1. Introduction
Advances in the treatment of acute lymphoblastic leukemia (ALL) have led to a marked improvement in the survival rate of patients, ranging from almost fatal to curable in 85-90% of patients [
1]. Nevertheless, ALL patients develop adverse effects during and after treatment [
2,
3,
4]; such effects include decreased bone mineral density (BMD), osteopenia/osteoporosis, osteonecrosis, fragility fractures, and vitamin D deficiency [
5,
6,
7,
8,
9].
Childhood and adolescence are critical stages for bone development, mineralization, and the attainment of peak bone mass [
10]. Bone remodeling is a tightly regulated process that involves the repair of microdamage and replacement of old bone with new bone through osteoclastic resorption and osteoblastic bone formation [
11]. The RANK/RANKL/OPG pathway controls osteoclastogenesis [
12] and bone resorption [
13]. The RANK/RANKL interaction induces the formation of multinucleated mature osteoclasts, leading to bone resorption, while the binding of OPG to RANKL inhibits osteoclastogenesis [
14,
15] and disruptions in this complex result in excessive or impaired bone remodeling [
13].
Corticosteroids are among the most potent osteotoxic drugs that are routinely prescribed to treat serious childhood illnesses, including leukemia [
16].16 Corticosteroids affect bone formation by decreasing the number and differentiation of osteoblastic lineage cells and stimulating osteoclast differentiation and function through increased RANKL production, decreased OPG production by osteoblasts, an increased RANKL/OPG ratio, and excessive osteocyte apoptosis, leading to increased bone resorption and increased fracture risk [
17,
18]. Additionally, low vitamin D levels are associated with an increase in RANKL expression and the RANKL/OPG ratio [
19]. Childhood ALL patients are at risk for impaired vitamin D status and bone metabolism, which could be explained by limited sun exposure, nutritional factors [
20], decreased physical activity, corticosteroids and methotrexate therapy [
21,
22]. In vivo studies have shown that corticosteroid administration downregulates the expression of the CYP24A7 and CYP27B1 mRNAs, both of which are essential for controlling the availability of the active metabolite of vitamin D, 1,25(HO)2D [
23,
24].
Some reports have explored the role of corticosteroids in modulating RANKL and OPG levels and the RANKL/OPG ratio in pediatric patients with chronic disorders, immobility, and corticosteroid exposure and its relationship with bone health [
19,
25,
26]. Nevertheless, studies evaluating the role of RANKL and OPG in bone health in pediatric patients during and after treatment are limited [
9,
27]. Muggeo et al. assessed the levels of bone turnover markers in ALL patients after the intensification phase. The authors reported higher levels of RANKL and OPG in the ALL group than in the control group [
9]. Hablas et al. reported higher concentrations of RANKL in ALL survivors than in healthy patients. Additionally, vitamin D levels were positively correlated with OPG and negatively correlated with RANKL levels [
27]. In this context, the exploration of vitamin D [25(OH)D] levels and the impact of the administration of corticoids on the RANKL/OPG ratio in pediatric patients with ALL is relevant since an imbalance in the expression or function of any component of this system can induce the deregulation of the remodeling cycle and generate modifications in BMD. These effects can increase the long-term risk of osteoporosis and fractures in pediatric patients with ALL.
Therefore, our aim was to analyze changes in RANKL, OPG and 25(OH)D levels, the RANKL/OPG ratio, and other bone turnover markers (BTM) [osteocalcin, tartrate-resistant acid phosphatase type 5b (TRACP-5b) and bone alkaline phosphatase (BAP)] from diagnosis to complete remission in children with ALL.
2. Materials and Methods
2.1. Study Design and Participants
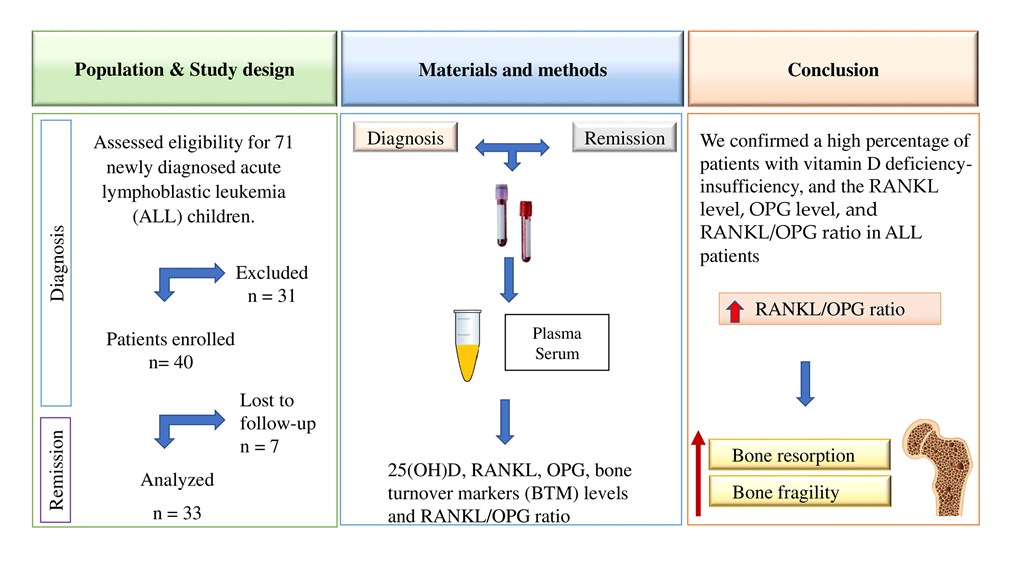
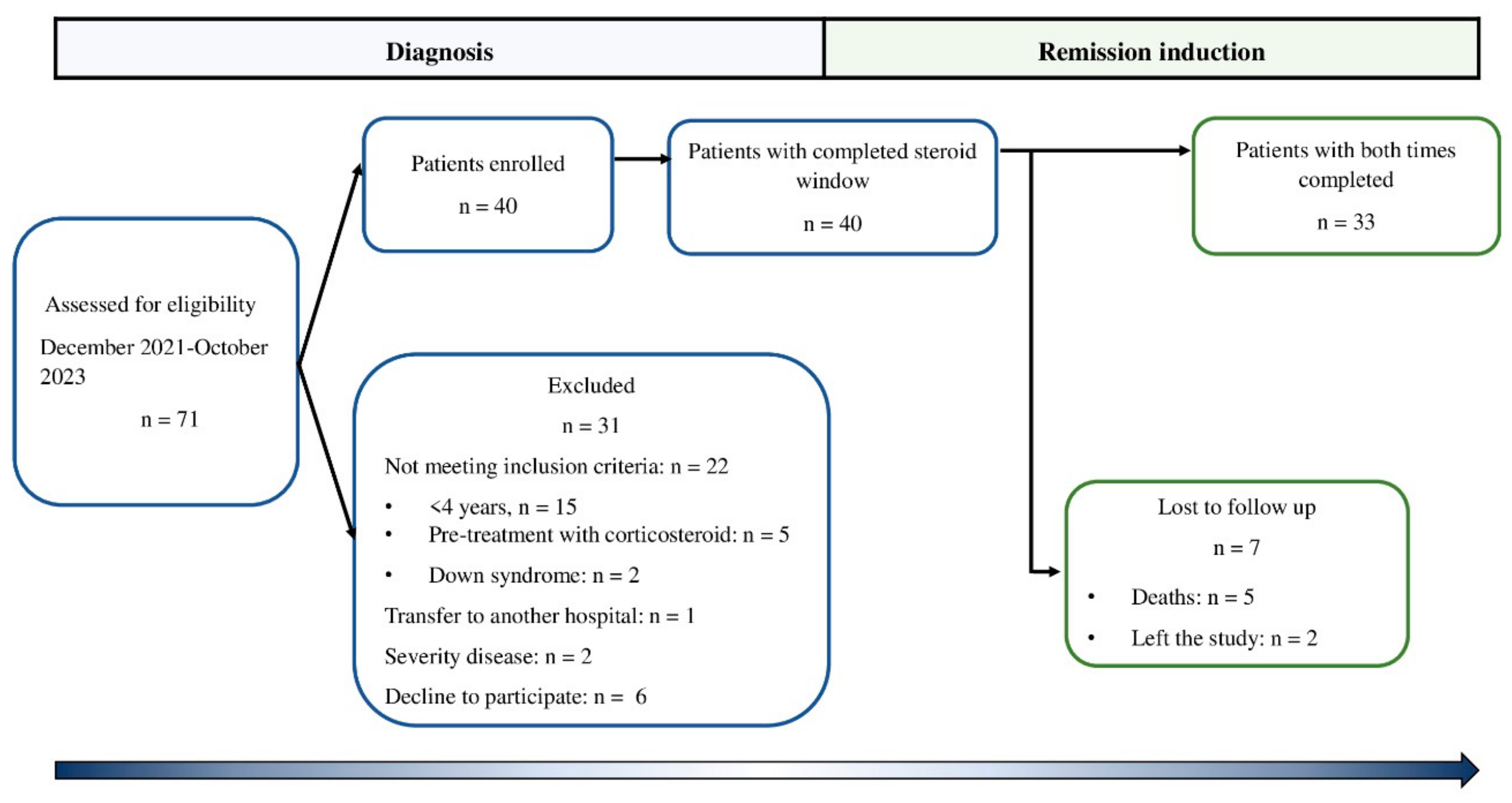
