Submitted:
18 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
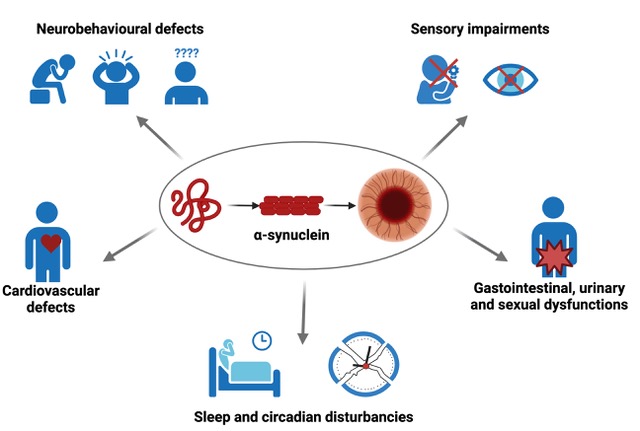
Keywords:
1. Introduction
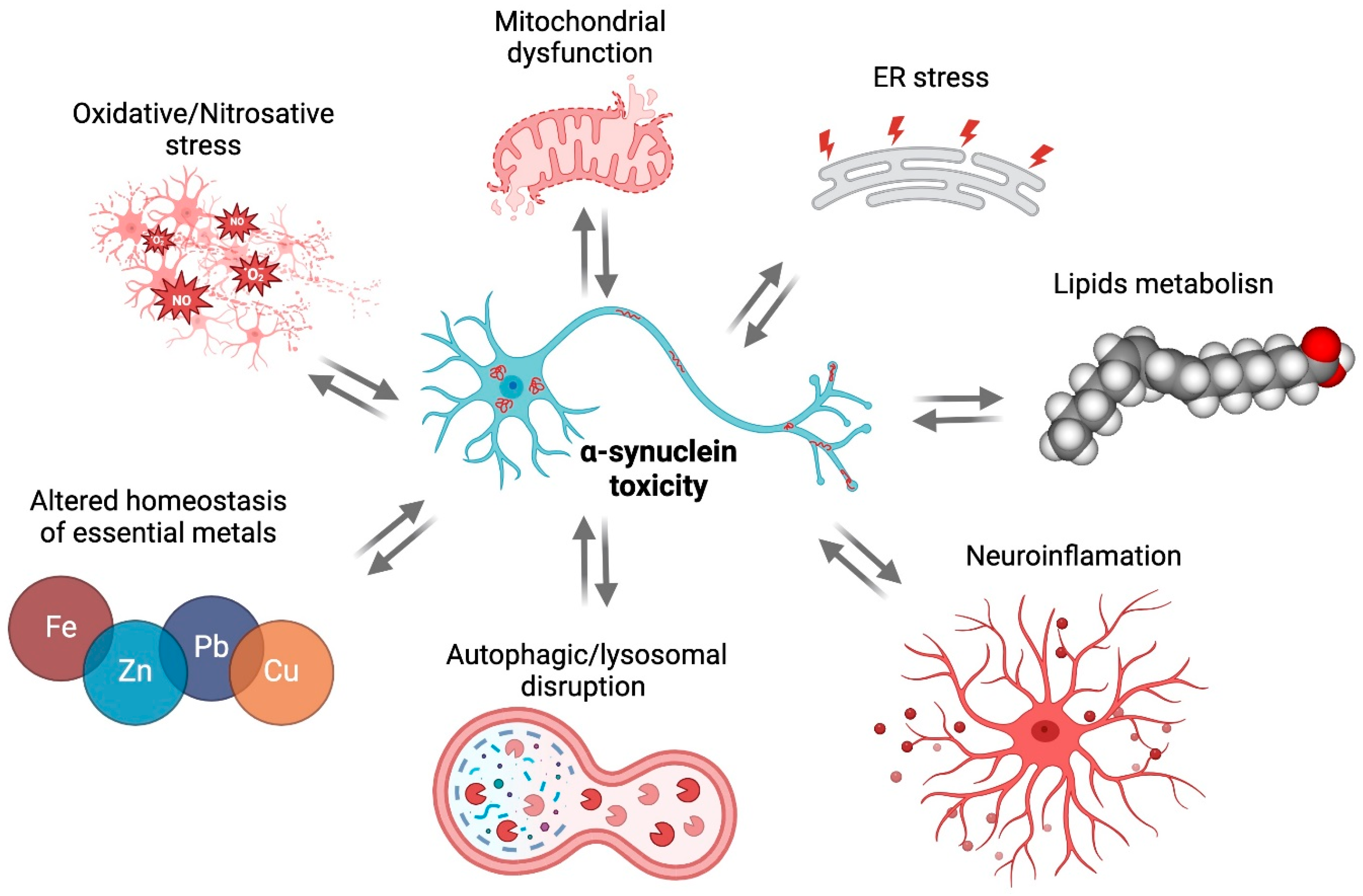
2. αSyn Toxicity
2.1. Oxidative/Nitrosative Stress
2.2. Mitochondrial Dysfunction
2.3. ER Stress
2.4. Lipids
2.5. Autophagic/Lysosomal Disruption
2.6. Metals
3. Distribution of αSyn in Tissues and Fluids in Parkinson’s Disease
4. Non-Motor Symptoms Associated with αSyn Pathology
4.1. Depression
4.2. Anxiety
4.3. Psychosis
4.4. Cognitive Impairment
4.5. Pain
4.6. Olfaction Dysfunction
4.7. Visual Impairment
4.8. Sleep and Circadian Dysfunctions
4.9. Gastrointestinal Symptoms
4.10. Sexual Dysfunction and Urinary Dysfunction
4.11. Cardiovascular Symptoms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Stevenson, T.J.; Murray, H.C.; Turner, C.; Faull, R.L.M.; Dieriks, B. V.; Curtis, M.A. α-Synuclein Inclusions Are Abundant in Non-Neuronal Cells in the Anterior Olfactory Nucleus of the Parkinson’s Disease Olfactory Bulb. Sci Rep 2020, 10, 6682. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front Neurosci 2018, 12. [Google Scholar] [CrossRef]
- Durcan, R.; Wiblin, L.; Lawson, R.A.; Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Brooks, D.J.; Pavese, N.; Burn, D.J. Prevalence and Duration of Non-motor Symptoms in Prodromal Parkinson’s Disease. Eur J Neurol 2019, 26, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat Rev Neurosci 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.C.G.; Silva, F.G.; Costa, L.O.P.; Freitas, S.M.S.F. Smell Tests Can Discriminate Parkinson’s Disease Patients from Healthy Individuals: A Meta-Analysis. Clin Neurol Neurosurg 2021, 211, 107024. [Google Scholar] [CrossRef] [PubMed]
- Iravani, B.; Arshamian, A.; Schaefer, M.; Svenningsson, P.; Lundström, J.N. A Non-Invasive Olfactory Bulb Measure Dissociates Parkinson’s Patients from Healthy Controls and Discloses Disease Duration. NPJ Parkinsons Dis 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Coulson, E.J.; Rajnarayanan, R.; Oster, H.; Videnovic, A.; Rawashdeh, O. Sleep and Circadian Rhythms in Parkinson’s Disease and Preclinical Models. Mol Neurodegener 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-C.; Shi, R.; Gao, C.; Yuan, X.-L.; Wu, Y.; Liu, Z.-G.; Wang, C.-D.; Zhao, S.-R.; Chen, X.; Yuan, C.-X.; et al. Autonomic Function and Motor Subtypes in Parkinson’s Disease: A Multicentre Cross-Sectional Study. Sci Rep 2023, 13, 14548. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Autonomic Failure: A Neglected Presentation of Parkinson’s Disease. Lancet Neurol 2021, 20, 781–782. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.A.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of Oligomeric Forms of A-synuclein Protein in Human Plasma as a Potential Biomarker for Parkinson’s Disease. The FASEB Journal 2006, 20, 419–425. [Google Scholar] [CrossRef]
- Kosaka, K. Latest Concept of Lewy Body Disease. Psychiatry Clin Neurosci 2014, 68, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Pritzkow, S. Protein Misfolding, Aggregation, and Conformational Strains in Neurodegenerative Diseases. Nat Neurosci 2018, 21, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Dauer, W.T.; Vonsattel, J.P.G. A Critical Evaluation of the Braak Staging Scheme for Parkinson’s Disease. Ann Neurol 2008, 64, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Pyatigorskaya, N.; Mongin, M.; Valabregue, R.; Yahia-Cherif, L.; Ewenczyk, C.; Poupon, C.; Debellemaniere, E.; Vidailhet, M.; Arnulf, I.; Lehéricy, S. Medulla Oblongata Damage and Cardiac Autonomic Dysfunction in Parkinson Disease. Neurology 2016, 87, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K. Del; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Dams, J.; Siebert, U.; Bornschein, B.; Volkmann, J.; Deuschl, G.; Oertel, W.H.; Dodel, R.; Reese, J. Cost-effectiveness of Deep Brain Stimulation in Patients with Parkinson’s Disease. Movement Disorders 2013, 28, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Geng, X.; Huang, B.; Chen, Y.; Jiang, H.; Zou, Y.; Wang, W.; Li, Y.; Li, Y.; Yin, L.; et al. Outcomes of STN-DBS in PD Patients With Different Rates of Disease Progression Over One Year of Follow-Up. Front Neurol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Fundament, T.; Eldridge, P.R.; Green, A.L.; Whone, A.L.; Taylor, R.S.; Williams, A.C.; Schuepbach, W.M.M. Deep Brain Stimulation for Parkinson’s Disease with Early Motor Complications: A UK Cost-Effectiveness Analysis. PLoS ONE 2016, 11, e0159340. [Google Scholar] [CrossRef] [PubMed]
- Lilleeng, B.; Gjerstad, M.; Baardsen, R.; Dalen, I.; Larsen, J.P. Motor Symptoms after Deep Brain Stimulation of the Subthalamic Nucleus. Acta Neurol Scand 2015, 131, 298–304. [Google Scholar] [CrossRef]
- Listik, C.; Cury, R.G.; Casagrande, S.C.B.; Listik, E.; Arnaut, D.; Santiago, N.; Da Silva, V.A.; Galhardoni, R.; Machado, J. de L.A.; Almeida, J.C. de; et al. Improvement of Non-Motor Symptoms and Quality of Life After Deep Brain Stimulation for Refractory Dystonia: A 1-Year Follow-Up. Front Neurol 2021, 12. [Google Scholar] [CrossRef]
- Eghlidos, Z.; Rahimian, Z.; Vadiee, G.; Jahangiri, S. Effects of Subthalamic Deep Brain Stimulation on Non-motor Symptoms of Parkinson’s Disease: A Meta-analysis. Acta Neurol Scand 2022, 146, 115–125. [Google Scholar] [CrossRef]
- Alonso-Frech, F.; Fernandez-Garcia, C.; Gómez-Mayordomo, V.; Monje, M.H.G.; Delgado-Suarez, C.; Villanueva-Iza, C.; Catalan-Alonso, M.J. Non-Motor Adverse Effects Avoided by Directional Stimulation in Parkinson’s Disease: A Case Report. Front Neurol 2022, 12. [Google Scholar] [CrossRef]
- El Ghazal, N.; Nakanishi, H.; Martinez-Nunez, A.E.; Al Sabbakh, N.K.; Segun-Omosehin, O.A.; Bourdakos, N.E.; Nasser, M.; Matar, R.H.; Than, C.; Danoun, O.A.; et al. The Effects of Deep Brain Stimulation on Mood and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Cureus 2023. [Google Scholar] [CrossRef]
- Gupta, S.; Shukla, S. Non-Motor Symptoms in Parkinson’s Disease: Opening New Avenues in Treatment. Current Research in Behavioral Sciences 2021, 2, 100049. [Google Scholar] [CrossRef]
- Maroteaux, L.; Campanelli, J.; Scheller, R. Synuclein: A Neuron-Specific Protein Localized to the Nucleus and Presynaptic Nerve Terminal. The Journal of Neuroscience 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Burré, J.; Vivona, S.; Diao, J.; Sharma, M.; Brunger, A.T.; Südhof, T.C. Properties of Native Brain α-Synuclein. Nature 2013, 498, E4–E6. [Google Scholar] [CrossRef]
- Cholak, E.; Bugge, K.; Khondker, A.; Gauger, K.; Pedraz-Cuesta, E.; Pedersen, M.E.; Bucciarelli, S.; Vestergaard, B.; Pedersen, S.F.; Rheinstädter, M.C.; et al. Avidity within the N-terminal Anchor Drives A-synuclein Membrane Interaction and Insertion. The FASEB Journal 2020, 34, 7462–7482. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takenouchi, T.; Mallory, M.; Masliah, E.; Takeda, A.; Culvenor, J.G.; McLean, C.A.; Campbell, B.C.V.; Masters, C.L.; Li, Q.-X.; et al. The Role of NAC in Amyloidogenesis in Alzheimer’s Disease. Am J Pathol 2000, 156, 734–735. [Google Scholar] [CrossRef]
