Submitted:
18 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Study Population
- Ethics Declaration
- Endocarditis
- Indication for Valve Surgery
- AKI
- Outcomes investigated
- Statistical Analysis
3. Results
- Baseline Characteristics
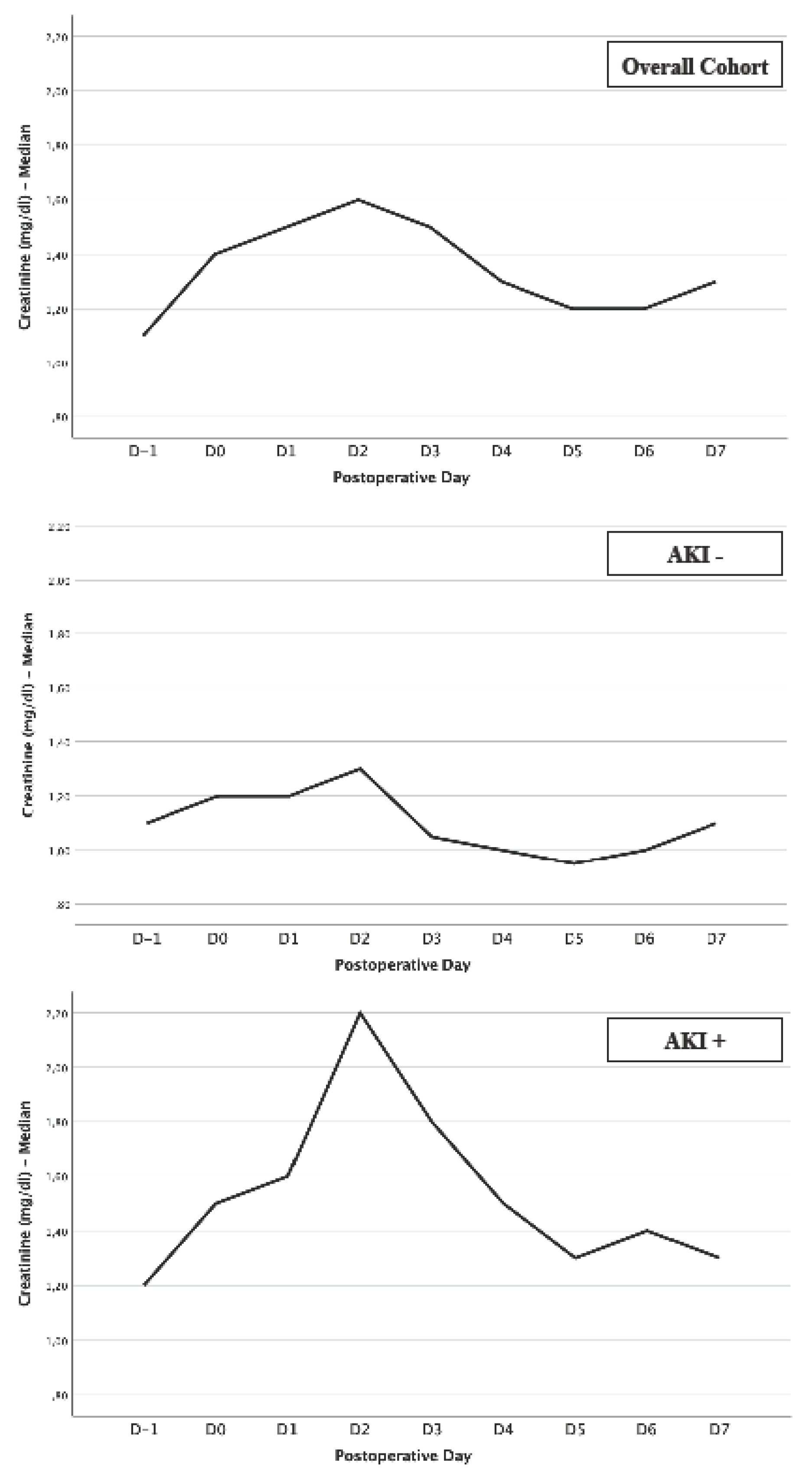
- Creatinine Levels: 2nd postoperative day as vulnerable day regarding kidney function
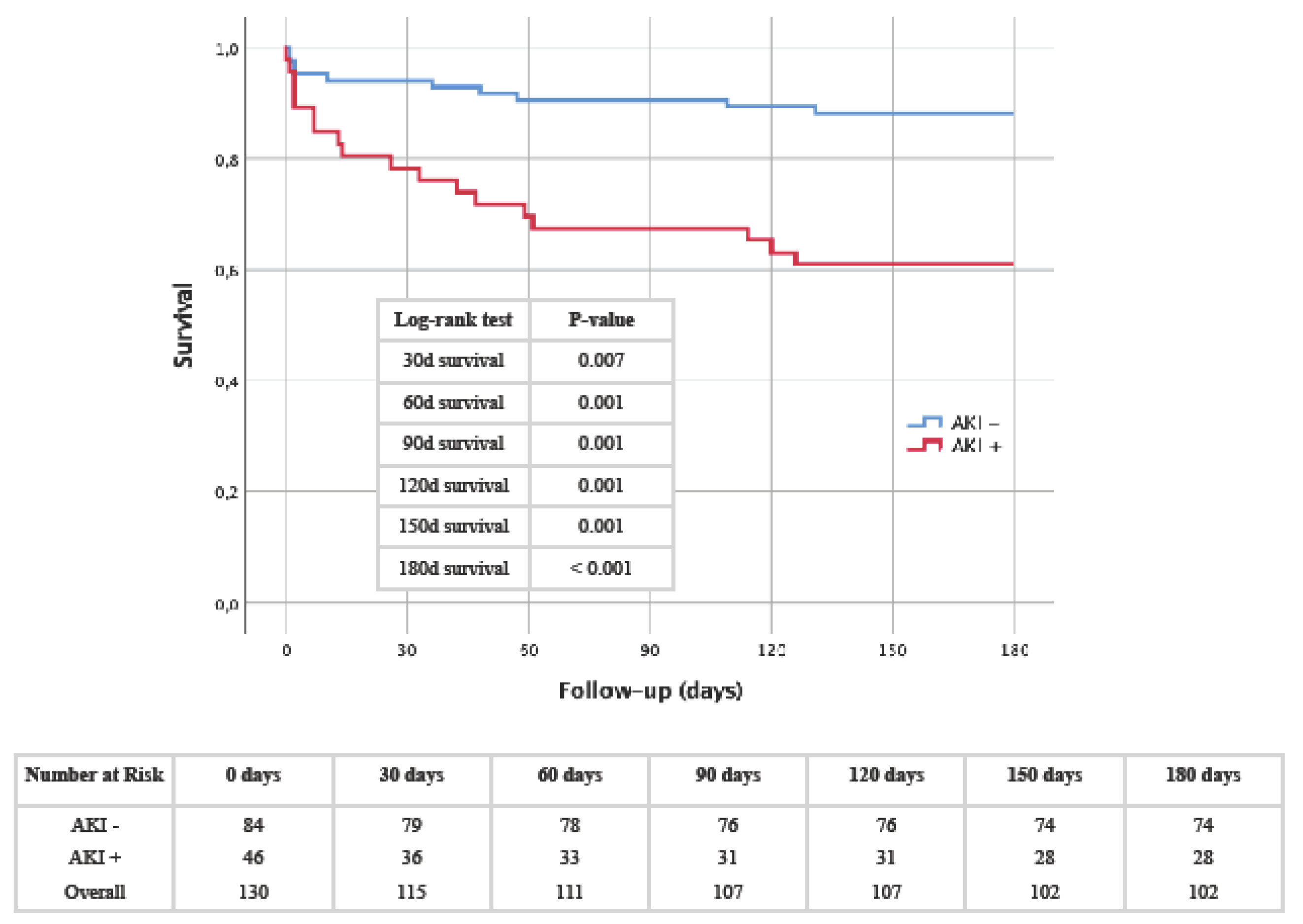
- Kaplan-Meier: AKI as a driving force for premature mortality
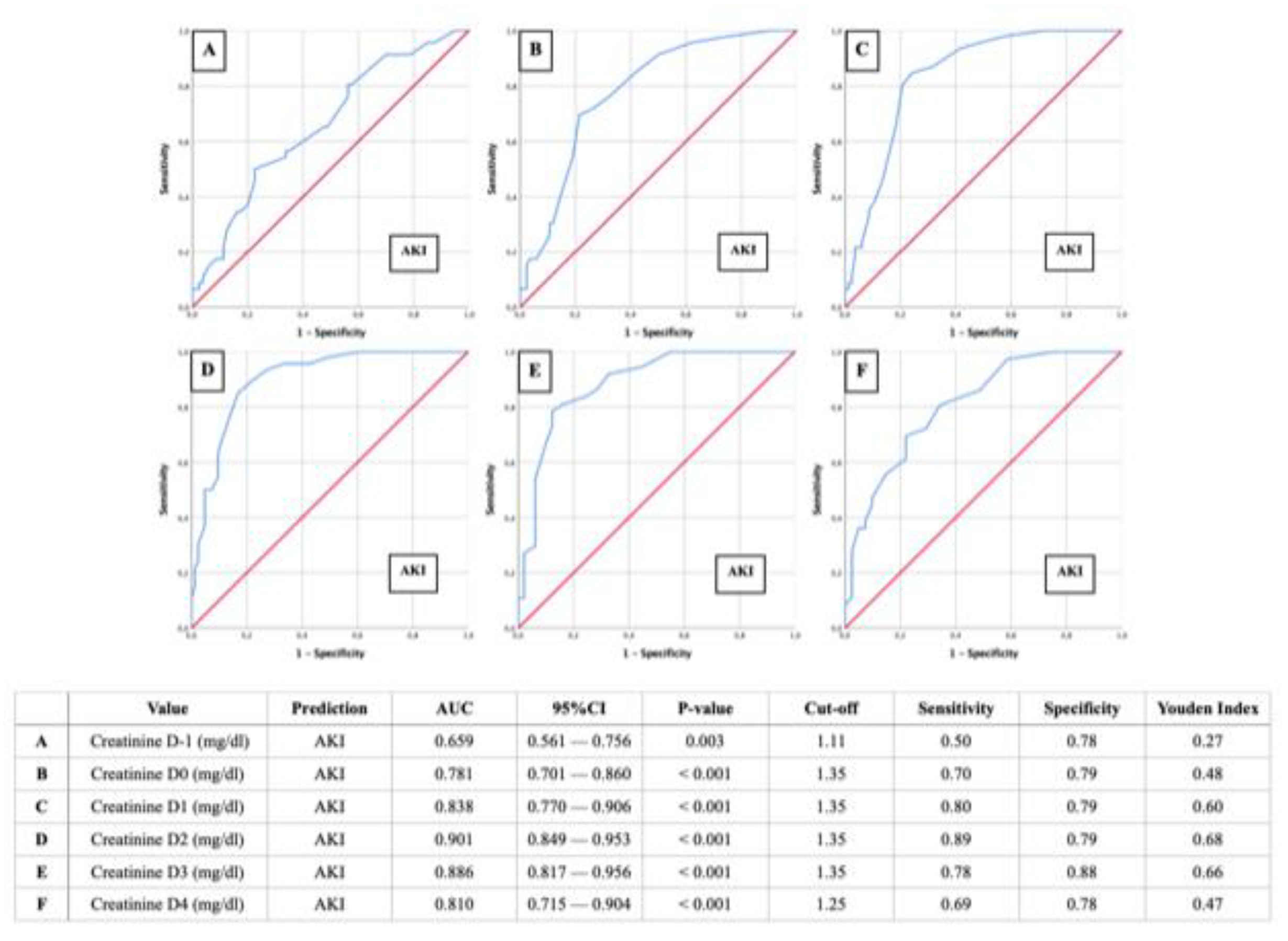
- AUROC-AKI: Creatinine of 1.35 mg/dl as relevant predictor for postoperative AKI
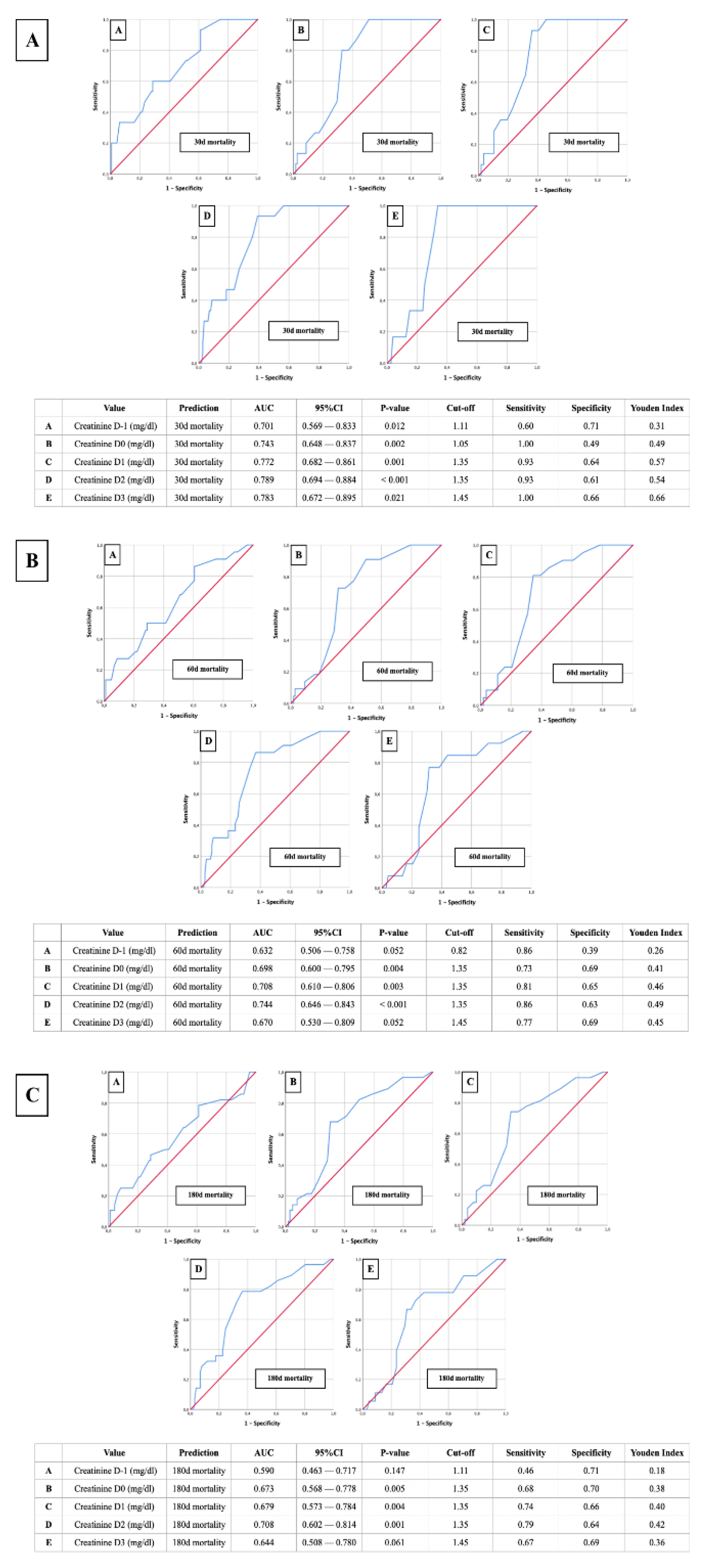
- AUROC-Mortality: Creatinine of 1.35 mg/dl as relevant predictor for postoperative mortality
- Binary Logistic Regression: Hemoglobin, CK-MB and renal excretion 2-3 hours after surgery as independent predictors for postoperative AKI
4. Discussion
- Postoperative frequency of AKI in patients undergoing valve surgery due to endocarditis
- Early detection of AKI: Overcoming the challenge of delay
- Consistancy of our creatinine cut-off value highlights AKI’s significance in short-term mortality prediction
- How can we prevent postoperative AKI following surgery for endocarditis?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Hubers, S.A.; DeSimone, D.C.; Gersh, B.J.; Anavekar, N.S. Infective Endocarditis: A Contemporary Review. Mayo Clin. Proc. 2020, 95, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Lichtenauer, M.; Haar, M.; Hödl, G.; Kern, J.M.; Zhou, Z.; Wuppinger, T.; Kraus, J.; Strohmer, B.; Hoppe, U.C.; et al. Infective endocarditis – A review of current therapy and future challenges. Hell. J. Cardiol. 2021, 62, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Arockiam, A.D.; Jamil, Y.; El Dahdah, J.; Honnekeri, B.; El Helou, M.C.; Kassab, J.; Wang, T.K.M. Contemporary risk models for infective endocarditis surgery: a narrative review. Ther. Adv. Cardiovasc. Dis. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, A.; Barbone, A.; Basciu, A.; Cuko, E.; Droandi, G.; Galbiati, D.; Romano, G.; Citterio, E.; Fumero, A.; Scarfò, I.; et al. Surgical Challenges in Infective Endocarditis: State of the Art. J. Clin. Med. 2023, 12, 5891. [Google Scholar] [CrossRef] [PubMed]
