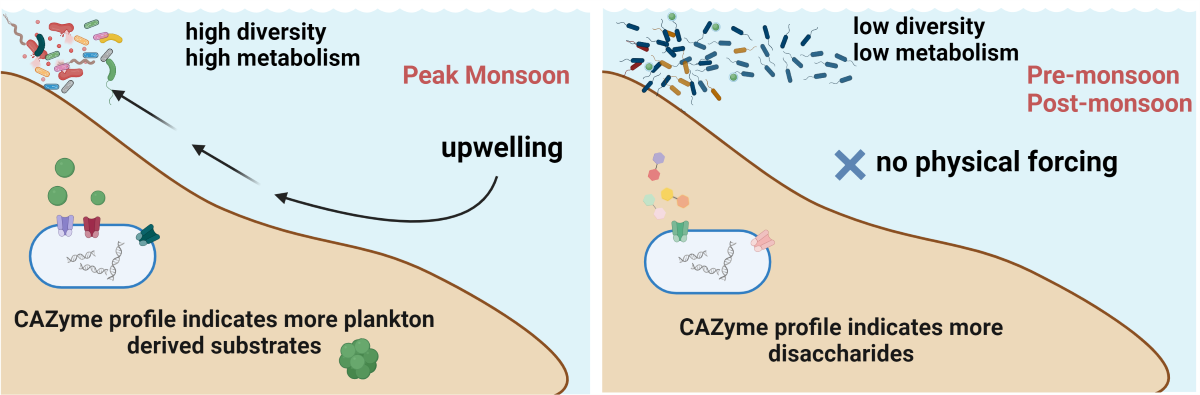
1. Introduction
The Arabian Sea (AS) stands out as one of the most productive marine regions globally, undergoing pronounced seasonal fluctuations in biological activity. During the pre-monsoon (PR) period in the AS, the surface waters retain an unstable warm state due to high solar insolation. Conversely, the monsoon (MN) season witnesses stable surface waters along the Kochi to Goa regions. The robust southwest monsoon triggers vigorous upwelling along the western Arabian Sea, enriching the photic zone with nutrients from deeper waters and fostering higher productivity [
1,
2,
3,
4]. However, during the post-monsoon (PM) period, generally unstable conditions prevail throughout the WCI [
5]. Higher chlorophyll
a concentrations have been reported during PM and MN with respect to monsoon [
6]. During MN and PM, various mechanisms govern water-column mixing, nutrient upwelling, with subsequent increase in phytoplankton biomass [
7,
8,
9,
10,
11,
12].
Variations in phytoplankton, nutrients and other environmental variables dynamically influence the abundance and composition of microbial communities over time and space [
13,
14,
15]. Anthropogenic inputs, such as riverine influx during the monsoon, activities like coastal urbanization, industries, maritime transport, oil extraction and refining, tourism, and aquaculture, affect both the seawater column and marine sediments through benthic-water flux (upwelling processes) [
16]. Pollution impacts on coastal microbial communities represent a complex interplay of multiple stressors, including natural and anthropogenic pollutants [
17]. These stressors can alter coastal ecosystems, potentially affecting marine microbes and ecosystem functioning negatively [
17]. However, investigations into microbial community dynamics are limited, despite their critical importance. Though observations such as Indian JGOFS extensively investigated the Arabian Se
a’s biological and chemical properties during the monsoon season, studies were limited in temporal scope and did not thoroughly address the taxonomic composition of microbial communities along the western coast of India in an annual cycle. Understanding microbial population
s’ seasonal variance is crucial, considering their pivotal roles in nutrient cycling, sulphate reduction, hydrocarbon degradation, and other ecological processes [
18]. On a global scale, studies of microbial communities in upwelling systems have revealed significant correlations between microbial diversity and primary production, underscoring the crucial role of bacteria in organic matter decomposition and transport [
19]. Nonetheless, there remains a scarcity of data on microbial community differences and functions along the eastern AS.
This study aims to investigate shifts in bacterial communities and their functional potential across seasons (PR, MN, PM) in the AS. Specifically, this study examines the taxonomic composition of the bacterial community in preceding (pre-monsoon, April-May), during (monsoon, August), and following the monsoon phenomenon (post-monsoon, January-February), along the western coast of India, spanning from 9.951°N to 22.22°N latitude (
Figure S1) using metagenomic approaches. This study also examines the variations in functional roles of bacterial communities during these three seasons.
2. Materials and Methods
2.1. Sampling Location and Sample Collection
Sampling was carried out during a multidisciplinary oceanographic expedition onboard FORV Sagar Sampada and Sagar Kanya along the WCI (2018-19). The research area spanned from 9.951°N to 22.22°N latitude and 68.940°E to 76.166°E longitude covering the south-Kochi (L1), central-Goa (L2) and north-Okha (L3) in the eastern Arabian Sea (
Figure S1). Each location consisted of 3 samples collected from coastal surface waters as part of MEDAS project of Ministry of Earth Science (MoES) during Summer Monsoon-MN (August; FORV SS375), Pre-monsoon-PR (April-May; FORV SK351), Post monsoon-PM (January; FORV SS382). Samples were collected using Niskin samplers (10 L, Hydrobios, Kiel) (Seabird Scientific, Bellevue, WA, USA) attached to a Conductivity Temperature Depth (CTD) profiler (SBE 19, Seabird Scientific, Bellevue, WA, USA).
2.2. Physiochemical Properties
The CTD profiler equipped with sensors was used to measure temperature, dissolved oxygen (DO), and salinity. The Winkler method was used to estimate dissolved oxygen (DO) in the water samples. The samples were titrated with standard thiosulphate utilising starch as an endpoint detector after being fixed with 0.5 mL of Winkler reagents [
20]. Using an auto analyser, dissolved inorganic nutrients such as nitrite (NO
2), phosphate (PO
43-), silicate (SiO
4), nitrate (NO
3-), ammonium (NH
4+)) were measured (Scalar SAN++, Netherlands) [
21,
22].
2.3. DNA Isolation
10L seawater samples collected using Niskin sampler was filtered through 0.22 μm Millipore filter papers (Millipore, MA, USA) to entrap the bacterial population after sequential filtration of 10L of water sample through a 20-μm and 1-μm pre-filters (Millipore, MA, USA). Genomic DNA was isolated using the NucleoSpin eDNA Water Kit from Macherey-Nagel. One fourth of the round filter was used to which 2ml buffer was added to dissolve the filter. Filter was treated with proteinase K and eDNA was extracted following manufactures instructions. The concentration of DNA was checked using a Qubit 3.0 fluorometer (Thermofisher Scientific, Massachusetts, USA). DNA purity was assessed using a Nanodrop 2000 (Thermofisher Scientific, Massachusetts, USA).
2.4. Whole Genome Library Preparation and Sequencing
Whole genome Library Preparation was done using KAPA HyperPlus Kit (Merck SA, Argentina). A 350-500ng DNA was used for library construction, as per manufacturers’ protocol, The prepared libraries were quantified using Qubit 3.0 fluorometer (Thermofisher Scientific, Massachusetts, USA) using DNA HS assay kit (Thermofisher Scientific, Massachusetts, USA). Size of DNA was checked on Agilent 2100 bioanalyzer using Agilent high sensitivity DNA assay kit. Based on the concentration and size of the fragments samples were pooled and pooled libraries were sequenced using NovaSeq 6000 Sequencing System S4 flow cell (Illumina, Inc. San Diego, CA, USA)
2.5. Diversity Analysis and Taxonomy Classification
The alpha diversity was calculated using Shannon and Simpson diversity metrics. For Alpha-diversity calculation vegan R package was used. For KEGG pathway classification studies, the high-quality reads compared against NCBI-NR- KEGG Database using Diamond tool based on sequence homology approach[
23]. The result file was viewed using MEGAN and the results were reported at two levels of KEGG classification. For taxonomy classification, the high-quality reads were aligned against NCBI-NR Database using diamond blastx tool. The result files were converted into MEGAN viewable format and based on MEGAN-LCA algorithm.
2.6. CAZy Annotation
Sequences were further annotated with dbCAN metaSever. The reads were compared against CAZy protein sequences using Diamond blastx tool to analyse the CAZymes.[
24]
2.7. Statistical Analysis
The Non-metric Multidimensional Scaling (NMDS) analysis was carried out to understand the physiochemical and biological disparities between seasons using Primer 7 for Windows software (Plymouth Marine Laboratory, Plymouth, United Kingdom)[
25]. A Bray-Curtis similarity matrix (4th root transformed to deemphasize the contribution of any one particular dominant cluster) was constructed from the clusters from each location. Additionally, statistical analyses using STAMP G-test (with Yate
s’ correction) and Fisher’s test underscore significant variation in diversity between the PR, MN and PM[
26]. Tukey-Kramer post-hoc plots ANOVA with Benjamini-Hochberg FDR correction using p-value cutoff < 0.05 and 0.95 confidence was done to analyse significant variation between CAZy profiles and KEGG functional profile.