- Gao, V.; Briano, J.A.; Komer, L.E.; Burré, J. Functional and Pathological Effects of α-Synuclein on Synaptic SNARE Complexes. J Mol Biol 2023, 435, 167714. [Google Scholar] [CrossRef]
- Diao, J.; Burré, J.; Vivona, S.; Cipriano, D.J.; Sharma, M.; Kyoung, M.; Südhof, T.C.; Brunger, A.T. Native α-Synuclein Induces Clustering of Synaptic-Vesicle Mimics via Binding to Phospholipids and Synaptobrevin-2/VAMP2. Elife 2013, 2. [Google Scholar] [CrossRef]
- Bell, R.; Thrush, R.J.; Castellana-Cruz, M.; Oeller, M.; Staats, R.; Nene, A.; Flagmeier, P.; Xu, C.K.; Satapathy, S.; Galvagnion, C.; et al. N-Terminal Acetylation of α-Synuclein Slows down Its Aggregation Process and Alters the Morphology of the Resulting Aggregates. Biochemistry 2022, 61, 1743–1756. [Google Scholar] [CrossRef]
- Horsley, J.R.; Jovcevski, B.; Pukala, T.L.; Abell, A.D. Designer D-Peptides Targeting the N-Terminal Region of α-Synuclein to Prevent Parkinsonian-Associated Fibrilization and Cytotoxicity. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2022, 1870, 140826. [Google Scholar] [CrossRef] [PubMed]
- Hmila, I.; Vaikath, N.N.; Majbour, N.K.; Erskine, D.; Sudhakaran, I.P.; Gupta, V.; Ghanem, S.S.; Islam, Z.; Emara, M.M.; Abdesselem, H.B.; et al. Novel Engineered Nanobodies Specific for N-terminal Region of Alpha-synuclein Recognize Lewy-body Pathology and Inhibit In-vitro Seeded Aggregation and Toxicity. FEBS J 2022, 289, 4657–4673. [Google Scholar] [CrossRef] [PubMed]
- Daida, K.; Shimonaka, S.; Shiba-Fukushima, K.; Ogata, J.; Yoshino, H.; Okuzumi, A.; Hatano, T.; Motoi, Y.; Hirunagi, T.; Katsuno, M.; et al. A-Synuclein V15A Variant in Familial Parkinson’s Disease Exhibits a Weaker Lipid-Binding Property. Movement Disorders 2022, 37, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Diaw, S.H.; Borsche, M.; Streubel-Gallasch, L.; Dulovic-Mahlow, M.; Hermes, J.; Lenz, I.; Seibler, P.; Klein, C.; Brüggemann, N.; Vos, M.; et al. Characterization of the Pathogenic α-Synuclein Variant V15A in Parkinson´s Disease. NPJ Parkinsons Dis 2023, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Emin, D.; Zhang, Y.P.; Lobanova, E.; Miller, A.; Li, X.; Xia, Z.; Dakin, H.; Sideris, D.I.; Lam, J.Y.L.; Ranasinghe, R.T.; et al. Small Soluble α-Synuclein Aggregates Are the Toxic Species in Parkinson’s Disease. Nat Commun 2022, 13, 5512. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Flagmeier, P.; Kumita, J.R.; Meisl, G.; Chirgadze, D.Y.; Bongiovanni, M.N.; Knowles, T.P.J.; Dobson, C.M. The Influence of Pathogenic Mutations in α-Synuclein on Biophysical and Structural Characteristics of Amyloid Fibrils. ACS Nano 2020, 14, 5213–5222. [Google Scholar] [CrossRef]
- Krzystek, T.J.; Banerjee, R.; Thurston, L.; Huang, J.; Swinter, K.; Rahman, S.N.; Falzone, T.L.; Gunawardena, S. Differential Mitochondrial Roles for α-Synuclein in DRP1-Dependent Fission and PINK1/Parkin-Mediated Oxidation. Cell Death Dis 2021, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Helwig, M.; Ulusoy, A.; Rollar, A.; O’Sullivan, S.A.; Lee, S.S.L.; Aboutalebi, H.; Pinto-Costa, R.; Jevans, B.; Klinkenberg, M.; Di Monte, D.A. Neuronal Hyperactivity–Induced Oxidant Stress Promotes in Vivo α-Synuclein Brain Spreading. Sci Adv 2022, 8. [Google Scholar] [CrossRef]
- Stojkovska, I.; Wani, W.Y.; Zunke, F.; Belur, N.R.; Pavlenko, E.A.; Mwenda, N.; Sharma, K.; Francelle, L.; Mazzulli, J.R. Rescue of α-Synuclein Aggregation in Parkinson’s Patient Neurons by Synergistic Enhancement of ER Proteostasis and Protein Trafficking. Neuron 2022, 110, 436–451. [Google Scholar] [CrossRef]
- Kang, N.; Han, X.; Li, Z.; Liu, T.; Mi, X.; Li, Y.; Guo, X.; Han, D.; Yang, N. Rapamycin Affects the Hippocampal SNARE Complex to Alleviate Cognitive Dysfunction Induced by Surgery in Aged Rats. Brain Sci 2023, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, D.; Cisternas-Olmedo, M.; Arcos, J.; Nassif, M.; Vidal, R.L. Contribution of Autophagy-Lysosomal Pathway in the Exosomal Secretion of Alpha-Synuclein and Its Impact in the Progression of Parkinson’s Disease. Front Mol Neurosci 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Homma, D.; Bloem, B.; Gibb, L.G.; Amemori, K.; Hu, D.; Delcasso, S.; Truong, T.F.; Yang, J.; Hood, A.S.; et al. Chronic Stress Alters Striosome-Circuit Dynamics, Leading to Aberrant Decision-Making. Cell 2017, 171, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front Mol Neurosci 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-Synuclein Oligomers: A New Hope. Acta Neuropathol 2017, 134, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Abarrategui, J.; Brimblecombe, K.R.; Roberts, R.F.; Velentza-Almpani, E.; Tilley, B.S.; Bengoa-Vergniory, N.; Proukakis, C. Selective Vulnerability in α-Synucleinopathies. Acta Neuropathol 2019, 138, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Kaufman, E.; Brundin, L.; Hall, S.; Surova, Y.; Hansson, O. Non-Motor Symptoms in Patients with Parkinson’s Disease – Correlations with Inflammatory Cytokines in Serum. PLoS ONE 2012, 7, e47387. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.; Shin, J.H.; Lee, C.Y.; Jeon, S.H.; Jeon, B. Serum Inflammatory Markers and Progression of Nonmotor Symptoms in Early Parkinson’s Disease. Movement Disorders 2022, 37, 1535–1541. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-Immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.D.; Lucassen, P.J.; van Dam, A.-M. Microglial Phenotypes and Toll-like Receptor 2 in the Substantia Nigra and Hippocampus of Incidental Lewy Body Disease Cases and Parkinson’s Disease Patients. Acta Neuropathol Commun 2014, 2, 90. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J. Mitochondrial Complex I Deficiency and Parkinson Disease. Nat Rev Neurosci 2023, 24, 193–193. [Google Scholar] [CrossRef]
- Li, X.; Xue, L.; Sun, J.; Sun, Y.; Xie, A. Single Nucleotide Polymorphisms in the Toll-like Receptor 2 (TLR2) Gene Are Associated with Sporadic Parkinson’s Disease in the North-Eastern Han Chinese Population. Neurosci Lett 2017, 656, 72–76. [Google Scholar] [CrossRef]
- Mazzotta, G.M.; Ceccato, N.; Conte, C. Synucleinopathies Take Their Toll: Are TLRs a Way to Go? Cells 2023, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-like Receptors in the Pathogenesis of Neuroinflammation. J Neuroimmunol 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Kouli, A.; Horne, C.B.; Williams-Gray, C.H. Toll-like Receptors and Their Therapeutic Potential in Parkinson’s Disease and α-Synucleinopathies. Brain Behav Immun 2019, 81, 41–51. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The Role of Toll-like Receptors and Neuroinflammation in Parkinson’s Disease. J Neuroinflammation 2022, 19, 135. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lin, Y.; Heo, Y.; Lee, Y.-H. Polymorphism in Alpha-Synuclein Oligomers and Its Implications in Toxicity under Disease Conditions. Front Mol Biosci 2022, 9. [Google Scholar] [CrossRef]
