1. Introduction
The selective catalytic reduction of nitrogen oxides with ammonia (NH
3-SCR-DeNO
x) is widely applied as an emission control process to eliminate NO
x from diesel engines as well as power plants. So far, many catalysts have been investigated in NH
3-SCR-DeNO
x, including vanadium pentoxide-based catalysts, transition metal-exchanged zeolites (e.g., Cu-Y or Cu-SSZ-13), or hybrid catalysts (e.g., Ce-Zn mixed oxides with Fe-ZSM-5 or Zn-Ti mixed oxides with Cu-SSZ-13, etc.) [
1,
2,
3]. Furthermore, besides catalysts based on SSZ-13 or ZSM-5, Cu-containing ERI revealed enhanced catalytic properties in NH
3-SCR-DeNO
x. For example, Zhu et al. [
4] proved that their fast-synthesized Cu-containing ERI achieved a catalytic activity comparable to the commercial Cu-SSZ-13. Moreover, Sultana et al. [
5] reported that Cu-ERI catalysts showed similar catalytic activity to Cu-ZSM-5, although with higher resistance against decane poisoning. Some researchers claimed that the activity and N
2 selectivity in both NH
3-SCR-DeNO
x and the selective catalytic oxidation of ammonia (NH
3-SCO) have been enhanced for the micro-/mesoporous materials (e.g., Cu-ZSM-5, Cu-SAPO-34, Fe-ZSM-5) [
6,
7,
8]. For example, Oord et al. [
9] and Wu et al. [
10] applied the post-synthetic modification of SSZ-13 with an aqueous solution of NaOH. They both found that the catalytic activity and N
2 selectivity can be improvead for Cu-SSZ-13 (with the support treated with 0.1 M solution). Otherwise, the post-synthetic modification above 0.1 M led to a drop in the catalytic properties of the materials. On the other hand, Jabłońska et al. found that the mesopores introduced into Cu-containing zeolite ZSM-5 [
11] or Y [
12] catalysts do not play any direct role in the activity of the NH
3-SCR-DeNO
x. Although Tekla et al. [
13] introduced mesoporosity in erionite using both acid and alkali leaching treatments, such materials have not been investigated in NH
3-SCR-DeNO
xor NH
3-SCO. Given the discrepancies mentioned above, further studies of micro-/mesoporous materials are required to understand the relationship between mesoporosity, catalytic activity, and N
2 selectivity in NH
3-SCR-DeNO
x. Thus, ERI and SSZ-13 zeolites were subjected to different post-synthetic treatments, depending on the zeolite topology, including treatment with NaOH or sequential treatment with HNO
3, followed by treatment with NaOH. The structure, texture, elemental analysis, and acidic properties of the copper-containing zeolites were characterized by X-ray diffraction (XRD), solid-state nuclear magnetic resonance (NMR), N
2 sorption, inductively coupled plasma optical emission spectroscopy (ICP-OES), and temperature-programmed desorption of NH
3 (NH
3-TPD). The nature of copper species was investigated by temperature-programmed reduction of H
2 (H
2-TPR), as well as diffuse reflectance UV-Vis (DR UV-Vis), extended X-ray absorption fine structure (EXAFS), and electron paramagnetic resonance (EPR) spectroscopy. The activity and N
2 selectivity were evaluated
via NH
3-SCR-DeNO
x (4 NH
3 + 4 NO + O
2 → 4 N
2 + 6 H
2O) and NH
3-SCO (4 NH
3 + 3 O
2 → 2 N
2 + 6 H
2O) for Cu-form of conventional zeolites and post-synthetically treated SSZ-13 and especially ERI for the first time in literature. Furthermore, the reaction pathways were investigated through
in situ Fourier-transform infrared (FT-IR) and DR UV-Vis spectroscopy and temperature-programed studies with
18O
2 isotope over both zeolite topologies to deepen our understanding of the reactive reaction intermediates and thus, maximize their potential in industrial applications.
4. In situ spectroscopic studies
When exposed to molecules with strong basicity (e.g., NH
3, H
2O), isolated Cu species are pulled away from their original position and migrate within the zeolite cages [
44]. This reaction-driven dynamic Cu motion reflects the low-temperature NH
3-SCR-DeNO
x and can be monitored by In situ FT-IR and In situ DR UV Vis spectroscopy.
Figure 9 and
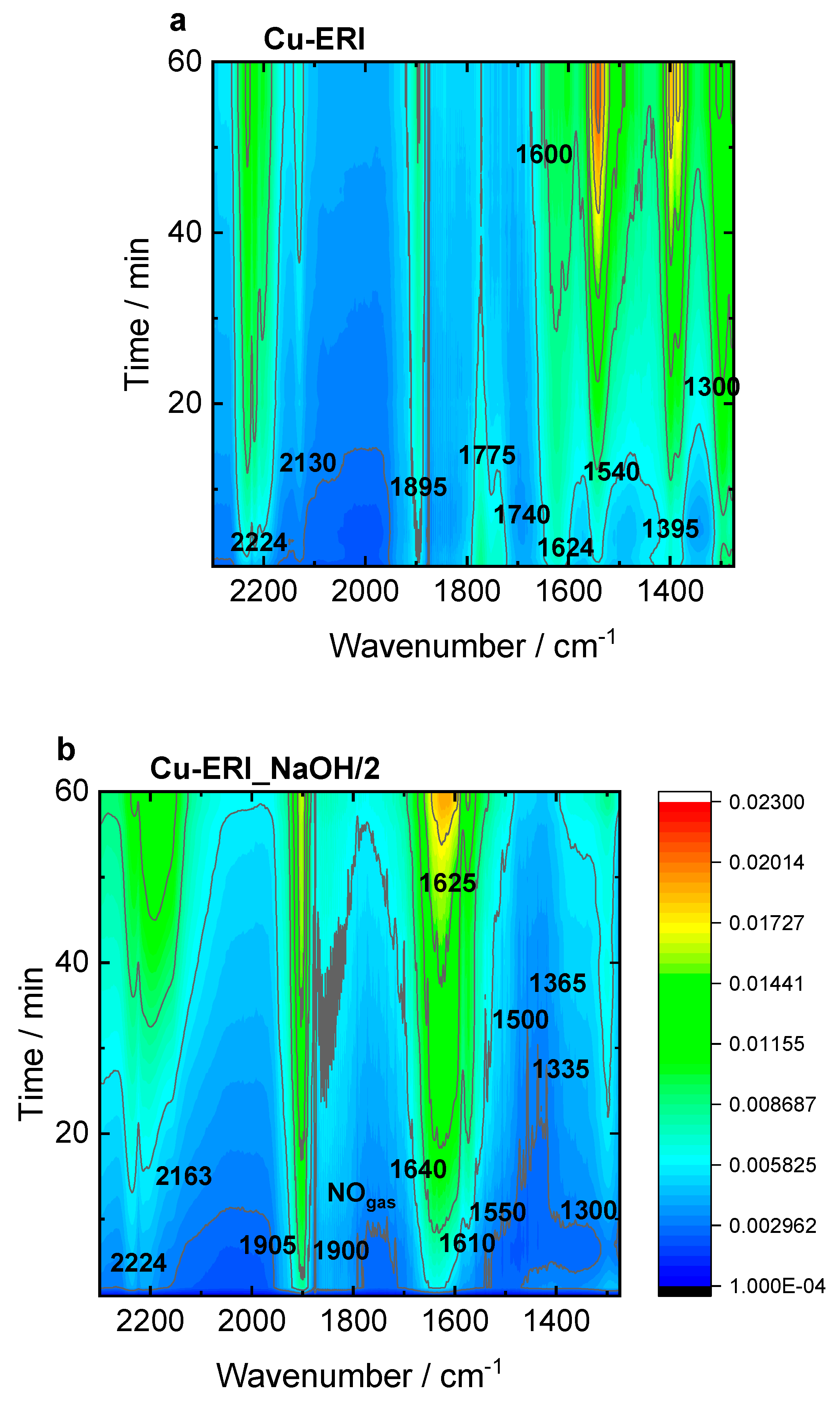
Figure S11 present the top-down projection of In situ FT-IR spectra collected for the Cu-containing ERI and SSZ-13 catalysts contacted with reactants for 1 h at 125 °C. In Cu-ERI (
Figure 9a), the bands of the Cu
2+(NO) mononitrosyl (1895 cm
-1), N
2O (2224 cm
-1) appeared immediately when the NO
3-/NO
2- bands (located at 1300, 1395, 1540, and 1600 cm
-1) accompanied by the formation of NO
+ ions (2130 cm
-1) started to dominate the spectrum in later SCR reaction course. The band at 1624 cm
-1 can be attributed to ammonia molecules in a coordination sphere of Cu
2+ ions consumed in favor of the production of nitrates/nitrites. The Cu
+(NO) species are identified by the 1775 and 1740 cm
-1 bands. Overall, the most dominant species detected for Cu-ERI, also in the presence of water vapor, are the nitrates (located at 1395 and 1540 cm
-1). The different positions of the Cu
2+(NO) mononitrosyls (1900 and 1905 cm
-1) formed on the surface of the Cu-ERI_NaOH/2 sample (
Figure 9b) than for Cu-ERI indicates the different nature of Cu
2+ ions ruled by both their different location in zeolite framework and the alteration of the ligand in copper coordination sphere induced by water presence. Nitrates (located at 1300 and 1550 cm
-1) appeared also to a limited extent on the surface of Cu-ERI_NaOH/2 than of Cu-ERI. The presence of water vapor in the reaction mixture (
Figure S11a,b) influences the SCR reaction what is detected as a decrease in the nitrate concentration (the lack of the bands at 1540 and 1395 cm
-1) in favour of water formation (1625 cm
-1). We also observed the NH
4+ species (1425 cm
-1), but their amount not being affected during the whole reaction course, points to them serving as spectator species under these reaction conditions, still, however serving as a reservoir of adsorbed NH
3 at higher temperature SCR stage [
45].
