Submitted:
18 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
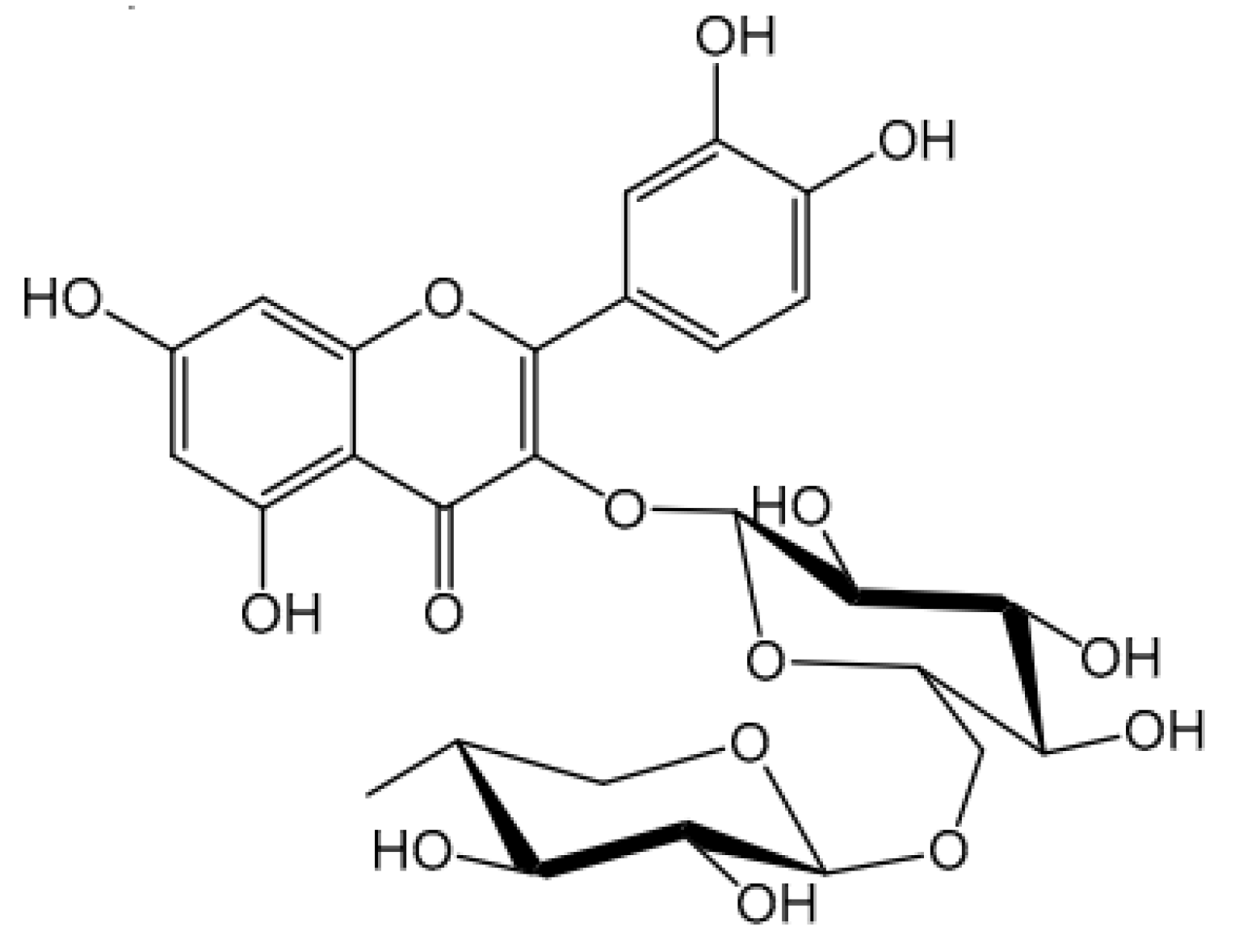
2. Physicochemical Stability of Cosmetic Preparations Containing Rutin
- (i)
- extrinsic factors—external conditions to which cosmetic products are exposed, such as temperature, light, oxygen, humidity, packaging materials, microorganisms, and movements, among others;
- (ii)
3. Cutaneous Permeability of Rutin: Relevance to Cosmetology and Photoprotection
4. Rutin and Sunscreen Systems: In Vitro and In Vivo Efficacy Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, J.; Verma, P.K. An Overview of Biosynthetic Pathway and Therapeutic Potential of Rutin. Mini-Reviews in Medicinal Chemistry 2023, 23, 1451–1460. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. Journal of Nutritional Biochemistry 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 1–24. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, 11. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier Inc., 2018; pp. 111–117 ISBN 9780128124918.
