Introduction
Cystic artery bleeding (CAB) is a rare entity arising from both traumatic and non-traumatic causes. The clinical presentation exhibits significant heterogeneity contingent upon the underlying aetiology. Manifestations can range from intraperitoneal haemorrhage to upper gastrointestinal bleeding, encompassing both haemobilia (blood in the biliary tree) and haemorrhagic shock.
Radiological imaging plays a pivotal role in the management of cystic artery haemorrhage. Its non-invasive nature allows for visualisation of the vasculature and identification of the bleeding source and offers the ability to differentiate cystic artery bleeding from other aetiologies of abdominal pain and haemorrhage, guiding appropriate treatment strategies.
In traumatic CAB, gallbladder injuries, while uncommon (approximately 2% in blunt abdominal trauma [
5,
6]) due to protective surrounding organs, can occur. These injuries are classified as contusion, avulsion, and laceration (perforation). Cystic artery trauma may or may not accompany a gallbladder injury. Penetrating trauma often involves direct vessel damage with coexisting lacerations of the liver parenchyma and gallbladder along the injury path. Blunt trauma can induce isolated cystic artery injury via shearing forces between the gallbladder and hepatic parenchyma [
5] [
7,
8], leading to the rupture of a post-traumatic pseudoaneurysm or direct avulsion, laceration, or transection of the cystic artery [
9].
Non-traumatic CAB typically involves a cystic artery pseudoaneurysm (CAP) as the primary pathogenetic mechanism. Primary CAP is infrequent [
10].
More commonly, CAP develops secondary to vascular injury following laparoscopic cholecystectomy, arterial access procedures, liver biopsy, ERCP, or liver transplantation. The median time to clinical presentation of CAP bleeding after cholecystectomy is around 50 days, with a range of eight days to three years [
11]. Additionally, it may arise secondary to inflammation-mediated processes like acute cholecystitis or pancreatitis and has been documented in association with gallbladder tumours. The precise pathogenesis remains unclear, although CAP formation is believed to be a consequence of pathological alterations that weaken and erode the muscular and elastic layers of the arterial wall. [
7,
11,
12]. This paper aims to educate radiologists on the key CT imaging features of CAB and CAP.
Vascular Anatomy
The cystic artery most commonly arises as a single vessel from the right hepatic artery, and it runs posteriorly to the cystic duct inside the hepatobiliary triangle. The hepatobiliary triangle is delineated superiorly by the inferior border of the liver, medially the common hepatic duct, and inferiorly the cystic duct and it contains the right hepatic artery and its branch; the cystic artery, the cystic node, lymphatics, and connective tissue[
1,
2]. As the cystic artery approaches the gallbladder, it divides into two branches, the larger anterior superficial that runs below the serosal tunic on the left side of the gallbladder and the smaller posterior deep branch that courses between the gallbladder and the gallbladder fossa[
3]. The two branches then form anastomoses in the gallbladder parenchyma[
2,
3]. Cystic artery can have a short or long length, or multiple cystic arteries can be found[
4]. Variations of cystic artery anatomy can be related to its origin and/or pathway and have been reported in up to 31.9% of patients[
4]. The cystic artery can rarely originate from other branches of the celiac axis or from a replaced or accessory right hepatic artery. Cystic artery pathway variants are related to its anomalous course anteriorly to the common hepatic or bile ducts, and inferiorly to the cystic duct[
2,
4].
Symptoms
The clinical presentation is extremely variable. In traumatic conditions, patients classically present signs of hemoperitoneum. Instead, the diagnosis of non-traumatic and iatrogenic conditions may be challenging. Patil et [
5] al reported that the classic Quincke’s triad of haemobilia (jaundice, right upper quadrant pain and upper gastrointestinal bleeding with hematemesis or melena) was present in 32.2
% of patients. In comparison, abdominal pain (77.9%) and upper GI bleed (64.4%) were the common presentation. Clinical presentation of pseudoaneurysms includes haemobilia, hematemesis, and abdominal pain[
6].
It’s worrying that Pali et al. reported that in 83% of patients, the diagnosis was at the time of CAP rupture, among them 19.4% presented with haemorrhagic shock. Laboratory usually shows hyperbilirubinemia and anaemia in almost half of the patients[
5,
7]
.
Imaging
Ultrasonography (US) can be the first line imaging modality to pose suspects of cystic artery damage and bleeding especially for the detection of cystic artery pseudoaneurysms. In cases of ruptured pseudoaneurysms, the gallbladder may exhibit hyperechoic fluid. Additionally, colour Doppler US has the potential to visualize the characteristic "Yin-Yang" flow pattern within the pseudoaneurysm itself.
In Trauma scenarios and non-sumbrative ones, triple-phase CT angiography offers high sensitivity for detecting cystic artery bleeding. This modality can frequently provide additional insight into the underlying aetiology, such as acute cholecystitis. Furthermore, it plays a critical role in the planning of percutaneous treatment by enabling the identification of aberrant vasculature and anatomical variations.[
8,
9].
At non-enhanced CT, characteristically, one key finding is the presence of hyperdense fluid that embraces the gallbladder, this anatomical disposition of the hemoperitoneum should alert radiologists [
10].
Following intravenous (IV) contrast administration, extravasation of contrast material may be evident within the gallbladder fossa, potentially along the course of the cystic artery’s anterior or posterior branches. A cystic artery pseudoaneurysm (CAP) can be identified as a hyperenhancing focus during the arterial phase along the cystic artery branches. This finding demonstrates a change in attenuation but not morphology in the venous and delayed phases (
Figure 1). In CAP active bleeding extravasation, is detected around the oval/round pseudo aneurysmatic sac. Because of the possible presence of pre-existing gallbladder stones or surgical clips for previous cholecystectomy, images should be examined carefully in different CT phases in order to differentiate hyperdense material appearing in an angiographic phase that should not be present at the same location in non-contrast images, delayed images can be useful in differentiating between active extravasation or CAP and relatively benign process [
10,
11,
12,
13,
14].
Treatment
Cystic artery bleeding related to or not to CAP is a rare event, and the therapeutical approach relies on the patient’s general condition and the anaesthesiologist’s risk. Surgery with cholecystectomy and proximal ligation or clipping of cystic artery is considered the gold standard but clinical scenarios such as in polytraumatized patients or poor general patient conditions may influence the treatment[
15] [
14]. Transarterial embolization (TAE) can be performed with high success rates and cessation of bleeding in up to 90% of patients[
11,
12,
13,
14,
15]. Percutaneous selective cystic artery embolization (CAE) has emerged as an efficacious treatment strategy in the acute setting for patients with CAP or CAB. Compared to surgical intervention, CAE offers advantages such as reduced mortality and morbidity, improved identification of the bleeding vessel, and higher rates of achieving haemorrhage control[
16].
