1. Introduction
A Morel–Lavallée lesion (MLL) is a traumatic, internal closed degloving injury that results when subcutaneous tissue shears away from the underlying fascia, usually in association with high-velocity trauma. Common causes of MLL are high-energy, crush injury or blunt force trauma.[
1,
2,
3,
4] Closed degloving occurs most frequently over the hip, flank and proximal thigh, but can occur anywhere on the trunk and extremities.[
5] Injury to areas with rich vascular and lymphatic supplies leads to the accumulation of hemolymphatic fluid (serosanguineous fluid) in this newly formed cavity generated by separation of the superficial and deep fascia.[
2] Necrotic material and blood products produce chronic inflammatory reactions. Over time, an encapsulated lesion lined by fibrous capsule develops, filled with necrotic fatty tissue, blood products, debris, lymph, and fibrin, potentially leading to bacterial colonization and infection.[
4]
MLL typically presents within hours to days after the causative trauma. However, up to one-third of cases present months to years later and may be missed initially or difficult to associate with a specific inciting trauma event.[
5,
6]The clinical presentation of MLL varies widely from obvious ecchymosis, edema, and abrasions to the absence of any external signs. MLL can be confirmed by ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI).[
5] Early identification of MLL is essential because neglected lesions can become infected and may progress to extensive soft tissue necrosis.
The treatment for MLL is related to a variety of factors, such as lesion size, stage, and severity, but no guidelines for the management of MLL have yet been developed. Although many studies have reported the efficacy of various treatment regimens, such as conservative treatment, percutaneous aspiration, sclerotherapy, minimally invasive surgery, open surgery, or negative pressure wound therapy (NPWT), high-quality evidence remains lacking.[
5,
7,
8,
9,
10] To the best of our knowledge, the number of cases of MLL treated with portable NPWT is very small and no reviews have been reported.
