Introduction
Fold change is widely used in biomedical research to quantify the magnitude of group differences in omics variables, initially mainly in gene expression studies but nowadays adopted in other "omics" fields and even non-omics research, as evidenced by a PubMed search for "fold change", where the term was found to be associated with a variety of fields beyond gene expression studies (
Figure 1). Fold-change calculation can significantly influence the interpretation of results and subsequent decision-making processes in biomedical research. Gene lists from microarray studies generated by fold-change ranking were more reproducible than those obtained by t-test p-value or other significance analyses [
1,
2], and fold-change is a potential criterion for univariate feature selection [
3], alone or as a complement to other machine learning-based methods for omics analysis [
4].
Fold change is often visualized in "volcano plots" [
5] using a
on the y-axis and log
2-ratio of signals between treatment and reference groups on the x-axis. However, the exact calculation method is often not reported, although several methods exist. The definition of “average” (such as arithmetic or geometric mean) to quantify group expression levels can lead to inconsistencies. Despite previous studies downplaying the consequences [
5,
6,
7], it seems crucial to carefully choose the most robust method against potential violations of standard assumptions about the data distribution and variance. This report reevaluates the influence of various fold-change calculation methods on fold-change values and aims to recommend a preferred approach.
Methods
Retrieval of Fold Change Reporting in Biomedical Publications
On March 23, 2024, a PubMed (
https://pubmed.ncbi.nlm.nih.gov/) search was performed using the query "("fold change" NOT (review[PT])". The R package "easyPubMed" (
https://cran.r-project.org/package=easyPubMed [
8]) was used to retrieve details of the papers, including titles, abstracts, and publication years. To identify the main topics where fold-change reporting is common, words in the abstracts were filtered against generic text using the R package ‘PubMedWordcloud’ (
https://cran.r-project.org/package=PubMedWordcloud [
9]). Recursive cABC analysis [
10], an item categorization technique, was then applied to the frequency of the remaining words using the R library "ABCanalysis" (
https://cran.r-project.org/package=ABCanalysis [
11]). Biomedical domain experts identified biomedical topics based on the relevant terms. The type of omics research covered was determined by applying another cABC analysis to the occurrences of words containing the substring "omics".
Common Basic Variants of the Fold-Change Calculation
There are numerous descriptions of fold change calculation, such as in [
12]. The calculation is based on the log ratio between the treatment and reference groups of a biological signal found in a dataset, i.e., the values of a variable of interest. One common way of calculating the signal log ratio is:
where
and
are the positive, non-zero expected values of variable
measured under two different conditions a and b, such as before (
) and after (
) a treatment, or control (
) versus patient (
). The value of fold change,
, is then obtained as [
12]:
Pairwise Test/Reference Ratio Calculation
As an alternative approach, fold-change can be calculated by taking the ratio of each paired value from variable
and variable
. Let
be a vector of length
and
be a vector of length
. Then, for all possible combinations of an element of
with an element of
, expressed as
, the log ratio can be calculated as:
If the experimental design involves matched samples (e.g., before-and-after measurements, case-control studies), the case-wise calculation naturally incorporates this pairing. If some cases have missing values in either the treatment or reference group, the case-wise calculation can still proceed with the available pairings.
Comparative Evaluation of Common Calculation Methods
Evaluations were coded in the R language [
15] using the R software package [
16], version 4.4.0 for Linux (
https://CRAN.R-project.org/), and in the matrix laboratory language using MATLAB (version 23.2.0.2485118 (R2023b)) and run on an AMD Ryzen Threadripper 3970X (Advanced Micro Devices, Inc., Santa Clara, CA, USA) desktop computer and an Intel® (Intel Corporation, Santa Clara, CA, USA) Core
TM i7-13700H notebook computer, both running on Ubuntu Linux 22.04.4 LTS (Canonical, London, UK). The equations used in the experiments are summarized in
Table 1, along with the abbreviations or acronyms of the methods used in this report.
Evaluation of the Role of the Data Distribution for the Correct Calculation of FC
Fold change calculations were evaluated on data generated to represent different distributions, including normal and log-normal, as well as identity, uniform, and mixed, where the latter is a random mixture of the four former distributions (Data set # 1;
Table 2). Two vectors
and
were created, with vector
with serving as the reference and the values of vector
being
times the values of vector
. Thus, the ratios between
and
were known at the time of data generation allowing comparison of the
values obtained by different calculation methods with the true values. Different combinations of
with different standard deviations of the sample sizes of
and
were performed using different calculation methods of
.
Evaluation of the Role of Variance Equality for the Correct Calculation of FC
Since the experiments above highlighted specific issues with the log-normal distribution for certain variants of
calculation, and considering the frequent log-normal distribution of biological datasets, including non-omics data such as psychophysical measurements [
17], and many others, the effect of different values of
and different standard deviations of treatment (
) and reference (
)) on the accuracy of fold change recovery was explored in log-normally distributed data (Data set # 2). Across a wide range of simulated
and
= scenarios, with values of
, we quantified the errors in fold change estimates as
Evaluation of the Relationship of FC Calculation to Statistical Outcomes
The two components of a volcano plot, fold change (
) respectively the log ratio of treatment and reference and the statistical significance (
), were further evaluated using a simulated dataset #3 containing variables with either normal or log-normal distributions. This dataset comprised 99 pairs of vectors,
(reference) and
(treatment), generated by randomly drawing values for means
, standard deviations
and
, and values of
from predefined ranges. Different combinations of
with different standard deviations
. Experiments were conducted on these 99 pairs of vectors
and
, employing different calculations for
(mean of logs, log of means, paired approach) in combination with both nonparametric (Wilcoxon-Mann-Whitney U test [
18,
19]) and parametric (t-test [
20]) statistical methods for comparing the vectors
and
in each of the d = 99 variables. The correlations of the absolute values of
with the values of
were assessed by calculating Spearman’s
[
21].
