1. Introduction
Gliomas are potentially lethal types of primary brain tumours that are characterized by aggressive capability and angiogenesis [
1].
Regional epidemiological data suggests that there are over 100, 000 cases of glioma diagnosed annually worldwide.
Data from CBTRUS and WHO, suggests that the incidence, predictive indicators and the prognosis of gliomas is different based on age, gender, ethnicity and genetic mutations [
2].
In South Africa the incidence of the central nervous system (CNS) cancers between males and females is 0,56% and 0,49% respectively based on the cancer registry which is available publicly.
Gliomas pose a medical and surgical challenge; they constitute 27% of all the primary CNS tumours and also account for 80% of all malignant primary CNS tumours [
1,
2,
3,
4,
5,
6,
7].
In general, the prognosis of diffuse gliomas in adults is poor based on a recent review on the therapeutic modalities utilized in the managing the disease. Therapy with curative intent in the management strategy is unlikely as gliomas tend to recur and have aggressive capabilities [
5].
Surgically, minimizing or extension of the extent of resection (EOR) either leads to residual tumour that will progress or resecting even the normal brain parenchyma thus leading to permanent neurological results that affect the quality of life (QoL) negatively.
Gliomas arise from glial cells which are non-neuronal, and are the supporting infrastructural components of the CNS and peripheral nervous system (PNS).
Glial cells are vital in maintaining cellular homeostasis and production of myelin, thereby contributing to the blood-brain-barrier and protection of the neurons. They can be found in the brain and spinal cord.
In the human and mammalian brain, glial cells constitute the microglia, ependymal cells, oligodendrocyte lineage and astrocytes. It was previously believed that gliomas arose from only astrocytoma and oligodendrocyte cell lineage, hence the earlier WHO criteria for classification based on morphology; however, various studies have shown that microglial cells are also part of glioma origin cells. Some of these cells, in particular microglia, are the resident immune cells of the brain that survey their environment and respond to pathogens, toxins, and tumors [
8,
9].
In the 2020 EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood, several important modifications were made i.e., glioblastoma (GBM) is now defined as a diffuse astrocytic glioma with no mutations in IDH genes nor histone H3 genes and is characterized by microvascular proliferation, necrosis and/or specific molecular features, including TERT promoter mutation, EGFR gene amplification and/or a +7/-10 cytogenetic signature, IDH-mutant glioblastoma is now referred to as IDH-mutant astrocytoma, WHO grade IV (4), homozygous H3.3 G34-mutant diffuse hemispheric gliomas constitute a novel glioma entity corresponding to WHO grade 4 (previously referred to as glioblastoma multiforme) [
10].
The notable hallmark of gliomas is integrated genome mutations and methylome that correlates with distinctive patterns of transcription that potentiates glioma heterogeneity, angiogenesis, poor prognosis and treatment response, however as previously believed oncologic medical interventions particularly bevacizumab does not prolong progression-free-survival (PFS) nor overall survival (OS).
The current gold standard for the treatment of glioblastoma is care that includes surgery, adjuvant radiotherapy and temozolomide (TMZ) chemotherapy. Unfortunately, these treatment strategies are not effective in curing glioblastoma, hence the poor prognosis.
Novel agents such as nivolumab (an immune-therapeutic agent) are not superior to bevacizumab nor superior to temozolomide in patients diagnosed with glioblastoma without MGMT promoter methylation.
This evidence suggests that it is imperative to understand all mechanisms underlying glioblastomagenesis to open a new avenue of effective treatment.
The current gold standard of glioma diagnosis is on histopathology, however this is not always feasible based on location of the lesion or comorbidities. Therefore non-invasive techniques such as MRI are often utilized preoperatively, postoperatively and during follow-up where there is suspected recurrence of the tumour.
MRI imaging of gliomas depends on various sequences and the administration of contrast agents i.e., gadolinium-enhanced (GE) MRI with T1 and T2-weighted sequences. Christy and colleagues found the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of detecting intra-axial gliomas to be 93%, 77%, 80% and 90% respectively, this suggests MRI as a useful and powerful imaging modality [
10,
11]. However, infiltrative tumour growth within non-enhancing regions of FLAIR signal abnormality is not readily visualised with conventional MR sequences (before and after therapy).
Other imaging techniques that are used for glioma management is ultrasonography with different modes e.g., elastography and DC. The drawback has been that it is user-depended and the advantage being that brain-shift can be accounted for when the craniotomy is performed [
10,
12].
Molecular imaging may hold the key or be a potential adjunct in differentiating and characterising gliomas e.g., low-grade (LGG) vs. high grade (HGG), pseudoprogression and recurrence. These tools are in the mainstream of Nuclear Medicine diagnostic armamentarium, single-photon emission tomography (SPECT) or Positron emission tomography (PET).
In this review, we will provide recent studies on nuclear medicine imaging techniques i.e., SPECT and PET. This review will aim to incorporate imaging techniques with the molecular and epigenetic aberrations demonstrated. Moreover, we will also recommend the recent potential use of PET 68[Ga]68Ga-labelled integrins (RGD) using integrins for the development of epigenetic-targeted therapy and theranostics. Nowadays, it is impractical to discuss gliomas in isolation of the molecular/genetic aberrations since the WHO has embarked on the change in classification and characterization of the disease.
The format taken is to understand the pathophysiology, link it with the current known molecular markers and introduce integrins and how Nuclear Medicine can be utilized.
In a recent review by Bolcaen et al. Novel Tyrosine Kinase Pathway inhibitors for targeted therapy of glioblastoma focussed on seven tyrosine kinase receptors, based on their role in GB [
11].