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-Like Mechanisms in Parkinson’s Disease. Front Neurosci 2019, 13. [Google Scholar] [CrossRef]
- Jan, A.; Gonçalves, N.P.; Vaegter, C.B.; Jensen, P.H.; Ferreira, N. The Prion-Like Spreading of Alpha-Synuclein in Parkinson’s Disease: Update on Models and Hypotheses. Int J Mol Sci 2021, 22, 8338. [Google Scholar] [CrossRef]
- Garcia, P.; Jürgens-Wemheuer, W.; Uriarte Huarte, O.; Michelucci, A.; Masuch, A.; Brioschi, S.; Weihofen, A.; Koncina, E.; Coowar, D.; Heurtaux, T.; et al. Neurodegeneration and Neuroinflammation Are Linked, but Independent of Alpha-synuclein Inclusions, in a Seeding/Spreading Mouse Model of Parkinson’s Disease. Glia 2022, 70, 935–960. [Google Scholar] [CrossRef]
- Dening, Y.; Straßl, T.; Ruf, V.; Dirscherl, P.; Chovsepian, A.; Stievenard, A.; Khairnar, A.; Schmidt, F.; Giesert, F.; Herms, J.; et al. Toxicity of Extracellular Alpha-Synuclein Is Independent of Intracellular Alpha-Synuclein. Sci Rep 2022, 12, 21951. [Google Scholar] [CrossRef] [PubMed]
- Vasili, E.; Dominguez-Meijide, A.; Flores-León, M.; Al-Azzani, M.; Kanellidi, A.; Melki, R.; Stefanis, L.; Outeiro, T.F. Endogenous Levels of Alpha-Synuclein Modulate Seeding and Aggregation in Cultured Cells. Mol Neurobiol 2022, 59, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Karpowicz, R.J.; Trojanowski, J.Q.; Lee, V.M.-Y. Transmission of α-Synuclein Seeds in Neurodegenerative Disease: Recent Developments. Laboratory Investigation 2019, 99, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Holper, L.; Ben-Shachar, D.; Mann, J. Multivariate Meta-Analyses of Mitochondrial Complex I and IV in Major Depressive Disorder, Bipolar Disorder, Schizophrenia, Alzheimer Disease, and Parkinson Disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Hu, X.; Luk, K.C.; Sanders, L.H.; Lee, V.M.; Greenamyre, J.T. Synthetic Alpha-Synuclein Fibrils Cause Mitochondrial Impairment and Selective Dopamine Neurodegeneration in Part via INOS-Mediated Nitric Oxide Production. Cellular and Molecular Life Sciences 2017, 74, 2851–2874. [Google Scholar] [CrossRef]
- Yamamoto, K.; Izumi, Y.; Arifuku, M.; Kume, T.; Sawada, H. α-Synuclein Oligomers Mediate the Aberrant Form of Spike-Induced Calcium Release from IP3 Receptor. Sci Rep 2019, 9, 15977. [Google Scholar] [CrossRef] [PubMed]
- Leandrou, E.; Chalatsa, I.; Anagnostou, D.; Machalia, C.; Semitekolou, M.; Filippa, V.; Makridakis, M.; Vlahou, A.; Anastasiadou, E.; Vekrellis, K.; et al. α-Synuclein Oligomers Potentiate Neuroinflammatory NF-ΚB Activity and Induce Cav3.2 Calcium Signaling in Astrocytes. Transl Neurodegener 2024, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Shvachiy, L.; Geraldes, V.; Outeiro, T.F. Uncovering the Molecular Link Between Lead Toxicity and Parkinson’s Disease. Antioxid Redox Signal 2023, 39, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein Binds to TOM20 and Inhibits Mitochondrial Protein Import in Parkinson’s Disease. Sci Transl Med 2016, 8. [Google Scholar] [CrossRef]
- Shan, L.; Heusinkveld, H.J.; Paul, K.C.; Hughes, S.; Darweesh, S.K.L.; Bloem, B.R.; Homberg, J.R. Towards Improved Screening of Toxins for Parkinson’s Risk. NPJ Parkinsons Dis 2023, 9, 169. [Google Scholar] [CrossRef]
- Won, S.J.; Fong, R.; Butler, N.; Sanchez, J.; Zhang, Y.; Wong, C.; Tambou Nzoutchoum, O.; Huynh, A.; Pan, J.; Swanson, R.A. Neuronal Oxidative Stress Promotes α-Synuclein Aggregation In Vivo. Antioxidants 2022, 11, 2466. [Google Scholar] [CrossRef]
- Almandoz-Gil, L.; Welander, H.; Ihse, E.; Khoonsari, P.E.; Musunuri, S.; Lendel, C.; Sigvardson, J.; Karlsson, M.; Ingelsson, M.; Kultima, K.; et al. Low Molar Excess of 4-Oxo-2-Nonenal and 4-Hydroxy-2-Nonenal Promote Oligomerization of Alpha-Synuclein through Different Pathways. Free Radic Biol Med 2017, 110, 421–431. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Villamil Giraldo, A.M.; Öllinger, K.; Hallbeck, M.; Civitelli, L. Lipid Vesicles Affect the Aggregation of 4-Hydroxy-2-Nonenal-Modified α-Synuclein Oligomers. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2018, 1864, 3060–3068. [Google Scholar] [CrossRef]
- Sahin, C.; Østerlund, E.C.; Österlund, N.; Costeira-Paulo, J.; Pedersen, J.N.; Christiansen, G.; Nielsen, J.; Grønnemose, A.L.; Amstrup, S.K.; Tiwari, M.K.; et al. Structural Basis for Dityrosine-Mediated Inhibition of α-Synuclein Fibrillization. J Am Chem Soc 2022, 144, 11949–11954. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Biasetti, L.; Blakeman, B.J.F.; Pollack, S.J.; Zibaee, S.; Abdul-Sada, A.; Thorpe, J.R.; Xue, W.-F.; Serpell, L.C. The Involvement of Dityrosine Crosslinking in α-Synuclein Assembly and Deposition in Lewy Bodies in Parkinson’s Disease. Sci Rep 2016, 6, 39171. [Google Scholar] [CrossRef]
- Wördehoff, M.M.; Shaykhalishahi, H.; Groß, L.; Gremer, L.; Stoldt, M.; Buell, A.K.; Willbold, D.; Hoyer, W. Opposed Effects of Dityrosine Formation in Soluble and Aggregated α-Synuclein on Fibril Growth. J Mol Biol 2017, 429, 3018–3030. [Google Scholar] [CrossRef]
- Buell, A.K.; Galvagnion, C.; Gaspar, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.J.; Linse, S.; Dobson, C.M. Solution Conditions Determine the Relative Importance of Nucleation and Growth Processes in α-Synuclein Aggregation. Proceedings of the National Academy of Sciences 2014, 111, 7671–7676. [Google Scholar] [CrossRef]
- Mehta, N.; Marwah, P.; Njus, D. Are Proteinopathy and Oxidative Stress Two Sides of the Same Coin? Cells 2019, 8, 59. [Google Scholar] [CrossRef]
- Stykel, M.G.; Humphries, K.M.; Kamski-Hennekam, E.; Buchner-Duby, B.; Porte-Trachsel, N.; Ryan, T.; Coackley, C.L.; Bamm, V. V.; Harauz, G.; Ryan, S.D. α-Synuclein Mutation Impairs Processing of Endomembrane Compartments and Promotes Exocytosis and Seeding of α-Synuclein Pathology. Cell Rep 2021, 35, 109099. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jangir, D.K.; Verma, G.; Shekhar, S.; Hanpude, P.; Kumar, S.; Kumari, R.; Singh, N.; Sarovar Bhavesh, N.; Ranjan Jana, N.; et al. S-Nitrosylation of UCHL1 Induces Its Structural Instability and Promotes α-Synuclein Aggregation. Sci Rep 2017, 7, 44558. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain Aging and Neurodegeneration: From a Mitochondrial Point of View. J Neurochem 2017, 143, 418–431. [Google Scholar] [CrossRef]
- Park, J.-S.; Koentjoro, B.; Davis, R.L.; Sue, C.M. Loss of ATP13A2 Impairs Glycolytic Function in Kufor-Rakeb Syndrome Patient-Derived Cell Models. Parkinsonism Relat Disord 2016, 27, 67–73. [Google Scholar] [CrossRef]
