Submitted:
18 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
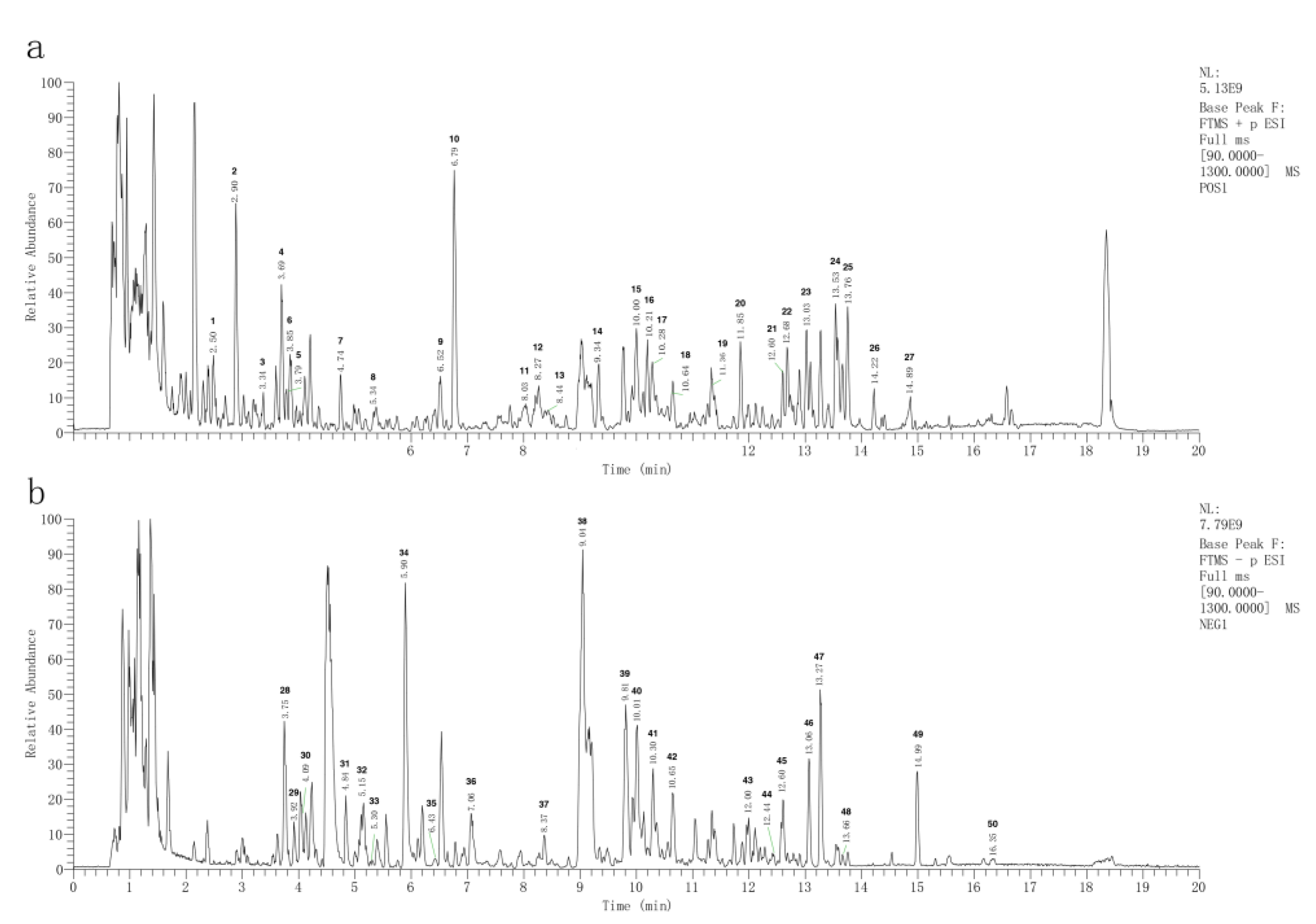
2.1. Main Compounds in CF Extract
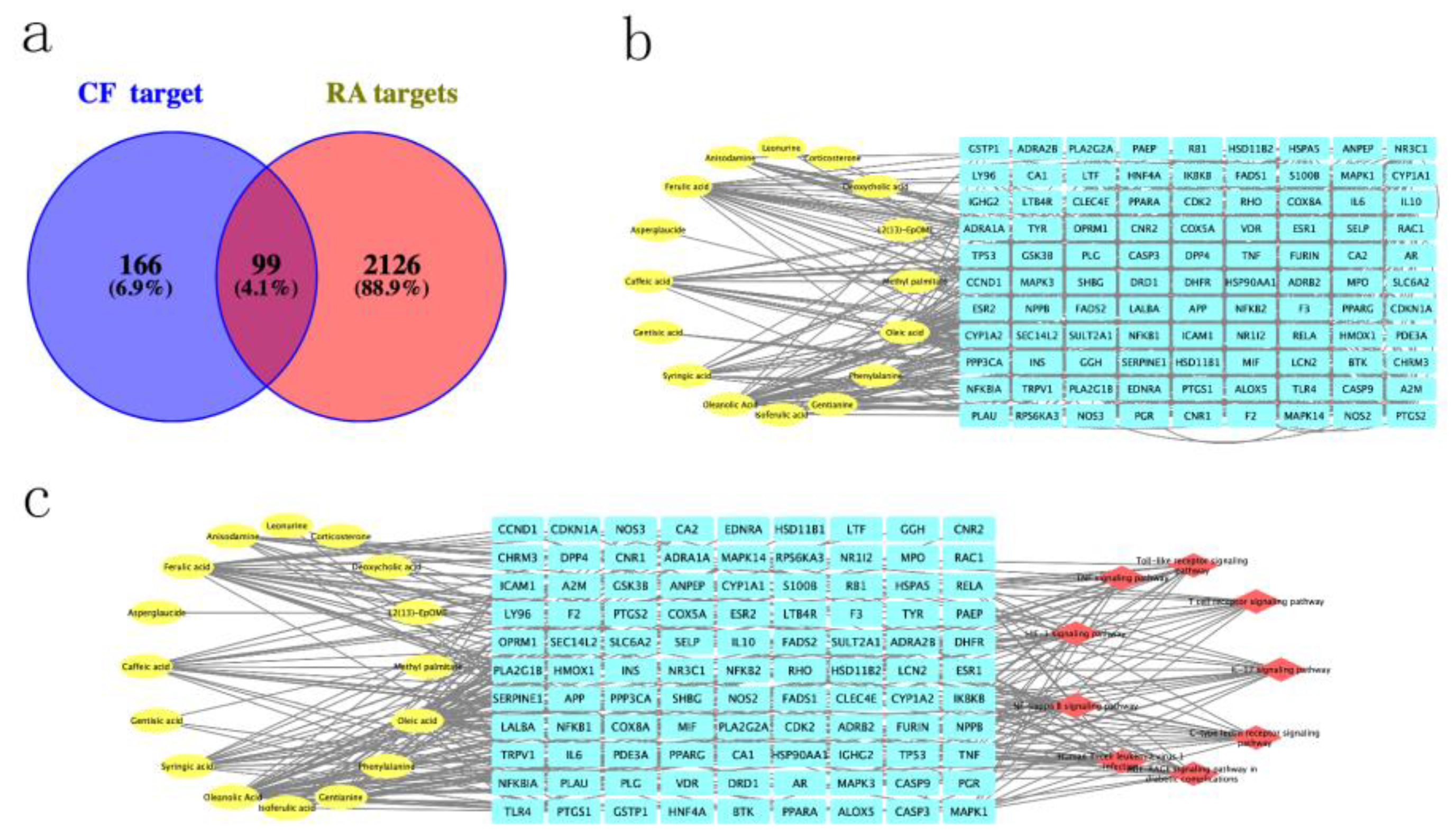
2.2. Construction of CT Network and Screening Core Compounds in CF
2.3. Screening of Main Targets
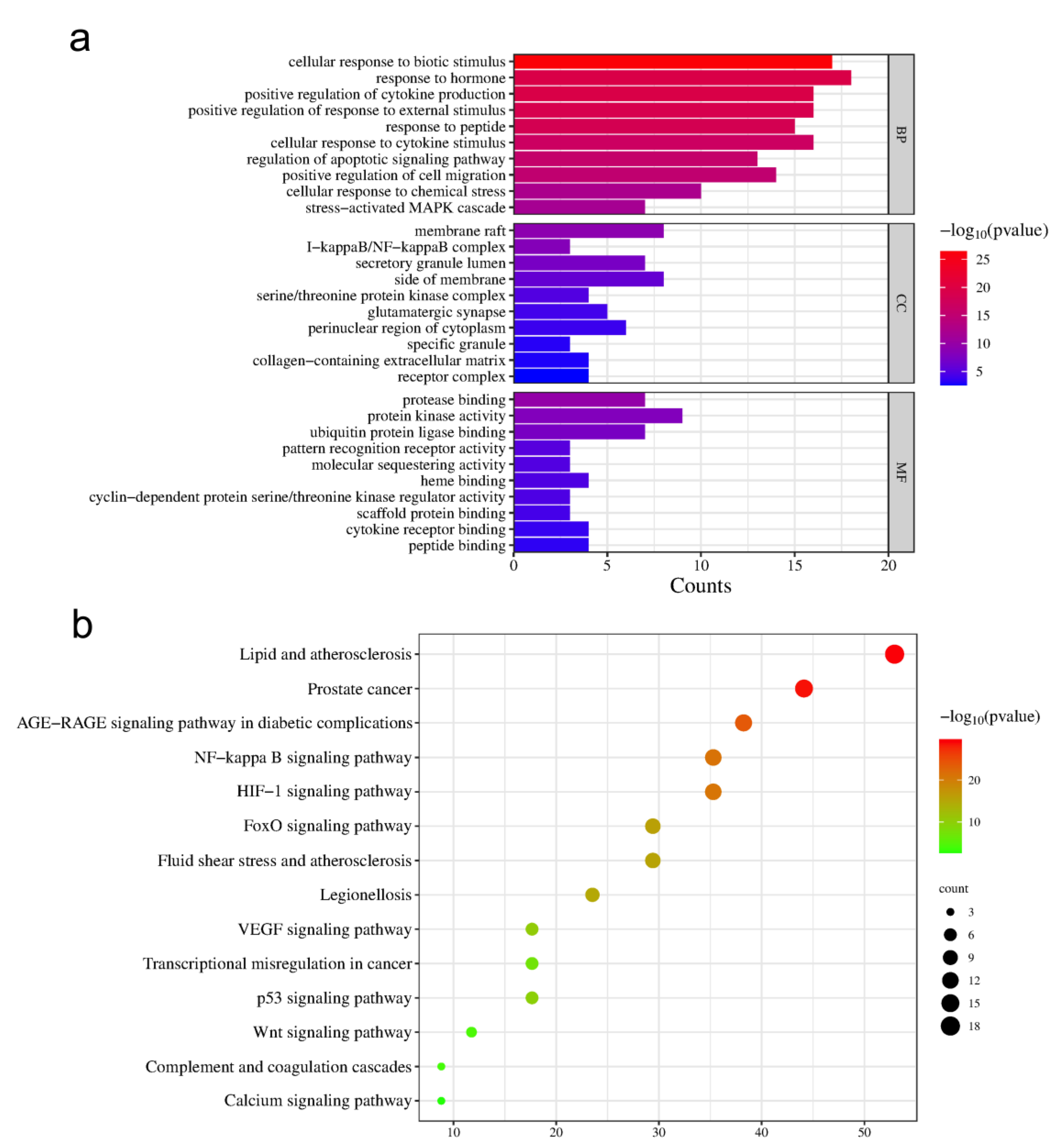
2.4. GO and KEGG Pathway Enrichment Analysis
2.5. Construction of CPS Network and Screening of Core Targets
2.6. Molecular Docking Effectiveness
2.7. HPLC Measurement of Quality Control Substance Oleanolic Acid Content
2.8. CF Alleviated Xylene-Induced Mouse Ear Edema
2.9. CF Showed Analgesic Activity in Acetic Acid-Induced Writhing Model
2.10. CF Alleviated the Severity of Arthritis in Rats
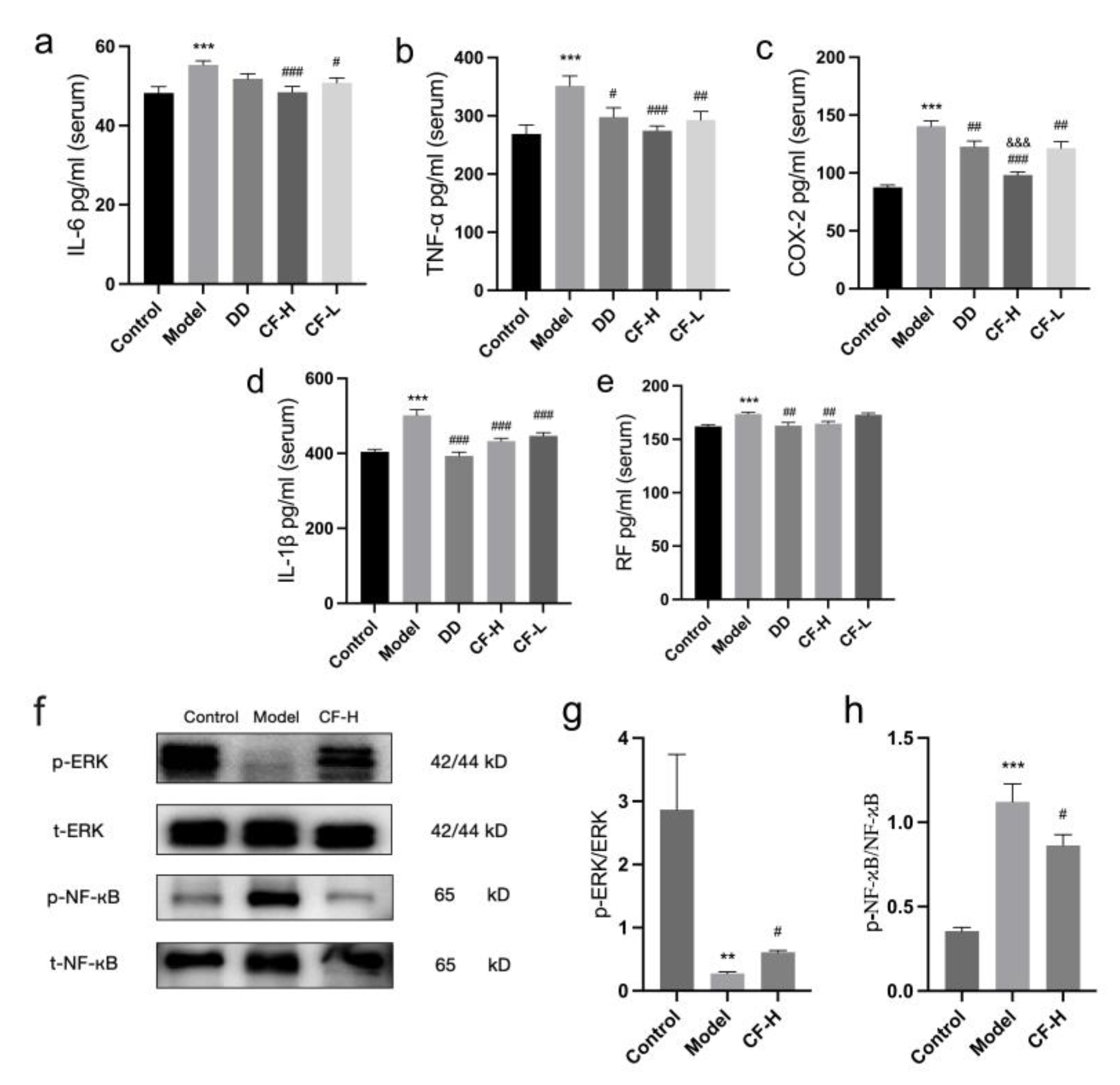
2.11. Effects of CF Extracts on Serum IL-6, COX-2, TNF-α, IL-1β and RF in RA Rats
2.12. Protein Expression of p-ERK, ERK, p-NF-κB and NF-κB in Rats
2.13. Histological Analysis of Rats
3. Discussion
