1. Introduction
The Cardiac resynchronization therapy (CRT) is a fundamental treatment for patients with heart failure and ventricular conduction abnormalities [
1]. Patients suitable for CRT typically exhibit cardiomyopathy along with an electrical substrate. This electrical substrate refers to a delayed electrical activation within the left ventricular (LV) lateral wall [
2], leading to disorganized ventricular contractions and diminished systolic function, thereby contributing to the progression of heart failure. Previous studies have shown that delayed activation of the LV lateral wall is more frequently observed in patients with a widened QRS complex and left bundle branch block (LBBB) compared to those without LBBB [
3]. This explains why patients with heart failure and LBBB have better outcomes following CRT and experience a considerable improvement in their quality of life [
4].
Various electrocardiographic [
5] and clinical parameters [
6] are thought to predict thequality-of-life improvement in patients undergoing such therapies. The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a reliable indicator used to quantify the quality of life in patients who have benefited from cardiac resynchronization [
7,
8]. It is widely regarded as the most comprehensive and extensively studied clinical tool for predicting overall well-being and clinical outcomes in real-world settings [
1]. Research suggests that the KCCQ may be more precise in assessing outcomes for patients with cardiomyopathy and reduced ejection fraction than the New York Heart Association (NYHA) functional class [
9]. A study conducted by Stephen Greene et al deems NYHA class poorly reproducible, as it evaluates a patient’s heart failure severity based on perceived difficulty in accomplishing daily tasks, which can vary widely among physicians. The cohort study, comprising 2872 patients evaluated over 12 months, shows that NYHA classification’s prognostic value is also less relevant compared to the KCCQ. An improvement of 5 or more points on KCCQ was independently associated with decreased mortality, whereas improvement in the NYHA class was not [
9].
However, there are some limitations: KCCQ relies on self-reported data from patients, which can introduce subjectivity and potential biases, as patients may underreport or overreport their symptoms and functional limitations based on personal perceptions or psychological factors. Patients with low literacy levels or cognitive impairments may struggle to understand and accurately respond to the questionnaire items. Additionally, the full KCCQ can be somewhat lengthy, which may be a burden for some patients, especially those with severe symptoms or fatigue. While the KCCQ is designed to be sensitive to changes in a patient’s condition over time, it may not be sensitive enough to detect very small but clinically important changes in health status or quality of life. Furthermore, by focusing on physical symptoms, social functioning, and quality of life related to heart failure, it may not capture other important aspects of a patient’s overall health.
According to M.B.Kronborg and J.C.Nielsen, about 30-50% of patients receiving CRT do not benefit from the treatment and thus are considered non-responders. Potential candidates for the procedure often have comorbidities such as ischemic heart disease, renal dysfunction, or diabetes. Despite its high success rate, CRT is riskier to establish compared to other implantable electronic devices. Their study shows that KCCQ might be very useful in identifying non-responders, who would benefit greatly from a more intensive post-intervention follow-up [
2].
In terms of factors influencing patient non-response, these can be categorized as pre-, peri- and post-operatory. Pre-implant factors include appropriate patient selection, baseline ECG morphology and QRS duration (patients with LBBB typically have better outcomes than those with RBBB), gender (women tend to respond better), etiology of heart failure, presence of myocardial scar, and disease severity. Peri-operatory factors encompass appropriate LV lead technology and optimization of the LV stimulation site. Post-operatory factors involve remote monitoring, frequency of biventricular pacing, and device programming [
6].
Further research is needed to identify potential prognostic markers and factors contributing to patient non-response, as well as to establish correlations between these markers and the KCCQ. The endpoint of the present study focused on assessing improvements in patient's hemodynamic status and well-being, quantified by the total score on the KCCQ [
8]. Our objective was to identify correlations between patient outcomes post-procedure and demographic, clinical, and electrocardiographic parameters, including systolic blood pressure, ECG morphology elements, QRS duration, QRS area, and associated comorbidities.
While QRS duration has been extensively studied as a predictor after CRT, other factors, such as QRS morphology elements [
10], QRS area [
11], and intraprocedural increase in blood pressure [
12] are emerging areas of research. The CRT algorithm categorizes each left ventricular pace as effective based on the detection of QS or QS-r morphology on the ECG; however, the relationship between percent effective CRT pacing and patient responses remains unclear [
10]. Concerning the QRS area, reduction following CRT has been linked to improved survival, yet current guidelines do not provide individual prognosis predictions, prompting further study in this direction [
11]. Systolic blood pressure (SBP) may also be an important predictor of favorable CRT outcomes: some studies suggest that a rapid SBP increase immediately after CRT is more prevalent in responders compared to non-responders [
12].
An interesting aspect we explored was whether a decrease in QRS area and duration following CRT could potentially signify an improvement in the electrical substrate and the long-term response of the patients, as well as verifying the possibility of a correlation with an increase in systolic blood pressure. This would help shorten hospitalization time and improve the efficiency of post-operatory follow-up that patients with CRT receive [
13]. Conversely, these correlations could be used as indicators for unfavorable outcomes, indicating patients that require monitoring and other treatment strategies.
2. Materials and Methods
2.1. Ethical Approval
The research was conducted in the department of Cardiology, Prof. Dr. Agrippa Ionescu Emergency Clinical Hospital in Balotesti, Ilfov, Romania, from 2017 to 2022, as a unicenter observational prospective study. Approval for the study protocol was obtained from the Ethics Committee of the Carol Davila University of Medicine and Pharmacy, Bucharest, Romania (15037/05.06.2017). Planning, data collection, and reporting involving human subjects were conducted following the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants in the study, following General Data Protection Regulation (GDPR) requirements. All participants in the study provided written informed consent in accordance with General Data Protection Regulation (GDPR) requirements.
2.2. Study Design
This prospective observational study included 69 individuals diagnosed with cardiac failure and dilatative cardiomyopathy with low ejection fraction (EF) and major LBBB who underwent CRT-device implantation at the Agrippa Ionescu Hospital in Balotesti, Ilfov, Romania between 2017 and 2022. Baseline data were collected from the hospital’s patient information system and details regarding heart failure etiology, comorbidities, and medication were gathered from patients' histories and other medical records. The KCCQ was evaluated by conducting anamnesis on the patients.
Patients were considered eligible for the implantation of a CRT device based on specific criteria such as symptomatic heart failure with LBBB QRS morphology, a baseline QRS duration over 130 milliseconds, and an EF below 35% despite optimal medical treatment [
14]. Heart failure etiology was categorized as ischemic if evidence of myocardial infarction, coronary artery disease, or coronary artery bypass grafting (CABG) was documented in the medical records.
The exclusion criteria were as follows: individuals with unipolar or bipolar pacemakers or those eligible for device replacement; patients with psychiatric disorders impeding their attendance at follow-up appointments; those unable to demonstrate satisfactory adherence to medical therapy; and individuals with recent infections or those deemed to be at a significantly elevated risk for device-related infections.
For the study we had both a baseline 12-lead ECG and a paced 12-lead ECG available post-implantation and at follow-up intervals of 6 months, 9 months, and 12 months after implantation. Patient selection, device implantation, and follow-up procedures were performed according to the local protocols at the time of enrollment. The analyzed outcome was the evolution of the total score on the KCCQ questionnaire, determined pre-procedure, and at three post-procedure time points: 6, 9, and 12 months.