Here, we describe the case of a middle-aged Japanese man with acute MLL successfully treated using portable NPWT. The aim of this study was to describe and evaluate the effectiveness and utility of using NPWT to treat MLL.
2. Case Presentation
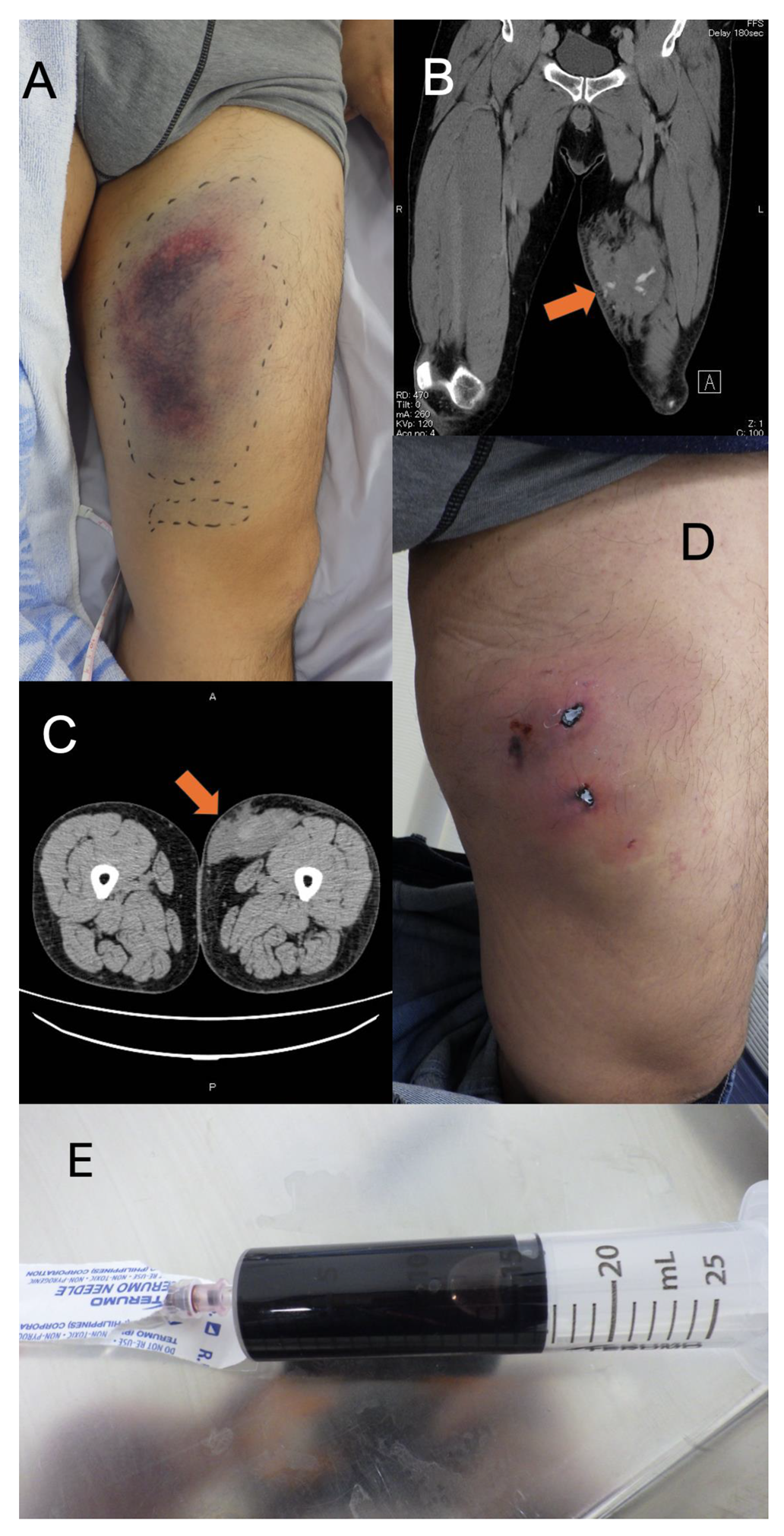
A 52-year-old Japanese man was transported to our hospital for further workup of a left thigh injury. He had fallen from a motorcycle after colliding with a car at a speed of around 30 km/h. He was initially transferred to another hospital, where contrast-enhanced computed tomography (CT) showed left thigh hematoma with extravasation from the superficial femoral artery. The patient was referred to our hospital for interventional radiology (IVR). The patient had a medical history of adjustment disorder with anxiety and had been prescribed sertraline hydrochloride and hydroxyzine hydrochloride once daily and clotiazepam as needed when he felt anxious. His habitual activities and family history were unremarkable. He was a salesman living with his parents. On arrival in the emergency room, vital signs were: temperature, 36.6°C; heart rate, 66 beats/min with regular rhythm; respiratory rate, 20 breaths/min; blood pressure, 118/94 mmHg; and oxygen saturation, 97% on room air. Glasgow Coma Scale score on arrival was 15 (E4V5M6). On examination, the patient was alert and complained of left thigh pain. The trachea was central, with neither crackles nor decreased breath sounds heard on auscultation. The abdomen was not distended. Examination of cranial nerves showed no abnormalities. Bruising was seen around the left thigh (
Figure 1A). Examination of the other limbs showed no abnormalities. Contrast-enhanced CT of the left thigh revealed hematoma with extravasation of a branch of the left superficial femoral artery, but no apparent aneurysms or fractures of the femur or pelvis (