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson, G. B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Pirracchio, R.; Rosa, A.; Petersen, M.L.; Van der Laan, M.; Fabiani, J.-N.; Fernandez-Gerlinger, M.-P.; Podglajen, I.; Safran, D.; Cholley, B.; et al. Incidence, risk factors and prediction of post-operative acute kidney injury following cardiac surgery for active infective endocarditis: an observational study. Crit. Care 2013, 17, R220–10. [Google Scholar] [CrossRef] [PubMed]
- Von Tokarski, F.; Lemaignen, A.; Portais, A.; Fauchier, L.; Hennekinne, F.; Sautenet, B.; Halimi, J.-M.; Legras, A.; Patat, F.; Bourguignon, T.; et al. Risk factors and outcomes of early acute kidney injury in infective endocarditis: A retrospective cohort study. Int. J. Infect. Dis. 2020, 99, 421–427. [Google Scholar] [CrossRef]
- Ortiz-Soriano, V.; Donaldson, K.; Du, G.; Li, Y.; Lambert, J.; Rudy, M.; Cleland, D.; Thornton, A.; Fanucchi, L.C.; Huaman, M.A.; et al. Incidence and Cost of Acute Kidney Injury in Hospitalized Patients with Infective Endocarditis. J. Clin. Med. 2019, 8, 927. [Google Scholar] [CrossRef]
- Ritchie, B.M.; Hirning, B.A.; Stevens, C.A.; Cohen, S.A.; DeGrado, J.R. Risk factors for acute kidney injury associated with the treatment of bacterial endocarditis at a tertiary academic medical center. J. Chemother. 2017, 29, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.; Cazorla, C.; Tulane, C.; Fuzellier, J.; Verhoeven, P.; Frésard, A.; et al. Acute kidney injury in infective endocarditis: A retrospective analysis. Med. Et Mal. Infect. 2019, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Delgado, V.; Marsan, N.A.; Marsan, N.A.; de Waha, S.; de Waha, S.; Bonaros, N.; Bonaros, N.; Brida, M.; Brida, M.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Hear. J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Borger, M.A. The value of an “Endocarditis Team”. Ann. Cardiothorac. Surg. 2019, 8, 621–629. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.N.; Nakazone, M.A.; Maia, L.N. Acute Kidney Injury Based on KDIGO (Kidney Disease Improving Global Outcomes) Criteria in Patients with Elevated Baseline Serum Creatinine Undergoing Cardiac Surgery. Rev. Bras. de Cir. Cardiovasc. 2014, 29, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.; Hernandez, A.; Parramon, F.; Estanyol, N.; Pardina, B.; Muñoz, A.; Alvarez, P.; Hernandez, C. Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int. J. Nephrol. Renov. Dis. 2019, 12, 153–166. [Google Scholar] [CrossRef]
