2. Case Description
2.1. History, Clinical Examination, Laboratory Analysis and Initial Management
An 8-year-old, 5.0 kg, entire male domestic shorthair cat presented to the emergency service at AniCura Djursjukhuset, Hässleholm with acute onset of dyspnoea. The cat’s medical history prior to adoption 3-years prior had not been known. The patient had had a long past medical history from different veterinary clinics and tested positive result on FIV antibody testing and underwent resection of the nasal plane. The patient’s medications at the time of presentation included artificial tear supplementation (Aptus SentrX Eye Drops, Orion Pharma) and gastrointestinal diet (Gastrointestinal, Royal Canin). Clinical examination revealed severely reduced demeanour, body temperature of 38,2 C and severe dyspnoea with paradoxical respiration and obvious abdominal ‘push’. Thoracic auscultation revealed bilateral crackles over the lung fields and heart rate of 200 bpm audible murmur or gallop or dysrhythmia. Haematological analysis showed mild anaemia (5.04 x 1012/L; ref 6.54-12.20 x 1012/L), mild neutrophilia (11.66 x 109/L; re: 2.30-10.23 x 109/L) and eosinopenia (0.14 x 109/L; ref: 0.17-1.57 x 109/L). Biochemical analysis revealed mild reduction in ALT (35 U/L; ref: 40-158 U/L), mild elevation in creatinine (168 umol/L; ref: 70-160 umol/L) and borderline low albumin concentrations (28 g/L; ref: 28-35 g/L). Subsequent biochemical analyses revealed further reduction in albumin concentration (25 g/L on day 2 and day 6 post-admission and 22 g/L on day 9 post-admission) with high normal total protein (80 g/L on day 2 post-admission; ref: 57-80 g/L). Neither protein electrophoresis nor urinalysis were performed at any point.
The cat received butorphanol 0.4 mg/kg i.m. (Butomidor Vet 10 mg/ml, Salfarm Scandinavia AB) for sedative purposes and underwent thoracic radiographic examination. The thoracic radiographs showed bilateral pleural effusion and subsequent thoracentesis yielded 147 ml of fluid from the right and 50 ml of fluid from the left hemithorax (the macroscopic character of the fluid not recorded in the clinical notes). The respiratory rate reduced from 64/min before to 40/min after thoracentesis. The cat received furosemide 1.0 mg/kg i.v. (Furix Injektionsvätska 10 mg/ml, Orifarm Generics AB), was placed in an oxygen cage and started on butorphanol CRI at 0.5 mg/kg/h (Butomidor Vet 10 mg/ml, Salfarm Scandinavia AB). Maropitant injection at 1.0 mg/kg s.c. (Prevomax 10 mg/ml, Dechra Veterinary Products) 10 mg/kg was added due to hypersalivation. The following day, analysis of the plural effusion revealed elevation in TNCC (5.96 x 109/L; with 69% small lymphocytes and 30% granulocytes) as well as triglycerides (4.82 mmol/L in the pleural fluid; 0.3 mmol/L in the serum), the findings were consistent with chylous effusion. A point-of-care (POC) thoracic and abdominal ultrasound examinations were performed and revealed atelectatic/consolidated parts of lung lobes ventrally surrounded by moderate amount of pleural fluid. Abdominal scan did not reveal any peritoneal fluid; there was moderate amount of echogenic bile within the gallbladder without concurrent dilation of the biliary tree. The kidneys showed moderate reduction in corticomedullary demarcation and there was moderate corrugation of the gastrointestinal tract.
As pneumonia could not been excluded at this point the patient was started on intravenous amoxicillin at 20 mg/kg every 6 hours (Doktacillin 1g vial, Viatris). Ondasentron (Ondansetron Accord 2 mg/ml, Accord Healthcare AB) at 0.5 mg/kg intravenously twice daily was also added to the therapeutic regimen and nasogastric feeding tube was placed.
2.2. Echocardiography and Computed Tomography