Figure 1B). IVR was therefore not indicated and observational management with compression bandages was applied. The patient was admitted to our hospital for pain management and discharged on hospital day 6. He left hospital able to walk unaided. The patient subsequently sought medical aid and visited our emergency department (ED) with left chest pain 2 days after discharge. He was advised to use acetaminophen for pain. Five days after discharge, he again visited our ED with the same left chest pain along with swelling in the left thigh and was again advised to use acetaminophen for pain. He revisited our ED 10 days after discharge complaining of pain, increased swelling, and loss of local sensation in the left thigh. CT of the thigh showed subcutaneous hematoma in the left thigh with no evidence of fracture (
Figure 1C). Gross inspection 10 days after discharge revealed two necrotic plaques of roughly the same size with induration of the skin in surrounding areas (
Figure 1D). Percutaneous aspiration was performed and 15 mL of bloody serosanguineous fluid was drained by needle aspiration (
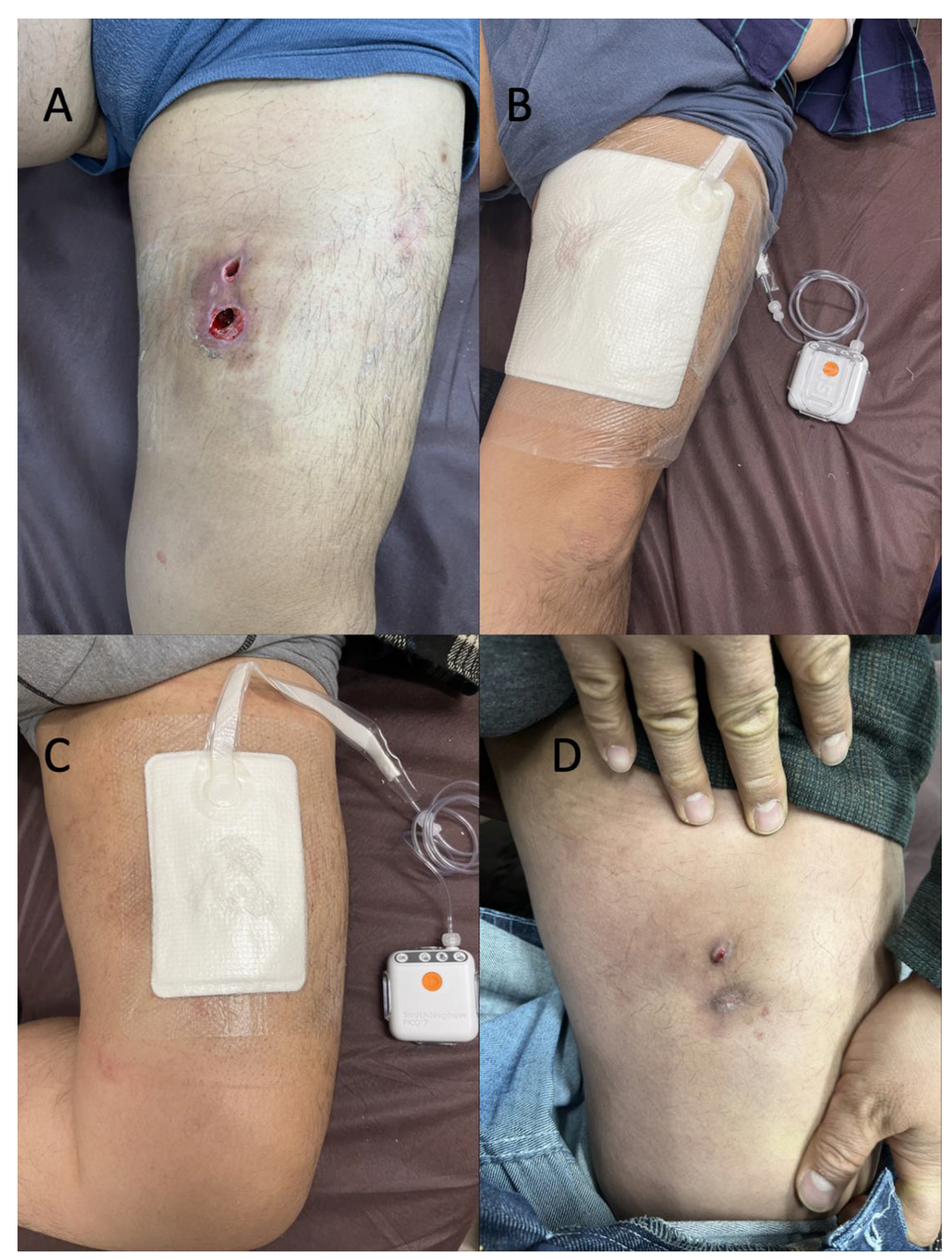
Figure 1E) along with a total of 100 mL of clots after rupture of the two necrotic plaques. Compression dressing was applied to the involved area using gauze and elastic bandages. A follow-up visit to another outpatient clinic was made 12 days after discharge, where wound care was provided. No necrotic tissue was observed, because follow-up treatment was provided relatively soon after the injury (
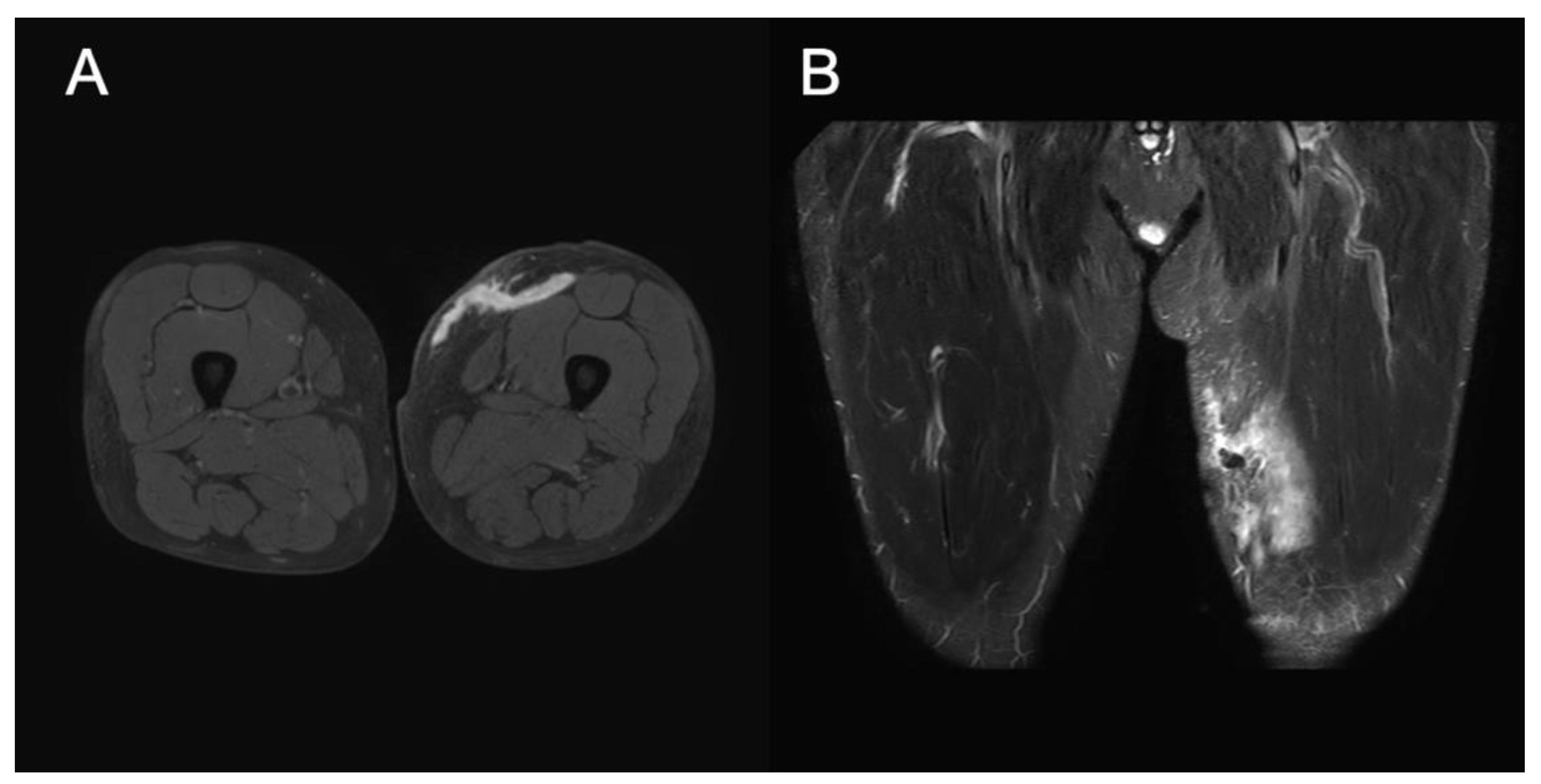
Figure 2A). MRI of the left thigh was performed 14 days after discharge. T2-weighted MRI revealed cystic masses under the subcutaneous fat layer without a peripheral ring or capsule, and MLL was confirmed (
Figure 3A,B). Considering that the patient was ambulant, we changed the treatment plan and applied a single-use disposable portable NPWT system (PICO system; Smith & Nephew, London, UK) after several weeks of compression treatment (
Figure 2B,C). NPWT dressings were changed with simultaneous serial debridement at 3- to 4-day intervals for 57 days. MLL completely resolved, but the patient felt discomfort and skin indentation over the site of MLL (
Figure 2D).