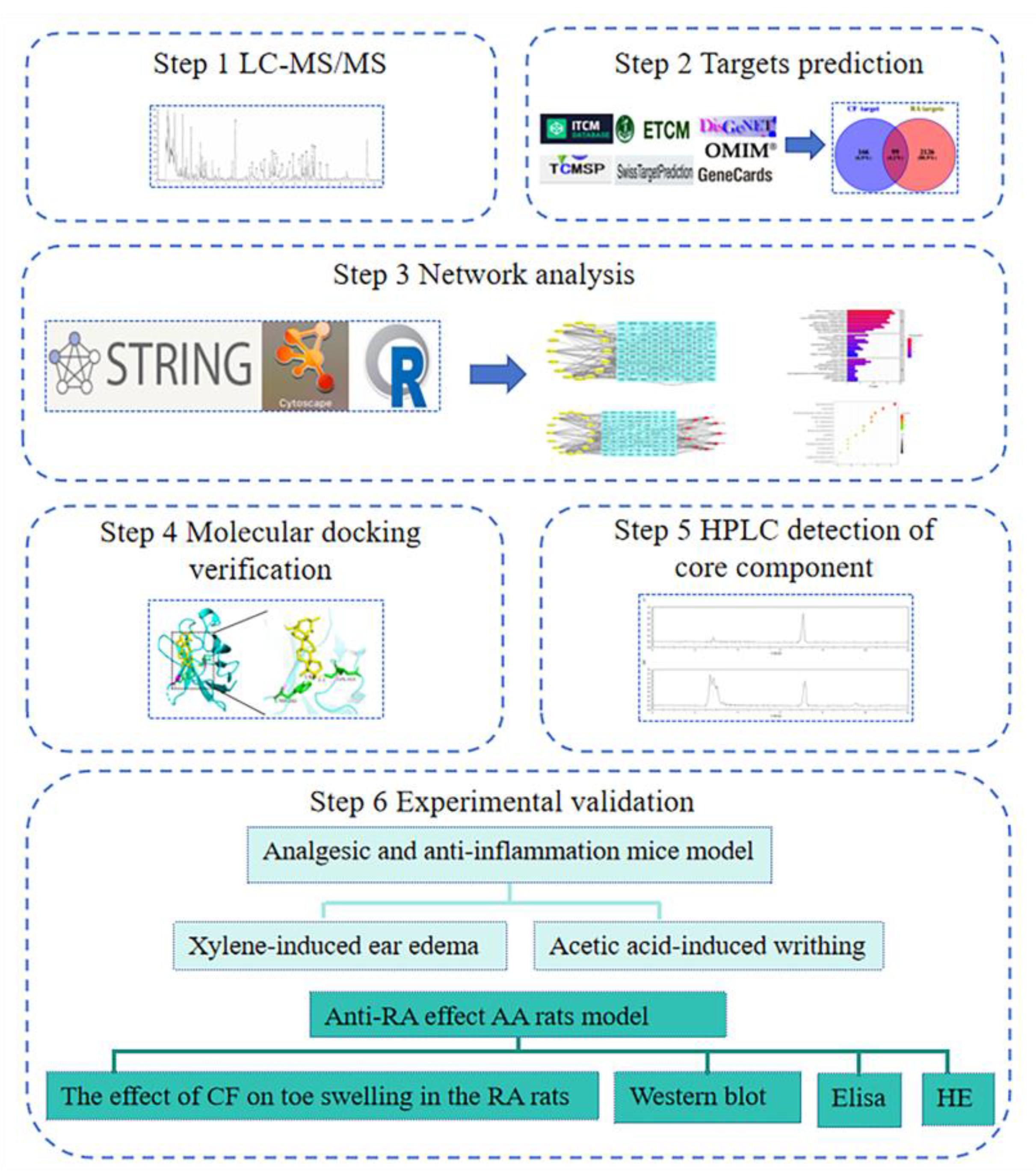
4. Materials and Methods
4.1. LC-MS/MS Analysis
4.1.1. Sample Preparation
4.1.2. Instruments and Analytical Conditions
4.1.3. Screening of Main Compounds of CF
4.2. Network Pharmacology Analysis
4.2.1. Candidate Therapeutic Targets of CF Screening
4.2.2. Construction of RA-Related Target Database
4.2.3. Intersection between Main Compounds and Disease Targets
4.2.4. Construction of CT Network
4.2.5. CPS Network Construction and Core Target Acquisition
4.2.6. GO and KEGG Enrichment Analysis
4.2.7. Molecular Docking
4.3. Quality Control of CF Extract
4.3.1. Sample and Standard Solution Preparation
4.3.2. Instruments and Analytical Conditions
4.4. Experimental Validation
4.4.1. Animals
4.4.2. Preparation of the CF Extract
4.4.3. Xylene-Induced Mouse Ear Edema
4.4.4. Acetic Acid-Induced Abdominal Writhing Response
4.4.5. AA Model Preparation
4.4.6. Experimental Grouping and Drug Administration
