1. Introduction
CD38 is expressed at relatively low levels on normal lymphoid and myeloid cells, and in some tissues of non-hematopoietic Origen. The specificity of this target has increased interest in new drugs and triggered the development of the CD38 monoclonal antibodies daratumumab, CD38 antibodies have pleiotropic mechanisms of action including Fc-dependent immune effector mechanisms, direct apoptotic activity, and immunomodulatory effects by the elimination of CD38+ immune suppressor cells.
Monoclonal antibody-based therapy has revolutionized MM therapy in the latest years increasing depth of response as we are observing this rate of cases [
1].
Based on their distinct mechanisms of action, favorable toxicity profile, and single agent activity, CD38 antibodies are attractive partners in combination regimens. Indeed, deep responses and prolonged progression- free survival can be achieved in relapsed refractory MM patients when CD38 antibodies are combined with immunomodulatory agents or proteosome inhibitors. In this report we have observed with surprise the benefit of daratumumab with durable and sustained responses in patients with various lines of treatment [
2,
3].
One study assessed the length of daratumumab use across lines of therapy and the probabilities of treatment discontinuation in patients with MM in the real-world. The duration of use was defined as the time between the first dose and discontinuation of daratumumab as a time-to-event outcome using the Kaplan-Meier method. A gap of more than 60 days between two consequent daratumumab claim dates was defined as daratumumab discontinuation. The median duration of continuous daratumumab use was 16.6 months, by 24 months, 33.1% of patients remained on daratumumab treatment. In a subgroup analysis of patients with 12 months or more continuous insurance coverage (n=1246) the median length of daratumumab use was 24.7 months; by 24 months, 51.8% remained on daratumumab treatment [
4].
2. Post-Maintenance Daratumumab Administration Schedule:
Daratumumab 16 mg/kg weekly for weeks 1-8, every two weeks for weeks 9-16, monthly weeks 17 and 18 (Total: 14 applications).
The characteristics of the patients are described in
Table 1.
3. Treatment Protocols:
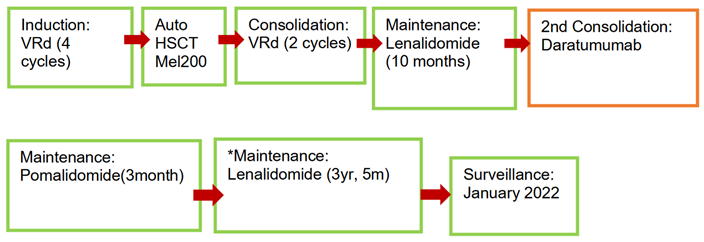
Patient 1
* Switch of lenalidomide to pomalidomide due to intolerance.
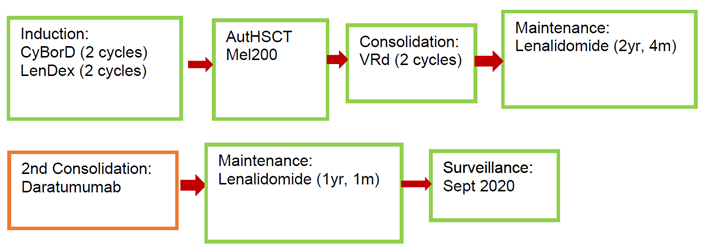
Patient 2
* CyBorD (Cyclofosfamide-Bortezomib-Dexamethasone), LenDex (Lenalidomide-Dexamethasone), Mel200 (Melphalan 200mg).
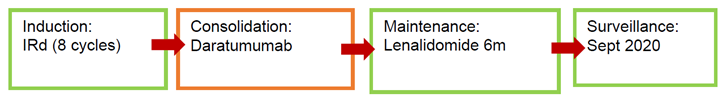
Patient 4
* Ixazomib-Lenalidomide-dexamethasone (IRd).
4. Outcomes
In the first patient complete response (CR) was achieved after induction. Post-maintenance consolidation with daratumumab began due to loss of CR after 10 months of maintenance with lenalidomide monotherapy.
Stringent CR (sCR) with negative Minimal Residual Disease negative (MRD-) has been documented since October 2020 while he was undergoing his second maintenance with lenalidomide. Surveillance begins in January 2022.
In the second patient, CR was achieved after induction. Post-maintenance consolidation with daratumumab in sCR begins after 2 years and 4 months of maintenance with lenalidomide monotherapy.
sCR with MRD (-) is documented since September 2020 while he was in his second maintenance with lenalidomide. Surveillance begins in September 2020.