- Schurle, A.; Koyner, J.L. CSA-AKI: Incidence, Epidemiology, Clinical Outcomes, and Economic Impact. J. Clin. Med. 2021, 10, 5746. [Google Scholar] [CrossRef]
- O’Neal, J.B.; Shaw, A.D.; Billings F.T., IV. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit. Care 2016, 20, 187. [Google Scholar] [CrossRef]
- Ramos, K.A.; Dias, C.B. Acute Kidney Injury after Cardiac Surgery in Patients Without Chronic Kidney Disease. Rev. Bras. de Cir. Cardiovasc. 2018, 33, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Eltzschig, H.K. Disease Mechanisms of Perioperative Organ Injury. Obstet. Anesthesia Dig. 2020, 131, 1730–1750. [Google Scholar] [CrossRef] [PubMed]
- Nadim, M.K.; Forni, L.G.; Bihorac, A.; Hobson, C.; Koyner, J.L.; Shaw, A.; Arnaoutakis, G.J.; Ding, X.; Engelman, D.T.; Gasparovic, H.; et al. Cardiac and Vascular Surgery–Associated Acute Kidney Injury: The 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J. Am. Hear. Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.; Alberts, T.; Preckel, B.; Strypet, M.; Eberl, S. Perioperative Complications in Infective Endocarditis. J. Clin. Med. 2023, 12, 5762. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.; Uddin, M.; Qureshi, W.T.; Regmi, N.; Tleyjeh, I.M.; Saydain, G. Predictors of Complications Secondary to Infective Endocarditis and Their Associated Outcomes: A Large Cohort Study from the National Emergency Database (2016–2018). Infect. Dis. Ther. 2022, 11, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Shaw, A.D.; Mythen, M.G.; Guzzi, L.; Reddy, V.S.; Crisafi, C.; Engelman, D.T. PeriOperative Quality Initiative and the Enhanced Recovery After Surgery Cardiac Workgroup. Adult Cardiac Surgery-Associated Acute Kidney Injury: Joint Consensus Report. J. Cardiothorac. Vasc. Anesthesia 2023, 37, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M. Serum creatinine role in predicting outcome after cardiac surgery beyond acute kidney injury. World J. Cardiol. 2014, 6, 1006–21. [Google Scholar] [CrossRef] [PubMed]