- Ganjam, G.K.; Bolte, K.; Matschke, L.A.; Neitemeier, S.; Dolga, A.M.; Höllerhage, M.; Höglinger, G.U.; Adamczyk, A.; Decher, N.; Oertel, W.H.; et al. Mitochondrial Damage by α-Synuclein Causes Cell Death in Human Dopaminergic Neurons. Cell Death Dis 2019, 10, 865. [Google Scholar] [CrossRef]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Lehtonen, Š.; Sonninen, T.-M.; Wojciechowski, S.; Goldsteins, G.; Koistinaho, J. Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front Neurosci 2019, 13. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front Pharmacol 2020, 11. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A. V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-Synuclein Oligomers Interact with ATP Synthase and Open the Permeability Transition Pore in Parkinson’s Disease. Nat Commun 2018, 9, 2293. [Google Scholar] [CrossRef]
- Wang, X.-L.; Feng, S.-T.; Wang, Y.-T.; Yuan, Y.-H.; Li, Z.-P.; Chen, N.-H.; Wang, Z.-Z.; Zhang, Y. Mitophagy, a Form of Selective Autophagy, Plays an Essential Role in Mitochondrial Dynamics of Parkinson’s Disease. Cell Mol Neurobiol 2022, 42, 1321–1339. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shin, J.Y.; Lee, J.E.; Kim, H.N.; Chung, S.J.; Yoo, H.S.; Kim, S.J.; Cho, H.J.; Lee, E.-J.; Nam, S.J.; et al. A Selective ER-Phagy Exerts Neuroprotective Effects via Modulation of α-Synuclein Clearance in Parkinsonian Models. Proceedings of the National Academy of Sciences 2023, 120. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Phan, K.; Bhatia, S.; Pickford, R.; Wu, P.; Dzamko, N.; Halliday, G.M.; Kim, W.S. Increased Unsaturated Lipids Underlie Lipid Peroxidation in Synucleinopathy Brain. Acta Neuropathol Commun 2022, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, T.; Li, J.; Xia, M.; Li, Y.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Kan, D.; et al. Oxidative Stress and 4-Hydroxy-2-Nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-Related Diseases. J Immunol Res 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- De Franceschi, G.; Frare, E.; Bubacco, L.; Mammi, S.; Fontana, A.; de Laureto, P.P. Molecular Insights into the Interaction between α-Synuclein and Docosahexaenoic Acid. J Mol Biol 2009, 394, 94–107. [Google Scholar] [CrossRef]
- De Franceschi, G.; Frare, E.; Pivato, M.; Relini, A.; Penco, A.; Greggio, E.; Bubacco, L.; Fontana, A.; de Laureto, P.P. Structural and Morphological Characterization of Aggregated Species of α-Synuclein Induced by Docosahexaenoic Acid. Journal of Biological Chemistry 2011, 286, 22262–22274. [Google Scholar] [CrossRef]
- Cai, Y.; Lendel, C.; Österlund, L.; Kasrayan, A.; Lannfelt, L.; Ingelsson, M.; Nikolajeff, F.; Karlsson, M.; Bergström, J. Changes in Secondary Structure of α-Synuclein during Oligomerization Induced by Reactive Aldehydes. Biochem Biophys Res Commun 2015, 464, 336–341. [Google Scholar] [CrossRef]
- Beal, M.F.; Chiluwal, J.; Calingasan, N.Y.; Milne, G.L.; Shchepinov, M.S.; Tapias, V. Isotope-Reinforced Polyunsaturated Fatty Acids Improve Parkinson’s Disease-like Phenotype in Rats Overexpressing α-Synuclein. Acta Neuropathol Commun 2020, 8, 220. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J Parkinsons Dis 2017, 7, 433–450. [Google Scholar] [CrossRef]
- Bonam, S.R.; Tranchant, C.; Muller, S. Autophagy-Lysosomal Pathway as Potential Therapeutic Target in Parkinson’s Disease. Cells 2021, 10, 3547. [Google Scholar] [CrossRef]
- Kinnart, I.; Manders, L.; Heyninck, T.; Imberechts, D.; Praschberger, R.; Schoovaerts, N.; Verfaillie, C.; Verstreken, P.; Vandenberghe, W. Elevated α-Synuclein Levels Inhibit Mitophagic Flux. NPJ Parkinsons Dis 2024, 10, 80. [Google Scholar] [CrossRef]
- Stykel, M.G.; Ryan, S.D. Nitrosative Stress in Parkinson’s Disease. NPJ Parkinsons Dis 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Bisi, N.; Feni, L.; Peqini, K.; Pérez-Peña, H.; Ongeri, S.; Pieraccini, S.; Pellegrino, S. α-Synuclein: An All-Inclusive Trip Around Its Structure, Influencing Factors and Applied Techniques. Front Chem 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, N.; Lemminger, L.; Pedersen, J.N.; Nielsen, S.B.; Otzen, D.E. The N-terminus of A-synuclein Is Essential for Both Monomeric and Oligomeric Interactions with Membranes. FEBS Lett 2014, 588, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Abeyawardhane, D.L.; Fernández, R.D.; Murgas, C.J.; Heitger, D.R.; Forney, A.K.; Crozier, M.K.; Lucas, H.R. Iron Redox Chemistry Promotes Antiparallel Oligomerization of α-Synuclein. J Am Chem Soc 2018, 140, 5028–5032. [Google Scholar] [CrossRef]
- Chang, C.J. Bioinorganic Life and Neural Activity: Toward a Chemistry of Consciousness? Acc Chem Res 2017, 50, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M.; Bubacco, L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef]
- Exley, C.; Mold, M.J. Aluminium in Human Brain Tissue: How Much Is Too Much? JBIC Journal of Biological Inorganic Chemistry 2019, 24, 1279–1282. [Google Scholar] [CrossRef]
- Mold, M.J.; Exley, C. Aluminium Co-Localises with Biondi Ring Tangles in Parkinson’s Disease and Epilepsy. Sci Rep 2022, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Andrews, H.; Amara, A.; Naito, A.; Alcalay, R.N.; Shaw, L.M.; Taylor, P.; Xie, T.; Tuite, P.; Henchcliffe, C.; et al. Cerebrospinal Fluid, Plasma, and Saliva in the BioFIND Study: Relationships among Biomarkers and Parkinson’s Disease Features. Movement Disorders 2018, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Chahine, L.M.; Beach, T.G.; Brumm, M.C.; Adler, C.H.; Coffey, C.S.; Mosovsky, S.; Caspell-Garcia, C.; Serrano, G.E.; Munoz, D.G.; White, C.L.; et al. In Vivo Distribution of α-Synuclein in Multiple Tissues and Biofluids in Parkinson Disease. Neurology 2020, 95. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Liu, G.-L.; Wang, D.-X.; Zhang, M.-M.; Kou, W.-Y.; Feng, T. Alpha-Synuclein in Peripheral Tissues in Parkinson’s Disease. ACS Chem Neurosci 2019, 10, 812–823. [Google Scholar] [CrossRef]
- Lee, J.M.; Derkinderen, P.; Kordower, J.H.; Freeman, R.; Munoz, D.G.; Kremer, T.; Zago, W.; Hutten, S.J.; Adler, C.H.; Serrano, G.E.; et al. The Search for a Peripheral Biopsy Indicator of α-Synuclein Pathology for Parkinson Disease. J Neuropathol Exp Neurol 2017, nlw103. [Google Scholar] [CrossRef]
- Chen, M.; Mor, D.E. Gut-to-Brain α-Synuclein Transmission in Parkinson’s Disease: Evidence for Prion-like Mechanisms. Int J Mol Sci 2023, 24, 7205. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, W.; Liu, P.; Wang, Z.; Zhou, Y.; Liu, X.; Li, A. α-Synuclein Aggregation in the Olfactory Bulb Induces Olfactory Deficits by Perturbing Granule Cells and Granular–Mitral Synaptic Transmission. NPJ Parkinsons Dis 2021, 7, 114. [Google Scholar] [CrossRef]