Embolization techniques typically involve coils, glue injection, or Gelfoam injection. Coil embolization is the preferred method due to its versatility in treating vessels of varying sizes and its ability to be introduced without the risk of increased pressure within the vascular lesion, a potential complication associated with injectable embolic agents like glue or Gelfoam[
17].
While established as a safe procedure, CAE is not without its risks. Non-target embolization of hepatic parenchyma can occur, exposing patients to the rare complications typically associated with hepatic artery embolization, such as ischemic hepatitis and abscess formation. Post-procedural monitoring is therefore crucial for identifying these potential complications.[
7]
Superselective embolization of the anterior or posterior branch of the cystic artery, when possible, reduces the risk of postoperative complication thanks to the anastomotic net.
In penetrating trauma or when computed tomography (CT) and angiography fail to provide a definitive diagnosis, laparoscopy becomes the preferred diagnostic and therapeutic modality [
21].
TRAUMATIC CYSTIC ARTERY BLEEDING
In trauma settings, multiphase CT represents the gold standard in stable patients as the first diagnostic step; CT can assess the presence of hemoperitoneum, the active bleeding and its source [
18,
19]. Isolated cystic artery trauma is an exceptional event, this may be explained by the protected anatomical location of the cystic artery course along the hepatobiliary triangle [
18,
20]. In blunt trauma, the more common presentation is hemoperitoneum with active extravasation due to cystic artery shear damage along its course within the gallbladder bed (
Figure 2,
Figure 3 and
Figure 4). Cystic artery trauma can manifest also as intraluminal gallbladder active bleeding when associated with gallbladder injuries[
10]. Post-traumatic development of CAP can be assessed by CT, and multiplanar reconstruction and maximum intensity projection are extremely useful for the recognition of focal millimetric pseudoaneurysm along the cystic artery course. Penetrating trauma and direct lesion of the cystic artery are extremely rare, few cases are reported in the literature of gallbladder penetrating trauma [
21,
22]. In penetrating stable trauma, laparoscopy is considered more sensitive than CT for exclusion of visceral injury, but CT is usually performed in stable patients as the first line imaging with identification of trauma lesion along the trajectory and hemoperitoneum around gallbladder with active extravasation of cyst artery determined by direct laceration or avulsion [
21].
Non-Traumatic and Iatrogenic Cystic Artery Bleeding
Cystic artery bleeding in non-traumatic and iatrogenic conditions is a rare condition commonly related to the presence of a pseudoaneurysm of the cystic artery. Pseudoaneurysms, also known as false aneurysms, develop from damage to an arterial wall resulting in a rupture that is contained by surrounding tissues [
6]. Cystic artery pseudoaneurysm can be secondary to surgical and non-surgical conditions. Iatrogenic causes of CAP are related to inadvertent vascular injury during procedures. CAP after cholecystectomy is related to vascular lesions during diathermy[