Evaluation of FC Calculation Method Dependency in Biomedical Data
Fold change calculations are widely available in the biomedical literature. Therefore, for the present reassessment of fold-change calculation methods, the analysis was limited to an extended multi-omics dataset (Data set # 3). It originates from recent rheumatologic research and consists of an ongoing omics study of a cohort clinically described in [
22]. This cross-sectional study of patients with rheumatic diseases was conducted in accordance with the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the Ethics Committee of the Medical Faculty of the Goethe University, Frankfurt am Main, Germany (approval number 19-492_5). Informed written consent was obtained from each participant. For the present analysis, a subset of cases consisting of n = 95 patients with psoriatic arthritis and n = 50 healthy controls have been used. The omics assessments included d = 680 plasma concentrations of d = 328 proteins from an inflammatory panel and d = 352 lipid markers.
Results
Reporting Styles of Fold-Change Calculation in Biomedical Publications
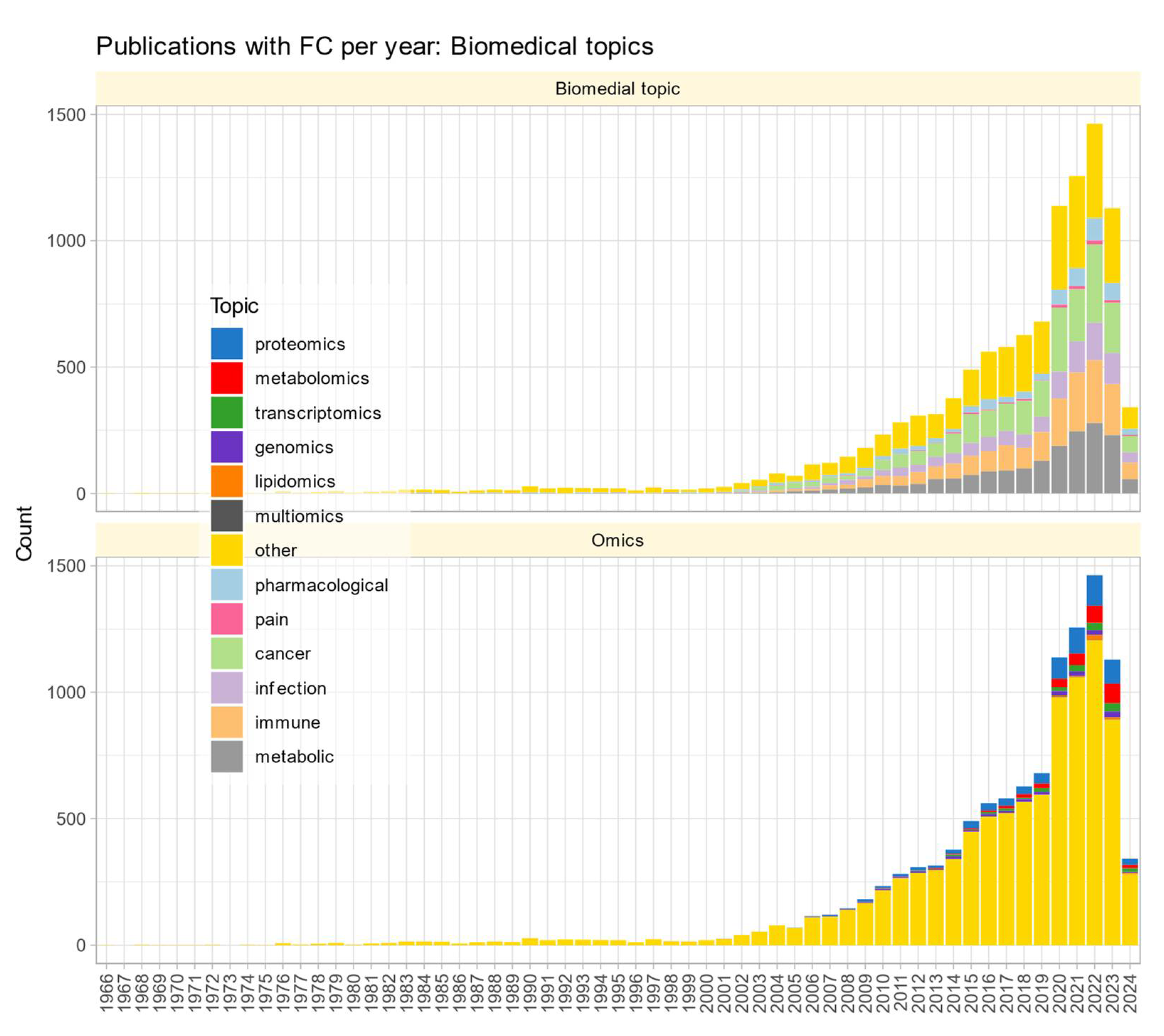
The search of the PubMed on March 23, 2024, using the query "("fold change" NOT (review[PT])" returned 10,978 results (
Figure 1). However, the true prevalence of fold change reporting is likely much higher, as researchers often employ fold change calculations and visualizations without explicitly using the term in titles, keywords, or abstracts. The number of publications per year has been increasing steadily since the turn of the century. An analysis of the context in which
reporting of omics research results is most common revealed five main biomedical topics (pharmacological research, cancer, infection, immune processes, metabolism) and seven variants of omics research (proteomics, metabolomics, transcriptomics, genomics, lipidomics, multiomics, toxicogenomics;
Figure 1).