In this review, we have selected specifically integrins, amino-acids and 18F-Fluorodeoxyglucose.
Systematic reviews for amino acids and FDG are available and the subject is well understood however integrins may be one of the key factors that needs further studies as mentioned by Li D et al. [
12].
2. The Pathology and Histological Classification of Gliomas
Gliomas are considerably heterogeneous tumours with subclones existing within tumour populations [
13].
Pathophysiologically, there are various deletions, amplifications and point mutations thus contributing to the tumour heterogeneity and pathogenesis.
Glioma cells tend to migrate away from the tumour mass through the brain parenchyma and infiltrate secondary structures i.e., structures of Scherer. Ultimately, the abnormal cells collect below the pial margin i.e., subpial spread, then surround neurons and vessels and migrate through the white matter tracts.
The causes, risk factors and associated factors leading to gliomas are numerous and continues to expand e.g., parasites (Toxoplasmosis gondii), viruses (Cytomegalovirus), monogenic disorders (Neurofibromatosis 1 & 2, tuberous sclerosis and Lynch Syndrome), exposure to ionizing and non-ionizing radiation, allergens, occupational exposure to pesticides, solvents and body mass index. Though some of these associations are inconsistent, it is important to note that there may be no singular causality to the pathogenesis of gliomas.
Gliomas are classified into astrocytoma, oligodendroglioma and oligo-astrocytoma, which are classically defined by WHO grades (I-IV) and cell-type origin and location. Astrocytoma and oligodendroglioma are derived from astrocytes and oligodendrocytes, respectively. Oligo-astrocytoma affect a mixture of both cell types. Gliomas are graded according to the exhibition and characteristics of histological features including circumscribed (WHO grade I), low grade anaplastic (WHO grade II) and high grade anaplastic and glioblastomic (WHO grades III and IV) astrocytomas.
According to the WHO, grade I gliomas are typically benign, slow-growing and are associated with long-term survival and are least likely to recur.
Grade II gliomas display an increased hypercellularity with no mitosis, necrosis or vascular proliferation.
Grade III gliomas have a high rate of hypercellularity and tumour recurrence.
Grade IV gliomas also have a higher rate of hypercellularity, mitosis and vascular proliferation. GBM multiforme (The term that fell away with the WHO 2016 revision)/Grade IV glioma is typically a disease that is adult-manifested, poorly differentiated, nuclear atypia, hypercellular, microvascular proliferatic and hypoxic malignancy. It is considerably haemorrhagic due to increased blood supply. The glioblastoma necrotic microtumour environment (MTE) is enriched with gross enlarged neutrophils associated with immunosuppression and poor survival and prognosis.
The genomic and epigenomic landscape of glioma delineates its classification and continues to advance physicians’ understanding about the triggers of cancer, prognosis and treatment response in each patient. These include isocitrate dehydrogenase (IDH), H3.3K27, telomerase reverse transcriptase (TERT) and ATRX mutations, 1p/19q co-deletion/non-co-deletion mutants, endothelial growth factor receptor (EGFR) amplification and promoter methylation of MGMT. This knowledge has been added in the WHO 2016 scheme for classification and led to major changes in the way glioma is being diagnosed and treated [
9]. IDH and ATRX with 1p/19q co-deleted mutants are mostly oligodendroglial morphology and are associated with the best prognosis; IDH-mutants and ATRX with 1p/19q non-co-deleted mutants are mostly astrocytic histology that are associated with intermediate outcome; and IDH wild-type are mostly detected in higher WHO grade (III or IV) tumours that are associated with poor prognosis.
These described features determine the success of treatment, which is currently standardised as surgery, chemotherapy with temozolomide and radiotherapy. For a well circumscribed and slowly growing WHO grade 1 pilocytic astrocytoma that is typically benign and occurs primarily in children, curative treatment is possible if completely removed through surgery. As for fast growing diffusely neighbouring cell infiltrating WHO grade II-IV gliomas, the treatment is a significant challenge and may be incurable [
14].
Table 1.
Recent Reviews and Findings in the imaging modalities used in the management of gliomas.
Table 1.
Recent Reviews and Findings in the imaging modalities used in the management of gliomas.
| Author |
Modality |
Year |
Title |
Findings |
| Kazerooni et al. |
MRI |
2019 |
Imaging signatures of glioblastoma molecular characteristics: A radiogenomics review |
Non-invasive genomics may play a role in the characterization of gliomas and may assist in the goal of designing patient-specific therapies |
| Alexiou et al. |
MRI, SPECT and PET |
2010 |
Assessment of glioma proliferation using imaging modalities |
MRS and PET may be complimentary in the assessment of Gliomas as they can provide insight into proliferation, monitoring therapy, biopsy guidance and estimation of overall prognosis |
| Bonm et al. |
MRI and PET |
2020 |
Clinical Imaging for Diagnostic Challenges in the Management of Gliomas: A Review |
Though MRI is the gold standard, molecular imaging techniques e.g., PET may assist in distinguishing true progression from pseudoprogression. |
| Bolcaen et al. |
SPECT and PET |
2021 |
Novel Receptor Tyrosine Kinase Pathways Inhibitors for Targeted Radionuclide Therapy of Glioblastomas |
Receptor Tyrosine Kinase inhibitors receptors e.g., EGFR, VEGFR, MET, PDGFR, FGFR, Eph and IGFR1 demonstrate promise in preclinical studies in order to improve prognosis and treatment outcome in Glioblastoma (GB) |
| Patel et al. |
MRI |
2017 |
MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis |
MRI has the disadvantage of not having standardised cut-off values/thresholds. More prospective work is needed in order to validate the results at an institution. |
3. The Link of Genome and Epigenome in Glioma-Mediated Angiogenic Switch
Gliomas are typically associated with angiogenic switch, which is a vital component of tumor growth that involves an alteration in the balance of pro-angiogenic and anti-angiogenic molecules that lead to tumor neovascularization.