23,
24,
25], moreover bile damages blood vessels, so intraoperatively simultaneous injury to biliary structures can delay the healing of an injured artery and predispose that artery to the formation of pseudoaneurysms [
6,
25]. CAP can be also a consequence of inflammatory hepatobiliary and pancreatic processes, long-standing infection and tumoral infiltration (
Figure 5,
Figure 6,
Figure 7 and
Figure 8) and it has been reported idiopathic in up to 8.9% of cases[
14]
Conclusions
Isolated cystic artery bleeding is a rare phenomenon. It can occur in traumatic and non-traumatic conditions and idiopathic cases have also been reported. CT is the imaging of choice in trauma settings and in suspected non-traumatic haemorrhages. In trauma settings, the typical anatomical location of the hemoperitoneum around the gallbladder and into the gallbladder bed with visualization of active contrast extravasation along the cystic artery or its branches allows a rapid diagnosis. In non-operative trauma management, treatment usually consists of trans-arterial embolization.
In non-traumatic and iatrogenic CAB, risk factors, such as previous cholecystectomy or inflammatory processes involving the hepatobiliary and pancreatic system, should be considered too rapidly suspect the presence of CAP. Furthermore, understanding the underlying causes (etiology) of CAP can empower radiologists to proactively identify these lesions during abdominal CT examinations performed for unrelated reasons. This ability to detect CAPs before they rupture or bleed has the potential to significantly improve patient outcomes by facilitating early intervention and preventing potentially life-threatening complications.
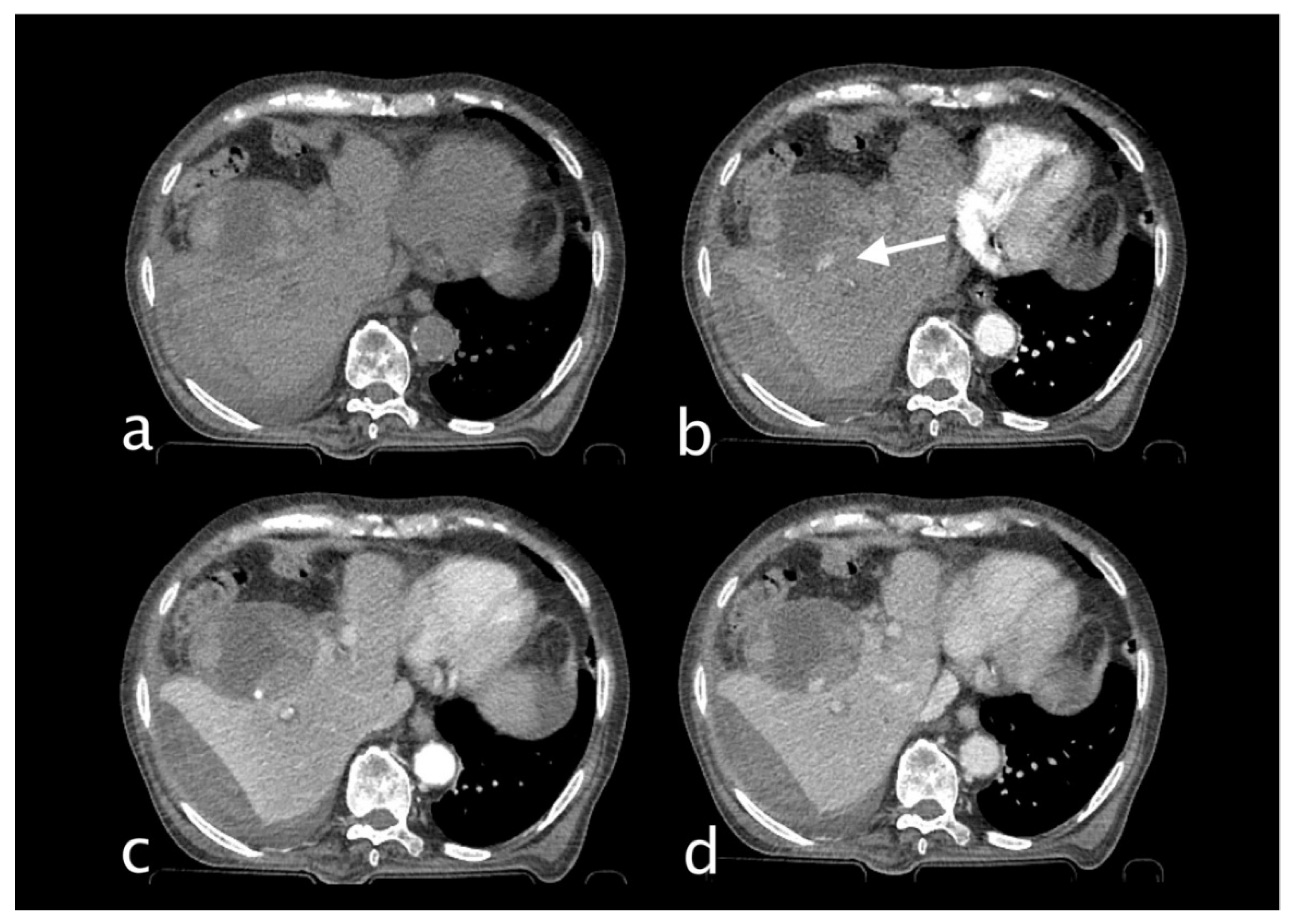
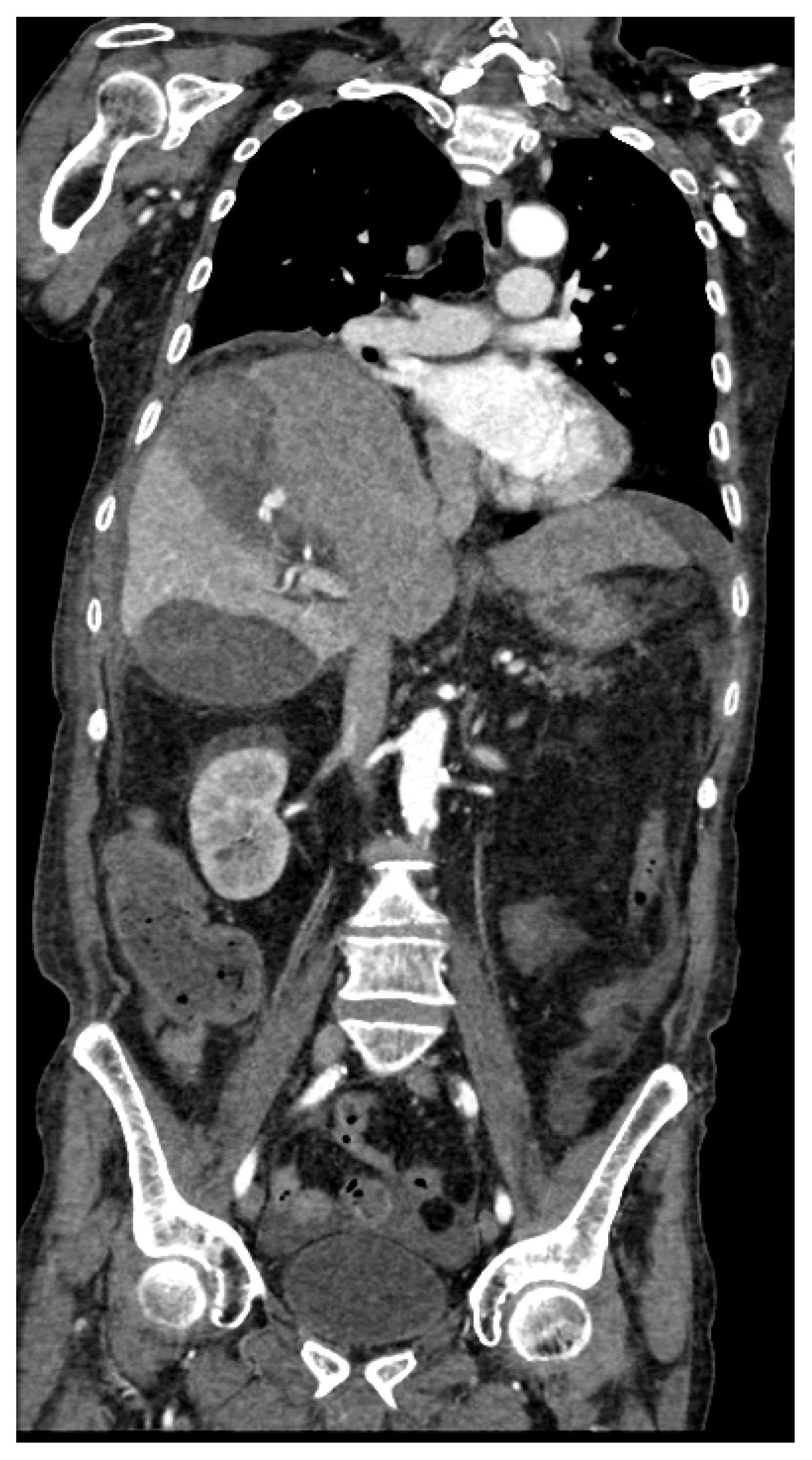
Figure 1.
74 years old male hospitalized for hip fracture who underwent arthroplasty (Hb 8 g/dl, n.v. 13-18; PRO B NP 39473 pg/ml, n.v. <375; PCR 5,04 mg/dl, n.v. 0.0-0.5; WBC 7,25 103/mm3 n.v. 4.2-10.5), who suddenly complained jaundice, right upper quadrant pain and melena. He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), which showed a distended gallbladder with slightly hyperdense content and a rounded hyperattenuating focus (white arrow) that did not change form and was isodense to blood pool in the venous and delayed phases. Diagnosis: CAP with no active bleeding. The patient underwent cholecystectomy and was discharged on.
Figure 1.
74 years old male hospitalized for hip fracture who underwent arthroplasty (Hb 8 g/dl, n.v. 13-18; PRO B NP 39473 pg/ml, n.v. <375; PCR 5,04 mg/dl, n.v. 0.0-0.5; WBC 7,25 103/mm3 n.v. 4.2-10.5), who suddenly complained jaundice, right upper quadrant pain and melena. He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), which showed a distended gallbladder with slightly hyperdense content and a rounded hyperattenuating focus (white arrow) that did not change form and was isodense to blood pool in the venous and delayed phases. Diagnosis: CAP with no active bleeding. The patient underwent cholecystectomy and was discharged on.