3. Results
3.1. Estimation of Physiochemical Parameters
The temperature during the PR and PM (30.51 ± 1.33°C and 26.42 ± 2.91°C) was higher compared to MN (23.31 ± 0.79°C). The highest temperature was observed at Station L1 during PR (31.69°C). The temperature decreased during MN to 22.42°C at Station L2 followed by Station L1 (23.58°C) and Station L3 (23.94°C). During PM, the temperatures gradually increased (26.42 ± 2.91°C) (
Figure S2a). Overall salinity was higher towards north (L3, av. 36.56 ± 0.11 and in L2, av. 34.89 ± 0.57) irrespective of seasonal changes. In south, L1 recorded a lower salinity during all seasons which was as low as 24.26 during MN (
Figure S2b). DO was also observed to be lower during MN in the south (L1: 106.51 µM and L2: 114.91 µM) (
Figure S2c). In the case of chlorophyll a, a, higher concentration was observed during MN (2.60 ± 1.9mg m−3) compared to PR and PM (1.27 ± 0.14 mg m−3 and 0.60 ± 0.29 mg m−3, respectively) (
Figure S2d). Nutrients showed higher concentration during MN (SiO
4:- 22.57 ± 10.35 µM; NO
3:- 7.22 ± 3.67 µM; NO
2:- 0.75 ± 0.85 µM; NH
4:- 0.66 ± 0.52 µM) compared to PR and PM (PR: SiO
4:- 4.49 ± 1.61 µM; NO
3:- 0.26 ± 0.17 µM; NO
2:- 0.41 ± 0.58 µM; NH
4:- 0.27 ± 0.04 µM and PM: SiO
4:- 5.28 ± 2.11 µM; NO
3:- 0.21 ± 0.24 µM; NO
2:- 0.18 ± 0.21 µM; NH
4:- 0.52 ± 0.25 µM) (
Figure S3).
3.2. Bacterial Diversity
The total reads ranged from 27256782 to 36467020. Using Illumina sequencing technology, for 9 samples, an average of 30.67 million raw data was generated where, 99.8% reads were retained as high-quality data (
Table S1,
Figure S4). The alpha diversity indices showed higher diversity during MN and least diversity at L2 PR and L1 PM (
Figure S5-S6,
Table S2).
After OTUs were categorised at 97% similarity, 6483 OTUs were found. 40 taxa were identified by phylum-level classification of OTUs, with Cyanobacteria and Proteobacteria dominating the metagenomes, followed by Bacteroidetes. Furthermore, in several metagenomes, Firmicutes, Actinomycetota, Tenericutes, and Planctomycetota had large relative abundances. Upon analysing the genus-level classification of the OTUs, 2,000 genera were identified, with Candidatus Pelagibacter, Synechococcus, Candidatus Nitrosopelagicus, Prochlorococcus, and Alteromonas showed a dominant position. However, 21.09 ± 8.9% of the reads were unable to be categorised to any specific genus.
3.3. Taxonomic Diversity at the Phylum Level
The phylum level analysis indicated Proteobacteria as a dominant phylum in L1 (53.59%) and L3 (55.00%) during PR. Whereas in L2, Cyanobacteria (74.44%) dominated. However, during MN, Proteobacteria emerged as the dominant phyla in L2 (78.73%) and Cyanobacteria declined to 0.86% in L2 and to 2.77% and 4.49% in L1 and L3, respectively (Figure 5). There was an increase in Firmicutes (2.57 to 5.35%) and Actinomycetota (2.36 to 8.36%) during MN in L1 & L2. Abundance of Firmicutes further increased during PM in L1 & L2 (6.91%), whereas Actinomycetota dropped to 3.18%. L3 showed no significant differences in relative abundance of Firmicutes and Actinomycetota among seasons. There observed variation in relative abundance of Bacteroidetes during PR (6.56%) to MN (12.94%) but dropped during PM (9.26%) (
Figure S7;
Table S3).
Classes Cyanophyceae (33.61%), Alphaproteobacteria (30.25%) and Gammaproteobacteria (20.66%) dominated in L1 during PR. The relative abundance of Cyanophyceae was twice more in L2 compared to other stations during PR (74.44%), followed by Alphaproteobacteria by 11.68%. In L3, Alphaproteobacteria dominated contributing to 36.44% of the total communities during PR. Following the onset of MN, there was a notable decrease in the relative abundance of Cyanophyceae across all stations, accompanied by a substantial increase in Betaproteobacteria, Flavobacteria, Bacilli, Actinomycetes, Clostridia, Epsilonproteobacteria, and Deltaproteobacteria (
Table S4). In PM, Cyanophyceae showed a relative increase to dominance in L1 and L2, along with Alphaproteobacteria. Conversely, in L3, Alpha and Gammaproteobacteria dominated, with no significant changes observed in the levels of other bacterial classes during the transition from PR to MN, except for Flavobacteria, which increased from 7.69% in PR to 14.45% during MN (
Figure S8;
Table S4)
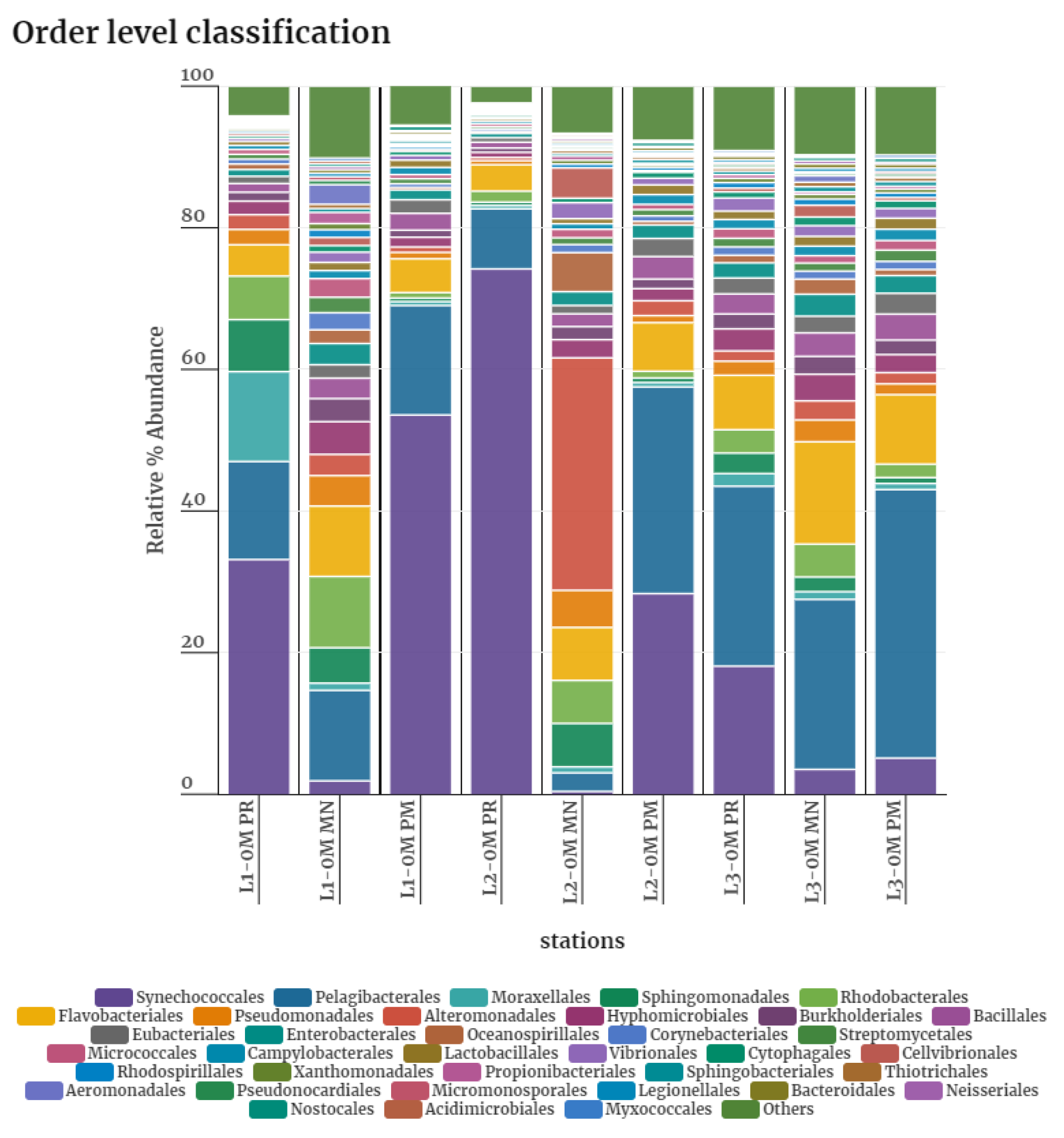
Synechococcales was predominant during PR, constituting 18.09-74.20% of the community, whereas it was considerably lower in the MN (3.51-0.40%), and their abundance increased again during PM in L1 (53.56%) and L2 (28.33%, whereas L3 retained similar level as in MN (5.10%). Pelagibacterales, although this order was present in all three seasons, its abundance varied among stations. In L1, they constituted 13.84% in PR, 12.78% in MN, and 15.46% in PM. But in L2, relatively lower percentage was observed during PR (8.76%) and it further dropped during MN (2.89%). In both L2 and L3, highest abundance of Pelagibacteriales was observed during PM (29.19% and 37.92% respectively). Abundance of Moraxellales was low in MN (1.02 and 1.09%) and PM (0.85 and 0.86%) compared to PR (12.70 and 1.79%), in L1 and L3. While the order Sphingomonadales was present in all three seasons in L1, its abundance was higher in PR (7.34%) compared to MN (5.00%) and PM (0.50%). Similar pattern is observed in L3 as well, a higher abundance was observed in PR (2.89%), but further reduction in relative abundance was observed in MN (2.07%) and PM (0.83). But in L2, a hike in relative abundance of Sphingomonadales (6.13%) was observed in MN, but the PR and PM levels were negligible (<0.7%). The percentage abundance of Rhodobacterales, Flavobacteriales, Pseudomonadales, Alteromonadales, Hyphomicrobiales, Burkholderiales orders showed considerable variation across seasons, with higher abundance in MN, specifically Rhodobacterales, Flavobacteriales in L1; Alteromonadales in L2; Flavobacteriales in L3. (
Figure 1;
Table S5)
Synechococcus (32.43%), Candidatus Pelagibacter (13.73%), Moraxella (11.70%) and Qipengyuania (3.50%) were dominant genus in L1 during PR. By the advent of monsoon Candidatus Pelagibacter (12.62%) Candidatus Nitrosopelagicus (5.17%) and Pseudomonas (2.19%) dominated over Synechococcus (1.59%). An observed increase in the relative abundance of genera Marinobacter, Flavobacterium, Nitrosopumilus was seen, during MN. Prochlorococcus (43.69%) dominated the system along with Candidatus Pelagibacter (15.31%) and Synechococcus (9.19%) in PM. Synechococcus (73.65%), Candidatus Pelagibacter (8.43%), dominated in L2 during PR. Alteromonas (25.84%), Pseudoalteromonas (4.73%), Qipengyuania (3.61%), Marinobacter (3.27%) were dominant during MN in L2. Candidatus Pelagibacter (28.94%) and Synechococcus (25.91%) codominated in L2 during PM. In L3, during all seasons Candidatus Pelagibacter and Synechococcus were consistently present as dominant genera. Apart from the above mentioned genera, PR and PM had the presence of Candidatus Nitrosopelagicus (3.99%; 1.83%) and Nitrosopumilus (2.02%; 2.41%). On the other hand, MN was much more diverse with Flavobacterium (2.64%), Pseudomonas (2.17%), followed by Clostridium, Vibrio, Sulfitobacter, Polaribacter (
Table S6).