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phytotherapy Research 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Kola, A.; Dudek, D.; Valensin, D. Metal Complexation Mechanisms of Polyphenols Associated to Alzheimer’s Disease. Curr Med Chem 2021, 28, 7278–7294. [Google Scholar] [CrossRef]
- Noon, J.; Mills, T.B.; Norton, I.T. The Use of Natural Antioxidants to Combat Lipid Oxidation in O/W Emulsions. J Food Eng 2020, 281. [Google Scholar] [CrossRef]
- Araújo, J.I.R. de; Oliveira, J.H.P. de; Silva, J.W.V. da; Silva, D.T.C. da; Soares, M.F. de L.R.; Soares-Sobrinho, J.L. Métodos Analíticos Para Avaliação Da Estabilidade de Rutina e Análise Da Formação de Seus Produtos de Degradação: Uma Revisão. Research, Society and Development 2022, 11, e399111234657. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and in Vitro Toxicological Profile Using 3d Reconstructed Human Epidermis Tissue and 2d Cells. Antioxidants 2021, 10, 1–21. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus Sinensis and Conventional Antibiotic Gentamicin on Pseudomonas Aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Kaneko, T.M.; Baby, A.R. Rutin Association with Ethylhexyl Methoxycinnamate and Benzophenone-3: In Vitro Evaluation of the Photoprotection Effectiveness by Reflectance Spectrophotometry. Latin American Journal of Pharmacy 2008, 27, 23–27. [Google Scholar]
- Rashidinejad, A.; Jameson, G.B.; Singh, H. The Effect of PH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 1–16. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic Sunscreens for Photoprotection. Green Chemistry 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Karunakaran, T.; Hamdan, M.R. Exploring the Potential Use of Hylocereus Polyrhizus Peels as a Source of Cosmeceutical Sunscreen Agent for Its Antioxidant and Photoprotective Properties. Evidence-based Complementary and Alternative Medicine 2020, 2020. [Google Scholar] [CrossRef]
- Franco, J.G.; Cefali, L.C.; Ataide, J.A.; Santini, A.; Souto, E.B.; Mazzola, P.G. Effect of Nanoencapsulation of Blueberry (Vaccinium Myrtillus): A Green Source of Flavonoids with Antioxidant and Photoprotective Properties. Sustain Chem Pharm 2021, 23. [Google Scholar] [CrossRef]
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. AAPS PharmSciTech 2018, 19, 1773–1780. [Google Scholar] [CrossRef]
- Chai, J.; Chen, X.; Jin, C.; Chai, F.; Tian, M. Selective Enrichment of Rutin in Sunscreen by Boronate Affinity Molecularly Imprinted Polymer Prior to Determination by High Performance Liquid Chromatography. Biochem Eng J 2023, 191. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv Pharmacol Sci 2018, 2018. [Google Scholar] [CrossRef]
- B, P.; SOMAN, A.; JOHNSON, J.; NARAYANAN, P.S.; JOHN, A.P. PLANTS AND PHYTOCONSTITUENTS HAVING SUNSCREEN ACTIVITY. World Journal of Current Medical and Pharmaceutical Research 2020, 02, 14–20. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa Oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 1–23. [Google Scholar] [CrossRef]
- Martins, R.M.; de Siqueira Martins, S.; Barbosa, G.L.F.; Fonseca, M.J.V.; Rochette, P.J.; Moulin, V.J.; de Freitas, L.A.P. Photoprotective Effect of Solid Lipid Nanoparticles of Rutin against UVB Radiation Damage on Skin Biopsies and Tissue-Engineered Skin. J Microencapsul 2022, 39, 668–679. [Google Scholar] [CrossRef]
