1. Introduction
Spinal cord injury (SCI) is often a devastating traumatic event, that leads to serious secondary health complications, including increased risks of diabetes and cardiovascular diseases[
1,
2]. Following SCI, significant muscle atrophy typically develops below the lesion site, primarily due to prolonged diminished contractile activity[
3]. This muscle atrophy is typically marked by a decrease in muscle mass and cross-sectional area, particularly affecting muscles innervated by the spinal cord segments below the injury level, resulting in pronounced weakness and diminished function[
4]. Additionally, these below injury level muscles display a higher proportion of fast glycolytic fibers, which, despite their capacity for quick, intense contractions, are implicated in reduced endurance and suboptimal muscle performance[
5]. However, when SCI affects all the lower motor neurons (LMNs), the atrophy of skeletal muscle groups becomes particularly severe[
6]. Therefore, there has been significant interest in the use of electrical stimulation to restore/maintain the structure and function of skeletal muscles in SCI individuals[
7,
8,
9]. Muscle atrophy caused by upper motor neuron lesions remains consistent from three to twenty years post-SCI [
10]. This situation contrasts starkly with SCIs that affect both upper and lower motor neurons (LMN), as such injuries result in actual denervation of the muscles. The condition is especially dire when a complete transverse SCI affects all LMNs of the involved muscles. These completely denervated muscles quickly become incapable of maintaining tension during tetanic contractions induced by electrical stimulation [
11]. Eventually, they lose excitability with conventional electrical stimulators, leading to severe, enduring atrophy as muscle fibers are replaced by adipocytes and collagen [
12,
13]. Current research literature does not adequately address the effects of neuromuscular electrical stimulation (NMES) on muscle health and metabolic functions in individuals with acute SCI who also have LMNLs. In this case report we report the efficacy of a novel Combined NMES (Comb-NMES) regimen on the distribution of muscle fiber types and the signaling pathways involved in muscle glucose uptake. The Comb-NMES regimen we have designed incorporates dynamic contractions through electrical stimulation at a high frequency (50 Hz trains with 450 µs biphasic pulses) for resistance training, with twitch contractions elicited by low-frequency electrical stimulation (5 Hz with a pulse duration/interval of 200/50 µs) for aerobic contraction, targeting the quadriceps muscle group. The protocol was designed to be manageable for individuals to perform on their own, whether seated in wheelchairs or lying in hospital beds. Therefore, the goal of this report is to document the effect of the Comb-NMES on muscle health and metabolic function in acute SCI individual with LMN lesions that exhibited markedly reduced muscle contractions with fibrillations in addition to a pronounced reduction in fasting insulin levels and glucose concentrations.
2. Case Presentation
A 32-year-old man had suffered a complete SCI (T9, AISA A) secondary to a gunshot wound. The patient stayed two weeks at the Traumatic Intensive Care Unit in UAB Hospital (Birmingham, Alabama). Then, he was transferred to the Spain Rehabilitation Center (SRC) to receive medical care after the traumatic injury. He remained in the intervention for 23 days with a total of 10 sessions given three times/week. He had no previous history of cardiovascular or metabolic disorders. The patient enrolled in the study after signing a consent form. The study protocol was approved by the University of Alabama in Birmingham’s (UAB) Institutional Review Board. This case report is part of a large study that investigated the effect of combined neuromuscular electrical stimulation on muscle health and metabolism after acute SCI. The study was registered at clinicaltrials.gov (NCT03204240).
In the course of the intervention with Comb-NMES, it was observed that the patient exhibited markedly reduced muscular contractions, which were palpable but not visually discernible in the lower extremities. Fibrillation, decreased reflexes, and tone were observed during the session. While standard diagnostic measures such as electromyography (EMG) to confirm LMN lesions were not employed, these clinical findings raise the possibility of an underlying LMN injury. It’s proposed that in the SCI population, 20-25% suffer LMN Lesions [
14]. This speculation is grounded in the understanding that LMN lesions can result in significant deficits in voluntary and reflexive muscle contractions, which aligns with the diminished responsiveness observed in the patient’s muscles to the electrical stimulation [
14]. However, it is important to note that this interpretation is put forward with caution and acknowledges the limitation of not having utilized standard tests that could offer a definitive diagnosis of LMN damage.