4.4.7. Detection the Degree of Toe Swelling in Rats
4.4.8. Determination of Serum Inflammatory Factor
4.4.9. Hematoxylin and Eosin Staining
4.4.10. Western Blotting
4.5. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat Rev Dis Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Singh, R.; Jadhav, K.; Vaghasiya, K.; Ray, E.; Shukla, R.; Verma, R.K. New Generation Smart Drug Delivery Systems for Rheumatoid Arthritis. Curr Pharm Des 2023, 29, 984–1001. [Google Scholar] [CrossRef]
- Mandal, A.K.; Sahoo, A.; Dwivedi, K.; Singh, R.; Kumar, V. Potential Therapeutic Application of Biophenols - Plants Secondary Metabolites in Rheumatoid Arthritis. Crit Rev Food Sci Nutr 2023, 63, 8900–8918. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Wilson, A.G.; Barton, A. DNA Methylation as a Marker of Response in Rheumatoid Arthritis. Pharmacogenomics 2017, 18, 1323–1332. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An Overview of Autoantibodies in Rheumatoid Arthritis. J Autoimmun 2020, 110, 102392. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, H.; Chen, G.; Huang, G. Synthesis and Biological Activities of Chemical Drugs for the Treatment of Rheumatoid Arthritis. Top Curr Chem (Cham) 2019, 377, 28. [Google Scholar] [CrossRef] [PubMed]
- Anita, C.; Munira, M.; Mural, Q.; Shaily, L. Topical Nanocarriers for Management of Rheumatoid Arthritis: A Review. Biomed Pharmacother 2021, 141, 111880. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Yang, F.; Zhu, F.; Hua, D. Effect of External Use of Qingluo San on Clinical Efficacy in Patients with Acute Gouty Arthritis. Eur J Med Res 2022, 27, 245. [Google Scholar] [CrossRef]
- Huang, C.; Chen,Yichong; Dong, R.; Yan, Y.; Wu, J.; Fang, zhi; Chen, J.; Shen, C.; Han, J.; Shen, T. Preliminary Study on Formulation Process Optimization and Pharmacody-Namics of She Medicine Clematis Florida Var. Plena Cream. Strait Pharmaceutical Journal 2023, 35, 10–16.