- Makris K (2018) The role of the clinical laboratory in the detection and monitoring of acute kidney injury. J Lab Precis Med. 3:4454. Accessed , 2024. https://jlpm.amegroups.org/article/view/4454.
- Cheruku, S.R.; Raphael, J.; Neyra, J.A.; Fox, A.A. Acute Kidney Injury after Cardiac Surgery: Prediction, Prevention, and Management. Anesthesiology 2023, 139, 880–898. [Google Scholar] [CrossRef]
- Hou, J.; Shang, L.; Huang, S.; Ao, Y.; Yao, J.; Wu, Z. Postoperative Serum Creatinine Serves as a Prognostic Predictor of Cardiac Surgery Patients. Front. Cardiovasc. Med. 2022, 9, 740425. [Google Scholar] [CrossRef]
- Ye, M.; Dai, Q.; Zheng, J.; Jiang, X.; Wang, H.; Lou, S.; Yu, K. The Significance of Post-operative Creatinine in Predicting Prognosis in Cardiac Surgery Patients. Cell Biochem. Biophys. 2014, 70, 587–591. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Haase, M.; Lewington, A.J.; O'Donoghue, D.J.; Wilson, F.P.; Nadim, M.K.; Silver, S.A.; Zarbock, A.; Ostermann, M.; et al. Quality Improvement Goals for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2019, 14, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Busse, L.W. New strategies to optimize renal haemodynamics. Curr. Opin. Crit. Care 2020, 26, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Fellahi, J.-L.; Futier, E.; Vaisse, C.; Collange, O.; Huet, O.; Loriau, J.; Gayat, E.; Tavernier, B.; Biais, M.; Asehnoune, K.; et al. Perioperative hemodynamic optimization: from guidelines to implementation—an experts’ opinion paper. Ann. Intensiv. Care 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kayilioglu, S.I.; Dinc, T.; Sozen, I.; Bostanoglu, A.; Cete, M.; Coskun, F. Postoperative fluid management. World J. Crit. Care Med. 2015, 4, 192–201. [Google Scholar] [CrossRef]
- Gumbert, S.D.; Kork, F.; Jackson, M.L.; Vanga, N.; Ghebremichael, S.J.; Wang, C.Y.; Eltzschig, H.K. Perioperative Acute Kidney Injury. Anesthesiology 2020, 132, 180–204. [Google Scholar] [CrossRef]
- Kalisnik, J.M.; Bauer, A.; Vogt, F.A.; Stickl, F.J.; Zibert, J.; Fittkau, M.; Bertsch, T.; Kounev, S.; Fischlein, T. Artificial intelligence-based early detection of acute kidney injury after cardiac surgery. Eur. J. Cardio-Thoracic Surg. 2022, 62. [Google Scholar] [CrossRef]
| Total | AKI+ | AKI- | P-value | |
|---|---|---|---|---|
| No. (%) | ||||