The exact calculation of fold-change (
) values is rarely reported in the literature. A review of over 200 papers found that only about 5% mentioned the
calculation method, often in the context of informatics approaches to differential expression analysis rather than the use of
in reporting biomedical findings. Among the few relevant papers, some used the arithmetic mean [
12,
23], while others mentioned log transformation, hinting at the use of the geometric mean [
24], though this was rarely stated explicitly. Additionally,
is sometimes calculated from pre-transformed data, such as in the
method [
25] as a standard in PCR data analysis [
26].
Role of the Data Distribution for the Correct Calculation of FC
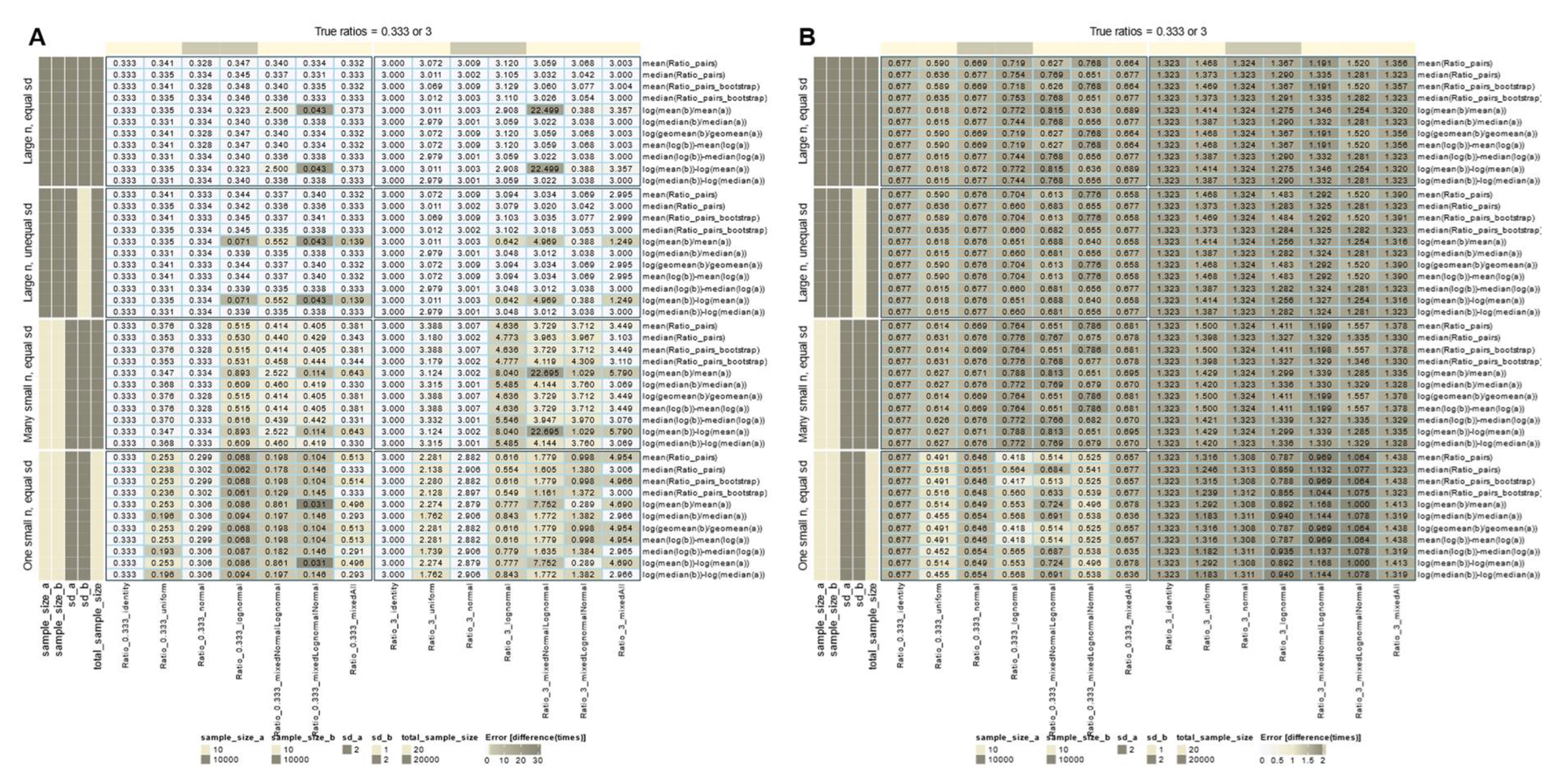
When the data distribution was normal, identity, or uniform, and the sample size was large (n = 10,000), all calculation methods accurately recovered the true treatment-to-reference ratios (
Figure 2 A). However, when lognormal data were included, recovery was confounded by various conditions: most methods succeeded when the standard deviations of the treatment and reference groups were equal, except for the ratio of arithmetic means when the treatment and reference distributions were different. In contrast, all methods except the ratio of arithmetic means were robust to unequal variances. Recovery deteriorated drastically with small sample sizes, especially with lognormal data, and none of the methods provided accurate results. Repeating the experiments with small sample sizes slightly improved recovery for normal or identity distributions.
The intuitive use of fold-change assumes absolute expression levels. However, a pitfall arises when using pre-transformed data from standard workflows, without realizing it. Applying additional log-transformations means log-transforming already log-transformed data (
Figure 2 B). Rankings of variables may be preserved, but calculated fold changes no longer represent "times expression", only arbitrary numbers, possibly better expressed as "times signal" as a more neutral description. When comparing studies or multi-omics data from different workflows, changes in fold change magnitude interpretation and comparability become relevant.
