1. Background
The late 20th century and early 21st centuries have highlighted the personal and communal burden as well as the scientific and societal challenges posed by emerging infectious diseases, predominantly zoonotic and highly pathogenic in humans [
1]. These viruses, an example of which is Ebola Virus Disease (EVD), cause severe infections with high mortality rates (50% to 90%) and can significantly spread from person to person [
2,
3].
Central Africa has experienced numerous outbreaks of EVD due to the fact that its ecosystem supports the establishment of various ecological niches for zoonotic illnesses. In 2021, Africa witnessed around 28 occurrences of EVD, as reported by Tshiani et al. [
4]. As of 11 June 2024, the Democratic Republic of Congo (DRC) is currently facing 16 outbreaks of EVD, with 8 provinces having already been affected by these epidemics. Between 1976 and 2022, there were a total of 4744 documented cases of EVD, resulting in 3207 deaths. This gives us an overall case–fatality rate of 67.6%, as stated in unpublished data. From 2018 to 2020, the most extended and lethal outbreak of EVD took place in the three provinces of North Kivu, South Kivu, and Ituri; this outbreak had a case–fatality rate of 66% [
5].
Several most promising medications, including the triple monoclonal antibody ZMapp, the antiviral agent Remdesivir, the triple monoclonal antibody REGN-EB3, and the single monoclonal antibody MAb114, have undergone testing to treat this condition. These four medications were used during the tenth EVD outbreak in the DRC. Some of them have proven to be effective in treating patients who are suspected or confirmed to have EVD. This represents a significant milestone in the treatment of EVD when used alongside other preventive measures [
6].
An EVD randomized controlled trial evaluated the safety and effectiveness of ZMapp, Remdesivir, REGN EB3, and MAb114, using the ZMapp group as control [
7]. Due to ethical considerations, the trial did not assess the efficacy of these regimens against standard supportive treatment. Additionally, while the delivery of several treatments enhanced survival rates (MAb114 and REGN-EB3 demonstrated greater effectiveness than ZMapp in lowering EVD mortality), the results did not satisfy the preset statistical threshold necessary to be considered effective [
8]. In the absence of comparisons to a standard treatment (supportive care without antiviral), it is impossible to determine if any of them are superior to the standard treatment or whether the observed effect is a result of the treatment itself or an external cause. Participants in this study also received an EVD vaccine (rVSV-ZEBOV Ebola vaccine), and the impact of the vaccine may have influenced the observed results. Does the comparison between these medicines and the standard treatment rely exclusively on statistical analysis, or do some of these pharmaceuticals fail to offer any further advantage in terms of survival compared to conventional treatment?
This secondary analysis aimed to examine the contribution of each regimen compared with the standard treatment on the survival of EVD patients and to assess whether the association between treatment type and survival rate was modified by vaccine status.