Macrophages, neutrophils and immature myeloid cells are essential pro-angiogenic immune cells in the glioma tumour microenvironment. Tumour-associated macrophages (TAMs) are the primary contributing factor in gliomas angiogenic switch. They promote angiogenesis mainly by secreting pro-angiogenic growth factors and inflammatory cytokines that facilitate the degradation of the perivascular extracellular matrix proteins (EMP) [
15,
16].
Distinct molecular pathways that activate tumour onset, growth and angiogenesis in gliomas have been identified and are attributable to complex crosstalk between “driver” genetic mutations, metabolic and epigenetic reprogramming [
17,
18]. This involves DNA and histone methylation mechanisms that perturb molecular pathways and favour glioma heterogeneity, progression and treatment resistance (e.g., chemotherapy, radiotherapy and theranostics) [
18]. An increased understanding of these genome anomalies is critical for the development of improved strategies in the fight against aggressive glioma.
3.1. Isocitrate Dehydrogenase Mutations and Aberrant Transcriptional Activities
IDH enzymes catalyse the oxidative decarboxylation of isocitrate into alpha-ketoglutarate (α-KG) that is utilised in the Krebs cycle and cellular homoeostasis. New emerging evidence demonstrate that IDHs are frequently mutated in a variety of human malignancies [
19].
IDH 1 and 2 mutations have been identified as the only valuable prognostic marker in glioma [
20].
They upregulate the transcription of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and 2-hydroxyglutaric acid (2-HG) to reprogramme metabolic and epigenetic mechanisms. This intercepts “normal” cellular functions causing glioma tumour cells to overproliferate and evade immune surveillance that normally prevents tumour growth and invasion [
21,
22].
Glioblastoma cells are also enriched with 5-hydroxymethylcytosine (5hmC) and ten-eleven translocation methyl cytosine dioxygenase (TET).
The D2HG induces hypermethylation of transformed cells by blocking TET and histone lysine demethylase genes (KDMs).
TET enzymes catalyse the hydroxylation of 5-methylcytosine into 5-hmC, and then further catalyse additional cytosine demethylation steps by transforming 5-hmC into 5-formylcytosine and 5-carboxylcytosine (5-caC). Most recently, two distinct methylation-based clusters of H3-Pons and H3-Medulla have also been identified, and demonstrate brainstem glioma subsets associated with tumor location and mutation landscape.
These clusters both harbor H3F3A mutations that are associated with differentially methylated and expressed genes with poor distinct prognosis [
23].
3.2. IDH-Mediated Gene Transcriptional Alterations
Hypoxia-Inducible Factor-1α
Tumour hypoxia is a predominant feature of aggressive gliomas, and is typically characterised by adequate oxygen deprivation in tumour cells.
Hypoxia stabilizes HIF proteins, and this confers resistance to chemotherapy and radiotherapy by activating various pathways such as EGFR, PI3K/Akt and MAPK/ERK pathways, autophagy and p53 leading to induction of cell cycle arrest, suppressed apoptosis and senescence of cells [
24].
Nucleic HIF1α binds to hypoxia-response element (HRE) in the promoter region and activates transcription of several hypoxia-associated genes including VEGF to promote angiogenesis and hostile tumour microenvironment.
O6-methylguanine methyltranferase (MGMT) is a DNA-repair enzyme that protects tumour cells by removing promutagenic alkyl adducts from 0(6) of guanine in DNA. Hypermethylation of MGMT and ultimate suppression of DNA repair and apoptosis have been observed in half of glioblastoma patients, and this is linked to increased chemotherapy and radiotherapy resistance.
In low grade oligodendrogliomas and astrocytomas, MGMGT methylation was in parallel with G:C to A:T transition of p53 mutation, 1p19q codeletion and IDH mutations [
25].
The prognostic biomarker value of MGMT methylation has resulted in a new standard of care for newly diagnosed glioblastoma [
26].
To minimise increased drug toxicities and unnecessary costs, patients whose tumors exhibit MGMT methylation are treated with temozolomide monotherapy. For those whose tumours lack MGMT methylation, radiotherapy is used as a treatment option [
27].
