1. Introduction
The temperature of average surface has been increasing with 0.19°C per decade [
1], as a consequence, the frequency and duration of the extreme weather events, especially the heatwaves and precipitation have significantly increased [
2]. As the ruminal fermentation process produces excessive heat, mammary gland conducts high anabolic activities, and cows is with low surface area to mass ratio, these factors render lactating cows are more venerable to heat stress [3, 4]. It has been reported that when the temperature-humidity index (THI) reached 72 (but recent research indicated this data as low as 68 or 70), the adverse influence of the heat stress on dairy cows will be shined through [5, 6]. The detrimental impacts of heat stress on dairy cows mainly including reduced feed intake, milk production metrics, milk protein metrics, and the following conception rate [
7], as well as the increased risk of ruminal acidosis and enteric methane (CH4) production [
8]. Therefore, heat stress becomes an obvious restrictive factor for dairy cow production, and will become worse with continuously increasing global temperature. Thus, finding out an effective cooling method to attenuate heat stress in cows is of significant importance.
The most critical point in attenuating heat stress is to re-establish the physiological thermal energy balance between the heat acquisition (including the maintenance, exercise, growth, lactation, gestation and feed intake) and the heat dissipation to the environment [9, 10]. During the production process, a number of cooling options, such as shading and air fans, are used to lower environment temperature and restore cow’s normal physiology [
11]. Further, nutritional strategies like supplementation of appropriate energy ingredients, minerals, vitamins, antioxidants, prebiotics, and probiotics are also applied to ameliorate heat stress [
12]. Compared with nutritional regulations, directly cooling the environment (such as cooling with sprinklers) appears to be the most economical and efficient method in relieving heat stress [
13]. It has been demonstrated that cooling with sprinklers was successful in reducing shell and body temperature, increase heat evaporation to lower respiration rate, enhance feed intake, and promote digestibility [
14]. As a consequence, increased milk production and milk protein production are achieved [15-18]. However, the underlying mechanism behind these positive effects of sprinklers cooling in attenuating heat stress is not been elucidated.
Ruminal microbiota is of vital importance for animal health and production performance since microorganisms can ferment feed ingredients to supply critical metabolites for the host [19-25]. For example, ruminal microbial communities can produce nutritional fermentation metabolites like vitamins and functional fatty acid [19, 26-29]. These metabolites can be adsorbed through rumen epithelial cells, and then transported through the blood to the target tissues to act regulate tissue functions. Recent researches have documented that the rumen microbial communities are directly regulated by the heat stress [30-33]. Specifically, Zhao et al (2019) found that heat stress led to ruminal bacterial composition alteration and functional deterioration, as well as lactate increasing and acetate-producing bacterial relative abundance reducing [
30]. Other researches disclosed that ruminal fiber degrading-bacteria
Fibrobacter population decreased while the starch degrading-bacteria including
Clostridium and
Streptococcus population increased [34, 35] in response to the heat stress. While heat stress relieving research, take live yeast as an example, found that live yeast supplementation positively ameliorated heat stress on dairy cows by regulating the microbiota composition and rumen fermentation in the rumen and hindgut [
36]. These evidences demonstrate that the response of ruminal microbiota maybe the underlying mechanism of one’s positive effects on heat stress in ruminal animals.
Therefore, the present study was to evaluate the effect of an automatic sprinkler on the production performances of cows and elucidate the potential mechanism from the ruminal microbiota perspective.
2. Materials and Methods
2.1. Experiment Animals and Management
The experiment was conducted in the Bengbu dairy farm, Modern Farming (Wuhe) Co. Ltd, Anhui Province, China (32.92N, 117.38E) from June 10th, 2023 to August 10th, 2023. Total of 1208 multiparous dairy cows with the average live weight of 683.6 ± 27.3 kg, the average lactation of 199.3±16.8 d, and the average lactating parities of 2.88±0.49 were used and randomly allocated into 6 barns. Three barns were equipped with automatic sprinklers (SP), each barn was considered as a replicate. The other three barns were received the routinely feeding procedure (CON) without sprinklers. The Schematic diagram and the actual equipment of the Springkler were shown in
Figure 1.
All cows were reared in a 312 m-long×96 m-wide shed to ensure the same feeding environment. Diets were formulated according to NRC (2001) to meet or exceed the energy requirements of Holstein dairy cows yielding 30 kg of milk/day with 3.5% milk fat and 3.0% true protein. The nutrient level and ingredient composition of the diet used were shown in
Table 1. Cows were fed three times per day at 06:00, 13:00, and 21:00, respectively. During the experimental period, all cows had free access to diet and water. Temperature and humidity were recorded every day, and THI were calculated through the following equation reported as previously reported [
37]. THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T −26)]. Where, T=temperature, RH = relative humidity. THI ≥70 was considered the heat stress occurs in high-yielding dairy cows (Pinto et al., 2020). Cows were milked three times per day (08:00, 14:00, and 20:00 h, respectively). Moreover, during the experimental time, when need, pregnant cows were induced using normal artificial insemination method [
38].
2.2. Feed Intake and Composition Analysis
Average daily intake was determined based on the dry matter intake (DMI). DMI was calculated as the difference between the feed offered and the residues on the dry matter basis. Feed samples were collected from each feeding time and then were mixed for analysis. Air-dried samples were obtained from the fresh feed which were dried using a forced-air oven (GZX-9246MBE, Shanghai Boxunshiye Co., Ltd., Shanghai, China) at 65°C for 48 h. Then absolute dried feed sample were obtained from the air-dried samples which were further dried at 105 °C for 3 h using the forced-air oven.