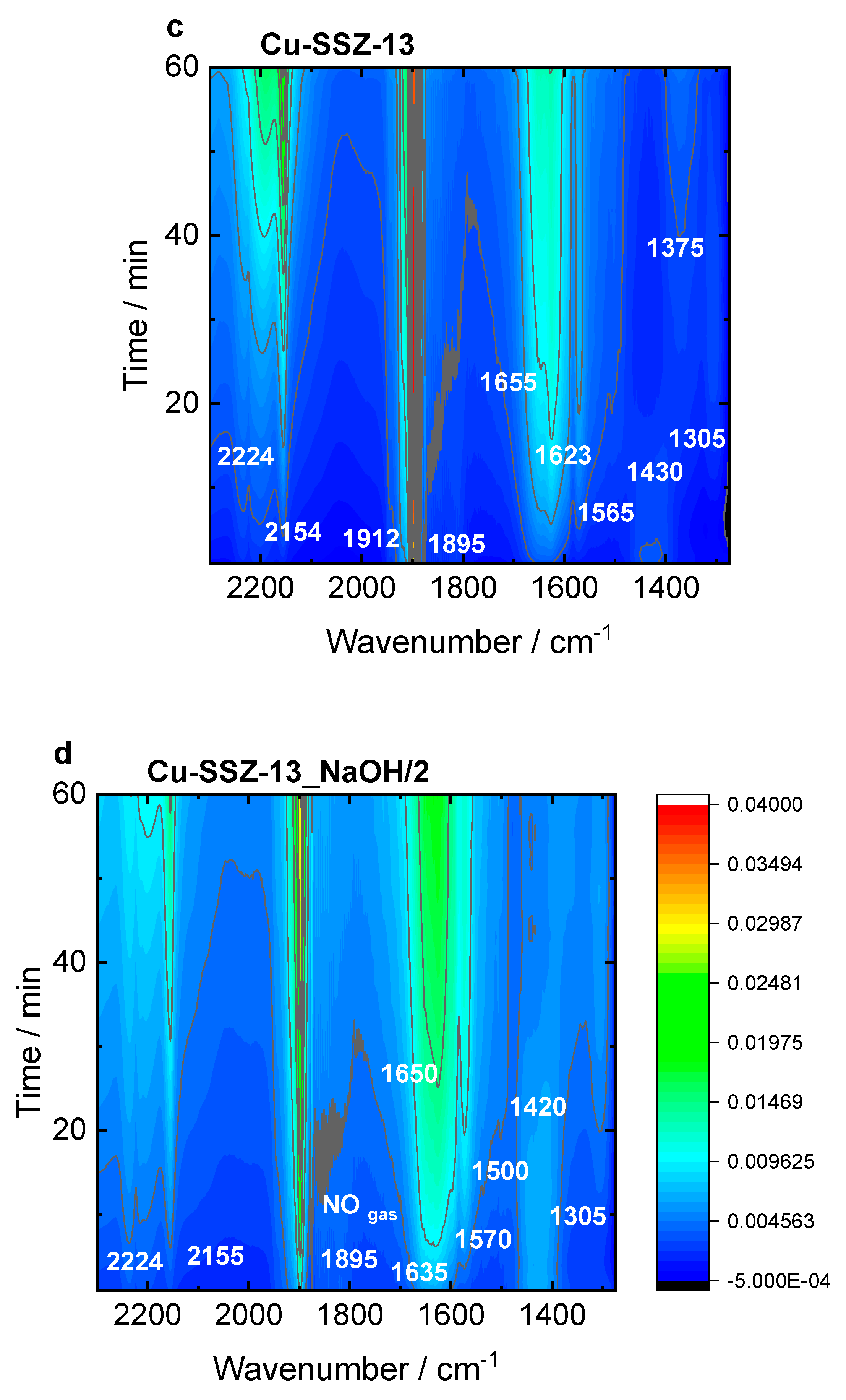
The Cu-SSZ-13 catalyst (
Figure 9b) is dominated by the Cu
2+(NO) mononitrosyls (1895 and 1912 cm
-1), N
2O (2224 cm
-1), and amine Cu
2+ adducts (1623 cm
-1). The latter are converted in water (1655 cm
-1) and NO
3-/NO
2- species (1375 and 1565 cm
-1), which is accompanied by the formation of NO
+ ions (2130 cm
-1). This weak intensity of nitrates, compared to Cu-ERI, could be indicative of a higher activity of the CHA-based catalysts. Still, the presence of water vapor in the feed results mainly in a decrease in the nitrate and Cu
2+(NO) mononitrosyls (
Figure S11c). Regarding the Cu-SSZ-13_NaOH/2 sample (
Figure 9d), the water (1650 cm
-1), Cu
2+(NO) mononitrosyl (1895 cm
-1), and nitrates (1500 and 1570 cm
-1) starts to be the dominant species. The appearance of NH
4+ species is also detected (1410 cm
-1). They are formed at the first reaction period and then disappear with the depletion of the Cu
2+-NH
3 (1635 cm
-1) and nitrates (1570 and 1500 cm
-1), indicating their intermediating role in the SCR process. The introduction of water in the gas mixture (
Figure S11d) results in changes in the position of the mononitrosyl band (1900 cm
-1), as previously detected for the Cu-containing ERI samples. A high number of NO
3-/NO
2- species (1575, 1520, 1350, 1310 cm
-1) is also confirmed. The results suggest the importance of the interplay between the copper sitting at different locations and various hydrothermal stability; thus, NH
3-SCR-DeNO
xactivity is forced by the zeolite framework type.
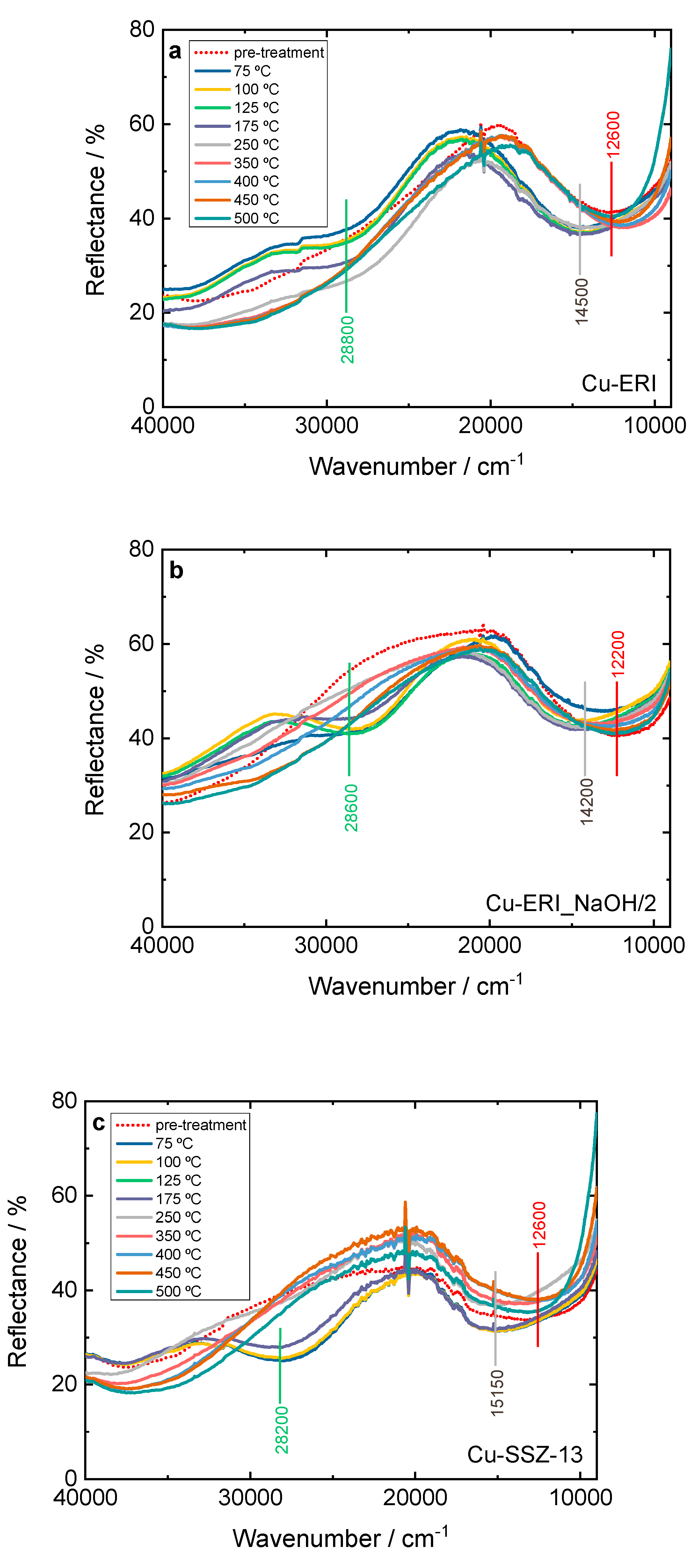
Furthermore, the NH
3-SCR-DeNO
x over the Cu-containing ERI and SSZ-13 samples was investigated
via In situ DR UV-Vis (
Figure 10 and
Figure S12). The bands previously mentioned related to the oxygen-to-metal charge transfer and d-d transition of Cu
2+ are visible. However, after the catalysts come into contact with the reaction gases, the d-d transition band experiences a shift to higher wavenumber values from 12200-12600 cm
-1 to 14200-15300 cm
-1. This reflects the interaction of NH
3 with copper species. Thus, the d-d transition band no longer belongs to isolated Cu
2+ but to the copper present in amine complexes (e.g., [Cu
II(NH
3)
4]
+) [
46]. In a further course of the reaction, a new band also appears at 28200-28800 cm
-1, which is assigned to the [Cu
II2(NH
3)
4O
2]
2+ intermediates with a side on μ-η
2,η
2-peroxo diammino dicopper(II) structure [
46,
47]. In the spectra of Cu-containing ERI (
Figure 10a,b), the [Cu
II2(NH
3)
4O
2]
2+ can be clearly visible below 250 °C. For Cu-ERI_NaOH/2, already at 75 °C a slight band at 28600 cm
-1 is present, but the band related to the d-d transition does not shift to higher wavenumbers. The intensity of the strong band at 28600 cm
-1 increases between 100-125 °C, while at the same time, the d-d transition band shifts to higher wavenumbers (i.e., indicating the presence of diamino dicopper species over this sample). Above 125 °C, the band at 28600 cm
-1 decreases in intensity until its disappearance at 250 °C. At progressively higher temperatures, the d-d transition signal returns to the wavenumber values previous to starting the reaction. The difference between 20000 and 30000 cm
-1 in the activation spectra of Cu-ERI and Cu-ERI_NaOH/2 (arising due to the different nature of copper species in these samples), explains why the band at ca. 28600 cm
-1 is more pronounced in Cu-ERI_NaOH/2 than in the other sample.
