1. Introduction
With the continuous development of imaging technology and multimodal ultrasound techniques, the detection rate and diagnostic accuracy of liver nodules have significantly improved [
1]. However, pathological examination is still necessary for the definitive diagnosis of liver nodules [
2,
3]. Percutaneous liver biopsy guided by ultrasound is one of the main methods for obtaining pathological specimens, and its effectiveness and safety have been confirmed in clinical practice [
4,
5].
However, liver nodules can be influenced by liver conditions such as hepatitis, cirrhosis, and fatty liver, as well as factors like lesion location, lung interference, and lesion size, making it difficult to visualize some lesions or achieve satisfactory imaging results during conventional ultrasound examinations. To improve the problem, computerized tomography or magnetic resonance -ultrasound (CT/MR-US) fusion imaging technology, contrast-enhanced ultrasound (CEUS), and high-frequency ultrasound techniques have been applied [
1]. Fusion imaging technology combines ultrasound with CT/MR images, providing more accurate localization and guidance [
6]. CEUS utilizes the backscattering and nonlinear effects of microbubble contrast agents to achieve real-time imaging of lesions and their perfusion information [
7]. High-frequency ultrasound, using a higher-resolution linear array transducer, significantly improves image quality and facilitates the visualization and observation of lesions within the detectable range [
8]. Previous studies have focused on evaluating the success rate of biopsies using ultrasound contrast or fusion imaging separately. Currently, there is a relative scarcity of research that comprehensively evaluates the success rate of biopsies using a combination of multiple ultrasound techniques. Therefore, the aim of this study is to summarize data from patients with non-visualized liver lesions on conventional ultrasound and, after applying multi-modal ultrasound, re-visualize these lesions and perform percutaneous liver biopsies. Furthermore, the study aims to explore the value of multimodal ultrasound techniques in guiding biopsy for liver nodules that are difficult to visualize using conventional ultrasound.
2. Materials and methods
2.1. Patients
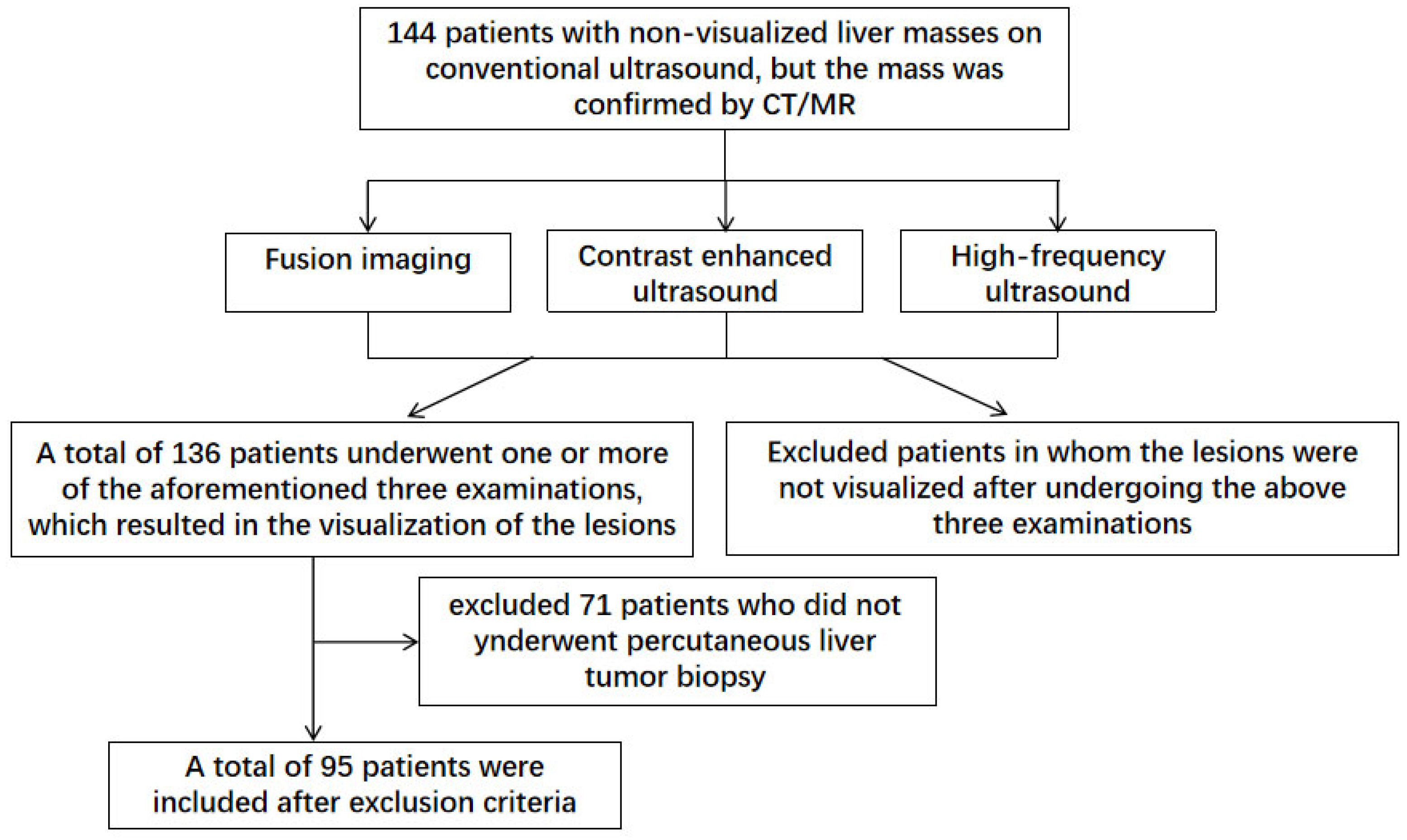
This retrospective study enrolled 144 patients with non-visualized liver masses on conventional ultrasound from the Nanjing Drum Tower Hospital from October 2018 to January 2024. The following inclusion criteria were applied: (1) Patients above the age of 18; (2) Presence of liver masses confirmed by MR or CT imaging; (3) Poor visualization of liver lesions during conventional ultrasound examinations; (4) Diffuse liver masses with unclear borders, requiring multimodal ultrasound techniques for correlation with CT/MR findings; (5) Visualization of liver lesions after underwent CT/MR-US