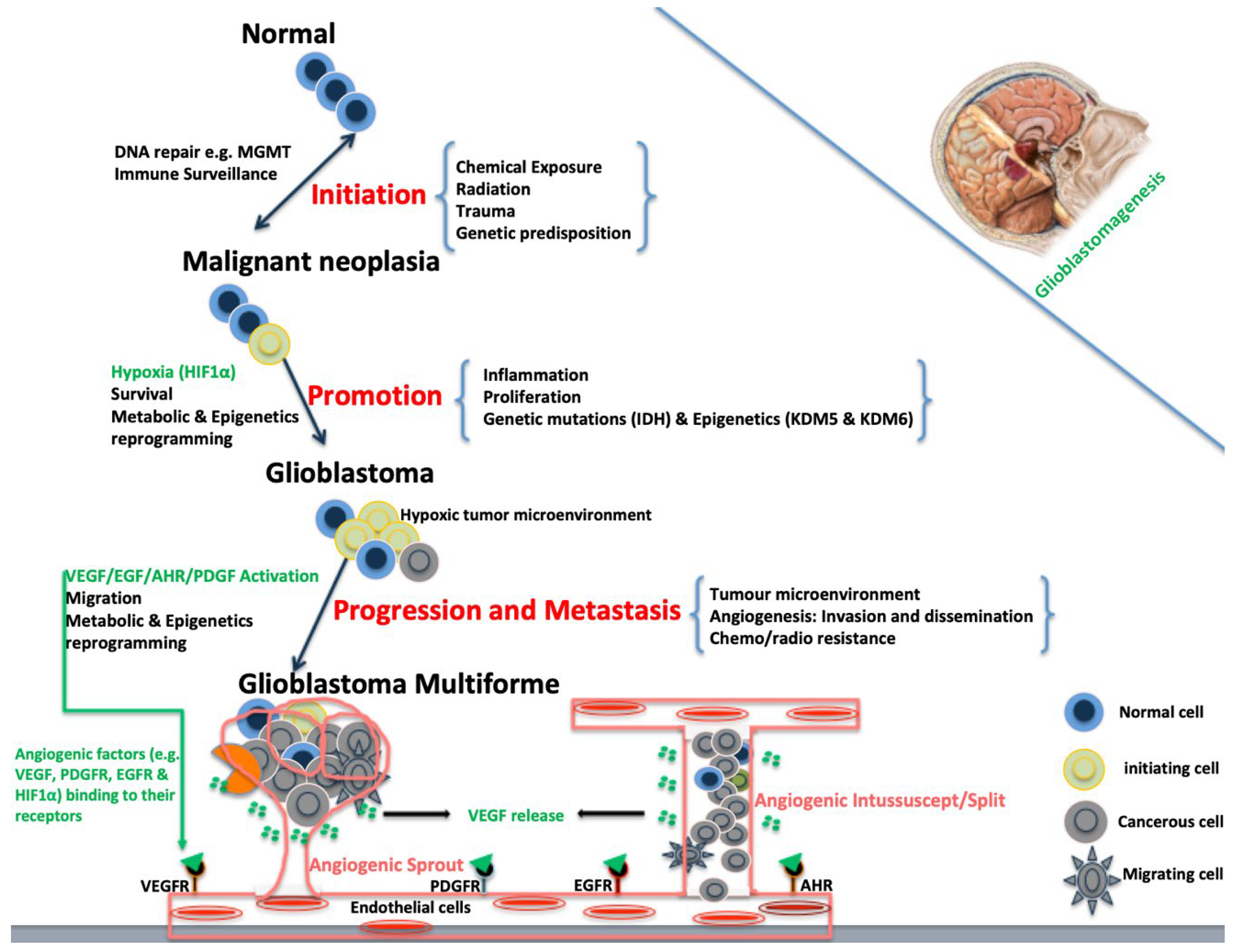
Figure 1.
Schematic diagram of Glioblastomagenesis. Glioblastomagenesis results from activation of angiogenic factors that, in turn, activate endothelial cells leading to cell migration and vascular formation that are associated with aggressive tumour.
Figure 1.
Schematic diagram of Glioblastomagenesis. Glioblastomagenesis results from activation of angiogenic factors that, in turn, activate endothelial cells leading to cell migration and vascular formation that are associated with aggressive tumour.
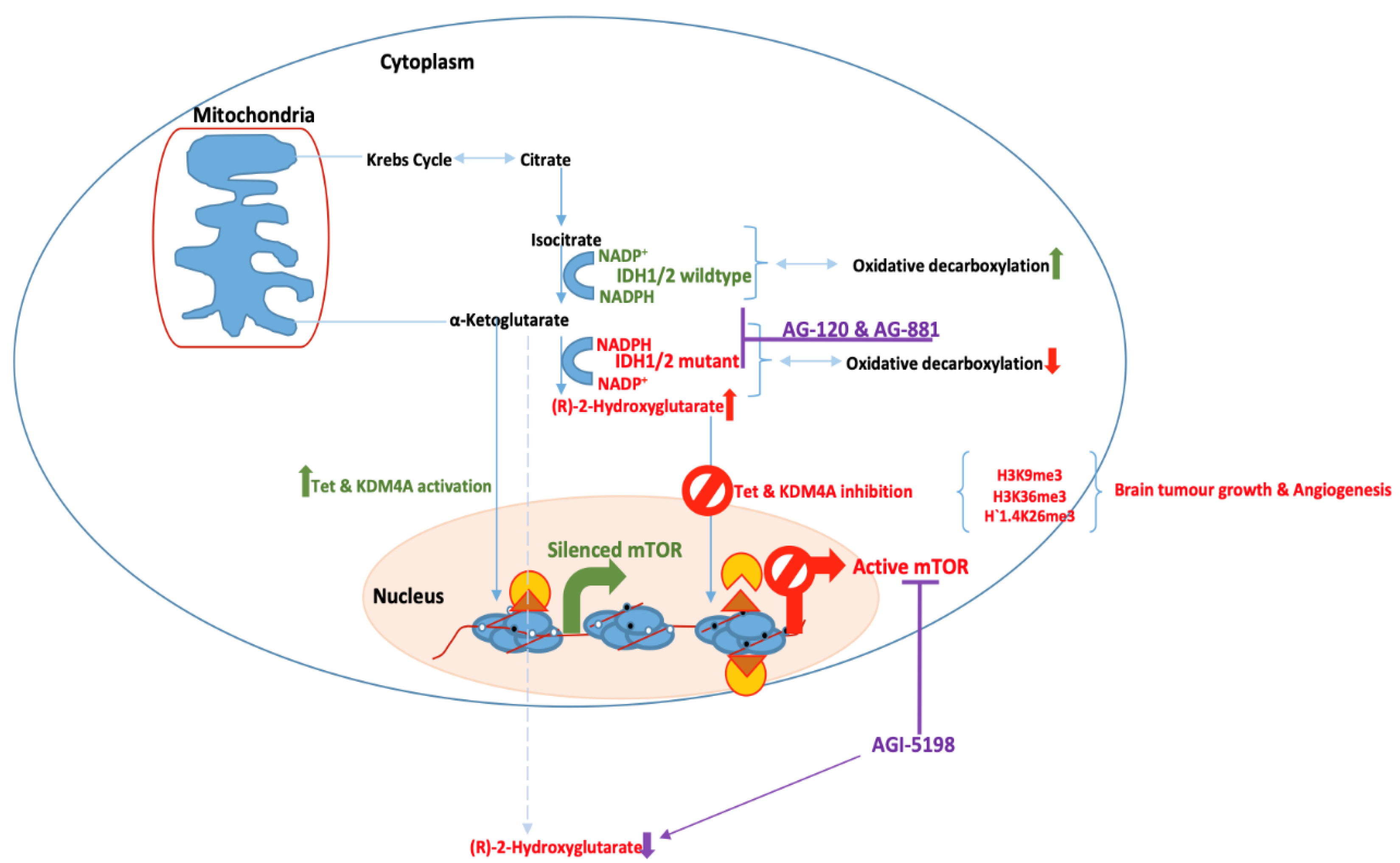
Figure 2.