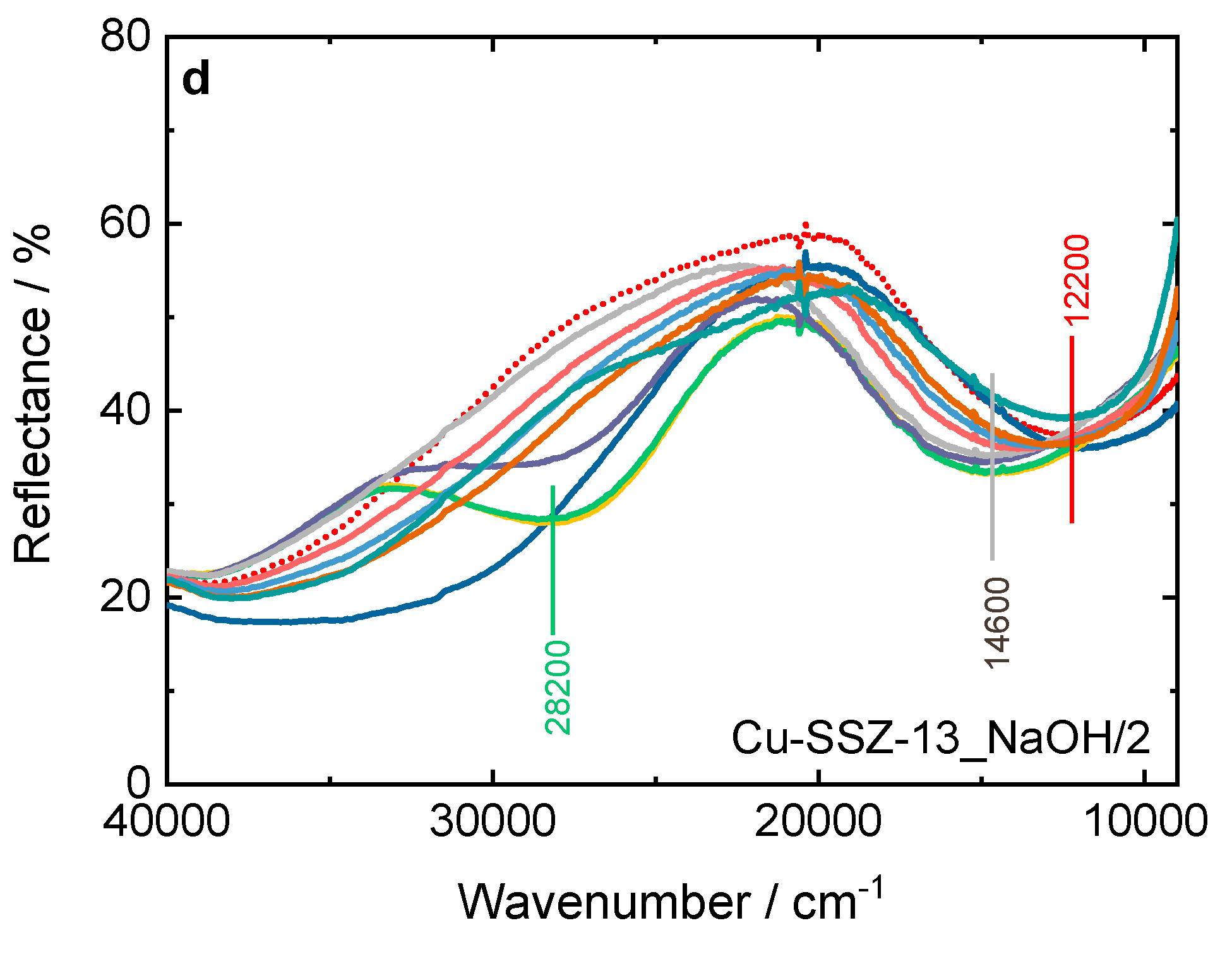
For Cu-SSZ-13 (
Figure 10c), as the reaction mixture comes into contact with the sample already at 75 °C, a strong band appears at 28200 cm
-1, and the d-d transition band shifts. At 250 °C, this band disappears, and the d-d transition band returns to its original position. On the contrary, for Cu-SSZ-13-NaOH/2, the bands related to the diamino dicopper complex become visible above 75 °C. Indeed, below 250 °C, Cu-SSZ-13 is more active than Cu-SSZ-13_NaOH/2 for NH
3-SCR-DeNO
x (
Figure 8). The formation of the [Cu
I(NH
3)
2]
+ intermediate can be hampered from Cu
2+ pairs coordinated with two Al sites [
47], which are the main species present in the Cu-SSZ-13_NaOH/2 sample. Above 250 °C, the solvation shell of ammonia around copper cations is not stable [
48], indicating that a different reaction mechanism is dominant at high temperatures [
25,
49,
50].
Furthermore, we investigated the influence of 5 vol.-% H
2O on forming the reaction intermediates. However, both Cu-containing ERI samples exhibit a similar behavior in the absence of water (Figure 12a,b). This aligns with the results of catalytic studies (
Figure S9b), which do not show any significant differences in NO conversion among these samples. Regarding the Cu-containing SSZ-13 samples under wet conditions (
Figure S12c,d), the band at 28800 cm
-1 recedes slowly below 250 °C, which could indicate that the reduction of the complex is slower than in the absence of water. At high temperatures (> 350 °C), the vertical asymptote present at low wavenumber range is caused by spurious black-body radiation emitted by the heating device.
The Cu-ERI_NaOH/2 and Cu-SSZ-13_NaOH/2 samples were also investigated for NH
3 oxidation. The spectra recorded for both samples (
Figure S13) are characteristic of the conventional activation of Cu-containing zeolites under an O
2 atmosphere [
46]. No appearance of the band related to the diammino dicopper intermediates was found. In contrast, the structure of the d-d transition band is less resolved which is unrelated to the reaction. Indeed, NO molecules are necessary to activate the copper ammine complexes that precede the diammino dicopper complex (e.g., [
51]).
Author Contributions
Alejandro Mollá Robles: Investigation, Data curation, Software, Writing - original draft, Writing - review & editing. Gabriel Deplano: Investigation, Data curation. Kinga Góra-Marek: Investigation, Data curation, Writing - review & editing. Marek Rotko: Investigation, Data curation. Anna Wach: Investigation, Data curation, Writing - review & editing. Muhammad Fernadi Lukman: Investigation, Data curation, Writing - review & editing. Marko Bertmer: Investigation, Data curation. Matteo Signorile: Investigation, Data curation. Silvia Bordiga: Review. Andreas Pöppl: Review. Roger Gläser: Review. Magdalena Jabłońska: Conceptualization, Methodology, Investigation, Data curation, Data management, Writing - original draft, Writing - review & editing, Supervision, Visualization, Project management.