Echocardiographic examination (Vivi i/q equipped with S12-RS 5.0-11.0 MHz phased-array transducer, GE Healthcare) with cardiologist on day 3 post-admission revealed subjective mild enlargement of the right atrium. The maximum velocity of the tricuspid regurgitant jet was mildly increased (TR VMax = 3.25 m/s) raising suspicion of mild pulmonary hypertension in the face of laminar flow of normal velocity through the pulmonic valve. Subsequently, the cat underwent a computed tomographic examination of the thoracic and abdominal cavities with an 80-row multidetector CT scanner (Aquilion Lightning, Canon Medical), before and after administration of intravenous contrast. Bilateral chest drains using a Seldinger technique (MILA International) were placed under the same anaesthetic episode. One hundred and twenty millilitres of chylous fluid were evacuated from the pleural cavity at the time of computed tomography. The right-sided chest drain required removal the following day, after it had got entrapped in the cage door. The medical management was continued with improvement in patient’s demeanour, appetite and respiratory rate. However, the abdominal dissension appeared to increase in severity based on daily abdominal palpations. Abdominal effusion was not sampled. Emptying of chest drain continued to yield chylous effusion, with volumes reaching 15 ml/kg/day.
The computed tomographic examination revealed bilateral pleural effusion and minimal (likely iatrogenic) pneumothorax. An oval (13.0 x 6.2 mm) area of fluid attenuation with peripheral contrast enhancement could be seen within the pleural cavity adjacent to the right eighth rib. This was thought to represent a compartmentalized pleural effusion or focal pleuritis, although, a cavitary lung nodule could not be excluded.
The eight right rib showed focal thickening proximally, with the seventh right intercostal space appearing narrower and the right eight intercostal space appearing wider than the remainder of the cats’ intercostal spaces. Moreover, there was a focal area of mineralization of the parietal pleural at the level of the eight intercostal space. These findings raised concerns about the possibility of previous thoracic wall injury. The intercostal muscles appeared subjectively thickened, bilaterally.
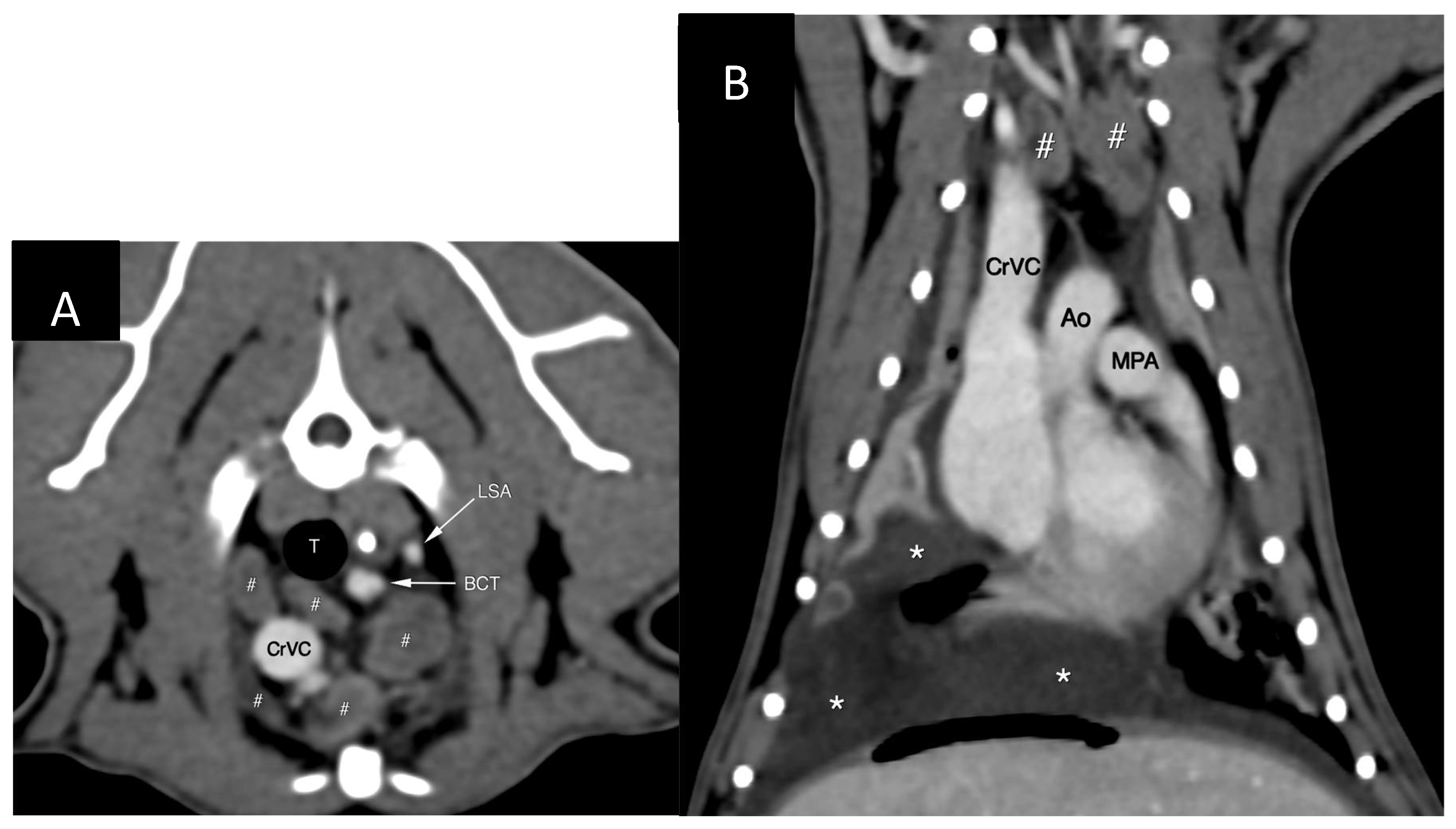
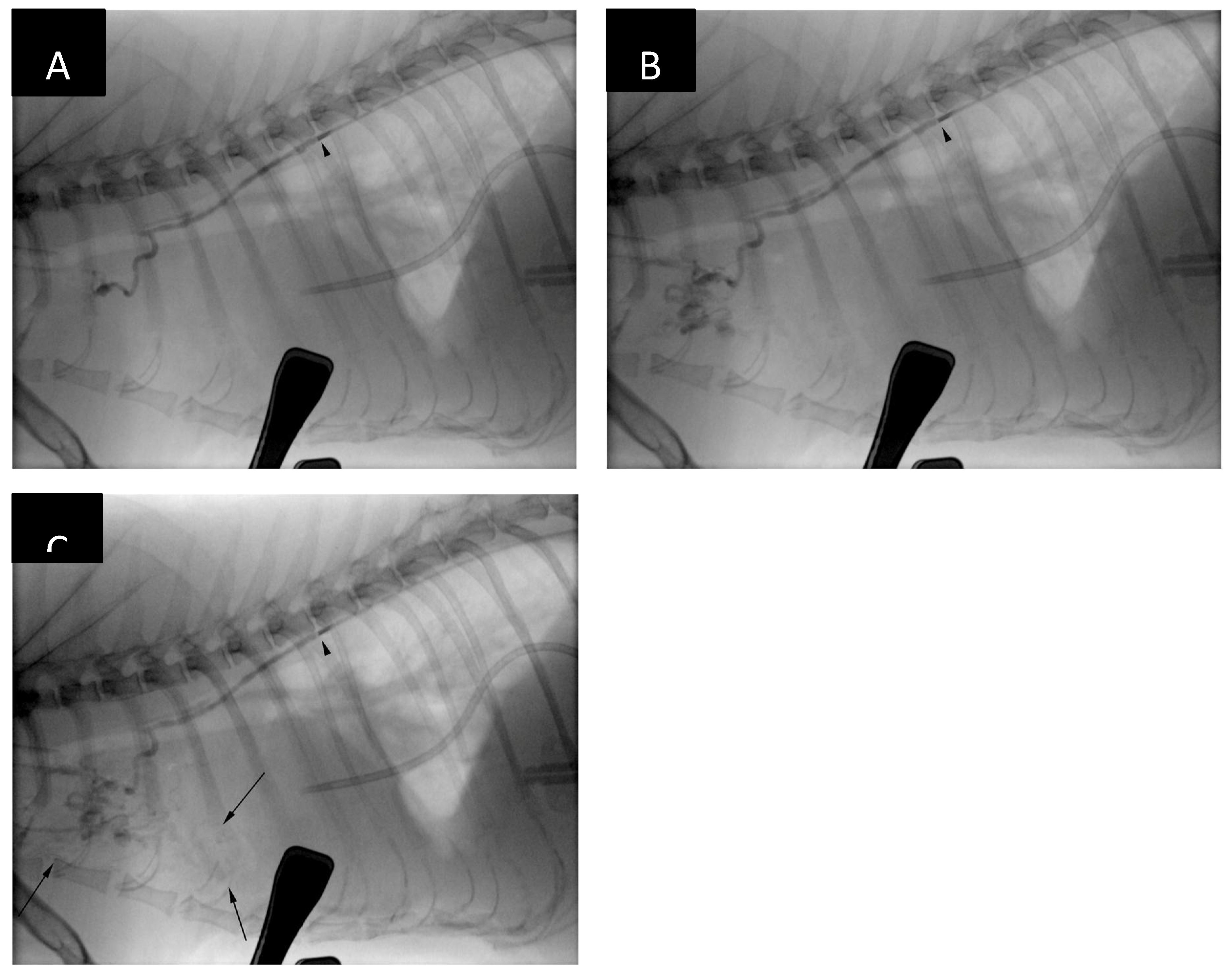
The cranial mediastinal, sternal and tracheobronchial lymph nodes were mildly- to-moderately enlarged (
Figure 1) and showed slightly heterogenous contrast enhancement. The axillary and cervical superficial lymph nodes also appeared prominent. There was presence of small amount fluid within the cranial mediastinum and mediastinal fat showed increased, wispy attenuation.
The lungs showed multitude of changes including rounding of the margins and volume reduction. There were multiple areas of atelectasis/consolidation and bronchial thickening and plugging. There was impression of cardiomegaly on CT images with thickening of the left ventricular wall and biatrial enlargement and dilatation of the pulmonary veins, the portal system and both venae cavae. Anaesthesia-related changes and use of dexmedetomidine as a premedicant were given priority as possible underlying causes for the above-mentioned cardiovascular changes as the echocardiography had not indicated such significant chamber and vessel dilatation.
Changes within the abdominal cavity included mild hepatosplenomegaly and mild ascites. The peritoneal fluid was not sampled for analysis. Multiple small nephroliths within the kidneys could be seen and there was an area of focal indentation of the cortex at the cranial pole of the right kidney. The wall of the terminal ileum appeared circumferentially thickened at the ileocolic junction. The abdominal lymph nodes appeared prominent.
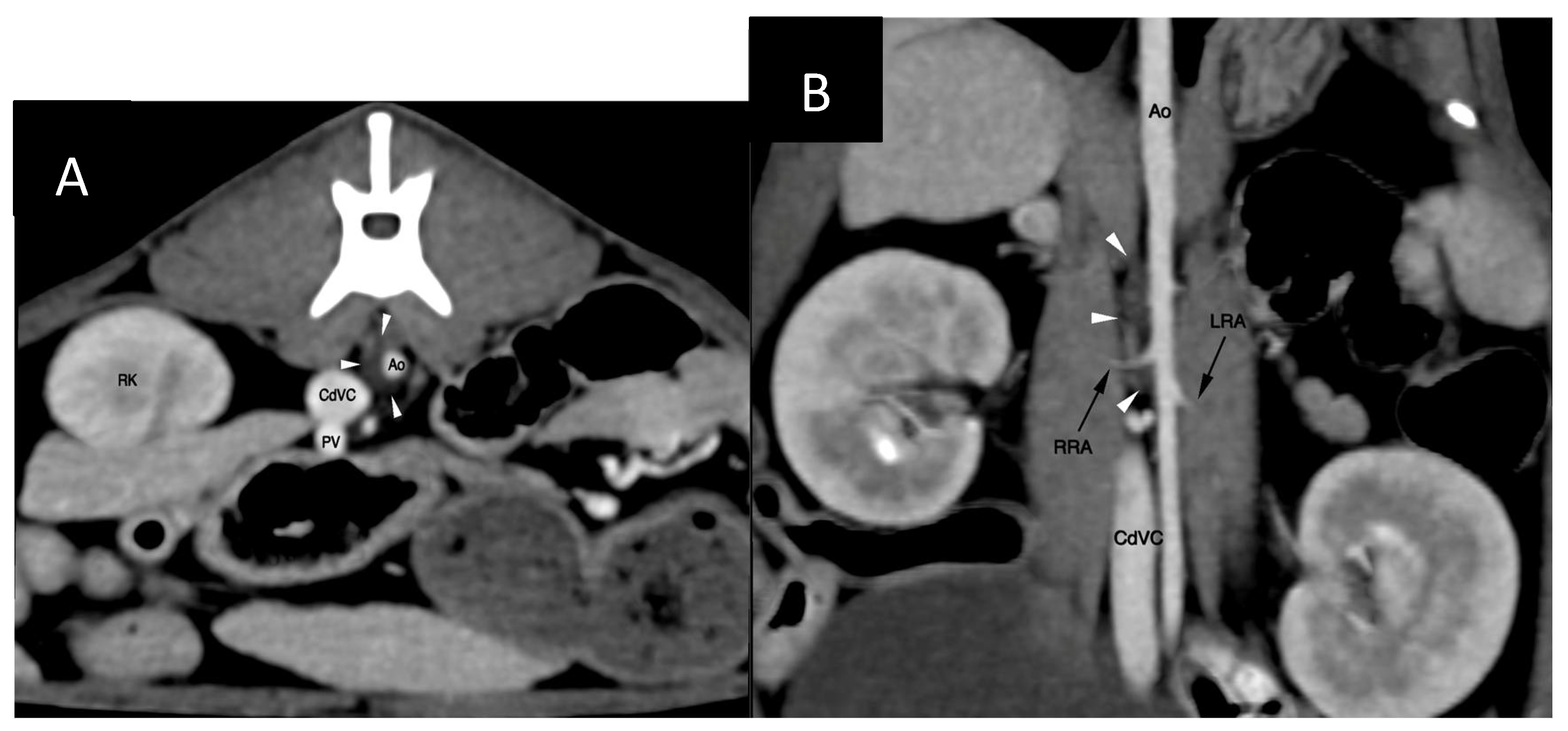
The cisterna chyli (CC) was visible at the level of the L2-L3 lumbar vertebrae, being crescent-shape and located slightly to the right of the aorta (
Figure 2). The proximal part of the thoracic duct was visible but quickly became indistinct cranially. No contrast opacification of the lymphatic system could be appreciated on post-intravenous contrast CT. Delayed post-intravenous contrast series as reported by Guarnera [
13] were not acquired, neither was computed tomography lymphangiogram (CTLA) performed.