This prospective observational cohort study (from diagnosis to complete remission) was carried out in the Medical Nutrition Research Unit and the Clinical Department of Hematology of the Pediatric Hospital of the Institute Mexicano del Seguro Social (IMSS) in Mexico City, Mexico. The eligibility criterion was patients who were newly diagnosed with ALL before the beginning of chemotherapy treatment between December 2021 and November 2023. Children who had previously been treated with chemotherapy at another institution, who had Down syndrome, or who were treated previously with corticosteroids were excluded. Seventy-one patients were newly diagnosed with B-cell ALL. Twenty-two patients were excluded because they did not meet the inclusion criteria, and others were excluded due to the severity of the disease (two patients). One patient was transferred to another hospital, and in six patients, the legal representative did not authorize their participation in the study. Thus, 40 boys were enrolled in the study. However, five patients died from complications inherent to treatment, and two patients left the study. Therefore, 33 patients (4-17 years old) were included in the analysis. Patients were followed from the time of diagnosis of leukemia to remission (
Figure 1). The patients were treated with the HP09 chemotherapy protocol based on the BFM 95 protocol [
28]. The patients were stratified according to the risk of relapse as follows: standard risk (SR) (> 1 year to < 7 years, initial leucocyte count of 20,000/mm3), intermediate risk (IR) (>7 years to <10 years, initial leucocyte count of >20,000/mm3 <50000/mm3) and high risk (HR) (<1 year and >10 years, initial leucocyte count of >50,000/mm3). Patients received monotherapy during the first seven days (50 mg/m2/d prednisone), multiple drugs during remission induction (29-33 days), prednisone 60 mg/m2/d for 28 days, and vincristine 1.5 mg/m2/d (4 doses), daunorubicin 30 mg/m2/d (2, 3 or 4 doses, to standard, intermediate and high risk, respectively), L-asparaginase 5000 IU/m2/d (8 doses to standard and intermediate, 6 doses to high risk), and 3 doses of intrathecal chemotherapy.
2.2. Clinical Data
The demographic and clinical characteristics of the patients were collected during recruitment (diagnosis) and follow-up (at complete remission). Body weight (kg) and body composition were measured by impedance using an InBody 230 (InBody USA, Cerritos, CA, USA) while the patients were wearing lightweight clothing. Height was measured with a wall-mounted stadimeter (Seca 222, Seca Corp., Oakland Center, Columbia, MD, USA). Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m); BMI scores were obtained from the World Health Organization (WHO) normative curves [
29].
2.3. Analytical Methods
2.3.1. Blood Samples
Peripheral blood samples at baseline and during complete remission were collected between 8:00 and 9:00 am after an 8–12-h overnight fast. Clotted blood samples were centrifuged for 15 min at 3000 rpm under cold conditions (4°C). Aliquots of serum and plasma were immediately frozen (-80°C) and used to determine the serum concentrations of 25(OH)D and the plasma concentrations of RANKL, OPG, osteocalcin, BAP, and TRACP-5b.
2.3.2. Biochemical Assays
Serum concentrations of 25(OH)D were determined by a chemiluminescent microparticle immunoassay using a kit from Abbott (Abbott Park, IL, USA). The 25(OH)D concentrations were classified according to the Endocrine Society as follows: vitamin D deficiency was defined as 25(OH)D < 20 ng/mL, vitamin D insufficiency was defined as 25(OH)D of 21–29 ng/mL, and sufficiency was defined as 25(OH)D ≥ 30 ng/mL [
30].
The concentrations of RANKL were determined using a Human RANKL Magnetic Bead assay (HRNKLMAG-51K-01) (Merck KGaA, Darmstadt, Germany) with an analytical sensitivity of 0.5 pg/mL and a range of 4.88-20000 pg/mL. OPG and osteocalcin concentrations were determined using a Human Bone Magnetic Bead Panel (HBNMAG-51K) (Merck KGaA, Darmstadt, Germany), with an analytical sensitivity of 1.9 pg/mL and a range of 7-30000 pg/mL for OPG and an analytical sensitivity of 68.5 pg/mL and a range of 146-600000 for osteocalcin. The RANKL/OPG ratio was calculated for each patient by dividing the value of RANKL by that of OPG.