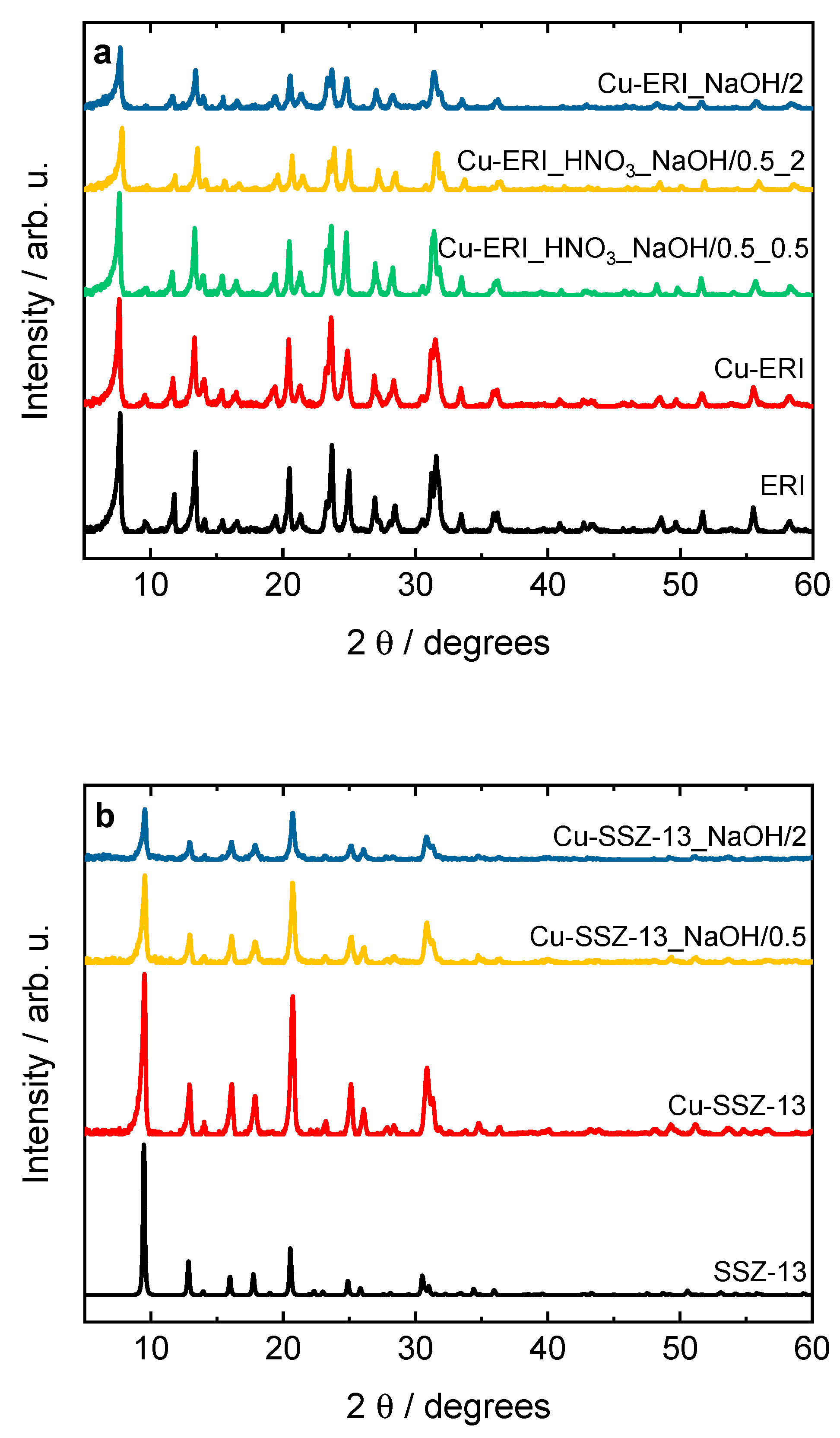
Figure 1.
XRD patterns of a) ERI, b) SSZ-13 samples and their Cu-containing forms (sample labels as in
Table 2).
Figure 1.
XRD patterns of a) ERI, b) SSZ-13 samples and their Cu-containing forms (sample labels as in
Table 2).
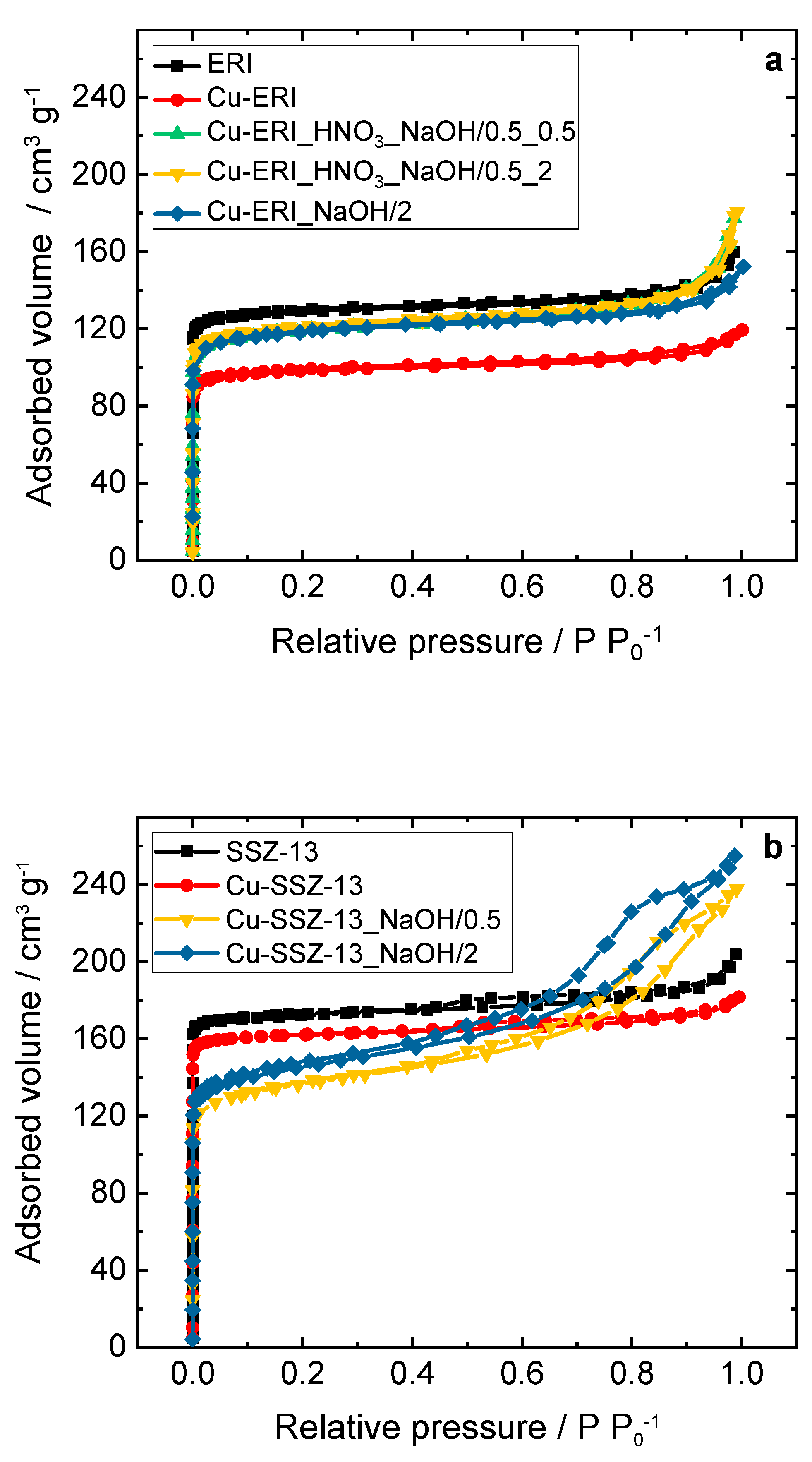
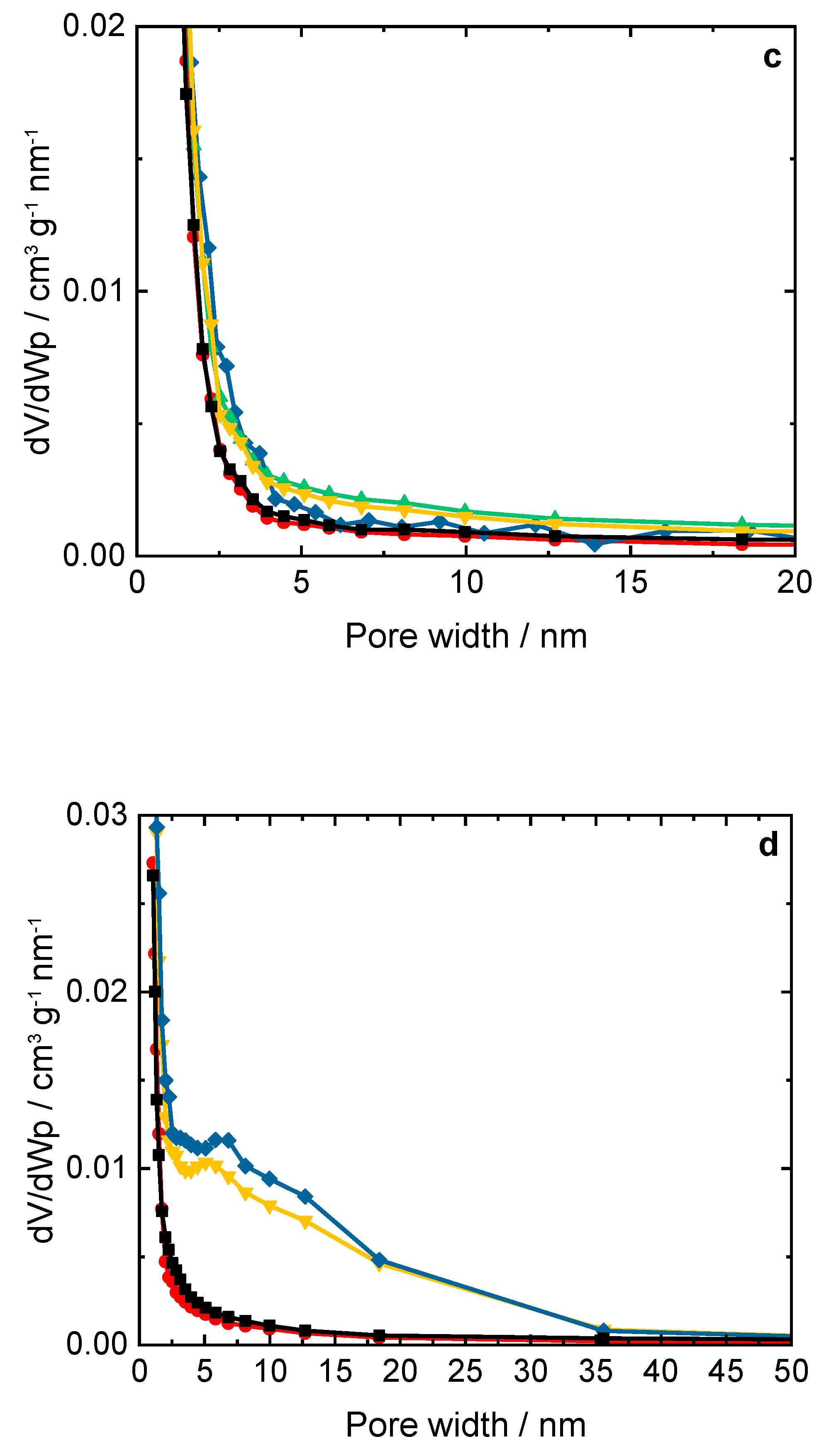
Figure 2.