3.4. KEGG Analysis
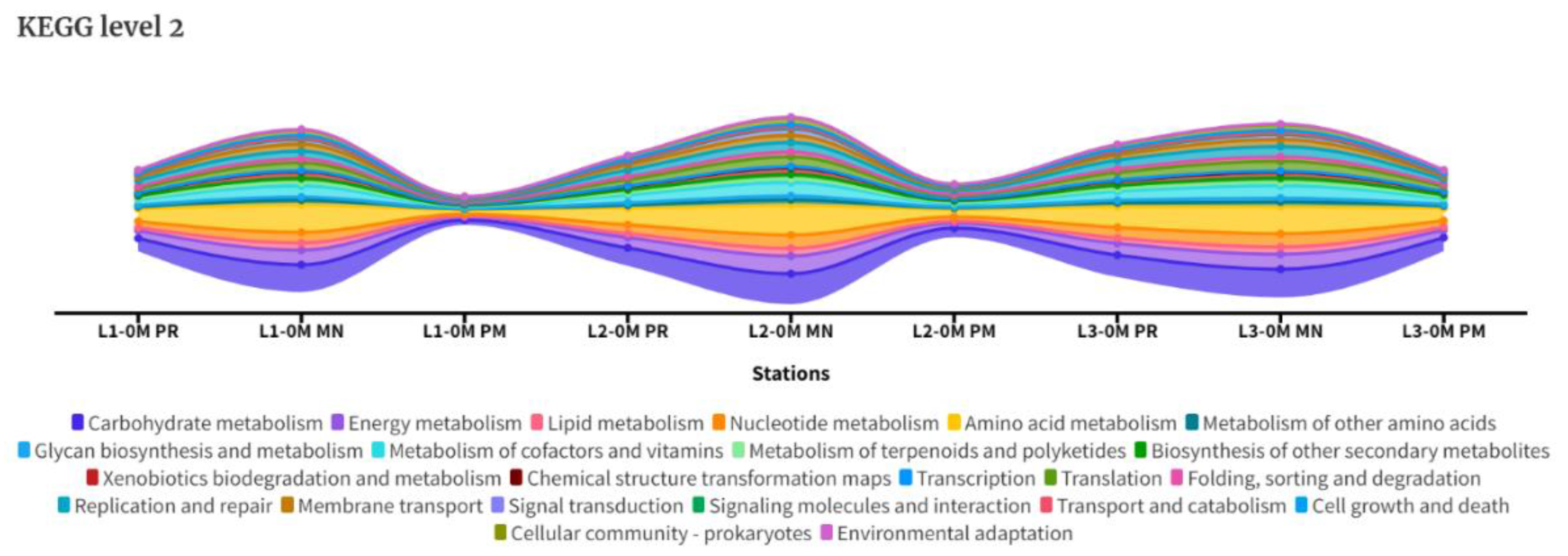
The assessment of functional potential was conducted by KEGG databases using MEGAN. The KEGG analysis unveiled notable disparities among seasons. KEGG level 1 revealed higher reads on metabolism in MN samples followed by PR (
Figure S9). KEGG level 2 analysis supported the above results were the MN samples were most functionally active especially in terms of carbohydrate metabolism, amino acid metabolism, energy metabolism, nucleotide metabolism, metabolism of co-factors and vitamins, lipid metabolism, xenobiotic degradation and metabolism, translation, replication and repair and membrane transport (Figure 9). Further PR showed lower functional profile and PM the lowest. Though the seasonal variation were significant, no significant difference between stations was observed functionally between 3 stations during PR and MN. During PM, the L3 (north) was more functionally active (in terms of metabolism
—carbohydrate, energy, nucleotide and translation) (
Figure 2).
3.5. CAZymes
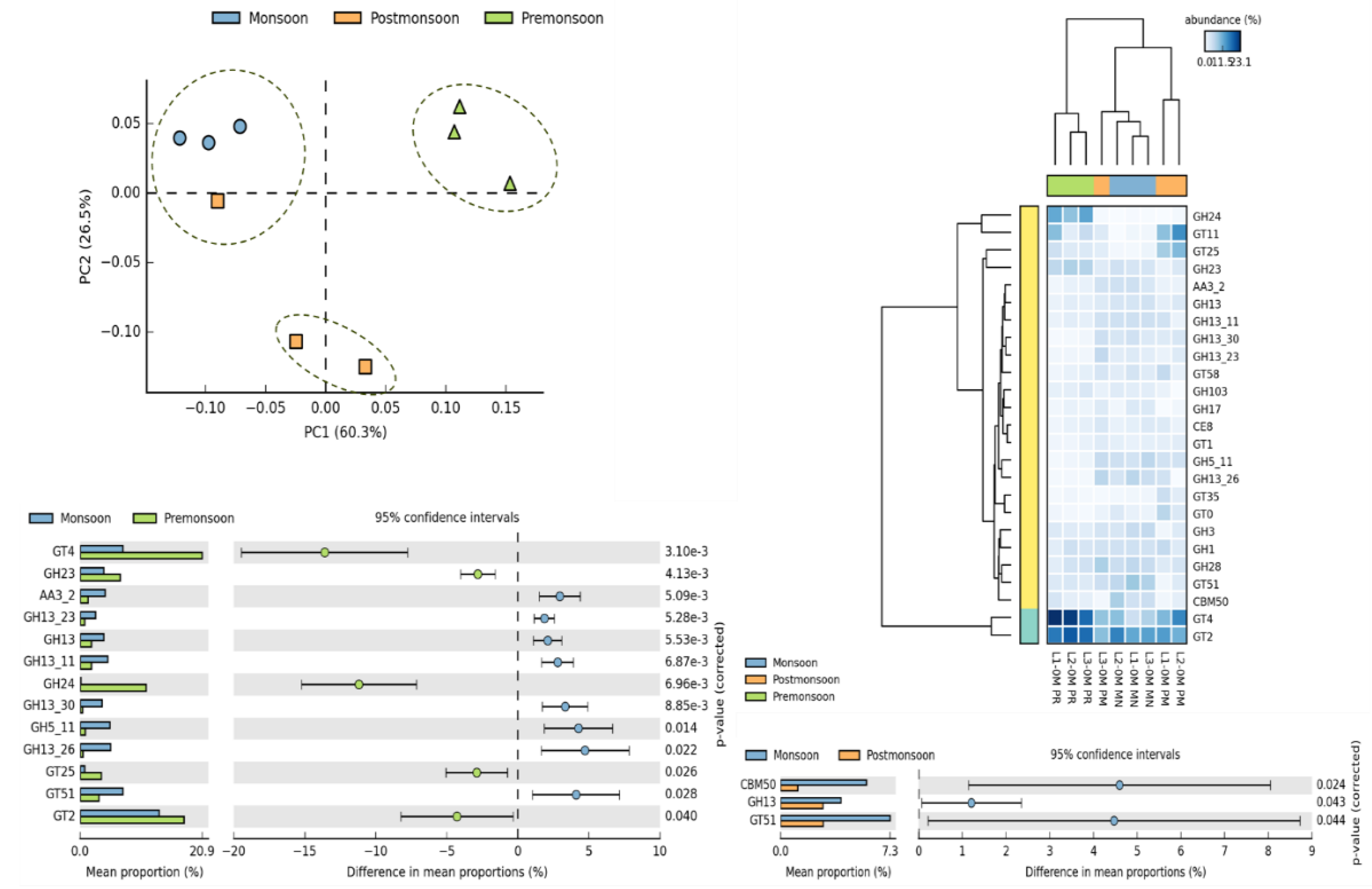
Significant variation in CAZymes distribution was observed between seasons. An increased abundance of GT4 (disaccharide-glucosyl transferases; retaining mechanism), GH23 (chitinase, peptidoglycan lyase), GH24 (Lysozymes), GT25 (galactosyl transferases; inverting mechanism), GT2 (disaccharide-glucosyl transferases; inverting mechanism) was observed in PR compared to MN. Whereas MN was observed to have increased levels of AA3_2 (GMC-oxidoreductase family; glycose 1-oxidse), GH13_23 (alpha glycosidase), GH13, GH13_11 (Amylomaltase, Isoamylase), GH13_30 (alpha glucosidases), GH5_11 (Xylan ß-1,4-Xylosidase, Endo ß-1,4 mannanase etc) and GT51 (Peptidoglycan glucosyl transferase) (
Figure 3). In comparison with PM, the levels of CBM50 (enzymes targeting Petidoglycan/chitin), GH13 and GT51 was higher during MN. Based on CAZymes profile a PCA and heatmap analysis was carried out in which PR, MN+L3PM and PM observed to have distinct profiles (
Figure 3).