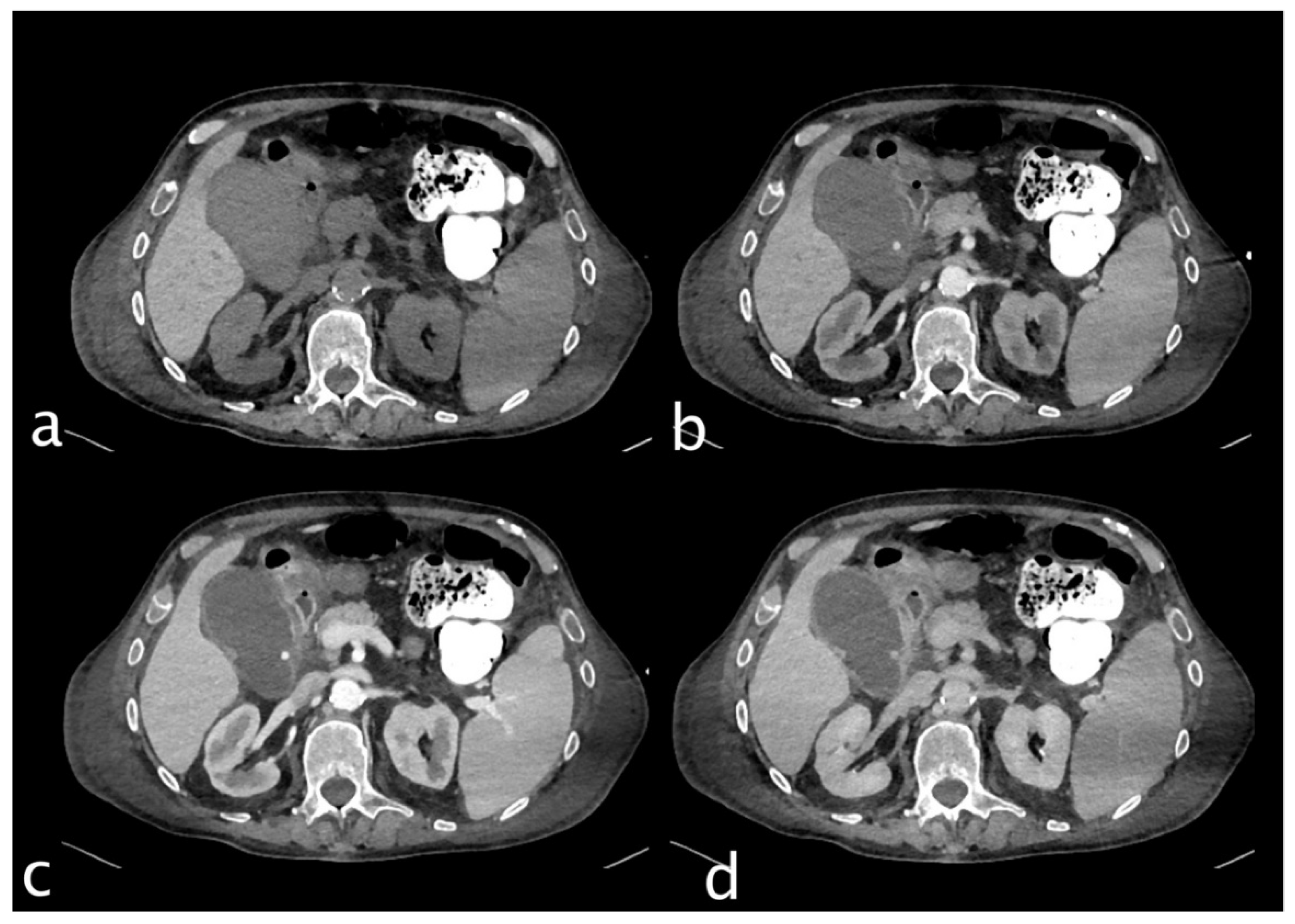
Figure 2.
Hemodynamically stable 78 years old male who underwent MVA trauma (Hb 8 g/dl, n.v. 13-18; PCR 2,04 mg/dl, n.v. 0.0-0.5; WBC 7,66 103/mm3 n.v. 4.2-10.5). He underwent CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase) that showed right hemothorax with multiple right rib fractures with hemothorax with no active bleeding, bifocal clavicle fracture, and hepatic contusion. Conspicuous perihepatic and pericholecystic hematoma with cystic artery laceration and active bleeding (c, white arrow) was detected on the anterior gallbladder profile that increases in the portal (c) and delayed phases (d) particularly evident adjacent to a subcapsular (pre-existing) cyst. The patient underwent TAE successfully with super selective embolization of the anterior branch of the cystic artery (spiral and contour embolization particles). Hepatic contusion and rib fractures were treated conservatively. Postoperatively, the patient was stable. He underwent orthopaedic surgery for a clavicle fracture and developed pneumonia. He was discharged on the 25th day because of medical complications.
Figure 2.
Hemodynamically stable 78 years old male who underwent MVA trauma (Hb 8 g/dl, n.v. 13-18; PCR 2,04 mg/dl, n.v. 0.0-0.5; WBC 7,66 103/mm3 n.v. 4.2-10.5). He underwent CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase) that showed right hemothorax with multiple right rib fractures with hemothorax with no active bleeding, bifocal clavicle fracture, and hepatic contusion. Conspicuous perihepatic and pericholecystic hematoma with cystic artery laceration and active bleeding (c, white arrow) was detected on the anterior gallbladder profile that increases in the portal (c) and delayed phases (d) particularly evident adjacent to a subcapsular (pre-existing) cyst. The patient underwent TAE successfully with super selective embolization of the anterior branch of the cystic artery (spiral and contour embolization particles). Hepatic contusion and rib fractures were treated conservatively. Postoperatively, the patient was stable. He underwent orthopaedic surgery for a clavicle fracture and developed pneumonia. He was discharged on the 25th day because of medical complications.