Catalysis of α-ketoglutarate and (R)-2-hydroxyglutarate (2HG) by IDH wildtype and mutant in glioblastoma. The 2HG is an oncometabolite and leads to aberrant induction of epigenetic programming that is consistent with tumour growth, cell migration and angiogenesis in glioblastoma.
Figure 2.
Catalysis of α-ketoglutarate and (R)-2-hydroxyglutarate (2HG) by IDH wildtype and mutant in glioblastoma. The 2HG is an oncometabolite and leads to aberrant induction of epigenetic programming that is consistent with tumour growth, cell migration and angiogenesis in glioblastoma.
4. Vascular Endothelial Growth Factor (VEGF)
In the early stages of tumour development, tumour cells are in hypoxic conditions and this stabilizes HIF as mentioned earlier. HIF proteins trigger the synthesis and release of proangiogenic factors including VEGF.
In cancer cells, tumour hypoxic cells activate VEGF to sprout or split out their own capillary branches through angiogenesis. This allows specific intracellular molecules to recognise and bind on the VEGF receptor, propagating the signals from the cell surface into the cells.
VEGF signals stimulate the formation of new vasculature growth towards the tumour hypoxic cells which supplies them with oxygen and nutrients that allow them to grow and metastasize. Activation of certain growth factor receptors is implicated in tumour cell growth. A classic example of this is epidermal growth receptor factor (EGFR), and its dysregulation which is a phenotypic feature of many cancers.
EGFR binds its receptors in the same manner as VEGF and stimulates a cascade of downstream signalling pathways such as the mitogen activated protein kinase/Ras (MAPK/Ras), FAK/paxillin, phosphatidylinositol 3-kinase (P13K/Akt) and PLCγ.
These pathways regulate abnormal gene transcription, cell proliferation and survival, cytoskeleton rearrangement and vascular permeability leading to tumour growth and metastases [
28,
29].
It has been demonstrated that VEGF initiates its activities via VEGFR2 that cooperates with integrins expressed by glioma cell [
30].
Integrins are a family of cell adhesion receptors that upregulate VEGF and drive an invasive phenotype of tumor cells.
Integrins αvβ3 and αvβ3 are vital mediators of endothelial cell invasion and hallmark of angiogenesis that are robustly produced by gliomas [
31,
32]. They selectively recognise ECM protein and immunoglobulin superfamily molecules as ligands and activate downstream signaling pathways upon interaction.
This promotes intracellular signal transduction, cell migration, invasion, proliferation and survival in angiogenesis [
30,
31,
32].
Given that the aberrant regulation of VEGF and its receptors stimulate angiogenesis that correlates with tumour progression and metastasis, VEGF blockage has been explored as treatment strategy. This involves the use of monoclonal antibodies (e.g., bevacizumab, temozolomide, sorafenib and sunitinib), and VEGF Trap, and these offered better clinical outcome and significantly transformed the treatment landscape of glioblastoma [22–24,
33,62–64].
5. Morphological Imaging
Magnetic Resonance Imaging (MRI)
Non-invasive imaging with contrast-enhanced (CE) MRI is still the gold standard, however the modality has limitations which impact management and diagnosis of true recurrence vs. pseudorecurrence remains a diagnostic conundrum.
Additional challenges with MRI imaging are several and have been documented e.g., post-irradiation on follow-up imaging where it may appear as though the lesion has increased in size, pseudo-progression seen with temozolomide use and features that are similar to recurrent tumour i.e., morphological features of radionecrosis (RN). In brief, MRI is not perfect, there are several factors that need to be addressed:
Currently, there is no morphological criterion to separate radionecrosis and recurrent glioblastoma with certainty.
Advanced techniques in MRI sequences e.g., perfusion, spectroscopy and diffusion may assist to differentiate radiation injury from tumour recurrence but may be time consuming.
The most reliable MR method to separate radionecrosis from tumour necrosis is perfusion imaging. With perfusion imaging, rCBV and PSR are reliable in the tumour assessment [
7,
8,
9,
34].
6. Nuclear Medicine Imaging
Nuclear medicine modalities e.g., single-emission (SPECT) and positron emission tomography (PET) are known modalities used in oncology that are now well-established to characterize, stage, prognosticate and follow-up pathology even before morphological aberrations are detected.
It is noted increasingly that more clinicians utilize these modalities complementary to other imaging techniques in order to study and manage patients.