| Total | 130 (100) | 46 (35.4) | 84 (64.6) | |
| Gender (male) | 92 (70.8) | 31 (67.4) | 61 (72.6) | 0.531 |
|
Age < 20 20 - 39 40 - 59 60 - 79 ≥ 80 |
1 (0.8) 14 (10.8) 29 (22.3) 81 (62.3) 5 (3.8) |
0 (0.0) 3 (6.5) 6 (13.0) 35 (76.1) 2 (4.3) |
1 (1.2) 11 (13.1) 23 (27.4) 46 (54.8) 3 (3.6) |
0.458 0.248 0.060 0.016 0.826 |
|
BMI < 18.5 18.5 - 24.9 25.0 - 29.9 30.0 - 34.9 35.0 - 39.9 ≥ 40.0 |
2 (1.5) 50 (38.5) 55 (42.3) 17 (13.1) 6 (4.6) 0 (0.0) |
0 (0.0) 14 (30.4) 21 (45.7) 7 (15.2) 4 (8.7) 0 (0.0) |
2 (2.4) 36 (42.9) 34 (40.5) 10 (11.9) 2 (2.4) 0 (0.0) |
0.292 0.164 0.568 0.592 0.101 - |
|
NYHA NYHA I NYHA II NYHA III NYHA IV |
78 (60.0) 29 (22.3) 12 (9.2) 11 (8.5) |
25 (54.3) 8 (17.4) 8 (17.4) 5 (10.9) |
53 (63.1) 21 (25.0) 4 (4.8) 6 (7.1) |
0.330 0.319 0.017 0.465 |
|
Microbiology Staphylococcus spp. - Staphylococcus aureus - Staphylococcus epidermidis Streptococcus spp. - Streptococcus mitis/oralis - Streptococcus sanguis/parasanguis Enterococcus spp. - Enterococcus faecalis HACEK group Candida spp. Mixed Infection Others Negative Blood Cultures and PCRs |
48 (36.9) 37 (28.5) 6 (4.6) 29 (22.3) 10 (7.7) 9 (6.9) 17 (13.1) 16 (12.3) 1 (0.8) 1 (0.8) 8 (6.2) 5 (3.8) 21 (16.2) |
22 (47.8) 18 (39.1) 2 (4.3) 4 (8.7) 2 (4.3) 1 (2.2) 7 (15.2) 7 (15.2) 0 (0.0) 0 (0.0) 4 (8.7) 0 (0.0) 9 (19.6) |
26 (31.0) 19 (22.6) 4 (4.8) 25 (29.8) 8 (9.5) 8 (9.5) 10 (11.9) 9 (10.7) 1 (1.2) 1 (1.2) 4 (4.8) 5 (6.0) 12 (14.3) |
0.057 0.046 0.914 0.006 0.290 0.114 0.592 0.455 0.458 0.458 0.372 0.458 0.434 |
|
Pre-existing Conditions Diabetes mellitus Arterial Hypertension CVD Previous Myocardial Infarction Atrial fibrillation Previous Aortocoronary Bypass Pacemaker (before Endocarditis) COPD Nicotine Consumption Hyperlipidemia Stroke (before Endocarditis) PAOD Chronic Kidney Disease Chronic Heart Failure |
19 (14.6) 66 (50.8) 40 (30.8) 8 (6.2) 35 (26.9) 15 (11.5) 9 (6.9) 8 (6.2) 15 (11.5) 53 (40.8) 11 (8.5) 8 (6.2) 18 (13.8) 22 (16.9) |
5 (10.9) 26 (56.5) 14 (30.4) 3 (6.5) 16 (34.8) 7 (15.2) 3 (6.5) 6 (13.0) 4 (8.7) 22 (47.8) 5 (10.9) 3 (6.5) 6 (13.0) 5 (10.9) |
14 (16.7) 40 (47.6) 26 (31.0) 5 (6.0) 19 (22.6) 8 (9.5) 6 (7.1) 2 (2.4) 11 (13.1) 31 (36.9) 6 (7.1) 5 (6.0) 12 (14.3) 17 (20.2) |
0.371 0.332 0.951 0.897 0.135 0.331 0.894 0.016 0.453 0.226 0.465 0.897 0.845 0.173 |