- Lin, T.-F.; Wang, L.; Zhang, Y.; Zhang, J.-H.; Zhou, D.-Y.; Fang, F.; Liu, L.; Liu, B.; Jiang, Y.-Y. Uses, Chemical Compositions, Pharmacological Activities and Toxicology of Clematidis Radix et Rhizome- a Review. J Ethnopharmacol 2021, 270, 113831. [Google Scholar] [CrossRef]
- Yang, N.-N.; Zhang, Y.-F.; Zhang, H.-T.; Ma, X.-H.; Shen, J.-H.; Li, P.; Zhong, T.-H.; Zhang, Y.-H. The in Vitro and in Vivo Anti-Inflammatory Activities of Triterpene Saponins from Clematis Florida. Nat Prod Res 2021, 35, 6180–6183. [Google Scholar] [CrossRef]
- Zhu, F.-R.; Li, Y.-N.; He, S.-L.; Chen, Q.-S.; Xu, X.-Y. Cytotoxic Activities of Total Saponins from Plena Clematis on Human Tumor Cell Lines In Vitro. Chin J Integr Med 2018, 24, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network Pharmacology. Nat Biotechnol 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network Pharmacology, a Promising Approach to Reveal the Pharmacology Mechanism of Chinese Medicine Formula. J Ethnopharmacol 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Liao, J.; Chen, Q.; Lu, X.; Fan, X. Network Pharmacology Approaches for Research of Traditional Chinese Medicines. Chin J Nat Med 2023, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.-H.; Ma, X.-H.; Zeng, X.-M.; Lin, Z.-Y.; Cai, Y.-M.; Zhang, H.-T.; Lin, X.-Y.; Feng, S.-B.; Zhong, T.-H.; Zhang, Y.-H. A New Indole-Type Alkaloid from the Roots of Clematis Florida Var. Plena. Nat Prod Res 2019, 33, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential Therapeutic Implications of Caffeic Acid in Cancer Signaling: Past, Present, and Future. Front Pharmacol 2022, 13, 845871. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Matsabisa, M.G.; Ibrahim, M.A.; Erukainure, O.L.; Chabalala, M.H.; Islam, M.S. Medicinal Plants with Concomitant Anti-Diabetic and Anti-Hypertensive Effects as Potential Sources of Dual Acting Therapies against Diabetes and Hypertension: A Review. J Ethnopharmacol 2019, 235, 329–360. [Google Scholar] [CrossRef]
- Kassa, T.; Whalin, J.G.; Richards, M.P.; Alayash, A.I. Caffeic Acid: An Antioxidant with Novel Antisickling Properties. FEBS Open Bio 2021, 11, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic Acid and Ellagic Acid Ameliorate Adjuvant-Induced Arthritis in Rats via Targeting Inflammatory Signals, Chitinase-3-like Protein-1 and Angiogenesis. Biomed Pharmacother 2019, 110, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Elamine, Y.; Lyoussi, B.; Miguel, M.G.; Anjos, O.; Estevinho, L.; Alaiz, M.; Girón-Calle, J.; Martín, J.; Vioque, J. Physicochemical Characteristics and Antiproliferative and Antioxidant Activities of Moroccan Zantaz Honey Rich in Methyl Syringate. Food Chem 2021, 339, 128098. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Chen, L.; Li, Y.; Zhao, S.; Liang, Y. Chemoprotective Effect of Syringic Acid on Cyclophosphamide Induced Ovarian Damage via Inflammatory Pathway. J Oleo Sci 2021, 70, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-Inflammatory Agents: An Update. Curr Med Chem 2021, 28, 3622–3646. [Google Scholar] [CrossRef] [PubMed]
- Creamer, P. Osteoarthritis Pain and Its Treatment. Curr Opin Rheumatol 2000, 12, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A Panoramic Review of IL-6: Structure, Pathophysiological Roles and Inhibitors. Bioorg Med Chem 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Vanamee, É.S.; Faustman, D.L. The Benefits of Clustering in TNF Receptor Superfamily Signaling. Front Immunol 2023, 14, 1225704. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front Immunol 2019, 10, 2925. [Google Scholar] [CrossRef]
- Li, J.; Tang, R.-S.; Shi, Z.; Li, J.-Q. Nuclear Factor-κB in Rheumatoid Arthritis. Int J Rheum Dis 2020, 23, 1627–1635. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Ya-nan, L.; Juan, N; Yu-shun, F; Tao, L; Hong-fei, T; Qing-song, Z Effects of Curcumin on Apoptosis and Proliferation of Inflammatory Chondrocytes Induced by Lipopolysaccharide. Chinese Journal of Tissue Engineering Research 2018, 22, 5157–5162.
- Xu, T.; Gu, J.; Li, C.; Guo, X.; Tu, J.; Zhang, D.; Sun, W.; Kong, X. Low-Intensity Pulsed Ultrasound Suppresses Proliferation and Promotes Apoptosis via P38 MAPK Signaling in Rat Visceral Preadipocytes. Am J Transl Res 2018, 10, 948–956.
- Ling, L.; Wei, T.; He, L.; Wang, Y.; Wang, Y.; Feng, X.; Zhang, W.; Xiong, Z. Low-Intensity Pulsed Ultrasound Activates ERK1/2 and PI3K-Akt Signalling Pathways and Promotes the Proliferation of Human Amnion-Derived Mesenchymal Stem Cells. Cell Prolif 2017, 50, e12383. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-J.; Liu, D.; Li, Y.-S.; Tian, J.; Yu, D.-J.; Li, H.-Z.; Zhang, F.-J. OPN Inhibits Autophagy through CD44, Integrin and the MAPK Pathway in Osteoarthritic Chondrocytes. Front Endocrinol (Lausanne) 2022, 13, 919366. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Liu, Y.; Wu, H.; He, Y.; Li, C.; Wang, Q.; Su, X.; Yan, S.; Su, W.; et al. A Novel Drug Combination of Mangiferin and Cinnamic Acid Alleviates Rheumatoid Arthritis by Inhibiting TLR4/NFκB/NLRP3 Activation-Induced Pyroptosis. Front Immunol 2022, 13, 912933. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, L.; Wu, J.; Zhou, X.; Li, X.; Sun, X.; Zhu, L.; Xia, T.-S.; Ding, Q. A Hederagenin Saponin Isolated from Clematis Ganpiniana Induces Apoptosis in Breast Cancer Cells via the Mitochondrial Pathway. Oncol Lett 2018, 15, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Cheng, T.-F.; Jia, Y.-R.; Li, P.; Li, F. Anti-Rheumatoid Arthritis Effects of Traditional Chinese Herb Couple in Adjuvant-Induced Arthritis in Rats. J Ethnopharmacol 2017, 205, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C; Li,X; Jie,H; Han,J; He,Z; Hu,J Experimental Study on the Acute Toxicity of the Decoction of the Clematis Florida Var. Plena D.Don. Chinese Journal of Ethnomedicine and Ethnopharmacy 2017, 26, 27–34.
- Zhou, C; Zhao,L; Jiang ,C; Hu,J Study on the Toxicity of Ethanolic Extracts from Clematis Florida Thunb. Var. Plena DE. Don Ointment by Skin Administration. Chinese Medical Sciences Journal 2020, 10, 69–71.
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Clares, B. Enhancing Topical Analgesic Administration: Review and Prospect for Transdermal and Transbuccal Drug Delivery Systems. Curr Pharm Des 2015, 21, 2867–2882. [Google Scholar] [CrossRef]
- Commission, N.P. Pharmacopoeia of the People’s Republic of China. China Medical Science Press, Beijing 2020; ISBN 978-7-5214-1574-2.