2.3. Percutaneous Thoracic Duct Embolization
Computed tomography lymphangiography and thoracic duct ligation +/- subtotal pericardiectomy (SPC) were discussed with the owner. These interventions were, however, declined due to economic constraints. Percutaneous thoracic duct embolization (TDE) was also offered, which was accepted by the owner due to its lower cost. The owner was informed about limited veterinary data regarding this approach, particularly in cats. Technical challenges (mainly inability to cannulate the CC/TD) and possible complications (such as glue embolization into the venous system with subsequent migration into the heart and pulmonary circulation; lack of resolution or recurrence of chylothorax, development of inflammatory/non-chylous pleural effusion or chyloabdomen) were discussed with the owner.
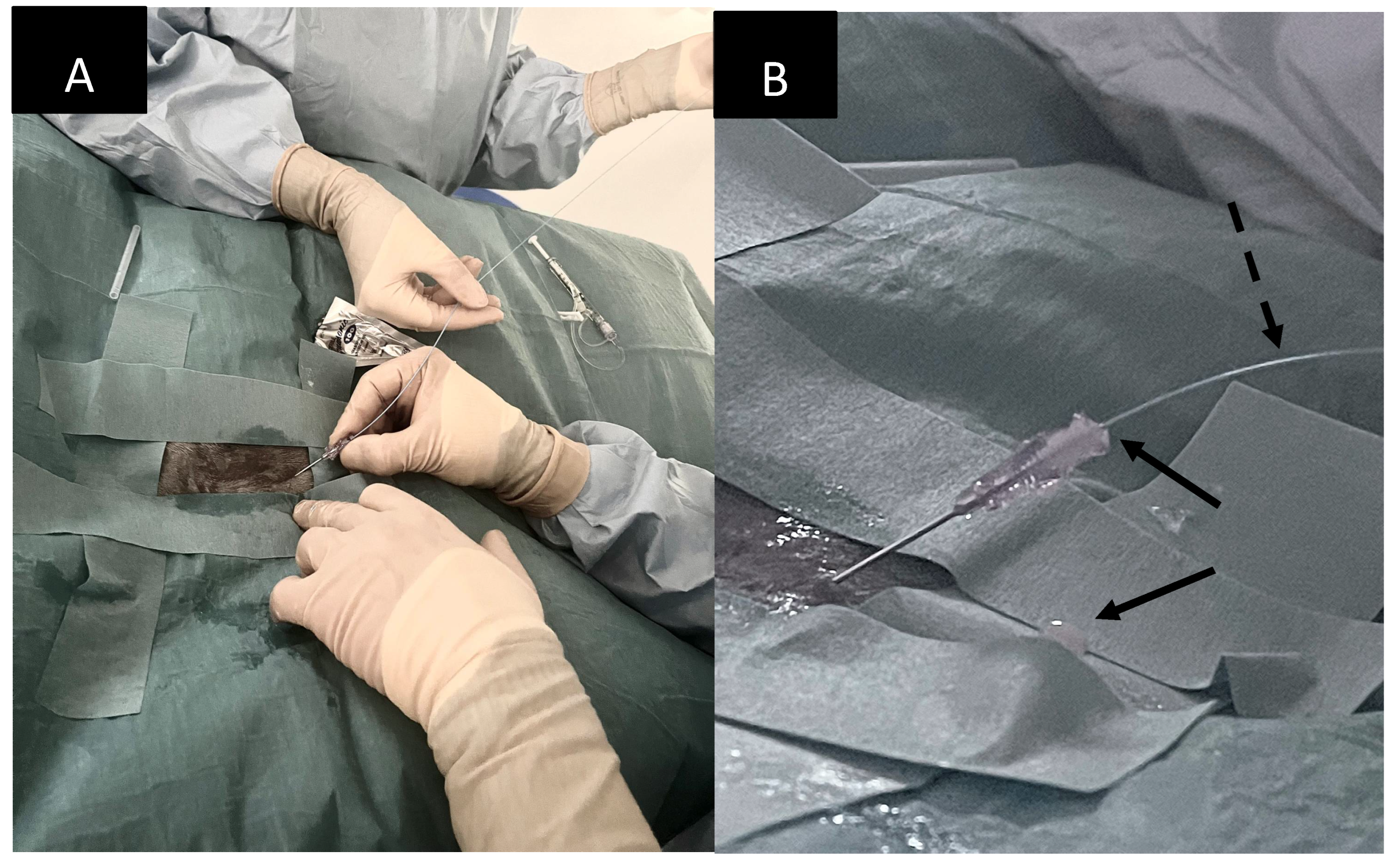
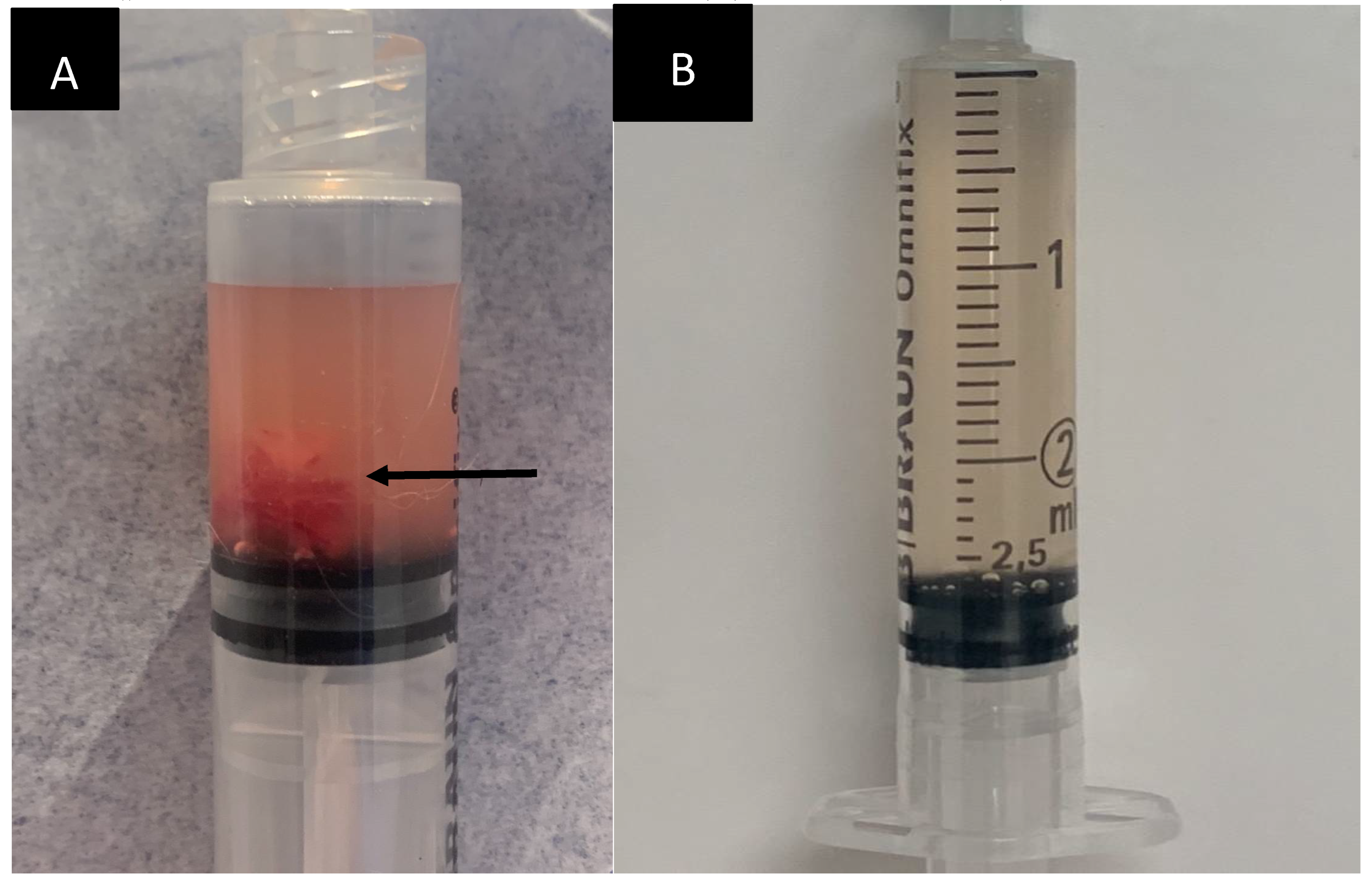
On day 8 post-admission the patient underwent percutaneous thoracic duct embolization. The patient was premeditated with methadone (Semfortan Vet 10 mg/ml, Dechra Veterinary Products) 0.2 mg/kg i.m. and preoxygenated. Anaesthesia was induced with midazolam and ketamine to effect and anaesthesia was maintained with isoflurane. Soon after induction systemic hypotension (MAP < 60 mm Hg, the exact value not recorded in the anaesthetic protocol) was detected and POC ultrasound showed signs consistent with hypovolaemia. A bolus of 38 ml of Ringer Acetate (Ringer-Acetate Baxter Viaflo, Baxter) was administered over 15 minutes followed by intravenous administration of ephedrine (Efedrin Unimedic 5 mg/ml, Unimedic Pharma) 0,02 mg/kg administered intravenously. Those intervention led to normalization of the blood pressure and blood pressure remained stable throughout the procedure. Emptying of the thoracostomy tube before draping yielded 35 ml of pinkish-white, turbid fluid.