|
Premedication Beta-Blocker Diuretics ACEI/ARB/ARNI Statins |
58 (44.6) 56 (43.1) 36 (27.7) 33 (25.4) |
23 (50.0) 25 (54.3) 17 (37.0) 16 (34.8) |
35 (41.7) 31 (36.9) 19 (22.6) 17 (20.2) |
0.361 0.055 0.081 0.068 |
|
Preoperative Conditions Elective Surgery Urgent Surgery Emergency Surgery Prosthetic Valve Endocarditis Cardiogenic shock |
9 (6.9) 97 (74.6) 24 (18.5) 35 (26.9) 1 (0.8) |
2 (4.3) 35 (76.1) 9 (19.6) 16 (34.8) 1 (2.2) |
7 (8.3) 62 (73.8) 15 (17.9) 19 (22.6) 0 (0.0) |
0.392 0.775 0.810 0.135 0.175 |
|
Intraoperative Conditions Endocarditis of One Heart Valve Endocarditis of Two Heart Valves Endocarditis of Three Heart Valves One Surgically Repaired Heart Valve Two Surgical Repaired Heart Valves Three Surgical Repaired Heart Valves Additional aortocoronary bypass Cardioplegia Blood Products |
111 (85.4) 19 (14.6) 0 (0.0) 79 (60.8) 43 (33.1) 8 (6.2) 11 (8.5) 124 (95.4) 94 (72.3) |
38 (82.6) 8 (17.4) 0 (0.0) 23 (50.0) 18 (39.1) 5 (10.9) 5 (10.9) 44 (95.7) 40 (87.0) |
73 (86.9) 11 (13.1) 0 (0.0) 54 (66.7) 25 (29.8) 3 (3.6) 6 (7.1) 80 (95.2) 54 (64.3) |
0.507 0.525 - 0.063 0.278 0.098 0.465 0.914 0.006 |
|
Postoperative Conditions ECMO Bleeding/Tamponade Stroke Valvular Complications Complicated Pneumonia Wound Healing Disorder Third-Degree Atrioventricular Block Sepsis Tracheostomy In-Hospital Death |
5 (3.8) 15 (11.5) 4 (3.1) 1 (0.8) 5 (3.8) 6 (4.6) 15 (11.5) 2 (1.5) 4 (3.1) 21 (16.2) |
3 (6.5) 6 (13.0) 2 (4.3) 1 (0.8) 4 (8.7) 4 (8.7) 4 (8.7) 0 (0.0) 2 (4.3) 15 (32.6) |
2 (2.4) 9 (10.7) 2 (2.4) 0 (0.0) 1 (1.2) 2 (2.4) 11 (13.1) 2 (2.4) 2 (2.4) 6 (7.1) |
0.240 0.691 0.535 0.175 0.033 0.101 0.453 0.292 0.535 < 0.001 |
| Mean ± SD | ||||
| Age (years) | 61.9 ± 14.4 | 65.7 ± 12.2 | 59.8 ± 15.1 | 0.023 |
| Height (cm) | 172.9 ± 7.8 | 171.9 ± 7.4 | 173.5 ± 8.0 | 0.265 |
| Weight (kg) | 79.4 ± 15.6 | 82.2 ± 15.5 | 77.9 ± 15.5 | 0.135 |
| BMI (kg/m2) | 26.5 ± 4.5 | 27.8 ± 4.6 | 25.8 ± 4.3 | 0.016 |
| BSA (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.403 |
| ACEF 2 | 3.1 ± 1.6 | 3.3 ± 1.5 | 3.0 ± 1.6 | 0.300 |
| EuroScore II | 10.4 ± 10.2 | 13.4 ± 9.6 | 8.8 ± 10.3 | 0.013 |
| Surgery Time (min) | 271.8 ± 114.8 | 316.4 ± 117.0 | 247.3 ± 106.6 | 0.001 |
| Clamping Time (min) | 104.0 ± 53.9 | 125.6 ± 58.9 | 92.1 ± 47.3 | 0.001 |