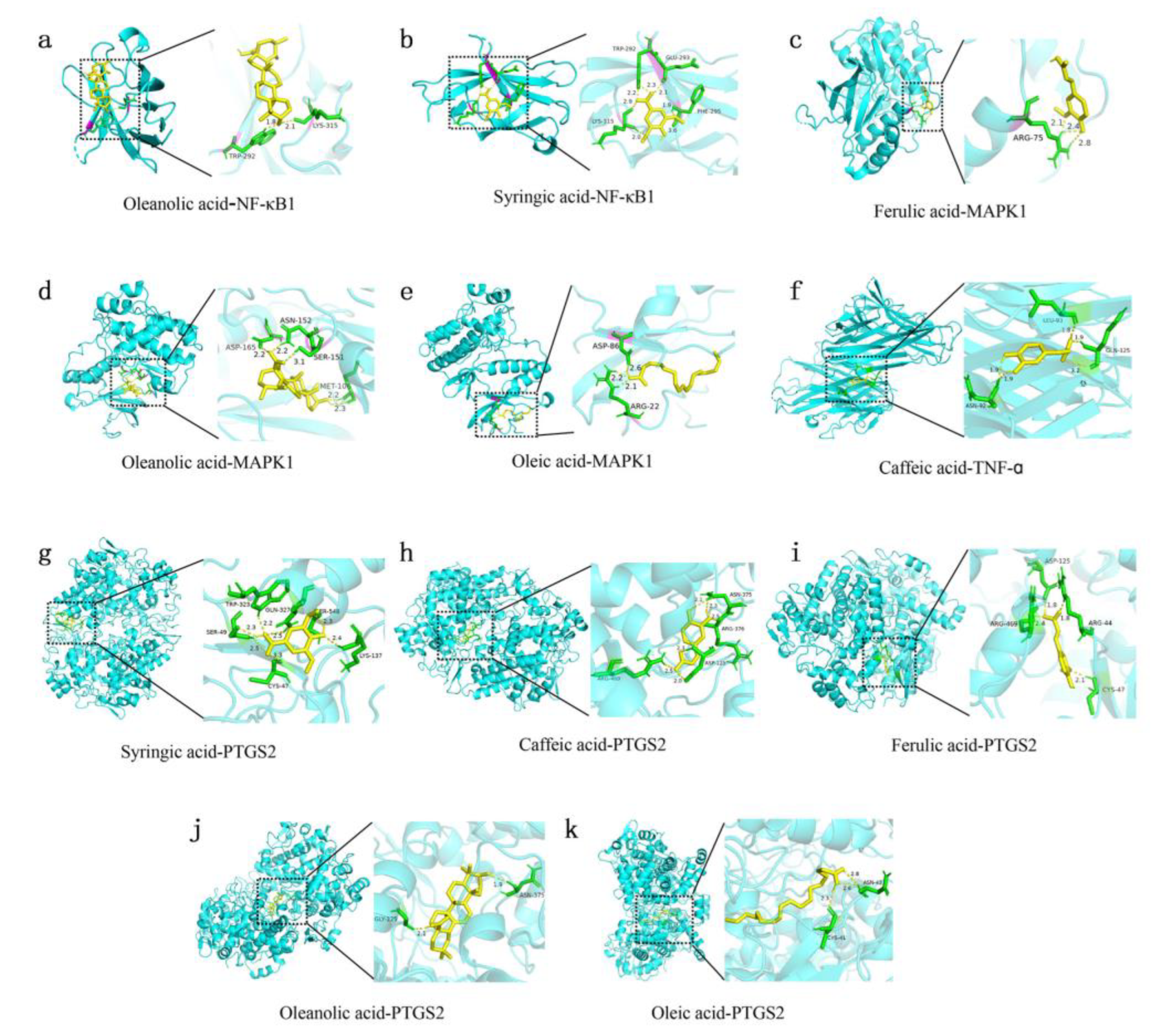
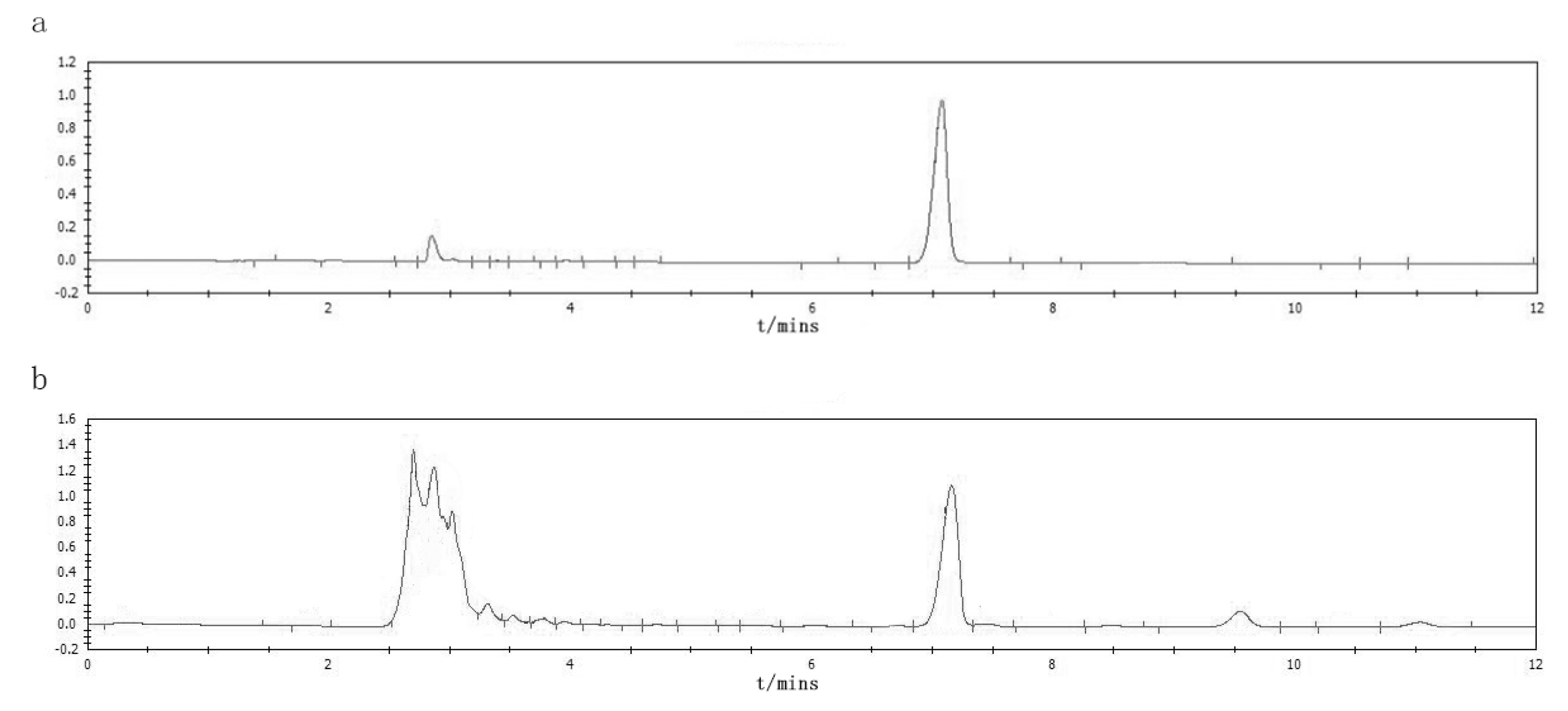
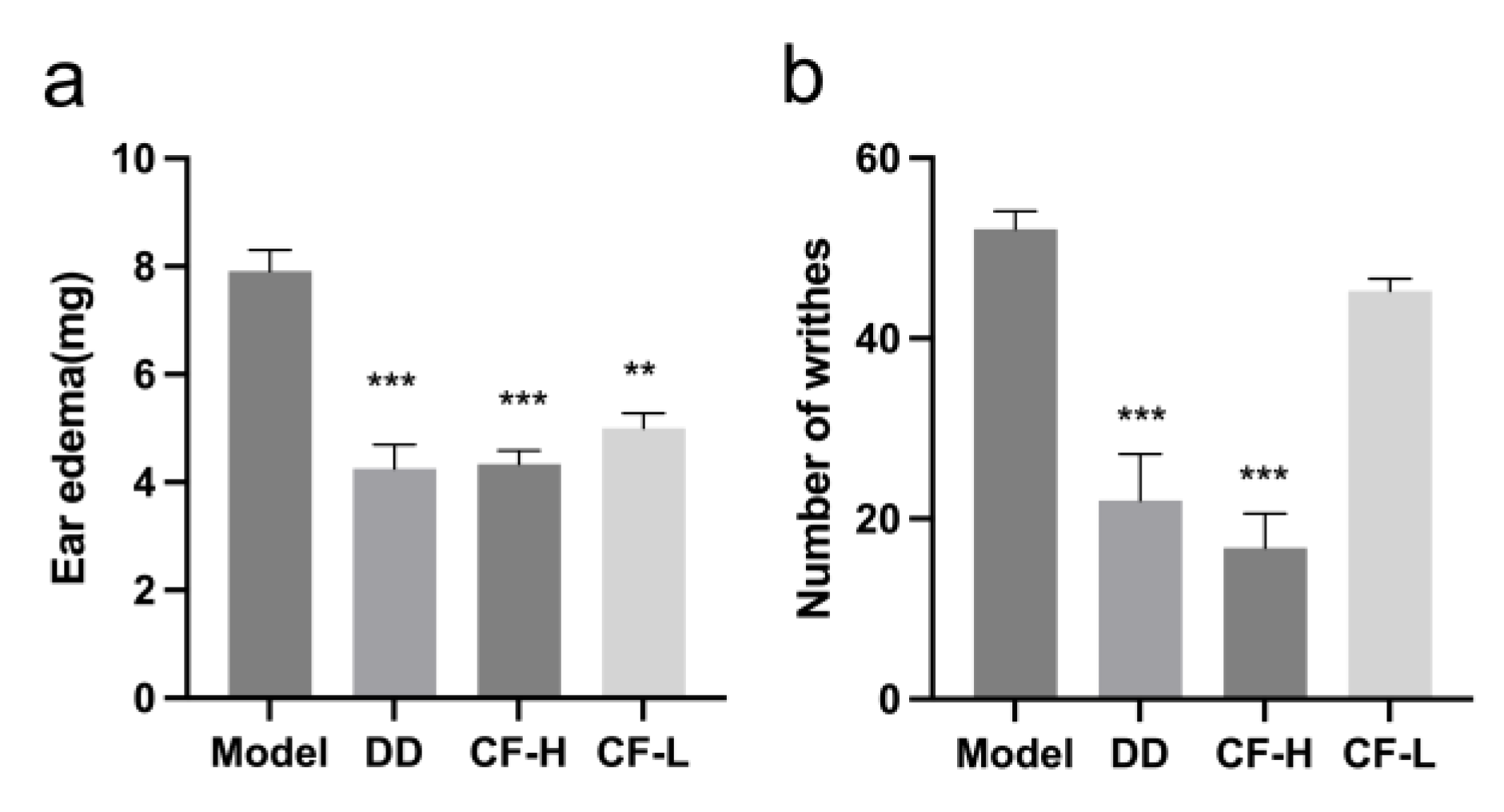
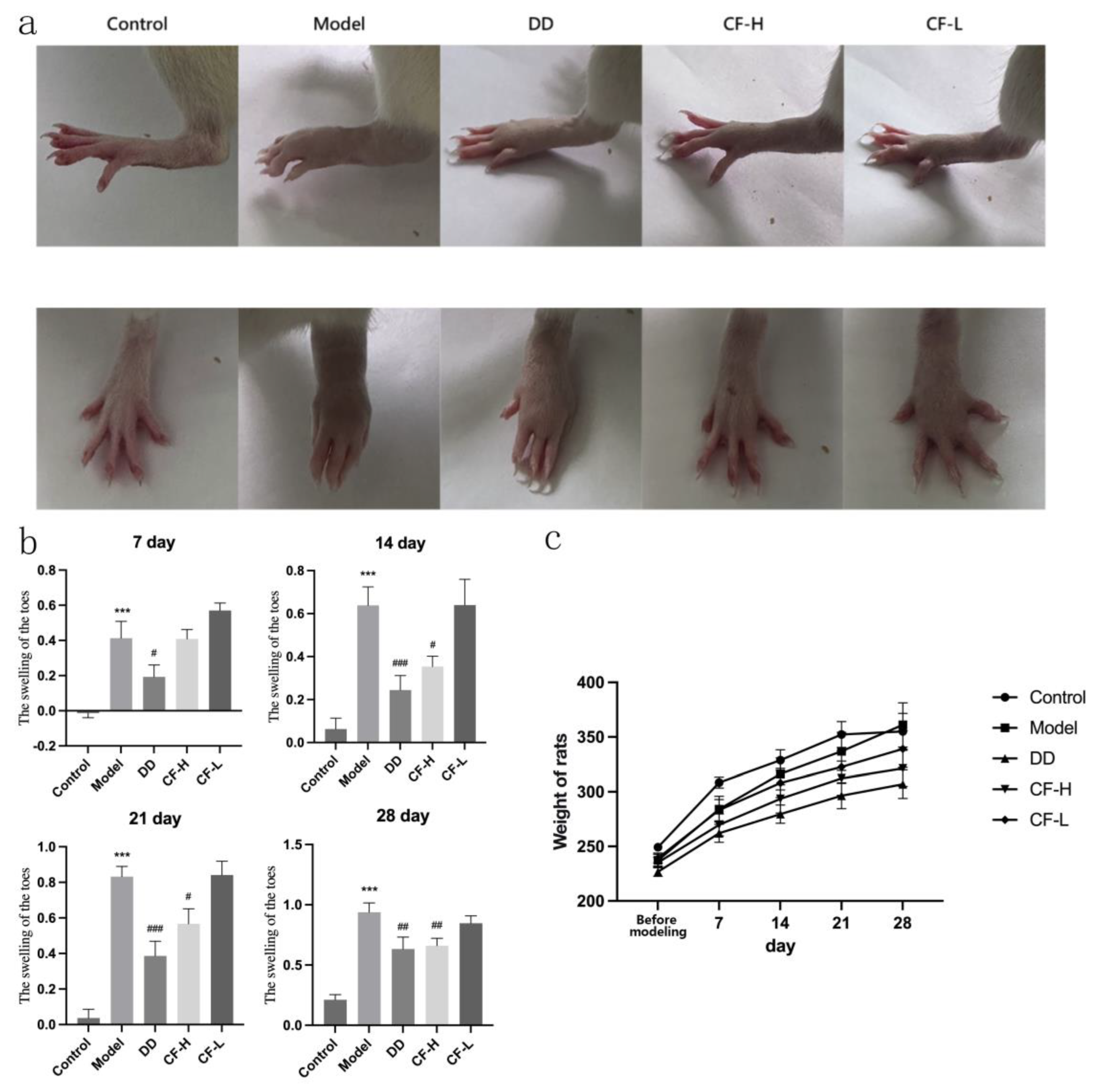

| Pathway | Target |
|---|---|
| IL-17 signaling pathway | LCN2, GSK3B, IL6, NF-κB1, TNF, PTGS2, CASP3, HSP90AA1, MAKP1, IKBKB, MAPK3, MAPK14, NF-κBIA, RELA |
| C-type lectin receptor signaling pathway | NF-κB1, IKBKB, NF-κBIA, RELA, PTGS2, PPP3CA, MAPK14, MAPK3, MAPK1, TNF, IL6, IL10, NF-κB2, CLEC4E |
| AGE-RAGE signaling pathway in diabetic complications | ICAM1, NOS3, F3, TNF, IL6, NF-κB1, SERPINE1, MAPK3, CASP3, RELA, MAPK1, CCND1, MAPK14 |
| Human T-cell leukemia virus 1 infection | CDK2, RB1, CCND1, CDKN1A, MAPK3, PPP3CA, TP53, NFKBIA, NF-κB1, TNF, MAPK1, RELA, IL6, IKBKB, NF-κB2, ICAM1 |
| NF-kappa B signaling pathway | PLAU, ICAM1, PTGS2, RELA, TNF, NF-κB1, NF-κB2, IKBKB, TLR4, NF-κBIA, LY96, BTK |