2. Materials and Methods
2.1. Data
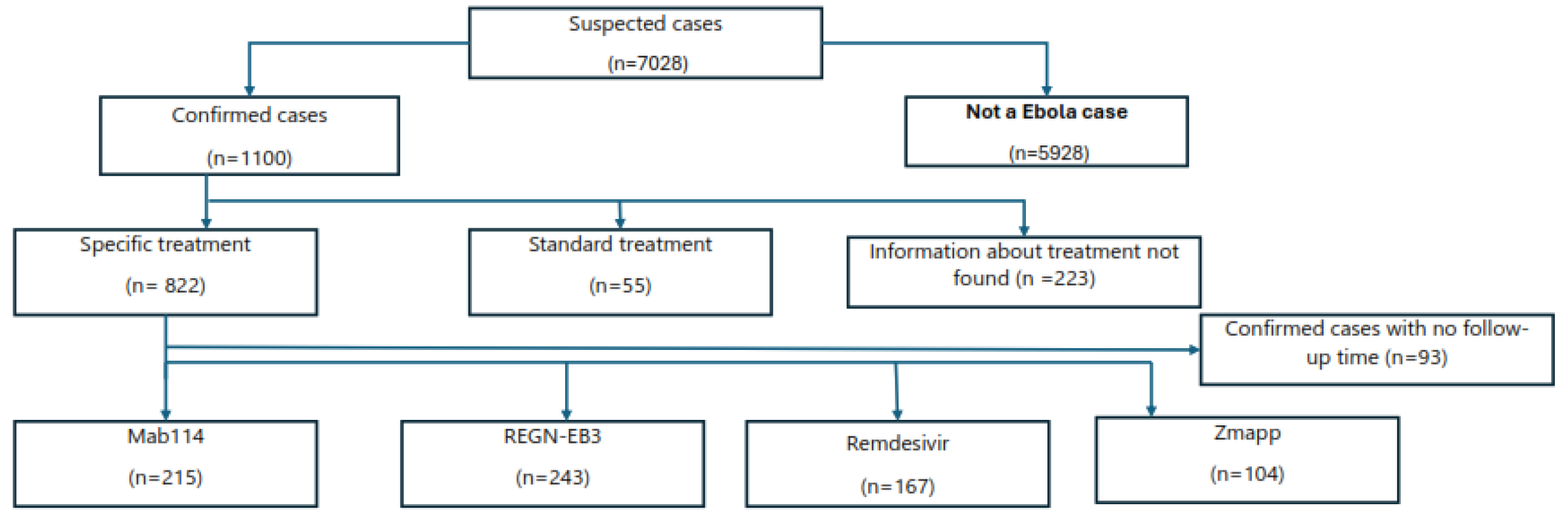
This is a study that examined existing data from the National Institute of Public Health (NPHI); we used secondary data obtained from four EVD treatment centers (ETCs) located in Katwa, Mangina, Butembo, and Beni in the North Kivu region. This analysis exclusively encompasses cases that were thoroughly reviewed and confirmed by the response team and that were subsequently admitted to an ETC. The timeframe for this analysis spans from 1 August 2018 to 14 February 2020. Furthermore, only cases that have explicit information regarding the treatment type and duration of observation in the patient’s record or database were considered. A total of 7,028 cases were admitted to the four ETCs, with 5,928 being non-cases (cases that did not turn out to be EBV, but another illness) and 1,100 being confirmed cases. Out of the 1,100 being confirmed cases. Out of the total of 1,100 confirmed cases, 822 received targeted treatment (Mab114, REGN-EB3, Remdesivir or ZMapp), 55 standard treatment and did not participate in the RCT for several reasons, and 223 cases had an unknown treatment status (see
Figure 1).
We used pre-existing data gathered from multiple ETCs, encompassing comprehensive information regarding the alert, such as ID number, alert number, age, gender, occupation, address, date of symptom onset, contact information, vaccination status, treatment particulars, date of treatment initiation, types of treatment administered, and end point (death, discharge, or end of the study). The data were transcribed into the Viral Hemorrhagic Fever (VHF) database, which utilizes an Epi-info input mask, and were subsequently exported to Excel and then to Stata for analyses.
2.2. Analysis
After inputting the database into Excel, we proceeded to export it to Stata version 17 (Stata Corp, College Station, TX, USA) for the purpose of processing, categorization, and analysis. Proportions and confidence intervals were computed for all the categorical variables. Quantitative variables were used to calculate the medians and interquartile ranges. The duration of follow-up for each patient was determined to start from the hospital admission. Descriptive data were used to characterize the EVD patients affected in each group.