6.1. SPECT Techniques and Tracers
Single-photon emission tomography has long history in Nuclear Medicine and has several advantages i.e., availability, technically easier to operate however has the limitation of reduced resolution.
Over a decade ago, Jia et al. [
29], trialed 3
99mTc-radiotracers in an animal study (mice) i.e., (SQ168)(EDDA), (SQ168)(tricine)(PDA) and (SQ168)(tricine)(TPPTS) and found that all three tracers were able to localize the glioma tumour. Moreso, there was high tumour uptake and retention. Of all the 3 TPPTS demonstrated superior binding to the integrin
.
More recently in 2020, SPECT is entering the theranostics space with in vivo work by Zhao et al.30using scorpion venom Chlorotoxin (CTX) and CTX like peptide Buthus martensii.
Radiolabeled iodine using
123I and
131I was also trialed by multiple investigators however resolution and translation into further development was attenuated [
32,
33,
34].
Dual imaging with 201Tl and 99mTc-HMPAO were used by Schwartz and colleagues33 in a study involving 15 patients; although the results appeared promising, the image quality i.e., target to background (TBR) now appears much inferior and also the radiation dose would be much criticized because of the advent of PET and the ALARA (as low as reasonably allowed) would make this technique difficult to perform. Although this study was able to distinguish radionecrosis (RN), the preparation of 99mTc-HMPAO which is a perfusion tracer that is still used today, PET imaging would be more superior because of the resolution and the rapid advances in PET systems that could be used with lower doses and shortening of the scanning time which would make the workflow of a physician much better.
6.2. PET Techniques and Tracers
Medical imaging with positron imaging in gliomas entered the fray in less-than-ideal circumstances. Although it was already established that PET yielded far superior imaging quality and localization better than SPECT, the utilization of PET in low-grade-gliomas (LGG) was controversial.33,34
6.3. 11C-methinionine, 18F-FDG, 18F-FTD
As
18F-FDG (Fluorodeoxyglucose) fast because the work-horse in PET, because of the biodistribution of this tracer in the brain, distinguishing lesions was superior to SPECT however imaging with amino acids appeared to demonstrate superior accuracy [
35,
36,
37].
There are multiple systematic reviews concluded that 11C-methionine and Fluorotyrosine (FET) were far superior to FDG although the half-life (T1/2) of FDG which is 110 minutes was better. 11C-MET has a t1/2 of 20 minutes which requires meticulous injection and imaging time.
Katsanos et al. [
35] demonstrated in a meta-analysis that when
11C-FET, MET and
18F-FDG PET were compared, imaging with amino acids was superior.
Amino acids are not readily available from the manufacturing and it also appears that theranostics will not be feasible.
The advantage that MRI holds over PET is the ionizing radiation from the emission of 68Gallium radioisotope. An added advantage that 68Ga-RGD can have over other techniques is that the dose of the activity can be reduced and the newer PET scans can perform a whole-body scan in under 15 minutes i.e., time of flight (TOF). This essentially means that if it is only the head and neck (H&N) region that requires imaging, the amount of time saved and throughput could be significant.
Increasingly, there are novel imaging technologies such as PET-MRI with improved TOF; this could also be a complementary modality therefore derivation of molecular and anatomical data can increase the diagnostic confidence of the nuclear physician and radiologist. In addition, this has the potential of being utilized for biopsy purposes.
6.4. [68]68Ga-RGD i.e., Arginine-Glycine-Aspartatic Acid PET Imaging
Integrins and the angiogenesis pathway can be imaged with modalities such as 68Gallium-RGD PET/CT to putatively characterize, state and detect recurrent gliomas.
Integrins belong to a large family of transmembrane receptors and it was established several years ago that the signaling of the integrins drives the glioblastogenesis. Integrins are comprised of non-covalently linked α and β chains, which form heterodimeric receptor complexes.
The integrin pathway is currently singled out because it can be targeted and expressed on endothelial cells which are most prominent on new blood vessel formation i.e., neo-vascularization. Integrins composed of heterodimeric proteins; RGD i.e., Galacto-Arginine-Glycine-Aspartic acid) has been displayed as a potential biomarker that can be imaged via PET in order to trace the angiogenetic pathway.37
Focus on integrin imaging is essential because there is existing data that suggest that the down regulation of integrins with Cilengitide, an arginine-glycine-aspartic acid leads to reduced angiogenesis and cell death. Multiple chemotherapeutic agents such as bevacizumab have not demonstrated good results in the management of gliomas. Li et al. conducted a prospective study in which they concluded that
imaging with 68Ga-PRGD PET was possible in humans however the study had limitations i.e., Second, the study lacked a sufficient number of control patients with other types of brain tumors. An additional study is required to recruit a broad variety of patients with brain-occupying lesions to determine the sensitivity, specificity, and accuracy of
68Ga-PRGD2 PET/CT in assessing glioma [
29,
36,
37].
In contrast to 18Fluorine which is produced in a cyclotron, 68Gallium is eluted from a germanium generator (Ge/Ga generator); the t1/2 of gallium is 68 minutes and has a biodistribution that is suitable for brain imaging. The generator can be kept in the Nuclear Medicine facility thus avoid supply or logistical delays.
7. Conclusions
Gliomas are a heterogeneous group of central nervous system tumours that require an appropriate understanding from a molecular perspective in order to provide guidance in its management. This understanding of the molecular markers and genetic aberrations and pathways will hopefully lead to the development of improved imaging tools and therapies that will impact overall survival positively in afflicted patients.
The molecular characterization with PET has an advantage over conventional methods pre and post-operatively or in the neo-adjuvant setting for treatment selection.