3. Discussion
We encountered a case of MLL that was diagnosed relatively early and successfully treated with portable NPWT. MLL results from a traumatic, internal closed degloving injury.[
1,
2,
3,
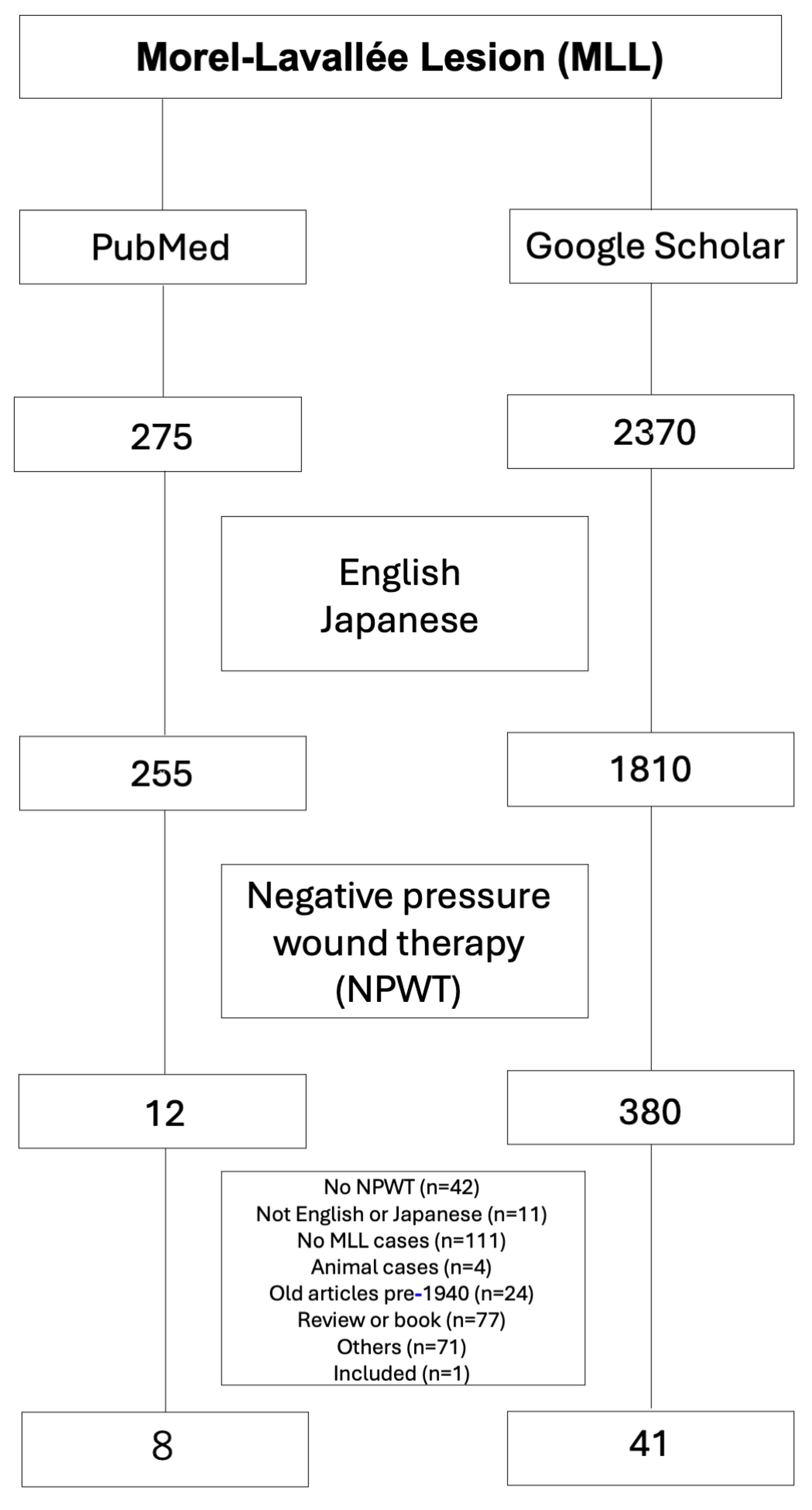
4] These lesions are easily missed initially and can also be difficult to associate with a specific inciting trauma event. Treatment with portable NPWT was non-invasive and effective in this case. Early identification of MLL and subsequent treatment with NPWT might be associated with shortening the period of treatment required for MLL. Early diagnosis of MLL is challenging because the lesion develops in subcutaneous tissues and does not cause early signs. MLL is often underestimated and undertreated. Prompt identification is key to successfully treating MLL. We were actually unable to correctly diagnose the patient with MLL during admission. Only 12 published articles were identified in a PubMed search, but 380 published articles were identified from a Google Scholar search of the English and Japanese literatures using the search terms “Morel–Lavallée lesion” and “negative pressure wound therapy”. Two reviewers manually screened the remaining titles and abstracts with full texts for relevance. Individual case reports were included unless they did not discuss NPWT. As a result, 12 full-text articles from the PubMed search and 380 full-text articles from the Google Scholar search were initially downloaded. Review of the reference lists from those articles provided one additional article for inclusion. Finally, 41 articles were included in the study (
Figure 3, Table 1, Supplemental Table 1). Table 1 shows these 41 studies and the 96 patients with MLL treated using NPWT (60 males, 36 females).
Etiology
MLL is a traumatic, internal closed degloving injury that results when subcutaneous tissue shears away from the underlying fascia, usually in association with high-velocity trauma.[
1,
2,
3,
4] The most common causes of MLL are high-velocity trauma, crush injuries, and blunt force trauma.[
2] Table 1 shows that about 50 of the 96 cases of MLL resulted from motor vehicle accidents, with falls as the second most common cause of MLL (10 of 96 cases). The shear force that separates underlying fascia from the subcutaneous tissue results in injury to trans-aponeurotic capillaries and lymphatics, leading to hemolymph accumulating within the newly created space.