a,b) N2 sorption isotherms collected at -196 °C and c,d) BJH pore width distribution of ERI, SSZ-13 samples, and their Cu-containing forms; a,c) and b,d) sample labels are identical.
Figure 2.
a,b) N2 sorption isotherms collected at -196 °C and c,d) BJH pore width distribution of ERI, SSZ-13 samples, and their Cu-containing forms; a,c) and b,d) sample labels are identical.
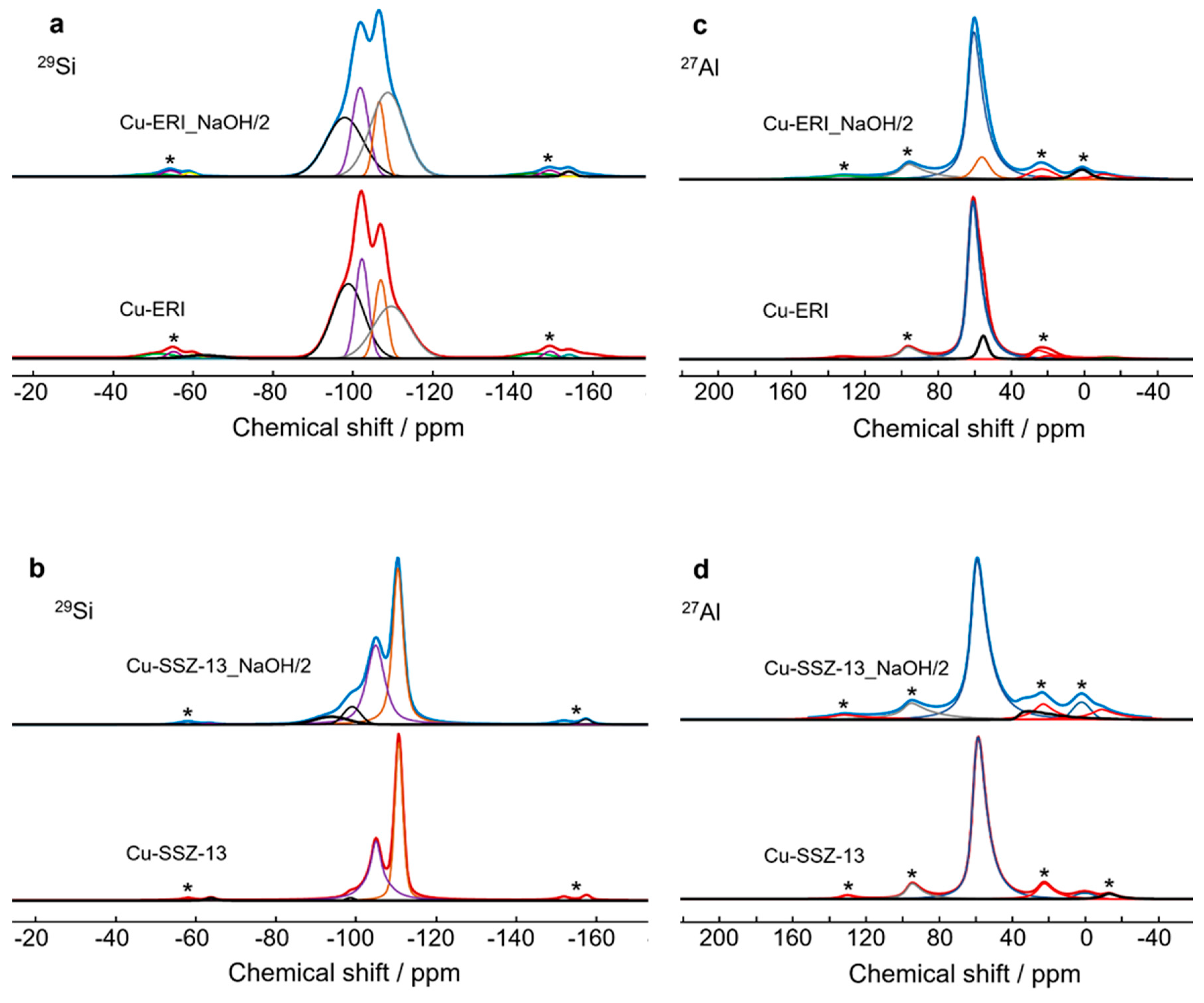
Figure 3.
a, b) 29Si and c,d) 27Al NMR spectra of Cu-containing ERI and Cu-containing SSZ-13 samples; * indicates spinning sidebands.
Figure 3.
a, b) 29Si and c,d) 27Al NMR spectra of Cu-containing ERI and Cu-containing SSZ-13 samples; * indicates spinning sidebands.
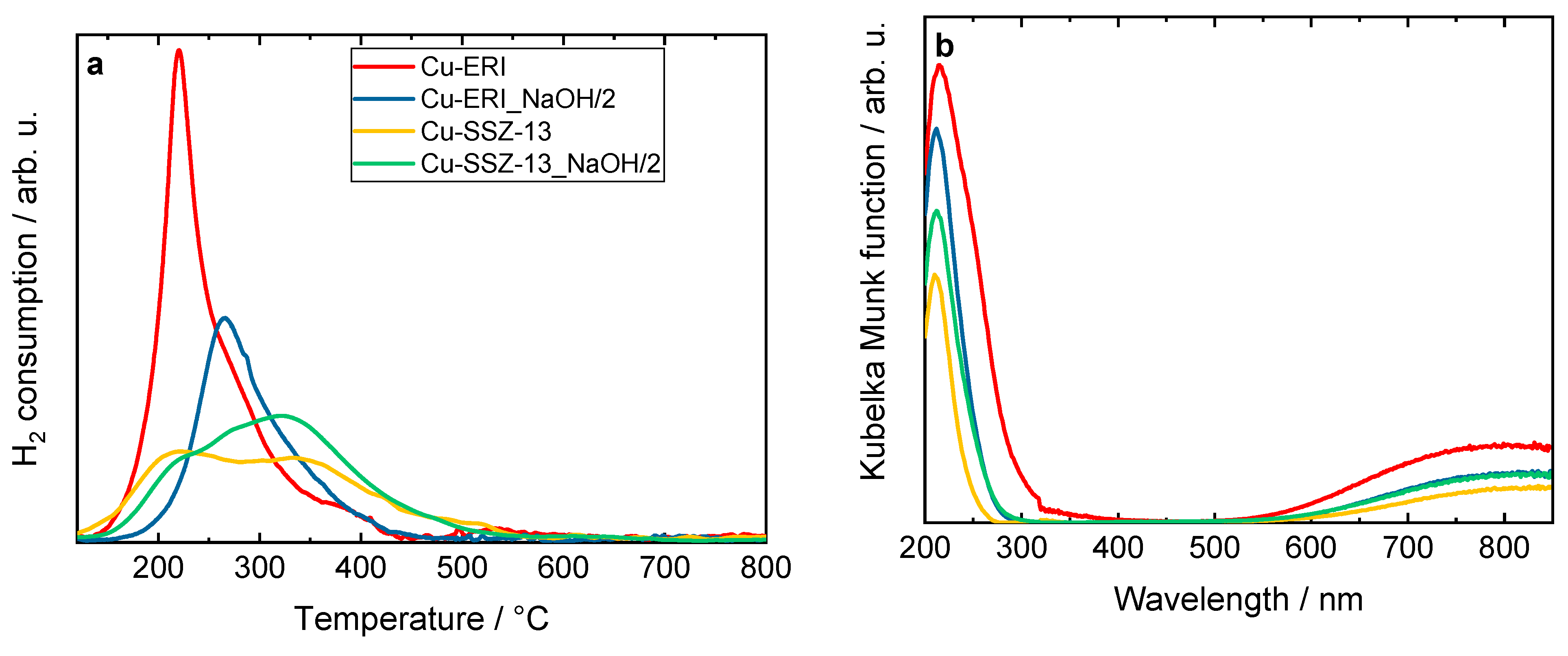
Figure 4.
a) H2-TPR profiles and b) DR UV-Vis spectra of Cu-containing ERI and SSZ-13 samples, a) and b) sample labels are identical.