2.3. KCCQ-The Main Outcome Variable
The KCCQ consists of 23 questions that evaluate various aspects, including physical and social function, symptoms, knowledge, self-efficacy, and overall quality of life. Scores on the KCCQ range from 0 to 100 points, with higher values indicating better health status [
15]. Changes in KCCQ scores span from a 5-point gain, indicating a small benefit, to a 10-point gain, signifying a moderate benefit, and up to a 22-point gain, indicating a large improvement. These changes reflect the indirect effects of therapy. Conversely, a decrease of 5, 17, or 25 points represents a small, moderate, or large deterioration in quality of life, respectively [
16].
There is a need to create a system to standardize the results of this test and implement a threshold to differentiate the clinically significant changes. Some studies have considered a 5-point change to be small, but clinically important, while a 10 to 20-point variation would be a large change [
16]. Kosiborod et al. stated that 5 points are usually significant, a 5-point increase correlating with a 10% reduction of cardiovascular risk, while a 5-point decrease of the KCCQ score would increase the risk by 10% [
17].
It is also important to determine which score value should be taken into account. A study conducted at the University of Missouri [
18] demonstrated that, among the previous value, the current value, and the difference between them, the current value holds the highest prognostic significance.
2.4. Electrocardiographic Data
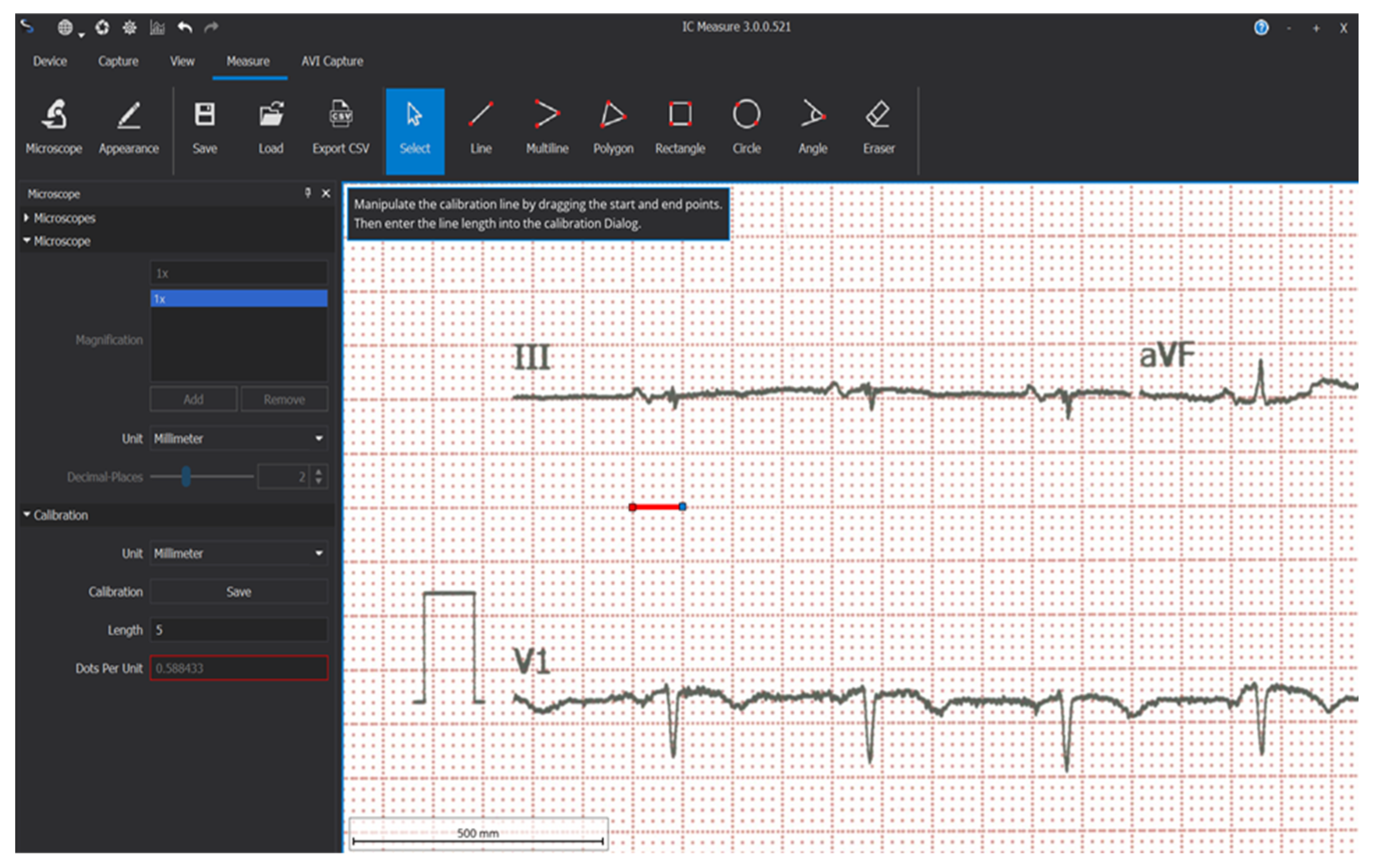
Study time points for ECG analysis were at the 6, 9, and 12 follow-up visits. Electrocardiographic data were acquired by scanning the baseline ECG paper and the ECGs recorded after CRT device implantation at every follow-up using a multifunctional scanner. The freeware software IC-Measure (22) was used for measuring ECGs. Calibration was performed for each ECG using the software’s microscope and caliper settings, with the calipers placed on the large squares of the ECG and set to a value of 5 mm (
Figure 1).
The next step involved accurately measuring the QRS complex in each of the 12 ECG leads provided by the ECGs. All measurements were conducted by well-trained physicians. Since each millimeter on the horizontal axis corresponds to 40 milliseconds (0.04 seconds) and each millimeter on the vertical axis corresponds to 0.1 millivolts, the QRS complex measurements were converted to millivolt-seconds.
2.5. Monitoring the Intraprocedural Systolic Blood Pressure
During each cardiac resynchronization procedure, arterial blood was continuously monitored using a 4 or 5 Fr radial or femoral-placed catheter to assess any changes following the initiation of biventricular pacing. This monitoring was prompted by the expectation that correcting myocardial asynchrony, previously confirmed via cardiac magnetic resonance, would improve cardiac output. While some authors indicated a 5mmHg threshold in their studies13, we included all systolic blood pressure values from our database to correlate them with changes in additional parameters such as QRS duration and QRS area.
2.6. Statistical Analysis
The measurements were recorded in our database datasheet and further analysis was performed using R software, version 4.3.3, Copyright (C) 2024 The R Foundation for Statistical Computing, R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [
19]. A repeated measures ANOVA test was employed to assess the statistical significance of differences, followed by post-hoc paired Bonferroni t-tests.
Additionally, a linear mixed effects model was utilized to examine the correlation between the KCCQ score and intraprocedural systolic blood pressure, R wave in V1, qs wave in aVL, QRS area, QRS duration, and biventricular pacing percentage. In this model, the KCCQ total score values served as the dependent variable, while demographic, clinical, and electrocardiographic parameters were treated as fixed effects. The model also incorporated random effects, specifically unique intercepts for each patient in the study.