3.6. Statistical Analysis
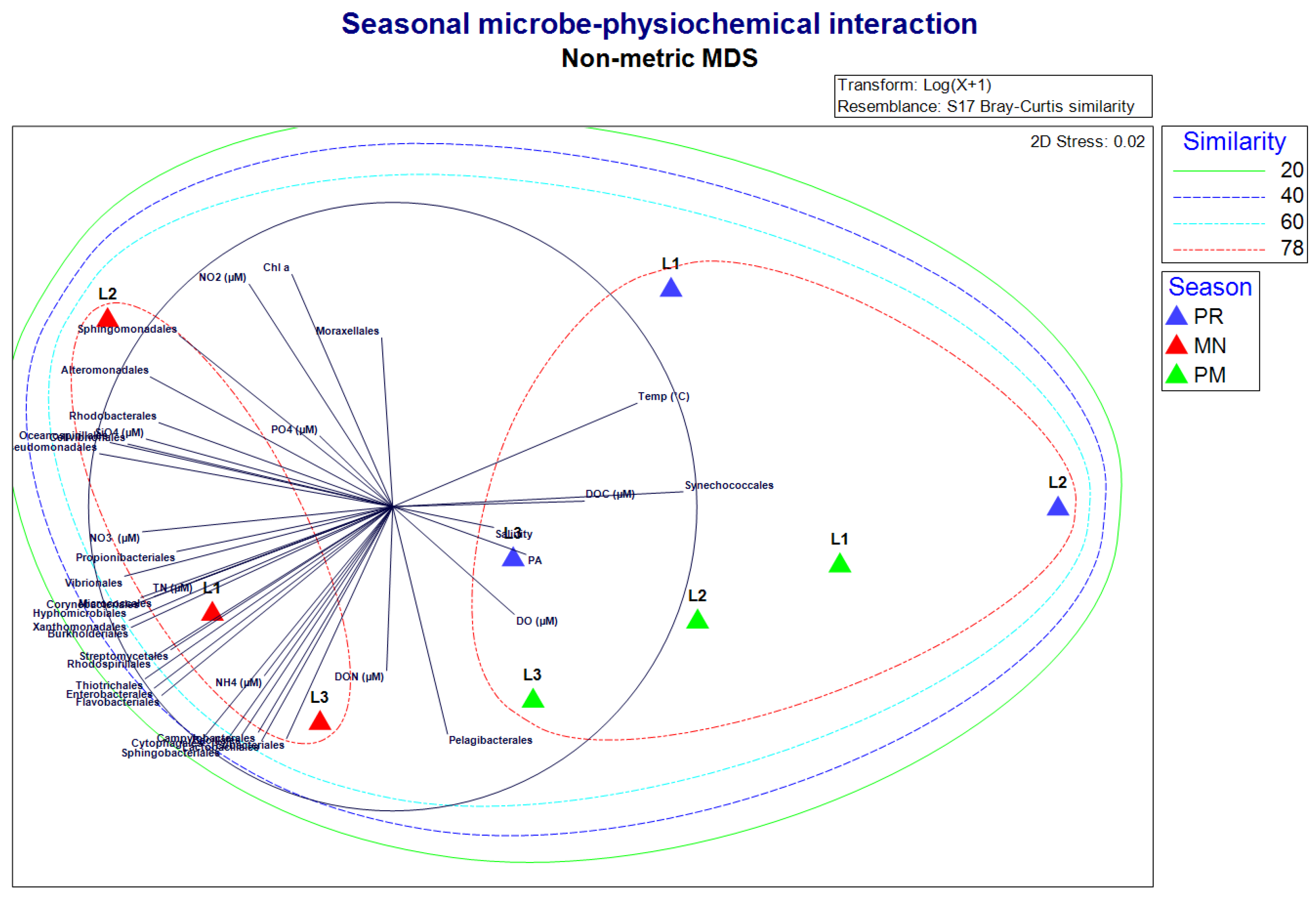
The Non-metric Multidimensional Scaling (NMDS) analysis revealed distinct physiochemical and biological disparities in the MN compared to the PR and PM. Cluster analysis revealed that PR and PM formed a distinct group with 78% similarity, while MN formed another cluster with a 60% similarity. The cluster analysis derived from NMDS distinctly delineated variations in prokaryotic diversity (Figure 11). Additionally, statistical analyses using STAMP G-test (with Yates’ correction) and Fisher’s test underscore validates the diversity variance between the PR, MN and PM (
Supplementary Figures 1–3). Tukey-Kramer post-hoc plots ANOVA with Benjamini-Hochberg FDR correction using p-value cutoff < 0.05 and 0.95 confidence identified the significant variation between CAZymes profiles (
Figure 3) and KEGG functional profile of MN, PR and PM (
Figure 2 and
Figure S9). The bacterial community composition analyzed using the n-MDS, indicated that the influencing factors of the bacterial community was predominantly nutrients whereas for PR and PM it was temperature, salinity and DO. And a higher diversity of bacterial community was observed in MN compared to PR and PM (
Figure 4).
4. Discussion
This study demonstrates the changes in bacterial diversity and variations in the functional activity of bacterial communities during three seasons in the eastern AS. To understand the dynamic nature of microbial communities, the community-level physiological and functional profiling was also carried out in the present study. This approach offers insights into how microorganisms respond and adapt to diverse stressors across different marine environments, as well as shed light on their coping up mechanisms and adaptive strategies. The dataset obtained from three sampling cruises across three transects from south to north examined the bacterial diversity in relation to various physicochemical parameters, including temperature, dissolved oxygen (DO), salinity, and nutrients (nitrate, nitrite, phosphate, and silicate).
4.1. Physicochemical Settings during the Study
There were observed changes in physicochemical parameters from PR to MN and MN to PM. The pre-monsoon (PR) period is marked by elevated surface temperatures and moderate nutrient levels, except for phosphate, which is lower in the northern region (1.85 µM) (
Figure S3). Previous studies across the entire Arabian Sea have noted very low phytoplankton biomass and primary production during PR. This is attributed to a uniform mixed layer (<30 m) influenced by increased solar insolation and strong stratification [
27]. Consistent with these observations, this study also found lower chlorophyll
a level during PR (
Figure S2d).
The temperature was considerably higher during PR (30.51±1.34˚C) which was dropped during MN due to monsonal showers and upwelling. This decline in temperature during MN could be attributed to the presence of upwelled waters, riverine influxes and precipitation [
28,
29,
30,
31,
32,
33]. A drop in salinity was observed in L1 during MN compared to other stations and seasons. This could possibly be due to riverine fresh water influx, comparatively less saline water inputs and the low salinity waters transported from the Bay of Bengal [
34]. The MN is also characterized by deoxygenation nutrient surge specifically nitrate, phytoplankton abundance and chlorophyll
a hike [
2,
35]. Similar to PR, PM is also characterized by warm temperature and high salinity and a lower chlorophyll
a concentration (
Figure S2). The nutrients were also very low (
Figure S3).