- Roquete Amparo, T.; Cherem Peixoto da Silva, A.; Brandão Seibert, J.; dos Santos da Silva, D.; Martins Rebello dos Santos, V.; Melo de Abreu Vieira, P.; Célio Brandão, G.; Henrique Bianco de Souza, G.; Aloise Maneira Corrêa Santos, B. In Vitro and in Silico Investigation of the Photoprotective and Antioxidant Potential of Protium Spruceanum Leaves and Its Main Flavonoids. J Photochem Photobiol A Chem 2022, 431. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Gonçalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In Vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus Persica (L.) Var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. 2021. [Google Scholar] [CrossRef]
- Oliveira, C.A. De; Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; Araújo, G.L.B. De; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. European Journal of Pharmaceutical Sciences 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Fonseca, M.; Rehman, M.; Soares, R.; Fonte, P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules 2023, 13, 1–32. [Google Scholar] [CrossRef]
- de Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Efficacy Evaluation of Gelatin-Based Nanoparticles Associated with UV Filters. Colloids Surf B Biointerfaces 2016, 140, 531–537. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF Enhancement Provided by Rutin in a Multifunctional Sunscreen. Int J Pharm 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Clementi, M.E. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Hansson, A.C.; Johansson, H.; Lemkine, G.F.; Thénot, J.P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (Nams). Cosmetics 2021, 8, 1–18. [Google Scholar] [CrossRef]
- Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes 2021, 9. [Google Scholar] [CrossRef]
- Argollo, G. De; Camila, M.; Hiraishi, F.; Ivo, P.; Macedo, D.S.; Aparecida, C.; Oliveira, S. De; João, P.; Catarina, G. HPLC- TBARS- EVSC ( High- Performance Liquid Thiobarbituric Acid Reactive Substances – Ex Vivo Stratum Corneum ) Protocol : Selection of the Subjects and Approach to Present the Results. Int J Cosmet Sci 2023, 45, 647–654. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-Aging and Sunscreens: Paradigm Shift in Cosmetics. Adv Pharm Bull 2019, 9, 348–359. [Google Scholar] [CrossRef]
- ANVISA, A.N. de V.S. Guia de Controle de Qualidade de Produtos Cosméticos: Uma Abordagem Sobre Os Ensaios Físicos e Químicos; 2008; ISBN 9788588233348.
- Badruddoza, A.Z.M.; Thean Yeoh, J.C.S.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J Pharm Sci 2023, 112, 1772–1793. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Maciel, C.R.M.; Sarruf, F.D.; Pinto, C.A.S.O.; Consiglieri, V.O.; Kaneko, T.M.; Baby, A.R. Desenvolvimento e Teste Preliminar Da Estabilidade de Formulaç Ões Cosméticas Acrescidas de Extrato Comercial de Trichilia Catigua Adr. Juss (e) Ptychopetalum Olacoides Bentham. Revista de Ciencias Farmaceuticas Basica e Aplicada 2008, 29, 179–194. [Google Scholar]
- Baby, A.R.; Migliato, K.F.; Maciel, C.P.M.; Zague, V.; Aparecida Sales de Oliveira Pinto, C.; Salgado, H.R.N.; Kaneko, T.M.; Velasco, M.V.R. Accelerated Chemical Stability Data of O/W Fluid Emulsions Containing the Extract of Trichilia Catigua Adr. Juss (and) Ptychopetalum Olacoides Bentham. Revista Brasileira de Ciencias Farmaceuticas/Brazilian Journal of Pharmaceutical Sciences 2007, 43, 405–412. [Google Scholar] [CrossRef]
- Maciel, C.P.M.; Kaneko, T.M. UV Spectrophotometric Determination of Bioflavonoids from a Semisolid Pharmaceutical Dosage Form Containing. 2006, 1–6.