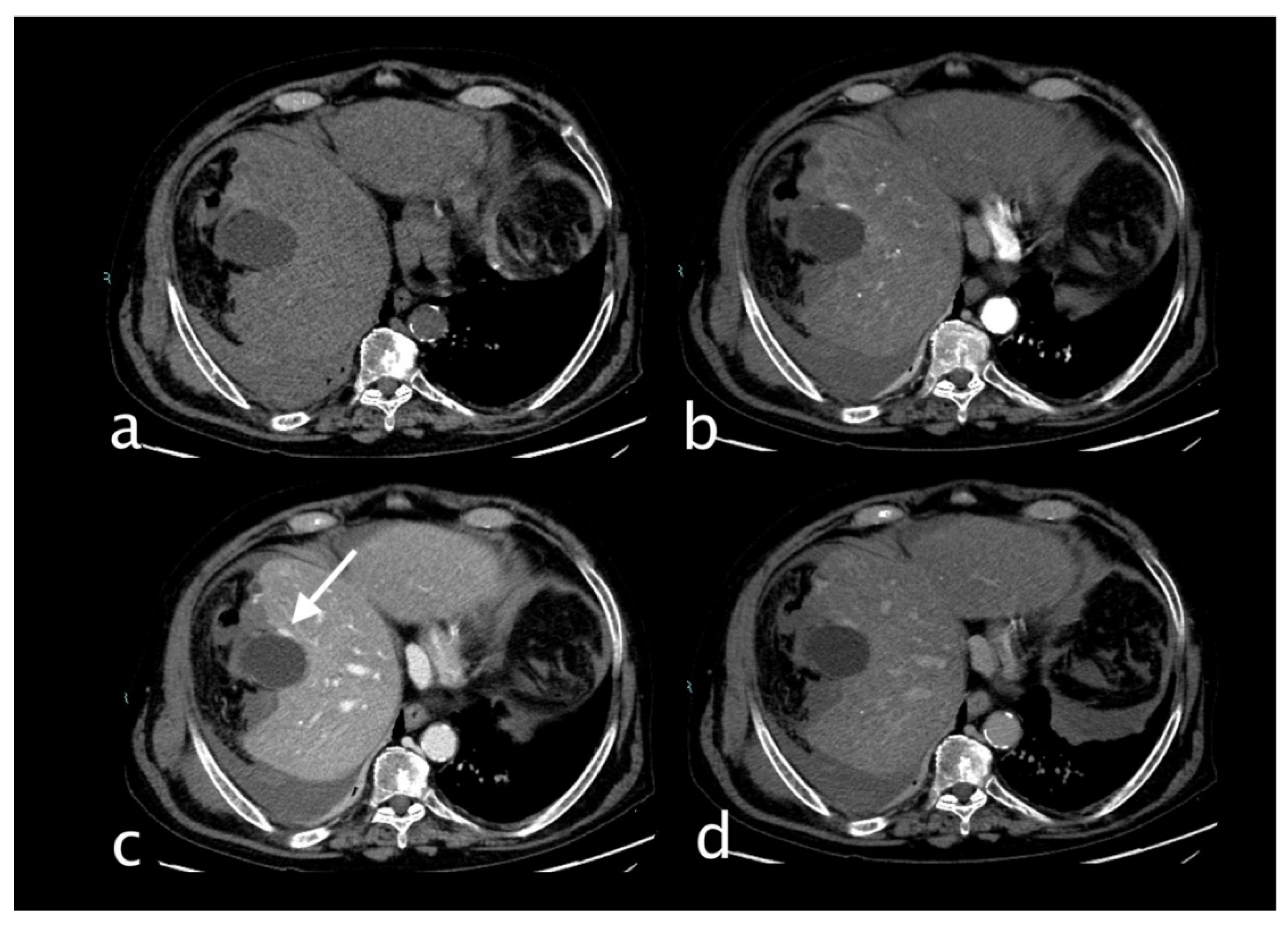
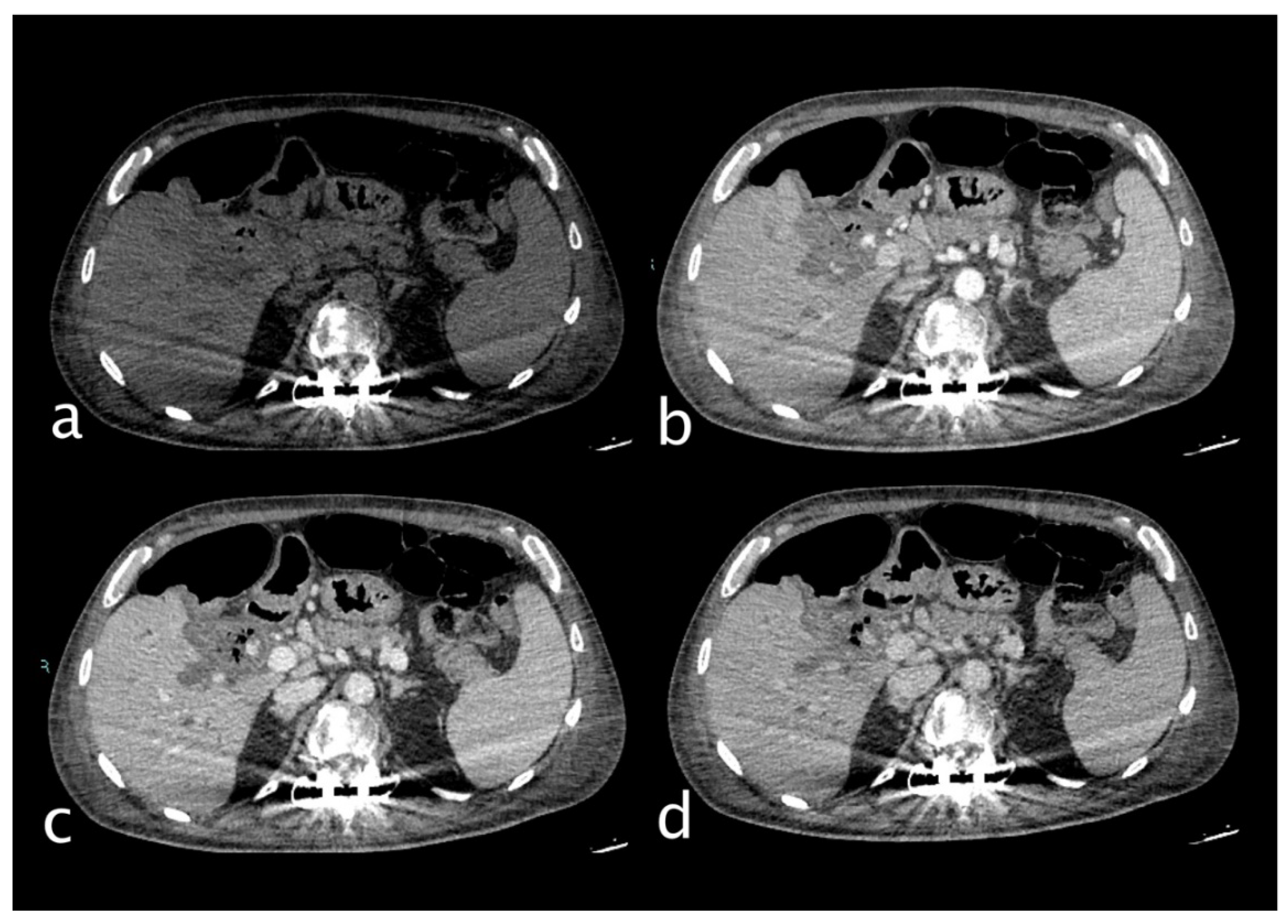
Figure 3.
Hemodynamically stable 30 years old male who underwent MVA trauma (Hb 11,9 g/dl, n.v. 13-18; PCR 0,43 mg/dl, n.v. 0.0-0.5; WBC 15,19 103/mm3 n.v. 4.2-10.5). He underwent CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase) that showed subcapsular and pericholecystic hematoma, laceration with non-active bleeding of the fourth hepatic segment and cystic artery active bleeding (a,white arrow) along the anterior gallbladder profile with conspicuous increase of contrast extravasation in portal (c)and delayed (d) phases.
Figure 3.
Hemodynamically stable 30 years old male who underwent MVA trauma (Hb 11,9 g/dl, n.v. 13-18; PCR 0,43 mg/dl, n.v. 0.0-0.5; WBC 15,19 103/mm3 n.v. 4.2-10.5). He underwent CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase) that showed subcapsular and pericholecystic hematoma, laceration with non-active bleeding of the fourth hepatic segment and cystic artery active bleeding (a,white arrow) along the anterior gallbladder profile with conspicuous increase of contrast extravasation in portal (c)and delayed (d) phases.