fusion imaging, CEUS, or high-frequency ultrasound examinations; (6) Preoperative evaluations consistent with indications for liver biopsy. The following exclusion criteria were applied: (1) Persistence of non-visualized lesions after CT/MR-US fusion imaging, CEUS, or high-frequency ultrasound examinations; (2) Failure to undergo percutaneous liver biopsy due to various reasons. After excluded 8 patients with non-visualized lesions under multimodal ultrasound and 71 patients who did not underwent percutaneous liver tumor biopsy, a total of 95 patients meeting the inclusion criteria were selected for analysis based on their clinical and radiological data (
Figure 1). All patients underwent complete blood routine, liver function test, coagulation test, infectious disease test, digestive tract test and electrocardiogram (ECG) before operation. Informed of the risks and potential complications of liver biopsy, and were signed with informed consent.
2.2. Multimodal Ultrasound Assessment
2.2.1. Conventional Ultrasound and Fusion Imaging Methods
Conventional examination was peformed using a GE LOGIQTM E9 machine (GE Healthcare, USA) with a 1–5 MHz convex probe. We first assessed the liver condition, evaluated the presence of hepatitis or liver cirrhosis, and determined the adequacy of lesion visualization. If the lesions were poorly visualized, the patient's CT/MR images were reviewed, and a preliminary assessment of the lesion location was performed before proceeding with fusion imaging. The specific steps were as follows: 1) DICOM-formatted CT or MR images were imported into the ultrasound machine; 2) The patient was positioned in a supine position. First, single-plane registration was performed, followed by selecting anatomical landmarks such as the portal vein and hepatic vein bifurcation for point-to-point registration. After completing the registration, the CT/MR images were displayed side by side with the ultrasound images. Subsequently, fine registration was performed based on smaller vascular branches. 3) After completing the registration, the synchronized ultrasound images were observed for the presence of lesions based on the location of the lesions on the CT or MR images. The locations (left lobe, right lobe, or liver lobe junction), sizes, and numbers (single, multiple, or diffuse) of the recurrent lesions were recorded.
2.2.2. High-Frequency Ultrasound Examination
We used a 9 MHz linear transducer for high-frequency ultrasound examination. The patient assumed a supine or left lateral position. The conventional ultrasound examination was performed first using a convex array transducer. If the lesion was not visualized by conventional ultrasound, but closer to the hepatic capsule (≤1.5 cm) in CT/MRI, a high-frequency ultrasound examination was conducted. When a lesion was identified, the depth and focus were adjusted one by one to display the lesion area as clearly as possible.