- L.C. Passos, M.; M.F.S. Saraiva, M.L. Detection in UV-Visible Spectrophotometry: Detectors, Detection Systems, and Detection Strategies. Measurement (Lond) 2019, 135, 896–904. [CrossRef]
- Haque, S.M. Optimized Box–Behnken Experimental Design Based Response Surface Methodology and Youden’s Robustness Test to Develop and Validate Methods to Determine Nateglinide Using Kinetic Spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc 2022, 268, 120712. [Google Scholar] [CrossRef]
- Civan, F.; Alarcon, L.J.; Campbell, S.E. Laboratory Confirmation of New Emulsion Stability Model. J Pet Sci Eng 2004, 43, 25–34. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The Photostability of Flavanones, Flavonols and Flavones and Evolution of Their Antioxidant Activity. J Photochem Photobiol A Chem 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Zagoskina, N. V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V. V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Morgenni, F.; Mazzi, G.; Vincieri, F.F. Evaluation of Chemical Stability of St. John’s Wort Commercial Extract and Some Preparations. Int J Pharm 2001, 213, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Banov, D.; Baby, A.R.; Del Bosco, L.M.; Kaneko, T.M.; Velasco, M.V.R. Caracterização Do Extrato Seco de Ginkgo Biloba L. Em Formulações de Uso Tópico. Acta Farmaceutica Bonaerense 2006, 25, 219–224. [Google Scholar]
- Nishikawa, D.O.; Zague, V.; Pinto, C.A.S.O.; Vieira, R.P.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Avaliação Da Estabilidade de Máscaras Faciais Peel-off Contendo Rutina. Rev. Ciênc. Farm. Básica Apl 2007, 28, 227–232. [Google Scholar]
- Valenta, C.; Nowack, E.; Bernkop-Schnürch, A. Deoxycholate-Hydrogels: Novel Drug Carrier Systems for Topical Use. Int J Pharm 1999, 185, 103–111. [Google Scholar] [CrossRef]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Tavante-Júnior, C.R.; Pinto, C.A.S. de O.; Zague, V.; Arêas, E.P.G.; Kaneko, T.M.; Velasco, M.V.R. Stability and in Vitro Penetration Study of Rutin Incorporated in a Cosmetic Emulsion through an Alternative Model Biomembrane. Revista Brasileira de Ciências Farmacêuticas 2008, 44. [Google Scholar] [CrossRef]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Pinto, C.A.S.D.O.; Kaneko, T.M.; Velasco, M.V.R. Influence of Urea, Isopropanol, and Propylene Glycol on Rutin in Vitro Release from Cosmetic Semisolid Systems Estimated by Factorial Design. Drug Dev Ind Pharm 2009, 35, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Carbone, C.; Maniscalco, C.; Lambusta, D.; Nicolosi, G.; Ventura, C.A.; Puglisi, G. In Vitro Evaluation of Quercetin-3-O-Acyl Esters as Topical Prodrugs. Int J Pharm 2007, 336, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wadher, K.; Trivedi, S.; Rarokar, N.; Umekar, M. Development and Assessment of Rutin Loaded Transfersomes to Improve Ex Vivo Membrane Permeability and in Vitro Efficacy. Hybrid Advances 2024, 5, 100144. [Google Scholar] [CrossRef]
- Hassan, A.S.; Soliman, G.M. Rutin Nanocrystals with Enhanced Anti-Inflammatory Activity: Preparation and Ex Vivo/In Vivo Evaluation in an Inflammatory Rat Model. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, R.; Huang, J.; Xia, Q. Development and Characterization of a New Non-Aqueous Self-Double-Emulsifying Drug Delivery System for Topical Application of Rutin. J Drug Deliv Sci Technol 2021, 61. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, C.; Wu, F.; Li, H.; Zhang, W.P.; Zhang, Q. Microencapsulated Nanostructured Lipid Carriers as Delivery System for Rutin. Materials Technology 2018, 33, 357–363. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Aisha, M.; Zhang, J.; Sun, M. A Rutin Nanocrystal Gel as an Effective Dermal Delivery System for Enhanced Anti-Photoaging Application. Drug Dev Ind Pharm 2021, 47, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9. [Google Scholar] [CrossRef]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin-Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (SmartCrystals). Cosmetics 2016, 3. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Salabat, A. Microemulsion and Microemulsion Gel Formulation for Transdermal Delivery of Rutin: Optimization, in-Vitro/Ex-Vivo Evaluation and SPF Determination. J Dispers Sci Technol 2022, 43, 1848–1857. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Kurzawa, M. Chitosan-Based Films Containing Rutin for Potential Cosmetic Applications. Polymers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Keck, C.M. Flavonoid Nanocrystals Produced by ARTcrystal®-Technology. Int J Pharm 2015, 482, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Georgetti, S.R.; Verri, W.A.; Borin, M.F.; Lopez, R.F. V; Fonseca, M.J. V In Vitro Evaluation of Quercetin Cutaneous Absorption from Topical Formulations and Its Functional Stability by Antioxidant Activity. 2007, 328, 183–190. [Google Scholar] [CrossRef]
- Ghazi, S. Do the Polyphenolic Compounds from Natural Products Can Protect the Skin from Ultraviolet Rays? Results Chem 2022, 4. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Animal Model Exp Med 2023, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Fatharani, R.M.; Fitrian, U.A.; Ishak, S.S.O.; Adlia, A. Sun Protection Factor and Tyrosinase Inhibitory Activity of Several Plant Secondary Metabolites. Current Research on Biosciences and Biotechnology 2023, 5, 315–319. [Google Scholar] [CrossRef]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D.; Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.; Peres, D.Da.; Rugno, C.M.; Kojima, M.; De Oliveira Pinto, C.A.S.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional Photostability and Cutaneous Compatibility of Bioactive UVA Sun Care Products. J Photochem Photobiol B 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Pasuch Gluzezak, A.J.; Dos Santos, J.L.; Maria-Engler, S.S.; Gaspar, L.R. Evaluation of the Photoprotective and Antioxidant Potential of an Avobenzone Derivative. Front Physiol 2024, 15. [Google Scholar] [CrossRef]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily Photoprotection to Prevent Photoaging. Photodermatol Photoimmunol Photomed 2021, 37, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Mercurio, D.G.; Catalan, E.; Bouhadanna, E.; Delaunay, C.; Miranda, D.F.; Passeron, T. Impact on Facial Skin Aging Signs of a 1-Year Standardized Photoprotection over a Classical Skin Care Routine in Skin Phototypes II–VI Individuals: A Prospective Randomized Trial. Journal of the European Academy of Dermatology and Venereology 2023, 37, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.E.; Hung, Y.T.; Fang, A.H.; Ching-Shuang, W. Effects of Irradiance on UVA-Induced Skin Aging. J Dermatol Sci 2019, 94, 220–228. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int J Mol Sci 2022, 23, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yamauchi, R.; Tsuji, T.; Krutmann, J.; Morita, A. The Expression of Matrix Metalloproteinase-1 MRNA Induced by Ultraviolet A1 (340-400 Nm) Is Phototherapy Relevant to the Glutathione (GSH) Content in Skin Fibroblasts of Systemic Sclerosis. Journal of Dermatology 2003, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Goss, G.; Hiratsuka, T.; Sipilä, K.H.; Kirk, T.; Kober, K.I.; Lui, P.P.; Tsang, V.S.K.; Hawkshaw, N.J.; Pilkington, S.M.; et al. Role of Distinct Fibroblast Lineages and Immune Cells in Dermal Repair Following UV Radiation Induced Tissue Damage. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Proteins Involved in the Antioxidant and Inflammatory Response in Rutin-Treated Human Skin Fibroblasts Exposed to UVA or UVB Irradiation. J Dermatol Sci 2018, 90, 241–252. [Google Scholar] [CrossRef]