- Oliveira, L.M.A.; Gasser, T.; Edwards, R.; Zweckstetter, M.; Melki, R.; Stefanis, L.; Lashuel, H.A.; Sulzer, D.; Vekrellis, K.; Halliday, G.M.; et al. Alpha-Synuclein Research: Defining Strategic Moves in the Battle against Parkinson’s Disease. NPJ Parkinsons Dis 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Sancesario, G.M.; Di Lazzaro, G.; Biticchi, B.; Colona, V.L.; Mercuri, N.B.; Bernardini, S.; Pisani, A. CSF α-Synuclein Inversely Correlates with Non-Motor Symptoms in a Cohort of PD Patients. Parkinsonism Relat Disord 2019, 61, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Météreau, E.; Tremblay, L.; Favre, E.; Klinger, H.; Lhommée, E.; Le Bars, D.; Castrioto, A.; Prange, S.; Sgambato, V.; et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Movement Disorders 2021, 36, 2888–2900. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Vahid-Ansari, F.; Sun, H.; Albert, P.R. Mood Disturbances in Parkinson’s Disease: From Prodromal Origins to Application of Animal Models. Neurobiol Dis 2023, 181, 106115. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.; Lim, J.; Choi, H.J. Recent Advances in the Pathology of Prodromal Non-Motor Symptoms Olfactory Deficit and Depression in Parkinson’s Disease: Clues to Early Diagnosis and Effective Treatment. Arch Pharm Res 2021, 44, 588–604. [Google Scholar] [CrossRef]
- Stocchi, F.; Angelo Antonini; Barone, P. ; Bellelli, G.; Fagiolini, A.; Ferini Strambi, L.; Sorbi, S.; Padovani, A. Exploring Depression in Parkinson’s Disease: An Italian Delphi Consensus on Phenomenology, Diagnosis, and Management. Neurological Sciences 2023, 44, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Banwinkler, M.; Theis, H.; Prange, S.; van Eimeren, T. Imaging the Limbic System in Parkinson’s Disease—A Review of Limbic Pathology and Clinical Symptoms. Brain Sci 2022, 12, 1248. [Google Scholar] [CrossRef]
- Prange, S.; Metereau, E.; Maillet, A.; Lhommée, E.; Klinger, H.; Pelissier, P.; Ibarrola, D.; Heckemann, R.A.; Castrioto, A.; Tremblay, L.; et al. Early Limbic Microstructural Alterations in Apathy and Depression in de Novo Parkinson’s Disease. Movement Disorders 2019, 34, 1644–1654. [Google Scholar] [CrossRef]
- Buchanan, A.M.; Mena, S.; Choukari, I.; Vasa, A.; Crawford, J.N.; Fadel, J.; Maxwell, N.; Reagan, L.; Cruikshank, A.; Best, J.; et al. Serotonin as a Biomarker of Toxin-Induced Parkinsonism. Molecular Medicine 2024, 30, 33. [Google Scholar] [CrossRef]
- Tong, Q.; Zhang, L.; Yuan, Y.; Jiang, S.; Zhang, R.; Xu, Q.; Ding, J.; Li, D.; Zhou, X.; Zhang, K. Reduced Plasma Serotonin and 5-Hydroxyindoleacetic Acid Levels in Parkinson’s Disease Are Associated with Nonmotor Symptoms. Parkinsonism Relat Disord 2015, 21, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Kamagata, K.; Nakatsuka, T.; Sakakibara, R.; Tsuyusaki, Y.; Takamura, T.; Sato, K.; Suzuki, M.; Hori, M.; Kumamaru, K.K.; Inaoka, T.; et al. Diagnostic Imaging of Dementia with Lewy Bodies by Susceptibility-Weighted Imaging of Nigrosomes versus Striatal Dopamine Transporter Single-Photon Emission Computed Tomography: A Retrospective Observational Study. Neuroradiology 2017, 59, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Butkovich, L.M.; Houser, M.C.; Chalermpalanupap, T.; Porter-Stransky, K.A.; Iannitelli, A.F.; Boles, J.S.; Lloyd, G.M.; Coomes, A.S.; Eidson, L.N.; De Sousa Rodrigues, M.E.; et al. Transgenic Mice Expressing Human α-Synuclein in Noradrenergic Neurons Develop Locus Ceruleus Pathology and Nonmotor Features of Parkinson’s Disease. The Journal of Neuroscience 2020, 40, 7559–7576. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, G.; Luo, H.; Pan, Y.; Xu, Q.; Ma, K. Hippocampal Alpha-Synuclein Mediates Depressive-like Behaviors. Brain Behav Immun 2021, 95, 226–237. [Google Scholar] [CrossRef]
- Miquel-Rio, L.; Alarcón-Arís, D.; Torres-López, M.; Cóppola-Segovia, V.; Pavia-Collado, R.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Casal, C.; Montefeltro, A.; et al. Human α-Synuclein Overexpression in Mouse Serotonin Neurons Triggers a Depressive-like Phenotype. Rescue by Oligonucleotide Therapy. Transl Psychiatry 2022, 12, 79. [Google Scholar] [CrossRef]
- Dissanayaka, N.N.W.; White, E.; O’Sullivan, J.D.; Marsh, R.; Silburn, P.A.; Copland, D.A.; Mellick, G.D.; Byrne, G.J. Characteristics and Treatment of Anxiety Disorders in Parkinson’s Disease. Mov Disord Clin Pract 2015, 2, 155–162. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abdelrahman, A.A.; Elserogy, Y.; Zaki, A.F.; Gamea, A. Depression and Anxiety among Patients with Parkinson’s Disease: Frequency, Risk Factors, and Impact on Quality of Life. Egypt J Neurol Psychiatr Neurosurg 2020, 56, 116. [Google Scholar] [CrossRef]
- Lai, T.T.; Gericke, B.; Feja, M.; Conoscenti, M.; Zelikowsky, M.; Richter, F. Anxiety in Synucleinopathies: Neuronal Circuitry, Underlying Pathomechanisms and Current Therapeutic Strategies. NPJ Parkinsons Dis 2023, 9, 97. [Google Scholar] [CrossRef]
- Stoyka, L.E.; Arrant, A.E.; Thrasher, D.R.; Russell, D.L.; Freire, J.; Mahoney, C.L.; Narayanan, A.; Dib, A.G.; Standaert, D.G.; Volpicelli-Daley, L.A. Behavioral Defects Associated with Amygdala and Cortical Dysfunction in Mice with Seeded α-Synuclein Inclusions. Neurobiol Dis 2020, 134, 104708. [Google Scholar] [CrossRef]
- Uemura, N.; Ueda, J.; Yoshihara, T.; Ikuno, M.; Uemura, M.T.; Yamakado, H.; Asano, M.; Trojanowski, J.Q.; Takahashi, R. A-Synuclein Spread from Olfactory Bulb Causes Hyposmia, Anxiety, and Memory Loss in BAC-SNCA Mice. Movement Disorders 2021, 36, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Taddei, R.N.; Cankaya, S.; Dhaliwal, S.; Chaudhuri, K.R. Management of Psychosis in Parkinson’s Disease: Emphasizing Clinical Subtypes and Pathophysiological Mechanisms of the Condition. Parkinsons Dis 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Polissidis, A.; Koronaiou, M.; Kollia, V.; Koronaiou, E.; Nakos-Bimpos, M.; Bogiongko, M.; Vrettou, S.; Karali, K.; Casadei, N.; Riess, O.; et al. Psychosis-Like Behavior and Hyperdopaminergic Dysregulation in Human α -Synuclein <scp>BAC</Scp> Transgenic Rats. Movement Disorders 2021, 36, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Pagonabarraga, J.; Bejr-Kasem, H.; Martinez-Horta, S.; Kulisevsky, J. Parkinson Disease Psychosis: From Phenomenology to Neurobiological Mechanisms. Nat Rev Neurol 2024, 20, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Mielke, M.M.; Rocca, W.A. Incidence and Time Trends of Drug-Induced Parkinsonism: A 30-Year Population-Based Study. Movement Disorders 2017, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Göverti, D.; Büyüklüoğlu, N.; Nazik Yüksel, R.; Kaya, H.; Yücel, Ç.; Göka, E. Decreased Serum Levels of A-synuclein in Patients with Schizophrenia and Their Unaffected Siblings. Early Interv Psychiatry 2023, 17, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Ö.F.; Cetin, İ.; Turan, Ş.; Sağlam, T.; Yıldız, N.; Duran, A. Decreased Expression of α-Synuclein, Nogo-A and UCH-L1 in Patients with Schizophrenia: A Preliminary Serum Study. Psychiatry Investig 2017, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Noori-Daloii, M.R.; Kheirollahi, M.; Mahbod, P.; Mohammadi, F.; Astaneh, A.N.; Zarindast, M.R.; Azimi, C.; Mohammadi, M.R. Alpha- and Beta-Synucleins MRNA Expression in Lymphocytes of Schizophrenia Patients. Genet Test Mol Biomarkers 2010, 14, 725–729. [Google Scholar] [CrossRef]