In a subsample of 20 patients, we analyzed BAP and TRACP-5b levels by ELISA. BAP was measured using a commercial kit (MBS60806) (MyBiosource Inc., USA, San Diego, CA), with an analytical sensitivity of 1.89 IU/L and a range of 3-900 IU/L, and high levels were defined as ≥ 75th percentile [
31]. TRACP-5b was assessed using a commercial kit (MBS045195) (MyBiosource Inc., USA, San Diego, CA), which has an analytical sensitivity of 0.1 IU/L and a range of 0.5-16 IU/L.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS Statistics version 23.0 software (IBM, Armonk, New York). The data were analyzed by total sample size (n = 33), vitamin D deficiency/insufficiency group, and risk of ALL relapse from diagnosis to complete remission. The data distribution was assessed with the Shapiro-Wilk test. The quantitative data are presented as the means ± standard deviations (SDs) for normally distributed data or as the medians (minimal, maximal). Categorical variables are presented as percentages. To analyze changes in 25(OH)D levels, the RANKL/OPG ratio, and the levels of MBT during the follow-up, we used a Wilcoxon test or paired Student’s t-test according to the data distribution. The differences between groups by relapse risk were evaluated by Student’s t-test or the Mann–Whitney U test. To examine the associations between 25(OH)D and corticosteroid dose and the RANKL/OPG ratio, Spearman’s correlation was performed. The sample size had a value of a=0.05, a β=0.1 and a statistical power of 1 - β=0.9. Statistical significance was defined as p<0.05.
3. Results
The demographic, anthropometric, body composition, clinical parameters, and BTM data of the ALL patients at diagnosis are presented in
Table 1.
3.1. Changes in Bone Turnover Markers and the RANKL/OPG Ratio
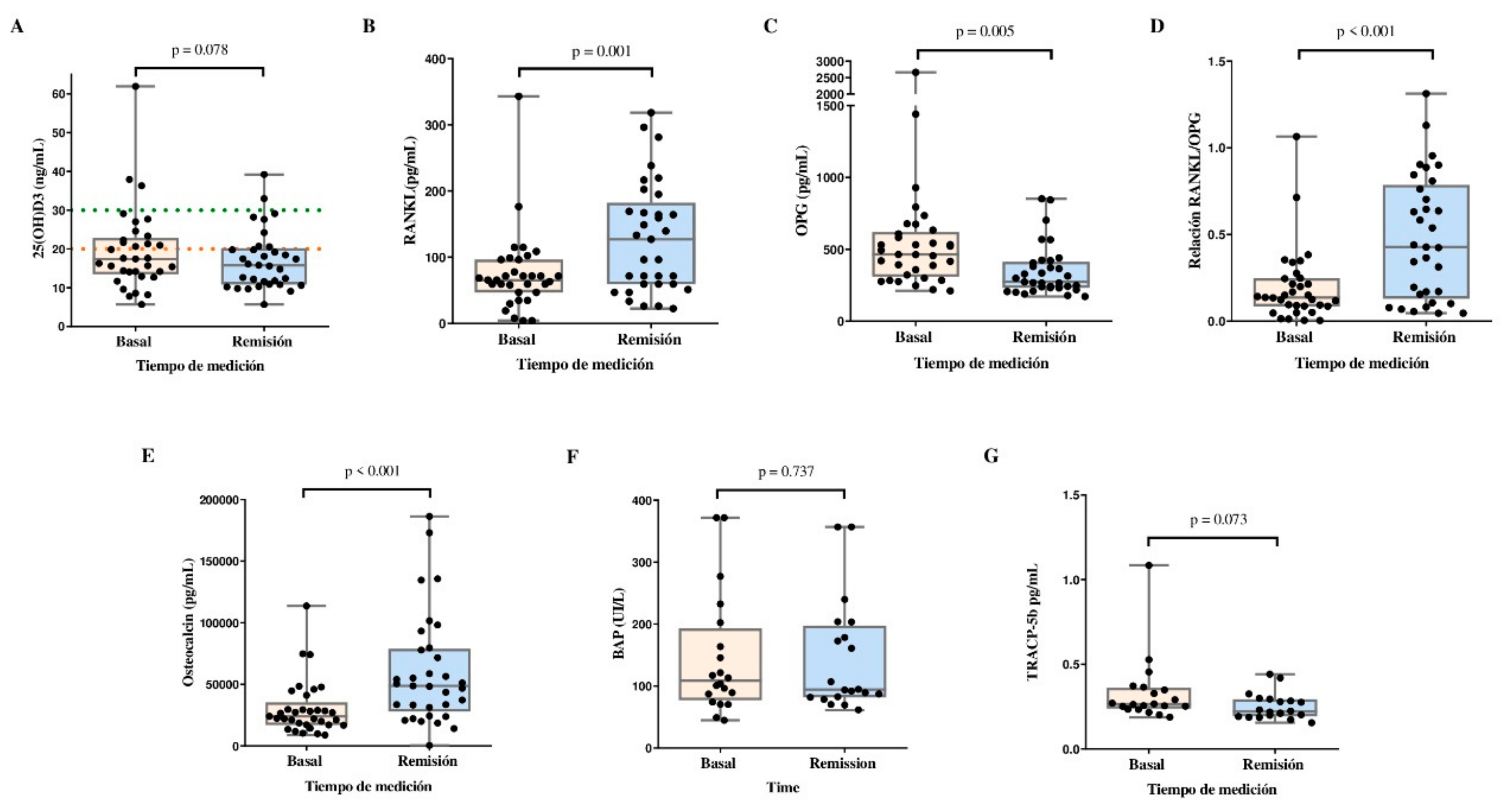
Figure 2 shows the changes in 25(OH)D and BTM concentrations in the study population. The 25(OH)D levels tended to decrease between baseline and remission. We observed an increase in the serum concentrations of RANKL (65.5 pg/mL vs. 127.6 pg/mL, p=0.001), and osteocalcin (24,098 pg/mL vs. 48,789 pg/mL, p<0.001), and in the RANKL/OPG ratio (0.137 vs. 0.427, p<0.001) between baseline and remission. OPG concentrations were decreased at remission compared with basal levels (463.9 pg/mL vs. 273.0 pg/mL, p=0.005). Moreover, the TRACP-5b concentration tended to decrease at remission compared with baseline (p = 0.073). BAP did not change during the follow-up.