The Kaplan–Meier method enabled us to analyze the likelihood of survival based on the duration between hospital admission and the occurrence of the final event (either death or the end of observation), while considering the presence of censored data. The main outcome measure was mortality within a 28-day period, which was utilized to compare our findings with a prior investigation involving the same group of patients [
7]. The Log-rank test allowed us to compare survival curves based on predictors, while the Cox model was used to identify predictors of survival in patients hospitalized with EVD. To assess how the association between survival and treatment group might differ according to vaccine status, an interaction term between the treatment group and vaccine status was included in the Cox multivariable model, and the log-likelihood ratio test was used to assess its significance. If it was found to be insignificant, then this term was removed in the final model. Mortality rates (hazard ratios) were adjusted for patient’s age, time from onset of illness to admission, gender, profession, vaccine status and treatment group
The proportionality test, using Schoenfeld residuals, confirmed adherence to the assumption of proportional risks. The proportional hazards test indicated that the assumption was not violated. Regarding the adequacy of the final model, we observed that the danger function roughly aligns with the 45-degree line, except for instances with longer time values. In summary, we can confidently state that the final model accurately aligns with the data. We evaluated the presence of multicollinearity by examining variance inflation factors (VIFs) that exceeded a threshold of 2.24. The tests were conducted using a two-tailed approach, with a confidence level of 95%; a result was deemed statistically significant if the p-value was less than 0.05.
2.3. Ethical Considerations
This study was authorized by the Ministry of Public Health, Hygiene, and Prevention, which includes the National Institute of Public Health (NPHI), who considered the fundamental ethical principles of respect for persons, beneficence, and justice. This study was carried out in accordance with the principles outlined in the Helsinki Declaration. The secondary analysis received approval from the ethics committee of the Kinshasa School of Public Health (reference number: ESP/CE/72B/2023). The study subjects were not subjected to any invasive procedures, and we did not have any direct interaction with them. All patient data were analyzed anonymously and confidentially. The principal investigator maintained the electronic and physical records in a secure location, with password-protected access for the computer.
3. Results
During the specified time frame, a total of 1064 patients diagnosed with EVD were included in the study. A total of 283 patient records, which had incomplete information on treatment status and/or duration of follow-up, were eliminated from the study. This resulted in 781 records that satisfied the requirements for analysis, 73% of the initial total. In general, the patients who were removed from the analyses were comparable to those who were included in terms of age distribution (with an average age of 31 years), gender (with 54% being female), and profession (with 5% being health professionals). Nevertheless, the patients who were omitted from the analysis were more inclined to be non-vaccinated. Among them, only 6% were vaccinated, while 20% of the patients included in the analysis were vaccinated.
Table 1 displays the characteristics of patients who were included and excluded in the analysis.
Out of the 781 individuals analyzed, approximately 31% were treated with REGN-EB3, 27% received MAb114, 21% were given Remdesivir, and 13% received a ZMapp-based regimen. Only 7% of the individuals included in the study had exclusively received the standard treatment. Vaccinated individuals had a shorter interval between admission and the onset of initial symptoms of a brief illness compared to non-vaccinated patients (3.70 days vs. 5.00 days; p = 0.0002).
The patients included in the studies exhibited a comparable age and gender distribution. The duration from the disease’s commencement to admission was the same in the groups who underwent the novel treatment (promising treatment), but was significantly longer in the group that only received the standard treatment. A very small proportion of healthcare professionals had not received the standard treatment. Patients who received the standard treatment exhibited a lower vaccination coverage in comparison to the other groups, as seen in
Table 2.