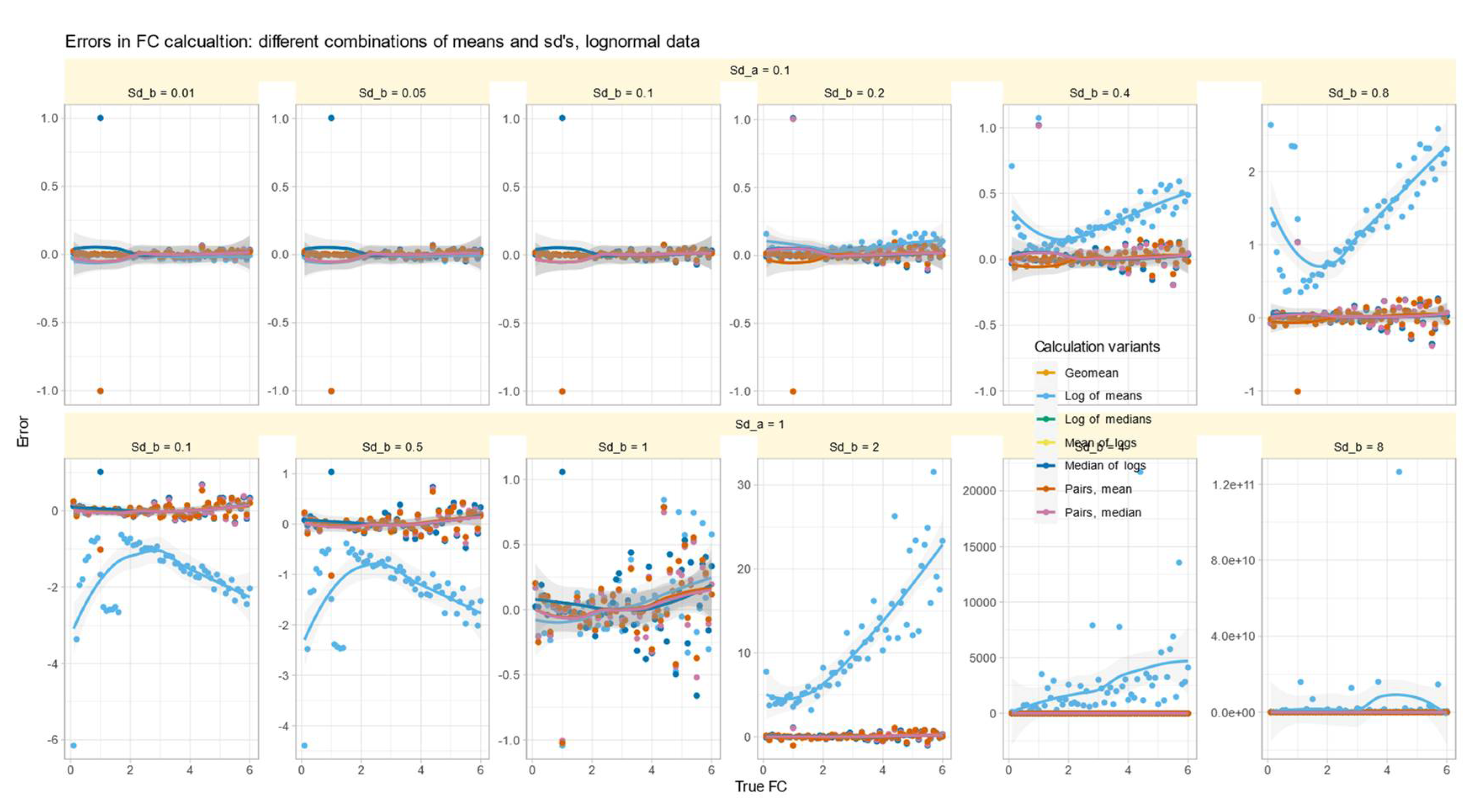
Role of Variance Equality for the Correct Calculation of FC
The errors in test/reference ratios calculated by major methods in Data Set #2 were analyzed for log-normal data with varying standard deviations. The results (
Figure 3) showed that all methods except those based on arithmetic means made small errors of about 0.1 times more or less than the true factor. The arithmetic mean-based method had varying effects depending on the fold change and standard deviation of the test relative to the reference. Specifically, when
and
were not equal, the error reversed direction at a value of
. That is, when
and
, the arithmetic mean-based method overestimated the value of
, was close to zero when
, and underestimated the error when
. Thus, the success of correctly estimating
by arithmetic means depended first on the similarity of the standard deviations. This recalls a precondition of
calculation mentioned in [
5], namely that
can be considered as the special case when the variances of all genes are equal.