The patient was placed in sternal recumbency for intrametatarsal pad injection of iohexol (Omnipaque 300 mg I / ml, GE Healthcare), 1.0 ml/kg divided in two sites (0.5 ml/kg/site), as described by Chiang et al. [
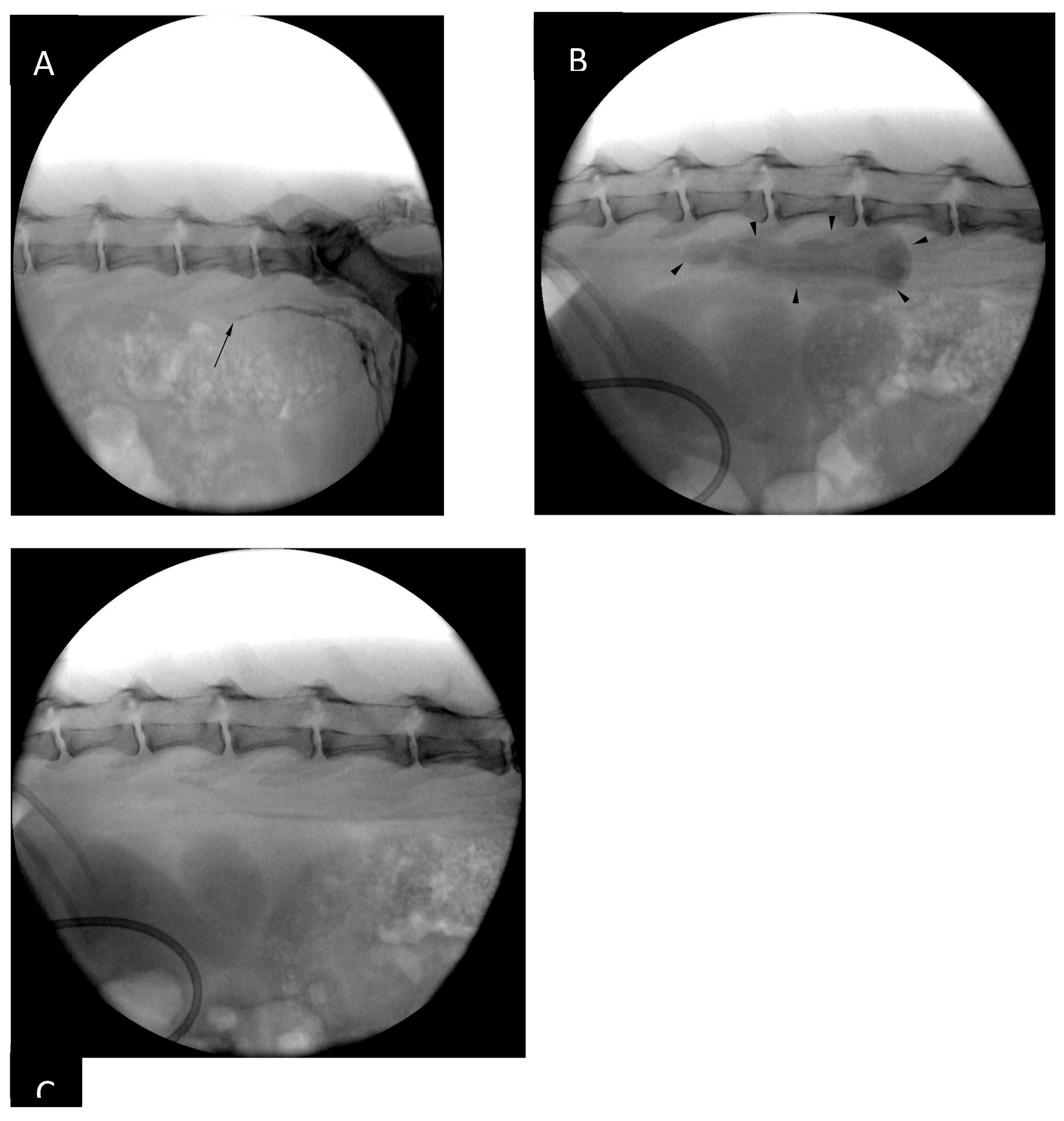
14]. Following the injection and gentle massage of the pads the patient was positioned in left lateral recumbency and draped for right lateral sublumbar approach to the cisterna chyli (
Figure 3). The contrast injection resulted in strong opacification of the lymphatics up to the level of the caudal lumbar vertebrae, however, the opacification was progressively less intense further cranially. By the time all the equipment was set up for CC puncture there was nearly no visible contrast within the CC (
Figure 3).