Predictors of Mortality among Cases of EVD
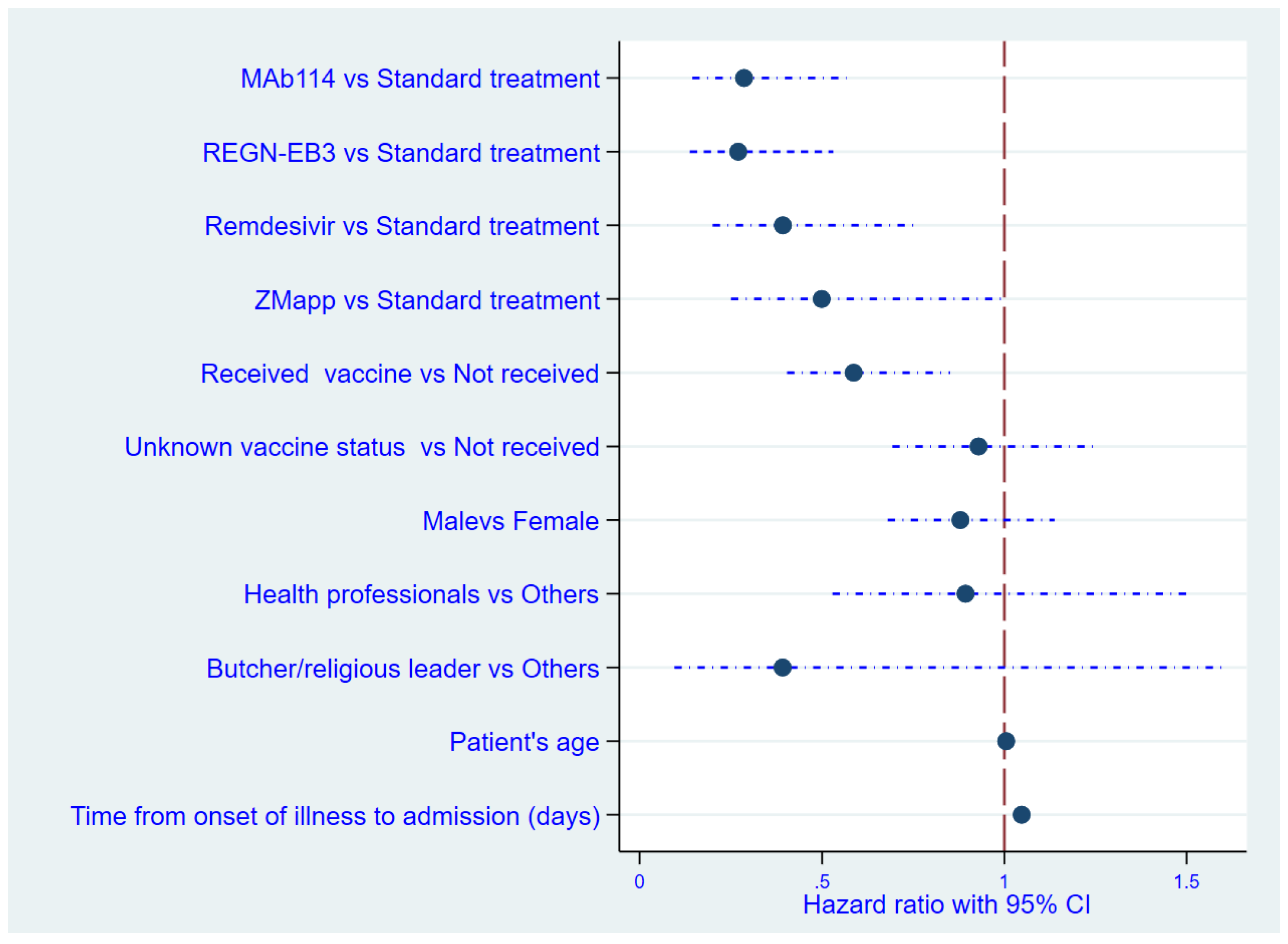
In this analysis, it was found that, among the patients with EVD, those who had received the vaccine were 1.7 times less likely to die compared to those who had not received the vaccine (relative risk: 0.59). A prolonged delay in receiving treatment (measured as the time elapsed from the onset of sickness to admission, in days) increased the likelihood of early mortality in patients, while a longer duration of symptoms prior to therapy was correlated with significantly poorer outcomes. The mortality risk escalated by 5% for every day following the onset of symptoms that the patient failed to present at the treatment facility. The mortality rate was decreased in the MAb114 and REGN-EB3 groups compared to patients who received the standard treatment, as shown in
Table 3.