Relationship between Calculated Fold Change and Statistical Significance
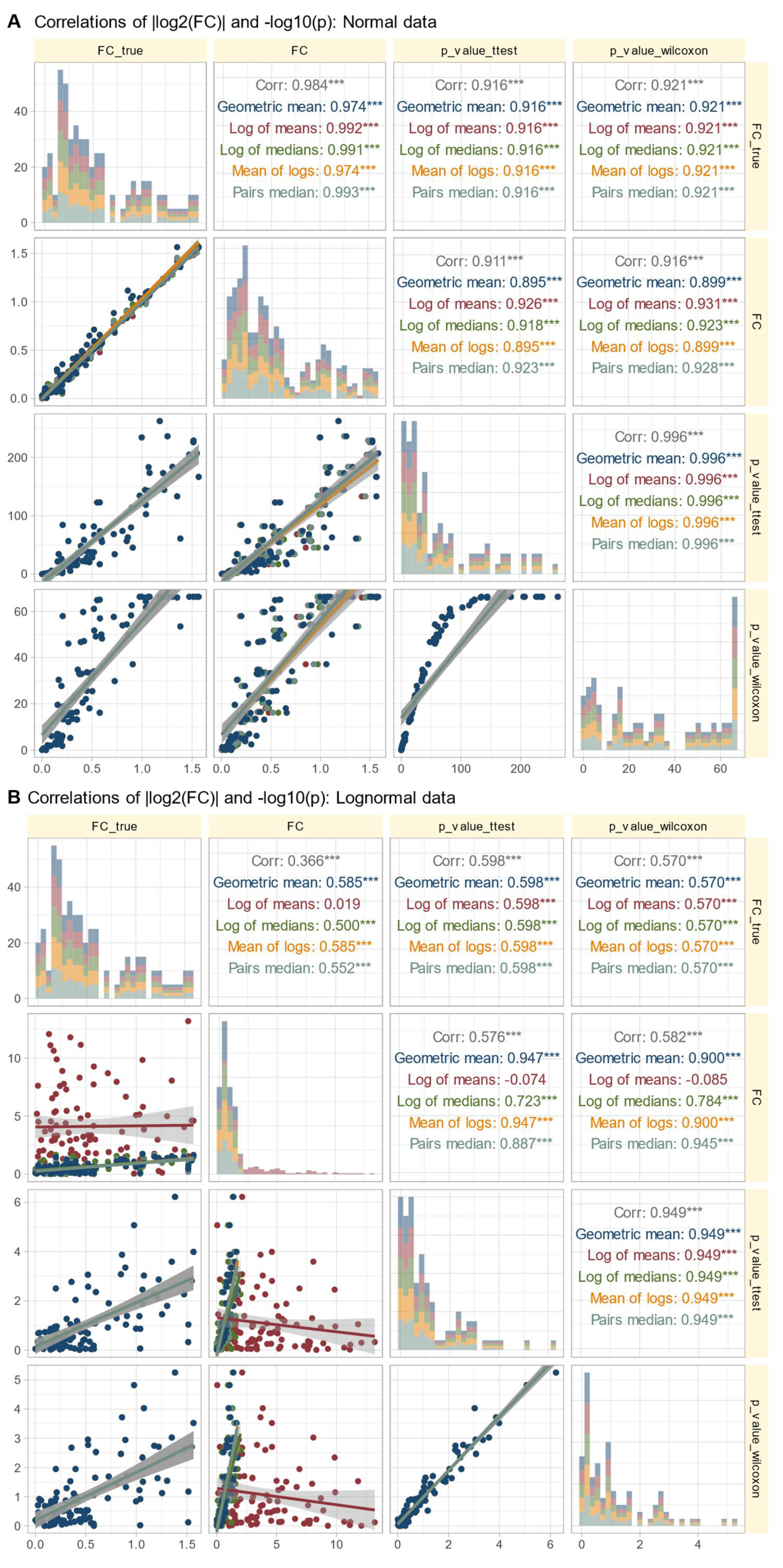
Intuitively, higher fold changes tend to be associated with higher statistical significance because larger expression differences between conditions are more likely to be statistically significant, assuming consistent variability within each condition. However, deviations from this intuitive expectation can occur due to high and different variability within and between conditions, leading to lower significance despite large fold changes.
Data Set #3 tested the robustness of fold change (FC) calculation by combining different fold changes with varying standard deviations in normally and log-normally distributed data In normal distributed data, the choice of
calculation method was nevertheless irrelevant to the results (
Figure 4 A). The obtained values of
correlated with the true values of
and with the statistical significance of the group comparisons, and the correlations were quite similar regardless of the
calculation method. The picture changed somewhat for log-normal data (
Figure 4 B), where it became clear that calculating
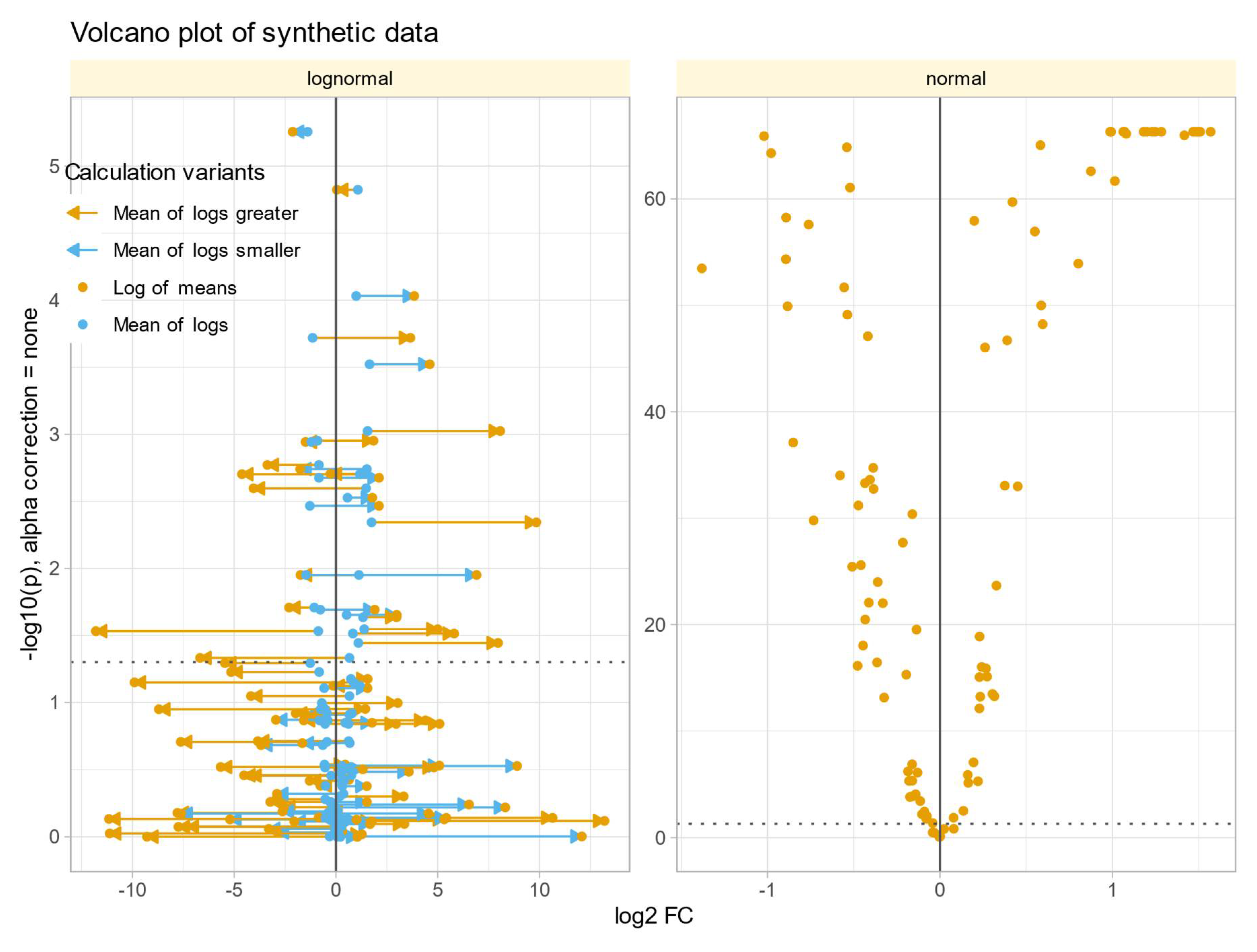