Pathophysiology
In patients with no symptoms other than swelling, the diagnosis of MLL may be confused with hematoma, soft tissue edema, or bursitis, delaying diagnosis. Blood within the cavity is reabsorbed and the hemosiderin-rich hemolymphatic fluid (serosanguineous fluid) remains surrounded by a hemosiderin layer. This layer induces inflammation in peripheral tissues and development of a fibrous capsule around the hemolymphatic fluid, preventing reabsorption. Cavity infection and skin necrosis have been observed in untreated chronic MLL.
Signs and symptoms
MLL typically presents within hours to days after trauma, but up to one-third of cases present months to years later and may be missed initially or may prove difficult to associate with a specific inciting trauma event.[
5,
6] Clinical findings resemble those of regional contusion. Patients may experience decreased cutaneous sensation because of shearing injury to the cutaneous afferent nerves and increased mobility of the overlying skin.[
6] Secondary changes to overlying skin can include ecchymosis, edema, cracking, drying, abrasions, frank necrosis, and even the absence of any external signs.[
6,
11] Some patients present with recurrence of the soft tissue lesion, particularly after minimally invasive treatments.[
12] Although MLL is admittedly rare, persistent subcutaneous fluid collection in the setting of trauma should raise the clinical suspicion of underlying MLL.
Evaluation
Imaging is an important adjunct used for diagnosis when MLL is suspected. Features of MLL on ultrasound (US) are non-specific. MLLs are usually compressible without flow on color Doppler US, displaying a focal heterogeneous appearance with irregular margins and a lobular shape in the acute or subacute stage.[
4,
11,
13] Chronic MLL is better defined, homogeneous and smoothly marginated on US, located between the subdermal fat and fascia.[
4,
11,
13] CT of MLL has limited value and often demonstrates fluid-fluid levels reflecting the internal content of immiscible lymphatic fluid and hemorrhage. The density of MLL on CT is usually lower than that of simple hematoma because of the mixing of low-density lymphatic fluid. In chronic lesions, a peripheral capsule may be present.[
4,
11,
13] MRI is the preferred imaging modality for the evaluation of MLL. Signal characteristics depend on the internal contents of the lesion. Acute MLL appears hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging. Subacute lesions may appear hyperintense on T1-weighted imaging due to the methemoglobin content. Chronic lesions appear homogeneously hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging. In chronic lesions, a peripheral ring or capsule that appears hypointense on T1- and T2-weighted imaging may be present, representing hemosiderin and fibrous tissue.[
11]
Management
No specific guidelines are available in the current literature regarding the management of MLL. Several small cohort studies have shown variable results from multiple treatment modalities, including conservative treatment, percutaneous aspiration, sclerotherapy, minimally invasive surgery, open surgery, and NPWT.
Conservative Treatment
Compression bandaging with analgesics has been advocated for small, acute lesions where no capsule is present, but effective compression is difficult in the trochanteric and pelvic regions where MLL is common. In cases of large or chronic MLL, conservative management is unsuitable and surgical intervention is required.
Percutaneous Aspiration
Some studies have shown effective results after percutaneous aspiration of MLL, but the recurrence rate was high, particularly for lesions with a volume >50 ml, and even more so for cases requiring multiple aspirations.[
5]
Sclerotherapy
This treatment modality has been successfully used in MLL, particularly in cases where percutaneous aspiration fails. Potential sclerosants include doxycycline, erythromycin, vancomycin, tetracycline, bleomycin, absolute ethanol, and talc. Fibrin glue has also been used with satisfactory results.[
14] The overall efficacy of sclerotherapy in managing MLL has been reported as 95.7%.[
12]
Minimally Invasive Surgery
This approach involves removal of the fibrotic pseudocapsule with obliteration of any dead space. A minimally invasive method through endoscopy is used to debride the fibrotic pseudocapsule. Various methods can be applied for obliteration of dead space, including doxycycline, fibrin glue, loop drainage, and percutaneous cutaneo-fascial suture technique.[
15,
16,
17,
18] NPWT was also applied in some cases with minimally invasive surgery, as described later.
Open Surgery
Most cases of MLL require open debridement with complete removal of necrotic tissue in acute cases showing overlying skin necrosis, and with excision of the pseudocapsule in chronic cases. The end goal in managing MLL is obliteration of the dead space within the lesion. Previous studies have found that the overall recurrence rate was lower in the surgical group than in the percutaneous aspiration group.[
2,
5] In cases where the skin overlying the lesion is necrotic with massive soft tissue loss, dead tissue needs to be debrided, followed by reconstruction with skin grafts.[
4] If open surgery failed, the last treatment would be en bloc resection of the lesion with surrounding intact tissues.[
12]
NPWT
MLL treated with open surgery management can recur in up to 15% of patients.[
5] In the setting of chronic or recurrent MLL, NPWT can offer several benefits regarding dead space management. NPWT applies contracting force on the wound to minimize dead space and create a stable wound environment by draining excessive fluid, in turn supporting the formulation of granulation tissue.[
19] The reduction of tissue edema within the wound can enhance cell proliferation and tissue perfusion.[
19]
Recent studies on 96 MLL patients (60 males, 36 females; average age, 40.0 years) described NPWT (Table 1, Supplemental Table 1). In these studies, after percutaneous aspiration or incisional debridement combined with vacuum-assisted closure, NPWT was applied. Favorable outcomes were reported for the majority of patients, besides three failure cases and 4 deaths. No evidence of lesion recurrence or infection was seen, suggesting the effectiveness of this treatment. The three cases of NPWT failure showed healing of the MLL after secondary intention.[
20,
21,
22] We also found several cases in which healing was achieved with additional debridement after NPWT.[
20,
21,
23,
24,
25,
26,
27]
The single-use disposable portable NPWT was used in one case for acute sacral MLL.[
9] The single-use ultraportable NPWT device delivers negative pressure at -80 mmHg in a continuous pattern and is a small, canister-free device that is easy to operate and less uncomfortable in daily life.[
28]
Limitations to this study need to be considered. The effectiveness of treatment using NPWT alone remains unclear. Percutaneous aspiration alone without the use of portable NPWT might also have induced different results (e.g., infection or lesion recurrence). However, we performed portable NPWT concurrently with aspiration drainage treatment to ensure healing and prevent recurrence.