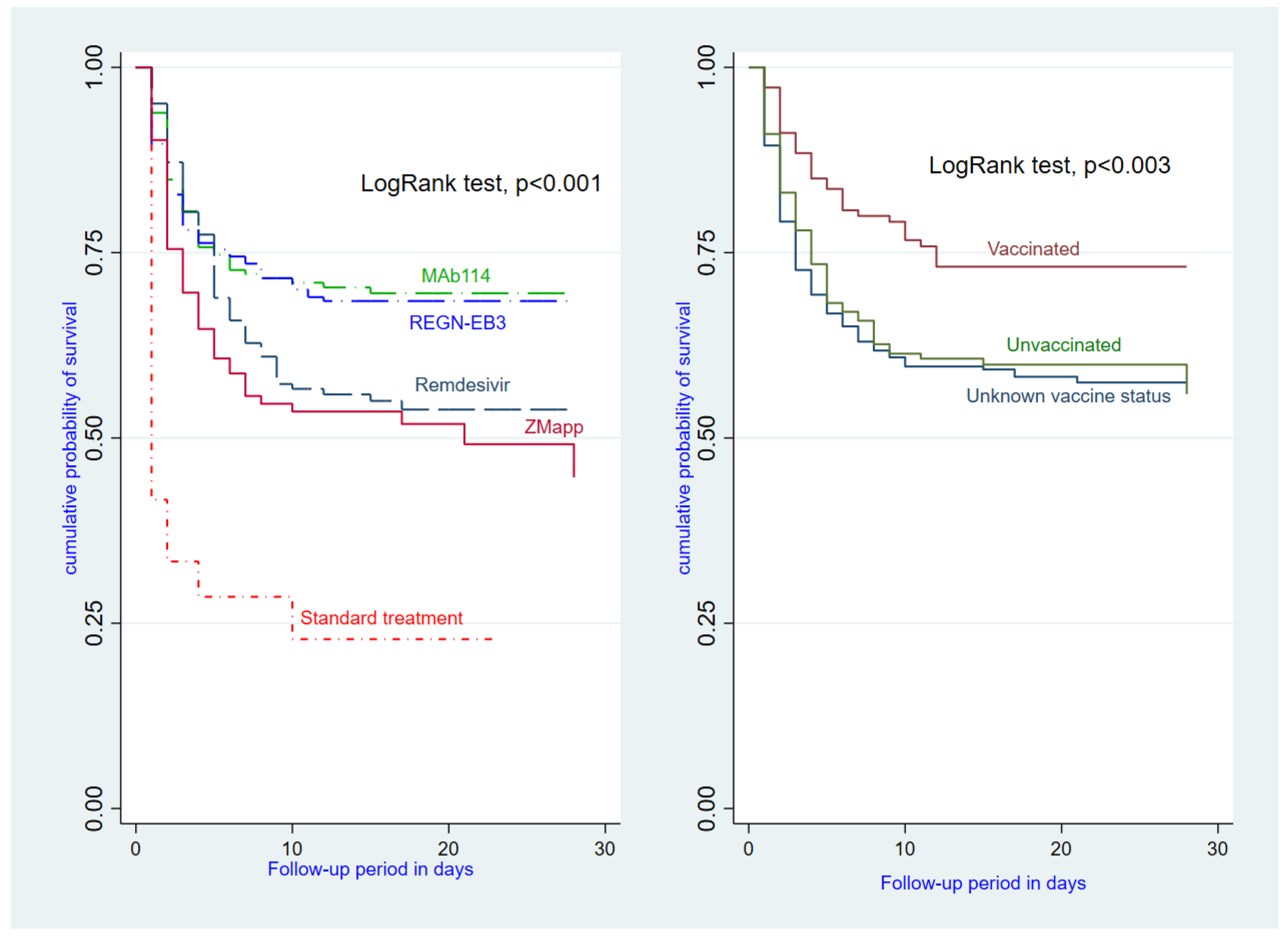
The survival benefits reported in the MAb114 and REGN-EB3 groups, relative to patients who received the standard treatment, are verified in
Figure 2 and
Figure 3 below.