The patient received the Comb-NMES training protocol which is published elsewhere [
15]. Briefly, training was performed while the patient was seated in a wheelchair or bed and knee flexed between 70-90 degrees. The training was conducted three times weekly under professional supervision, using the TheraTouch 4.7 (Rich-Mar, Inola, OK, USA) stimulation device with self-adhesive 7.6 x 13 cm electrodes (Axelgaard ValuTrode, Fallbrook, CA, USA) for quadriceps stimulation. The Comb-NMES intervention combined resistance and aerobic exercises. Resistance exercises involved 4 sets of 10 quadriceps contractions using 50 Hz, 450 µs pulses, with intensity modulated from 0 to 200 mA to achieve full knee extension, and ankle weights adjusted as needed. Aerobic exercises started with 10 minutes of 2 Hz twitch stimulation and increased to 30 minutes at 6 Hz, with intensity set at 175 mA.
3. Clinical and Laboratory Procedures
Muscle samples were obtained from the vastus lateralis using a Bergstrom-type needle under local anesthesia (1% lidocaine). For immunohistochemistry, small muscle tissue samples (50-70 mg) were mounted cross-sectionally and quick-frozen in nitrogen-cooled isopentane, with the remaining tissues snap-frozen for subsequent biochemical analyses.
Frozen muscle samples were sectioned at 6 µm using a cryostat. Myofiber types I, IIa, and IIax/IIx were identified through immunohistochemistry. Sections were stained with primary antibodies (NCL-MHCs for MHC I, NCL-MHCf for MHC II, and anti-laminin) and secondary antibodies (ALEXA Fluor 594 and 488) following standard protocols. Hybrid IIax fibers were combined with type IIx fibers due to their prevalence (
Figure 1).
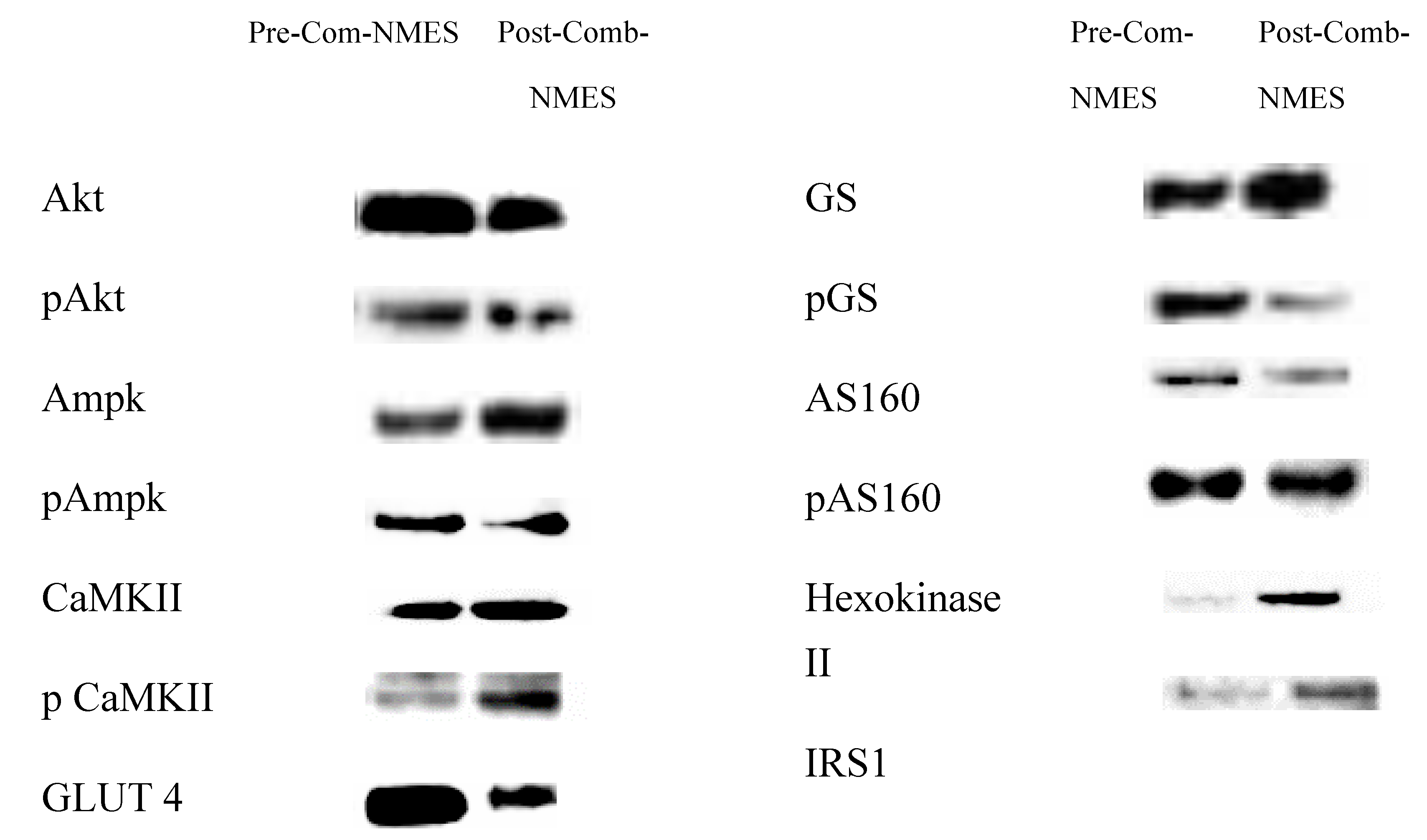