The net energy (NE) level of the feed was calculated using the methodology described as previous [
39]. The feed compositions were determined according the the AOAC (2007) method. Specifically, a Kjeldahl nitrogen analyzer (SKD-1800, Shanghai Peiou Analytical Instrument Co., Ltd., Shanghai, China) was used to determine crude protein (CP) level. Ether extract (EE) was determined using a
A semi-automatic fiber analyzer (A200i, ANKOM, USA) was used to determine the neutral detergent fiber (NDF) and acid detergent fiber (ADF) levels. Calcium (Ca), and phosphorus (P) levels were determined by a near-infrared spectroscopy (NIRS) method.
2.3. Milk Production and Composition
Daily milk yield was automatically recorded through the rotary milking facilities (9JRP-50P2100, Delaval, Israel). Milk samples were collected from each treatment during the last three consecutive days. They were stored in 100-mLvials with 2-bromo-2-nitropropan-1,3-diol at 4℃ for subsequent analysis.
Milk protein and milk fat were measured using a near-infrared analyzer (MilkoScanTM 7 RM, Foss Electric, Denmark). Somatic cell count (SCC) was measured using a SCC rapid analyzer (Fossomatic 7/7 DC, FOSS).
2.4. Body Temperature Measurement
Body temperature of cows was measured once a week using a thermometer (VT 1831; microLife, Switzerland) rectally.
2.5. The Initial Conception Rate (ICR)
Pregnancy was induced using the standard artificial insemination when the cows were ready to be pregnant. The initial conception rate (ICR) of cows in the both SP and CON groups was calculated as the percentage of pregnant cows relevant to the total cows who were subjected to the standard artificial insemination.
2.6. Rumen Content Collection and Fermentation Parameter Analysis
Three hours after the morning feeding, 100 mL rumen contents of 18 dairy cows (9 cows from one barn, that is 9 cows from the CON and SP group, respectively) were collected using an oesophageal tube on the last day of the experiment [
40]. In order to avoid the potential saliva contamination, the first 200 mL contents were discarded. All rumen samples were divided into two parts. One part was analyzed for the pH, volatile fatty acid (VFAs) and ammonia-N (NH3-N). The pH of each rumen fluid sample was measured immediately using a portable pH meter (Testo 205, Testo AG, Lenzkirch, Germany). Individual and total VFAs (TVFA) in the aliquots of ruminal fluid were determined by gas chromatograph (GC-2010, Shimadzu, Kyoto, Japan). Concentration of NH3-N was determined by the indophenol method and the absorbance value was measured using a UV-2600 ultraviolet spectrophotometer (Tianmei Ltd., China) [
41]. The other part was rapidly frozen with liquid nitrogen and then stored at -80℃ for further analysis.
2.7. Rumen Content Collection and Fermentation Parameter Analysis
Rumen microbial DNA was extracted from approximate 1.0 mL rumen content the MagBind® Soil DNA Kit (M5636, Omega, Norcross, GA, USA). DNA concentration, purity, and quality were assessed using a spectrophotometer and agarose gel electrophoresis. The V4 and V3 region of the 16S rRNA gene was amplified using the universal primers (F: ACTCCTACGGGAGGCAGCAG and R: GGACTACHVGGGTWTCTAAT). The mixture of PCR products was purified with Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany). Sequencing was conducted on an Illumina MiSeq PE300 platform /NovaSeq PE250 platform (Illumina, San Diego, CA, USA) in a commercial laboratory. Quality filtering of raw tags was performed under standard filtering conditions to obtain the high-quality clean tags according to the Quantitative Insights into Microbial Ecology (QIIME, V1.7.0) quality controlling process. Sequences within similarity >97% were assigned into the same operational taxonomic unit (OTU). For each representative sequence, the GreenGene Database was used based on SILVA classifier algorithm to annotate taxonomic information. Then, the α-diversity index, β-diversity index, and species abundances were analyzed at different taxonomic level.
2.8. Statistical Analysis
Data was presented as mean ± SEM. Statistical analysis was performed using an unpaired two-tailed Student’s T-test procedures of SAS (v.9.2, SAS Institute, Cary, NC, USA), except where indicated. P-value < 0.05 was considered significant, and 0.05 ≤P≤ 0.10 was considered to indicate a trend.