4. Discussion
This study aimed to assess the patient survival rate of four promising therapies (MAb114, REGN-EB3, Remdesivir, and ZMapp) compared to standard supportive treatment (no antiviral). Adjusted patient survival rates were highest with MAb114 and REGN-EB3, followed by Remdesivir. The ZMapp group showed a reduction, albeit with borderline statistical significance.
Vaccinated patients had a lower-case fatality rate than non-vaccinated individuals, confirming findings from Neil et al.’s study [
9]. Findings from the WHO indicate that the efficacy of the vaccine in preventing EBD onset ten days or more after vaccination is 97.5%, and its efficacy in preventing EBD onset at any time is 88.1% [
10]. Out of the 726 patients included in this analysis, 150 (28% of 541) reported being vaccinated, based on the available information. Vaccinated patients were more likely to enroll in the study sooner after experiencing symptoms and generally had more positive prognostic characteristics at the beginning of the study. This suggests a connection between vaccination and the tendency to seek medical attention promptly, which in turn led to improved outcomes.
An unfavorable therapeutic response was linked to delayed therapy initiation after symptom onset (a 5% increase in mortality risk for each day that symptoms persisted before therapy initiation). Thus, the duration between the onset of the disease and admission to the treatment center was a reliable indicator of mortality risk. Patients receiving standard treatment were hospitalized 8.84 days after the initial manifestation of symptoms and had a case-fatality rate of 89.1%. These statistics emphasize the necessity of raising community awareness regarding the correlation between early diagnosis, prompt treatment, and improved survival rates. Our results align with findings reported by Malvy et al. [
11] who suggested considering cultural factors is crucial for establishing trust within communities.
The high efficacy of MAb114 and REGN-EB3 compared to ZMapp and Remdesivir in this analysis may partially explained by the fact that MAb114 and REGN-EB3 were administered as single doses, while ZMapp and Remdesivir required multiple infusions [
7], which may have been delayed due to staff shortages or other operational barriers.
It is worth noting that 97% of deaths in this study occurred within ten days of enrollment. While most baseline characteristics were similar among the five groups, patients who received the standard treatment had a lower vaccination rate (9%) compared to the other four groups. Additionally, patients who received Remdesivir began treatment slightly later than the other groups, suggesting that these patients may have been more ill on average. This disparity in health status could potentially account for the study's findings. Remdesivir underwent clinical trials for Ebola in 2014 but did not demonstrate sufficient efficacy to be considered an effective treatment for Ebola infection [
8].
While the administration of ZMapp seemed to improve survival rates, the outcome did not meet the predetermined statistical threshold required to be deemed effective [
8].
Nevertheless, the trial played a crucial role in facilitating additional research on monoclonal antibodies as potentially effective treatments for EVD. This investigation validates the efficacy of REGN-EB3 and mAb114 as monoclonal antibody-based therapies for EVD and provides further support for the updated guidelines by the World Health Organization [
12].
This secondary analysis had limitations due to the use of previously collected data. Challenges included incomplete or even erroneous information. These results are additionally constrained by the patient's self-reported vaccination status. Since the primary trial did not consider vaccination status in randomization, conclusive statements about its impact on mortality cannot be made. The absence of data from laboratory tests, such as viral load, poses challenges in interpreting our Cox regression results, as these metrics are crucial for assessing disease stage and severity.
5. Conclusions
The Democratic Republic of the Congo (DRC) experienced its eleventh outbreak of the Ebola virus since its initial identification in 1976. The outbreak occurred in a region affected by armed conflict, adding challenges to containment and management. Historical instances of Ebola outbreaks and actions taken have demonstrated that prompt identification and treatment, together with enhanced supportive care (including replenishing fluids and electrolytes and addressing symptoms), significantly enhance survival chances. Today, administering vaccines and using mAb114, REGN-EB3, and, to some extent, Remdesivir will further enhance patient survival rates. Our analysis underscores the critical importance of consistently using EVD vaccines during every outbreak, guaranteeing an ample vaccine supply, maintaining a strategic reserve of mAb114 or REGN-EB3, enhancing public awareness about seeking prompt medical attention at the onset of symptoms.