Muscle protein lysates were prepared, homogenized with protease and phosphatase inhibitors, and centrifuged. Protein content was determined using the bicinchoninic acid method. Proteins, including glucose transporter 4 (GLUT 4), total and phosphorylated AMP-activated protein kinase-α (AMPK-α), calcium/calmodulin-stimulated protein kinase II (CaMKII), Akt substrate of 160 kDa (AS160), Akt, Hexokinase II, Glycogen Synthase (GS), and insulin receptor substrate 1 (IRS-1), were separated via SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked, probed with specific antibodies, and analyzed using densitometry. Myofiber type distribution of VL was determined for the total number of 925 fibers (type I: 394, type IIa: 521, type IIx: 10) pre-training and for 873 fibers (type I: 406, type IIa: 443, type IIx: 24) post-training.
Blood Glucose, Insulin, and Lipid Measurements:
Serum glucose, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were measured using automated analyzers, with LDL calculated via the Friedewald method. Serum insulin levels were determined by immunofluorescence.[This methodological approach is adapted from previously published work (Alharbi et al., 2023)].
4. Statistical Analysis
Due to the case report nature of this study, conventional statistical methods were not applicable. Descriptive analysis was used to detail changes in the patient’s measurements pre- and post-Comb-NMES intervention, with percent change calculations and visual representations through protein blots and microscopic imaging.
5. Results:
5.1. Fasting Insulin and Glucose Levels
A significant reduction (74.7%) was observed in fasting insulin levels (pre: 49.50 µIU/mL, post: 12.50 µIU/mL ) and a moderate decrease (28.2%) in fasting glucose levels were observed (pre: 117.00 mg/dL, post: 84.00 mg/dL).
5.2. Fasting Lipid Profile
There were slight changes in the lipid profiles. For example, fasting triglyceride levels increased by 8% (pre: 125.0 mg/dL, post: 135.0 mg/dL). LDL and HDL levels declined by 1.6% and 8.9%, respectively (pre LDL: 191.0 mg/dL, post LDL:188.0 mg/dL; pre HDL: 45 mg/dL, post HDL: 41mg/dL). Total cholesterol levels showed a slight increase (2.8%; pre: 247 mg/dL, post: 254 mg/dL).