This sets the prelude for more focussed prospective studies using molecular and genetic markers in imaging for the evaluation of adult gliomas in all stages and also assessing whether PET and MRI can be used in a complimentary manner in order to evaluate, follow-up gliomas in an improved manner. Other hybrid modalities e.g., PET/MR though in the early stages of research should theoretically provide better information that will characterize gliomas better.
Author Contributions
All authors contributed equally. MMS, MV and LP were the main supervisors.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study i.e., “Not applicable” as it was a review article.
Informed Consent Statement
Not applicable for the paper.
Acknowledgments
The authors would like to acknowledge the great contribution of Siemens, the University of Pretoria, the Nuclear Medicine, Radiology, Anatomical Pathology and Neurosurgery Staff at Steve Biko Academic Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Laug, Dylan, Stacey M. Glasgow, and Benjamin Deneen. “A glial blueprint for gliomagenesis.” Nature Reviews Neuroscience 19, no. 7 (2018): 393-403. [CrossRef]
- Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424 (2018). [CrossRef] [PubMed]
- Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and Molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019 Jul; 16(7): 405 -17. [CrossRef]
- Jäkel S, Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front Cell Neurosci. 2017:11:24. Published 2017 Feb 13. [CrossRef]
- Weller M, van den Bent M, Preusser M, Rhun EL, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. (2020). March 2021, 18. . www.nature.com/nrclinoc [accessed April 2021].
- Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. Br J Cancer. 2016 Dec 6;115(12):1445-1450. Epub 2016 Nov 10. [CrossRef] [PubMed] [PubMed Central]
- Zong, Hui, Roel GW Verhaak, and Peter Canoll. “The cellular origin for malignant glioma and prospects for clinical advancements.” Expert review of molecular diagnostics 12, no. 4 (2012): 383-394. [CrossRef]
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. [CrossRef]
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J. Neuroimmunol. (1990) 30, 81 -93.
- Chishty IA, Rafique MZ, Hussain M, Akhtar W, Ahmed MN, Sajjad Z, et al. MRI characterization and histopathological correlation of primary intra-axial brain glioma. `j Liaquat Uni Med Health Sci. 2010;9(02):64-9.
- Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood [published correction appears in Nat Rev Clin Oncol. 2022 May;19(5):357-358]. Nat Rev Clin Oncol. 2021;18(3):170-186. [CrossRef]
- Scodeller P, Simón-Gracia L, Kopanchuk S, et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci Rep. 2017;7(1):14655. Published 2017 Nov 7. [CrossRef]
- Gusyatiner, O., & Hegi, M. E. (2018, August). Glioma epigenetics: from subclassification to novel treatment options. In Seminars in cancer biology (Vol. 51, pp. 50-58). Academic Press. [CrossRef]
- Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018 Aug 4,392(10145):432-46. Epub 2018 Jul 27. [CrossRef] [PubMed]
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. [CrossRef]
- Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front Oncol. 2019;9:506. Published 2019 Jun 12. [CrossRef]
- Huang LE. Friend or foe-IDH1 mutations in glioma 10 years on. Carcinogenesis. 2019;40(11):1299-1307. [CrossRef]
- Raimbault A, Cazals X, Lauvin MA, Destrieux C, Chapet S, Cottier JP. Radionecrosis of malignant glioma and cerebral metastasis: a diagnostic challenge in MRI. Diagnostic and Interventional imaging. 2014 Oct 1;95(10):985-1000. [CrossRef]
- Zhang, Guilong, Chen, Lukui, Khan, Ahsan, et al. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int. j. cancer. 2018; [CrossRef]
- Mosrati MA, Malmström A, Lysiak M, et al. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6(18):16663-16673. [CrossRef]
- Evans IM, Yamaji M, Britton G, et al. Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol Cell Biol. 2011;31(6):1174-1185. [CrossRef]
- Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177-185. [CrossRef]
- Masi, A., Becchetti, A., Restano-Cassulini, R. et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer 93, 781–792 (2005). [CrossRef]
- Bota, Daniela A., et al. “Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis.” Journal of neurosurgery119.6 (2013): 1415-1423.
- Erasmus JJ, Homer A, Macapinlac. Low-Sensitivity FDG-PET Studies: Less Common Neoplasms. Seminars in Nuclear Medicine. July 2012: 255 – 60.
- Bolcaen J, Nair S, Driver C, Boshomane TMG et al. Novel Tyrosine Pathway Inhibitors for targeted Radionuclide Therapy for Glioblastoma. Pharmaceuticals, 2021, 14, 626. [CrossRef]
- Ferris SP, Hofmann JW, Solomon DA, Perry A. Characterization of gliomas: from morphology to molecules. Virchows Arch. 2017 Aug;417(2):257 – 69. [CrossRef]
- Perry A, Wesseling P. Histologic classification of Gliomas. Handb Clin Neurol. 2016;134:71-95.
- Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconjugate chemistry. 2006 Jul 19;17(4):1069-76.
- Zhao L, Zhu J, Wang T, Liu C, Song N, Wu S, Qiao W, Yang J, Zhu M, Zhao J. A novel Buthus martensii Karsch chlorotoxin derivative for glioma SPECT imaging. New Journal of Chemistry. 2020;44(35):14947-52. [CrossRef]
- Cheng Y, Zhu J, Zhao L, Xiong Z, Tang Y, Liu C, Guo L, Qiao W, Shi X, Zhao J. 131I-labeled multifunctional dendrimers modified with BmK CT for targeted SPECT imaging and radiotherapy of gliomas. Nanomedicine. 2016 May;11(10):1253-66. [CrossRef]
- Kuwert T, Woesler B, Morgenroth C, Lerch H, Schäfers M, Palkovic S, Matheja P, Brandau W, Wassmann H, Schober O. Diagnosis of recurrent glioma with SPECT and iodine-123-α-methyl tyrosine. Journal of Nuclear Medicine. 1998 Jan 1;39(1):23-7.