2.2.3. Contrast-Enhanced Ultrasound Examination
The patient assumed a supine or left lateral position. The optimal view was selected, and the CEUS mode was activated using a convex array transducer. Ultrasound contrast agents used sulfur hexafluoride microbubbles (Sonovue) or perfluorobutane microspheres (Sonazoid), with doses of 1.2 ml or 0.6 ml, respectively. The contrast agents were administered via peripheral venous bolus injection, followed by a 5ml saline flush. And the lesion was observed in real-time dynamic mode for 2 minutes, with images being stored. After 2 minutes, the entire liver was scanned again using the convex array transducer to check for the presence of any other lesions and store the images. We observed the arterial phase (10-20 seconds after contrast injection), portal venous phase (30-45 seconds after contrast injection), and delayed phase (starting from 120 seconds after contrast injection). In Sonazoid, liver Kupffer phase were observed 10 minutes after injection. The enhancement degree was classified as high enhancement, iso-enhancement, low enhancement, or no enhancement, based on the comparison with the echogenicity of the liver parenchyma. The location, size, number of recurrent lesions, the degree of enhancement and enhancement patterns (homogeneous enhancement, inhomogeneous enhancement, peripheral rim enhancement, synchronous enhancement) were recorded.
2.2.4. High-Frequency Contrast-Enhanced Ultrasound Examination
For superficial and small lesions (diameter ≤ 1cm), after completing the conventional contrast-enhanced ultrasound examination, we still had difficulty in observing the internal blood perfusion information of the lesions. Therefore, after performing local burst, we used a linear array transducer to activate the CEUS mode for reperfusion observation. We observed the lesions in real-time for 3-5 minutes and stored the dynamic images.
2.3. Percutaneous liver biopsy
After excluding contraindications for liver biopsy, the lesion location was determined through multimodal ultrasound to ensure the puncture point and pathway, while avoiding blood vessels and vital organs. Freehand puncture method was used to complete the operation. Standard disinfection and draping were carried out, and after local anesthesia, a 17G cannula needle was punctured into the tumor under ultrasound guidance. Then, an 18G biopsy needle was inserted into the tumor through trocar, and 3-5 samples were obtained by cutting. The tissue was fixed in formalin. After biopsy, color Doppler ultrasound was observed to judge if there was any bleeding. The patient was instructed to fasting for 4 hours, and bed rest with eletrocardiograph monitoring was maintained for 8 hours.
2.4. Statistical Analysis
IBM SPSS Statistics 27.0.1 were used for statistical analysis. Demographic data were analyzed using descriptive statistics. Quantitative data is displayed as the mean standard deviation or median values (interquartile ratio), and categorical data is shown as absolute (n) and relative (%) frequencies. The accurate rate of puncture was defined as the number of true positive samples/total number of puncture cases. The final diagnosis of the patients was based on the comprehensive judgment of clinical history, laboratory examination, imaging, pathological results and follow-up. True positivity was defined if the pathological diagnosis was consistent with the final diagnosis. If not, it was defined as negative.
3. Results
3.1. General Results
In this study, a total of 95 patients were enrolled, with 67 males and 28 females. The average age was 57.6 years, ranging from 33 to 83 years. Among the study subjects, 5 cases had concomitant liver cirrhosis, 38 cases had a history of hepatitis B, 1 case had autoimmune liver disease, 1 case had a history of schistosomiasis, and 29 cases had a history of extrahepatic malignancies. Additionally, 7 cases had a history of liver tumor resection, 2 cases had undergone ablation therapy, and 7 cases had a history of other non-liver surgical treatments. Laboratory tests showed that 32 patients were positive for HBsAg, 36 cases had elevated AFP levels, 48 cases had elevated CA199 levels, and 11 cases had elevated CEA levels.
Table 1 contained more detailed demographic and clinical data. Among the study subjects, there were 38 cases of solitary lesions, 34 cases of multiple lesions, and 23 cases of diffuse lesions. The average maximum diameter of the lesions was 2.7 cm (ranging from 0.7 to 10.0 cm). In this study, the statistical definition of lesion size referred to the maximum diameter of the first observed mass in multimodal ultrasound that was not detected in conventional ultrasound examination. The lesion size of the 23 cases with diffuse hepatocellular carcinoma was not included in the statistics. While for patients with multiple lesions, the maximum diameter of the mass was selected for calculation. Among the 32 patients underwent MR-US fusion imaging examination, the lesion recurred in 16 cases, with 2 cases undergoing liver biopsies guided by MR-CEUS fusion imaging. Additionally, among the 23 patients underwent CT-US fusion imaging examination, the lesion recurred in 11 cases, with 1 case undergoing liver biopsy guided by CT-CEUS fusion imaging. Contrast-enhanced ultrasound examination was conducted for all 95 patients, with 68 cases using Sonovue, 21 cases using Sonazoid, and 6 cases using a combination of Sonovue and Sonazoid. Furthermore, high-frequency ultrasound examination was performed for 21 patients, including 2 cases guided by CEUS-high-frequency ultrasound for biopsies. No serious complications or deaths occurred in any of the patients after biopsy.