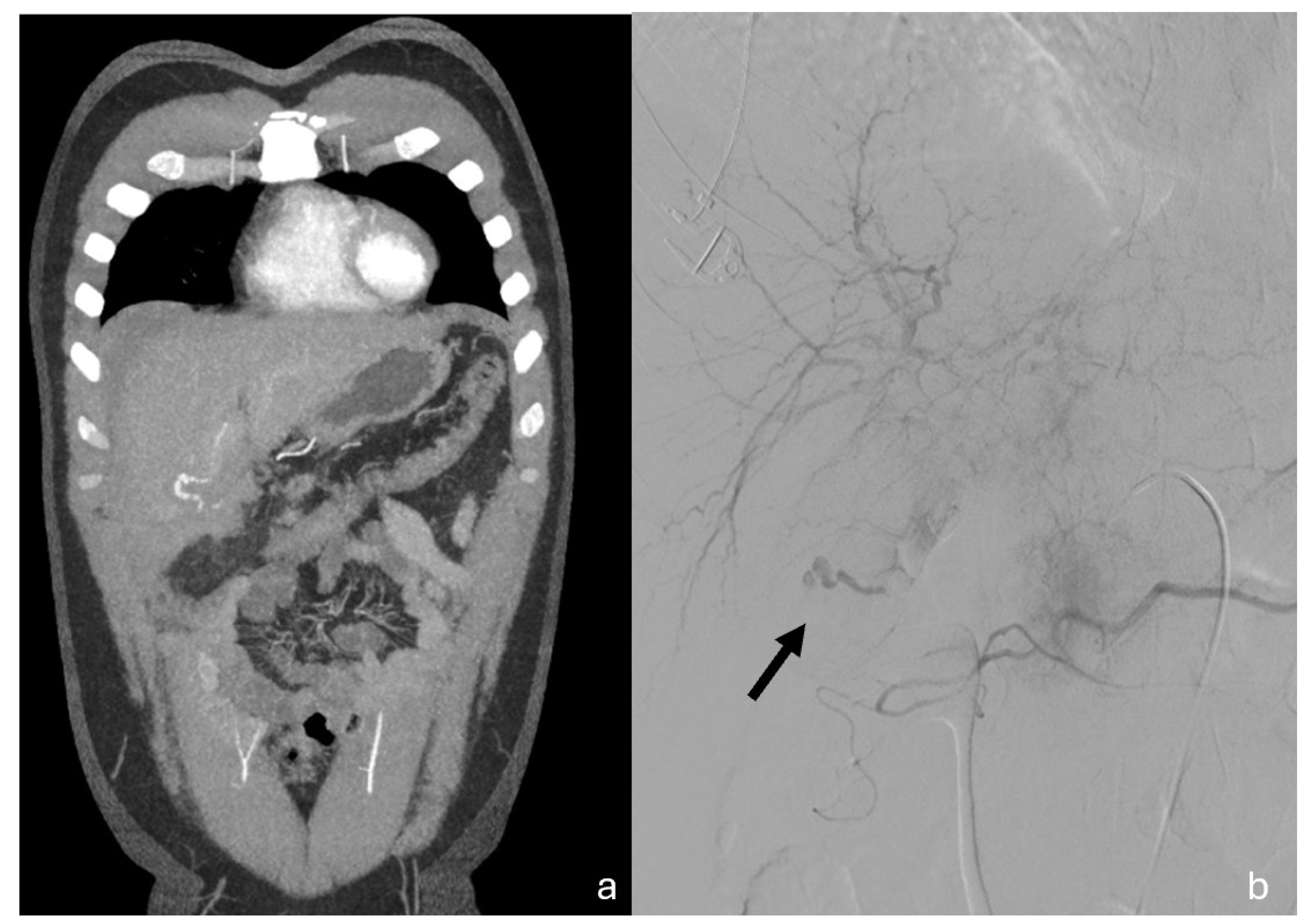
Figure 4.
Coronal MPR CT images (a) and Angiography (b) both showing cystic artery conspicuous bleeding. (b)Digital subtraction angiography demonstrates the entity of the bleeding; black arrow pointing towards the cystic artery and the extravasation. The patient underwent TAE successfully with super selective embolization of the anterior branch of the cystic artery (spiral and embolic material). Hepatic laceration was treated conservatively. Postoperatively, the patient was stable and with good recovery and discharged on 14th day.
Figure 4.
Coronal MPR CT images (a) and Angiography (b) both showing cystic artery conspicuous bleeding. (b)Digital subtraction angiography demonstrates the entity of the bleeding; black arrow pointing towards the cystic artery and the extravasation. The patient underwent TAE successfully with super selective embolization of the anterior branch of the cystic artery (spiral and embolic material). Hepatic laceration was treated conservatively. Postoperatively, the patient was stable and with good recovery and discharged on 14th day.