Figure 4.
a) H2-TPR profiles and b) DR UV-Vis spectra of Cu-containing ERI and SSZ-13 samples, a) and b) sample labels are identical.
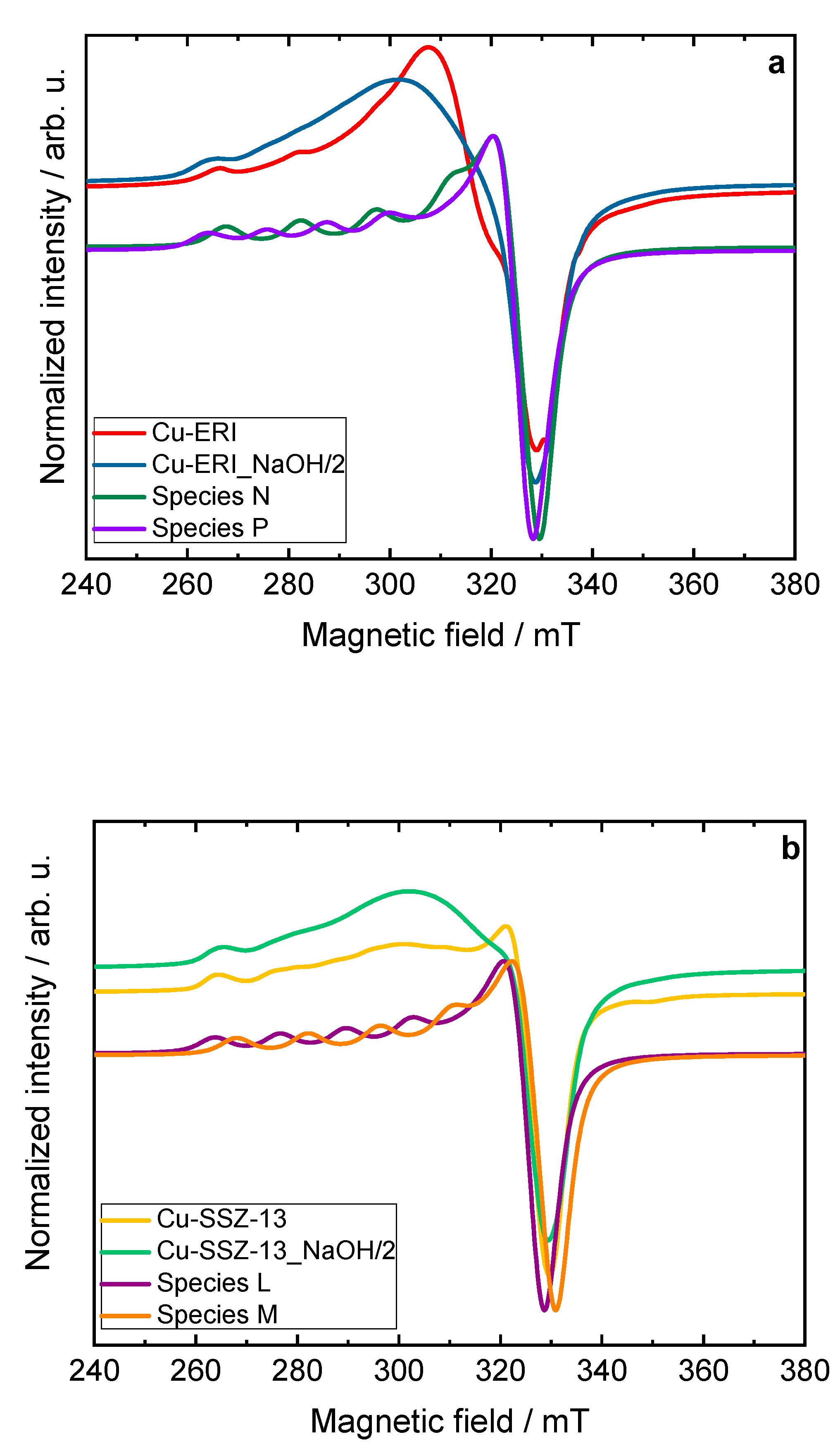
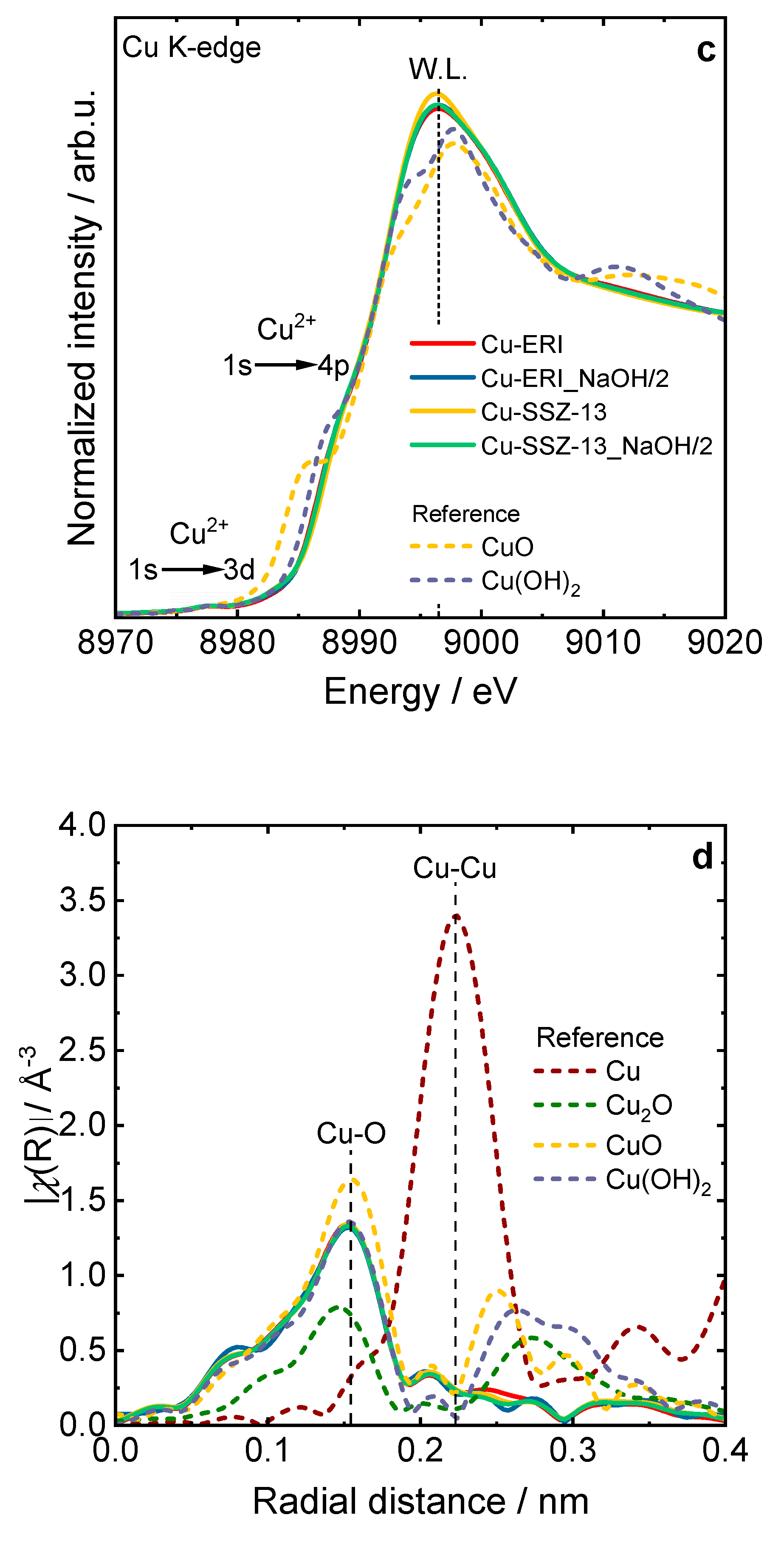
Figure 5.
Experimental and simulated CW-EPR spectra of dehydrated a) Cu-containing ERI and b) SSZ-13 samples at X-band, including the spectral simulation that adds the contribution of their respective species, c) XANES spectra, and d) EXAFS Fourier transforms of the Cu-containing zeolite samples compared with copper references, c) and d) sample labels are identical.
Figure 5.