4.2. Taxonomic Distribution during PR
During PR, Proteobacteria was the dominant phylum across all locations, but its abundance varied significantly between L1, L2, and L3. The highest abundance was observed in L3. The dominance of Proteobacteria in marine environments is well-documented [
36,
37,
38,
39,
40]. They can adapt in most environments mostly due to their ability to utilise wide range of compounds such as carbon, sulfur, aromatics, fatty acids, carbohydrates etc [
40]. The higher abundance of the phylum Proteobacteria, particularly the class Alphaproteobacteria, in L3 compared to L1 and L2, suggests a location-specific prevalence. A recent study conducted in the marine alphaproteobacterial HIMB59 identified one of the key factors of adaptation as phosphate concentration [
41]. An increased phosphate concentration was observed in L3 in particular during the study could be a factor for this increased abundance. Comparable levels of diversity within the Proteobacteria phylum have been documented in the coastal zone of the Arabian Sea [
42] as well as in various other regions globally [
43]. Alphaproteobacteria is widely recognized for their diversity and ecological roles in marine environments [
44]. In detail, the order Pelagibacterales contributed most significantly to this class in L3. Initially, this order known solely from metagenomic data as the SAR11 clade [
45,
46], are notably small with small genome sizes and limited metabolic functions [
47]. Some are oligotrophs, relying on dissolved organic carbon and nitrogen, and are unable to fix carbon or nitrogen themselves. The higher abundance could indicate limited nutrient availability in surface waters during PR, particularly in L3. Such a nutrient limited condition can be observed during this study (
Figure S3). Gamma proteobacteria also exhibited variation, with higher abundance in L1 compared to L2 and L3. Such higher abundance of Gammaproteobacteria has also been reported previously in SEAS [
48]. Gammaproteobacteria included versatile marine taxa with roles in carbon cycling [
49]. Betaproteobacteria, Epsilonproteobacteria, and Deltaproteobacteria classes exhibited relatively lower abundances across all locations, with some minor variations between sampling sites. These classes mostly comprise of nitrifying and sulfate reducers [
50] and reduced nutrient levels during PR (
Figure S3) could be the reason for lower abundance in all stations.
Cyanobacteria are known for their role in primary production and can bloom under nutrient enrichment [
51]. The higher abundance in L2 could be related to localized factors promoting cyanobacterial growth. Among which, Prochlorococcus thrives in warm oligotrophic waters but is notably absent in eutrophic coastal regions. In the present study, Prochlorococcus fluorished during PR and declined in MN where eutrophic condition existed (
Figure S3). Recent research indicates that it originated from an ancestral cyanobacterium through a process involving reductions in cell and genome sizes. Environmental pressures evidently played a significant role in shaping the evolution of Prochlorococcus. Its diminutive size confers advantages for thriving in nutrient-poor environments [
52]. Conversely, Synechococcus tends to dominate picocyanobacterial communities in eutrophic coastal areas and mesotrophic open ocean waters [
53]. Nutrient data during this study [
54] points to a clear mesotrophic condition during PR (
Figure S3). Our study aligns with this pattern, indicating higher abundance of Synechococcus during PR.
Bacteroidetes and Firmicutes exhibited a slight increase in abundance in L3 compared to L1 and L2. Bacteroidetes and Firmicutes play important roles in organic matter degradation and are influenced by nutrient inputs [
55]. The increased abundance in L3 might be due to the increased phosphate level at L3 (
Figure S3) [
54]. Flavobacteriia and Bacilli are involved in organic matter degradation and nutrient cycling in marine environments [
56]. Actinomycetota, Tenericutes, Planctomycetota, Fusobacteria, Spirochaetes, Deinococcus-Thermus phylas exhibit relatively lower abundances across all locations, with some minor variations between sampling sites during PR.
4.3. Taxonomic Distribution during PM
Proteobacteria exhibited varying abundances across the stations with higher abundance in north experiencing winter monsoon (L3-PM, 62.38%). Cyanobacteria displayed substantial variability across stations L1 (54.73%), L2 (29.34%), and L3 (6.15%). A reduced abundance towards north could be due to an unfavourable condition brought out by the winter monsoon. The PM sampling was carried out during January-February were north experienced substantial mixing [
57]. This influence can be observed in the temperature variance during the study (
Figure S2a) L3-23.06˚C. On order and genus level analysis indicate abundance of Prochlorococcus in L1 and Synechococcus in L2. The presence of elevated Prochlorococcus abundance observed during the period of Postmonsoon (L1 PM) may be attributed to oligotrophic conditions and warmer temperature (28.10 ± 0.31 ˚C) in the south. To date, there are no reports on the abundance of Prochlorococcus in the coastal waters of Kochi [
58]. Similar community structure has been previously observed during winter monsoon period of Bay of bengal in which Proteobacteria represented the highest diversity and largest fractions [
59]. Candidatus Nitrosopelagicus, Nitrosopumilus show increased abundance from south to north. Increased abundance of Candidatus Nitrosopelagicus and Nitrosopumilus across locations suggests their role in nitrogen cycling, potentially responding to varying nutrient inputs, specifically ammonium (
Figure S3) [
60]. Flavobacterium, Clostridium, Vibrio display relatively consistent abundances across locations, with slight variations.
4.4. Taxonomic Distribution during MN
The Monsoon season (MN) lead to significant shifts in bacterial composition, particularly Cyanophyceae, Alphaproteobacteria, and Gammaproteobacteria. Cyanophyceae (Cyanobacteria) drastically declined during MN, while certain classes like Gammaproteobacteria increased sharply during this period. A notable decrease in Synechococcales abundance during MN (0.40-3.51%) compared to PR (74.20-18.09%) and PM (53.56-5.10%) samples, suggest a shift away from cyanobacterial dominance during the MN. The factors governing the abundance of Synechococcus remain poorly understood, particularly given that even in the most nutrient-depleted regions of the central gyres, population growth rates are often high and not limited [
53]. Despite these uncertainties, there is likely a relationship between ambient nitrogen concentrations and Synechococcus abundance [
61]. Nitrogen source has a stronger influence on ligand concentration compared to the growth phase, while phosphorus limitation has a more pronounced effect on the cellular and extracellular properties of Synechococcus than nitrogen limitation does [
62]. Prochlorococcus is believed to be at least 100 times more abundant than Synechococcus in warm oligotrophic waters [
53]. Although there are ambient nutrients available during the MN period, the cyanobacterial population failed to flourish, potentially due to the colder surface water temperatures characteristic of MN due to upwelling. Research conducted in upwelling systems along the east coast of Hainan Island in the South China Sea also revealed a decrease in the abundance of Cyanobacteria in upwelling surface waters, suggested that upwelling conditions may not be favorable for Cyanobacteria growth [
63]. Relatively stable abundance of Pelagibacterales across seasons in L1, indicating consistent representation in bacterial communities. Significant increase in Rhodobacterales abundance during MN (L1-MN), potentially reflecting favorable conditions for this order during the Monsoon season. Pelagibacterales exhibited relatively stable abundance across seasons, indicating persistence or resilience in bacterial community representation. Flavobacteriales, Pseudomonadales, Alteromonadales, Hyphomicrobiales, Burkholderiales, Enterobacterales, Corynebacteriales showed varying degrees of abundance across seasons, highlighting shifts in bacterial community composition. During MN, a notable increase in the abundance of Alteromonadales, particularly Pseudoalteromonas, was observed. These bacteria are commonly found in sea ice and cold waters, recognized for their production of glycolipid-type extracellular polymeric substances (EPS) that exhibit a diverse range of biological activities [
64]. This surge in population abundance is perhaps influenced by the temperature of upwelling waters. Reports have documented the presence of this bacterial order even in Arctic ice [
65].
4.5. Functional Analysis
The KEGG analysis highlights the increased functional potential during the MN, compared to PR and PM. The MN was marked with higher metabolic rates in terms of carbohydrate, energy, nucleotide as well as aminoacids. The higher xenobiotic degradation potential was observed during the MN which was not present in PR and PM points to the presence of organisms adapted to the deeper waters [
66].The distinct profiles of CAZyme indicates that the bacterial bioavailable carbohydrates in each season was different which might contribute to the diversity difference in PR, MN, and PM. This supports the findings presented in taxonomic and KEGG variation. The CAZyme profile of north L3 PM showed similarity to MN profiles of all stations due to the effect of North east monsoon in the north (
Figure 3). The GH13 enzymes were the most prevalent glycoside hydrolases (GHase) during MN. The GH13 family is consistently one of the most abundant enzyme families across various metagenomic studies [
67,
68]. Among the GH13 family detected in the heterotrophic communities associated with this environment were sucrose phosphorylase and glucosidase, known for their involvement in breaking down glucan deposits derived from phytoplankton. In oceanic culture studies from South China Sea, the majority of genes encoding GH13 family CAZymes were linked to Rhodobacteraceae and Flavobacteriaceae, which were relatively more abundant during MN (6.93% and 10.62%, respectively) (
Figure 3). Conversely, in other studies, Alteromonadaceae order bacteria were the predominant ones encoding GH23 family enzymes such as peptidoglycan lyase, which was notably abundant in PR and MN (
Figure 3) [
69]. Additionally, glycosyltransferases (GT) of the GT4 and GT2 families were the most abundant in all datasets. GH13 and GT4 families encompass CAZymes involved in the uptake and utilization of trehalose. The high prevalence of these CAZymes in oceanic environments could be attributed to trehalos
e’s characteristics as a stable, non-reducing disaccharide capable of withstanding various pH ranges (3.5–10) and playing a crucial role in energy storage and cellular protection under different stress conditions [
70,
71]. The Glycosyl hydrolase family 5 (GH5) is recognized as one of the largest and diverse families of GH [
72]. GH5 enzymes exhibit specificity towards various substrates, such as chitin, mannan, cellulose, Xylan, glucan, and lichenin [
73]. The prevalence of GH5 subfamily 11 was notably high during MN, indicating the presence of chitin, mannan, cellulose, Xylan, glucan, and lichenin in the system. On culture-based studies widely show that GH5 endoglucanases are mostly presented by Gammaproteobacteria [
74,
75]. The abundance of Gammaproteobacteria was higher during MN in the present study (
Figure 3).