In the third patient CR was documented after induction treatment with VRd so maintenance with lenalidomide was started. Post-maintenance consolidation with daratumumab was initiated due to loss of complete response after 18 months of monotherapy with lenalidomide. After post-maintenance consolidation, sCR was documented with MRD (-) at the sixth month of the second maintenance period with lenalidomide. It is decided to start surveillance in January 2020.
Patient number 4 achieved CR after induction and subsequently went on to consolidation with daratumumab. He remains in sCR so he is given maintenance with lenalidomide for 6 months. It has been under surveillance since September 2020.
In this series of 4 cases 100% of the patients achieved a strict complete response with undetectable MRD after post-maintenance consolidation with daratumumab regardless of the scheme used as induction/consolidation, as well as whether they had received HSCT. Until the last follow-up the patients are under surveillance with no evidence of progression. It should be noted that 3 out of 4 of these patients debuted with high-risk disease (ISS-R III) as presented in
Table 2.
5. Discussion
The present work shows the benefit derived from adding monoclonal antibodies as consolidation post-maintenance with lenalidomide. Although this strategy is not performed in a standard way the results are promising, achieving deep responses in 100% of patients.
As already well described these responses translated into excellent post-treatment prognoses keeping patients without evidence of disease progression even after discontinuation of treatment.
The surveillance period was observed in a range of 9-33 months with no relapse events, follow-up was performed monthly with serum and urinary electrophoresis and immunofixation measurements, as well as minimal residual disease, Positron Emission Tomography (PET), serum free light chains, and bone marrow biopsy.
Anti CD38 monoclonal antibodies such as daratumumab and isatuximab have been protagonists in improving the depth of the response [
5,
6]. Its use in induction and consolidation has been widely documented in studies, that evaluated the results of bortezomib, thalidomide and dexamethasone (VTD) with or without daratumumab showed an increase in Minimal Residual Disease negativity of 64% vs 44%in patients who received the quadruplet , this increase translated into a 53% decrease in the risk of progression or death as well as in PFS, with a follow-up of 44.5 months the median PFS had not been reached in the Daratumumab -VTD arm while in the VTD arm was 51.5 months,[
7]. the impact was also demonstrated in other study that compared Bortezomib-Lenalidomide-Dexametasone (VRd) versus daratumumab plus VRd, the quadruplet achieve negative MRD in 51% of patients compared to 20.4% in patients in the VRd arm directly impacting PFS al 24 months (95.8 vs 89.8%) [
8].
One of the most important achievements in the outcomes of the grou was the negativization on MRD. Minimal residual disease detection represents a great advancement in multiple myeloma. The International Myeloma Working Group recommends using next-generation flow cytometry (NGF) or next-generation sequencing (NGS) to search for MRD in clinical trials. Best sensitivity thresholds must be confirmed, as well as timing to detect it. MRD has proven as the best prognosticator in many trials and promises to enter also in clinical practice to guide future therapy. Each improvement in the depth of MRD testing has led to superior discrimination of outcomes, and sustained MRD negativity seems to be paramount to durable responses. Peripheral blood assays to assess for MRD are still under investigation but hold promise as complementary tools to bone marrow MRD assays such as next-generation sequencing and flow cytometry. In our work we were able to demonstrate that some patients breach and maintain depth in the response [
9,
10,
11].
In a retrospective study, was presented the outcomes from a multinational and multicenter series of 400 patients with MRD monitoring during front-line therapy with the aim of exploring how clinical decisions made based on those MRD results affected outcomes. As expected, achievement of MRD negativity at any point was associated with improved PFS versus persistent MRD positivity (median PFS 104 vs. 45 months, p < 0.0001). In addition, however, 67 out of 400 patients underwent a clinical decision (treatment discontinuation, intensification, or initiation of a new therapy) based on MRD results. Those patients in whom a treatment change was made showed a prolonged PFS in comparison with those 333 patients in which MRD results were not acted upon (respectively, PFS 104 vs. 62 months, p = 0.005). In patients who achieved MRD negativity during maintenance (n = 186) on at least one occasion, stopping therapy in 24 patients vs. continuing in 162 did not alter PFS (PFS 120 months vs. 82 months, p = 0.1) [
12].
In our study practically 100% of the patients present with negative MRD in different periods of the use of daratumumab, supporting the idea that the use of the monoclonal antibody helps in get a profound response even when the initial response to the treatment line wasn´t that good.