3.2. Changes in Bone Turnover Markers and the RANKL/OPG Ratio According to the Risk of Relapse
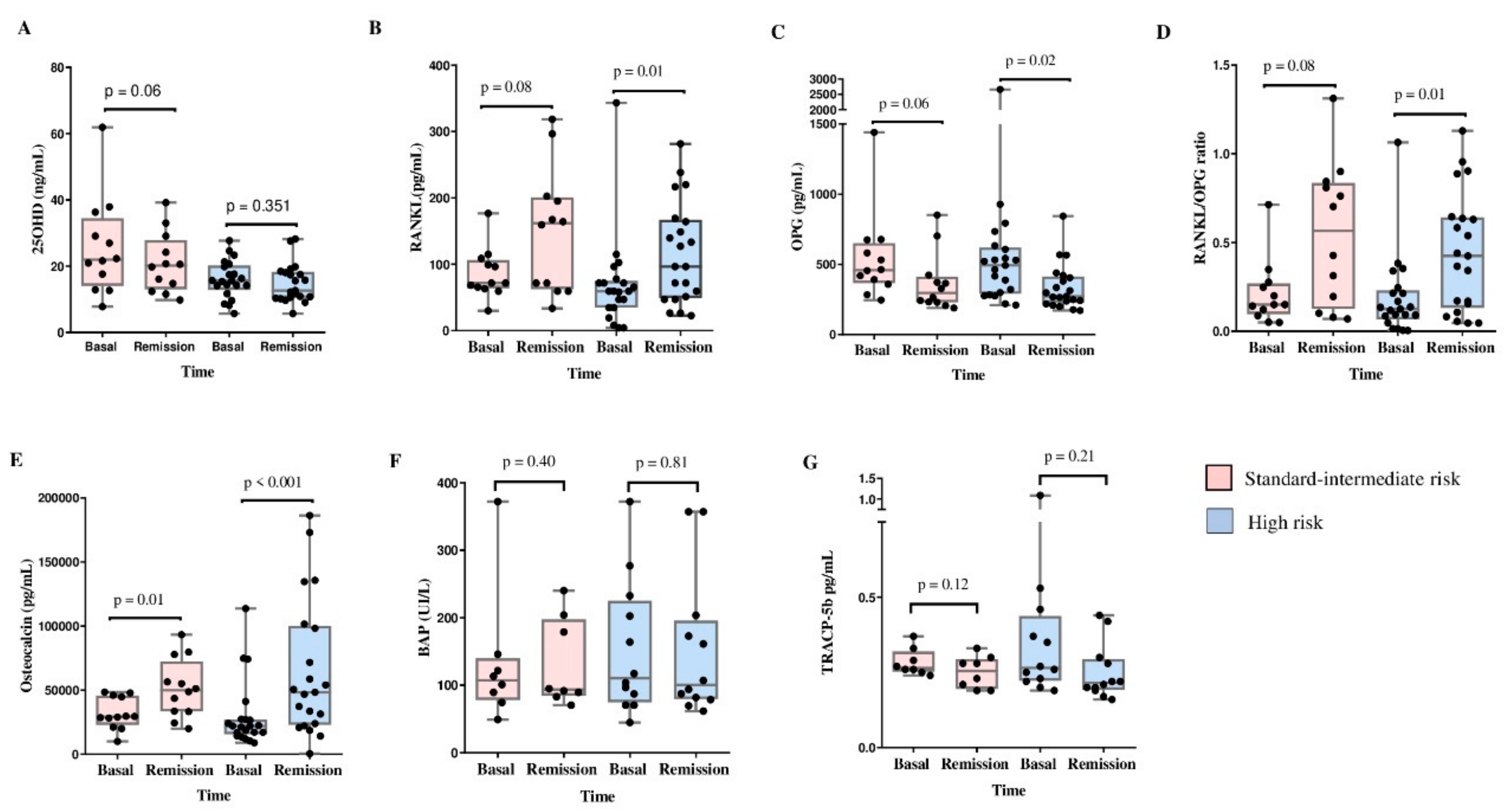
Comparisons of 25(OH)D and BTM levels between baseline and remission according to the risk of relapse [standard-intermediate-risk (SI-R) vs. HR] were performed. In the SI-R group, we observed increased osteocalcin concentrations (29,227.2 pg/mL vs. 49,993.5 pg/mL, p=0.01) between baseline and remission, with a tendency toward increased RANKL levels and RANKL/OPG ratio and decreased 25(OH)D and OPG concentrations at remission. However, we did not observe changes in BAP or TRACP-5b concentrations.
The HR group showed an increase in the levels of RANKL (59.3 ng/mL vs. 96.3 ng/mL, p=0.01), osteocalcin (22090 pg/mL vs. 48739 pg/mL, p<0.001), and RANKL/OPG ratio (0.125 vs. 0.424, p=0.01), moreover, the OPG level (493.5 pg/mL vs. 272.0 pg/mL, p=0.02) decreased at remission compared with baseline. There were no changes in the 25(OH)D, BAP, or TRACP-5b concentrations (
Figure 3).
As expected, a difference in the cumulative dose of prednisone between the SI-R and HR groups was observed (1906.1 ± 460.4 mg/m2 vs. 2972.6 ± 1003.3 mg/m2, p=0.002, respectively).
3.3. Changes in Biochemical Bone Turnover Markers and the RANKL/OPG Ratio According to Vitamin D Status