5.3. Skeletal Muscle Intracellular Signaling
AKT and pAKT_Ser473 levels decreased (55% and 17% respectively). In addition, AMPK and pAMPK_Thr172 slightly decreased (9% and 2.6%, respectively). CaMKII and pCaMKII _Thr286 levels significantly increased (76%, and 159%, respectively). GLUT 4 expression was reduced by 46.7%. Glycogen synthase increased by 42%, and phosphorylated GS_Ser641 markedly decreased by 72%. AS160 increased by 0.4% and pAS160_Ser318 decreased by 29%. Remarkably, Hexokinase II increased by 451% and IRS1 increased by 85% post-Comb-NMES.
5.4. Myofiber-Type Distribution
There was a slight increase in MHC I fibers (3%), and a drastic increase in MHC IIx fibers (140%). Conversely, MHC IIa fibers slightly decreased (15%).
Figure 2.
Immunoblots of studied proteins.
Figure 2.
Immunoblots of studied proteins.
6. Discussion
A pronounced reduction in fasting insulin levels (pre: 49.5 µIU/mL, post: 12.5 µIU/mL), and glucose concentrations (pre: 117.0 mg/dL, post: 84.0 mg/dL) indicates a meaningful improvement in metabolic control following Comb-NMES training. Insulin, a hormone responsible for controlling blood glucose levels, plays a crucial role in metabolic health. Lower fasting insulin levels may reflect increased insulin sensitivity, while reduced fasting glucose levels can denote better glucose utilization [
16]. Both measures are essential indicators of improved metabolic function. These findings suggest that Comb-NMES enhances glucose utilization, potentially through increased muscular uptake and utilization, possibly due to enhanced insulin sensitivity. The overall lack of change in the lipid profiles indicate that Comb-NMES was not effective in altering circulating lipid levels. The lack of significant changes may be attributed to the short duration of the intervention. Further research with longer intervention periods is necessary to better understand the potential effects of Comb-NMES on lipid metabolism.
The Comb-NMES intervention influenced several key proteins involved in muscle glucose uptake and metabolism. The observed substantial increases in CaMKII and p CaMKII _Thr286 levels (76% and 159%, respectively), highlight the activation of this pathway via Comb-NMES. CaMKs are critical regulators of muscle metabolism, responding to changes in intracellular calcium (Ca²⁺) levels. The increase in Ca²⁺ levels is a fundamental aspect of muscle contraction [
17]. CaMKs can regulate glucose uptake independently of AMPK signaling [
17]. The marked increase in CaMKII phosphorylation suggests that Comb-NMES effectively stimulates this signaling pathway in the denervated muscle. In addition, this enhanced response could be attributed to the altered muscle fiber composition in SCI, which tends to have a higher proportion of type II fibers that are more responsive to Ca²⁺ mediated signaling [
18,
19].
IRS-1’s robust response can be attributed to its pivotal role in mediating the effects of insulin on muscle cells. During and post-exercise, there is an increase in the phosphorylation of IRS-1, which enhances its activity, thereby amplifying downstream signaling that promotes glucose uptake and glycogen synthesis [
20]. Electrical muscle stimulation can mimic the effects of physical exercise, therefore, Comb-NMES could lead to increased insulin receptor activation and subsequent upregulation of IRS-1. In addition, both acute and chronic exercise can enhance the expression and activation of insulin signaling molecules to improve insulin sensitivity [
20]. As a result, the upregulating response of IRS-1 likely reflects an adaptive mechanism to optimize glucose uptake and utilization in response to increased metabolic demand in the denervated muscle.