3.2. Results of Lesion Display by Multimodal Ultrasound
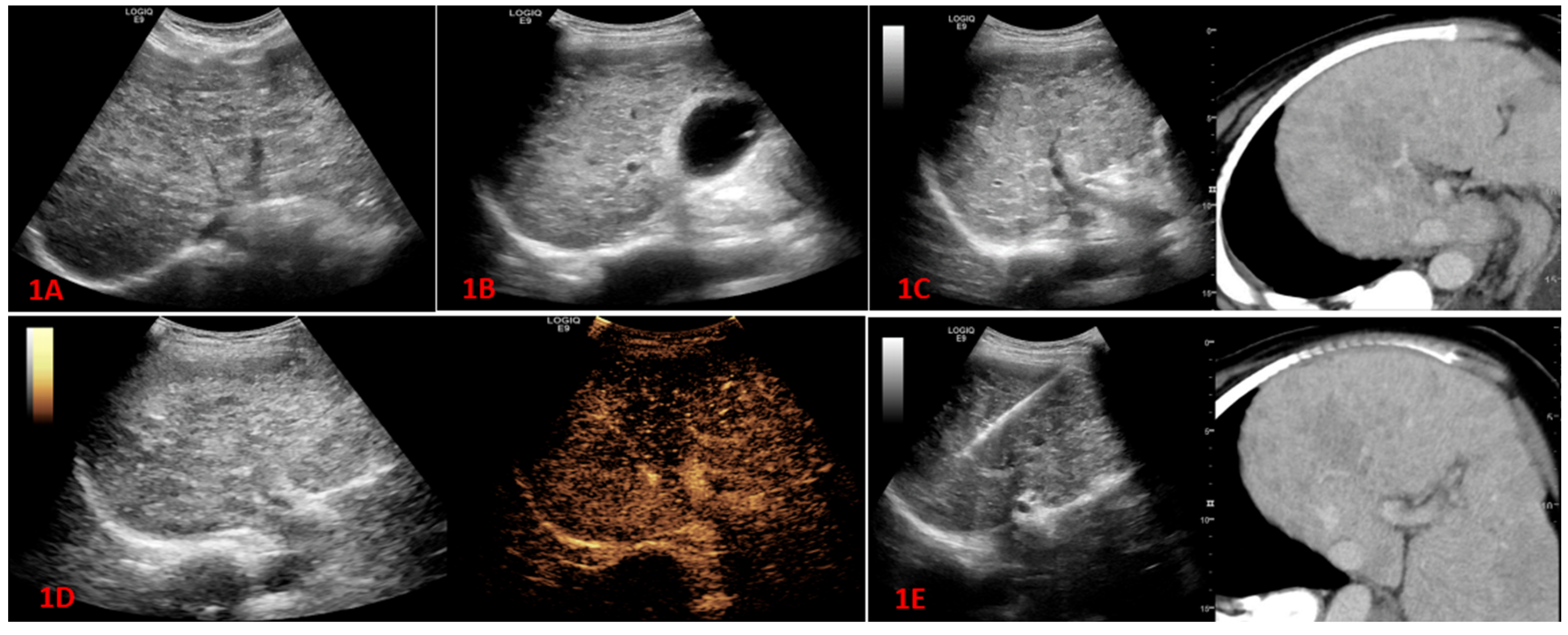
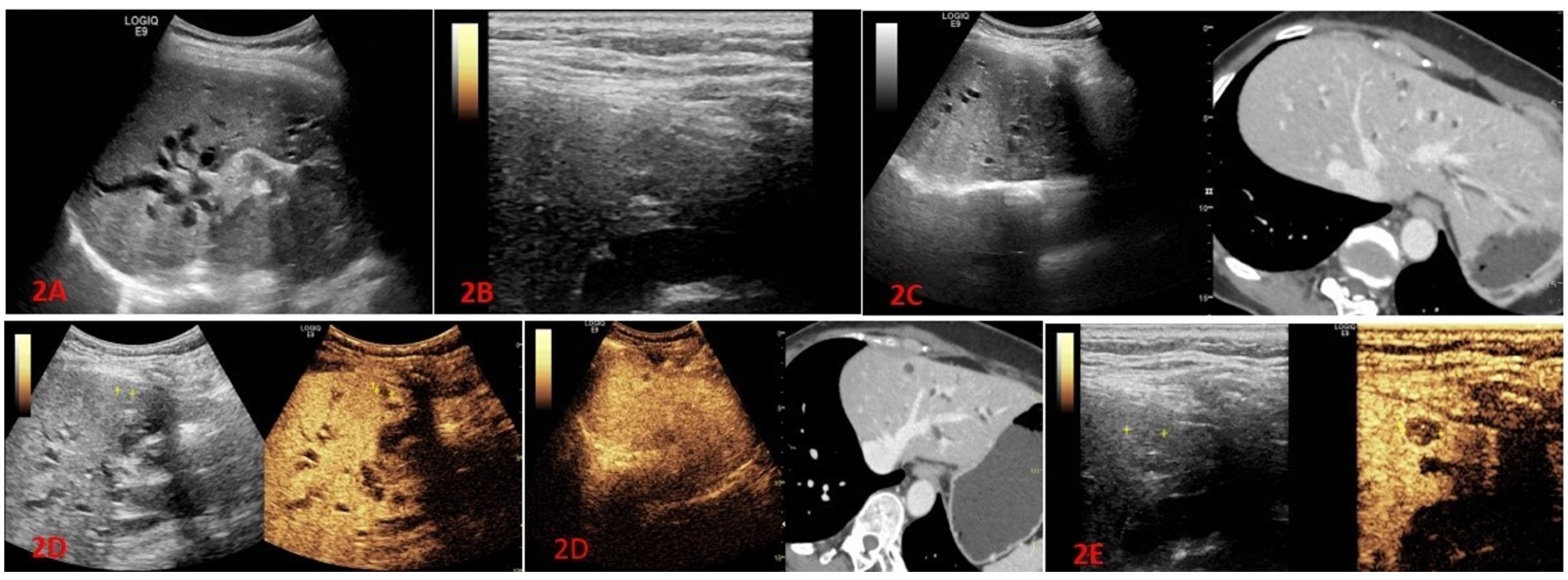
In this study, difficulties were encountered in visualizing lesions during conventional ultrasound examinations for 95 patients. Among them, lesions were unable to be visualized in 82 cases, and in an additional 13 cases, the lesions were partially unable to be visualized or had unclear boundaries. Among the 55 patients who experienced difficulties in lesion visualization during conventional ultrasound examinations, 27 patients had their lesions visualized after undergoing fusion imaging, resulting in a CT/MR-US visualization rate of 49.1%. Furthermore, CEUS were performed for all 95 patients, and lesions were visualized in 92 cases, yielding a CEUS visualization rate of 96.8%. Additionally, for the 20 patients who had difficulties in lesion visualization during conventional ultrasound examinations, 16 patients had their lesions visualized after undergoing high-frequency ultrasound examinations, resulting in a high-frequency ultrasound visualization rate of 76.2% (
Table 2).
3.3. Methods of Percutaneous Liver Biopsy and Pathological Results
In this study, after the target was determined by multimode ultrasound, the appropriate biopsy methods were chosen. A total of 24 cases underwent biopsy under grayscale ultrasound guidance. Among them, 23 cases were guided by conventional grayscale ultrasound after the lesion location was clearly identified through CT/MR fusion imaging, and 1 