- Schwartz RB, Carvalho PA, Alexander ED, Loeffler JS, Folkerth R, Holman BL. Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. American journal of neuroradiology. 1991 Nov 1;12(6):1187-92.
- Minn H. PET and SPECT in low-grade glioma. European journal of radiology. 2005 Nov 1;56(2):171-8. [CrossRef]
- Katsanos AH, Alexiou GA, Fotopoulos AD, Jabbour P, Kyritsis AP, Sioka C. Performance of 18F-FDG, 11C-methionine, and 18F-FET PET for glioma grading: a meta-analysis. Clinical nuclear medicine. 2019 Nov 1;44(11):864-9. [CrossRef]
- Li D, Zhao X, Zhang L, Li F et al. 68Ga-PRGD2 PET/CT in the Evaluation of Glioma: A Prospective Study. Mol Pharm. 2014 Nov 3; 11(11): 3923-29. [CrossRef]
- Chen H, Niu G, Wu H, Chen X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics. 2016;6(1):78-92. [CrossRef]
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastomas. Nat Med. 2019;25(3): 477- 86. [CrossRef]
- Woernle CM, Péus D, Hofer S, Rushing EJ, et al. Efficacy of Surgery and Further Treatment of Progressive Glioblastoma. World Neurosurgery. 2015; 84(2):301-7. [CrossRef]
- Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Annals of Oncology. 2017; 28(6): 1457-72. [CrossRef]
- Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumours of the central nervous system: a summary. Acta Neuropathol 2016; 131:803 – 20. [CrossRef]
- Prados MD, Byron SA, Tran NL et al. Toward Precision medicine in glioblastoma: the promise and the challenges. Neuro Oncol 2015; 17:1051 – 63. [CrossRef]
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000; 60: 1383-87.
- David J. Pisapia. The Updated World Health Organization Glioma Classification: Cellular and Molecular Origins of Adult Infiltrating Gliomas. Archives of Pathology & Laboratory Medicine 2017, Vol.141,No. 12, 1633 – 45. [CrossRef]
- Weigel, Maya, Wang, Lin, Fu, Meng-meng. Microtubule organization and dynamics in oligodendrocytes, astrocytes, and microglia. Developmental Neurobiology. 2020. [CrossRef]
- Nguyen, Thanh, Melkus, Gerd, Taccone, Michael, et al. Preoperative Determination of Isocitrate Dehydrogenase Mutation in Gliomas Using Spectral Editing MRS: A Prospective Study. Journal of Magnetic Resonance Imaging. 2020; [CrossRef]
- Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer cell 17, 510–522 (2010). [CrossRef]
- Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–483. (2012). [CrossRef]
- Horbinski, C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol 125, 621–636. (2013). [CrossRef]
- Bello L, Francolini M, Marthyn P, et al. α(v)β3 and α(v)β5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–9. discussion 90.
- Abdollahi A, Griggs DW, Zieher H, et al. Inhibition of α(v)β3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11 (17):6270–9. [CrossRef]
- Hegi ME, Diserens AC, Gorlia T, et al.: MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352(10):997-1003. [CrossRef]
- Weller M, Stupp R, Reifenberger G, et al.: MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 2010;6:39-51. [CrossRef]
- Wick W, Platten M, Meisner C, et al.: Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707-715. [CrossRef]
- 4. Malmstrom A, Gronberg BH, Marosi C, et al.: Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916-926. [CrossRef]
- Mansouri A, Hachem LD, Mansouri S, et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol; Published 5 September 2018. [CrossRef]
- Szenker E., Ray-Gallet D., Almouzni G. The double face of the histone variant H3.3. Cell Res. 2011;21:421–434. [CrossRef]
- Jones C., Baker S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer. 2014;14:651–661. [CrossRef]
- Bjerke L., Mackay A., Nandhabalan M., Burford A., Jury A., Popov S., Bax D.A., Carvalho D., Taylor K.R., Vinci M., et al. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov. 2013;3:512–519. [CrossRef]
- 44. Bender S., Tang Y., Lindroth A.M., Hovestadt V., Jones D.T.W., Kool M., Zapatka M., Northcott P.A., Sturm D., Wang W., et al. Reduced H3K27me3 and DNA Hypomethylation Are Major Drivers of Gene Expression in K27M Mutant Pediatric High-Grade Gliomas. Cancer Cell. 2013;24:660–672. [CrossRef]
- 45. Hashizume R., Andor N., Ihara Y., Lerner R., Gan H., Chen X., Fang D., Huang X., Tom M.W., Ngo V., et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 2014;20:1394–1396. [CrossRef]
- Lee S, Chen T, Barber C, Jordan M, Murdock J, Desai S, Ferrara N, Nagy A, Roos K and Iruela-Arispe M (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007 August 24; 130(4): 691–703.
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003 Dec;31(Pt 6):1171-7. [CrossRef]
- Ji Y, Lu X, Zhong Q, Liu P, An Y, Zhang Y, Zhang S, Jia R, Tesfamariam IG, Kahsay AG, Zhang L, Zhu W, Zheng Y (2013). Transcriptional profiling of mouse uterus at pre-implantation stage under VEGF repression. PLoS One. 8(2):e57287. [CrossRef]
- Szekanecz Z, Besenyei T, Paragh G, Koch AE (2009) Angiogenesis in rheumatoid arthritis. Autoimmunity. 42(7):563-73.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).