using the logarithm of the mean was associated with a higher risk of inaccuracy than the alternatives. This was evident in the volcano plot (
Figure 5), where extreme cases showed crossover from downregulation to upregulation or vice versa.
FC Calculation Method Dependency in Biomedical Data
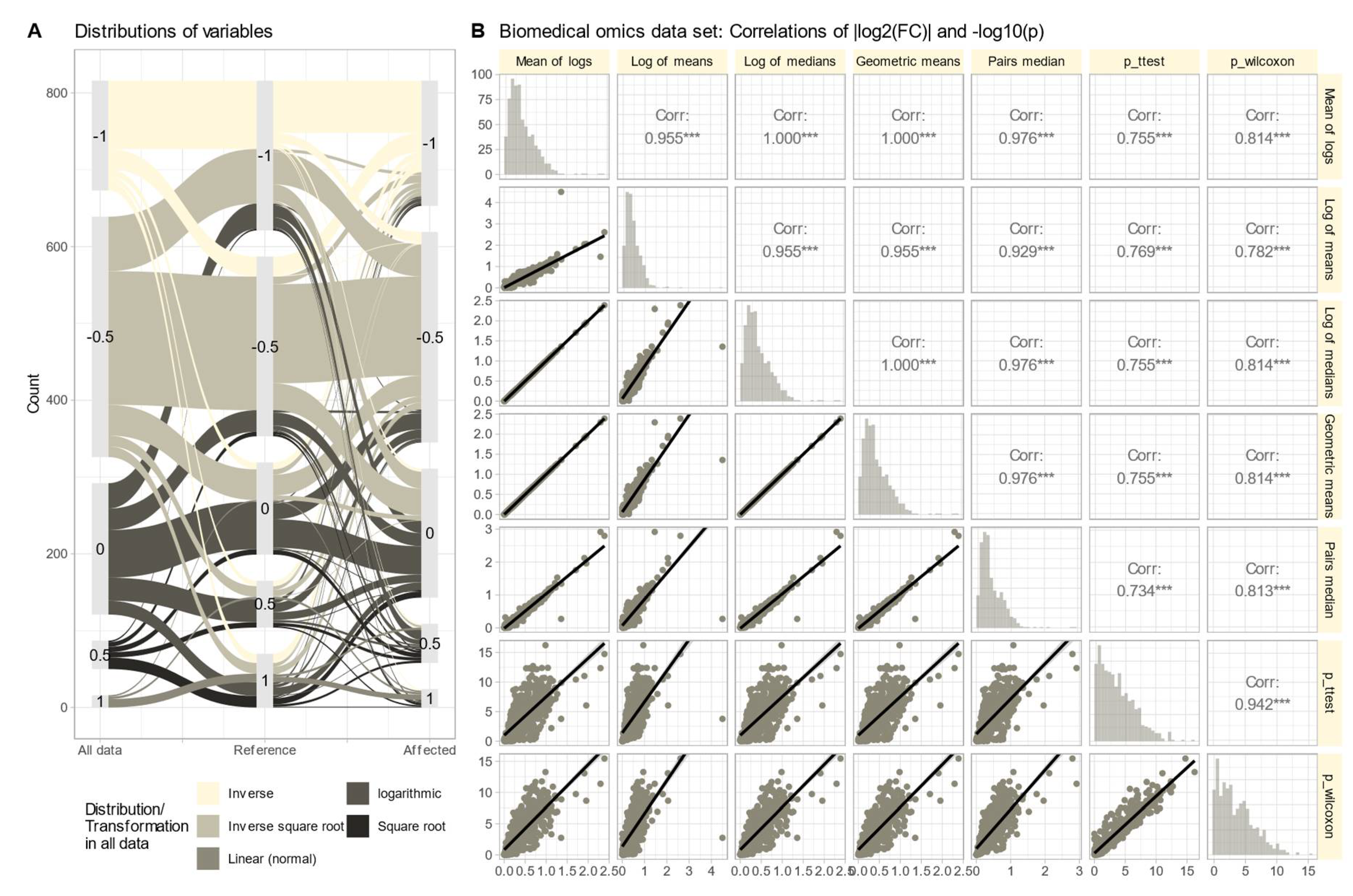
The omics data set #4 from rheumatology research showed diverse distributions, with only 14.3% of variables normally distributed in the raw data (
Figure 6 A). Log-transforming the data increased normality to 48.1%. Therefore, Box-Cox transformation [
27] was used with aligning the obtained values of
with the steps of Tukey’s ladder of power [
28]. The calculation of FC using different methods (logarithm of means, means of logs, etc.;
Figure 6 B) yielded similar results, with high correlations between FC values and statistical significances in parametric and nonparametric tests. Further stressing of the calculations by complete permutation of the entire data matrices in the x- and y-directions resulted in highly skewed data (skewness = 23.4, kurtosis = 837.6). Then, the correlations between methods decreased, and the pairwise method showed the strongest correlation with nonparametric test results (
Figure 7).