case was guided by high-frequency grayscale ultrasound after the lesion location was clearly identified through fusion imaging. Additionally, 71 cases underwent biopsy under CEUS guidance. Among them, 56 cases were solely guided by CEUS, 3 cases were guided by the combination of fusion imaging and ultrasound contrast, and 12 cases were guided by high-frequency contrast ultrasound. (
Table 3)
After performing needle biopsies, we analyzed the pathological results of 95 patients. The results showed that 82 patients had positive pathological findings (82/95, 86.3%), The biopsy pathology included 39 cases of hepatocellular carcinoma (HCC), 16 cases of intrahepatic cholangiocarcinoma (ICC), 22 cases of metastatic adenocarcinoma, 3 cases of hepatic adenoma (HA), 1 case of vascular sarcoma, and 1 case of mixed HCC-ICC. Additionally, no malignant tumor cells were observed in the pathological results of 13 patients. Combining imaging studies and laboratory examinations, 8 cases were considered false negatives. Among the 5 negative cases, 2 cases were attributed to post-chemotherapy changes, and 3 cases were diagnosed as inflammatory lesions with no progression observed during follow-up. The overall accuracy rate of our group's biopsies was 91.6% (87/95), with a positive predictive value of 91.1% (82/90) and a false-negative rate of 8.9% (8/90). In addition, 9 patients with recurrent liver cancer after liver biopsy were guided by ultrasound radio-frequency ablation. No serious complications such as death and bleeding occurred.
