Submitted:
18 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Surgical Technique
2.2. Perioperative Management
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Proofreading
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
References
- Keum, N.; Giovannucci, E. Global Burden Of Colorectal Cancer: Emerging Trends, Risk Factors And Prevention Strategies. Nat Rev Gastroenterol Hepatol 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Marcellinaro, R.; Spoletini, D.; Grieco, M.; Avella, P.; Cappuccio, M.; Troiano, R.; Lisi, G.; Garbarino, G.M.; Carlini, M. Colorectal Cancer: Current Updates And Future Perspectives. J Clin Med 2023, 13. [Google Scholar] [CrossRef]
- Rocca, A.; Avella, P.; Scacchi, A.; Brunese, M.C.; Cappuccio, M.; De Rosa, M.; Bartoli, A.; Guerra, G.; Calise, F.; Ceccarelli, G. Robotic Versus Open Resection For Colorectal Liver Metastases In A "Referral Centre Hub&Spoke Learning Program". A Multicenter Propensity Score Matching Analysis Of Perioperative Outcomes. Heliyon 2024, 10, E24800. [Google Scholar] [CrossRef]
- Van Leersum, N.J.; Snijders, H.S.; Henneman, D.; Kolfschoten, N.E.; Gooiker, G.A.; Ten Berge, M.G.; Eddes, E.H.; Wouters, M.W.; Tollenaar, R.A.; Bemelman, W.A.; et al. The Dutch Surgical Colorectal Audit. Eur J Surg Oncol 2013, 39, 1063–1070. [Google Scholar] [CrossRef]
- Hannan, E.; Feeney, G.; Ullah, M.F.; Ryan, C.; Mcnamara, E.; Waldron, D.; Condon, E.; Coffey, J.C.; Peirce, C. Robotic Versus Laparoscopic Right Hemicolectomy: A Case-Matched Study. J Robot Surg 2022, 16, 641–647. [Google Scholar] [CrossRef]
- Schwenk, W.; Haase, O.; Neudecker, J.; Müller, J.M. Short Term Benefits For Laparoscopic Colorectal Resection. Cochrane Database Syst Rev 2005, 2005, Cd003145. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines For Perioperative Care In Elective Colorectal Surgery: Enhanced Recovery After Surgery (Eras(®)) Society Recommendations: 2018. World J Surg 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.; Schoetz, D.; Marcello, P.; Read, T.; Hall, J.; Roberts, P.; Ricciardi, R. What Is The Risk Of Clinical Anastomotic Leak In The Diverted Colorectal Anastomosis? J Gastrointest Surg 2014, 18, 1812–1816. [Google Scholar] [CrossRef]
- Pronio, A.; Di Filippo, A.; Narilli, P.; Mancini, B.; Caporilli, D.; Piroli, S.; Vestri, A.; Montesani, C. [Anastomotic Dehiscence In Colorectal Surgery. Analysis Of 1290 Patients]. Chir Ital 2007, 59, 599–609. [Google Scholar] [PubMed]