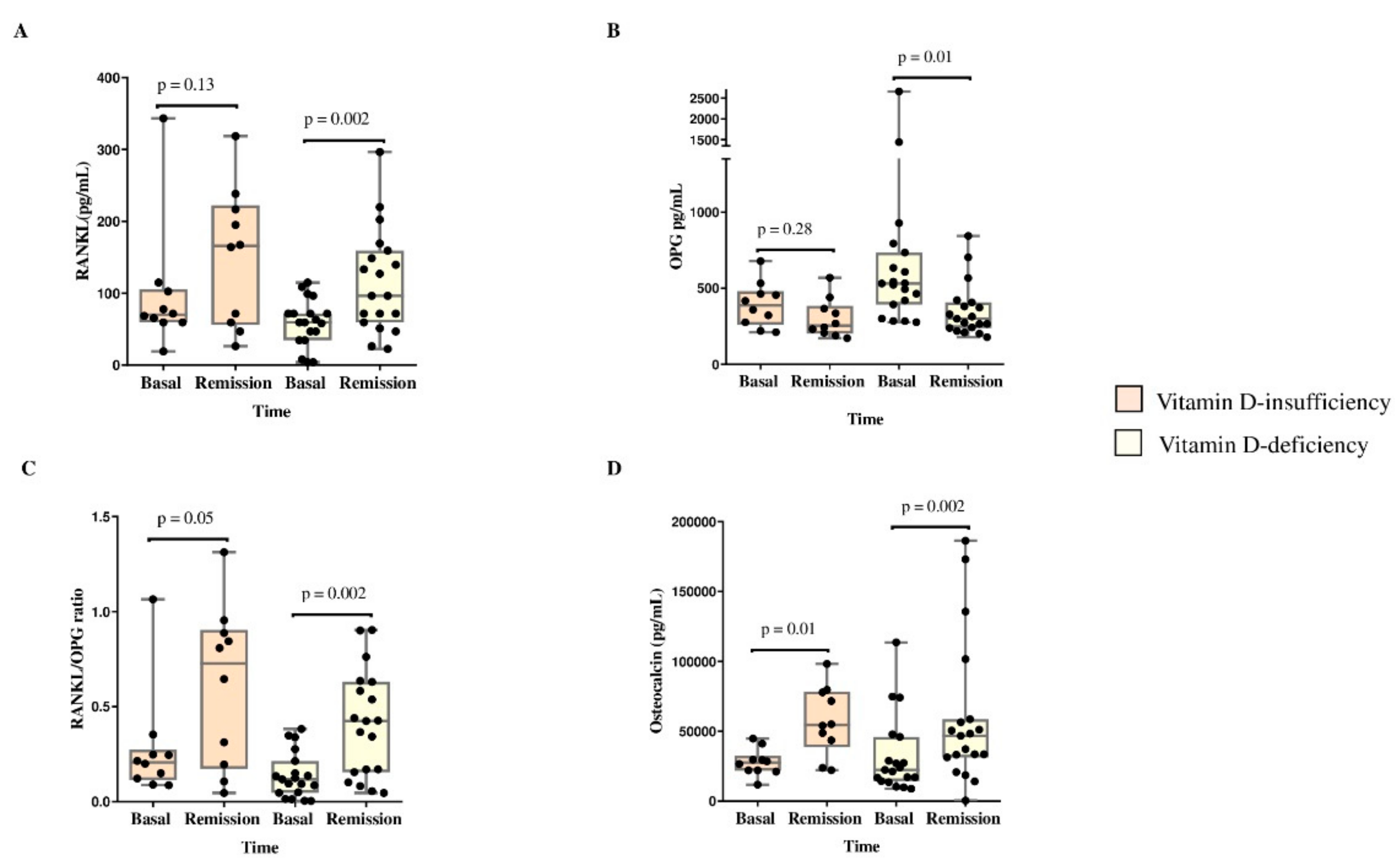
BTM and the RANKL/OPG ratio were compared between baseline and at remission according to vitamin D deficiency status (
Figure 4). The vitamin D-deficient group presented increases in RANKL (54.3 pg/mL vs. 96.3 pg/mL, p=0.002), osteocalcin (22413 pg/mL vs. 46816 pg/mL, p=0.002) and the RANKL/OPG ratio (0.118 vs. 0.424, p=0.002) and decreased OPG (530.6 pg/mL vs. 299.0 pg/mL, p=0.01). Moreover, the vitamin D-insufficient group presented increases in osteocalcin levels (27,575.5 pg/mL vs. 57,215.0 pg/mL, p=0.01) and the RANKL/OPG ratio (0.207 vs. 0.727, p=0.05) (
Figure 4).
3.4. Associations among Bone Turnover Markers, 25(OH)D Concentration, and Corticosteroid Dose
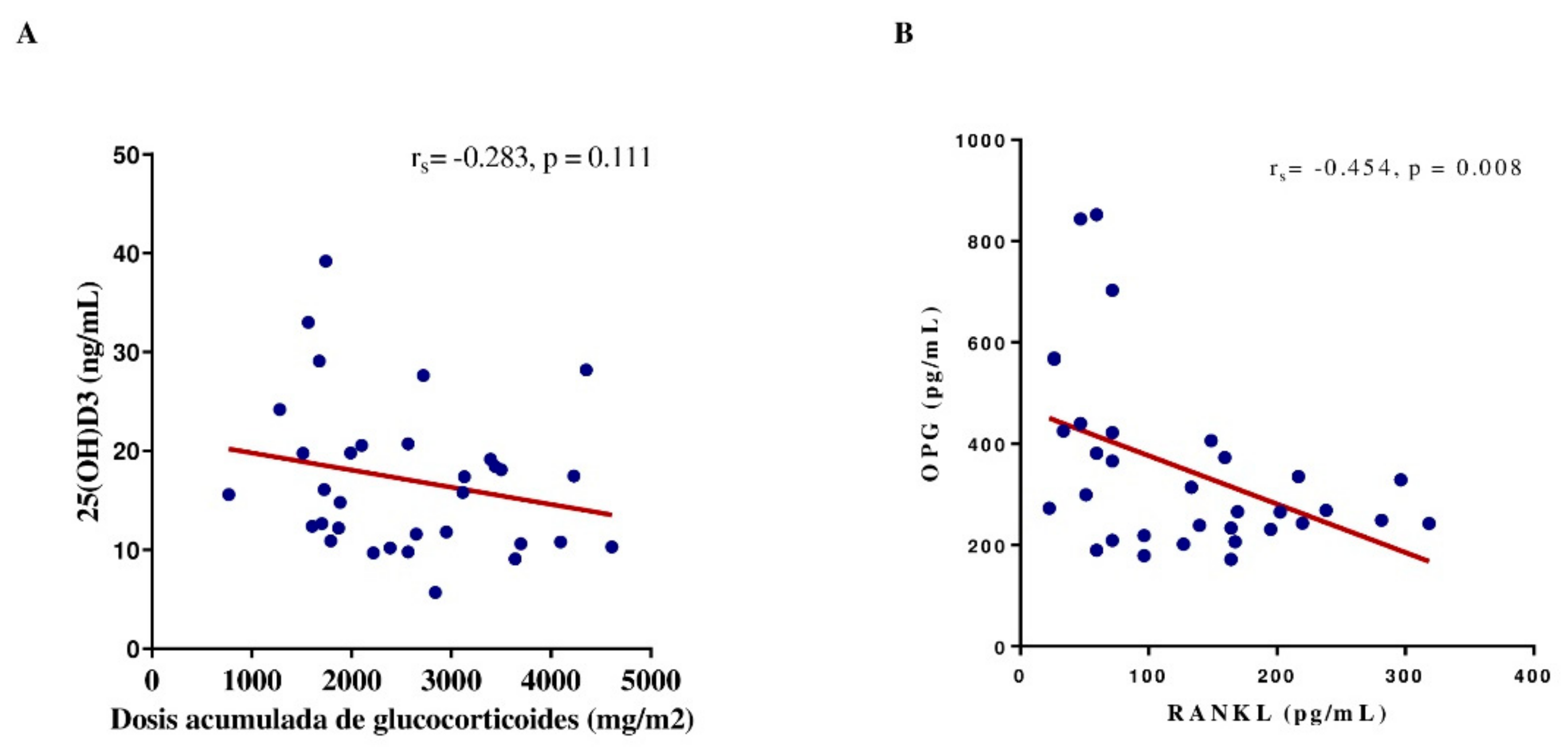
The correlations among the BTM concentration, 25(OH)D concentration, and corticosteroid dose were analyzed during remission. A negative correlation was observed between RANKL and OPG plasma levels (r=-0.454, p=0.008). The dose of prednisone did not correlate with the serum 25(OH)D, RANKL, OPG, osteocalcin, BAP, or TRACP-5b concentrations or the RANKL/OPG ratio (r=-0.283, r=0.183, =-0.240, r=0.178, r=0.213, r=-0.283, r=0.182, respectively; p≥0.99) (
Figure 5).