3. Results
3.1. Effects of Automatic Spraying on Body Temperature, Milk Yield, Milk Content and Pregnant Rate under the Heat Stress Condition
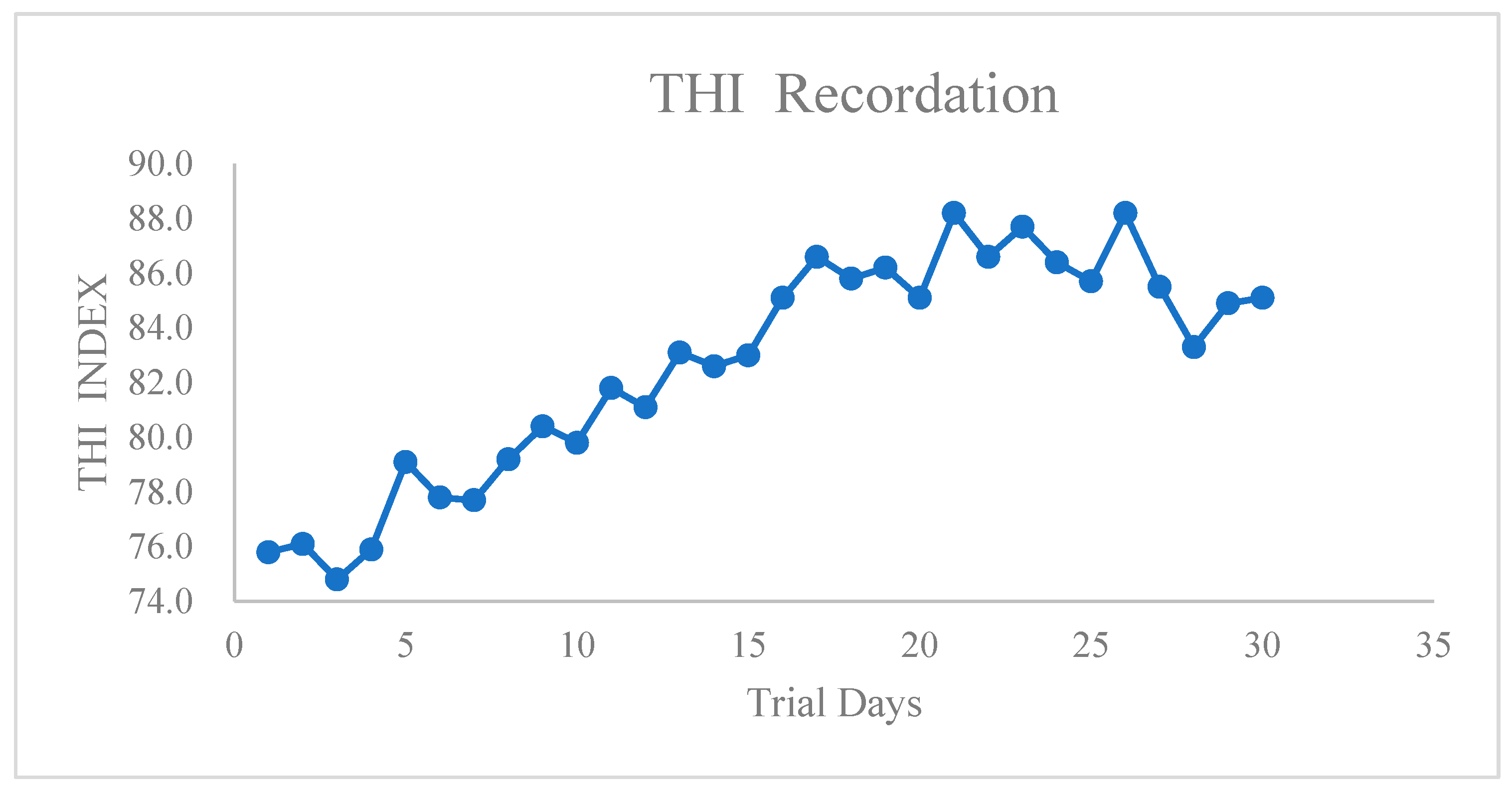
In this study, temperature and relative humidity of feeding lair were recorded at 5 different locations, and THI was recorded throughout the whole experiment (
Figure 2). During all the experimental period, environmental THI exceeded 74, indicating that the heat stress occurred throughout the whole experiment.
Body temperature and the productive performances of dairy cows received automatic spraying and the control treatment were further detected and the results are shown in
Table 2. Body temperature of cows that received SP treatment significantly reduced (
P<0.05), which implied the successfully achievement of cooling effect. In addition, DMI and milk yield were significantly increased (
P<0.05) after spraying treatment under the heat stress condition, which demonstrated that automatic spraying effectively ameliorated the detrimental effects of heat stress on cows. Besides, the milk fat content tended to increase (
P = 0.056). Although it was not significant, milk protein levels were numerically slightly elevated (
P = 0.412). For the SCC, it was remarkably decrease in the SP group (
P<0.05). Moreover, total of 167 cows (n
SP=91, n
CON =76) were received the artificial insemination, and the initial conception rate (ICR) was showed in
Table 2. Although the ICR was still lower than normal fertilization rate, it significantly improved from 30% to 35% after automatic spraying treatment. Collectively, these data suggested that automatic spraying effectively alleviated the adverse effects of heat stress on cows, improving their lactating performance and pregnant rate.
3.2. Effects of Automatic Spraying on Rumen Fermentable Parameters under the Heat Stress Condition
Rumen fermentation parameters including pH, NH
3-N and VFAs were presented in
Table 3. The automatic spraying had no effects on the rumen pH and NH
3-N levels (
P > 0.05), but significantly increased rumen acetate, isobutyrate, butyrate levels (
P < 0.05), and tended to increase propionate (
P = 0.054) level of cows from the SP groups compared to the CON group. Consistently, the TVFA level was significantly higher in the SP groups compared to the CON group (
P < 0.05). While the ratio of acetic to propionic was not changed between two groups (
P > 0.05). Collectively, these data suggested that automatic spraying effectively enhanced rumen fermentation functions.
3.3. Effects of Automatic Spraying on Rumen Microbiome
Rumen microbiota is critical significant for its rumen fermentation functions, and cow health and production performances. Since automatic spraying alleviated the adverse effects of heat stress on cows and enhanced rumen fermentation functions, we next investigated the underlying mechanism of automatic spraying’s positive regulation effects from the rumen microbiota’s perspective. Rumen microbiota of cows from the CON and SP group was evaluated. A total of 5800 OTUs, 17phyla, 290 genera, and nearly 2000 species were identified after quality control, and all the taxonomic information is displayed in
Table S1. All identified bacteria were chosen for further analysis to investigate the effects of SP treatment on α-diversity parameters of ruminal bacteria. Results showed that the α-diversity was significantly increased in the SP group as indicated by the significantly increased ACE index and observed species (
P < 0.05), as well as the tended increased Chao1 index (
P = 0.062), suggesting that automatic spraying improved rumen microbiota species richness and diversity (
Table 4).