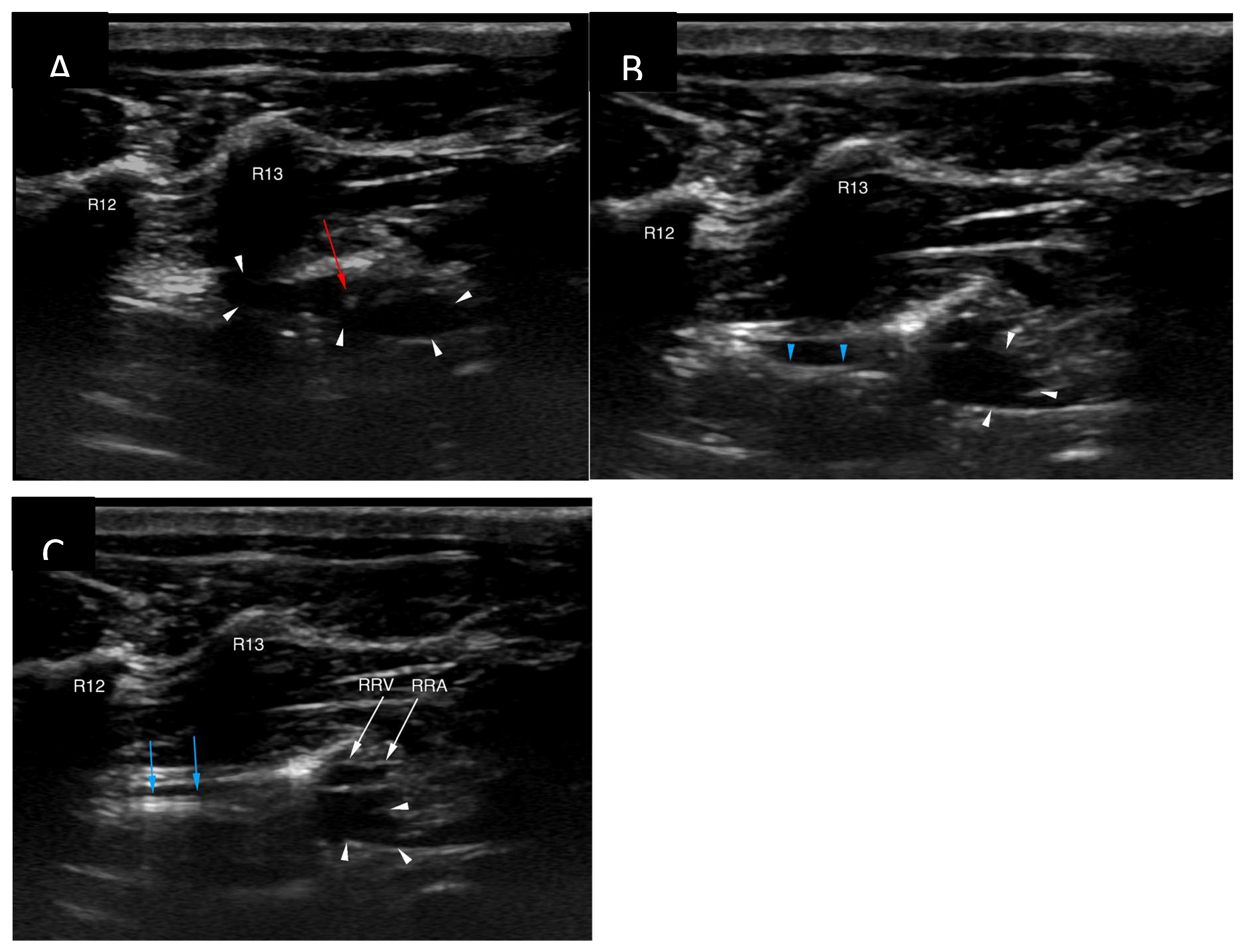
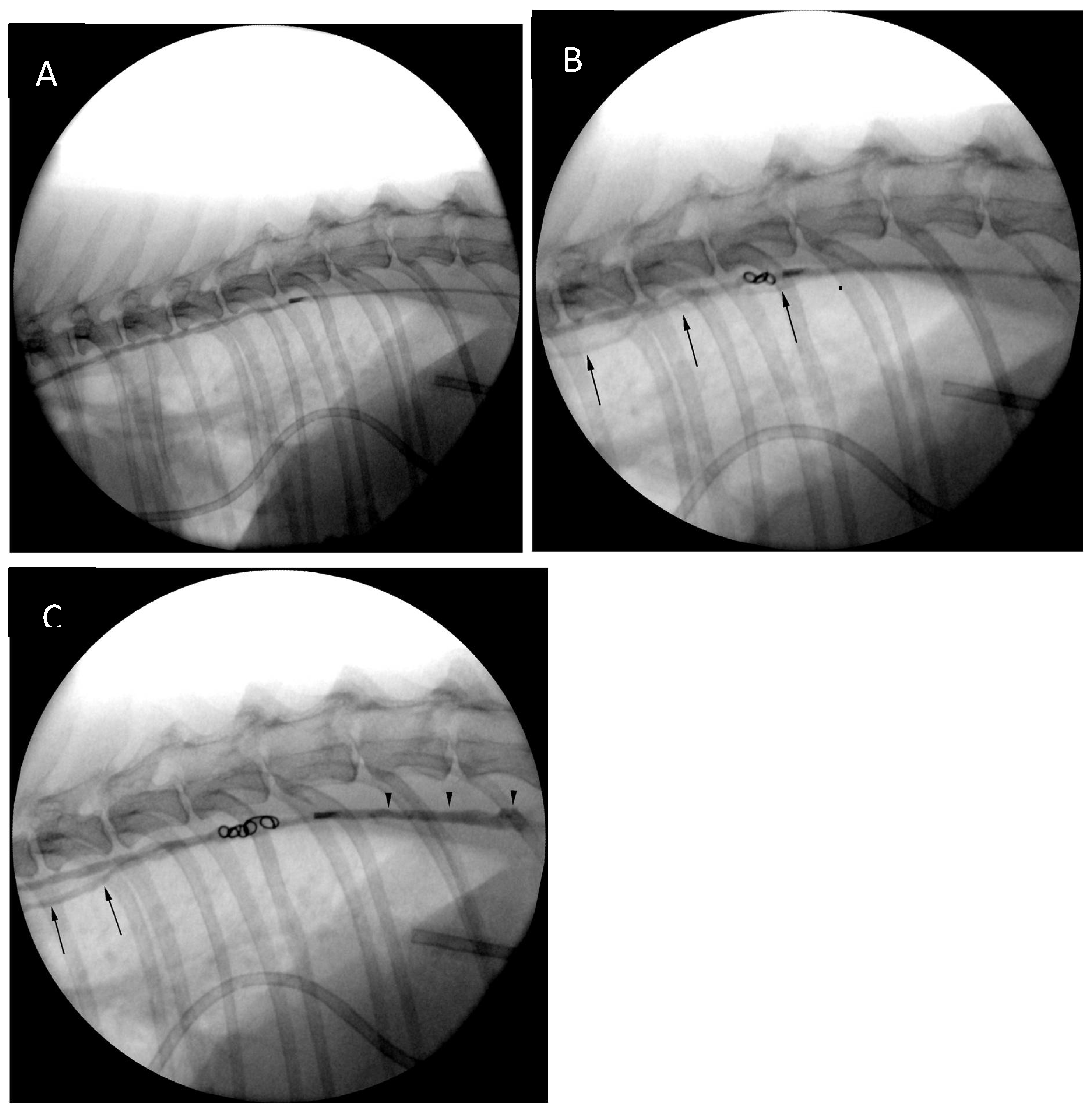
The cisterna chyli was punctured with an 18G, five-centimetre-long vascular access needle (Merit Advance, Merit Medical) under ultrasonographic guidance (Logic S7, GE Healthcare). A 3.8-10.8 MHz linear transducer (11L, GE Healthcare) was used, and the needle trajectory was from caudal to cranial (
Figure 4). Care was taken to avoid iatrogenic injury to the aorta and the right renal artery and vein. Although the initial puncture appeared successful, the combination of the 3.0 Fr microcatheter (Slip-Cath Infusion Catheter, Cook Medical) and a 0.014-inch guidewire (Runthrough NS, Terumo) could not be advanced into the central lymphatic system. The author suspects that either the initial access was lost when the ultrasound probe was removed from the patient's skin for fluoroscopic monitoring, or the angle of the initial puncture was too steep precluding easy advancement of the equipment. The CC access was repeated with a flatter angle and both the puncture as well as the advancement of the equipment were monitored only with ultrasound to minimize risk for needle displacement. On this occasion introduction of the guidewire followed by the catheter could be accomplished without problems (
Figure 5).