4. Discussion
In this report, we present an analysis of changes in RANKL and OPG levels and the RANKL/OPG ratio in children with ALL from diagnosis to remission without any intervention in addition to hematological treatment. In this study, we demonstrated an increase in RANKL, osteocalcin levels, and the RANKL/OPG ratio, as well as a decrease in OPG levels at remission. When relapse risk and vitamin D were considered, patients with an HR of relapse or vitamin D deficiency showed these changes. These changes could reflect accelerated bone remodeling.
The RANKL/RANK/OPG axis plays an essential role in the regulation of bone metabolism, especially in bone remodeling. This lifelong process is characterized by an equilibrium between the activity of osteoclasts and that of osteoblasts [
12]. Several studies have evaluated RANKL and OPG levels and the RANKL/OPG ratio in different pediatric diseases [
19,
25,
26,
32,
33], but few studies have evaluated changes in bone health parameters in patients with ALL during treatment and without any intervention. In this sense, Solmanz et al. [
34]. evaluated the effect of a combination of vitamin K2 (menaquinone-7 100 µg/day) and vitamin D3 (calcitriol 10 µg/day) on BMD, RANKL, OPG levels, and other biochemical markers of bone turnover in twenty-nine patients with ALL (1.0-17 years) who were randomized (study group, n = 15, and control group, n = 14). Patients were evaluated from diagnosis to the first, second, third, and sixth months of treatment in this study. Nevertheless, they did not find differences in RANKL or OPG concentrations between groups, or within groups during the follow-up. The OPG/RANKL ratio differed between groups in the first month. In contrast, even though we had a shorter follow-up time than Solmaz et al. [
34]., and without any intervention with vitamin D, we observed an increase in the RANKL concentration and the RANKL/OPG ratio and a decrease in OPG level between diagnosis and remission, suggesting that during the early phase of ALL treatment, glucocorticoid administration mainly increased osteoclastic activity and consequently bone resorption in these patients. Similarly, we observed the same changes in the HR and vitamin D deficiency groups. This observation could be explained, in part, by the higher cumulative glucocorticoid dose in the HR group than in the SI-R group; consequently, this group presented an increase in RANKL levels and a decrease in OPG levels during follow-up [
18]. In addition, low vitamin D levels are associated with increased RANKL [
27], which could explain the increase in RANKL in the vitamin D-deficient group of patients.
According to the 25(OH)D serum levels, there was a high prevalence of hypovitaminosis D at diagnosis (~90%) and at remission (94%). Remarkably, this percentage was higher than that reported by ENSANUT 2018 for healthy Mexican preschoolers and schoolchildren (73.2% and 75%, respectively) [
35]. These results are consistent with those reported previously in several studies on patients with ALL receiving early-phase treatment [
21,
36,
37]. In contrast to studies that have reported a decrease in 25(OH)D levels during the treatment of children with ALL, we only found a trend toward a decrease in the levels of 25(OH)D during remission.
In addition, we observed a negative correlation between RANKL and OPG plasma levels, which could explain the increase in the RANKL/OPG ratio. This is important because a high RANKL/OPG ratio has been associated with increased bone resorption [
38]. Additionally, the increase in RANKL may be associated with an initial increase in the signaling and activity of osteoclasts, which could lead to a predisposition to osteopenia and osteoporosis [
33]. We hypothesized that the increase in both molecules could result in bone abnormalities, such as decreased bone mineral density; however, more research is required to further understand the impact on healthy bone.
TRAP-5b is an enzyme that is highly expressed in osteoclasts and is a regulator of bone resorption [
39]. In pediatric patients with ALL, circulating TRACP-5b concentrations are reportedly greater than those in healthy individuals [
9]. Solmaz et al. [
34]. did not observe changes during the 6 months of follow-up. Although we observed a significant increase in RANKL levels at remission, we found a tendency toward a decrease in TRACP-5b levels only between diagnosis and remission. A decrease in TRACP-5b has been reported in patients with myeloma who underwent induction treatment with vincristine-doxorubicin-dexamethasone-pamidronate [
40]. Especially, long-term glucocorticoid reduces TRACP-5b concentrations. These results could be partly explained by disruptions in the cytoskeleton of osteoclasts generated by long-term glucocorticoids that reduce their activity [
41].
Moreover, BAP and osteocalcin are considered bone formation markers. BAP is an isoenzyme of alkaline phosphatase which is produced from bone and is an indicator of osteoblastic activity. Osteocalcin is a protein synthesized by mature osteoblasts that plays an important role in metabolic regulation, bone mineralization, and calcium homeostasis. Orgel et al. [
42]. evaluated bone structure, density, and circulating BTM at diagnosis and changes during induction therapy in preadolescents, adolescents, and young adults (n=38) with ALL and sex-matched healthy controls (n=38). The authors reported a decrease in bone structure and BMD. However, there were no changes in BAP or osteocalcin levels during follow-up, but the levels of C-telopeptide, a resorption marker, increased. Our BAP results are consistent with those of Orgel et al.; in contrast, we reported increased osteocalcin levels in pediatric patients with ALL in complete remission; this could be explained by the increase in RANKL levels because RANKL is a central player in osteoclast activation and bone destruction [
43]. Osteocalcin can bind to the bone matrix because it contains three glutamate residues that can be carboxylated; this modification allows it to bind to calcium and hydroxyapatite. Hence, the resorption of the bone matrix by osteoclasts can result in the release and decarboxylation of bound carboxylated osteocalcin, which could increase osteocalcin levels [
44,
45]. Previously, a modification of bone metabolism generated by ALL-B cells was demonstrated [
43], which explains the presence of vertebral fractures at diagnosis [
46]. We did not observe differences in BAP levels, which could be explained by the fact that 40% of patients had elevated concentrations of BAP (≥ 75th percentile) at diagnosis, and 45% had elevated concentrations of BAP at remission. Most likely, this is compensatory to disorders of bone metabolism present in these patients because high levels of BAP and its association with decreased lumbar BMD have been reported, especially in older women [
47]. Additionally, our study population was largely composed of schoolchildren, and studies have reported the highest levels of BAP in this population [
31,
48].
Nevertheless, even though we detected changes in the RANKL level, OPG level, and RANKL/OPG ratio in the deficient and HR groups, Spearman correlation analysis of the bivariate BTM did not reveal a significant association between vitamin D concentration and corticoid dose. Similarly, Muggeo et al. [
9]. did not observe significant correlations between bone turnover marker levels and vitamin D levels. In contrast, Hablas et al. reported a negative correlation between circulating vitamin D and RANKL levels and a positive correlation with OPG levels in 60 ALL survivors; this is probably due to differences in sample size, population, and mainly, the proportion of patients with >30 ng/mL vitamin D (30% vs. ~9% [two patients]).