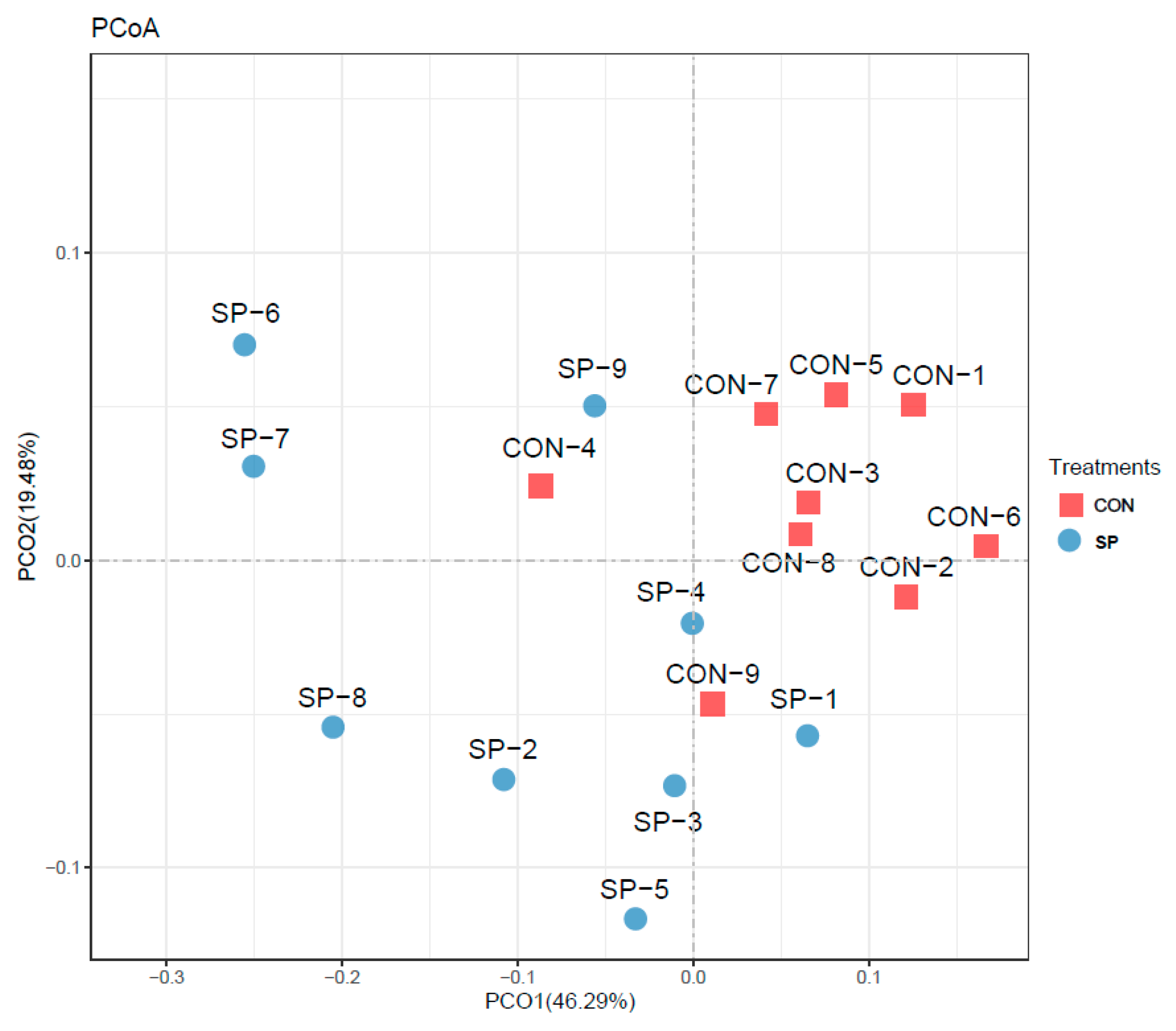
Following, PCoA analysis was performed to assess the β–diversity. PCoA results clarified the monolithic discrepancy of microbial profiles between SP and CON treatments. As shown in
Figure 3, PCoA axes 1 and 2 accounted for 46.29% and 19.48%, respectively. Bacterial communities in SP group could be separated from those in CON group, indicating that automatic spraying modulated the composition of rumen microbiota under the heat stress condition.
At the phylum level,
Firmicutes,
Bacteroidetes and
Tenericutes were the top abundant microorganism identified in the rumen under the heat stress condition (
Table 5). The relative abundance of rumen
Fibrobacters significantly increased and
Bacteroidetes tended to increase in the SP group compared to that in the CON group.
At the genera level, Prevotella, Ruminococcaceae, Succiniclasticum, Lachnospiraceae, and Eubacterium located the top 5 abundant genera. The relative abundance of Succiniclasticum, Butyrivibrio, Pseudobutyrivibrio, Bifidobacterium, and Streptococcus significantly increased (P < 0.05) in the SP treatment compared with CON. On the contrary, the relative abundance of Ruminococcaceae and Succinivibrio significantly decreased (P < 0.05) in the rumen from the SP group compared to that from the CON group.