Nonsurgical treatments such as aspiration drainage are often prioritized in the treatment of MLL. Aspiration of more than 50 mL of fluid from the lesion warrants surgical intervention.[
5]
While the results for the present case were promising, future research needs to expand the number of patients to validate the present findings for NPWT. Future studies would preferably involve a controlled study design, but this may be complicated by the current absence of a “gold standard” for MLL treatment.
4. Conclusions
MLL is a traumatic, internal closed degloving injury that results from subcutaneous tissue shearing away from the underlying fascia. We encountered a patient presenting with MLL who was successfully treated by single-use portable NPWT without invasive treatment. MLL can be treated with portable NPWT when the space is relatively small and the patient is compliant with treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplemental table 1 The supplemental table 1 includes all data regarding MLL with NPWT. Supplemental table 2 The supplemental table 2 is summary of table 1, which shows duration of NPWT.
Author Contributions
KoO, KS, and KaO treated the patient and made the clinical diagnosis. KoO, KS, and KaO interpreted the MRI data. KoO, KaO, KS and AT wrote and revised the manuscript. All authors have read and approved the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to case report..
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, R.; Rymer, B.; Youssef, B.; Lim, J. The Morel-Lavallée lesion and its management: A review of the literature. J Orthop. 2018, 15, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, T. The Morel-Lavallée Lesion: Review and Update on Diagnosis and Management. Orthop Surg. 2023, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, H.Y. Morel–Lavallée Lesion. New England Journal of Medicine. 2021, 385, 2179–2179. [Google Scholar] [CrossRef]
- Molina, B.J.; Ghazoul, E.N.; Janis, J.E. Practical Review of the Comprehensive Management of Morel-Lavallée Lesions. Plast Reconstr Surg Glob Open. 2021, 9, E3850. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, T.P.; Zielinski, M.D.; Jenkins, D.H.; Schiller, H.J. The Mayo clinic experience with morel-lavallée lesions: Establishment of a practice management guideline. Journal of Trauma and Acute Care Surgery. 2014, 76, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Yoon, I.; Masih, S.; Patel, D.B.; White, E.A.; Levine, B.D.; Chow, K.; Gottsegen, C.J.; Matcuk, G.R. The Morel-Lavallée lesion: Pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2014, 21, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kage, T.; Hirota, J.; Yamamoto, N.; Kawasaki, Y.; Asai, S.; Zhang, L.; Ugawa, S.; Seichi, A. Arthroscopic treatment for Morel-Lavallée lesion of the thigh: A case report and literature review. Int J Surg Case Rep. 2021, 78, 58–61. [Google Scholar] [CrossRef]
- Tatman, L.M.; Gajari, V.; Obremskey, W.T. Application of Negative Pressure Wound Therapy. J Orthop Trauma. 2021, 35, S32–S33. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Shin, D.S.; Cheon, J.S.; Son, K.M.; Choi, W.Y. Effective Single-Use Portable Negative Pressure Therapy Used in Acute Morel-Lavallee Lesions: A Case Report. Journal of Wound Management and Research. 2022, 18, 58–61. [Google Scholar] [CrossRef]
- Ning, T.; Zha, Z.G. Treatment of Morel-Lavallée Lesions (MLLs) with Mesh Incisions Combined with Quilting Sutures and Negative Pressure Wound Therapy (NPWT). Altern Ther Health Med. 2023, 29, 810–815. [Google Scholar]
- Diviti, S.; Gupta, N.; Hooda, K.; Sharma, K.; Lo, L. Morel-lavallee lesions-review of pathophysiology, clinical findings, imaging findings and management. Journal of Clinical and Diagnostic Research. 2017, 11, TE01–TE04. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Tiwari, V. Morel Lavallee Lesion.; 2023.
- Hussein, K.; White, B.; Sampson, M.; Gupta, S. Pictorial review of Morel-Lavallée lesions. J Med Imaging Radiat Oncol. 2019, 63, 212–215. [Google Scholar] [CrossRef]
- Pan, C.-H.; Tu, C.-P.; Ou, S.-Y.; Tung, K.-Y.; Huang, W.-C.; Yu, C.-M.; Tsai, M.-F.; Yao, W.-T.; Chen, Y.-F. Percutaneous Debridement of and Fibrin Glue Injection into a Pretibial Morel-Lavallée Lesion: A Case Report and Literature Review. Ann Plast Surg. 2021, 86, S123–S126. [Google Scholar] [CrossRef]
- Kim, S. Endoscopic treatment of Morel-Lavallee lesion. Injury. 2016, 47, 1064–1066. [Google Scholar] [CrossRef]
- Koc, B.B.; Somorjai, N.; Kiesouw, E.P.; Vanderdood, K.; Meesters-Caberg, M.; Draijer, F.W.; Jansen, E.J. Endoscopic debridement and fibrin glue injection of a chronic Morel-Lavallée lesion of the knee in a professional soccer player: A case report and literature review. Knee. 2017, 24, 144–148. [Google Scholar] [CrossRef]