The significance level (α) was set at 0.05, with p-values below 0.05 indicating statistical significance.
3. Results
3.1. The Comparative Mean KCCQ Values Over Time
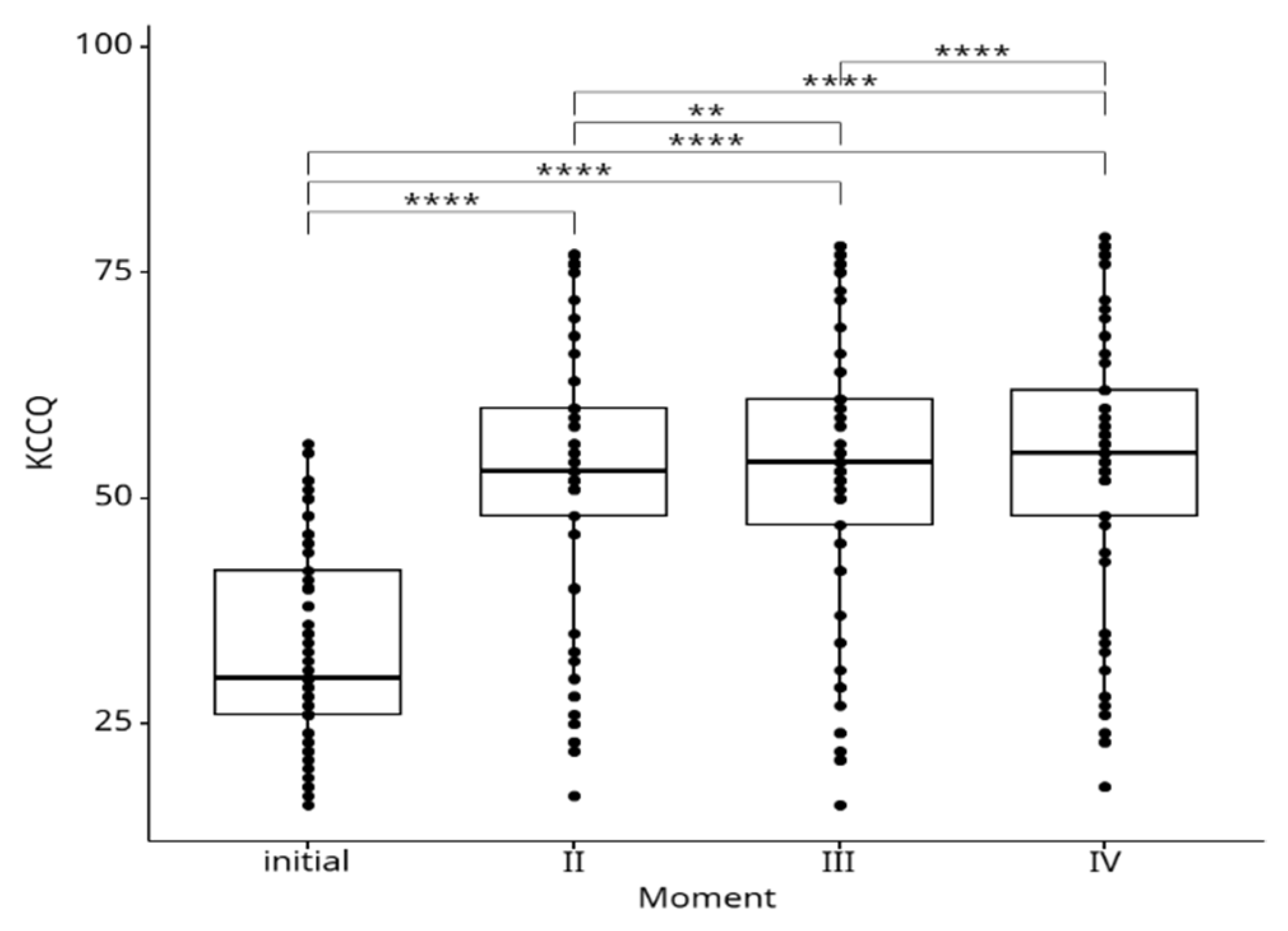
The mean KCCQ values over time are depicted in the
Figure 2. We observed a statistically significant increase of KCCQ scores during the follow-up period at all time points relative to baseline: at 6 months (33.7±11 vs. 52.9±15.7, p˂0.0001), 9 months (33.7±11 vs. 53.4±16, p<0.0001), and 12 months (33.7±11 vs. 54,5±16, p<0.0001), respectively.
3.2. Correlations between various Predictors and KCCQ Score at 12 Months
Table 1 reveals the main predictors considered in the study and their impact on KCCQ score at the 12-month follow-up. N represents the number of measurements (the number of patients/observations is N/4).
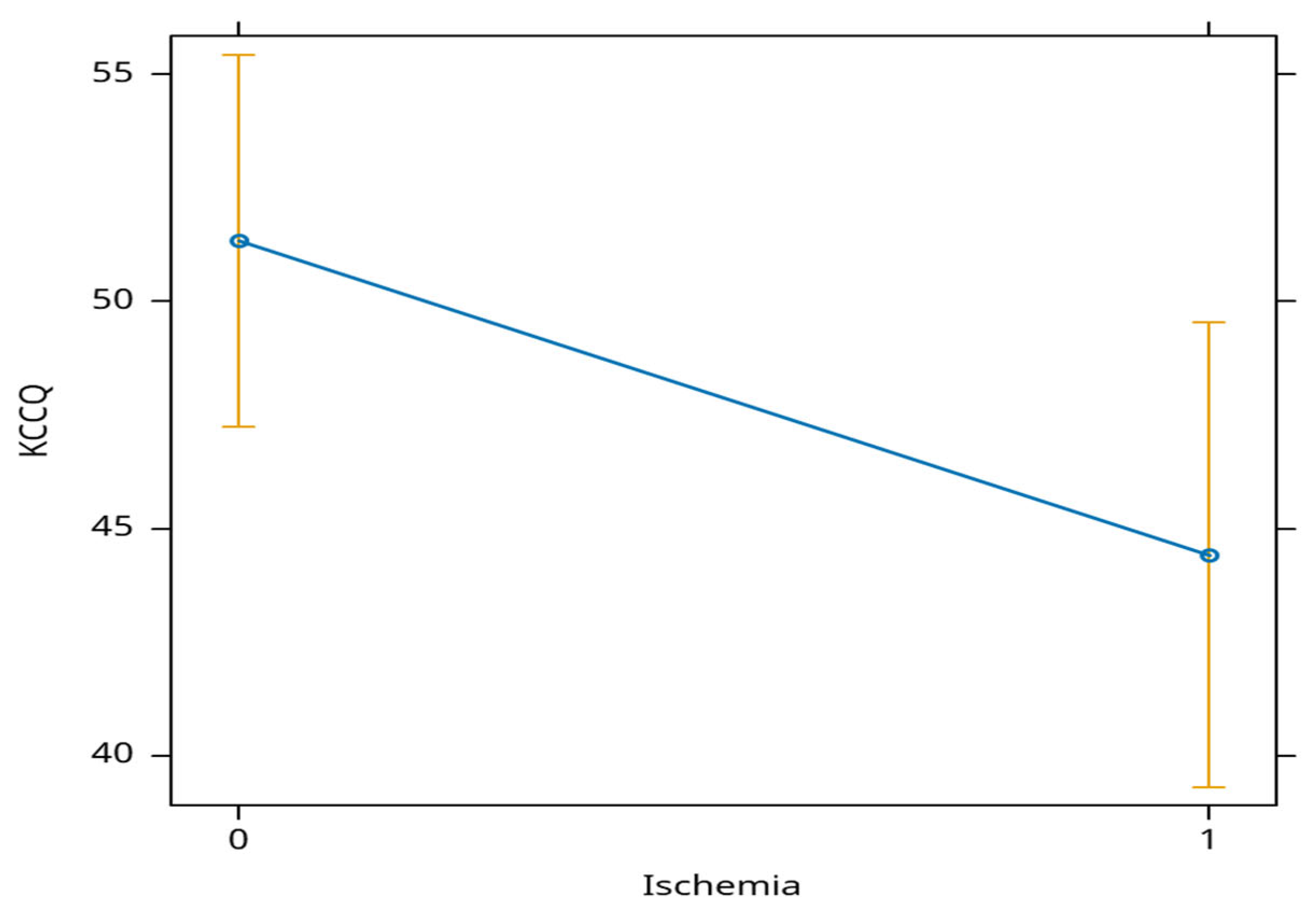
Patients with ischemia had an average decrease in KCCQ score of almost 7 points at 12 months (
Figure 3).