- Khan, A.A.; Wheeler, J.M.; Cunningham, C.; George, B.; Kettlewell, M.; Mortensen, N.J. The Management And Outcome Of Anastomotic Leaks In Colorectal Surgery. Colorectal Dis 2008, 10, 587–592. [Google Scholar] [CrossRef]
- Marcellinaro, R.; Grieco, M.; Spoletini, D.; Troiano, R.; Avella, P.; Brachini, G.; Mingoli, A.; Carlini, M. How To Reduce The Colorectal Anastomotic Leakage? The Miracle Protocol Experience In A Cohort In A Single High-Volume Centre. Updates Surg 2023, 75, 1559–1567. [Google Scholar] [CrossRef]
- Leourier, P.; Pellegrin, A.; Regimbeau, J.M.; Sabbagh, C. Is Early Ct In Cases Of Elevated Postoperative Crp The Best Option For The Diagnosis Of Colorectal Anastomotic Leakage? Int J Colorectal Dis 2023, 38, 278. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.E.; Peterknecht, E.; Hajibandeh, S.; Torrance, A.W. C-Reactive Protein Can Predict Anastomotic Leak In Colorectal Surgery: A Systematic Review And Meta-Analysis. Int J Colorectal Dis 2021, 36, 1147–1162. [Google Scholar] [CrossRef]
- Tamini, N.; Bernasconi, D.; Ripamonti, L.; Lo Bianco, G.; Braga, M.; Nespoli, L. Clinical Validation Of The Comprehensive Complication Index In Colon Cancer Surgery. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Stephensen, B.D.; Reid, F.; Shaikh, S.; Carroll, R.; Smith, S.R.; Pockney, P. C-Reactive Protein Trajectory To Predict Colorectal Anastomotic Leak: Predict Study. Br J Surg 2020, 107, 1832–1837. [Google Scholar] [CrossRef]
- Giaccaglia, V.; Salvi, P.F.; Antonelli, M.S.; Nigri, G.; Pirozzi, F.; Casagranda, B.; Giacca, M.; Corcione, F.; De Manzini, N.; Balducci, G.; et al. Procalcitonin Reveals Early Dehiscence In Colorectal Surgery: The Predics Study. Ann Surg 2016, 263, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Perrella, A.; Giuliani, A.; De Palma, M.; Castriconi, M.; Molino, C.; Vennarecci, G.; Antropoli, C.; Esposito, C.; Calise, F.; Frangiosa, A. C-Reactive Protein But Not Procalcitonin May Predict Antibiotic Response And Outcome In Infections Following Major Abdominal Surgery. Updates Surg 2022, 74, 765–771. [Google Scholar] [CrossRef]
- Platt, J.J.; Ramanathan, M.L.; Crosbie, R.A.; Anderson, J.H.; Mckee, R.F.; Horgan, P.G.; Mcmillan, D.C. C-Reactive Protein As A Predictor Of Postoperative Infective Complications After Curative Resection In Patients With Colorectal Cancer. Ann Surg Oncol 2012, 19, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Müller, S.A.; Ulrich, A.; Kischlat, A.; Hinz, U.; Kienle, P.; Büchler, M.W.; Schmidt, J.; Schmied, B.M. C-Reactive Protein As Early Predictor For Infectious Postoperative Complications In Rectal Surgery. Int J Colorectal Dis 2007, 22, 1499–1507. [Google Scholar] [CrossRef]