4. Discussion
Percutaneous liver biopsy involves the insertion of a needle through the skin and into the liver to obtain a tissue sample for diagnostic purposes, staging, or treatment planning for various liver conditions, and remains an important diagnostic procedure for liver mass [
9]. With the advancements in imaging technologies like ultrasound, CT, and MRI, percutaneous liver biopsy can now be more precisely targeted towards specific lesions, enhancing the accuracy of the biopsy and reducing the overall risk of complications [
10]. Conventional gray-scale ultrasonography is the first choice for percutaneous liver biopsy because of its convenience, real-time and low cost. Its diagnostic accuracy is above 90% [
11]. However, percutaneous echo-guided biopsy for hepatic tumor diagnosis faces certain limitations in its performance. Factors such as tumor type, size, and location significantly influence its effectiveness. Moreover, the background of the liver, including conditions like severe fatty liver, hepatitis, and cirrhosis, can impact the visualization of the lesion [
12]. The diagnosis of diffuse tumors in the liver is often constrained by several factors. These tumors tend to exhibit a fusion pattern between lesions with blurred borders, making it difficult to differentiate them from liver cirrhosis and severe liver damage through conventional ultrasound examinations. Larger tumors may have internal necrosis that is challenging to visualize using conventional ultrasound before liquefaction occurs, potentially leading to false-negative results in biopsies. Moreover, tumors located at the top of the liver, smaller tumors, and those adjacent to vital organs are difficult to clearly visualize under conventional ultrasound, making it challenging to accurately target them during the biopsy procedure, thereby potentially affecting the accuracy of the biopsy. Therefore, new techniques are needed to improve the visualization capabilities of ultrasound examinations and enhance the success rate of biopsies for such tumors.
With the continuous advancement of imaging technology, imaging techniques such as ultrasound, CT, and MRI can display real-time images simultaneously from different angles through transducers. CT/MR-US fusion imaging technology improves the visualization of lesions that cannot be seen with conventional ultrasound, helping us understand the three-dimensional relationship between the hepatic vascular system and the lesions [
13]. There have been multiple literature reports on the clinical research of MR/CT-US fusion imaging technology, which is currently primarily focused on improving the success rate of ablation. However, there are relatively few reports on the application of fusion imaging for liver biopsies of lesions that cannot be visualized with conventional ultrasound [
14,
15,
16,
17]. In our study, a total of 95 patients with unclear lesions on routine ultrasound were included, and the accuracy rate of biopsy reached 91.6%. The lesion visualization rate after CT/MR-CT fusion imaging was 49.1% (27/55), and after lesion visualization, the percutaneous liver biopsy success rate under the guidance of fusion imaging and grayscale ultrasound reached 85.2% (23/27). This enabled the targeting of tumors that were not visualized under conventional ultrasound to be clearly identified and successfully biopsied under the guidance of fusion imaging.
CEUS is a specialized form of ultrasound imaging that involves the intravenous injection of microbubble contrast agents. Due to its higher safety profile and real-time visualization of vascular perfusion within lesions, CEUS has been widely used for the evaluation of focal liver lesions, and has been added to the American College of Radiology Liver Imaging Reporting and Data System (CEUS LI-RADS) [
18,
19]. CEUS greatly enhances the diagnostic accuracy of ultrasound in detecting and characterizing focal liver lesions [
20]. There have been numerous literature reports on clinical studies involving CEUS technology, with current focus primarily on characterizing differences among different tumor types [
21,
22,
23]. Meanwhile, there are relatively fewer reports on the application of fusion imaging to visualize lesions that cannot be detected with conventional ultrasound and guide liver biopsies. In our study, 96.8% (92/95) of the lesions were detected after CEUS, and 60.8% (56/92) of the lesions were detected after percutaneous liver biopsy guided only by contrast-enhanced ultrasound. It is worth mentioning that, for larger tumors or diffuse tumors in liver segments, ultrasound-guided biopsy under contrast-enhanced ultrasound (CEUS) was particularly important. In these patients, two-dimensional ultrasound examination often showed scattered echoes, unclear tumor boundaries, and merging of diffuse tumors. After contrast-enhanced ultrasound, the boundaries of the lesions and the presence of necrotic areas within the tumor could be clearly determined. By avoiding the necrotic areas during biopsy under ultrasound guidance, satisfactory results could be obtained. In our study, out of 23 patients with diffuse liver cancer, 17 patients (73.9%) obtained positive pathological results under ultrasound guidance. Among the 8 patients with larger tumors (maximum lesion diameter ≥ 5cm), 5 patients (62.5%) obtained positive pathological results under ultrasound guidance.