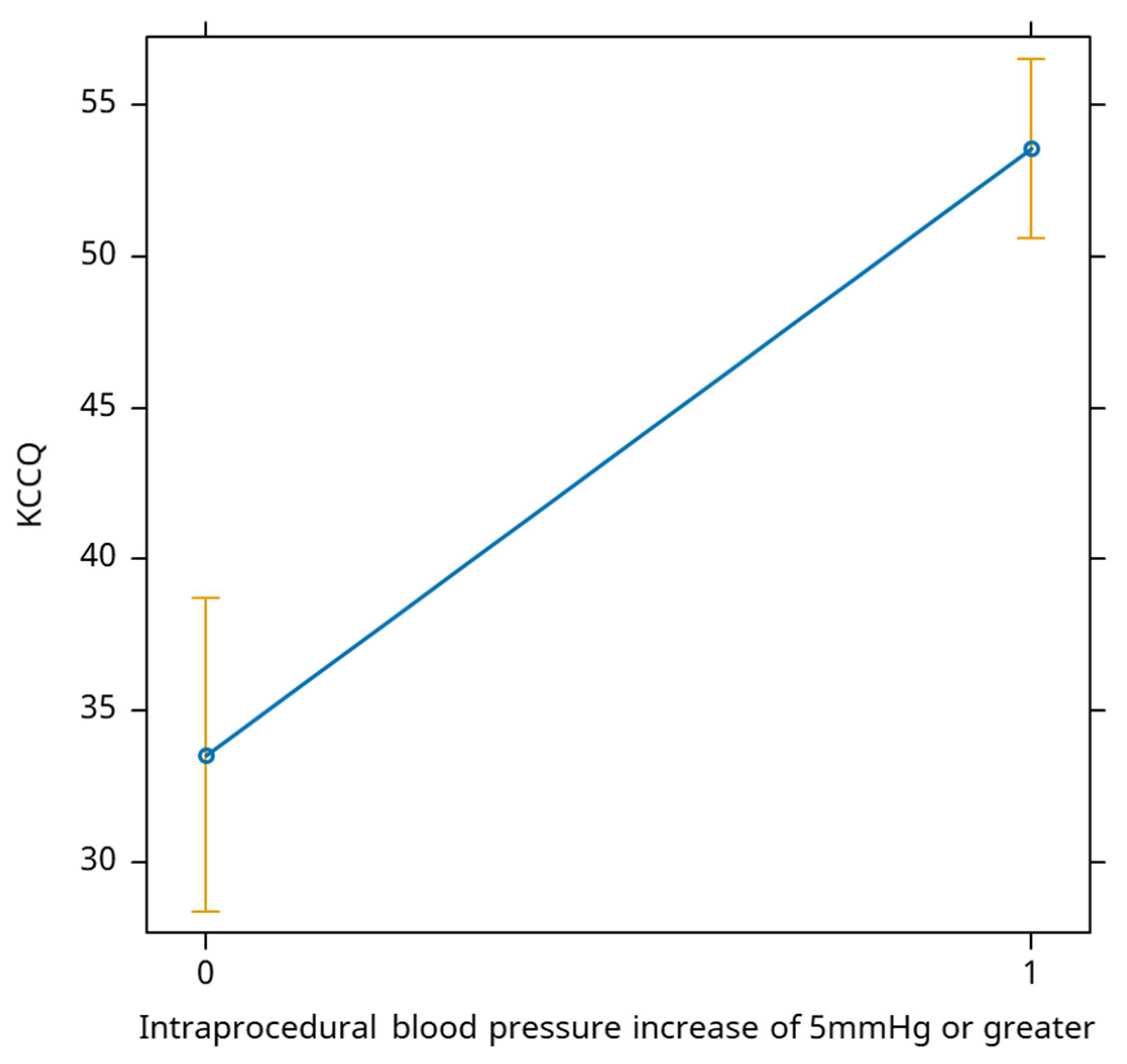
An increase of more than 5 mm in the monitored intra-procedural blood pressure is positively associated with the evolution of the KCCQ score, leading to an average increase of 20 points in the score (
Figure 4).