| Perfusion Time (min) | 158.4 ± 86.2 | 194.0 ± 96.5 | 138.9 ± 73.6 | < 0.001 |
| Hospitalization Days (d) | 20.6 ± 20.3 | 23.7 ± 22.6 | 19.0 ± 18.9 | 0.208 |
| Postoperative Days (d) | 24.5 ± 21.7 | 27.2 ± 24.8 | 23.1 ± 19.9 | 0.302 |
| Ventilation Period (h) | 55.5 ± 87.7 | 91.7 ± 99.9 | 35.7 ± 73.6 | 0.001 |
| ICU stay (h) | 240.8 ± 390.5 | 353.4 ± 493.8 | 179.1 ± 306.5 | 0.033 |
| Red Blood Cell Concentrates (No.) | 1.9 ± 2.7 | 2.2 ± 1.8 | 1.7 ± 3.1 | 0.402 |
| Platelet Concentrate (No.) | 0.4 ± 0.8 | 0.6 ± 0.9 | 0.3 ± 0.8 | 0.060 |
| FFPs (No.) | 1.1 ± 2.1 | 1.9 ± 2.5 | 0.7 ± 1.7 | 0.004 |
| Median ± IQR | ||||
| LVEF (%) | 55.0 ± 5.0 | 55.0 ± 4.5 | 55.0 ± 5.0 | 0.746 |
| Min. Hb — intraop. (g/dl) | 7.5 ± 1.0 | 7.4 ± 1.1 | 7.6 ± 1.3 | 0.056 |
| Min. Hb — 6h postop. (g/dl) | 9.1 ± 1.7 | 8.4 ± 1.3 | 9.5 ± 1.7 | 0.077 |
| Min. Hb — 24h postop. (g/dl) | 8.7 ± 1.5 | 7.9 ± 1.1 | 8.9 ± 1.2 | 0.075 |
| Max. Lactate — 6h postop. (mmol/l) | 2.6 ± 2.5 | 3.8 ± 3.3 | 2.3 ± 1.4 | 0.006 |
| Max. Lactate — 24h postop. (mmol/l) | 2.9 ± 2.8 | 4.6 ± 2.1 | 2.4 ± 2.2 | 0.023 |
| Max. Troponin T — 24h postop. (ng/l) | 800.0 ± 1380.5 | 1024.0 ± 3619.5 | 728.5 ± 798.3 | 0.007 |
| Max. CK-MB - 24h postop (U/l) | 57.8 ± 54.8 | 91.7 ± 117.1 | 53.6 ± 28.3 | < 0.001 |
| Min. MAP — intraop. (mmHg) | 47.0 ± 7.1 | 47.0 ± 7.1 | 49.0 ± 7.5 | 0.439 |
| Max. NOR — intraop. (ml/min/kg) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.149 |
| ⌀ NOR — intraop. (ml/min/kg) | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.282 |
| Renal Excretion 1 - 2h (ml) | 40.0 ± 65.0 | 30.0 ± 22.5 | 45.0 ± 103.8 | 0.001 |
| Renal Excretion 2 - 3h (ml) | 40.0 ± 52.5 | 20.0 ± 30.0 | 50.0 ± 70.0 | < 0.001 |
| Drainage Volume — 6h postop. (ml) | 230.0 ± 285.0 | 350.0 ± 215.0 | 150.0 ± 145.0 | < 0.001 |
| Drainage Volume — 12h postop. (ml) | 270.0 ± 350.0 | 350.0 ± 287.5 | 150.0 ± 312.5 | < 0.001 |
| Drainage Volume — 24h postop. (ml) | 550 ± 587.5 | 800.0 ± 512.5 | 400 ± 548.8 | 0.008 |
| Fluide Volume — intraop. (l) | 3.2 ± 1.6 | 3.2 ± 1.6 | 3.2 ± 1.6 | 0.134 |
| Total | AKI- | AKI+ | P-value | |
|---|---|---|---|---|
| Median ± IQR | ||||
| Creatinine D-1 (mg/dl) | 1.1 ± 0.7 | 1.1 ± 0.6 | 1.2 ± 0.7 | 0.003 |
| Creatinine D0 (mg/dl) | 1.4 ± 0.8 | 1.2 ± 0.7 | 1.5 ± 0.9 | < 0.001 |