To conclude, the MN supported a highly diverse bacterial diversity compared to PR and PM. The changes during the MN could be attributed to several factors:
Increased nutrient availability: During the spring intermonsoon period (PR), surface layers of the Arabian Sea are nutrient-depleted, but during summer monsoon, phytoplankton growth is fueled by upwelling events. This upwelling brought on nutrient-rich cold subsurface water into the euphotic zone, with reported three-fold increases in nitrate compared to surrounding areas (
Figure S3). Consequently, high productivity occurs in the Arabian Sea during this season, providing increased organic material for bacterial metabolism [
76]. Earlier research conducted in the South China Sea [
63], Western Subtropical Pacific [
77], and Arabian Sea [
78]corroborated these findings.
Riverine Influxes with Anthropogenic Inputs: August correspond24s to one of the peak riverine runoff months [
79]. Our study corroborates previous findings [
80,
81] as it demonstrates a higher nutrient concentrations during the MN period and these could also be anthropogenic driven. Studies shows a 4-6 fold increase in anthropogenic nutrient flux in the past 50 years [
82,
83] (
Figure S3). Research indicates that riverine ecosystems exhibit higher bacterial abundance compared to intertidal and ocean systems [
84]. Thus the riverine run off during monsoon could positively contribute to the bacterial diversity.
Chlorophyll
a (Chl
a) as a Proxy for Phytoplankton Biomass: High Chl a levels indicate elevated productivity and organic matter availability, supporting increased bacterial growth [
85]. Enhanced levels of nitrates, nitrites, and silicates directly promote phytoplankton growth [
86], which contributes significantly during the MN. Such enhanced levels of nutrients were observed during this study. Moreover, Upwelling positively influence the phytoplankton blooms [
87] and researchers have investigated for decades, the relationship between bacteria and algae involving the assimilation and remineralization of phytoplankton-derived organic matter by bacteria [
88,
89]. All these attributes to the conclusion that phytoplankton blooms due to upwelled nutrients could be a reason for higher bacterial diversity during MN in the study region.
Deeper Water Communities: Bacterial communities adapted to deeper waters ascend to the surface during the MN, sustained by optimal conditions created during upwelling—lower temperatures, higher nutrients, and salinity [
86]. These communities revert to their native compositions when surface water conditions return to normal physicochemical levels. Recent studies in ocean systems indicate that deeper waters harbour more diverse bacterial community than surface waters [
90]. During upwelling events, these diverse communities ascend to the surface and possibly attempt to adapt to the environment.
In summary, the observed high bacterial diversity during the MN season in the Western Coast of India can be attributed to the interplay of increased nutrient availability, riverine influxes with anthropogenic inputs, elevated phytoplankton biomass, and the influence of deeper water communities brought to the surface during upwelling events.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, H.S. and P.A.; methodology, H.S. and P.A; software, H.S. and A.K.T; validation, H.S., P.A and P.R.A.S; formal analysis, H.S.,N.R., and A.K.T., ; investigation, H.S. and P.A.; resources, P.A., P.R.A.S., J.R., and G.V.M.G; data curation, H.S.,N.R., and A.K.T.; writing—original draft preparation, H.S. and P.A.; writing—review and editing, H.S., P.A., and P.R.A.S; visualization, H.S. and P.A.; supervision, P.A. and P.R.A.S; project administration, P.A., J.R., and G.V.M.G; funding acquisition, P.A., J.R., and G.V.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study formed a part of the projects, DST-SERB-Marine microbiome to ascertain the role of microbes in biogeochemical cycling in the Eastern Arabian Sea (SRG/2022/001545) and Marine Ecosystem Dynamics of Eastern Arabian Sea (MEDAS) GAP 3274, funded by the Ministry of Earth Sciences (MoES), Government of India.
Data Availability Statement
NCBI Sequence Read Archive (SRA) Nucleotide sequence accession number XXXXXX under the bio project number, PRJNAXXXXX
Acknowledgments
The authors acknowledge the Directors and Scientist-in-Charges, CSIR- National Institute of Oceanography, India for facilities and support. Authors express their gratitude to the Secretary of the Ministry of Earth Sciences; Director and the former Directors, Centre for Marine Living Resources and Ecology, and the Director, National Centre for Coastal Research, Chennai India for the great support. This study was carried out as part of the project Marine Ecosystem Dynamics of eastern Arabian Sea (MEDAS) funded by MoES, India and Science and Engineering Research Board (SERB). The data presented are archived at the MoES repository
www.incois.gov.in. We also acknowledge all participants of various MEDAS cruises and all colleagues at CSIR-NIO (RC), Kochi for their support and advice. HS acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi and NR acknowledges Science and Engineering Research Board (SERB), for funding research fellowship grants. This is the NIO contribution No. XXX; CMLRE contribution No. XXX.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- S. P. Kumar, R. P. Roshin, J. Narvekar, P. D. Kumar, and E. Vivekanandan, ‘What drives the increased phytoplankton biomass in the Arabian Sea?’, Current Science, pp. 101–106, 2010.
- J. Retnamma, K. Chinnadurai, J. Loganathan, A. Nagarathinam, P. Singaram, and A. K. Jose, ‘Ecological responses of autotrophic microplankton to the eutrophication of the coastal upwelling along the Southwest coast of India’, Environmental Science and Pollution Research, vol. 28, pp. 11401–11414, 2021.
- S. Parab, A. S. Jagtap, R. M. Meena, and C. S. Manohar, ‘Bacterial dynamics along the west coast of India during the non-monsoon and monsoon season’, Continental Shelf Research, vol. 251, p. 104876, Dec. 2022. [CrossRef]
- S. Prakash and R. Ramesh, ‘Is the Arabian Sea getting more productive?’, CURRENT SCIENCE, vol. 92, no. 5, p. 5, 2007.
- V. Narayanan Nampoothiri S, C. V. Ramu, K. Rasheed, Y. V. B. Sarma, and G. V. M. Gupta, ‘Observational evidence on the coastal upwelling along the northwest coast of India during summer monsoon’, Environ Monit Assess, vol. 194, no. 1, p. 5, Dec. 2021. [CrossRef]
- K. J. Albin et al., ‘Distinctive phytoplankton size responses to the nutrient enrichment of coastal upwelling and winter convection in the eastern Arabian Sea’, Progress in Oceanography, vol. 203, p. 102779, Apr. 2022. [CrossRef]
- C. K. Sherin et al., ‘Nutriclines and nutrient stoichiometry in the eastern Arabian Sea: Intra-annual variations and controlling mechanisms’, Progress in Oceanography, vol. 215, p. 103048, Jul. 2023. [CrossRef]
- R. T. Barber et al., ‘Primary productivity and its regulation in the Arabian Sea during 1995’, Deep Sea Research Part II: Topical Studies in Oceanography, vol. 48, no. 6–7, pp. 1127–1172, 2001.
- J. I. Goes, P. G. Thoppil, H. do R. Gomes, and J. T. Fasullo, ‘Warming of the Eurasian landmass is making the Arabian Sea more productive’, Science, vol. 308, no. 5721, pp. 545–547, 2005.
- S. Smith, ‘The Arabian Sea of the 1990s: New biogeochemical understanding’, Progress in Oceanography, vol. 2, no. 65, pp. 113–115, 2005.
- C. Bird, J. Martinez Martinez, A. G. O’Donnell, and M. Wyman, ‘Spatial distribution and transcriptional activity of an uncultured clade of planktonic diazotrophic γ-proteobacteria in the Arabian Sea’, Applied and environmental microbiology, vol. 71, no. 4, pp. 2079–2085, 2005.
- J. Barcelos e Ramos et al., ‘Nutrient-specific responses of a phytoplankton community: a case study of the North Atlantic Gyre, Azores’, Journal of Plankton Research, vol. 39, no. 4, pp. 744–761, 2017.
- M. P. Sison-Mangus, S. Jiang, R. M. Kudela, and S. Mehic, ‘Phytoplankton-Associated Bacterial Community Composition and Succession during Toxic Diatom Bloom and Non-Bloom Events’, Frontiers in Microbiology, vol. 7, 2016, Accessed: Feb. 18, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fmicb.2016.01433.
- N. Arandia-Gorostidi et al., ‘Novel Interactions Between Phytoplankton and Bacteria Shape Microbial Seasonal Dynamics in Coastal Ocean Waters’, Frontiers in Marine Science, vol. 9, 2022, Accessed: Feb. 18, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fmars.2022.901201.