- Hoek, V.T.; Sparreboom, C.L.; Wolthuis, A.M.; Menon, A.G.; Kleinrensink, G.J.; D'hoore, A.; Komen, N.; Lange, J.F. C-Reactive Protein (Crp) Trajectory As A Predictor Of Anastomotic Leakage After Rectal Cancer Resection: A Multicentre Cohort Study. Colorectal Dis 2022, 24, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Pockney, P.; Holmes, R.; Doig, F.; Attia, J.; Holliday, E.; Carroll, R.; Draganic, B. Biomarkers And Anastomotic Leakage In Colorectal Surgery: C-Reactive Protein Trajectory Is The Gold Standard. Anz J Surg 2018, 88, 440–444. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; Mackenzie, C.R. A New Method Of Classifying Prognostic Comorbidity In Longitudinal Studies: Development And Validation. J Chronic Dis 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Mazaki, J.; Kasahara, K.; Udo, R.; Tago, T.; Nagakawa, Y. Learning Curve Of Intracorporeal Anastomosis In Laparoscopic Colectomy For Right Side Colon Cancer: A Cumulative Sum Analysis. Anticancer Res 2023, 43, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification Of Surgical Complications: A New Proposal With Evaluation In A Cohort Of 6336 Patients And Results Of A Survey. Ann Surg 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- The Impact Of Enhanced Recovery Protocol Compliance On Elective Colorectal Cancer Resection: Results From An International Registry. Ann Surg 2015, 261, 1153–1159. [CrossRef] [PubMed]
- Ceresoli, M.; Pedrazzani, C.; Pellegrino, L.; Muratore, A.; Ficari, F.; Polastri, R.; Scatizzi, M.; Totis, M.; Tamini, N.; Ripamonti, L.; et al. Early Postoperative Low Compliance To Enhanced Recovery Pathway In Rectal Cancer Patients. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Capretti, G.; Beretta, L.; Gemma, M.; Pecorelli, N.; Braga, M. Enhanced Recovery Program In Colorectal Surgery: A Meta-Analysis Of Randomized Controlled Trials. World J Surg 2014, 38, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Irani, J.L.; Hedrick, T.L.; Miller, T.E.; Lee, L.; Steinhagen, E.; Shogan, B.D.; Goldberg, J.E.; Feingold, D.L.; Lightner, A.L.; Paquette, I.M. Clinical Practice Guidelines For Enhanced