| HIF-1 signaling pathway | NOS2, NOS3, NF-κB1, INS, CDKN1A, SERPINE1, HMOX1, TLR4 MAPK3, MAPK1, RELA, IL6 |
| TNF signaling pathway | TNF, IL6, RELA, NF-κB1, CASP3, IKBKB, PTGS2, MAPK1, MAPK3 , ICAM1, MAPK14, NF-κBIA |
| Toll-like receptor signaling pathway | TNF, NF-κB1, LY96, TLR4, RELA, MAPK1, MAPK3, MAPK14, IL6, IKBKB, NF-κBIA |
| T cell receptor signaling pathway | IL10, IKBKB, TNF, NF-κB1, NF-κBIA, GSK3B, MAPK14, RELA, MAPK1, MAPK3, PPP3CA |
| Target | Full Name of Target | Target Alias | Degree |
|---|---|---|---|
| PTGS2 | 2Prostaglandin G/H Synthase 2 | COX-2 | 15 |
| MAPK1 | Mitogen-Activated Protein Kinase 1 | ERK2 | 11 |
| NFκB1 | Nuclear factor kappa-B | P50 | 11 |
| TNF | Tumor necrosis factor | TNF-alpha | 11 |
| RELA | V-rel reticuloendotheliosis viral oncogene homolog A | NFκB P65 | 11 |
| MAPK3 | Mitogen-activated protein kinase 3 | ERK1 | 9 |
| PTGS1 | Prostaglandin-endoperoxide synthase 1 | COX-1 | 9 |