More sophisticated and less invasive studies to measure are already in practice, this is the case of the spectrometry which have enabled reliable and highly sensitive detection of low abundance serum biomarkers making it a viable and significantly less invasive approach. Mass spectrometry can achieve dynamic monitoring of MRD and identify therapeutic monoclonal antibodies as well as oligoclonal proteins. In this review we summarize mass spectrometry methods in M protein detection and their applications of MRD detection in MM.
Mass Spectrometry was established as a sensitive assay for disease monitoring. In different studies was evaluate the performance of EasyM-a noninvasive, sensitive, MS-based assay for M-protein monitoring [
13,
14,
15].
Although recent data have demonstrated very promising results in Daratumumab clinical practice and trials, some patients may do get a complete response or may experience a relapse. Several mechanisms contribute to the development of Daratumumab resistance, including CD38 reduction, the antibody dependent cell-mediated cytotoxicity, the antibody-dependent cellular phagocytosis, the complement-dependent cytotoxicity, and immune-mediated processes [
16].
The reduction in CD38 cell surface expression is a transient phenomenon, because CD38 levels are restored to baseline levels on the MM cells 6 months after the last daratumumab infusion.[
17,
18]. Daratumumab-mediated CD38 reduction is also observed on non-tumor cells, such as normal B-cells, T-cells, NK-cells, and monocytes [
19]. In addition, recent studies showed that daratumumab treatment results in the clustering of CD38 molecules into distinct polar aggregates, which can subsequently be released as tumor-derived microvesicles [
20]. Direct internalization may also contribute to loss of CD38. Finally, active transfer of CD38-daratumumab complexes and accompanying cell membrane from MM cells to monocytes and granulocytes also contributes to CD38 reduction. This process of trogocytosis is in part FcγR-dependent.[
20,
21].
.
In conclusion, the addition of daratumumab at any time during treatment improves its results and therefore improves the survival of patients, with little impact on their quality of life, giving the opportunity to develop more flexible therapeutic schemes in real life that allow clinicians to do use of medications at times other than those strictly indicated based on clinical trials, which are sometimes not so easy to adhere to due to the peculiar characteristics of each patient. Today there are different study methods to measure MRD and be able to design personalized treatment strategies.
At this moment no daratumumab resistance has been demonstrated in our patients.
Author Contributions
All authors approved the final version of the manuscript. Authorship All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics approval and consent to participate this study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
This study does not contain any personal information that could lead to the identification of the patient. Consent for Publication. Written informed consent to publish this information was obtained from the patient of the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gozzetti A, Ciofini S, Simoncelli M, Santoni A, Pacelli P, Raspadori D, Bocchia M. Anti CD38 monoclonal antibodies for multiple myeloma treratment. Hum Vaccine Immunother. 2022;18(5):2052658.
- van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018, 131, 13–29. [CrossRef]
- Stork, M.; Spicka, I.; Radocha, J.; Minarik, J.; Jelinek, T.; Jungova, A.; Pavlicek, P.; Pospisilova, L.; Sedlak, F.; Straub, J.; et al. Daratumumab with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma patients – real world evidence analysis. Ann. Hematol. 2023, 102, 1501–1511. [CrossRef]
- Goldsmith SR, Foley N, Schroeder MA. Daratumumab for the treatment of multiple myeloma. Drugs Today. 2021;57(10):591-605.
- Morè, S.; Corvatta, L.; Manieri, V.M.; Olivieri, A.; Offidani, M. Current Main Topics in Multiple Myeloma. Cancers 2023, 15, 2203. [CrossRef]
- Moreau P., Attal M., Hulin C., Arnulf B., Belhadj K., Benboubker L., Béné M.C., Broijl A., Caillon H., Caillot D., et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomized, open-label, phase 3 study. Lancet. 2019; 394:29–38.
- Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, Chari A, Silbermann R, Costa LJ, Anderson LD Jr, Nathwani N, Shah N, Efebera YA, Holstein SA, Costello C, Jakubowiak A, Wildes TM, Orlowski RZ, Shain KH, Cowan AJ, Murphy S, Lutska Y, Pei H, Ukropec J, Vermeulen J, de Boer C, Hoehn D, Lin TS, Richardson PG. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant elegible newly diagnosed multiple myeloma: the GRIFFIN trial Blood. 2020;136(8):936-945.
- Fonseca R, Chinaeke EE, Gupta-Werner N, Fu AZ, Kaila S. Real word duration of use and dosing frequency of daratumumab in patients with multiple myeloma in the United States. Mayo Clin Proc Innov Qual Outcomes. 2023;7(5):430-436.
- Gozzetti A, Bocchia M. Minimal residual disease in Multiple Myeloma. Rev Recent Clin Trials. 2022;17(1):9-10.