Recovery After Colon And Rectal Surgery From The American Society Of Colon And Rectal Surgeons And The Society Of American Gastrointestinal And Endoscopic Surgeons. Surg Endosc 2023, 37, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Ishizawa, T.; Cohen, R.; Chand, M. Indocyanine Green Fluorescence Imaging In Colorectal Surgery: Overview, Applications, And Future Directions. Lancet Gastroenterol Hepatol 2017, 2, 757–766. [Google Scholar] [CrossRef]
- Singh, P.P.; Zeng, I.S.; Srinivasa, S.; Lemanu, D.P.; Connolly, A.B.; Hill, A.G. Systematic Review And Meta-Analysis Of Use Of Serum C-Reactive Protein Levels To Predict Anastomotic Leak After Colorectal Surgery. Br J Surg 2014, 101, 339–346. [Google Scholar] [CrossRef]
- Milone, M.; Desiderio, A.; Velotti, N.; Manigrasso, M.; Vertaldi, S.; Bracale, U.; D'ambra, M.; Servillo, G.; De Simone, G.; De Palma, F.D.E.; et al. Surgical Stress And Metabolic Response After Totally Laparoscopic Right Colectomy. Sci Rep 2021, 11, 9652. [Google Scholar] [CrossRef] [PubMed]
- Van Oostendorp, S.; Elfrink, A.; Borstlap, W.; Schoonmade, L.; Sietses, C.; Meijerink, J.; Tuynman, J. Intracorporeal Versus Extracorporeal Anastomosis In Right Hemicolectomy: A Systematic Review And Meta-Analysis. Surg Endosc 2017, 31, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, Y.; Mao, W. Meta-Analysis Of Randomized Controlled Trials Comparing Intracorporeal Versus Extracorporeal Anastomosis In Minimally Invasive Right Hemicolectomy: Upgrading The Level Of Evidence. Int J Colorectal Dis 2023, 38, 147. [Google Scholar] [CrossRef] [PubMed]
| CHARACTERISTICS | Overall | EA | IA | p-value |
|---|---|---|---|---|
| n (%) | 340 (100) | 238 (70) | 102 (30) | |
| Median age, years (IQR) | 73 (64-80) | 73 (63-79) | 73 (65-81) | 0.348 |
| Sex, Male, n (%) | 173 (50.9) | 120 (50.4) | 53 (52) | 0.795 |
| Previous surgery, n (%) | 107 (31.5) | 63 (26.5) | 44 (43.1) | 0.333 |
| Diabetes mellitus, n (%) | 49 (14.4) | 35 (14.7) | 14 (13.7) | 0.031 |
| CKD, n (%) | 24 (7.1) | 16 (6.7) | 8 (7.8) | 0.232 |
| Arterial Hypertension, n (%) | 122 (35.9) | 59 (24.8) | 63 (61.8) | 0.600 |
| COPD, n (%) | 16 (4.7) | 8 (3.4) | 8 (7.8) | 0.702 |
| Charlson Comorbidity Index, median (IQR) | 5 (4-7) | 5 (4-7) | 6 (5-7) | 0.522 |
| OPERATIVE CHARACTERISTICS | TOTAL | EA (n=238) | IA (n=102) | p-value |
|---|---|---|---|---|
| Operative time, min, median (IQR) | 179 (141.5-215) | 172.5 (140-210) | 188 (150-228) | 0.059 |