| Creatinine D1 (mg/dl) | 1.5 ± 0.8 | 1.2 ± 0.6 | 1.6 ± 0.9 | < 0.001 |
| Creatinine D2 (mg/dl) | 1.6 ± 1.0 | 1.3 ± 0.7 | 2.2 ± 1.0 | < 0.001 |
| Creatinine D3 (mg/dl) | 1.5 ± 1.0 | 1.1 ± 0.7 | 1.8 ± 1.0 | < 0.001 |
| Creatinine D4 (mg/dl) | 1.3 ± 0.9 | 1.0 ± 0.7 | 1.5 ± 1.0 | < 0.001 |
| Creatinine D5 (mg/dl) | 1.2 ± 0.7 | 1.0 ± 0.7 | 1.3 ± 0.9 | < 0.001 |
| Creatinine D6 (mg/dl) | 1.2 ± 1.0 | 1.0 ± 0.7 | 1.4 ± 1.2 | < 0.001 |
| Creatinine D7 (mg/dl) | 1.3 ± 0.8 | 1.1 ± 0.7 | 1.3 ± 1.6 | 0.003 |
| AKI Binary Logistic Regression | Univariate | Multivariable | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
| Age | 1.601 (1.057 — 2.423) | 0.026 | 0.656 (0.062 — 6.982) | 0.727 |
| BMI | 1.572 (1.079 — 2.291) | 0.019 | 2.035 (0.579 — 7.149) | 0.268 |
| EuroScore II | 1.564 (1.080 — 2.265) | 0.018 | 1.329 (0.636 — 2.775) | 0.449 |
| Renal Excretion 0 - 1h (postoperative) | 0.331 (0.117 — 0.942) | 0.038 | 0.445 (0.073 — 2.703) | 0.379 |
| Renal Excretion 2 - 3h (postoperative) | 0.324 (0.141 — 0.744) | 0.008 | 0.003 (0.000 — 0.275) | 0.012 |
| Hb minimal (intraoperative) | 0.437 (0.212 — 0.900) | 0.025 | 0.203 (0.044 — 0.926) | 0.039 |
| Surgery Time | 1.873 (1.268 — 2.766) | 0.002 | 0.225 (0.007 — 7.564) | 0.405 |
| Clamping Time | 1.964 (1.292 — 2.985) | 0.002 | 0.220 (0.019 — 2.499) | 0.222 |
| Perfusion Time | 2.003 (1.316 — 3.048) | 0.001 | 1.260 (0.447 — 3.552) | 0.662 |
| Ventilation Time | 1.954 (1.285 — 2.972) | 0.002 | 3.096 (0.118 — 81.191) | 0.498 |
| Intensive Care Unit Time | 1.660 (1.036 — 2.660) | 0.035 | 0.421 (0.023 — 7.817) | 0.561 |
| Blood Products (intraoperative) | 3.704 (1.408 — 9.743) | 0.008 | 0.094 (0.006 — 1.524) | 0.096 |
| FFP (intraoperative) | 1.795 (1.232 — 2.616) | 0.002 | 2.388 (0.945 — 6.030) | 0.066 |
| Lactate maximum (6h postoperative) | 1.607 (1.058 — 2.441) | 0.026 | 00.461 (0.158 — 1.344) | 0.156 |
| Troponin T maximum (24h postoperative) | 2.722 (1.381 — 5.365) | 0.004 | 1.193 (0.050 — 28.343) | 0.913 |
| CK-MB maximum (24h postoperative) | 5.483 (1.965 — 15.300) | 0.001 | 10.671 (1.733 — 65.723) | 0.011 |
| Quantity of Surgically Treated Heart Valves | 1.885 (1.045 — 3.400) | 0.035 | 0.629 (0.045 — 8.755) | 0.730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