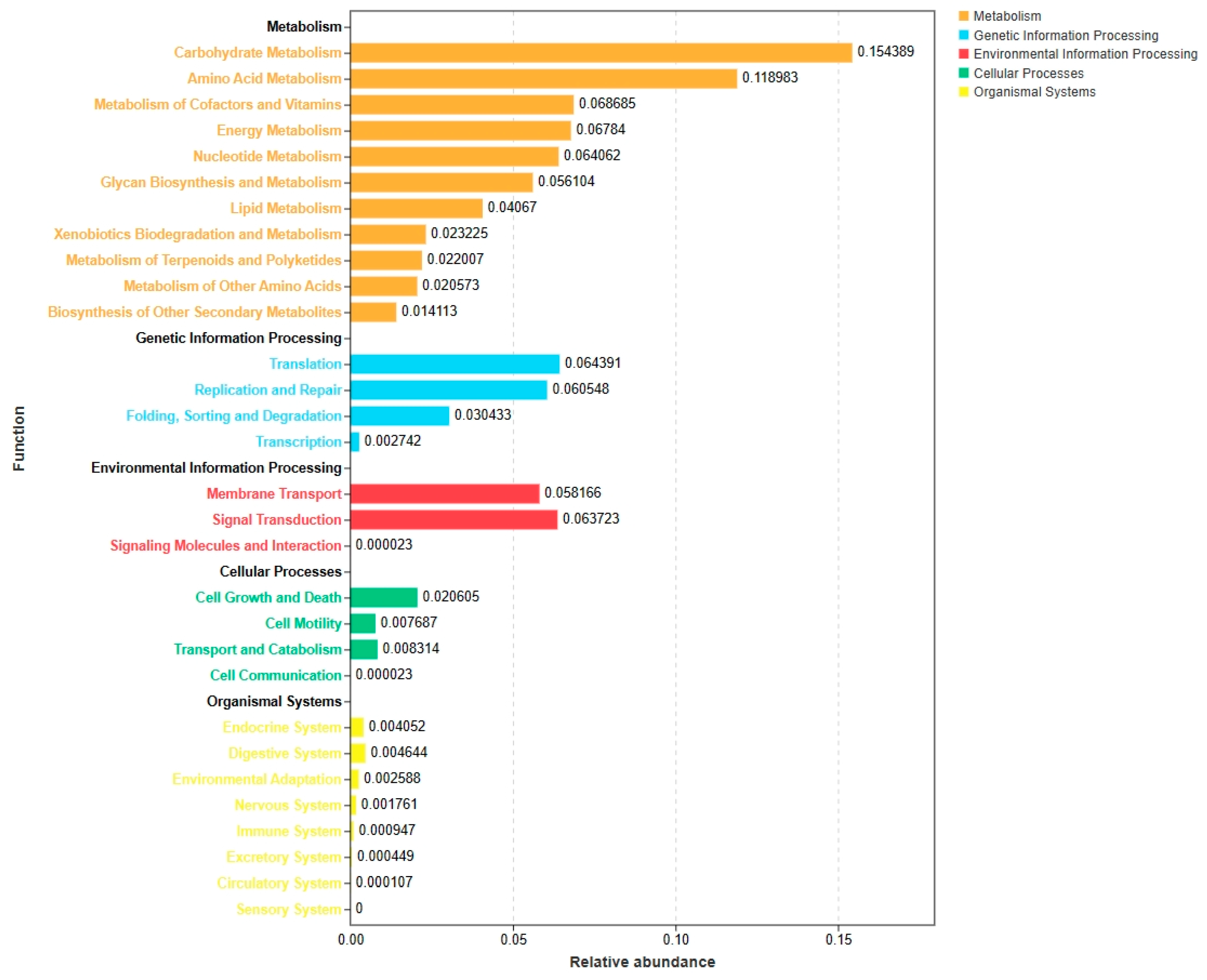
Functions that potentially presented in the differentially identified microbiota were predicted using the Tax4Fun method, and the results are shown in
Figure 4. Metabolic processes which mainly including the carbohydrate metabolism, amino acids metabolism energy metabolism and cofactors, and genetic information processing which mainly including the translation process, replication and repair process were the predominant functional pathways. Specifically, the functions of differentially abundant bacteria were mostly enriched in carbohydrates, amino acids, energy, cofactors, and vitamin metabolism. On the contrary, lower relative abundances are observed on lipid metabolism and secondary metabolites. Genetic information processing methods such as the translation, replication and repair processes, and environmental information processing methods including membrane transport and signal transduction are also enriched by the differential abundant microbiota.
Table 6.
Effects of springkling on relative rumen bacteria diversities during heat stress conditions (level of phyla).
Table 6.