| Manual sutures, n (%) | 198 (58.2) | 198 (83.2) | 0 (0) | <0.001 |
| Mechanical sutures, n (%) | 142 (41.8) | 40 (16.8) | 102 (100) | <0.001 |
| Abdominal drainage, n (%) | 168 (49.4) | 141 (59.2) | 27 (26.5) | <0.001 |
| Lymph nodes, median (IQR) | 20 (15-26) | 20 (15-26) | 19 (15-25) | 0.523 |
| POSTOPERATIVE OUTCOMES | Overall | EA (n=238) | IA (n=102) | p-value |
|---|---|---|---|---|
| Overall morbidity, n (%) | 94 (27.6) | 75 (31.5) | 36 (35.2) | 0.464 |
| Major complications, n (%) | 26 (7.6) | 20 (8.4) | 6 (5.9) | 0.475 |
| Clavien-Dindo grade | ||||
| IIIB, n (%) | 15 (4.4) | 13 (5.5) | 2 (2) | 0.167 |
| IVA, n (%) | 6 (1.8) | 5 (2.1) | 1 (1) | 0.496 |
| IVB, n (%) | 5 (1.5) | 2 (0.8) | 3 (2.9) | 0.127 |
| V, n (%) | 0 | 0 | 0 | |
| Gastrointestinal bleeding, n (%) | 8 (2.3) | 2 (0.8) | 6 (5.9) | 0.218 |
| Anastomotic leak, n (%) | 16 (4.6) | 12 (5) | 4 (3.9) | 0.718 |
| Superficial SSI, n (%) | 18 (5.3) | 12 (5) | 6 (5.9) | 0.677 |
| Deep SSI, n (%) | 12 (3.6) | 10 (4.2) | 2 (2) | 0.338 |
| Reoperation, n (%) | 25 (7.3) | 19 (8) | 6 (5.9) | 0.567 |
| LOS, days, median (IQR) | 7 (6-9) | 7 (6-9) | 7 (5-9) | 0.236 |
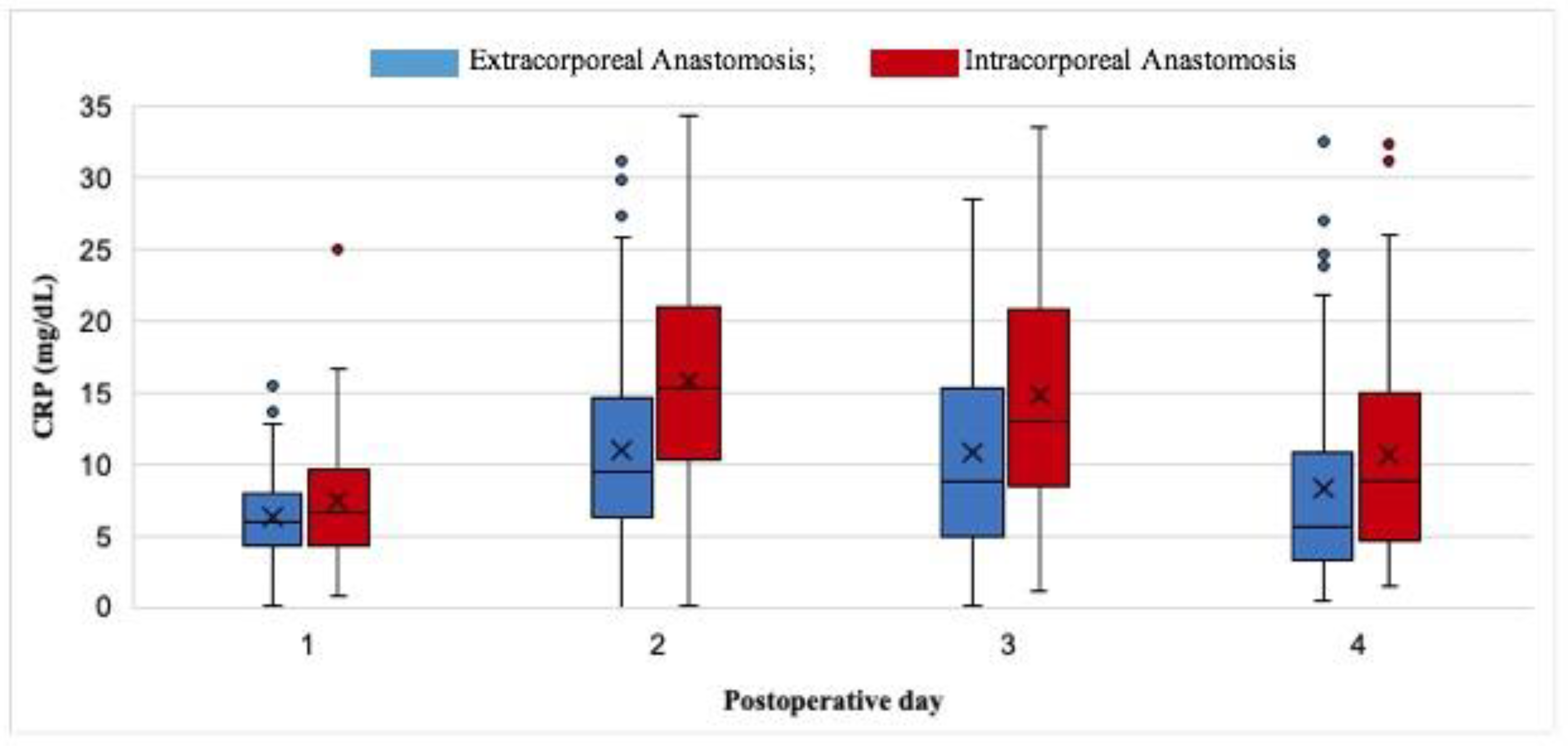
| CRP LEVELS, OVERALL | ||||
|---|---|---|---|---|
| POSTOPERATIVE DAY | Overall | EA (n=238) | IA (n=102) | p-value |
| I median (IQR) | 6.2 (4.3-8.4) | 6 (4.4-8) | 6.7 (4.3-9.5) | 0.122 |
| II median (IQR) | 11.4 (6.8-16.7) | 9.5 (6.3-14.6) | 15.2 (10.5-20.9) | <0.001 |
| III median (IQR) | 11 (6.5-17.7) | 8.7 (5-15) | 12.9 (8.5-20.7) | <0.001 |
| IV median (IQR) | 7 (4.1-13.1) | 5.6 (3.3-10.6) | 8.8 (4.6-15) | 0.006 |
| CRP LEVELS IN PATIENTS WITH MAJOR COMPLICATIONS (CD≥IIIa) | ||||
| I median (IQR) | 7.5 (6.5-11.1) | 7.9 (6.6-10.8) | 7.5 (4.8-11.9) | 0.968 |
| II median (IQR) | 16.6 (15.1-23.1) | 15.9 (14.8-21) | 22.5 (19-31.9) | 0.142 |
| III median (IQR) | 19.2 (13.2-27.9) | 18.3 (7.4-26.7) | 24.4 (18.9-31.9) | 0.153 |
| IV median (IQR) | 14 (9.6-24.7) | 14 (8.5-24.7) | 16.8 (11.9-31.1) | 0.733 |
| CRP LEVELS IN PATIENTS WITHOUT MAJOR COMPLICATIONS | ||||
| I median (IQR) | 6 (4.2-8.2) | 5.8 (4.2-7.7) | 6.6 (4.1-9.3) | 0.068 |
| II median (IQR) | 10.9 (6.5-16.4) | 8.7 (6.1-13.3) | 14.6(10.1-20.5) | <0.001 |
| III median (IQR) | 9.8 (6-16.6) | 8.6 (4.6-13) | 12.6 (8.1-20.4) | <0.001 |
| IV median (IQR) | 6.1 (4-12.3) | 5.3 (3.2-9.3) | 8.4 (4.6-14.3) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