High-frequency ultrasound is a technique that utilizes a high-frequency linear ultrasound probe (5-12MHz) to image superficial suspicious lesions in the liver. During the imaging process, continuous adjustments of depth and focus are necessary to ensure clear imaging of the suspicious lesions [
24]. According to related research, the additional use of a high-frequency transducer during conventional abdominal examinations can detect new liver lesions in a significant number of patients. Importantly, this additional use does not significantly extend the overall examination time. Although there is currently limited clinical research on high-frequency ultrasound, the focus primarily lies in enhancing the detection rate of superficial lesions using high-frequency ultrasound contrast imaging. In our study, after undergoing high-frequency ultrasound examination, a total of 16 patients had a lesion visualization rate of 76.2% (16/21). All of these 16 patients received additional ultrasound techniques following lesion visualization. Among them, 9 patients (56.2%) underwent CEUS examination, while 7 patients (43.8%) underwent a combination of fusion imaging technique and CEUS examination. The reason for this result is due to the characteristics of high-frequency ultrasound, where the higher the frequency, the faster the attenuation, making it less sensitive to blood flow detection. Therefore, in clinical practice, it is common to combine high-frequency ultrasound with contrast-enhanced ultrasound to more clearly obtain the internal blood perfusion information of superficial liver lesions.
The limitations of this study are as follows: (1) The inclusion criteria of this study excluded patients who did not show lesions even after undergoing multimodal ultrasound and did not undergo percutaneous liver biopsy. This may lead to a higher lesion detection rate after conducting the three ultrasound examinations in the included patients. (2) Due to considerations of clinical time and patient costs, when the lesion location and biopsy method can be determined through one or two ultrasound examinations, additional examinations are usually not performed, resulting in inconsistent sample sizes for the three ultrasound examination methods among patients. (3) This study is a single-center summary with a small sample size, and no control group using only grayscale ultrasound-guided biopsy was established.
5. Conclusions
This study aims to evaluate the potential of multimodal ultrasound technology in improving the visibility of inconspicuous liver lesions under routine ultrasound. Specifically, the study explores the application of fusion imaging, CEUS, and high-frequency ultrasound in enhancing the visibility of liver masses and accurately locating them for percutaneous tissue biopsy. The study compares the biopsy results with the final diagnosis, and assess whether multimodal imaging techniques can improve the success rate of percutaneous liver biopsy for lesions that are not clearly visible under conventional ultrasound. Furthermore, the application of multimodal ultrasound technology has the potential to impact the development of personalized treatment strategies. It can assist in guiding microwave ablation therapy for lesions and facilitate precise treatment planning in collaboration with clinicians. In conclusion, this study investigates the potential of multimodal ultrasound technology in diagnosing and treating liver lesions, offering new methods and insights for improving the management of liver diseases.
Consent for publication
All authors and hospitals agreed to publish this article. All authors and hospitals agreed to publish this article. Informed consent for publication of images were obtained from all participants.
Availability of data and materials
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Competing interests
All authors declare no conflict of interest.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Gulou Hospital affiliated to Nanjing University School of Medicine (approval number: 2022-140-01). Patients were consented by an informed consent process that was reviewed by the Ethics Committee of Gulou Hospital and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinkl. Written informed consent has been obtained from the patients, and their anonymous information will be published in this article.
Author Contributions
Conceptualization, X.Y.L., S.Y.T., and Y.H.H.; Methodology and Data curation, X.Y.L. and W.T.K.; Investigation. X.Y.L., S.Y.T., and Y.H.H.; Writing- Original draft preparation, X.Y.L.; Writing- Reviewing and Editing, K.K.C. and W.T.K.; All authors reviewed the manuscript.
Acknowledgments
Not applicable.
References
- Hu, J.; Zhou, Z.-Y.; Ran, H.-L.; Yuan, X.-C.; Zeng, X.; Zhang, Z.-Y. Diagnosis of liver tumors by multimodal ultrasound imaging. Medicine 2020, 99, e21652. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. La Radiol. medica 2022, 127, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, M.; Terracciano, L.; Di Tommaso, L.; David, E.; Colombo, M. Liver precancerous lesions and hepatocellular carcinoma: The histology report. Dig. Liver Dis. 2011, 43, S361–S372. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Manini, M.A.; Sangiovanni, A.; Fraquelli, M.; Forzenigo, L.V.; Di Tommaso, L.; Aghemo, A.; Roncalli, M.; Ronchi, G.; Colombo, M. Contrast-enhanced computed tomography and ultrasound-guided liver biopsy to diagnose dysplastic liver nodules in cirrhosis. Dig. Liver Dis. 2013, 45, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.V.C.; Moura, E.A.; Santos, J.A.d.M.d.; Lima, M.V.A. US-guided percutaneous core liver BIOPSY: analysis of 171 cases from a single oncology service. Arq. de Gastroenterol. 2018, 55, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography 2014, 33, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.H.; Fetzer, D.T.; Dillman, J.R. Practical Contrast Enhanced Liver Ultrasound. Radiol. Clin. North Am. 2022, 60, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Horrow, M.M. High-Frequency US in Hepatobiliary Imaging. RadioGraphics 2023, 43, e230062. [Google Scholar] [CrossRef] [PubMed]
- Rustagi T, Newton E, Kar P. Percutaneous liver biopsy. Trop Gastroenterol. 2010;31(3):199-212.