Author Contributions
Conceptualization, EKM and APZ.; methodology, EKM and APZ.; software, EKM and APZ.; validation, EKM, TNT and APZ.; formal analysis EKM and APZ.; investigation, EKM.; resources, EKM and APZ.; data curation, EKM and APZ.; writing—original draft preparation, EKM, TNT, MKP, ASM, MTJ and APZ.; writing—review and editing, EKM, TNT, MKP, ASM, MTJ and APZ.; visualization, EKM, TNT and APZ.; supervision APZ project administration, EKM.; funding acquisition, EKM and APZ. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have received no specific funding for this work.
Institutional Review Board Statement
Ethical clearance was obtained from the Ethics Committee of the KSPH (reference number: ESP/CE/72B/2023). Consent was obtained from each respondent during data collection. Privacy and confidentiality were maintained throughout the study.
Informed Consent Statement
All co-authors consented to the publication of the latest version of the present article.
Data Availability Statement
The dataset used for analysis can be obtained upon reasonable request by writing an email to the corresponding author.
Acknowledgments
We thank all individuals who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malvy, D.; Gaüzère, B.A.; Migliani, R. Epidemic and emerging prone-infectious diseases: Lessons learned and ways forward. Presse Medicale 2019, 48, 1536–1550. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd. Ebola Virus Disease, Nature Reviews Disease Primers; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- WHO Ebola Response Team. Ebola virus disease in West Africa--the first 9 months of the epidemic and forward projections. N Engl J Med. 2014, 371, 1481–1495. [Google Scholar] [CrossRef]
- Tshiani Mbaya, O.; Mukumbayi, P.; Mulangu, S. Review: Insights on Current FDA-Approved Monoclonal Antibodies against Ebola Virus Infection. Front. Immunol. 2021, 12, 721328. [Google Scholar] [CrossRef] [PubMed]
- Kiiza, P.; Mullin, S.; Teo, K.; Adhikari, N.K.J.; Fowler, R.A. Treatment of Ebola-related critical illness. Intensive Care Med. 2020, 46, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Keita, A.K.; Koundouno, F.R.; Faye, M.; Düx, A.; Hinzmann, J.; Diallo, H.; Ayouba, A.; Le Marcis, F.; Soropogui, B.; Ifono, K.; et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature 2021, 597, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- PREVAIL II Writing Group; Multi-National PREVAIL II Study Team; Davey, R.T., Jr.; Dodd, L.; Proschan, M.A.; Neaton, J.; Neuhaus Nordwall, J.; Koopmeiners, J.S.; Beigel, J.; Tierney, J.; et al. A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N. Engl. J. Med. 2016, 375, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Rupani, N.; Ngole, M.E.; Lee, J.A.; Aluisio, A.R.; Gainey, M.; Perera, S.M.; Ntamwinja, L.K.; Matafali, R.M.; Muhayangabo, R.F.; Makoyi, F.N.; Laghari, R.; Levine, A.C.; Kearney, A.S. Effect of Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus Vaccination on Ebola Virus Disease Illness and Death, Democratic Republic of the Congo. Emerg Infect Dis. 2022, 28, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilunga Kalenga, O.; Moeti, M.; Sparrow, A.; Nguyen, V.K.; Lucey, D.; Ghebreyesus, T.A. The Ongoing Ebola Epidemic in the Democratic Republic of Congo, 2018-2019. N Engl J Med. 2019, 25, 381, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Malvy D; Groupe d'étude JIKI¥. Évaluation des antiviraux dans la maladie à viru. Ébola, Guinée, 2014-2015: Enjeux et perspectives. Bull Acad Natl Med. 2014, 198, 1515–1527. [PubMed]
- WHO makes new recommendations for Ebola treatments, calls for improved. Available online: https://www.who.int/news/item/19-08-2022-who-makes-new-recommendations-for-ebola-treatments-----calls-for-improved-access (accessed on 10 January 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).