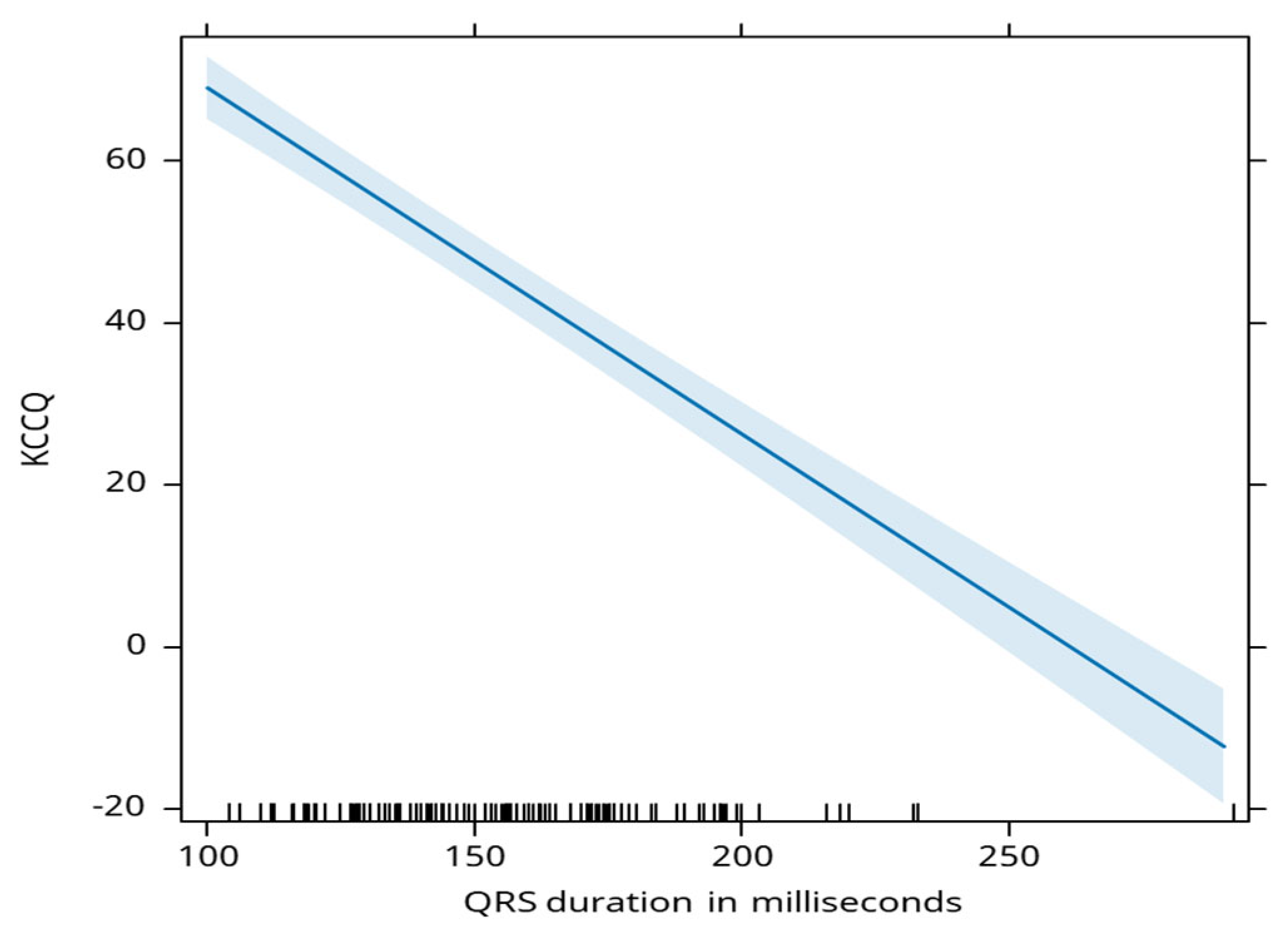
QRS duration is negatively associated with the KCCQ score, where a 1 millisecond decrease in duration is linked to an average increase in KCCQ score by 0.43 points (
Figure 5).
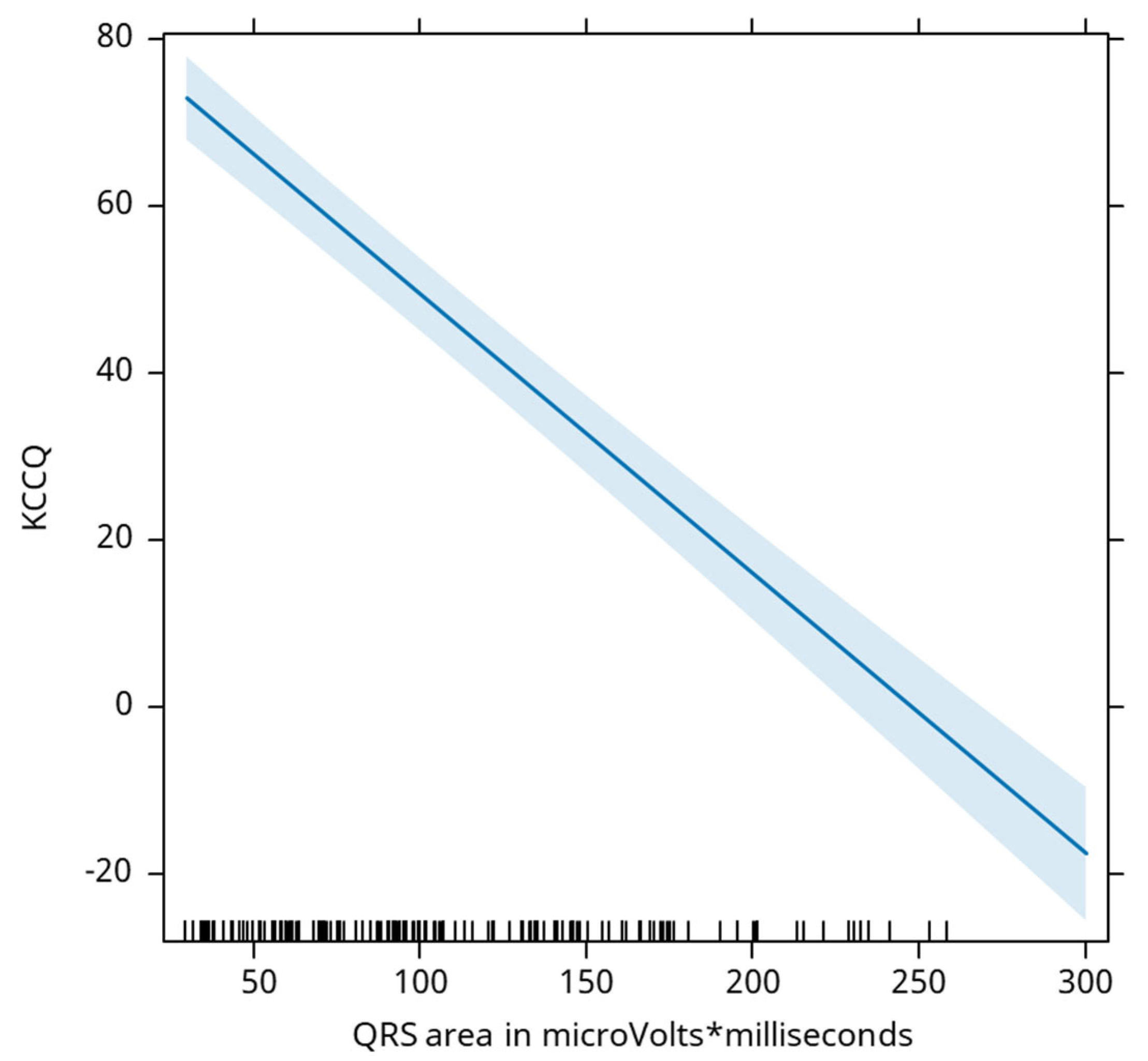
QRS area is negatively associated with the KCCQ score, where a decrease of 1 mm2 is associated with an average increase in the KCCQ score by 0.33 points (
Figure 6).