Experimental and simulated CW-EPR spectra of dehydrated a) Cu-containing ERI and b) SSZ-13 samples at X-band, including the spectral simulation that adds the contribution of their respective species, c) XANES spectra, and d) EXAFS Fourier transforms of the Cu-containing zeolite samples compared with copper references, c) and d) sample labels are identical.
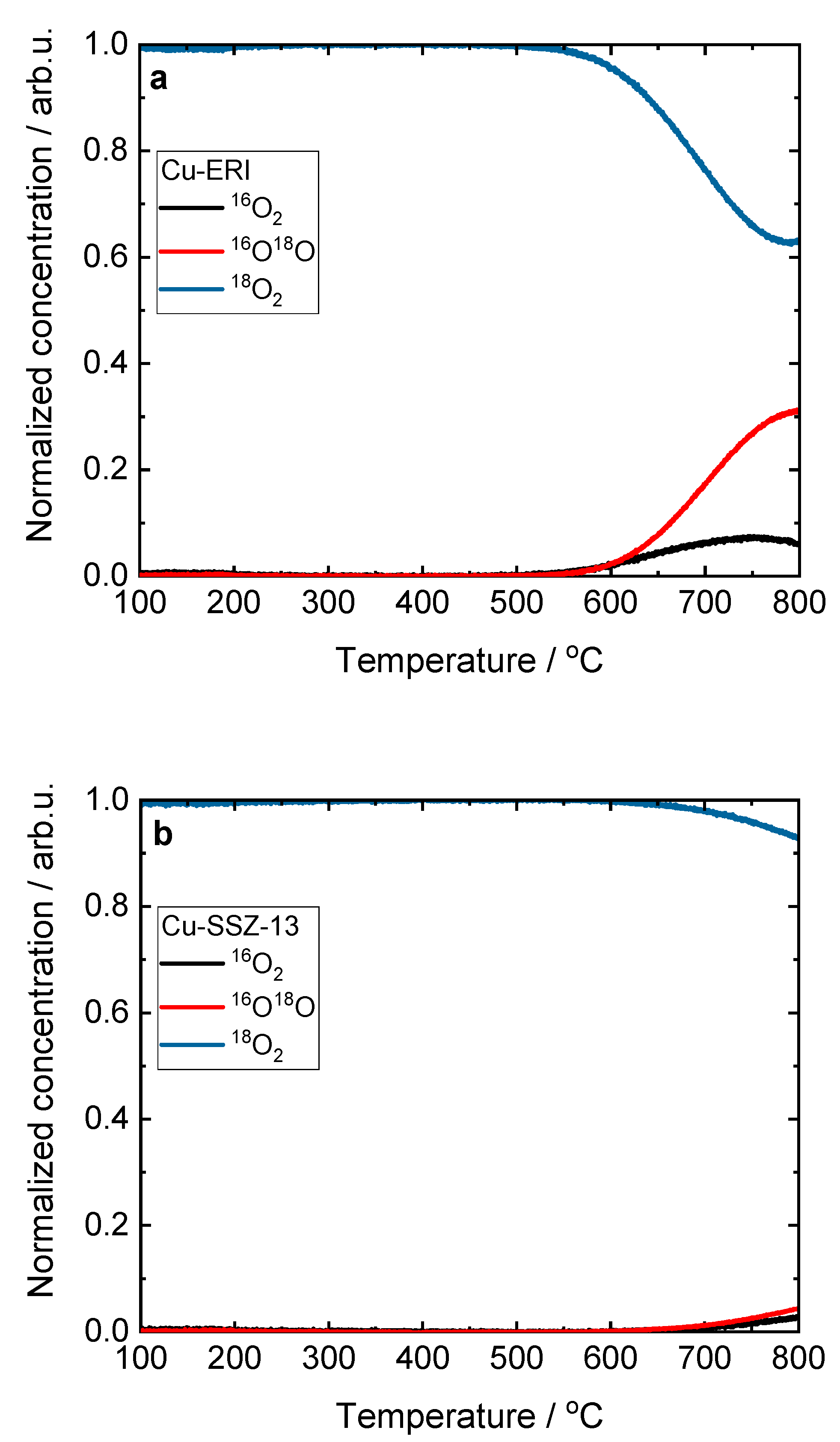
Figure 7.
Results of the TPIE experiment obtained for a) Cu-ERI and b) Cu-SSZ-13.
Figure 7.
Results of the TPIE experiment obtained for a) Cu-ERI and b) Cu-SSZ-13.
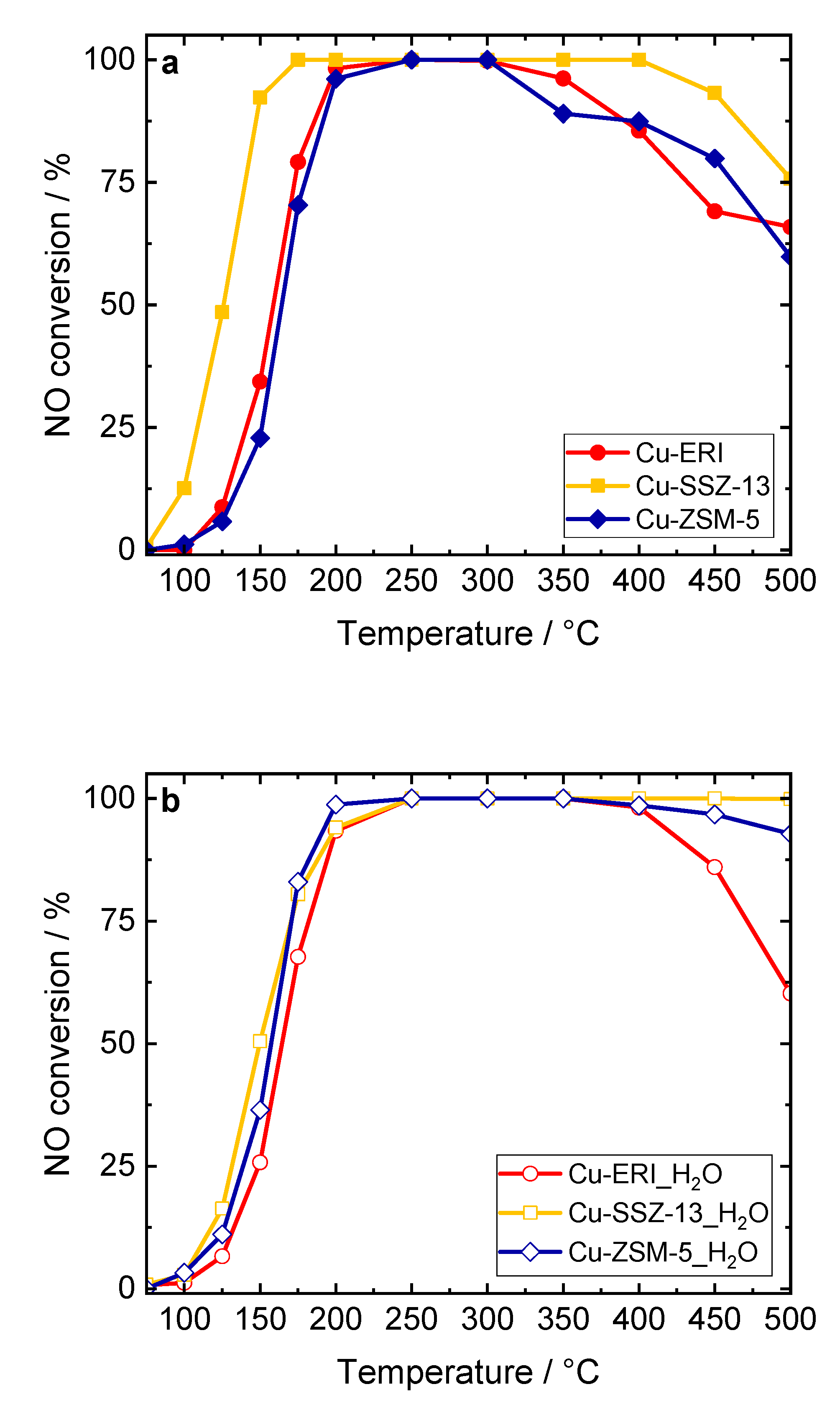
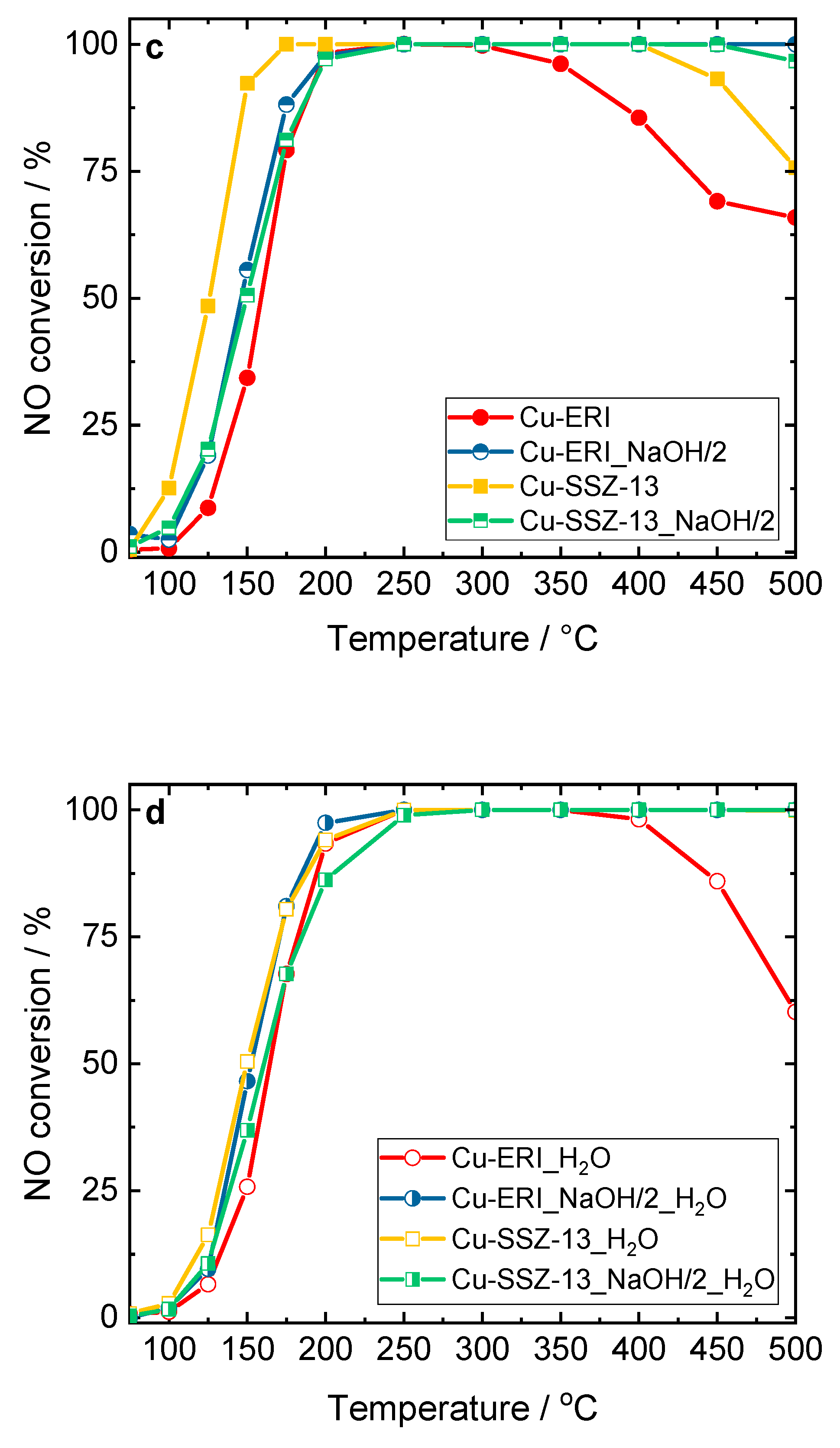
Figure 8.