- Chan M, Navarro VJ. Percutaneous Liver Biopsy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; , 2023. 10 April.
- Howlett, D.C.; Drinkwater, K.J.; Lawrence, D.; Barter, S.; Nicholson, T. Findings of the UK National Audit Evaluating Image-guided or Image-assisted Liver Biopsy. Part I. Procedural Aspects, Diagnostic Adequacy, and Accuracy. Radiology 2012, 265, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Sparchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Grigorescu, I.; Botis, G.; Badea, R. Usefulness of contrast enhanced ultrasound guidance in percutaneous biopsies of liver tumors. . 2011, 20, 191–6. [Google Scholar] [PubMed]
- Minami Y, Kudo M. Ultrasound fusion imaging technologies for guidance in ablation therapy for liver cancer. J Med Ultrason (2001). 2020;47(2):257-263.
- Xu, E.; Long, Y.; Li, K.; Zeng, Q.; Tan, L.; Luo, L.; Huang, Q.; Zheng, R. Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int. J. Hyperth. 2018, 35, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Minami Y, Kudo M. Ultrasound fusion imaging technologies for guidance in ablation therapy for liver cancer. J Med Ultrason (2001). 2020;47(2):257-263.
- Sheng, Y.; Sun, X.; Sun, H.; Qi, J.; Li, H.; Luan, J.; Zhai, D. Fusion imaging versus ultrasound-guided percutaneous thermal ablation of liver cancer: a meta-analysis. Acta Radiol. 2023, 64, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Xu, E.; Zeng, Q.; Ju, J.; Huang, Q.; Liang, P.; Zheng, R.; Li, K. Intra-procedural real-time ultrasound fusion imaging improves the therapeutic effect and safety of liver tumor ablation in difficult cases. 2020, 10, 2174–2184.
- Lyshchik, A.; Kono, Y.; Dietrich, C.F.; Jang, H.-J.; Kim, T.K.; Piscaglia, F.; Vezeridis, A.; Willmann, J.K.; Wilson, S.R. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom. Imaging 2017, 43, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Terranova, M.C.; Gagliardo, C.; Taibbi, A. CEUS LI-RADS: a pictorial review. Insights into Imaging 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.H.; Fetzer, D.T.; Dillman, J.R. Practical Contrast Enhanced Liver Ultrasound. Radiol. Clin. North Am. 2022, 60, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Niu, Y.; Luo, Y.; Ma, X. Characteristic contrast-enhanced ultrasound findings of hepatic epithelioid haemangioendothelioma: A case report and literature review. Oncol. Lett. 2023, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dong Y, Wang WP, Mao F, et al. Imaging Features of Fibrolamellar Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound. Bildgebungsmerkmale des fibrolamellären hepatozellulären Karzinoms im kontrastverstärkten Ultraschall. Ultraschall Med. 2021;42(3):306-313.
- Dong, Y.; Wang, W.-P.; Cantisani, V.; D’onofrio, M.; Ignee, A.; Mulazzani, L.; Saftoiu, A.; Sparchez, Z.; Sporea, I.; Dietrich, C.F. Contrast-enhanced ultrasound of histologically proven hepatic epithelioid hemangioendothelioma. World J. Gastroenterol. 2016, 22, 4741–4749. [Google Scholar] [CrossRef] [PubMed]
- Schacherer, D.; Wrede, C.; Obermeier, F.; Schölmerich, J.; Schlottmann, K.; Klebl, F. Comparison of low and high frequency transducers in the detection of liver metastases. Dig. Liver Dis. 2006, 38, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Schacherer, D.; Schuh, C.; Strauch, U.; Ehrenstein, B.; Wiest, R.; Schölmerich, J.; Schlottmann, K.; Klebl, F. Improvement in the routine diagnostic assessment of the liver by high-resolution sonography: An analysis of 999 cases. Scand. J. Gastroenterol. 2007, 42, 366–373. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).