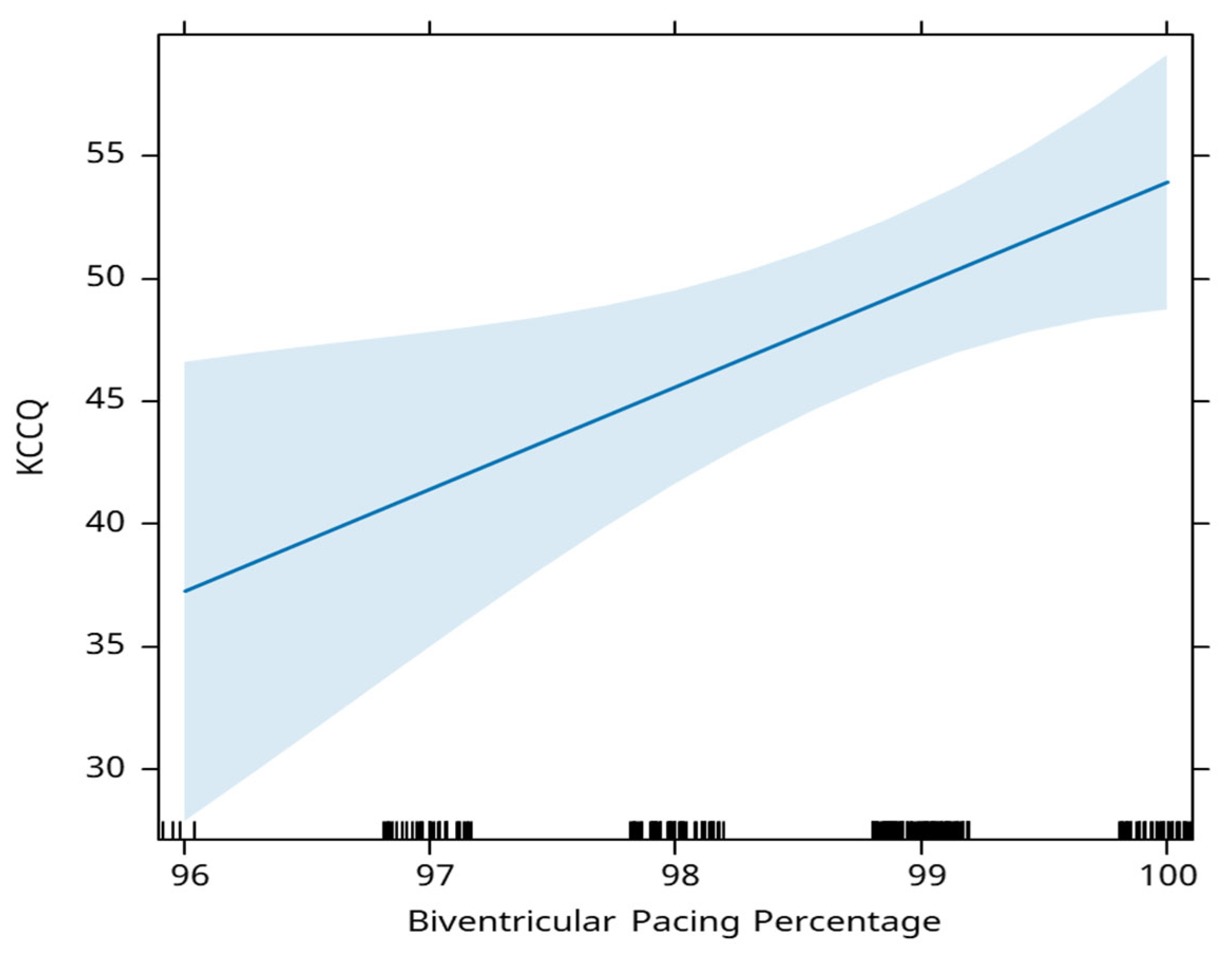
The percentage of biventricular pacing is positively associated with the KCCQ score. A 1% increase in this percentage, is associated with an average increase in the KCCQ score of 4.2 points (
Figure 7).
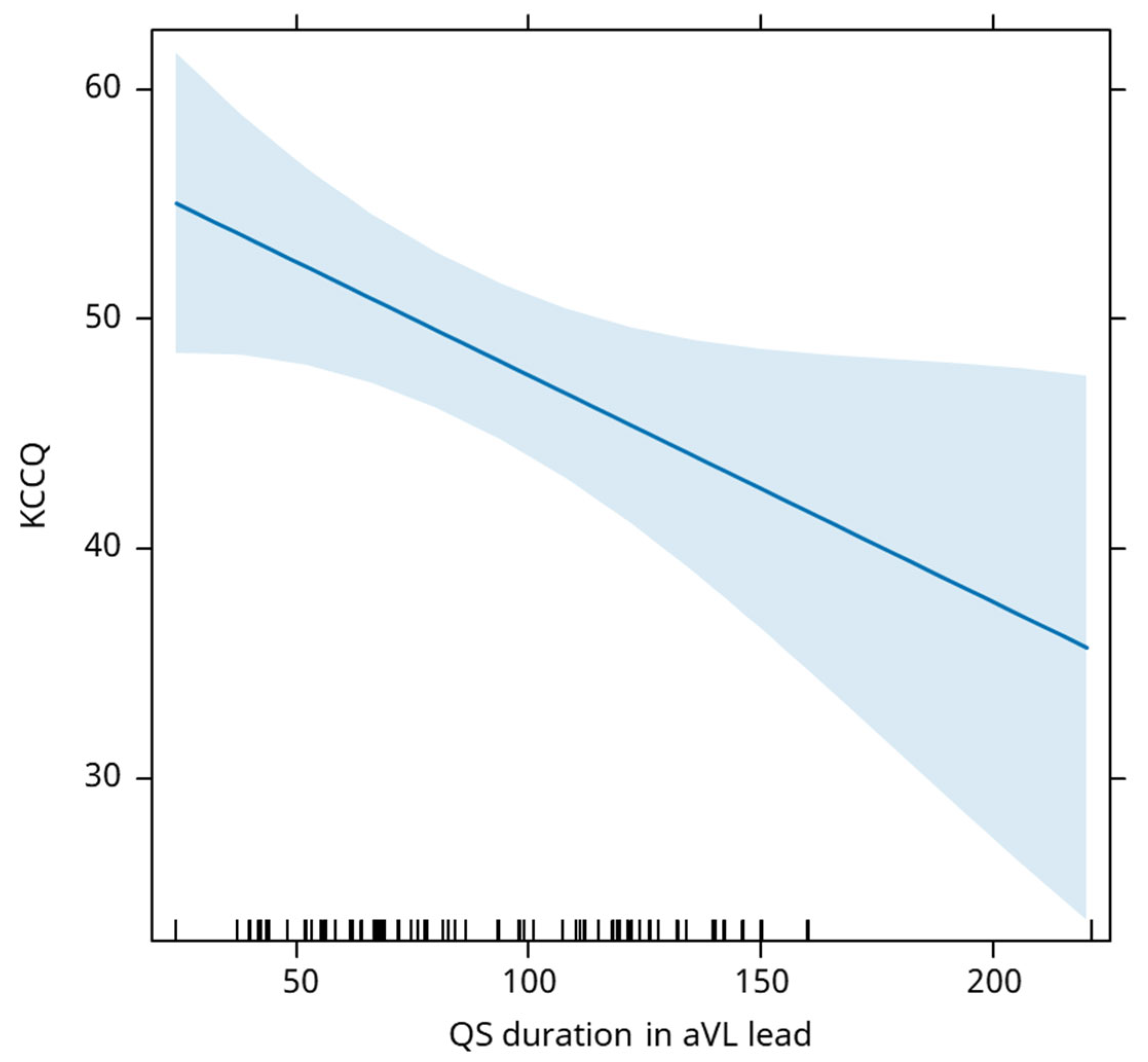
QS duration in aVL lead is negatively associated with the evolution of the KCCQ score, an increase of 1ms being associated with an average decrease of the KCCQ score by 0.10 (
Figure 8).