- Takamura, S.; Ikeda, A.; Nishioka, K.; Furuya, H.; Tashiro, M.; Matsushima, T.; Li, Y.; Yoshino, H.; Funayama, M.; Morinobu, S.; et al. Schizophrenia as a Prodromal Symptom in a Patient Harboring SNCA Duplication. Parkinsonism Relat Disord 2016, 25, 108–109. [Google Scholar] [CrossRef]
- Mosquera, F.E.C.; Guevara-Montoya, M.C.; Serna-Ramirez, V.; Liscano, Y. Neuroinflammation and Schizophrenia: New Therapeutic Strategies through Psychobiotics, Nanotechnology, and Artificial Intelligence (AI). J Pers Med 2024, 14, 391. [Google Scholar] [CrossRef]
- Gerlach, M.; Sharma, M.; Romanos, M.; Lesch, K.-P.; Walitza, S.; Conzelmann, H.A.; Krüger, R.; Renner, T.J. Family-Based Association Study on Functional α-Synuclein Polymorphisms in Attention-Deficit/Hyperactivity Disorder. ADHD Attention Deficit and Hyperactivity Disorders 2019, 11, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Erskine, D.; Thomas, A.J.; Taylor, J.-P.; Savage, M.A.; Attems, J.; McKeith, I.G.; Morris, C.M.; Khundakar, A.A. Neuronal Loss and A-Synuclein Pathology in the Superior Colliculus and Its Relationship to Visual Hallucinations in Dementia with Lewy Bodies. The American Journal of Geriatric Psychiatry 2017, 25, 595–604. [Google Scholar] [CrossRef]
- Doxakis, E. Post-Transcriptional Regulation of α-Synuclein Expression by Mir-7 and Mir-153. Journal of Biological Chemistry 2010, 285, 12726–12734. [Google Scholar] [CrossRef]
- Yang TT, L.C.G.S.Z.Y.W.P. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed Environ Sci 2018. [Google Scholar]
- Han, L.; Tang, Y.; Bai, X.; Liang, X.; Fan, Y.; Shen, Y.; Huang, F.; Wang, J. Association of the Serum MicroRNA-29 Family with Cognitive Impairment in Parkinson’s Disease. Aging 2020, 12, 13518–13528. [Google Scholar] [CrossRef]
- Li, S.; Lei, Z.; Sun, T. The Role of MicroRNAs in Neurodegenerative Diseases: A Review. Cell Biol Toxicol 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Stabile, F.; Torromino, G.; Rajendran, S.; Del Vecchio, G.; Presutti, C.; Mannironi, C.; De Leonibus, E.; Mele, A.; Rinaldi, A. Short-Term Memory Deficit Associates with MiR-153-3p Upregulation in the Hippocampus of Middle-Aged Mice. Mol Neurobiol 2024, 61, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, S.; Rossi, M.; Hall, S.; Quadalti, C.; Mattsson-Carlgren, N.; Dellavalle, S.; Tideman, P.; Pereira, J.B.; Nilsson, M.H.; Mammana, A.; et al. Cognitive Effects of Lewy Body Pathology in Clinically Unimpaired Individuals. Nat Med 2023, 29, 1971–1978. [Google Scholar] [CrossRef]
- Manchinu, M.F.; Pala, M.; Palmas, M.F.; Diana, M.A.; Maschio, A.; Etzi, M.; Pisanu, A.; Diana, F.I.; Marongiu, J.; Mansueto, S.; et al. Region-Specific Changes in Gene Expression Are Associated with Cognitive Deficits in the Alpha-Synuclein-Induced Model of Parkinson’s Disease: A Transcriptomic Profiling Study. Exp Neurol 2024, 372, 114651. [Google Scholar] [CrossRef]
- Ford, B. Pain in Parkinson’s Disease. Movement Disorders 2010, 25. [Google Scholar] [CrossRef]
- Buhidma, Y.; Hobbs, C.; Malcangio, M.; Duty, S. Periaqueductal Grey and Spinal Cord Pathology Contribute to Pain in Parkinson’s Disease. NPJ Parkinsons Dis 2023, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Gonçalves, N.P.; Jan, A.; Jensen, N.M.; van der Laan, A.; Mohseni, S.; Vægter, C.B.; Jensen, P.H. Trans-Synaptic Spreading of Alpha-Synuclein Pathology through Sensory Afferents Leads to Sensory Nerve Degeneration and Neuropathic Pain. Acta Neuropathol Commun 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Möser, C. V.; Weiß, U.; Niederberger, E. The Role of AlphαSynuclein in Mouse Models of Acute, Inflammatory and Neuropathic Pain. Cells 2022, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Chao, D.; Sandhu, H.K.; Liao, X.; Zhao, J.; Wen, G.; Xia, Y. δ-Opioid Receptor Activation Reduces α-Synuclein Overexpression and Oligomer Formation Induced by MPP+ and/or Hypoxia. Exp Neurol 2014, 255, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; White, C.L.; Hladik, C.L.; Sabbagh, M.N.; Connor, D.J.; Shill, H.A.; Sue, L.I.; Sasse, J.; Bachalakuri, J.; Henry-Watson, J.; et al. Olfactory Bulb α-Synucleinopathy Has High Specificity and Sensitivity for Lewy Body Disorders. Acta Neuropathol 2009, 117, 169. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.M.; Wang, Y.; Bhatia, T.N.; Miner, K.M.; Trbojevic, S.A.; Stolz, J.F.; Luk, K.C.; Leak, R.K. The Center of Olfactory Bulb-seeded A-synucleinopathy Is the Limbic System and the Ensuing Pathology Is Higher in Male than in Female Mice. Brain Pathology 2019, 29, 741–770. [Google Scholar] [CrossRef]
- Johnson, M.E.; Bergkvist, L.; Mercado, G.; Stetzik, L.; Meyerdirk, L.; Wolfrum, E.; Madaj, Z.; Brundin, P.; Wesson, D.W. Deficits in Olfactory Sensitivity in a Mouse Model of Parkinson’s Disease Revealed by Plethysmography of Odor-Evoked Sniffing. Sci Rep 2020, 10, 9242. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lopez, E.; Vidyadhara, D.J.; Liberia, T.; Meller, S.J.; Harmon, L.E.; Hsu, R.M.; Spence, N.; Brennan, B.; Han, K.; Yücel, B.; et al. α-Synuclein Pathology and Reduced Neurogenesis in the Olfactory System Affect Olfaction in a Mouse Model of Parkinson’s Disease. The Journal of Neuroscience 2023, 43, 1051–1071. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, K.; Deng, X.-H.; Wei, Y.-H.; Guo, R.; Liu, S.-F.; Zhu, Y.-F.; Zhong, J.-J.; Zheng, J.-Y.; Wang, M.-D.; et al. Amelioration of Olfactory Dysfunction in a Mouse Model of Parkinson’s Disease via Enhancing GABAergic Signaling. Cell Biosci 2023, 13, 101. [Google Scholar] [CrossRef]
- Nieto-Escamez, F.; Obrero-Gaitán, E.; Cortés-Pérez, I. Visual Dysfunction in Parkinson’s Disease. Brain Sci 2023, 13, 1173. [Google Scholar] [CrossRef]