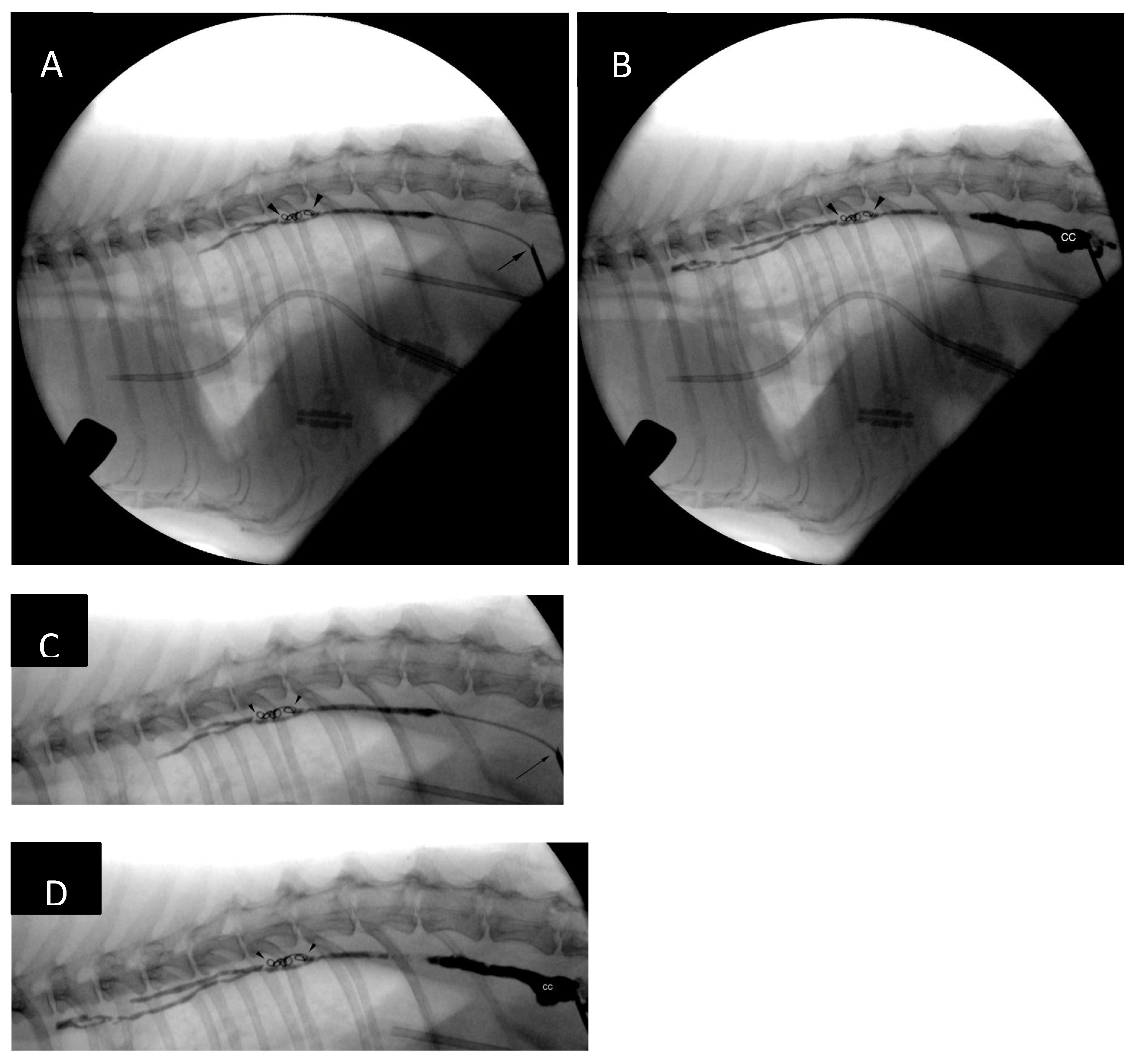
The ultrasound probe was removed from the patient’s skin and further stages of the procedure were performed with guidance of a portable fluoroscopy unit (Zenition 70, Philips). The guidewire was removed, and a lymphangiogram was performed with 50:50 iohexol:0.9% sodium chloride mixture revealing presence of lymphangiectasia and leakage of contrast medium at the level of the cranial mediastinum (
Figure 6).
After the catheter had been retracted to the lever of the cranial endplate of the T11, the image was magnified, and another lymphangiogram performed. Two 0.018-inch 2 mm x 2 cm hydrogel coils (Azur CX and Azur HydroCoil, Terumo) were deployed in the caudal thoracic duct with control lymphangiograms after each deployment. Opacification of additional thoracic duct branches could be appreciated (
Figure 7). The operator's intention was to deploy an additional 0.018-inch pushable, fibered coil, unfortunately, an 0.035-inch coil was inserted into the catheter instead by mistake. This resulted in the coil being stuck in the proximal portion of the catheter. As further injections, including the injection of neat contrast (Omnipaque 300, GE Healthcare) the procedure was continued without catheter exchange. The catheter was flushed with 5% dextrose in water and embolization of the thoracic duct and cranial portion of the cisterna chyli was performed with combination of n-butyl-2-cyanoacrylate glue (Histoacryl, B Braun) and ethiodized oil (Lipiodol Ultra Fluid 480 mg I/ml, Guerbet) in 1:2 (glue-to-oil) ratio. The embolic mixture was injected under continuous fluoroscopic monitoring while retracting the microcatheter (a ‘pull-back’ technique) to obtain adequate filling of the TD and CC (
Figure 7). No evidence of glue embolization into the venous circulation could be appreciated. Subsequently, the access needle was removed, and sterile dressing (Sorbact, Abigo Medical AB) applied. No skin sutures were needed. No dorsoventral imaging was performed at the time of the TDE due to limitations in room setup, although, it might have provided additional anatomical information [
15].
Emptying of the chest drain immediately post-TDE yielded 48 ml of fluid of the similar character as pre-procedure. Anaesthetic recovery was uneventful.
2.4. Post-TDE Management
The chest drain was not being evacuated overnight post-procedure and POC thoracic ultrasound the day after TDE showed only minimal amount of pleural fluid. Drain evacuation after ultrasound examination yielded only 1.0 ml of pinkish fluid that macroscopically did not appear to be chylous, although it was not submitted for laboratory analysis. The following day additional 13 ml of relatively translucent, non-chylous fluid were evacuated from the pleural cavity.
On day 3 post-TDE only 1.6 ml of pinkish and moderately flocculent fluid could be aspirated from the thoracic drain (
Figure 9) and POC ultrasound showed minimal amount of pleural fluid in the ventral thorax, bilaterally. The chest tube appeared to be surrounded by mild amount of echogenic material, thought to represent fibrinous strands. Biochemical analysis of the evacuated pleural fluid revealed significant (i.e. 17-fold) reduction in triglyceride concentration in comparison to pre-TDE values (0.27 mmol/l on day 3 post-TDE vs 4.82 mmol/l pre-TDE). Neither cytological analysis of the pleural fluid nor biochemical analysis of serum sample were performed due to financial constraints.
The chest tube was removed on day 4 post-TDE as no pleural fluid could be evacuated. At this time point the patient’s demeanour and appetite were normal and no respiratory symptoms could be appreciated. Detailed thoracic auscultation was hindered by purring. The cat was discharged from the hospital on oral Amoxicillin (Amoxival Vet 200 mg tablets) 20 mg/kg three times daily and Mirtazapine (Mirataz transdermal ointment 20 mg/g, Dechra Veterinary Products) as needed.
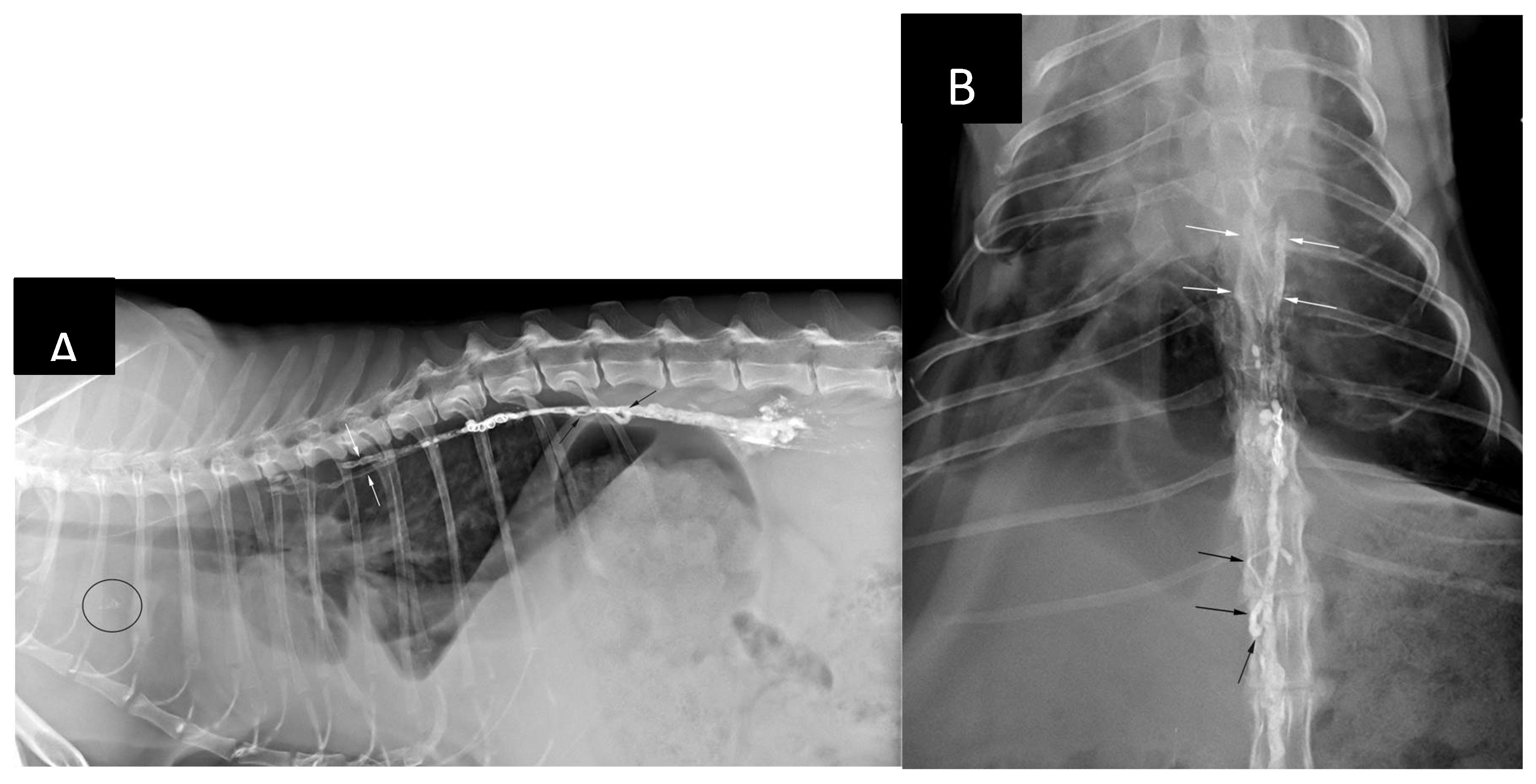
During a follow-up consultation on day 8 post-TDE an increase in respiratory rate (40/min) with abdominal ’push’ were noted. Abdominal cavity appeared distended. Thoracic x-rays (
Figure 10) revealed mild to moderate pleural effusion and distension of the caudal vena cava. There was no evidence of presence of embolic material within the pulmonary circulation. However, there was mild amount of radiopaque material visible cranial to the cardiac silhouette on the lateral view which was presumed to represent either non-target mediastinal embolization or accumulation of the ethiodized oil within of the mediastinal lymph node(s). Presence of the ‘embolus’ in the periaortic and cranial mediastinal lymph nodes was noted in all dogs on 12 weeks follow-up CTLA in Carvajal’s et al. study [
16].