Discussion
Fold change calculation is widely used to describe the effect of a treatment on a measurement or group differences. Despite its ease of calculation, the exact method used is often not reported. Although different methods exist, they appear to give similar results. However, a re-evaluation of these methods found that one method, using the arithmetic mean, is less robust to anomalies in the data distribution and can result in incorrect fold change values more frequently than others. Unfortunately, this is often cited as the standard method for
calculation [
7,
29].
The present experiments highlight the importance of sample size, distribution, and variance for accurate fold change calculation. For small sample sizes (e.g., n = 10), none of the methods could accurately reproduce true ratios. This limitation has been addressed in studies on sample size calculation for differential expression [
30,
31], but variance estimation from small samples can be generally unreliable [
5]. In addition, if the
calculation is considered as a special case when the variances of all genes are equal, the calculation via the supposed standard equation can go wrong. The present biomedical data showed different distributions of the test and reference data subsets (
Figure 6 A), albeit to a degree that induced moderate consequences, but nevertheless show the possibility of such a scenario. The arithmetic mean fold change calculation was most sensitive to violations of assumptions, and the logarithmic calculation performed worst when data were log-normally distributed. In settings with unequal distributions, the arithmetic mean calculation diverged from true values, while alternative equations provided accurate results and maintained correlation with statistical significance.
In the present experiments, different mathematically identical calculation equation variants were used to improve clarity and serve as internal validation. However, we could not reproduce the distinction between
and
made in previous work [
7], as it seemed to interpret a difference as a ratio [
32]. Here, we interpreted fold change exclusively as a ratio. Evaluations focused on calculating numerical fold change values without further refinement of up- or downregulation [
33]. Comprehensive assessments highlight the need for more precise methods, especially for single-cell RNA-seq data [
34], as the chosen statistical or fold change cutoff can provide multiple answers for microarray analysis [
35].
Finally, the recommendation to use the median or geometric mean to quantify the expected value or group mean must be combined with the warning that even these values are not insensitive to unusual data constellations. The geometric mean is usually appropriate for lognormal data. The median is more general but can also be misleading. For example, in the analysis of right-skewed distributions observed in the analysis of social dynamics, the median can often be more misleading than the mean [
36], and such scenarios are also not excluded in biomedical data. Above all, this simple example emphasizes that careful data exploration during preprocessing including the adequate visualization of raw data [
37], as an essential part of the omics data analysis workflow, cannot be replaced by an unquestioned standard procedure rigidly applied to the data at hand.
The recommendation to use the median or geometric mean must be combined with the warning that these values are not insensitive to unusual data constellations. The geometric mean is usually appropriate for lognormal data, while the median can be misleading, especially for right-skewed distributions [
36]. This emphasizes the importance of careful data exploration during preprocessing, including adequate visualization of raw data [
37], as an essential part of the omics data analysis workflow that cannot be replaced by unquestioned standard procedures.
Conclusions
Fold change reporting is widely used to summarize differential expression patterns, but the exact calculation method is often unclear. Different equations can produce different results, especially when data distributions are unequal. To ensure accurate interpretation and reproducibility, it is crucial to use methods less sensitive to data distribution and accurately report the calculation methods used [
38]. The inferior arithmetic mean-based method is often perceived as the standard, despite mathematically different equations being possible that mainly differ in the estimation of the expected value. In conclusion, the choice of fold-change calculation method can significantly influence the interpretation of results and subsequent decision-making processes in biomedical research. Adopting less vulnerable methods and transparent reporting is a reasonable practice to ensure correct interpretation and reproducibility.
Declarations
Ethics approval
The biomedical dataset has been acquired in a study that followed the recommendations of the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the Ethics Committee of the Medical Faculty of the Goethe University, Frankfurt am Main, Germany (approval number 19-492_5)
Consent for Publication
Written informed consent, including anonymized publication, was obtained from all participants.
Author Contributions
Background, programming, execution of the experiments, writing of the manuscript, data analysis and preparation of figures, acquisition of funding. DK - Literature research and manuscript writing AU - Scientific advice, mathematical supervision, programming and execution of experiments, writing the manuscript.
Funding
JL was supported by the Deutsche Forschungsgemeinschaft (DFG Lo 612/16-1).
Data Availability Statement
The biomedical dataset used in the experiments in this report is available from the first author upon reasonable request and subject to approval by the appropriate ethics committee.
Conflicts of Interest
None declared.
References
- Guo, L.; Lobenhofer, E.K.; Wang, C.; Shippy, R.; Harris, S.C.; Zhang, L.; Mei, N.; Chen, T.; Herman, D.; Goodsaid, F.M.; et al. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol 2006, 24, 1162–1169. [Google Scholar] [CrossRef]
- Dembélé, D.; Kastner, P. Fold change rank ordering statistics: a new method for detecting differentially expressed genes.