- Li, P.; Ning, X.; Jia, L.; Du, G.; Jiang, S.; Gong, Z.; Song, K.; Wang, Z.; Zhang, K. A minimally invasive incision and loop drainage technique for the treatment of lower limb Morel-Lavallée lesions: Nose ring drainage technique. Injury. 2020, 51, 570–573. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Zhou, X.; Wu, L.; Wang, J.; Qi, L.; Cai, C. A Novel Surgical Technique for treatment of Morel-Lavallée Lesion: Endoscopic debridement combined with percutaneous cutaneo-fascial suture. Injury. 2018, 49, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Hefner, D.W.; Dye, M.; Lantz, R. A Complex Surgical Case of a Morel-Lavallee Lesion. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.P.; Kondewar, P.; Gadod, L.; Kamble, M.; Gund, A. Morel-Lavallee Lesion Associated with Subtrochanteric Femur Fracture in a Young Female Resulting in Extensive Soft-Tissue Necrosis and Hypertrophic Non-union Managed with Staged Surgeries and Exchange Nailing: A Rare Injury Pattern and Review of Literatur. J Orthop Case Rep. 2022, 12, 47–50. [Google Scholar] [CrossRef]
- Miura, K. Outpatient Treatment of Refractory Morel-Lavallée Lesion Infection With Retention Sutures: A Case Report. Cureus. 2023, 15, 1–9. [Google Scholar] [CrossRef]
- Phillips, T.J.; Jeffcote, B.; Collopy, D. Bilateral morel-lavallée lesions after complex pelvic trauma: A case report. Journal of Trauma - Injury, Infection and Critical Care. 2008, 65, 708–711. [Google Scholar] [CrossRef]
- Weiss, N.A.; Johnson, J.J.; Anderson, S.B. Morel-lavallee lesion initially diagnosed as quadriceps contusion: Ultrasound, MRI, and importance of early intervention. Western Journal of Emergency Medicine. 2015, 16, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Dodwad, S.N.M.; Niedermeier, S.R.; Yu, E.; Ferguson, T.A.; Klineberg, E.O.; Khan, S.N. The Morel-Lavallée lesion revisited: Management in spinopelvic dissociation. Spine Journal. 2015, 15, e45–e51. [Google Scholar] [CrossRef]
- Eldenburg, E.; Pfaffenberger, M.; Gabriel, A. Closure of a Complex Lower Extremity Wound With the Use of Multiple Negative Pressure Therapy Modalities. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Nica, O.; Grecu, A.; Dincă, E.A.; Marinescu, D.; Ciurea, M.E. A Rare Case of Upper Calf Swelling and Necrosis - The Morel-Lavallée Lesion. Curr Health Sci J. 2018, 44, 311–315. [Google Scholar] [CrossRef]
- Hudson, D.A.; Adams, K.G.; Van Huyssteen, A.; Martin, R.; Huddleston, E.M. Simplified negative pressure wound therapy: Clinical evaluation of an ultraportable, no-canister system. Int Wound J. 2015, 12, 195–201. [Google Scholar] [CrossRef]
- Archer, A.D.; Horsley, N.B.; Lawson, C.M.; Burns, J.B. Lower Extremity Sandblast Injury: A Rarely Seen Injury Mechanism in the Civilian Population. Am Surg. 2023, 89, 3316–3318. [Google Scholar] [CrossRef]
- Blome-Eberwein, S.A. Morel-Lavallée Lesion with Friction Burn: Management Using Veraflo Vac Dressing, Preserving Body Contour. Plast Reconstr Surg Glob Open. 2020, 8, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Lu, K.J.G.; Chang, K.; Levin, J.; Schulz, J.T.; Goverman, J. A rare case of severe third degree friction burns and large Morel-Lavallee lesion of the abdominal wall. Burns Trauma. 2018, 6, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.J.; Olla, D.R.; Feimster, J.W.; Woldanski, L.M.; Hart, K.D.; Reid, A.; Schwartz, B.; Berry, N.N. Transretroperitoneal Pedicled Omental Flap for Coverage of Traumatic Sacral Defect: A Case Report. Plast Reconstr Surg Glob Open. 2022, 10, E4298. [Google Scholar] [CrossRef]
- Choi, E.S.; Yang, J.Y.; Ahn, B.H. Limited Incisional Drainage and Negative-Pressure Wound Therapy in an Acute Morel-Lavallée Lesion. Journal of Trauma and Injury. 2021, 34, 75–78. [Google Scholar] [CrossRef]
- Cormican, M.T.; Creel, N.J.; Bosque, B.A.; Dowling, S.G.; Rideout, P.P.; Vassy, W.M. Ovine Forestomach Matrix in the Surgical Management of Complex Volumetric Soft Tissue Defects: A Retrospective Pilot Case Series. Eplasty. 2023, 23, e66. [Google Scholar]
- Sönmez Ergün, S.; Erözgen, F.; Akdemir, O.C.; Ziyade, S.; İçten, S.; Egeli, Ü. Missed closed degloving injury of the sacro-gluteal region. Eur J Plast Surg. 2010, 33, 41–44. [Google Scholar] [CrossRef]
- Evin, N. Vacuum-assisted surgical treatment of large and complex Morel-Lavallée lesions in lower extremities. Experimental Biomedical Research. 2023, 6, 349–358. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, A.B.; Samuel, V.; Chase, S.; Nayak, S. Oxytetracycline as a sclerosant in the management of Morel-Lavallee lesions. Trauma (United Kingdom). 2016, 18, 146–149. [Google Scholar] [CrossRef]
- Gunay, M.; Mollavelıoglu, B.; Gok, A.F.K.; Ilhan, M.; Ertekın, C. A Rare Cause of Septic Shock Secondary to Trauma: Morel-Lavallée Lesion—Case Report. SN Compr Clin Med. 2022, 4, 2–5. [Google Scholar] [CrossRef]
- Haydon, N.; Zoumaras, J. Surgical management of morel-lavallee lesion. Eplasty. 2015, 15, ic14 http://wwwncbinlmnihgov/pubmed/25834694. [Google Scholar]