- Baby, A.R.; Balogh, T.S.; Pedriali, C.A.; Kaneko, T.M.; Velasco, M.V.R. Uva I-Protection Effectiveness of Bioactive Compound and Organic Uv Filters: An in Vitro Assessment. Quim Nova 2009, 32, 1321–1323. [Google Scholar] [CrossRef]
- STATES, U. Labeling and Effectiveness Testing; Sunscreen Drug Products for over-the-Conter Human Use; Final Rule; 2011; Vol. 76, p. 46;
- El-Boury, S.; Couteau, C.; Boulande, L.; Paparis, E.; Coiffard, L.J.M. Effect of the Combination of Organic and Inorganic Filters on the Sun Protection Factor (SPF) Determined by in Vitro Method. Int J Pharm 2007, 340, 1–5. [Google Scholar] [CrossRef]
- Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L.J.M. Study of the Photostability of 18 Sunscreens in Creams by Measuring the SPF in Vitro. J Pharm Biomed Anal 2007, 44, 270–273. [Google Scholar] [CrossRef]
- Nadia, M.A.; Zulkarnain, A.K.; Sulaiman, T.N.S. Determination of Photoprotective Capacity of Topical Gel Formulations Containing Bioactive Compound Rutin and Evaluation of Physicochemical Stability. Tropical Journal of Natural Product Research 2023, 7, 3923–3931. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T. Plant Polyphenols: Recent Advances in Epidemiological Research and Other Studies on Cancer Prevention; 1st ed.; Copyright © 2013 Elsevier B.V. All rights reserved., 2013; Vol. 39; ISBN 9780444626158.
- Her, Y.; Lee, T.K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.C.; Ahn, J.H.; Park, J.H.; Lee, J.W.; Hong, J.; et al. Topical Application of Aronia Melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Sarruf, F.D.; Salgado-Santos, I.M.N.; Haroutiounian-Filho, C.A.; Kaneko, T.M.; Baby, A.R. Broad Spectrum Bioactive Sunscreens. Int J Pharm 2008, 363, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Monsalve-bustamante, Y.A.; Puertas-mejia, M.A.; Mejia-giraldo, J.C. Comparison of the Photoprotective Effect between Hydrolyzed and Aglycones Flavonoids as Sunscreen : A Systematic Review. 2020, 10, 116–123. [Google Scholar] [CrossRef]
- Opriş, O.; Soran, M.L.; Lung, I.; Stegarescu, A.; Guţoiu, S.; Podea, R.; Podea, P. Optimization of Extraction Conditions of Polyphenols, Antioxidant Capacity and Sun Protection Factor from Prunus Spinosa Fruits. Application in Sunscreen Formulation. Journal of the Iranian Chemical Society 2021, 18, 2625–2636. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J Cosmet Dermatol 2022, 21, 5409–5444. [Google Scholar] [CrossRef] [PubMed]
- He, hailun; Li, anqi; Li, shiqin; Tang, jie; Li, li; Xiong, lidan Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomedicine and Pharmacotherapy 2021, 134.
- Dondi, D.; Albini, A.; Serpone, N. Interactions between Different Solar UVB/UVA Filters Contained in Commercial Suncreams and Consequent Loss of UV Protection. Photochemical and Photobiological Sciences 2006, 5, 835–843. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J Drug Deliv Sci Technol 2023, 86. [Google Scholar] [CrossRef]
- Kamel, R.; Mostafa, D.M. Rutin Nanostructured Lipid Cosmeceutical Preparation with Sun Protective Potential. J Photochem Photobiol B 2015, 153, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Kuthi, N.; Basar, N.; Chandren, S. Nanonutrition- and Nanoparticle-Based Ultraviolet Rays Protection of Skin. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier, 2022; pp. 227–280 ISBN 9780323884501.
- Kumar, N.; Jose, J. Current Developments in the Nanomediated Delivery of Photoprotective Phytochemicals. [CrossRef]
- Gonçalo das Neves e Silva, E.; Ferreira Barbosa, G.L.; Alves Confessor, M.V.; Bacalhau de Sousa, W.J.; Lia Fook, M.V.; Siqueira-Júnior, J.P.; Pedro de Freitas, L.A.; Martins, R.M. Artemia Salina Leach in Photoprotection: A New Model to Evaluate the Potential of Nanoparticles for Topical Application. J Drug Deliv Sci Technol 2023, 90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