- Bertamini, L.; D’agostino, M.; Gay, F. MRD Assessment in Multiple Myeloma: Progress and Challenges. Curr. Hematol. Malign- Rep. 2021, 16, 162–171. [CrossRef]
- Derman, B.A.; Fonseca, R. Measurable Residual Disease and Decision-Making in Multiple Myeloma. Hematol. Clin. North Am. 2024, 38, 477–495. [CrossRef]
- Martinez-Lopez, J.; Alonso, R.; Wong, S.W.; Rios, R.; Shah, N.; Ruiz-Heredia, Y.; Sanchez-Pina, J.M.; Sanchez, R.; Bahri, N.; Zamanillo, I.; et al. Making clinical decisions based on measurable residual disease improves the outcome in multiple myeloma. J. Hematol. Oncol. 2021, 14, 1–4. [CrossRef]
- Liyasova, M.; McDonald, Z.; Taylor, P.; Gorospe, K.; Xu, X.; Yao, C.; Liu, Q.; Yang, L.; Atenafu, E.G.; Piza, G.; et al. A Personalized Mass Spectrometry–Based Assay to Monitor M-Protein in Patients with Multiple Myeloma (EasyM). Clin. Cancer Res. 2021, 27, 5028–5037. [CrossRef]
- Langerhorst, P.; Noori, S.; Zajec, M.; De Rijke, Y.B.; Gloerich, J.; van Gool, A.J.; Caillon, H.; Joosten, I.; Luider, T.M.; Corre, J.; et al. Multiple Myeloma Minimal Residual Disease Detection: Targeted Mass Spectrometry in Blood vs Next-Generation Sequencing in Bone Marrow. Clin. Chem. 2021, 67, 1689–1698. [CrossRef]
- Wijnands, C.; Langerhorst, P.; Noori, S.; Keizer-Garritsen, J.; Wessels, H.J.; Gloerich, J.; Bonifay, V.; Caillon, H.; Luider, T.M.; van Gool, A.J.; et al. M-protein diagnostics in multiple myeloma patients using ultra-sensitive targeted mass spectrometry and an off-the-shelf calibrator. cclm 2023, 62, 540–550. [CrossRef]
- Saltarella, I.; Desantis, V.; Melaccio, A.; Solimando, A.G.; Lamanuzzi, A.; Ria, R.; Storlazzi, C.T.; Mariggiò, M.A.; Vacca, A.; Frassanito, M.A. Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma. Cells 2020, 9, 167. [CrossRef]
- van de Donk, N.W.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [CrossRef]
- Nijhof, I.S.; Casneuf, T.; van Velzen, J.; van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.W.J.; van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [CrossRef]
- Krejcik J, Frerichs KA, Nijhof IS, van Kessel B, van Velzen JF, Bloem AC, et al.Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res. 2017;23:7498–511.
- Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zito, A.; Roato, I.; Morandi, F.; Marimpietri, D.; Bolzoni, M.; Toscani, D.; Oldham, R.J.; et al. NAD+-Metabolizing Ectoenzymes in Remodeling Tumor–Host Interactions: The Human Myeloma Model. Cells 2015, 4, 520–537. [CrossRef]
- Barabas, A.Z.; Cole, C.D.; Graeff, R.M.; Morcol, T.; Lafreniere, R. A novel modified vaccination technique produces IgG antibodies that cause complement-mediated lysis of multiple myeloma cells carrying CD38 antigen. Hum. Antibodies 2017, 24, 45–51. [CrossRef]
| Gender |
Age at Diagnosis |
MM Type |
ISS-R |
Genetic Profile |
| Male |
53 yr |
IgA Lambda |
I |
46, XY |
| Male |
50 yr |
IgG Kappa |
III |
Monosomy Cr. 13, del(3), t(4;14) FGFR3-IGH |
| Fem |
65 yr |
IgA Kappa |
III |
46, XX |
| Fem |
67 yr |
Kappa |
III |
t(14;16), monosomy cr13, hypodiploidy (gain Crs.9, 11, and 15) |
| Gender |
Age at Diagnosis |
MM Type |
ISS-R |
Surveillance Time (Free Treatment Period) |
| Male |
53 yr |
IgA Lambda |
I |
9 months (january 2022) |
| Male |
50 yr |
IgG Kappa |
III |
25 months (september 2020) |
| Fem |
65 yr |
IgA Kappa |
III |
33 months (january 2020) |
| Fem |
67 yr |
Kappa |
III |
25 months (september 2020) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).