- Heo, Y.; Kim, D.H. Traumatic abdominal wall hernia with Morel-Lavallée lesion: a case report. Trauma Image and Procedure. 2020, 5, 17–20. [Google Scholar] [CrossRef]
- Howell, M.; Loera, S.; Tickner, A.; Maydick-Youngberg, D.; Faust, E.; Martin, S.; Teleten, O.; Bryant, R.; Sandman, D.; Greenstein, E.; et al. Practice dilemmas: Conditions that mimic pressure ulcers/injuries- to be or not to be? Wound Manag Prev. 2021, 67, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Ma, L.; Huang, F.; Cai, Q. The treatment of a Morel-Lavallée lesion of the thigh with incision and drainage along with tissue debridement and a surgically placed drain: A case report and literature review. Front Surg. [CrossRef]
- Kage, T.; Hirota, J.; Yamamoto, N.; Kawasaki, Y.; Asai, S.; Zhang, L.; Ugawa, S.; Seichi, A. Arthroscopic treatment for Morel-Lavallée lesion of the thigh: A case report and literature review. Int J Surg Case Rep. 2021, 78, 58–61. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.; Jang, M.J.; Choi, K.K. Rare complication of skin necrosis after endoscopic debridement and cutaneo-fascial suture for a massive Morel-Lavallée lesion in Korea: a case report. Journal of Trauma and Injury. 2023, 36, 304–309. [Google Scholar] [CrossRef]
- Labler, L.; Trentz, O. The use of vacuum assisted closure (VACTM) in soft tissue injuries after high energy pelvic trauma. Langenbecks Arch Surg. 2007, 392, 601–609. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.H.; Kim, J.Y.; Han, H.H. Can a Morel-Lavallée lesion be misdiagnosed as a mass like lesion? Int Wound J. 2017, 14, 1258–1261. [Google Scholar] [CrossRef]
- Marangi, G.F.; Segreto, F.; Morelli Coppola, M.; Arcari, L.; Gratteri, M.; Persichetti, P. Management of chronic seromas: A novel surgical approach with the use of vacuum assisted closure therapy. Int Wound J. 2020, 17, 1153–1158. [Google Scholar] [CrossRef]
- Mettu, R.; Surath, H.V.; Chayam, H.R.; Surath, A. Chronic Morel-Lavallée Lesion: A Novel Minimally Invasive Method of Treatment. Wounds. 2016, 28, 404–407. [Google Scholar]
- Mooney, M.; Gillette, M.; Kostiuk, D.; Hanna, M.; Ebraheim, N. Surgical Treatment of a Chronic Morel-Lavallée Lesion: A Case Report. J Orthop Case Rep. 2020, 9, 15–18. [Google Scholar]
- Mulcahy, M.J.; Ball, J.R. The Morel-Lavallée lesion in thoracolumbar spine trauma—two index cases. Journal of Spine Surgery. 2018, 4, 654–657. [Google Scholar] [CrossRef]
- Nakajima, T.; Tada, K.; Nakada, M.; Matsuta, M.; Tsuchiya, H. Two Cases of Morel-Lavallée Lesion Which Resulted in a Wide Skin Necrosis from a Small Laceration. Case Rep Orthop 2020, 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Abbas, L.; Prado, A.; Takemura, R.E.; Wada, A.; Gomez, D.S.; Gemperli, R. Case Report: Stage VI Morel-Lavallée Lesion with a Large Challenging Defect. Plast Reconstr Surg Glob Open. 2021, 9, E3502. [Google Scholar] [CrossRef]
- Ozer, M.T.; Coskun, A.K.; Ozerhan, I.H.; Ersoz, N.; Yildiz, R.; Sinan, H.; Demirbas, S.; Kozak, O.; Uzar, A.I.; Cetiner, S. Use of vacuum-assisted closure (VAC TM) in high-energy complicated perineal injuries: Analysis of nine cases. Int Wound J. 2011, 8, 599–607. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H. Morel-Lavallée Lesion with Intramuscular Extension: A Case Report. Journal of Wound Management and Research. 2022, 18, 119–123. [Google Scholar] [CrossRef]
- Phillips, T.J.; Jeffcote, B.; Collopy, D. Bilateral morel-lavallée lesions after complex pelvic trauma: A case report. Journal of Trauma - Injury, Infection and Critical Care. 2008, 65, 708–711. [Google Scholar] [CrossRef]
- Row, E.; Rizkalla, J.; Holderread, B.; Fritz, J.K.; Jones, A. Management of a Close-Range High-Velocity Gunshot Wound to the Pelvis with Posterior Pelvic Plating: A Case Report. JBJS Case Connect. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Stiff, K.M.; Vargas, C.; Bates, M.; Somach, S.C. Chronic Morel-Lavallée lesion: Presentation as a pseudotumor. JAAD Case Rep. 2022, 27, 75–78. [Google Scholar] [CrossRef]
- Takahara, S.; Oe, K.; Fujita, H.; Sakurai, A.; Iwakura, T.; Lee, S.Y.; Niikura, T.; Kuroda, R.; Kurosaka, M. Missed Massive Morel-Lavallee Lesion. Case Rep Orthop. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Thoppanahalli Venkatesh, R.; Galagali, D.A.; Bhatia, A. Negative pressure therapy in a scenario of distal femur fracture with internal degloving injury. BMJ Case Rep. 2023, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watfa, W.; Campisi, C.; Ryan, M.; Matter, M.; Cherix, S.; Sörelius, K.; Raffoul, W.; di Summa, P.G. Lymphatic leaks of the thigh and inguinal region combined plastic surgery approaches for an effective treatment algorithm. Ann Plast Surg. 2020, 85, 661–667. [Google Scholar] [CrossRef]
- Weiss, N.A.; Johnson, J.J.; Anderson, S.B. Morel-lavallee lesion initially diagnosed as quadriceps contusion: Ultrasound, MRI, and importance of early intervention. Western Journal of Emergency Medicine. 2015, 16, 438–441. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).