- V. Fernandes and K. Bogati, ‘Analysis of Bacteria–Phytoplankton relationships at three discrete locations in the Eastern Arabian Sea during winter’, Continental Shelf Research, vol. 243, p. 104751, 2022.
- B. S. Halpern et al., ‘A global map of human impact on marine ecosystems’, science, vol. 319, no. 5865, pp. 948–952, 2008.
- B. Nogales, M. P. Lanfranconi, J. M. Piña-Villalonga, and R. Bosch, ‘Anthropogenic perturbations in marine microbial communities’, FEMS Microbiology reviews, vol. 35, no. 2, pp. 275–298, 2011.
- V. Sachithanandam et al., ‘Microbial diversity from the continental shelf regions of the Eastern Arabian Sea: A metagenomic approach’, Saudi Journal of Biological Sciences, vol. 27, no. 8, pp. 2065–2075, Aug. 2020. [CrossRef]
- F. Muller-Karger et al., ‘Annual cycle of primary production in the Cariaco Basin: Response to upwelling and implications for vertical export’, Journal of Geophysical Research: Oceans, vol. 106, no. C3, pp. 4527–4542, 2001.
- K. Grasshoff, ‘Determination of nutrients’, Methods of seawater analysis, pp. 125–187, 1983.
- E. García-Robledo, A. Corzo, and S. Papaspyrou, ‘A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes’, Marine Chemistry, vol. 162, pp. 30–36, 2014.
- E. Yakushev, E. Vinogradova, A. Dubinin, A. Kostyleva, N. Men’shikova, and S. V. Pakhomova, ‘On determination of low oxygen concentrations with Winkler technique’, Oceanology, vol. 52, Feb. 2012. [CrossRef]
- M. Kanehisa and S. Goto, ‘KEGG: kyoto encyclopedia of genes and genomes’, Nucleic Acids Res, vol. 28, no. 1, pp. 27–30, Jan. 2000. [CrossRef]
- E. Drula, M.-L. Garron, S. Dogan, V. Lombard, B. Henrissat, and N. Terrapon, ‘The carbohydrate-active enzyme database: functions and literature’, Nucleic Acids Research, vol. 50, no. D1, pp. D571–D577, Jan. 2022. [CrossRef]
- M. Anderson, ‘PERMANOVA+ for PRIMER: guide to software and statistical methods.’, Primer-E Limited., 2008.
- D. H. Parks, G. W. Tyson, P. Hugenholtz, and R. G. Beiko, ‘STAMP: statistical analysis of taxonomic and functional profiles’, Bioinformatics, vol. 30, no. 21, pp. 3123–3124, 2014.
- J. Marra and R. T. Barber, ‘Primary productivity in the Arabian Sea: A synthesis of JGOFS data’, Progress in Oceanography, vol. 65, no. 2, pp. 159–175, May 2005. [CrossRef]
- S. Bauer, G. L. Hitchcock, and D. B. Olson, ‘Influence of monsoonally-forced Ekman dynamics upon surface layer depth and plankton biomass distribution in the Arabian Sea’, Deep Sea Research Part A. Oceanographic Research Papers, vol. 38, no. 5, pp. 531–553, 1991.
- C. Jayaram, N. Chacko, K. A. Joseph, and A. Balchand, ‘Interannual variability of upwelling indices in the southeastern Arabian Sea: a satellite based study’, Ocean Science Journal, vol. 45, pp. 27–40, 2010.
- R. Jyothibabu et al., ‘Impact of freshwater influx on microzooplankton mediated food web in a tropical estuary (Cochin backwaters – India)’, Estuarine, Coastal and Shelf Science, vol. 69, no. 3, pp. 505–518, Sep. 2006. [CrossRef]
- M. Madhupratap et al., ‘Arabian Sea oceanography and fisheries of the west coast of India’, Current Science, vol. 81, no. 4, pp. 355–361, 2001.
- P. Muraleedharan and S. Prasannakumar, ‘Arabian Sea upwelling-A comparison between coastal and open ocean regions’, 1996.
- B. Smitha, V. Sanjeevan, K. Vimalkumar, and C. Revichandran, ‘On the upwelling off the southern tip and along the west coast of India’, Journal of Coastal Research, no. 24, pp. 95–102, 2008.
- H. L. Roman-Stork, B. Subrahmanyam, and V. S. N. Murty, ‘The Role of Salinity in the Southeastern Arabian Sea in Determining Monsoon Onset and Strength’, Journal of Geophysical Research: Oceans, vol. 125, no. 1, p. e2019JC015592, 2020. [CrossRef]
- G. Gupta et al., ‘The world’s largest coastal deoxygenation zone is not anthropogenically driven’, Environmental Research Letters, vol. 16, no. 5, p. 054009, 2021.
- R. J. Newton et al., ‘Genome characteristics of a generalist marine bacterial lineage’, The ISME journal, vol. 4, no. 6, pp. 784–798, 2010.
- T. O. Delmont et al., ‘Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes’, Nature microbiology, vol. 3, no. 7, pp. 804–813, 2018.
- A. L. Hauptmann et al., ‘Bacterial diversity in snow on North Pole ice floes’, Extremophiles, vol. 18, pp. 945–951, 2014.
- J. A. Huber et al., ‘Microbial population structures in the deep marine biosphere’, science, vol. 318, no. 5847, pp. 97–100, 2007.
- Z. Zhou, P. Q. Tran, K. Kieft, and K. Anantharaman, ‘Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation’, ISME J, vol. 14, no. 8, pp. 2060–2077, Aug. 2020. [CrossRef]
- C. Molina-Pardines, J. M. Haro-Moreno, and M. López-Pérez, ‘Phosphate-related genomic islands as drivers of environmental adaptation in the streamlined marine alphaproteobacterial HIMB59’, MSystems, vol. 8, no. 6, pp. e00898-23, 2023.
- H. P. Nair, R. M. Puthusseri, H. Vincent, and S. G. Bhat, ‘16S rDNA-based bacterial diversity analysis of Arabian Sea sediments: A metagenomic approach’, Ecological Genetics and Genomics, vol. 3–5, pp. 47–51, Nov. 2017. [CrossRef]
- X. Lai, X. Zeng, S. Fang, Y. Huang, L. Cao, and S. Zhou, ‘Denaturing gradient gel electrophoresis (DGGE) analysis of bacterial community composition in deep-sea sediments of the South China Sea’, World Journal of Microbiology and Biotechnology, vol. 22, pp. 1337–1345, 2006.
- M. A. Moran et al., ‘Ecological genomics of marine Roseobacters’, Appl Environ Microbiol, vol. 73, no. 14, pp. 4559–4569, Jul. 2007. [CrossRef]
- R. M. Morris et al., ‘SAR11 clade dominates ocean surface bacterioplankton communities’, Nature, vol. 420, no. 6917, pp. 806–810, Dec. 2002. [CrossRef]
- J. C. Thrash et al., ‘Phylogenomic evidence for a common ancestor of mitochondria and the SAR11 clade’, Sci Rep, vol. 1, p. 13, Jun. 2011. [CrossRef]
- M. S. Rappé, S. A. Connon, K. L. Vergin, and S. J. Giovannoni, ‘Cultivation of the ubiquitous SAR11 marine bacterioplankton clade’, Nature, vol. 418, no. 6898, pp. 630–633, Aug. 2002. [CrossRef]
- J. Vijayan, P. Ammini, and V. K. Nathan, ‘Diversity pattern of marine culturable heterotrophic bacteria in a region with coexisting upwelling and mud banks in the southeastern Arabian Sea’, Environ Sci Pollut Res, vol. 29, no. 3, pp. 3967–3982, Jan. 2022. [CrossRef]
- M. T. Cottrell and D. L. Kirchman, ‘Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low-and high-molecular-weight dissolved organic matter’, Applied and environmental microbiology, vol. 66, no. 4, pp. 1692–1697, 2000.
- V. Sharma et al., ‘Phylogenomics of the Phylum Proteobacteria: Resolving the Complex Relationships’, Curr Microbiol, vol. 79, no. 8, p. 224, Jun. 2022. [CrossRef]
- H. W. Paerl and T. G. Otten, ‘Harmful cyanobacterial blooms: causes, consequences, and controls’, Microbial ecology, vol. 65, pp. 995–1010, 2013.
- Z. Chen, X. Wang, Y. Song, Q. Zeng, Y. Zhang, and H. Luo, ‘Prochlorococcus have low global mutation rate and small effective population size’, Nat Ecol Evol, vol. 6, no. 2, pp. 183–194, Feb. 2022. [CrossRef]
- F. Partensky, J. Blanchot, and D. Vaulot, ‘Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review’, Bulletin de l’Institut Océanographique - Special issue: Marine cyanobacteria, vol. 19, pp. 457–476, Jul. 1999.
- H. Cheng et al., ‘Influence of phosphorus fertilization patterns on the bacterial community in upland farmland’, Industrial Crops and Products, vol. 155, p. 112761, Nov. 2020. [CrossRef]
- P. R. Gómez-Pereira et al., ‘Genomic content of uncultured Bacteroidetes from contrasting oceanic provinces in the North Atlantic Ocean’, Environmental Microbiology, vol. 14, no. 1, pp. 52–66, 2012.