NO conversion of the Cu-containing ERI, SSZ-13, and ZSM-5 samples: a,c) without H2O in the feed, and b,d) in the presence of H2O in the feed. Reaction conditions: mK = 0.1 g, c(NO) = 0.05 vol.-%, c(NH3) = 0.0575 vol.-%, c(O2) = 4 vol.-%, (c(H2O) = 5 vol.-% when used), He balance, FTOT = 120 ml min-1, GHSV = 30,000 h-1.
Figure 8.
NO conversion of the Cu-containing ERI, SSZ-13, and ZSM-5 samples: a,c) without H2O in the feed, and b,d) in the presence of H2O in the feed. Reaction conditions: mK = 0.1 g, c(NO) = 0.05 vol.-%, c(NH3) = 0.0575 vol.-%, c(O2) = 4 vol.-%, (c(H2O) = 5 vol.-% when used), He balance, FTOT = 120 ml min-1, GHSV = 30,000 h-1.
Figure 9.
In situ FT-IR spectra of a,b) Cu-containing ERI and c,d) Cu-containing SSZ-13 samples, recorded during NH3-SCR-DeNOx at 125 °C. Reaction conditions: mK = 0.1 g, c(NO) = 0.1 vol.-%, c(NH3) = 0.1 vol.-%, c(O2)= 10 vol.-%, He balance, FTOT = 120 ml min-1.
Figure 9.
In situ FT-IR spectra of a,b) Cu-containing ERI and c,d) Cu-containing SSZ-13 samples, recorded during NH3-SCR-DeNOx at 125 °C. Reaction conditions: mK = 0.1 g, c(NO) = 0.1 vol.-%, c(NH3) = 0.1 vol.-%, c(O2)= 10 vol.-%, He balance, FTOT = 120 ml min-1.
Figure 10.
In situ DR UV-Vis DR spectrum of a,b) Cu-containing ERI and c,d) Cu-containing SSZ-13 samples during NH3-SCR-DeNOx at different temperatures. Reaction conditions: mK = 0.1 g, c(NO) = 0.1 vol.-%, c(NH3) = 0.1 vol.-%, c(O2) = 10 vol.-%, He balance, FTOT = 120 ml min-1; a,b) and c,d) sample labels are identical.
Figure 10.
In situ DR UV-Vis DR spectrum of a,b) Cu-containing ERI and c,d) Cu-containing SSZ-13 samples during NH3-SCR-DeNOx at different temperatures. Reaction conditions: mK = 0.1 g, c(NO) = 0.1 vol.-%, c(NH3) = 0.1 vol.-%, c(O2) = 10 vol.-%, He balance, FTOT = 120 ml min-1; a,b) and c,d) sample labels are identical.
Table 1.
Sample labels and treatment conditions.
Table 1.
Sample labels and treatment conditions.
| Sample |
Applied treatment |
| ERI_HNO3_NaOH/0.5_0.5 |
0.3 M HNO3 at 65 °C for 0.5 h followed by 0.2 M NaOH at 65 °C for 0.5 h |
| ERI_HNO3_NaOH/0.5_2 |
0.3 M HNO3 at 65 °C for 0.5 h followed by 0.2 M NaOH at 65 °C for 2 h |
| ERI_NaOH/2 |
0.2 M NaOH at 65 °C for 2 h |
| SSZ-13_NaOH/0.5 |
0.2 M NaOH at 65 °C for 0.5 h |
| SSZ-13_NaOH/2 |
0.2 M NaOH at 65 °C for 2 h |
Table 2.
The elemental analysis results of ERI, SSZ-13 samples and their Cu-containing forms (ωi: mass fractions).
Table 2.
The elemental analysis results of ERI, SSZ-13 samples and their Cu-containing forms (ωi: mass fractions).
| Sample |
ωAl / wt.-% |
ωSi / wt.-% |
ωCu / wt.-% |
n(Si)/n(Al) |
n(Cu)/n(Al) |
| ERI |
8.8 |
31.5 |
- |
3.4 |
- |
| Cu-ERI |
8.2 |
29.9 |
6.1 |
3.5 |
0.3 |
| Cu-ERI_NaOH/2 |
7.9 |
23.7 |
3.7 |
2.9 |
0.2 |
| Cu-ERI_HNO3_NaOH/0.5_0.5 |
7.7 |
25.5 |
3.4 |
3.2 |
0.2 |
| Cu-ERI_HNO3_NaOH/0.5_2 |
7.6 |
21.5 |
3.1 |
2.7 |
0.2 |
| SSZ-13 |
6.1 |
38.3 |
- |
6.0 |
- |
| Cu-SSZ-13 |
5.8 |
36.2 |
4 |
6.0 |
0.3 |
| Cu-SSZ-13_NaOH/0.5 |
6.6 |
24.6 |
3 |
3.6 |
0.2 |
| Cu-SSZ-13_NaOH/2 |
6.7 |
24.2 |
3.3 |
3.5 |
0.2 |
Table 3.
The results of the textural properties of ERI, SSZ-13, and their Cu-containing forms: specific surface area (As(BET)), micropore pore volume (V(MIC)), mesopore pore volume (V(MES)), total pore volume (VTOT) and average pore width (dWp).
Table 3.
The results of the textural properties of ERI, SSZ-13, and their Cu-containing forms: specific surface area (As(BET)), micropore pore volume (V(MIC)), mesopore pore volume (V(MES)), total pore volume (VTOT) and average pore width (dWp).
| Sample |
As(BET) / m2 g-1
|
V(MIC) / cm3 g-1
|
V(MES) / cm3 g-1
|
V(TOT) / cm3 g-1
|
dWp
/ nm |
| ERI |
525 |
0.19 |
0.04 |
0.24 |
2.7 |
| Cu-ERI |
396 |
0.14 |
0.04 |
0.18 |
2.6 |
| Cu-ERI_HNO3_NaOH/0.5_0.5 |
466 |
0.17 |
0.10 |
0.27 |
3.9 |
| Cu-ERI_HNO3_NaOH/0.5_2 |
479 |
0.17 |
0.09 |
0.28 |
4.0 |
| Cu-ERI_NaOH/2 |
467 |
0.18 |
0.05 |
0.23 |
2.7 |
| SSZ-13 |
716 |
0.26 |
0.06 |
0.32 |
4.0 |
| Cu-SSZ-13 |
672 |
0.25 |
0.03 |
0.28 |
3.2 |
| Cu-SSZ-13_NaOH/0.5 |
526 |
0.14 |
0.23 |
0.37 |
4.9 |
| Cu-SSZ-13_NaOH/2 |
560 |
0.13 |
0.26 |
0.39 |
4.8 |