- Ortuño-Lizarán, I.; Esquiva, G.; Beach, T.G.; Serrano, G.E.; Adler, C.H.; Lax, P.; Cuenca, N. Degeneration of Human Photosensitive Retinal Ganglion Cells May Explain Sleep and Circadian Rhythms Disorders in Parkinson’s Disease. Acta Neuropathol Commun 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, E.; Indrieri, A.; Esposito, F.; Tarallo, V.; Carboncino, A.; Alvino, F.G.; De Falco, S.; Franco, B.; De Risi, M.; De Leonibus, E. α-Synuclein Overexpression in the Retina Leads to Vision Impairment and Degeneration of Dopaminergic Amacrine Cells. Sci Rep 2020, 10, 9619. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, M.-A.; Fallah, H.; Toomarisahzabi, M.; Rezaei, F.; Rahimi-Danesh, M.; Akhondzadeh, S.; Vaseghi, S. Parkinson’s Disease: A Narrative Review on Potential Molecular Mechanisms of Sleep Disturbances, REM Behavior Disorder, and Melatonin. Brain Sci 2023, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Roguski, A.; Rayment, D.; Whone, A.L.; Jones, M.W.; Rolinski, M. A Neurologist’s Guide to REM Sleep Behavior Disorder. Front Neurol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-C.; Lee, H.-H.; Hong, C.-T.; Hu, C.-J.; Wu, D. REM Sleep Behavior Disorder (RBD) in Dementia with Lewy Bodies (DLB). Behavioural Neurology 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Schaffrath, A.; Schleyken, S.; Seger, A.; Jergas, H.; Özdüzenciler, P.; Pils, M.; Blömeke, L.; Cousin, A.; Willbold, J.; Bujnicki, T.; et al. Patients with Isolated REM-Sleep Behavior Disorder Have Elevated Levels of Alpha-Synuclein Aggregates in Stool. NPJ Parkinsons Dis 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Ikuno, M.; Hondo, M.; Parajuli, L.K.; Taguchi, K.; Ueda, J.; Sawamura, M.; Okuda, S.; Nakanishi, E.; Hara, J.; et al. α-Synuclein BAC Transgenic Mice Exhibit RBD-like Behaviour and Hyposmia: A Prodromal Parkinson’s Disease Model. Brain 2020, 143, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Fernández-Arcos, A.; Tolosa, E.; Serradell, M.; Molinuevo, J.L.; Valldeoriola, F.; Gelpi, E.; Vilaseca, I.; Sánchez-Valle, R.; Lladó, A.; et al. Neurodegenerative Disorder Risk in Idiopathic REM Sleep Behavior Disorder: Study in 174 Patients. PLoS ONE 2014, 9, e89741. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and Circadian Rhythm Regulation in Early Parkinson Disease. JAMA Neurol 2014, 71, 589. [Google Scholar] [CrossRef]
- Diaconu, Ş.; Irincu, L.; Ungureanu, L.; Ciopleiaș, B.; Țînț, D.; Falup-Pecurariu, C. Restless Legs Syndrome in Parkinson’s Disease. J Pers Med 2023, 13, 915. [Google Scholar] [CrossRef]
- Azmin, S.; Khairul Anuar, A.M.; Nafisah, W.Y.; Tan, H.J.; Raymond, A.A.; Hanita, O.; Shah, S.A.; Norlinah, M.I. Restless Legs Syndrome and Its Associated Risk Factors in Parkinson’s Disease. Parkinsons Dis 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Chung, S.; Bohnen, N.I.; Albin, R.L.; Frey, K.A.; Müller, M.L.T.M.; Chervin, R.D. Insomnia and Sleepiness in Parkinson Disease: Associations with Symptoms and Comorbidities. Journal of Clinical Sleep Medicine 2013, 09, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the Development of Parkinson’s Disease-Related Pathology. Cell Tissue Res 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Lu, J.; Sherman, D.; Devor, M.; Saper, C.B. A Putative Flip–Flop Switch for Control of REM Sleep. Nature 2006, 441, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.M.S.; Andersen, M.L.; Reksidler, A.B.; Vital, M.A.B.F.; Tufik, S. The Role of the Substantia Nigra Pars Compacta in Regulating Sleep Patterns in Rats. PLoS ONE 2007, 2, e513. [Google Scholar] [CrossRef] [PubMed]
- Willison, L.D.; Kudo, T.; Loh, D.H.; Kuljis, D.; Colwell, C.S. Circadian Dysfunction May Be a Key Component of the Non-Motor Symptoms of Parkinson’s Disease: Insights from a Transgenic Mouse Model. Exp Neurol 2013, 243, 57–66. [Google Scholar] [CrossRef]
- McDowell, K.A.; Shin, D.; Roos, K.P.; Chesselet, M.-F. Sleep Dysfunction and EEG Alterations in Mice Overexpressing Alpha-Synuclein. J Parkinsons Dis 2018, 4, 531–539. [Google Scholar] [CrossRef]
- Gajula Balija, M.B.; Griesinger, C.; Herzig, A.; Zweckstetter, M.; Jäckle, H. Pre-Fibrillar α-Synuclein Mutants Cause Parkinson’s Disease-Like Non-Motor Symptoms in Drosophila. PLoS ONE 2011, 6, e24701. [Google Scholar] [CrossRef]
- Dhanushkodi, N.R.; Abul Khair, S.B.; Ardah, M.T.; Haque, M.E. ATP13A2 Gene Silencing in Drosophila Affects Autophagic Degradation of A53T Mutant α-Synuclein. Int J Mol Sci 2023, 24, 1775. [Google Scholar] [CrossRef]
- Abul Khair, S.B.; Dhanushkodi, N.R.; Ardah, M.T.; Chen, W.; Yang, Y.; Haque, M.E. Silencing of Glucocerebrosidase Gene in Drosophila Enhances the Aggregation of Parkinson’s Disease Associated α-Synuclein Mutant A53T and Affects Locomotor Activity. Front Neurosci 2018, 12. [Google Scholar] [CrossRef]
- Ito, K.; Kawasaki, H.; Suzuki, T.; Takahara, T.; Ishida, N. Effects of Kamikihito and Unkei-to on Sleep Behavior of Wild Type and Parkinson Model in Drosophila. Front Psychiatry 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A. The Two-process Model of Sleep Regulation: Beginnings and Outlook. J Sleep Res 2022, 31. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Nat Rev Mol Cell Biol 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurol 2014, 71, 463. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, T.; Schäfer, K.-H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal Involvement in Parkinson’s Disease: Pathophysiology, Diagnosis, and Management. NPJ Parkinsons Dis 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Camerucci, E.; Mullan, A.F.; Bower, J.H.; Bharucha, A.E.; Turcano, P.; Stang, C.D.; Benarroch, E.E.; Boeve, B.F.; Ahlskog, J.E.; Savica, R. Lifelong Constipation in Parkinson’s Disease and Other Clinically Defined Alpha-Synucleinopathies: A Population-Based Study in Southeast Minnesota. Parkinsonism Relat Disord 2023, 107, 105244. [Google Scholar] [CrossRef] [PubMed]
- Borghammer, P.; Horsager, J.; Andersen, K.; Van Den Berge, N.; Raunio, A.; Murayama, S.; Parkkinen, L.; Myllykangas, L. Neuropathological Evidence of Body-First vs. Brain-First Lewy Body Disease. Neurobiol Dis 2021, 161, 105557. [Google Scholar] [CrossRef] [PubMed]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Borghammer, P.; Just, M.K.; Horsager, J.; Skjærbæk, C.; Raunio, A.; Kok, E.H.; Savola, S.; Murayama, S.; Saito, Y.; Myllykangas, L.; et al. A Postmortem Study Suggests a Revision of the Dual-Hit Hypothesis of Parkinson’s Disease. NPJ Parkinsons Dis 2022, 8, 166. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Q.; Wang, Y.; Cheng, Y.; Geng, Y.; Tian, T.; Yuan, Y.; Fan, Y.; Lu, M.; Zhang, K. Phosphorylated α-Synuclein Deposited in Schwann Cells Interacting with TLR2 Mediates Cell Damage and Induces Parkinson’s Disease Autonomic Dysfunction. Cell Death Discov 2024, 10, 52. [Google Scholar] [CrossRef]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective Targeting of the TLR2/MyD88/NF-ΚB Pathway Reduces α-Synuclein Spreading in Vitro and in Vivo. Nat Commun 2021, 12, 5382. [Google Scholar] [CrossRef]
- Conte, C.; Ingrassia, A.; Breve, J.; Bol, J.J.; Timmermans-Huisman, E.; van Dam, A.-M.; Beccari, T.; van de Berg, W.D.J. Toll-like Receptor 4 Is Upregulated in Parkinson’s Disease Patients and Co-Localizes with PSer129αSyn: A Possible Link with the Pathology. Cells 2023, 12, 1368. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Sichetti, M.; Traina, G. Gut–Brain Axis: Focus on Neurodegeneration and Mast Cells. Applied Sciences 2020, 10, 1828. [Google Scholar] [CrossRef]