| IL6 | Interleukin 6 | HGF | 9 |
| NFκBIA | NF-Kappa-B Inhibitor Alpha Antibody | IKBA | 8 |
| IKBKB | Inhibitor of nuclear factor kappa-B kinase subunit beta | IKK2 | 8 |
| MAPK14 | Recombinant Human Mitogen-Activated Protein Kinase 14 | CSPS | 8 |
| ADRB2 | beta-2 adrenergic receptor | ADRB2R | 8 |
| Target | PDB ID | Compound | Affinity/(kcal.mol-1) |
|---|---|---|---|
| NF-κB1 | 1U36 | Oleanolic acid | -6.6 |
| NF-κB1 | 1U36 | Syringic acid | -3.87 |
| MAPK1 | 4S31 | Ferulic acid | -4.57 |
| MAPK1 | 4S31 | Oleanolic acid | -8.14 |
| MAPK1 | 4S31 | Oleic acid | -3.15 |
| TNF-ɑ | 2AZ5 | Caffeic acid | -5.37 |
| PTGTS2 | 5F19 | Syringic acid | -5.6 |
| PTGTS2 | 5F19 | Caffeic acid | -5.83 |
| PTGTS2 | 5F19 | Ferulic acid | -5.2 |
| PTGTS2 | 5F19 | Oleanolic Acid | -10.31 |
| PTGTS2 | 5F19 | Oleic acid | -5.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





