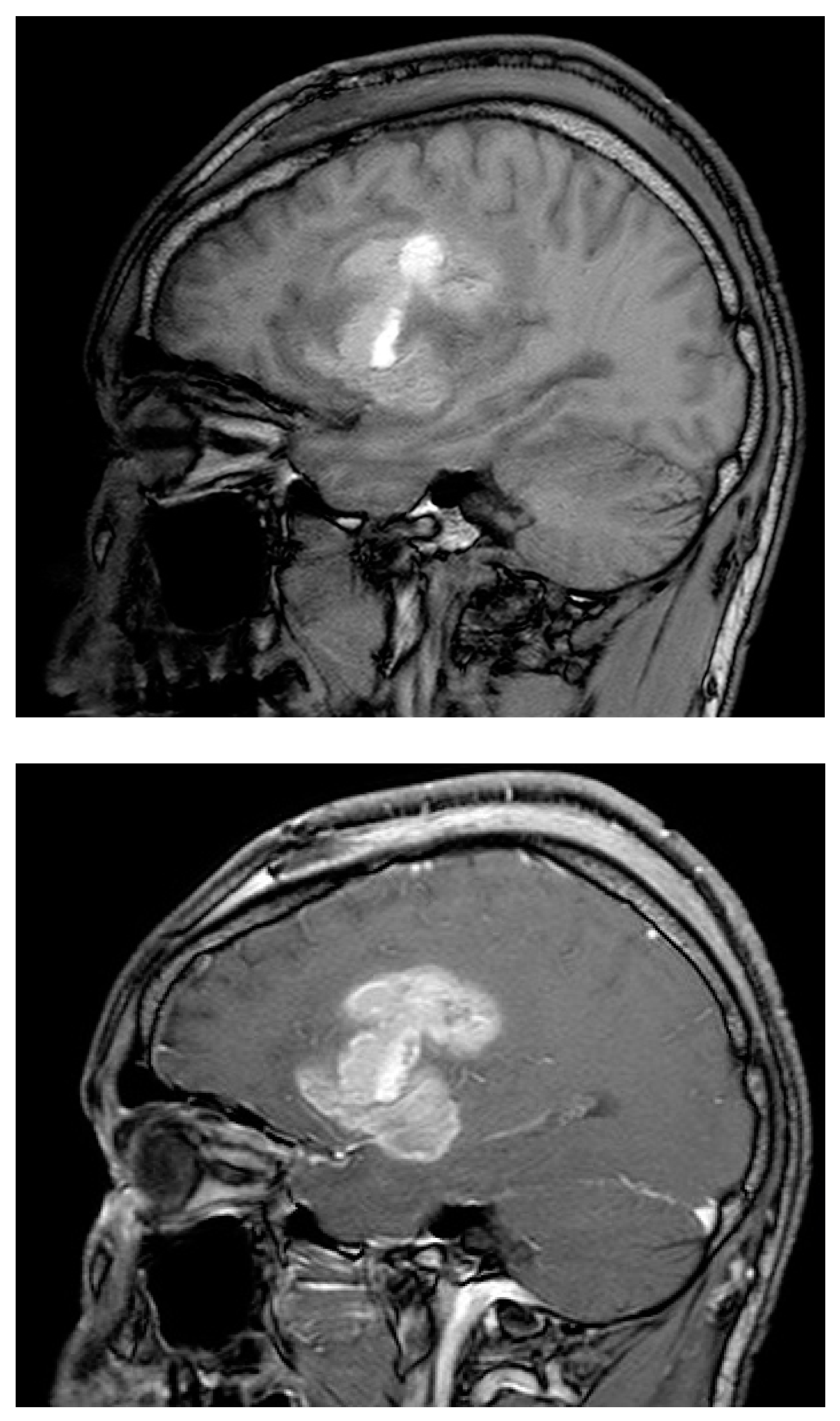
Figure C & D.
W image before (top image) and after contrast enhancement (bottom image), acquired 7 days postoperatively. Intrinsic T1 hyperintensity within the resection cavity is related to haemostatic agent and haemorrhage on the nonenhanced image, with peripheral enhancement after contrast administration. The presence of residual enhancing tumour is confounded by the resection cavity intrinsic hyperintensity, and the acquisition beyond the recommended early postoperative window, which adds postoperative reactive enhancement.
Figure C & D.