Effects of springkling on relative rumen bacteria diversities during heat stress conditions (level of phyla).
| Items |
SP(n=9) |
CON(n=9) |
SE |
P-value |
| g__Prevotella |
18.66 |
16.75 |
1.239 |
0.064 |
| g__Ruminococcaceae |
15.64 |
20.20 |
0.189 |
0.005 |
| g__Succiniclasticum |
11.42 |
9.76 |
0.321 |
0.029 |
| g__Lachnospiraceae |
5.38 |
5.41 |
0.282 |
0.623 |
| g__Eubacterium |
4.49 |
4.07 |
0.206 |
0.141 |
| g__Ruminococcus |
4.37 |
4.95 |
0.256 |
0.015 |
| g__Shuttleworthia |
1.95 |
1.37 |
0.182 |
0.272 |
| g__Prevotellaceae |
1.28 |
1.25 |
0.237 |
0.371 |
| g__Acetitomaculum |
0.992 |
0.741 |
0.121 |
0.063 |
| g__Lachnoclostridium |
0.713 |
0.751 |
0.012 |
0.137 |
| g__Butyrivibrio |
0.534 |
0.418 |
0.034 |
0.035 |
| g__Ruminiclostridium |
0.258 |
0.284 |
0.041 |
0.108 |
| g__Pseudobutyrivibrio |
0.253 |
0.182 |
0.014 |
0.009 |
| g__Selenomonas |
0.074 |
0.041 |
0.021 |
0.167 |
| g__Lactobacillus |
0.069 |
0.061 |
0.010 |
0.089 |
| g__Bifidobacterium |
0.036 |
0.021 |
0.013 |
0.048 |
| g__Escherichia-Shigella |
0.024 |
0.033 |
0.008 |
0.271 |
| g__Bacteroides |
0.022 |
0.024 |
0.006 |
0.345 |
| g__Succinivibrio |
0.014 |
0.071 |
0.012 |
0.033 |
| g__Streptococcus |
0.019 |
0.014 |
0.003 |
0.029 |
| g__Butyricicoccus |
0.011 |
0.011 |
0.006 |
0.132 |
| others |
34.06 |
33.64 |
3.346 |
0.421 |
4. Discussion
Heat stress detrimentally influence the dairy production. When the heat stress occurs, dairy cows manifest reduced feed intake, dairy production and milk quality, increased evaporated water loss, as well as metabolic disturbance. During the experiments, THI remained over 72, indicating that cows were all under the heat stress. Under the heat stress conditions, high temperature improves body temperature, which extends the feed duration in the rumen and in turn activates rumen sensor for stomach stretch [
42]. This alteration impacted the hypothalamic anorexia center, resulting in a lowered appetite and feed intake [
43]. As e results, DMI and energy intake are reduced under this condition. By using the automatic spraying cooling method, we found that cow body temperature was reduced, DMI, milk yield, milk fat and ICR were increased, SCC was lowered, as well as rumen fermentation functions were enhanced, which forcefully demonstrated that this method could effectively alleviated heat stress on cows.
Rumen microbial communities are critical to the dairy health and production performances. One important function of rumen microorganisms is to provide the host with energy and functional metabolites by metabolizing nutrients residues. In the rumen, carbohydrates are usually converted to pyruvate and acetyl-CoA by microorganisms through the glycolytic pathway and pentose phosphate pathway, and finally metabolized to VFAs, especially acetate and propionate to provide energy for productions. It has been reported that approximate 70% to 80% energy absorbed by the cows is provided by the rumen fermentation processes [
19]. Acetate was further transported into mammary and synthesized into milk fat while butyrate was utilized by the intestinal tract for the epithelial development. In the present study, VFAs including acetate, propionate and butyrate were significantly increased in rumen fluid under SP treatment. TVFA reflects energy balance and utilization in the cows, and the VFA composition and proportions are good indicators for the rumen fermentation capacity [
44]. More specifically, acetate provide lipid synthesis in adipose and mammary tissues of ruminants with primary carbon source [
45], which partially accounted for the increased milk fat level. In addition, heat stress can trigger toll-like receptor pathways and cause inflammatory responses. Deteriorated inflammation statues will cause mastitis, and lead to milk SCC content increase [
46]. Butyrate serves as critical immune regulator, helpful in inhibiting mastitis [
47], which may explain the reduced SCC in milk by the increased butyrate content. It should be noteworthy although that rumen fermentation was enhanced by the automatic spraying, the ratio of rumen acetate level to propionate level was not different between the SP group and the CON group, suggested that rumen fermentation pattern was not changed.
The ruminal homeostasis provided an ideal environment for nutrients digestion and transportation, energy generation, and the microbial proliferation [48, 49]. Rumen microbial diversity, composition and function significantly altered under the heat stressed environment, which might disrupt the primary homeostasis and further caused the reduction of feed intake and milk production. Ruminal microbial community composition and changes present important breakthroughs in understanding how heat stress affects dairy farming technology. We found that the rumen microbial richness and composition were modulated by the automatic spraying, indicating rumen microbial might implicated in the automatic spraying’s positive effects under the heat stress conditions. Zhao et al. (2019) and Uyeno et al. (2010) reported that the relative abundance of Spirochaeta, Streptococcus, and Ruminobacter were increased in the heat stress condition and parallel with the increased with the decreased acetic acid [30, 50]. In consistent with this data, we found that the relevant abundance of Streptococcus tended to decrease, and these results was also parallel with increased rumen acetic acid level. In addition, some functional microorganism abundances were modulated by the automatic spraying treatment. For example, the relative abundance of Prevotella and Pseudobutyrivibrio were increased. Prevotella is important in starch utilization, and Pseudobutyrivibrio is a stressor-related genera. Indeed, these changes accommodated to the reductive degradability of fiber content and the enhancement of starch degradability [34, 35], which ensured the abundant energy supply for body production and thus yield enhance production.
What’s more, we delighted to find that the ICR was enhanced by the automatic spraying treatment. Ambient temperatures that lead to body temperatures reach over the thermo-neutral zone for cows result in reduced reproductive efficiencies. This is because that during the heat stress condition, adrenocorticotropic hormone stimulates glucocorticoids synthesis and secretion, which detrimentally affects the hypothalamic-pituitary-gonadal axis, and thus disturb the female estrous cycle. In this study, automatic spraying reduced body temperature, may accounted for the improved ICR. But due to lack hormone information and detailed inspection on reproductive systems, the specific mechanism behind this should be investigated further.