POC ultrasound showed moderate pleural effusion, minimal amount of free abdominal fluid and subjective hepatomegaly. The following day POC cardiac ultrasound revealed subjectively moderate enlargement of the right ventricle and right atrium. There was no impression of systolic dysfunction of the right ventricle. Diagnostic thoracentesis yielded 2,5 ml of a translucent, straw-coloured fluid (
Figure 9). Cytological analysis revealed TNCC of 1.51 x 10
9/L, with 72 % segmented neutrophils, 26 % small agranulocytes and 2% large agranulocytes. Pleural fluid triglyceride and cholesterol concentration were low based on serum reference interval, 0.14 mmol/l (ref: < 2.00 mmol/L) and 1.3 mmol (ref: 2.00-6.00 mmol/L), respectively. Total protein concentration in the fluid was not assessed. The findings were consistent transudative effusion
. No bacteria were visible cytologically; however, the fluid was not submitted for culture. Due to suspicion of right-sided heart failure the cat was discharged with oral pimobendan (Vetmedin 1.25 mg, Boehringer Ingelheim Animal Health) 0.25 mg/kg twice daily and furosemide (Libeo Vet 10 mg, Ceva Animal Health) 1.0 mg/kg twice daily. The cat represented three days later with exacerbation of respiratory distress and was ultimately humanely euthanized the following day (day 14 post-TDE) due to uncertain prognosis and financial constrains limiting further investigation and treatment.
3. Discussion
Chylothorax is diagnosed by performing cholesterol and triglyceride analysis on pleural fluid and serum. In chylothorax, triglyceride levels in pleural fluid are significantly higher than in serum, and cholesterol levels are lower than serum’s concentration. A cholesterol/triglyceride ratio <0.2 in pleural fluid is considered diagnostic for chylothorax [
1]. Pleural effusions with triglyceride concentrations greater than 1.12 mmol/L (100 mg/dL) are always chylous [
17].
The surgical treatments for idiopathic chylothorax include thoracic duct ligation or embolization, cisterna chili ablation, subtotal pericardiectomy, omentalization, chronic percutaneous thoracostomy drainage, chronic pleuroperitoneal shunting, or combinations of these [
2].
Interventional thoracic duct embolization has received little attention in veterinary literature, although it was described already in 1989 [
3]. Detailed description of the technique can be found elsewhere [
3,
11,
12], but briefly, a laparotomy is required to expose and catheterize abdominal efferent lymphatics that are subsequently injected with n-butyl cyanoacrylate: ethiodized oil mixture. The embolic material is being pushed into the TD and CC by continued injection from the operator to cause permanent occlusion of the TD and CC as the nBCA polymerizes and solidifies.
The interventional approach to management of chylothorax offers several theoretical advantages over surgical ligation. Glue embolization could theoretically allow occlusion of smaller thoracic duct tributaries that cannot otherwise be easily visualized [
11]. Intraluminal occlusion of the thoracic duct offers the possibility of migration of the embolic to other (initially patent) lymphatic channels once the initial branch has become occluded. Some of the thoracic duct branches may still be missed during TDL procedure even when a thorough preoperative anatomical duct mapping using CTLA [
7,
18] and use of intraoperative near-infrared fluorescence imaging [
7]. The role of so-called sleeping ducts (ducts present but taking up inadequate contrast to be visible on pre-operative CTLA) as a potential cause of surgical failure is still unknown at this stage [
7,
18]. Lymphatic embolization could still lead to occlusion of those sleeping (i.e., initially non-opacified) lymphatic channels to overcome limitations associated with TDL (refer to
Figures 6, 7, 8 and 10).
Theoretically, when performing thoracic duct ligation, chyle leakage can occur caudal to the level of the ligation [
12]. Embolization of the entire duct from the cisterna chyli cranially would address this concern. Moreover, nBCA acts as a vessel irritant and has sclerotising properties [
3]. This should lead to permanent damage to lymphatic vessels potentially reducing the risk of recanalization and recurrence. In a study by Pardo et al. [
3] injection of isobutyl-2-cyanoacrylate and an oily contrast agent iophendylate mixture resulted in permanent, complete obstruction of the thoracic duct with a sclerosing granulomatous response surrounding the lymphatic embolus at week 6. However, at 6 months the pyogranulomatous reaction regressed, leaving a mature fibrous wall and occasional nests of lymphocytes, plasma cells and multinucleate giant cells with refractile intraluminal glue emboli. Lymphatic embolization has recently been described in conjunction with thoracoscopic TDL and pericardiectomy [
16]. The procedure was clinically successful in five out of six (83 %) dogs and produced a robust embolus that prevented antegrade continuation of the radiocontrast on follow-up CTLA 12 weeks post-surgery. The authors postulated that lymphatic embolization has a potential to reduce surgical failure by occlusion of missed branches of the thoracic duct and preventing the development of collaterals [
16].
Technically successful percutaneous transabdominal thoracic duct glue embolization, equivalent to the technique described in human medicine [
19] has been presented in abstract form and the data has recently been submitted for publication (Dana Clarke, personal communication). The first veterinary report on percutaneous CC access in dogs for embolization was published in 2011 [
20]. The authors of this report used a left-sided sublumbar needle puncture guided by injection of iodinated contrast medium into surgically exposed efferent mesenteric lymphatics. The technique was considered challenging and not recommended for routine use, as successful (defied as absence of flow across the embolized TD segment at postmortem examination) TDE could be performed in only 4 out of 15 dogs.
To overcome the technical challenges mentioned by Singh et al. [
20] the author decided to apply certain modifications. Ultrasound guidance was used to puncture the cisterna chyli and patient positioning and needle trajectory were inferred from the computed tomography study. The dorsoventral dimension of the CC was advantageous in this patient (
Figure 2) and allowed for a relatively uncomplicated access, although great care needed to be maintained to prevent iatrogenic injury/laceration to the aorta and the right renal artery and vein (
Figure 4). Ultrasound guidance provided real-time monitoring of the trajectory of the needle and continued fanning of the ultrasound probe allowed avoidance of the neighbouring vascular structures. No vascular sheath was used in this case to minimize number of exchanges and reduce the risk for loss of access. Instead, the microcatheter was inserted through the needle, a concept that is utilized during placement of epidural catheters.
The needle was left in place to provide additional stability and pushability to the microcatheter. The chosen craniocaudal angulation enabled easy advancement of the instrumentation. Entrapment of the 0.035 coil in the proximal portion of the microcatheter was a technical mistake on the operator’s side, however, it did not preclude the procedure from being continued. Should presence of the coil have precluded the injection of the embolic mixture, the microcatheter could have been replaced through the access needle.