W image before (top image) and after contrast enhancement (bottom image), acquired 7 days postoperatively. Intrinsic T1 hyperintensity within the resection cavity is related to haemostatic agent and haemorrhage on the nonenhanced image, with peripheral enhancement after contrast administration. The presence of residual enhancing tumour is confounded by the resection cavity intrinsic hyperintensity, and the acquisition beyond the recommended early postoperative window, which adds postoperative reactive enhancement.
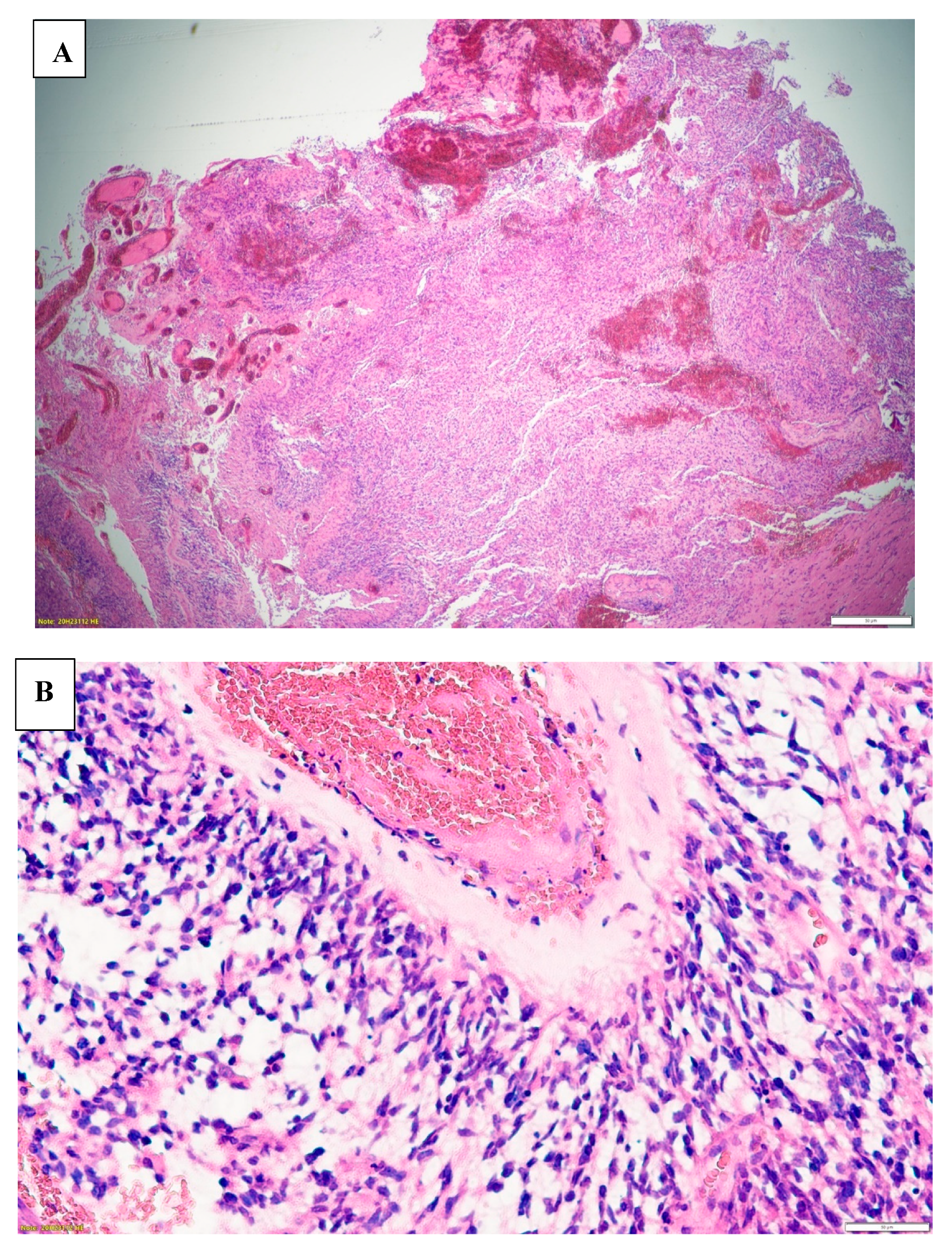
Images A and B.
H&E staining of the excisional biopsy elcetromicrograph images demonstrating the typical. A – Glioma cells spreading through normal cells. B- Glioma cells infiltrating the blood vessels i.e., endothelio-vascular proliferation.
Images A and B.
H&E staining of the excisional biopsy elcetromicrograph images demonstrating the typical. A – Glioma cells spreading through normal cells. B- Glioma cells infiltrating the blood vessels i.e., endothelio-vascular proliferation.