5. Conclusions
Heat stress detrimentally affects cows, resulting in economic losses in dairy farming production. The aim of this study was to evaluate the effects of an automatic spraying method on alleviating heat stress in cows and dissecting the potential mechanisms. Body temperature, dry matter intake (DMI), milk production and quality, rumen fermentation parameters and microbiota, and initial conception rate (ICR) of cows with or without spraying treatment were investigated. Results showed that spraying treatment reduced body temperature, milk yield and milk quality. Rumen fermentation and microbiota were modulated by the spraying treatment, and rumen changes may have implications for the phenotypic changes. In summary, this study demonstrated that automatic spraying modulated the rumen microbiota composition and fermentation function, and simultaneously effectively meliorated the adverse effects of heat stress on dairy cows, providing potential mechanism of how automatic spraying alleviating heat stress.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: 16S rRNA results of rumen microbiota.
Author Contributions
Fuguang Xue and Mingren Qu designed the study. En Liu, Liping Liu, and Zhili Zhang conducted the experiment. Kang Mao analyzed the data. Long Wang measured the fermentable parameters. En Liu and Fuguang Xue wrote and revised the manuscript.
Funding
This study was funded by the National Key Research and Development Program of China (2018YFD0501804), and supported by China Agriculture Research System of MOF and MARA (CARS-37)
Institutional Review Board Statement
Animal care and procedures abides by The Chinese Guidelines for Animal Welfare, and were approved by the Animal Care and Use Committee of Jiangxi Agricultural University (the approval number: JXAULL-20230609).
Informed Consent Statement
“Informed consent was obtained from all subjects involved in the study.”.
Data Availability Statement
Acknowledgments
We thank the Jiangxi Province Key Laboratory of Animal Nutrition/Engineering Research Center of Feed Development, Jiangxi Agricultural University.
Conflicts of Interest
“The authors declare no conflicts of interest.”
References
- Tokarska, K.B., et al., Past warming trend constrains future warming in CMIP6 models. Sci Adv, 2020. 6(12): p. eaaz9549. [CrossRef]
- Ebi, K.L., et al., Hot weather and heat extremes: health risks. Lancet, 2021. 398(10301): p. 698-708. [CrossRef]
- Tajima, K., et al., Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe, 2007. 13(2): p. 57-64. [CrossRef]
- Baumgard, L.H. and R.J. Rhoads, Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci, 2013. 1: p. 311-37. [CrossRef]
- Koester, L.R., et al., Influence of a sodium-saccharin sweetener on the rumen content and rumen epithelium microbiota in dairy cattle during heat stress. J Anim Sci, 2023. 101.. [CrossRef]
- Pinto, S., et al., Critical THI thresholds based on the physiological parameters of lactating dairy cows. J Therm Biol, 2020. 88: p. 102523. [CrossRef]
- Dahl, G.E., S. Tao and J. Laporta, Heat Stress Impacts Immune Status in Cows Across the Life Cycle. Front Vet Sci, 2020. 7: p. 116. [CrossRef]
- Kaufman, J.D., et al., Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J Dairy Sci, 2018. 101(1): p. 386-395. [CrossRef]
- Polsky, L. and M. von Keyserlingk, Invited review: Effects of heat stress on dairy cattle welfare. J Dairy Sci, 2017. 100(11): p. 8645-8657. [CrossRef]
- Rhoads, M.L., Effects of periconceptional heat stress on primiparous and multiparous daughters of Holstein dairy cows. Theriogenology, 2020. 150: p. 458-463. [CrossRef]
- Li, G., et al., Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J Dairy Sci, 2020. 103(6): p. 5466-5484. [CrossRef]
- Guo, J., et al., Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas J Anim Sci, 2018. 31(1): p. 47-53.. [CrossRef]
- West, J.W., Effects of heat-stress on production in dairy cattle. J Dairy Sci, 2003. 86(6): p. 2131-44. [CrossRef]
- Tresoldi, G., K.E. Schütz and C.B. Tucker, Cooling cows with sprinklers: Effects of soaker flow rate and timing on behavioral and physiological responses to heat load and production. J Dairy Sci, 2019. 102(1): p. 528-538.. [CrossRef]
- Igono, M.O., et al., Physiological, productive, and economic benefits of shade, spray, and fan system versus shade for Holstein cows during summer heat. J Dairy Sci, 1987. 70(5): p. 1069-79. [CrossRef]
- Gallardo, M.R., et al., Diet and cooling interactions on physiological responses of grazing dairy cows, milk production and composition. Int J Biometeorol, 2005. 50(2): p. 90-5.. [CrossRef]
- Honig, H., et al., Performance and welfare of high-yielding dairy cows subjected to 5 or 8 cooling sessions daily under hot and humid climate. J Dairy Sci, 2012. 95(7): p. 3736-42. [CrossRef]
- Chen, J.M., K.E. Schütz and C.B. Tucker, Cooling cows efficiently with water spray: Behavioral, physiological, and production responses to sprinklers at the feed bunk. J Dairy Sci, 2016. 99(6): p. 4607-4618. [CrossRef]
- Bergman, E.N., Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev, 1990. 70(2): p. 567-90. [CrossRef]