- Guyon, I. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Cheng, J.; Liu, H.-P.; Lin, W.-Y.; Tsai, F.-J. Machine learning compensates fold-change method and highlights oxidative phosphorylation in the brain transcriptome of Alzheimer’s disease. Scientific Reports 2021, 11, 13704. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Volcano plots in analyzing differential expressions with mRNA microarrays. J Bioinform Comput Biol 2012, 10, 1231003. [Google Scholar] [CrossRef]
- Dembélé, D.; Kastner, P. Fold change rank ordering statistics: a new method for detecting differentially expressed genes. BMC Bioinformatics 2014, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Witten, D.; Tibshirani, R. A comparison of fold-change and the t-statistic for microarray data analysis. 1–17. 2007.
- Fantini, D. easyPubMed: Search and Retrieve Scientific Publication Records from PubMed, 2019.
- Fan, F.Y. PubMedWordcloud: ’Pubmed’ Word Clouds_. R package version 0.3.6, <https://CRAN.R-project.org/package=PubMedWordcloud>, 2019.
- Lötsch, J.; Ultsch, A. Recursive computed ABC (cABC) analysis as a precise method for reducing machine learning based feature sets to their minimum informative size. Sci Rep 2023, 13, 5470. [Google Scholar] [CrossRef]
- Ultsch, A.; Lötsch, J. Computed ABC Analysis for Rational Selection of Most Informative Variables in Multivariate Data. PLoS One 2015, 10, e0129767. [Google Scholar] [CrossRef] [PubMed]
- Tadros, S.F.; D’Souza, M.; Zhu, X.; Frisina, R.D. Gene expression changes for antioxidants pathways in the mouse cochlea: relations to age-related hearing deficits. PLoS One 2014, 9, e90279. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.; Johnson, W.D.; Marshall, G.D. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol 2008, 100, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Thatte, J.; Braciale, T.; Ley, K.; O’Connell, M.; Lee, J.K. Local-pooled-error test for identifying differentially expressed genes with a small number of replicated microarrays. Bioinformatics 2003, 19, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics 1996, 5, 299–314. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2008.
- Fechner, G.T. Elemente der Psychophysik; Breitkopf and Härtel: Leipzig, 1860. [Google Scholar]
- Wilcoxon, F. Individual comparisons by ranking methods. Biometrics 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Student. The Probable Error of a Mean. Biometrika 1908, 6. [Google Scholar]
- Spearman, C. The proof and measurement of association between two things. Am J Psychol 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Rischke, S.; Poor, S.M.; Gurke, R.; Hahnefeld, L.; Köhm, M.; Ultsch, A.; Geisslinger, G.; Behrens, F.; Lötsch, J. Machine learning identifies right index finger tenderness as key signal of DAS28-CRP based psoriatic arthritis activity. Sci Rep 2023, 13, 22710. [Google Scholar] [CrossRef]
- Kumar, N.; Hoque, M.A.; Sugimoto, M. Robust volcano plot: identification of differential metabolites in the presence of outliers. BMC Bioinformatics 2018, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Hauber, A.L.; Rosenblatt, M.; Timmer, J. Uncovering specific mechanisms across cell types in dynamical models. PLoS Comput Biol 2023, 19, e1010867. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Hu, J.; Spencer, T.; Carroll, R.; Wu, G. Statistical models in assessing fold change of gene expression in real-time RT-PCR experiments. Comput Biol Chem 2006, 30, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Cox, D.R. An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological) 1964, 211–252. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, Mass, 1977. [Google Scholar]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001, 98, 5116–5121. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Su, P.F.; Shyr, Y. Sample size calculation based on exact test for assessing differential expression analysis in RNA-seq data. BMC Bioinformatics 2013, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Liu, P. Sample size calculation while controlling false discovery rate for differential expression analysis with RNA-sequencing experiments. BMC Bioinformatics 2016, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.E.; Boutros, M.; Michelson, A.M.; Church, G.M.; Halfon, M.S. Preferred analysis methods for Affymetrix GeneChips revealed by a wholly defined control dataset. Genome Biology 2005, 6, R16. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.A.; Kendziorski, C.M.; Richmond, C.S.; Blattner, F.R.; Tsui, K.W. On Differential Variability of Expression Ratios: Improving Statistical Inference about Gene Expression Changes from Microarray Data. Journal of Computational Biology 2001, 8, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, B.; Nelson, C.E.; Nabavi, S. Comparative analysis of differential gene expression analysis tools for single-cell RNA sequencing data. BMC Bioinformatics 2019, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinformatics 2012, 13 Suppl 2, S11. [Google Scholar] [CrossRef]
- Kämpke, T. The use of mean values vs. medians in inequality analysis. Journal of economic and social measurement 2010, 35, 43–62. [Google Scholar] [CrossRef]
- Lötsch, J.; Ultsch, A. Comments on the importance of visualizing the distribution of pain-related data. European journal of pain (London, England). [CrossRef]
- National Academies of Sciences, E.; Medicine; Policy; Global, A.; Committee on Science, E.M.; Public, P.; Board on Research, D.; Information; Division on, E.; Physical, S.; et al. In Reproducibility and Replicability in Science; National Academies Press (US): Washington (DC), 2019.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag New York: 2009.
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Schloerke, B.; Crowley, J.; Cook, D.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Larmarange, J. GGally: Extension to ’ggplot2’, 2018.
- Pedersen, T.L. ggforce: Accelerating ’ggplot2’, 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).