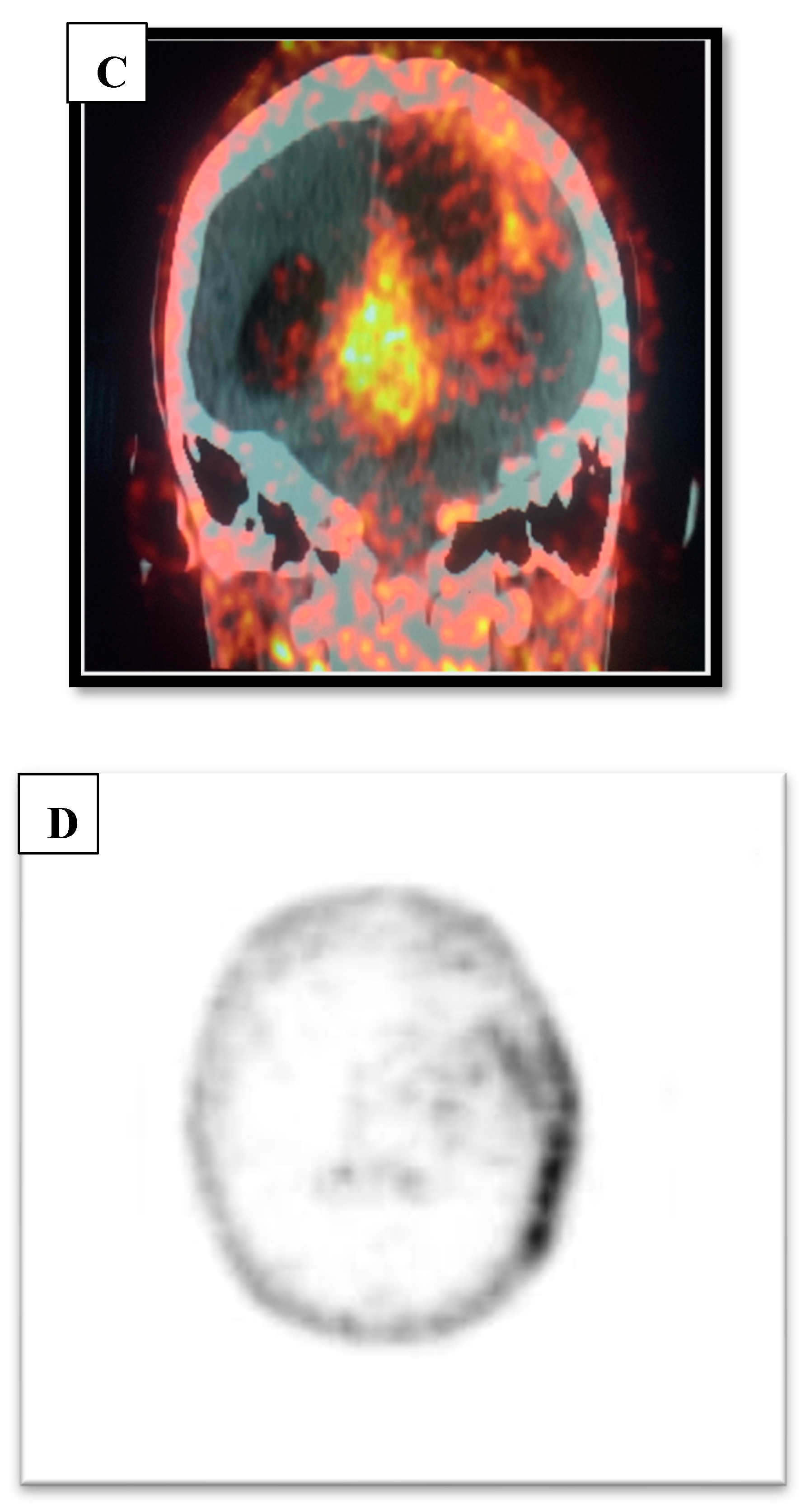
Figure C & D.
Ga-RGD PET/CT and PET only axial image demonstrating 68Ga-RGD avidity on the left parieto-temporal area i.e., recurrence.
Figure C & D.
Ga-RGD PET/CT and PET only axial image demonstrating 68Ga-RGD avidity on the left parieto-temporal area i.e., recurrence.
3. Discussion
Pseudo-progression (PsP) and radionecrosis (RN) are a known disadvantage of Magnetic Resonance Imaging technique when evaluating patients post-operatively and it has been also understood that MRI is not perfect. [
13,
14] Gliomas are heterogeneous and remain recalcitrant to conventional best care and are fatal; i.e., even post-resect, recurrence is almost a certainty.