- Ryman, S.; Vakhtin, A.A.; Richardson, S.P.; Lin, H.C. Microbiome–Gut–Brain Dysfunction in Prodromal and Symptomatic Lewy Body Diseases. J Neurol 2023, 270, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Chuah, K.H.; Beh, Y.Y.; Schee, J.P.; Mahadeva, S.; Lim, S.-Y. Gastrointestinal Dysfunction in Parkinson’s Disease: Neuro-Gastroenterology Perspectives on a Multifaceted Problem. J Mov Disord 2023, 16, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Parkinson’s Disease from the Gut. Brain Res 2018, 1693, 201–206. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Chen, X.; Le, W. New Understanding on the Pathophysiology and Treatment of Constipation in Parkinson’s Disease. Front Aging Neurosci 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Rota, L.; Pellegrini, C.; Benvenuti, L.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Cattaneo, A.; Colla, E. Constipation, Deficit in Colon Contractions and Alpha-Synuclein Inclusions within the Colon Precede Motor Abnormalities and Neurodegeneration in the Central Nervous System in a Mouse Model of Alpha-Synucleinopathy. Transl Neurodegener 2019, 8, 5. [Google Scholar] [CrossRef]
- Qiao, C.-M.; Sun, M.-F.; Jia, X.-B.; Li, Y.; Zhang, B.-P.; Zhao, L.-P.; Shi, Y.; Zhou, Z.-L.; Zhu, Y.-L.; Cui, C.; et al. Sodium Butyrate Exacerbates Parkinson’s Disease by Aggravating Neuroinflammation and Colonic Inflammation in MPTP-Induced Mice Model. Neurochem Res 2020, 45, 2128–2142. [Google Scholar] [CrossRef]
- Mahbub, N.U.; Islam, M.M.; Hong, S.-T.; Chung, H.-J. Dysbiosis of the Gut Microbiota and Its Effect on α-Synuclein and Prion Protein Misfolding: Consequences for Neurodegeneration. Front Cell Infect Microbiol 2024, 14. [Google Scholar] [CrossRef]
- Bhattacharyya, K.B.; Rosa-Grilo, M. Sexual Dysfunctions in Parkinson’s Disease: An Underrated Problem in a Much Discussed Disorder. In; 2017; pp. 859–876.
- Duan, W.-X.; Wang, F.; Liu, J.-Y.; Liu, C.-F. Relationship Between Short-Chain Fatty Acids and Parkinson’s Disease: A Review from Pathology to Clinic. Neurosci Bull 2024, 40, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of Gut Microbiota, Short-Chain Fatty Acids, Inflammation, and the Gut Barrier in Parkinson’s Disease. Mol Neurodegener 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Kakoty, V.; K C, S.; Dubey, S.K.; Yang, C.-H.; Taliyan, R. Neuroprotective Effects of Trehalose and Sodium Butyrate on Preformed Fibrillar Form of α-Synuclein-Induced Rat Model of Parkinson’s Disease. ACS Chem Neurosci 2021, 12, 2643–2660. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R.; Tateno, F.; Yamamoto, T.; Uchiyama, T.; Yamanishi, T. Urological Dysfunction in Synucleinopathies: Epidemiology, Pathophysiology and Management. Clinical Autonomic Research 2018, 28, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G.; Liu, J. Autonomic Dysfunction in Parkinson’s Disease: Implications for Pathophysiology, Diagnosis, and Treatment. Neurobiol Dis 2020, 134, 104700. [Google Scholar] [CrossRef]
- Shalash, A.; Hamid, E.; Elrassas, H.; Abushouk, A.I.; Salem, H.H. Sexual Dysfunction in Male Patients with Parkinson’s Disease: Related Factors and Impact on Quality of Life. Neurological Sciences 2020, 41, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Varanda, S.; Ribeiro da Silva, J.; Costa, A.S.; Amorim de Carvalho, C.; Alves, J.N.; Rodrigues, M.; Carneiro, G. Sexual Dysfunction in Women with Parkinson’s Disease. Movement Disorders 2016, 31, 1685–1693. [Google Scholar] [CrossRef]
- Hasan, S.; Mielke, M.M.; Ahlskog, J.E.; Bower, J.; Turcano, P.; Savica, R. Erectile Dysfunction Preceding Clinically Diagnosed Synucleinopathies: A Case-Control Study in Olmsted County. Parkinsons Dis 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Shaltiel-Karyo, R.; Davidi, D.; Menuchin, Y.; Frenkel-Pinter, M.; Marcus-Kalish, M.; Ringo, J.; Gazit, E.; Segal, D. A Novel, Sensitive Assay for Behavioral Defects in Parkinson’s Disease Model Drosophila. Parkinsons Dis 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Hatate, J.; Miwa, K.; Matsumoto, M.; Sasaki, T.; Yagita, Y.; Sakaguchi, M.; Kitagawa, K.; Mochizuki, H. Association between Cerebral Small Vessel Diseases and Mild Parkinsonian Signs in the Elderly with Vascular Risk Factors. Parkinsonism Relat Disord 2016, 26, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Potashkin, J.; Huang, X.; Becker, C.; Chen, H.; Foltynie, T.; Marras, C. Understanding the Links Between Cardiovascular Disease and Parkinson’s Disease. Movement Disorders 2020, 35, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, M.; Fang, Q.; Huang, J. Relationship between Parkinson’s Disease and Cardio-Cerebrovascular Diseases: A Mendelian Randomized Study. Sci Rep 2023, 13, 20428. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, S. Association between Parkinson’s Disease and the Risk of Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Holmes, C.; Wu, T.; Goldstein, D.S. Long-Term Trends in Myocardial Sympathetic Innervation and Function in Synucleinopathies. Parkinsonism Relat Disord 2019, 67, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Goldstein, D.S. Cardiovascular Dysautonomia in Parkinson Disease: From Pathophysiology to Pathogenesis. Neurobiol Dis 2012, 46, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Isonaka, R.; Sullivan, P.; Goldstein, D.S. Pathophysiological Significance of Increased α-Synuclein Deposition in Sympathetic Nerves in Parkinson’s Disease: A Post-Mortem Observational Study. Transl Neurodegener 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Goldstein, D.S. Cardiovascular Dysautonomia in Parkinson Disease: From Pathophysiology to Pathogenesis. Neurobiol Dis 2012, 46, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Cardiac Abnormalities in Parkinson’s Disease and Parkinsonism. Journal of Clinical Neuroscience 2018, 53, 1–5. [Google Scholar] [CrossRef]
- Park, D.G.; Kang, J.; An, Y.-S.; Chang, J.; Yoon, J.H. Association of Plasma α-Synuclein with Cardiac 123I-MIBG Scintigraphy in Early Parkinson’s Disease. Neurosci Lett 2022, 770, 136399. [Google Scholar] [CrossRef]
- Orimo, S.; Uchihara, T.; Nakamura, A.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Axonal -Synuclein Aggregates Herald Centripetal Degeneration of Cardiac Sympathetic Nerve in Parkinson’s Disease. Brain 2008, 131, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Javanshiri, K.; Drakenberg, T.; Haglund, M.; Englund, E. Sudden Cardiac Death in Synucleinopathies. J Neuropathol Exp Neurol 2023, 82, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Grosu, L.; Grosu, A.; Crisan, D.; Zlibut, A.; Perju-Dumbrava, L. Parkinson’s Disease and Cardiovascular Involvement: Edifying Insights (Review). Biomed Rep 2023, 18, 25. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