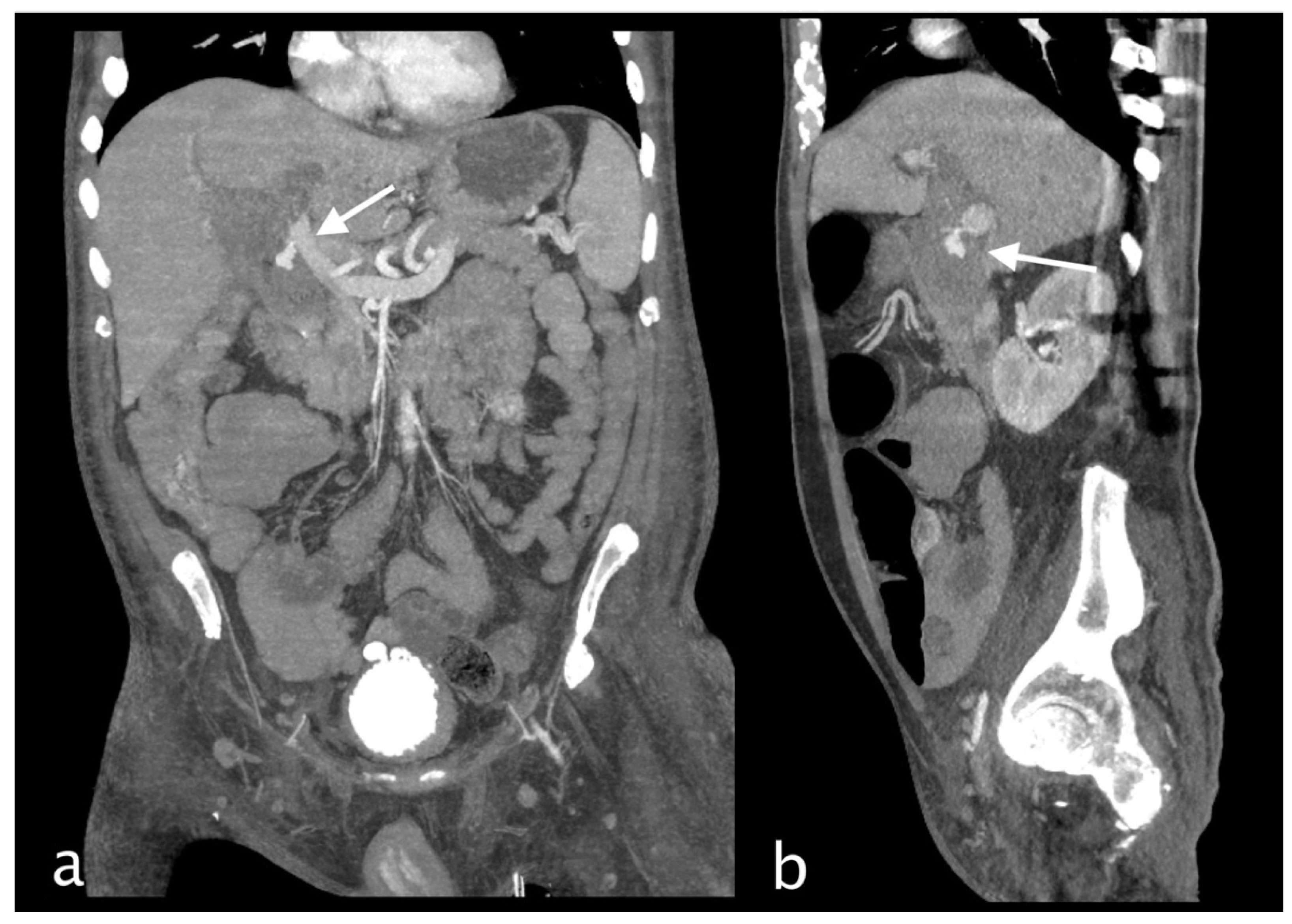
Figure 5.
82 years old man hospitalized for pneumonia who suddenly complained of jaundice, right upper quadrant pain and sudden decline of Hb 7,1 g/dl (PRO B NP 39473 pg/ml, n.v. <375; PCR 9,9 mg/dl, n.v. 0.0-0.5; WBC 23,78 103/mm3 n.v. 4.2-10.5). He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), that showed subcapsular and pericholecystic hematoma with endoluminal active bleeding originating from a cystic artery pseudoaneurysm (b, white arrow). CAP changed in shape and enhancement characteristic of pseudoaneurysm active bleeding. The patient underwent TAE successfully (spiral).
Figure 5.
82 years old man hospitalized for pneumonia who suddenly complained of jaundice, right upper quadrant pain and sudden decline of Hb 7,1 g/dl (PRO B NP 39473 pg/ml, n.v. <375; PCR 9,9 mg/dl, n.v. 0.0-0.5; WBC 23,78 103/mm3 n.v. 4.2-10.5). He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), that showed subcapsular and pericholecystic hematoma with endoluminal active bleeding originating from a cystic artery pseudoaneurysm (b, white arrow). CAP changed in shape and enhancement characteristic of pseudoaneurysm active bleeding. The patient underwent TAE successfully (spiral).
Figure 6.
Coronal CT image in arterial phase that clearly shows CAP with irregular margins.
Figure 6.
Coronal CT image in arterial phase that clearly shows CAP with irregular margins.
Figure 7.
58-year-old man hospitalized who underwent more than once ERCP for choledocholithiasis. He complained of severe right upper quadrant pain and sudden decline of Hb 7,3 g/dl (PCR 10,82 mg/dl, n.v. 0.0-0.5; WBC 18,76 103/mm3 n.v. 4.2-10.5). He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), which showed dilated intrahepatic bile ducts and markedly dilated common bile duct that present inhomogeneous content and air bubble for previous procedure and sphincterotomy. Within the common bile duct a cystic artery pseudoaneurysm was detected with active extravasation. (white arrow). The patient underwent TAE successfully.
Figure 7.
58-year-old man hospitalized who underwent more than once ERCP for choledocholithiasis. He complained of severe right upper quadrant pain and sudden decline of Hb 7,3 g/dl (PCR 10,82 mg/dl, n.v. 0.0-0.5; WBC 18,76 103/mm3 n.v. 4.2-10.5). He underwent abdominal CT with IV contrast (a non-contrast, b arterial, c venous, d delayed phase), which showed dilated intrahepatic bile ducts and markedly dilated common bile duct that present inhomogeneous content and air bubble for previous procedure and sphincterotomy. Within the common bile duct a cystic artery pseudoaneurysm was detected with active extravasation. (white arrow). The patient underwent TAE successfully.
Figure 8.
MPR coronal (a) and sagittal (b) clearly demonstrate cystic branch pseudoaneurysm bleeding.
Figure 8.
MPR coronal (a) and sagittal (b) clearly demonstrate cystic branch pseudoaneurysm bleeding.