The principal limitations of the study were the lack of a control group and the evaluation of BMD. However, our study has a larger sample than Solmaz et al. [
34]., with good statistical power. In addition, we explored the changes in a cohort from diagnosis to complete remission according to 25(OH)D status, risk of relapse, and cumulative doses of corticosteroids. We analyzed the evolution of the same group of patients at diagnosis and remission, in contrast with other studies [
9]. Another strength of this study was evaluating the changes in BTM and the RANKL/OPG ratio in a cohort of patients with ALL without any intervention in addition to early hematological treatment.
5. Conclusions
During remission, we observed an increase in RANKL and osteocalcin levels and a decrease in OPG levels. When relapse risk and vitamin D levels were considered, patients with an HR of relapse or vitamin D deficiency experienced these changes. The RANKL/OPG ratio could be a good marker of bone remodeling in patients with ALL. Hypovitaminosis D and increased RANKL/OPG may predispose in adult life to the development of bone health disorders such as osteopenia, osteoporosis, and increased bone fragility in this population. To better understand bone remodeling markers in children with leukemia, it is necessary to conduct future studies.
Author Contributions
Conceptualization, SAM and LBC; Methodology, SAM, LBC, ROM, JMH and JAMT; Validation, SAM, LBC, RAGR and ACA; Formal Analysis, SAM and LBC; Investigation, SAM, LBC, ROM, JMH, JAMT, MRC, KASL, BABM, AJM, ZHP, RAGR, ACA, REVA, JMDS, JVM, MERP; Resources, LBC and JMH; Data Curation SAM, LBC, RAGR and ACA; Writing – Original Draft Preparation, SAM; Writing – Review & Editing, LBC, ROM, JMH, JAMT and LBH; Visualization, SAM, LBC, ROM, JMH, JAMT, and LBH; Supervision, LBC, ROM and JMH.; Project Administration, LBC; Funding Acquisition, SAM and ROM.
Funding
This work was supported in part by the Departamento de Ciencias de la Salud, División de Ciencias Biológicas y de la Salud, and Programa de Doctorado en Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana, Ciudad de México, México. UAM740101AR1. apoyotdcbs@xanum.uam.mx.
Institutional Review Board Statement
This study was conducted by the Helsinki Declaration and was approved by the Research and Ethics Committee of the Pediatric Hospital (R-2021-3603-063), IMSS, in Mexico City, Mexico.
Informed Consent Statement
All parents, legal guardians, and patients (≥ 8 years old) provided written informed consent before participation.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to the Consejo Nacional de Humanidades Ciencia y Tecnología (CONAHCyT) Mexico, through a grant for doctoral studies (number 778461) awarded to Salvador Atilano Miguel.
Conflicts of Interest
The authors have no conflict of interest relevant to this article to disclose.
References
- Pui, C.H.; Robison, L.L.; Look, A.T. Acute lymphoblastic leukaemia. Lancet. 2008, 371(9617), 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Samoilenko, M.; Morel, S.; England, J.; Amre, D.; Bertout, L.; Drouin, S.; Laverdière, C.; Krajinovic, M.; Sinnett, D.; et al. Cardiometabolic Risk Factors in Childhood, Adolescent and Young Adult Survivors of Acute Lymphoblastic Leukemia - A Petale Cohort. Sci Rep. 2017, 7, 17684. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Léveillé, P.; Samoilenko, M.; Franco, A.; England, J.; Malaquin, N.; Tu, V.; Cardin, G.B.; Drouin, S.; Rodier, F.; et al. Biomarkers of cardiometabolic complications in survivors of childhood acute lymphoblastic leukemia. Sci Rep. 2020, 10, 21507. [Google Scholar] [CrossRef]
- Sheng, X.; Mittelman, S.D. The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front Pediatr. 2014, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Cao, X.; Han, A. Q.; Panetta, J. C.; Ness, K. K.; Metzger, M. L.; Rubnitz, J. E.; Ribeiro, R. C.; Sandlund, J. T.; Jeha, S; et al. Bone mineral density in children with acute lymphoblastic leukemia. Cancer. 2018, 124, 1025–1035. [CrossRef]
- van der Sluis, I.M.; van den Heuvel-Eibrink, M.M.; Hählen, K.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Watsky, M.A.; Carbone, L.D.; An, Q.; Cheng, C.; Lovorn, E.A.; Hudson, M.M.; Pui, C.H.; Kaste, S.C. Bone turnover in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014, 61, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.L.; Zhu, L.; Kaste, S.C.; Srivastava, K.; Barnes, L.; Nathan, P.C.; Wells, R.J.; Ness, K.K. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, P.; Grassi, M.; D’Ascanio, V.; Brescia, V.; Fontana, A.; Piacente, L.; Di Serio, F.; Giordano, P.; Faienza, M.F.; Santoro, N. Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature. Cancers. 2023, 15, 2554. [Google Scholar] [CrossRef] [PubMed]
- Maggioli, C.; Stagi, S. Bone modeling, remodeling, and skeletal health in children and adolescents: mineral accrual, assessment and treatment. Ann Pediatr Endocrinol Metab. 2017, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; D’Amato, G.; Chiarito, M.; Tullo, A.; Colaianni, G.; Colucci, S.; Grano, M.; Faienza, M.F. An update on the role of RANKL-RANK/osteoprotegerin and WNT-ß-catenin signaling pathways in pediatric diseases. World J Pediatr. 2019, 15, 4–11. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen 2020, 40, 2. [CrossRef]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007, 9 Suppl 1(Suppl 1):S1. [CrossRef]
- Ward, L.M. Glucocorticoid-Induced Osteoporosis: Why Kids Are Different. Front Endocrinol 2020, 11, 576. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: an update. Endocrine. 2018, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Velentza, L.; Zaman, F.; Sävendahl, L. Bone health in glucocorticoid-treated childhood acute lymphoblastic leukemia. Crit Rev Oncol Hematol 2021, 168, 103492. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Hammad, A.; El-Nahrery, E.; Hamdy, N.; Elhawary, A.K.; Eid, R. Serum RANKL, osteoprotegerin (OPG) and RANKL/OPG ratio in children with systemic lupus erythematosus. Lupus. 2019, 28, 1233–1242. [Google Scholar] [CrossRef]
- Oosterom, N.; Dirks, N. F.; Heil, S. G.; de Jonge, R.; Tissing, W. J. E., Pieters, R.; van den Heuvel-Eibrink, M. M.; Heijboer, A. C.; Pluijm, S. M. F. A decrease in vitamin D levels is associated with methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. Support Care Cancer 2019, 27, 183–190. [CrossRef]