Hexokinase II and GS both play critical roles in glucose metabolism. Hexokinase II is responsible for phosphorylating glucose to glucose-6-phosphate, a necessary step for its utilization and storage [
19]. Its 451% increase suggests a heightened capacity for glucose phosphorylation, possibly as a response to increased glucose uptake stimulated by Comb-NMES. Glycogen synthase, which showed a 42% increase, is crucial for glycogen synthesis. This aligns with exercise-induced adaptations where the muscle stores more glycogen to prepare for future energy demands. The increase in both enzymes suggests an enhanced capacity for glucose utilization and storage, supporting the muscle’s energetic needs during and after exercise [
21].
GLUT 4 is crucial for glucose uptake in skeletal muscle and is typically upregulated in response to exercise [
17]. However, in this study we did not observe an increase in GLUT 4 levels. This might be due to the specific type of electrical stimulation used (Comb-NMES) and the short nature of the training.
One of the most common changes in muscle after SCI is the transformation of muscle fibers from slow-twitch to fast-twitch fibers [
22]. Slow-twitch fibers, also known as type I fibers, are adapted for endurance and sustained activity, using aerobic metabolism to efficiently produce energy with a high fatigue resistance. In contrast, fast-twitch fibers, or type II fibers, are designed for quick bursts of speed and power, relying more on anaerobic metabolism [
23]. Our findings showed a substantial increase in type IIx fibers (140%) and a minimal increase in type I fibers (3%), while type IIa fibers slightly decreased (15%) (
Figure 1). Previous work demonstrated that NMES resistance training successfully shifted type IIx to type IIa in chronic SCI individuals [
9]. Therefore, we assume that the increase in type IIx and decrease in type IIa in our study may be related to the NMES resistance training duration being inadequate for the denervated muscle to enhance such a transition, as seen in other studies. Additionally, NMES aerobic training has been shown to increase the distribution of type I fibers in chronic SCI [
24]. In our current case, type I fibers did not change significantly; therefore, we speculate that the training intensity and/or duration was insufficient to increase type I fiber distribution in a denervated muscle.
7. Conclusions
The current case report demonstrates that Comb-NMES training improves fasting glucose and insulin levels in a patient with acute SCI and LMNLs, indicating enhanced metabolic control. These improvements are likely driven by the upregulation of key proteins involved in muscle glucose uptake signaling pathways. While the Comb-NMES intervention did not significantly alter lipid profiles or increase type I muscle fiber distribution, future research with extended intervention periods is warranted to fully elucidate the long-term effects of Comb-NMES.
Author Contributions
Conceptualization, C.Y.-F.; methodology, C.Y.-F., E.W., A.A.; formal analysis, C.Y.-F, A.A.; investigation, C.Y.-F., A.A.; resources, X.X.; data curation, X.X.; writing—original A.A.; writing—review and editing, C.Y.-F.; visualization, C.Y.-F., A.A.; supervision, C.Y.-F.; project administration, C.Y.-F.; funding acquisition, C.Y.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development, federal award identification number K01HD087463.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB) (IRB-170131007, 15 July 2017).
Informed Consent Statement
The participant gave written, informed consent after hearing a thorough explanation of study procedures and risks, and after having an opportunity to ask questions.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [CYF].
Acknowledgments
We sincerely thank the participant for his tireless dedication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Baligand, C.; Chen, Y.-W.; Ye, F.; Pandey, S.N.; Lai, S.-H.; Liu, M.; Vandenborne, K. Transcriptional Pathways Associated with Skeletal Muscle Changes after Spinal Cord Injury and Treadmill Locomotor Training. BioMed Research International 2015, 2015, 387090. [Google Scholar] [CrossRef]
- Budd, M.A., Jr.; D. R.G.; Channell, I. Psychosocial consequences of spinal cord injury: a narrative review. Journal of personalized medicine 2022, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Drasites, P.; Shams, R.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. Pathophysiology, biomarkers, and therapeutic modalities associated with skeletal muscle loss following spinal cord injury. Brain Sciences 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.W.; Cornwell, E.W.; Wu, C.L.; Kandarian, S.C. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Experimental physiology 2013, 98, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Ijaz, S.; Gholaminejhad, M.; Hassanzadeh, G. Clinical Complications Associated with Spinal Cord Injury: A Narrative Review. Journal of Contemporary Medical Sciences 2021, 7. [Google Scholar] [CrossRef]
- Kern, H.; Carraro, U.; Adami, N.; Biral, D.; Hofer, C.; Forstner, C.; Mödlin, M.; Vogelauer, M.; Pond, A.; Boncompagni, S.; et al. , Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair 2010, 24, 709–21. [Google Scholar] [CrossRef] [PubMed]
- Bochkezanian, V.; Newton, R.U.; Trajano, G.S.; Blazevich, A.J. Effects of neuromuscular electrical stimulation in people with spinal cord injury. 2018.