This case succinctly demonstrates that the angiogenic pathway in gliomas can be detected with RGD PET therefore serving as an important complimentary marker to MRI. Whilst integrins are heterodimeric, they can be uniquely be labelled with a radioactive metal i.e.,
68Gallium which has a half-life of 68 minutes proving to be ideal in the clinical scenario. [
15,
16,
17]
Katsanos et al [
14] managed to highlight that Magnetic Resonance Spectroscopy (MRS) and
68Ga-RGD PET can be utilized successfully in order to give more data that will be relevant clinically and complimentary in the evaluation of gliomagenesis.
4. Conclusions
When assessing patients with Glioma (LGG to HGG) it may be beneficial to perform a 68Ga-RGD PET in conjunction with MRI. Since molecular aberrations in gliomas trigger the angiogenic switch/pathway, integrin imaging may give additional information that may influence management.
PET scans are becoming readily available therefore hybrid imaging with PET/MR may enhance the evaluation of low grade to high grade gliomas. The uptake of 68Ga-RGD by glioma is non-equivocal and this may also assist in the choice for therapy i.e., surgical by limiting blood loss and chemotherapy by selection of anti-angiogenesis agents e.g., Bevacizumab. Avenues for targeted therapy i.e., theranostic may in the future become a possibility. With newer digital and time-of-flight PET scans, the risk of ionizing radiation may soon be insignificant due to the validated requirements of much lower injected radiation doses with no compromise in image quality. Nuclear Medicine techniques are poised to be crucial non-invasive modalities that will yield important information that can be used for diagnosis, characterization, classification, ability to distinguish between true progression (TP) vs Pseudo-progression (PsP) and recurrence determination.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, TMG. and MM; methodology, LP.; software, MM and MV.; validation, MM., MV. and MB.; formal analysis, MM.; investigation, FJ.; resources, MB, FB, MM.; data curation, TMG.; writing—original draft preparation, TMG.; writing—review and editing, TMG; visualization, TMG and MK.; supervision, MM and MV.; project administration, TMG.; funding acquisition, TMG. All authors have read and agreed to the published version of the manuscript.
Funding
This case report received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent for publication was obtained from participating patient.
Acknowledgments
We acknowledge the Departments of Anatomical pathology, Neurosurgery and Radiology for the images provided.
Conflicts of Interest
No conflict of interest noted.
References
- Laug, D.; Glasgow, S.M.; Deneen, B. A glial blueprint for gliomagenesis. Nat. Rev. Neurosci. 2018, 19, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 16, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Llaguno, S.R.A.; Parada, L.F. Cell of origin of glioma: biological and clinical implications. Br. J. Cancer 2016, 115, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Verhaak, R.G.; Canoll, P. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev. Mol. Diagn. 2012, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J. Neuroimmunol. 1990, 30, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Chishty IA, Rafique MZ, Hussain M, Akhtar W, Ahmed MN, Sajjad Z, et al. MRI characterization and histopathological correlation of primary intra-axial brain glioma. `j Liaquat Uni Med Health Sci. 2010, 9, 64–69. [Google Scholar]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2022, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Scodeller, P.; Simón-Gracia, L.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A.; et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; A Butowski, N. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Katsanos AH, Alexiou GA, Fotopoulos AD, Jabbour P, Kyritsis AP, Sioka C. Performance of 18F-FDG, 11C-methionine, and 18F-FET PET for glioma grading: a meta-analysis. Clinical nuclear medicine 2019, 44, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Schwartz RB, Carvalho PA, Alexander ED, Loeffler JS, Folkerth R, Holman BL. Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. American journal of neuroradiology. 1991, 12, 1187–1192. [Google Scholar]
- Bello L, Francolini M, Marthyn P, et al. α(v)β3 and α(v)β5 integrin expression in glioma periphery. Neurosurgery 2001, 49, 380–389. [Google Scholar]
- Abdollahi A, Griggs DW, Zieher H, et al. Inhibition of α(v)β3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005, 11, 6270–6279. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, E.G.O.; Baldoncini, M.; Cómbita, A.L.; Payán-Gómez, C.; Gómez-Amarillo, D.F.; Hakim, F.; Figueredo, L.F.; Forlizzi, V.; Rangel, C.C.; Luzzi, S.; et al. Understanding the molecular profiling of diffuse gliomas classification: A brief overview. Surg. Neurol. Int. 2023, 14, 225. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).