- D. L. Kirchman, ‘The ecology of Cytophaga–Flavobacteria in aquatic environments’, FEMS microbiology ecology, vol. 39, no. 2, pp. 91–100, 2002.
- P. G. Thoppil, A. J. Wallcraft, and T. G. Jensen, ‘Winter convective mixing in the northern Arabian Sea during contrasting monsoons’, Journal of Physical Oceanography, vol. 52, no. 3, pp. 313–327, 2022.
- L. Alonso-Sáez et al., ‘Transcriptional Mechanisms of Thermal Acclimation in Prochlorococcus’, mBio, vol. 14, no. 3, pp. e03425-22, Apr. 2023. [CrossRef]
- C. Wu et al., ‘Diversity, structure, and distribution of bacterioplankton and diazotroph communities in the Bay of Bengal during the winter monsoon’, Front. Microbiol., vol. 13, Nov. 2022. [CrossRef]
- A. Vuillemin, ‘Nitrogen cycling activities during decreased stratification in the coastal oxygen minimum zone off Namibia’, Front Microbiol, vol. 14, p. 1101902, Feb. 2023. [CrossRef]
- J. Blanchot, M. Rodier, and A. Le Bouteiller, ‘Effect of El Niño Southern Oscillation events on the distribution and abundance of phytoplankton in the Western Pacific Tropical Ocean along 165°E’, Journal of Plankton Research, vol. 14, no. 1, pp. 137–156, Jan. 1992. [CrossRef]
- Y. Liu et al., ‘Cell surface acid-base properties of the cyanobacterium Synechococcus: Influences of nitrogen source, growth phase and N:P ratios’, Geochimica et Cosmochimica Acta, vol. 187, pp. 179–194, Aug. 2016. [CrossRef]
- X. Liu, N. Xie, J. Li, M. Bai, B. Sen, and G. Wang, ‘Potential Contribution of Coastal Upwelling to Carbon Sink through Interaction between Cyanobacteria and Microbial Eukaryotes’, Water, vol. 14, no. 19, Art. no. 19, Jan. 2022. [CrossRef]
- C. Nikolova and T. Gutierrez, ‘Chapter 6 - Novel approaches in the use of biosurfactants in the oil industry and environmental remediation’, in Biosurfactants, G. Soberón-Chávez, Ed., in Foundations and Frontiers in Enzymology., Academic Press, 2023, pp. 107–128. [CrossRef]
- F. Bian et al., ‘Genome sequences of six Pseudoalteromonas strains isolated from Arctic sea ice’, J Bacteriol, vol. 194, no. 4, pp. 908–909, Feb. 2012. [CrossRef]
- D. Li, J. O. Sharp, and J. E. Drewes, ‘Microbial genetic potential for xenobiotic metabolism increases with depth during biofiltration’, Environ. Sci.: Processes Impacts, vol. 22, no. 10, pp. 2058–2069, Oct. 2020. [CrossRef]
- E. Cardenas, J. Kranabetter, G. Hope, K. R. Maas, S. Hallam, and W. W. Mohn, ‘Forest harvesting reduces the soil metagenomic potential for biomass decomposition’, The ISME Journal, vol. 9, no. 11, pp. 2465–2476, 2015.
- L. Manoharan, S. K. Kushwaha, K. Hedlund, and D. Ahrén, ‘Captured metagenomics: large-scale targeting of genes based on ‘sequence capture’reveals functional diversity in soils’, DNA Research, vol. 22, no. 6, pp. 451–460, 2015.
- X. Xia et al., ‘Distinct metabolic strategies of the dominant heterotrophic bacterial groups associated with marine Synechococcus’, Science of The Total Environment, vol. 798, p. 149208, Dec. 2021. [CrossRef]
- L. Jiang et al., ‘Identification and characterization of a novel trehalose synthase gene derived from saline-alkali soil metagenomes’, PLoS One, vol. 8, no. 10, p. e77437, 2013.
- M. Walmagh, R. Zhao, and T. Desmet, ‘Trehalose analogues: latest insights in properties and biocatalytic production’, International Journal of Molecular Sciences, vol. 16, no. 6, pp. 13729–13745, 2015.
- T. Collins, C. Gerday, and G. Feller, ‘Xylanases, xylanase families and extremophilic xylanases’, FEMS microbiology reviews, vol. 29, no. 1, pp. 3–23, 2005.
- M. T. Cottrell, L. Yu, and D. L. Kirchman, ‘Sequence and expression analyses of Cytophaga-like hydrolases in a Western arctic metagenomic library and the Sargasso Sea’, Applied and environmental microbiology, vol. 71, no. 12, pp. 8506–8513, 2005.
- L. E. Taylor, B. Henrissat, P. M. Coutinho, N. A. Ekborg, S. W. Hutcheson, and R. M. Weiner, ‘Complete cellulase system in the marine bacterium Saccharophagus degradans strain 2-40T’, Journal of bacteriology, vol. 188, no. 11, pp. 3849–3861, 2006.
- S. Violot, R. Haser, G. Sonan, D. Georlette, G. Feller, and N. Aghajari, ‘Expression, purification, crystallization and preliminary X-ray crystallographic studies of a psychrophilic cellulase from Pseudoalteromonas haloplanktis’, Acta Crystallographica Section D: Biological Crystallography, vol. 59, no. 7, pp. 1256–1258, 2003.
- B. Kumar and V. Sarma, ‘Variations in concentrations and sources of bioavailable organic compounds in the Indian estuaries and their fluxes to coastal waters’, Continental Shelf Research, vol. 166, pp. 22–33, 2018.
- A.-Y. Tsai, G.-C. Gong, R. W. Sanders, C.-F. Chao, and K.-P. Chiang, ‘Microbial dynamics in an oligotrophic bay of the western subtropical Pacific: Impact of short-term heavy freshwater runoff and upwelling’, Journal of oceanography, vol. 66, pp. 873–883, 2010.
- A. Malik et al., ‘Interactions between trophic levels in upwelling and non-upwelling regions during summer monsoon’, Journal of sea research, vol. 95, pp. 56–69, 2015.
- A. Shivaprasad, J. Vinita, C. Revichandran, N. T. Manoj, K. V. Jayalakshmy, and K. R. Muraleedharan, ‘Ambiguities in the classification of Cochin Estuary, West Coast of India’, Hydrology and Earth System Sciences Discussions, vol. 10, no. 3, pp. 3595–3628, Mar. 2013. [CrossRef]
- W. Shi and M. Wang, ‘Phytoplankton biomass dynamics in the Arabian Sea from VIIRS observations’, Journal of Marine Systems, vol. 227, p. 103670, 2022.
- P. N. M. Vinayachandran et al., ‘Reviews and syntheses: Physical and biogeochemical processes associated with upwelling in the Indian Ocean’, Biogeosciences, vol. 18, no. 22, pp. 5967–6029, Nov. 2021. [CrossRef]
- M. Krishna, M. Prasad, D. Rao, R. Viswanadham, V. Sarma, and N. Reddy, ‘Export of dissolved inorganic nutrients to the northern Indian Ocean from the Indian monsoonal rivers during discharge period’, Geochimica et Cosmochimica Acta, vol. 172, pp. 430–443, 2016.
- G. Martin et al., ‘Eutrophication induced changes in benthic community structure of a flow-restricted tropical estuary (Cochin backwaters), India’, Environmental monitoring and assessment, vol. 176, pp. 427–438, 2011.
- Y. Wang et al., ‘Comparison of the Levels of Bacterial Diversity in Freshwater, Intertidal Wetland, and Marine Sediments by Using Millions of Illumina Tags’, Appl Environ Microbiol, vol. 78, no. 23, pp. 8264–8271, Dec. 2012. [CrossRef]
- A. Debbab, A. H. Aly, W. H. Lin, and P. Proksch, ‘Bioactive compounds from marine bacteria and fungi’, Microbial biotechnology, vol. 3, no. 5, pp. 544–563, 2010.
- P. S. Kumar et al., ‘Influence of nutrient fluxes on phytoplankton community and harmful algal blooms along the coastal waters of southeastern Arabian Sea’, Continental Shelf Research, vol. 161, pp. 20–28, Jun. 2018. [CrossRef]
- A. Ferreira, V. Brotas, C. Palma, C. Borges, and A. C. Brito, ‘Assessing phytoplankton bloom phenology in upwelling-influenced regions using ocean color remote sensing’, Remote Sensing, vol. 13, no. 4, p. 675, 2021.
- A. Buchan, G. R. LeCleir, C. A. Gulvik, and J. M. González, ‘Master recyclers: features and functions of bacteria associated with phytoplankton blooms’, Nature Reviews Microbiology, vol. 12, no. 10, pp. 686–698, 2014.
- C. Costas-Selas et al., ‘Linking the impact of bacteria on phytoplankton growth with microbial community composition and co-occurrence patterns’, Marine Environmental Research, vol. 193, p. 106262, Jan. 2024. [CrossRef]
- M. Bandekar, N. Ramaiah, A. Jain, and R. M. Meena, ‘Seasonal and depth-wise variations in bacterial and archaeal groups in the Arabian Sea oxygen minimum zone’, Deep Sea Research Part II: Topical Studies in Oceanography, vol. 156, pp. 4–18, 2018.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).