- Bhattacharya, S.; Verma, N.; Kumar, A. Prevalence of vitamin D deficiency in childhood acute lymphoblastic leukemia and its association with adverse outcomes during induction phase of treatment. Nutr Cancer. 2020, 72, 1321–1325. [Google Scholar] [CrossRef]
- Kızılocak, H.; Okcu, F. Late Effects of Therapy in Childhood Acute Lymphoblastic Leukemia Survivors. Turk J Haematol. 2019, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zayny, A.; Almokhtar, M.; Wikvall, K.; Ljunggren, Ö.; Ubhayasekera, K.; Bergquist, J.; Kibar, P.; Norlin, M. Effects of glucocorticoids on vitamin D3-metabolizing 24-hydroxylase (CYP24A1) in Saos-2 cells and primary human osteoblasts. Mol Cell Endocrinol 2019, 496, 110525. [Google Scholar] [CrossRef] [PubMed]
- Shymanskyi, I.; Lisakovska, O.; Mazanova, A.; Labudzynskyi, D.; Veliky, M. Vitamin D3 Modulates Impaired Crosstalk Between RANK and Glucocorticoid Receptor Signaling in Bone Marrow Cells After Chronic Prednisolone Administration. Front Endocrinol 2018, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Rybi-Szuminska, A.; Zoch-Zwierz, W. Serum RANKL, osteoprotegerin (OPG), and RANKL/OPG ratio in nephrotic children. Pediatr Nephrol. 2010, 25, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Akhtar Ali, S.; Kang, H., Olney, R.; Ramos-Platt, L.; Ryabets-Lienhard, A.; Cheung, C.; Georgia, S.; Pitukcheewanont, P. Evaluating RANKL and OPG levels in patients with Duchenne muscular dystrophy. Osteoporos Int 2019, 30, 2283–2288. [CrossRef] [PubMed]
- Hablas, N.M.; Keshk, W.A. OPG/RANK/RANKL Axis in Egyptian Children with Acute Lymphoblastic Leukemia After Maintenance Therapy: Relationship to Bone Mineral and Vitamin D Status. J Pediatr Hematol Oncol. 2023, 45, e733–e738. [Google Scholar] [CrossRef]
- Möricke, A.; Reiter, A.; Zimmermann, M.; Gadner, H.; Stanulla, M.; Dördelmann, M.; Löning, L.; Beier, R.; Ludwig, W. D., Ratei, R.; et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008, 111, 4477–4489. [CrossRef]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Fischer, D.C.; Mischek, A.; Wolf, S.; Rahn, A.; Salweski, B.; Kundt, G.; Haffner, D. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem 2012, 49, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Sands, D.; Gajewska, J.; Chelchowska, M.; Laskowska-Klita, T. Bone turnover markers, osteoprotegerin and RANKL cytokines in children with cystic fibrosis. Adv Med Sci. 2013, 58, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Tsentidis, C.; Gourgiotis, D.; Kossiva, L.; Doulgeraki, A.; Marmarinos, A.; Galli-Tsinopoulou, A.; Karavanaki, K. Higher levels of s-RANKL and osteoprotegerin in children and adolescents with type 1 diabetes mellitus may indicate increased osteoclast signaling and predisposition to lower bone mass: a multivariate cross-sectional analysis. Osteoporos Int. 2016, 27, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, I.; Ozdemir, M.A.; Unal, E.; Abdurrezzak, U.; Muhtaroglu, S.; Karakukcu, M. Effect of vitamin K2 and vitamin D3 on bone mineral density in children with acute lymphoblastic leukemia: a prospective cohort study. J Pediatr Endocrinol Metab. 2021, 34, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.E.; Rivera-Pasquel, M.; Valdez-Sánchez, A.; De la Cruz-Góngora, V.; Contreras-Manzano, A.; Shamah-Levy, T.; Villalpando, S. Vitamin D status in Mexican children 1 to 11 years of age: an update from the Ensanut 2018-19. Salud Publica Mex. 2021, 63, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Maddheshiya, S.; Singh, S.K.; Kumar, I.; Aggarwal, P.; Gupta, V. Bone Mineral Metabolism During Chemotherapy in Childhood Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2021, 43, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; N Qureshi, R.; S Shamsi, T.; Mahboob, T. Vitamin D levels in patients of acute leukemia before and after remission-induction therapy. Pak J Med Sci. 2013, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- ura-Półtorak A, Szeremeta A, Olczyk K, Zoń-Giebel A, Komosińska-Vassev K. Bone Metabolism and RANKL/OPG Ratio in Rheumatoid Arthritis Women Treated with TNF-α Inhibitors. J Clin Med. 2021, 10, 2905. [CrossRef] [PubMed]
- Lei, Y.; Fu, S.; Yang, Y.; Chen, J.; Li, B.; Guo, Z; Ye, J. Identification and Functional Analysis of Tartrate-Resistant Acid Phosphatase Type 5b (TRAP5b) in Oreochromis niloticus. Int J Mol Sci 2023, 24, 7179. [CrossRef] [PubMed]
- Terpos, E.; de la Fuente, J.; Szydlo, R.; Hatjiharissi, E., Viniou, N.; Meletis, J.; Yataganas, X.; Goldman, J. M.; Rahemtulla, A. Tartrate-resistant acid phosphatase isoform 5b: a novel serum marker for monitoring bone disease in multiple myeloma. Int J Cancer 2003, 106, 455–457. [CrossRef] [PubMed]
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Orgel, E., Mueske, N. M., Wren, T. A., Gilsanz, V., Butturini, A. M., Freyer, D. R., & Mittelman, S. D. Early injury to cortical and cancellous bone from induction chemotherapy for adolescents and young adults treated for acute lymphoblastic leukemia. Bone 2016, 85, 131–137. [CrossRef]
- Rajakumar, S.A.; Papp.; Rajakumar, S. A.; v Papp, E.; Lee, K. K.; Grandal, I., Merico, D.; Liu, C. C.; Allo, B.; Zhang, L.; Grynpas, M. D.; Minden, M. D.; Hitzler, J. K.; Guidos, C. J.; Danska, J. S. Lee KK, et al. B cell acute lymphoblastic leukemia cells mediate RANK-RANKL-dependent bone destruction. Sci Transl Med 2020, 12, eaba5942. [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Battafarano, G.; Pepe, J.; Minisola, S.; Del Fattore, A. The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases. Int J Mol Sci. 2019, 20, 4502. [Google Scholar] [CrossRef] [PubMed]
- Halton, J.; Gaboury, I.; Grant, R.; Alos, N.; Cummings, E.A.; Matzinger, M.; Shenouda, N.; Lentle, B.; Abish, S.; Atkinson, S.; et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009, 24, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, H.; Wang, Y.; Yao, X. Associations between bone turnover markers and bone mineral density in older adults. J Orthop Surg (Hong Kong) 2021, 29, 2309499020987653. [Google Scholar] [CrossRef] [PubMed]
- Ladang, A.; Rousselle, O.; Huyghebaert, L.; Bekaert, A.C.; Kovacs, S.; Le Goff, C.; Cavalier, E. Parathormone, bone alkaline phosphatase and 25-hydroxyvitamin D status in a large cohort of 1200 children and teenagers. Acta Clin Belg. 2022, 77, 4–9. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).