The anatomy of the feline thoracic duct is variable [
15,
21,
22] and CTLA has been considered an essential component of chy lothorax diagnosis and presurgical planning [
23]. CTLA allows for visualization of thoracic duct morphology, and delineation of duct branching that may alter surgical approach and/or technique. Thoracic duct branches are frequently identified contralateral to the ‘main branch’, complicating surgical occlusion and making an accurate pre-operative imaging essential [
23].CTLA has become a common place before surgical intervention for IC [
7,
16], although it may not be technically successful in all patients [
7,
14]. Historically, both popliteal [
6,
21] and mesenteric lymph nodes [
22] have been used for contrast injection in cats. Recently CTLA via intrametatarsal pad injection [
14] has been described in this species. This technique did not prove to be useful to guide fluoroscopic access to the cisterna chyli in our cat, as the opacification of the CC was very short-lived. Ultrasound, on the other hand, provided adequate imaging in the presented patient and was a critical step of the procedure, even without access to prior CTLA. Subsequent lymphangiogram performed with the microcatheter positioned in the thoracic duct (
Figure 6) allowed clear visualization of the dilated and tortuous lymphatics, a finding consistent with lymphangiectasia. Ultrasound appearance of canine and feline cisterna chyli has been described [
24] the author’s experience indicate that this structure can often be visualized during routine abdominal ultrasonographic examinations, particularly in non-fasted individuals. Interestingly, the shape and size of the cisterna chyli in the individual animal can be variable during the same ultrasound examination and between different examinations [
24].
The author believes that direct access to the cisterna chyli and the thoracic duct also allows for more controlled injection of the embolic mixture decreasing the risk of glue embolization into the systemic circulation. This complication has been reported as an a clinically silent finding in a dog [
12] and as a fatal consequence of TDE in a cat [
11]. Injection of the embolic mixture in a ‘pull-back’ manner, starting in proximity of the target occlusion site, enables use of higher glue concentrations. This translates into shorter polymerization time and likely limiting too cranial migration of the embolic mixture. Some authors [
3,
11] suggest use of positive pressure ventilation to hinder cranial migration of lymphatic embolus toward the cranial vena cava. This was not required in our patient.
The role of feline immunodeficiency virus (FIV) in the development of chylothorax in our patient is unknown. FIV is known to infect a wide range of cell types in vitro and in vivo, including lymphocytes, monocytes/macrophages and follicular dendritic cells [
25]. Some authors recommend assessing the FIV/FeLV status in every cat diagnosed with chylothorax [
26]. The lymph nodes of experimentally FIV-infected cats may be hyperplastic during the acute phase of infection, and those in the terminal phase of infection may have disruption of normal architecture with loss of follicles and cellular depletion [
25]. Neoplasia is a common reason that FIV-infected cats are brought to a veterinary clinic [
25]. The cat from this report had mild-to-moderate enlargement of the intrathoracic lymph nodes based on the computed tomographic examination. These lymph nodes were, unfortunately, not sampled for cytology/histopathology nor was laboratory analysis of abdominal fluid performed. The limited diagnostics was dictated by financial constrains at the owner’s side. Therefore, it is not possible to ascertain truly idiopathic nature of the chylothorax in this cat, as it might have been secondary to other, yet not identified, medical condition.
Data surrounding the treatment of feline chylothorax is more limited when compared to their canine counterparts. However, it is generally accepted that cats have poorer outcome than dogs [
23]. Intraoperative identification of the thoracic duct and its branches is oftentimes extremely difficult in cats [
27] and intraoperative lymphangiography can be more difficult in small animals [
16,
27]. The procedure could not be successfully performed in any of the cats from Fossum’s et al. study [
27]. Percutaneous, ultrasound-guided needle access into the CC allowed clear delineation of the thoracic duct and mediastinal lymphangiectasia. Subsequent embolization provided rapid resolution of chyle leak in our patient. Unfortunately, non-chylous effusion (classified as transudate based on the total nucleated cell count) persisted, likely due to development of congestive heart failure (CHF) in our patient. It is plausible that the cat developed (or its presence had not been identified initially) constrictive pericardial physiology (CPP), which limited response to medical management of CHF. Chronic chylothorax can induce pleural fibrosis that can entrap lungs in a fibrous peel or induce a constrictive pericarditis [
2].
Cardiac catheterization and measurement of right-sided heart pressures at the time of TDE were considered, but unfortunately not performed due to financial constraints. For the same reasons subtotal pericardiectomy post-TDE could not be pursued. It has been postulated that impaired diastolic filling from a fibrosed and thickened (i.e., constrictive) pericardium can cause central venous hypertension. It has been hypothesized that this increase in right-sided venous pressures could act to impede the drainage of chyle via lymphaticovenous communications after TDL [
27]. Serosanguineous effusions that occur after TDL could also be potentially treated or prevented by pericardiectomy [
27]. However, the role of pericardiectomy has been questioned by other authors, as its positive effect on lowering the central venous pressure could not be demonstrated [
28]. This randomized study by McAnulty involving 23 dogs with idiopathic chylothorax revealed a higher resolu tion rate following TDL plus CCA (83%) than follow ing TDL plus SPC (60%), although this difference did not reach significance.
According to analysis of veterinary literature performed by Reeves et al. there is limited quality and quantity of data to support one treat ment over another for IC in dogs and cats [
29]. Moreover, pericardiectomy is not entirely without risks. In a study by Mayhew et al. [
7] perioperative mortality for TDL-SP cases was 11% (1/9), whereas TDL cases had 0% (0/17) perioperative mortality. A recent article [
30] described 16 canine cases of periprocedural ventricular fibrillation (VF) after pericardiectomy, seven of the pericardiectomies were performed due to IC. Development of VF was associated with high mortality in this cases series. The author is not aware of similar data with regards to feline pericardiectomy. Stockdale et al. [
31] described treatment results in 22 cats treated with either thoracic duct ligation and subtotal pericardiectomy (TDL+SPC) or thoracic duct ligation, subtotal pericardiectomy and cisterna chyli ablation (TDL+SPC+CCA). Pericardial histopathology performed in 82% (18/22) of cases and revealed evidence of pericarditis with various degrees of fibrosis. Despite pericardiectomy being performed in all subjects, 29% (5/17) of the patients with available long-term follow-up still had persistent chylous effusion 28 days after surgery. In two of those cats, however, the effusion resolved by three months post-intervention. Four out of 22 cats (18%) died within 4 weeks after surgical intervention (one TDL+SPC and three TDL+SPC+CCA), with two cats (both TDL+SPC+CCA) being euthanized 9 days after surgery due to persistent chylous effusion. Additionally, two cats (one from each treatment group) suffered from recurrence of chylothorax and one of those cats developed congestive heart failure. This data confirms challenges in management of feline chylothorax and underlines the fact that implementation of pericardiectomy does not always guarantee a positive outcome.
In constrictive pericarditis loss of elasticity impairs ventricular filling in mid- and late diastole, thereby limiting increases in ventricular volume after the end of the early filling period. The end-diastolic pressures become equalized or nearly equalized in all 4 cardiac chambers [
32]. Two main hemodynamic features of CP are exaggerated ventricular interdependence and dissociation between intrathoracic and intracardiac pressures [
33].
Diagnosis of constrictive pericarditis (CP) is challenging and invasive hemodynamic assessment with elevation of simultaneous recordings of the right and left ventricular pressures is the gold standard diagnostic test [
33]. This is because in patients with constrictive pericarditis, enhancement of ventricular interdependence leads to discordant changes in RV and LV pressures during respiration [
32]. This discordance manifests as reciprocal changes in peak systolic pressure, stroke volume, and pulse pressure in both ventricles during respiration [
32]. However, hemodynamic changes consistent with CPP may only become apparent after fluid loading [
34,
35].
Echocardiographic findings in two cats with constrictive pericarditis and chylothorax have been reported [
36,
37]. None of those changes were noted on the initial echocardiographic examination of our patient. In a study by Murphy et al. [
37] cardiac catheterization revealed elevated right atrial and right ventricular pressures, however, no data regarding possible temporal (i.e., dynamic respiratory) changes in pressure tracings were discussed by the authors. In their patient, the pleural effusion persisted, although to a lesser degree than initially, even though both subtotal pericardiectomy and epicardial decortication were performed.
The potential role of mildly elevated systolic right ventricular pressure (based on initial TR velocity) in the development of chylothorax in our patient could not be investigated further but warrants some consideration. FIV Infected cats can develop lymphoid interstitial pneumonitis [
25] but the author is not aware of association between lymphoid interstitial pneumonitis and development of pulmonary hypertension in cats. Interestingly, 4 cats in the study by Stockdale et al. underwent lung lobectomy due to suspected lung fibrosis, which was ultimately confirmed histopathologically in 2 out of 3 submitted lung samples. An additional cat with recurrence of chylothorax was euthanized due to suspected pulmonary fibrosis 774 days after surgery. Another interesting fact is that right ventricular ‘peak pressure’ in some of the patients from Mayhew’s et al. publication [
7] reached values up to 48.3 mmHg, which is higher than expected values in anaesthetized veterinary subjects [
38,
39].