4. Discussion
In this study, the KCCQ scale was used to assess the effectiveness of therapy in specific patient groups. By evaluating symptom severity, physical limitations, and social impact, the KCCQ proves to be a robust framework for evaluating health status outcomes and serves as a valuable indicator of improvements in quality of life. This score incorporates patient’s perceptions of how symptoms affect their daily activities, offering clinicians insights into their clinical status. Moreover, KCCQ score reflects the dynamic evolution of the health status over time, helping to monitor patients and identify changes that may require clinical action. Considering these aspects, the KCCQ score is an essential tool for ongoing monitoring and clinical management, particularly in the context of multidisciplinary healthcare teams and the expanding reach of telemedicine networks [
20].
Cardiac ischemia significantly impedes the outcomes of cardiac resynchronization therapy. Ischemic heart disease often results in myocardial scarring and reduced viability of heart tissue, which diminishes the effectiveness of electrical stimulation required for resynchronization and increase the perioperative mortality, especially after major abdominal surgery [
21]. Patients with ischemic cardiomyopathy typically exhibit lower response rates to CRT because scar tissue hinders the effective conduction of electrical impulses, leading to suboptimal clinical outcomes [
22]. Additionally, ischemia increases the risk of arrhythmias, which can disrupt the synchronization of ventricular contractions. The location and extent of myocardial infarction also play crucial roles; if the left ventricular lead is positioned within or near scarred tissue, therapy efficacy is compromised. Furthermore, patients with ischemic heart disease often present with multiple comorbidities such as diabetes and hypertension, which further diminish CRT's effectiveness. Persistent myocardial stress and impaired cardiac function due to ischemia can blunt the anticipated hemodynamic benefits of CRT, reducing improvements in cardiac output and symptom relief. Our observations indicate that despite CRT implementation, patients with cardiac ischemia commonly experience symptoms like dyspnea, fatigue, and palpitations, which significantly impact their quality of life. Conversely, coronary revascularization procedures such as percutaneous coronary intervention or coronary artery bypass grafting may enhance CRT outcomes by potentially improving myocardial perfusion and viability. Therefore, proper patient selection, precise lead placement, and comprehensive management of ischemic heart disease, including optimized medical therapy, are essential to maximize CRT benefits in patients with ischemic cardiomyopathy [
23].
Biventricular pacing optimizes the synchronization of the heart's ventricles and enhances cardiac efficiency, thereby reducing the frequency of heart failure exacerbations and subsequent hospitalizations. The improved ventricular synchrony achieved through biventricular pacing mitigates symptoms such as dyspnea and fatigue, enabling patients to engage more fully in daily activities and physical exercise. Furthermore, the therapy has a positive impact on mental health by reducing anxiety and depression associated with chronic heart failure. Our study demonstrated that biventricular pacing leads to medium- and long-term improvements in quality of life, as evidenced by an average increase of 4.2 points in the KCCQ score for every percentage increase in biventricular pacing effectiveness.
We also assessed here systolic blood pressure variation during the surgery and QRS duration to correlate them with the outcome. While changes in blood pressure and QRS duration were observed after CRT initiation, there isn't necessarily a direct correlation between the two in the short term. The relationship between blood pressure and QRS duration is influenced by various factors. However, as previously shown in Figure 12, patients who experienced an acute rise in systolic blood pressure also displayed a greater reduction in QRS duration.
Furthermore, we have observed that variations in electrocardiographic parameters such as QRS duration and QRS complex area following CRT therapy are linked to changes in the KCCQ score.
QRS duration is widely recognized as one of the most studied parameters for predicting response after CRT and is a crucial element in the inclusion criteria for identifying candidates who may benefit from this therapy [
17]. Our analysis revealed a correlation between QRS duration and KCCQ score: patients with wider QRS durations and more severe ventricular dyssynchrony tend to experience more severe symptoms and poorer quality of life, resulting in lower KCCQ scores.
Conversely, patients who achieve significant enhancement in ventricular synchrony and cardiac function with CRT report reduction in symptoms and improvements in quality of life, leading to higher KCCQ scores. However, it is important to note that while QRS duration is an important predictor of CRT response, individual patient factors and clinical assessment also play significant roles in determining the suitability of CRT and predicting outcomes. Therefore, while QRS duration and KCCQ score may be correlated in some patients, they represent distinct aspects of heart failure management and should be interpreted in conjunction with other clinical data. Ar mai fi de adaugat referinte in paragraful asta
QRS area derived from vectorcardiography (VCG) has been recognized in recent studies as a potentially superior indicator compared to QRS duration and morphology for predicting outcomes following CRT [
24]. It has been suggested that a larger QRS area correlates closely with delayed activation of the LV lateral wall, regardless of QRS morphology, and inversely correlates with myocardial scar size. Moreover, substantial evidence demonstrates a robust association between QRS area and both clinical outcomes and echocardiographic response. These findings suggest that QRS area reflects the electrical substrate suitable for CRT treatment and could serve as a criterion for identifying heart failure patients who would benefit from CRT [
25]. Our results demonstrate an association of ∆QRS area with an increase in the KCCQ score following CRT, supporting the hypothesis that changes in QRS area reflect alterations in the electrical substrate. Therefore, targeting a greater reduction in QRS area after CRT may offer additional benefits to patients.
Taken together, the here presented data reveal that modifications in QRS and acute systolic blood pressure significantly influence the KCCQ. A reduction in QRS area post-CRT is associated with a good prognostic, while a prompt rise in SBP might indicate the opposite. These easily reproducible parameters provide information about the response to treatment, helping classify patients early on as CRT responders or non-responders.
5. Study Limitations
There are several limitations and challenges in this research that should be noted. In the literature, both baseline and post-CRT implantation ECG data have been obtained from digitally stored information. However, some healthcare facilities lack access to digitally stored ECGs. This prompted us to investigate whether there is any correlation between measurements performed on paper ECGs and post-CRT outcomes. Digitizing the entire process could save valuable time. Although the software we used for the current measurements (i.e. IC Measure18) is very accurate, it is important to note that our method is still user-dependent. Additionally, a significant challenge is the numerous observations and measurements required to verify the response to CRT therapy. Optimization occurs hours or days after the implant, and interventions that require cardiac remodeling, such as CRT, affect the patient's overall health status. The QRS duration may remain unchanged even after 6 months. All these factors that affect measurements and timing should be taken into consideration when evaluating the KCCQ score.
An important limitation is the small number of patients enrolled in the study over a 6-year timeline. Nonetheless, the data obtained have reached statistical significance and provide a solid basis for future research, highlighting the need for larger-scale studies to validate these initial findings. Another impediment the study faced was related to patient education and willingness to complete the form, as it is yet not so widespread. Personalized healthcare and tailored treatment are in their infancy in many parts of the world. A possible solution to encourage the daily use of the KCCQ score would be to create a platform that patients can access individually before each visit, allowing them to familiarize themselves with it at their own pace.
6. Conclusions
The ECG trace, hemodynamic parameters and the severity of ventricular asynchrony are clinically and easy to follow hotspots, which impair the quality of life after CRT therapy. Moving forward and understanding these correlations will guide personalized treatment strategies, optimize patient selection for interventions, and ultimately improve outcomes in individuals with cardiovascular disease.