- Hess, M., et al., Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science, 2011. 331(6016): p. 463-7. [CrossRef]
- Morgavi, D.P., et al., Rumen microbial (meta)genomics and its application to ruminant production. Animal, 2013. 7 Suppl 1: p. 184-201.. [CrossRef]
- Weimer, P.J., Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front Microbiol, 2015. 6: p. 296. [CrossRef]
- Paz, H.A., et al., Rumen bacterial community structure impacts feed efficiency in beef cattle. J Anim Sci, 2018. 96(3): p. 1045-1058. [CrossRef]
- Xue, M.Y., et al., Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome, 2020. 8(1): p. 64. [CrossRef]
- Zhou, M., et al., Distinctive roles between rumen epimural and content bacterial communities on beef cattle feed efficiency: A combined analysis. Curr Res Microb Sci, 2021. 2: p. 100085. [CrossRef]
- Ragaller, V., et al., Effects of folic acid supplementation to rations differing in the concentrate to roughage ratio on ruminal fermentation, nutrient flow at the duodenum, and on serum and milk variables of dairy cows. Arch Anim Nutr, 2010. 64(6): p. 484-503. [CrossRef]
- Seck, M., et al., Apparent ruminal synthesis of B vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J Dairy Sci, 2017. 100(3): p. 1914-1922. [CrossRef]
- Averianova, L.A., et al., Production of Vitamin B2 (Riboflavin) by Microorganisms: An Overview. Front Bioeng Biotechnol, 2020. 8: p. 570828.. [CrossRef]
- Beckett, L., et al., Rumen volatile fatty acid molar proportions, rumen epithelial gene expression, and blood metabolite concentration responses to ruminally degradable starch and fiber supplies. J Dairy Sci, 2021. 104(8): p. 8857-8869. [CrossRef]
- Zhao, S., et al., Effect of Heat Stress on Bacterial Composition and Metabolism in the Rumen of Lactating Dairy Cows. Animals (Basel), 2019. 9(11).. [CrossRef]
- Kim, S.H., et al., Heat Stress: Effects on Rumen Microbes and Host Physiology, and Strategies to Alleviate the Negative Impacts on Lactating Dairy Cows. Front Microbiol, 2022. 13: p. 804562.. [CrossRef]
- Wang, Z., et al., Novel insights into heat tolerance using metabolomic and high-throughput sequencing analysis in dairy cows rumen fluid. Animal, 2022. 16(3): p. 100478. [CrossRef]
- Feng, L., et al., Altered rumen microbiome and correlations of the metabolome in heat-stressed dairy cows at different growth stages. Microbiol Spectr, 2023. 11(6): p. e0331223. [CrossRef]
- Zhong, S., et al., Temperature and humidity index (THI)-induced rumen bacterial community changes in goats. Appl Microbiol Biotechnol, 2019. 103(7): p. 3193-3203. [CrossRef]
- Correia, S.G., et al., Heat stress influence the microbiota and organic acids concentration in beef cattle rumen. J Therm Biol, 2021. 97: p. 102897. [CrossRef]
- Li, Z., et al., Live yeast supplementation altered the bacterial community's composition and function in rumen and hindgut and alleviated the detrimental effects of heat stress on dairy cows. J Anim Sci, 2023. 101.. [CrossRef]
- Tucker, C.B., A.R. Rogers and K. Schütz, Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Applied Animal Behaviour Science, 2008. 2-4(109): p. 141-154.
- Saini, G., et al., Intensity of estrus expression - valuable obvious determinant of fertility in Bos indicus cows. Anim Biotechnol, 2023. 34(8): p. 3867-3876. [CrossRef]
- Institute Of Animal Husbandry, C.A.O.A., Beef Cattle Feeding Standards. NY/T, 2004(815-2004, 30P, A4).
- Shen, J.S., et al., Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J Dairy Sci, 2012. 95(10): p. 5978-84. [CrossRef]
- Xue, F., et al., Effects of Partial Replacment of Dietary Forage Using Kelp Powder (Thallus laminariae) on Ruminal Fermentation and Lactation Performances of Dairy Cows. Animals (Basel), 2019. 9(10). [CrossRef]
- Wang, H., et al., Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Int J Mol Sci, 2022. 23(14). [CrossRef]
- Min, L., et al., Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J Zhejiang Univ Sci B, 2015. 16(6): p. 541-8. [CrossRef]
- Li, Y., et al., Effects of bamboo leaf extract on the production performance, rumen fermentation parameters, and rumen bacterial communities of heat-stressed dairy cows. Anim Biosci, 2021. 34(11): p. 1784-1793. [CrossRef]
- Cui, X., et al., Selenium Yeast Dietary Supplement Affects Rumen Bacterial Population Dynamics and Fermentation Parameters of Tibetan Sheep (Ovis aries) in Alpine Meadow. Front Microbiol, 2021. 12: p. 663945. [CrossRef]
- Dado-Senn, B., et al., RNA-Seq reveals novel genes and pathways involved in bovine mammary involution during the dry period and under environmental heat stress. Sci Rep, 2018. 8(1): p. 11096. [CrossRef]
- Zhao, C., et al., Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep, 2022. 41(8): p. 111681. [CrossRef]
- Martens, H., et al., Magnesium homeostasis in cattle: absorption and excretion. Nutr Res Rev, 2018. 31(1): p. 114-130. [CrossRef]
- Xue, Y., et al., Disruption of ruminal homeostasis by malnutrition involved in systemic ruminal microbiota-host interactions in a pregnant sheep model. Microbiome, 2020. 8(1): p. 138. [CrossRef]
- Uyeno, Y., et al., An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe, 2010. 16(1): p. 27-33. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).