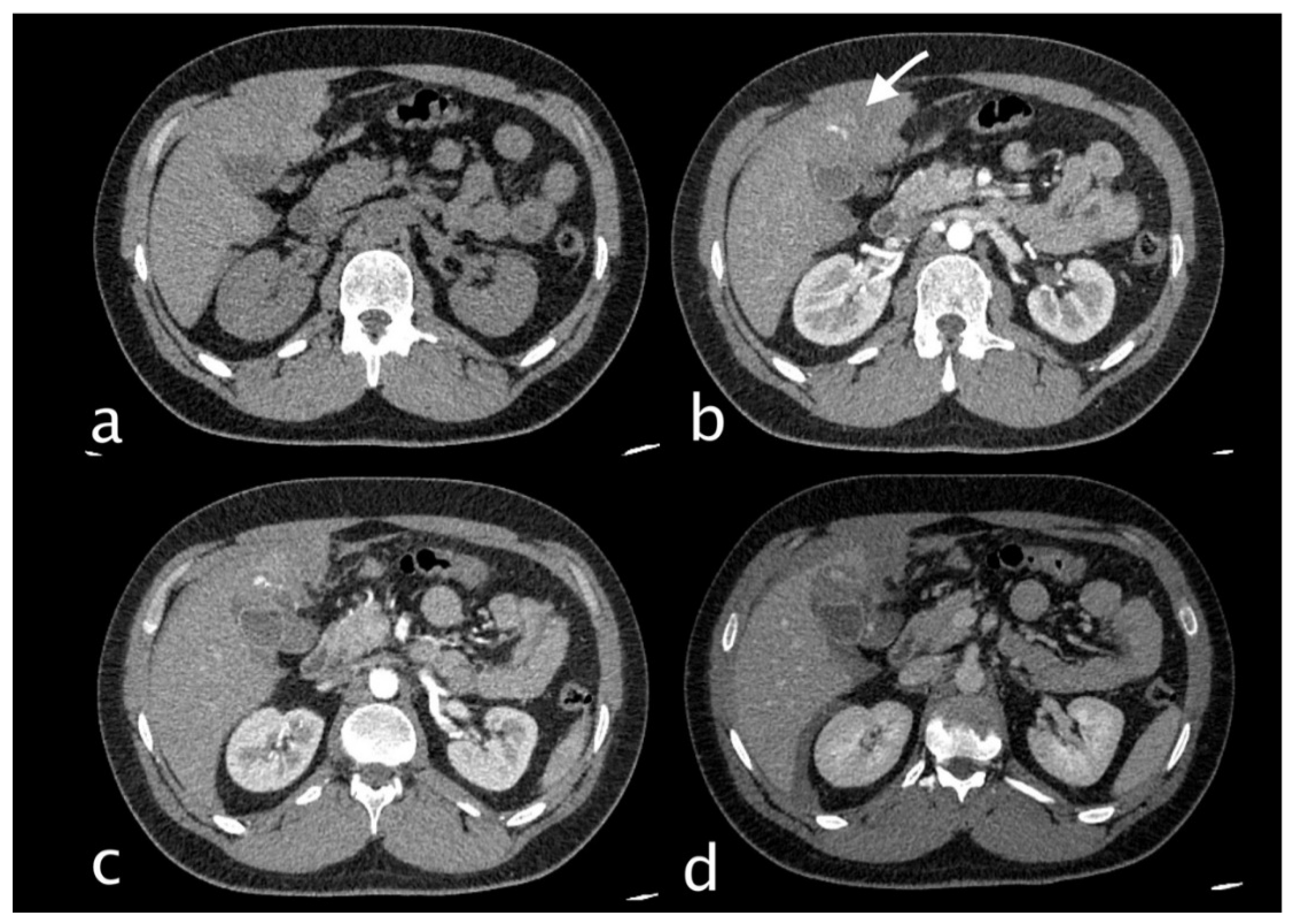
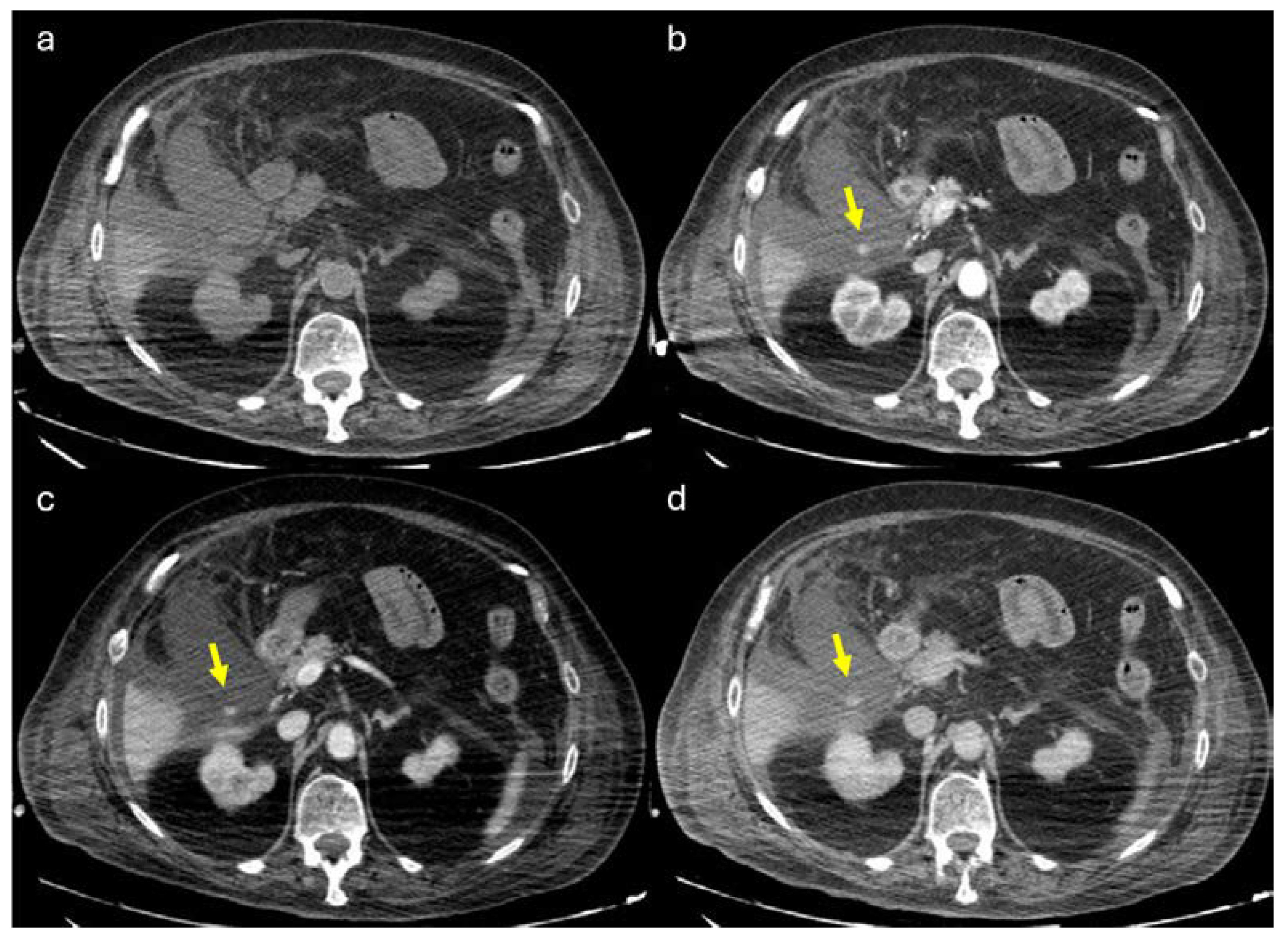
Figure 9.
80 y.o. woman with lung infection (candidiasis) with worsening of inflammatory markers. (a) Non contrast axial CT imaging demonstration of perihepatic, perisplenic fluid collections and the presence of fluid in the gallbladder bed. The gallbladder appears increased in size. (b) Axial CT image shows the presence of globular enhancing vascular image (yellow arrow) along the gallbladder wall in arterial phase. The globular vascular image did not change form and was isodense to blood pool in the venous and delayed phases (c-d). Diagnosis: CAP with no active bleeding.
Figure 9.
80 y.o. woman with lung infection (candidiasis) with worsening of inflammatory markers. (a) Non contrast axial CT imaging demonstration of perihepatic, perisplenic fluid collections and the presence of fluid in the gallbladder bed. The gallbladder appears increased in size. (b) Axial CT image shows the presence of globular enhancing vascular image (yellow arrow) along the gallbladder wall in arterial phase. The globular vascular image did not change form and was isodense to blood pool in the venous and delayed phases (c-d). Diagnosis: CAP with no active bleeding.