Author Contributions
Conceptualization, A.-M.R., L.-F.T., T.-G.B., and E.-S.R; methodology, A.-L.R., L.-N.B., A.V., and M.-D.T.; software, A.-G.B., S.I., T.I., and C.-J.S.; validation, R.-O.D., C.-L.A., T.A., and O.-A.P.; formal analysis, A.-M.R., T.-G.B., A.-L.R., and A.V.; investigation, A.-G.B., T.I., R.-O.D., and T.A.; resources, L.-F.T., E.-S.R., L.-N.B., and M.-D.T.; data curation, S.I., C.-J.S., C.-L.A., and O.-A.P.; writing- original draft preparation, A.-M.R., O.-A.P., L.-F.T., and T.A.; writing—review and editing, S.I., T.I., A.V., and A.-G.B.; visualization, T.-G.B., E.-S.R., R.-O.D., and C.-L.A.; supervision, A.-L.R., L.-N.B., M.-D.T., and C.-J.S.; project administration, A.-M.R.; funding acquisition, O.-A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Carol Davila University of Medicine and Pharmacy (15037/05.06.2017).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nakai T, Ikeya Y, Kogawa R, Okumura Y. Cardiac resynchronization therapy: Current status and near-future prospects. J Cardiol. 2022 Mar;79(3):352–7. [CrossRef]
- Kronborg MB, Frausing MHJP, Svendsen JH, Johansen JB, Riahi S, Haarbo J, et al. Does targeted positioning of the left ventricular pacing lead towards the latest local electrical activation in cardiac resynchronization therapy reduce the incidence of death or hospitalization for heart failure? Am Heart J. 2023 Sep;263:112–22.
- van Stipdonk AMW, Rad MM, Luermans JGLM, Crijns HJ, Prinzen FW, Vernooy K. Identifying delayed left ventricular lateral wall activation in patients with non-specific intraventricular conduction delay using coronary venous electroanatomical mapping. Neth Heart J. 2016 Jan;24(1):58–65.
- Strik M, Regoli F, Auricchio A, Prinzen F. Electrical and mechanical ventricular activation during left bundle branch block and resynchronization. Vol. 5, Journal of Cardiovascular Translational Research. 2012. p. 117–26.
- Végh EM, Kandala J, Januszkiewicz L, Ren J, Miller A, Orencole M, et al. A new simplified electrocardiographic score predicts clinical outcome in patients treated with CRT. Europace. 2018 Mar 1;20(3):492–500.
- Nath *, Kumar R, Athar K, Neeraj P. ELECTROCARDIOGRAPHIC PREDICTORS RESYNCHRONIZATION THERAPY. 2020.
- Spertus JA, Jones PG, Sandhu AT, Arnold S V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Nov 17;76(20):2379–90.
- Stogios N, Fezza G, Wong J V, Ross HJ, Farkouh ME, Nolan RP. Current challenges for using the Kansas City Cardiomyopathy Questionnaire to obtain a standardized patient-reported health status outcome. Eur J Heart Fail. 2021 Feb;23(2):205–7. [CrossRef]
- Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, Devore AD, et al. Comparison of New York Heart Association Class and Patient-Reported Outcomes for Heart Failure With Reduced Ejection Fraction. JAMA Cardiol [Internet]. 2021 May 1 [cited 2024 May 8];6(5):522–31. Available from: https://jamanetwork.com/journals/jamacardiology/fullarticle/2777640. [CrossRef]
- Oka S, Ueda N, Ishibashi K, Noda T, Miyazaki Y, Wakamiya A, et al. Significance of effective cardiac resynchronization therapy pacing for clinical responses: An analysis based on the effective cardiac resynchronization therapy algorithm. Heart Rhythm [Internet]. 2023 Sep 1 [cited 2024 Apr 9];20(9):1289–96. Available from: https://pubmed.ncbi.nlm.nih.gov/37307884/-fostul. [CrossRef]
- Marinko S, Platonov PG, Carlson J, Borgquist R. Baseline QRS Area and Reduction in QRS Area Are Associated with Lower Mortality and Risk of Heart Failure Hospitalization after Cardiac Resynchronization Therapy. Cardiology. 2022;147(3):298–306. [CrossRef]
- Tanaka Y, Tada H, Yamashita E, Sato C, Irie T, Hori Y, et al. Change in Blood Pressure Just After Initiation of Cardiac Resynchronization Therapy Predicts Long-Term Clinical Outcome in Patients With Advanced Heart Failure. [CrossRef]
- Tanaka Y, Tada H, Yamashita E, Sato C, Irie T, Hori Y, et al. Change in blood pressure just after initiation of cardiac resynchronization therapy predicts long-term clinical outcome in patients with advanced heart failure. Circ J. 2009 Feb;73(2):288–94.
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur Heart J [Internet]. 2021 Sep 14 [cited 2024 Apr 9];42(35):3427–520. [CrossRef]
- Huo X, Pu B, Wang W, Peng Y, Li J, Lei L, et al. New York Heart Association Class and Kansas City Cardiomyopathy Questionnaire in Acute Heart Failure Key Points Question How is clinician-assigned New York Heart Association (NYHA) classification concordant with patient-reported Kansas City Cardiomyopathy + Supplemental content. JAMA Netw Open. 2023;6(10):2339458.
- Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am Heart J. 2005 Oct 1;150(4):707–15.
- Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007 Apr;115(15):1975–81.
- Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold S V., et al. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2017 Dec 1;2(12):1315.
- The Imaging Source Europe GmbH. IC Measure - https://www.theimagingsource.com/en-us/product/software/icmeasure. Überseetor 18 | 28217 Bremen | Germany: The Imaging Source Europe GmbH;
- Spertus JA, Jones PG, Sandhu AT, Arnold S V. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Nov 17;76(20):2379–90.
- Mihalcea L, Sebastian I, Simion-Cotorogea M, Klimko A, Droc G. Reverse Takotsubo Cardiomyopathy after Orthotopic Liver Transplantation. A Case Report. J Crit Care Med (Targu Mures). 2022 May 12;8(2):117-122. [CrossRef] [PubMed] [PubMed Central]
- Mele D, Agricola E, Galderisi M, Rigo F, Citro R, Monte AD, et al. Echocardiographic Myocardial Scar Burden Predicts Response to Cardiac Resynchronization Therapy in Ischemic Heart Failure. Journal of the American Society of Echocardiography. 2009 Jun;22(6):702–8. [CrossRef]
- Bleeker GB, Kaandorp TAM, Lamb HJ, Boersma E, Steendijk P, De Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation [Internet]. 2006 Feb [cited 2024 May 14];113(7):969–76. Available from: https://pubmed.ncbi.nlm.nih.gov/16476852/.
- Eerenberg F, Luermans J, Lumens J, Nguyên UC, Vernooy K, van Stipdonk A. Exploring QRS Area beyond Patient Selection in CRT-Can It Guide Left Ventricular Lead Placement? J Cardiovasc Dev Dis. 2024 Jan 11;11(1).
- Tokavanich N, Prasitlumkum N, Mongkonsritragoon W, Trongtorsak A, Cheungpasitporn W, Chokesuwattanaskul R. QRS area as a predictor of cardiac resynchronization therapy response: A systematic review and meta-analysis. Pacing Clin Electrophysiol. 2022 Mar;45(3):393–400. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).