- Erickson, M.L.; Ryan, T.E.; Backus, D.; McCully, K.K. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle & nerve 2017, 55, 669–675. [Google Scholar]
- Yarar-Fisher, C.; Polston, K.F.; Eraslan, M.; Henley, K.Y.; Kinikli, G.I.; Bickel, C.S.; Windham, S.T.; McLain, A.B.; Oster, R.A.; Bamman, M.M. Paralytic and nonparalytic muscle adaptations to exercise training versus high-protein diet in individuals with long-standing spinal cord injury. Journal of applied physiology 2018, 125, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kern, H.; Hofer, C.; Mödlin, M.; Mayr, W.; Vindigni, V.; Zampieri, S.; Boncompagni, S.; Protasi, F.; Carraro, U. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3-to 20-year SCI. Spinal cord 2008, 46, 293–304. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Gage, P.W. Excitation-contraction coupling and charge movement in denervated rat extensor digitorum longus and soleus muscles. The Journal of Physiology 1985, 358, 75–89. [Google Scholar] [CrossRef]
- Kern, H.; Boncompagni, S.; Rossini, K.; Mayr, W.; Fanò, G.; Zanin, M.E.; Podhorska-Okolow, M.; Protasi, F.; Carraro, U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? Journal of Neuropathology & Experimental Neurology 2004, 63, 919–931. [Google Scholar]
- Biral, D.; Kern, H.; Adami, N.; Boncompagni, S.; Protasi, F.; Carraro, U. Atrophy-resistant fibers in permanent peripheral denervation of human skeletal muscle. Neurol Res 2008, 30, 137–44. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G.; Gorgey, A.S. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regeneration Research 2020, 15, 1397–1407. [Google Scholar] [PubMed]
- Alharbi, A.; Li, J.; Womack, E.; Farrow, M.; Yarar-Fisher, C. The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Signaling for Glucose Utilization, Myofiber Distribution, and Metabolic Function after Spinal Cord Injury. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Carnevale Schianca, G.P., A. Rossi, P.P. Sainaghi, E. Maduli, and E. Bartoli, The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes care 2003, 26, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Röckl, K.S.; Witczak, C.A.; Goodyear, L.J. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB life 2008, 60, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yarar-Fisher, C.; Bickel, C.S.; Windham, S.T.; McLain, A.B.; Bamman, M.M. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. Journal of Applied Physiology 2013, 115, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake — regulation and implications for glycaemic control. Nature Reviews Endocrinology 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A. Novel regulatory mechanisms in muscle metabolism during exercise. Experimental Physiology 2014, 99. [Google Scholar] [CrossRef]
- Long, Y.C.; Kostovski, E.; Boon, H.; Hjeltnes, N.; Krook, A.; Widegren, U. Differential expression of metabolic genes essential for glucose and lipid metabolism in skeletal muscle from spinal cord injured subjects. Journal of Applied Physiology 2011, 110, 1204–1210. [Google Scholar] [CrossRef]
- Dudley-Javoroski, S.; Shields, R.K. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. Journal of rehabilitation research and development 2008, 45, 283. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Erickson, M.L.; Young, H.-J.; McCully, K.K. Case report: endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Archives of physical medicine and rehabilitation 2013, 94, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).