1. Introduction
The choice of energy substrates for cell culture in vitro determines the cellular metabolic pathways. While high glucose levels in culture media promote cell proliferation, other monosaccharides like fructose and galactose also serve as good energy sources, influencing cell metabolism. Galactose, for instance, needs to be converted to glucose through the Leloir pathway, and its oxidation to pyruvate does not generate net ATP. Therefore, cells cultured with galactose as the main carbon source rely on mitochondrial oxidative phosphorylation (OXPHOS) to produce ATP, leading to a metabolic switch where glycolysis slows down and OXPHOS is activated, unlike cells cultured in high glucose media that tend to favor glycolysis [
1,
2,
3,
4]. Importantly, the absence of glucose and serum in the medium can quickly decrease cell viability, whereas cells grown in the presence of galactose can maintain their viability over longer periods [
5].
The essential role of mitochondrial OXPHOS in endothelial physiology is well-established [
6]. Endothelial cell mitochondria are highly coupled and operate at submaximal capacity, showing significant bioenergetic flexibility [
7]. This plasticity is crucial for responding to stimuli like blood flow, hypoxia, angiogenesis, and inflammation. Additionally, endothelial cells express PGC-1α, a key regulator of mitochondrial function, which plays a critical role in endothelial processes such as proliferation, migration, flow response, and nitric oxide production [
8,
9,
10,
11].
Mitochondria form networks and undergo fusion and fission cycles in various cell types, including endothelial cells, closely linked to their metabolic function. Fusion is associated with “coupling efficiency” and low superoxide production [
12], while fission is involved in removing damaged mitochondria, response to growth factors, and cell proliferation [
13,
14]. Additionally, studies have shown that mitochondrial fission serves as an adaptive response to cellular stress and can impact the control of mitochondrial-dependent apoptosis [
15]. Endothelial cells undergoing oxidative stress exhibit mitochondrial fission [
16,
17,
18]. As the mitochondrial network is crucial for endothelial function, the choice of energy substrate for studying mitochondrial function in these cells is vital [
5].
Transcription factors (TFs) that respond to the intracellular redox status, such as FOXO1/3 and Nrf2, are likely to influence metabolic processes, promoting the creation of highly energetic fused mitochondria while maintaining low levels of reactive oxygen species (ROS) and preserving cell viability, particularly in the absence of growth factors [
19,
20]. Currently, few studies have considered these aspects, making it challenging to reconcile cell culture findings with in vivo data on these phenomena.
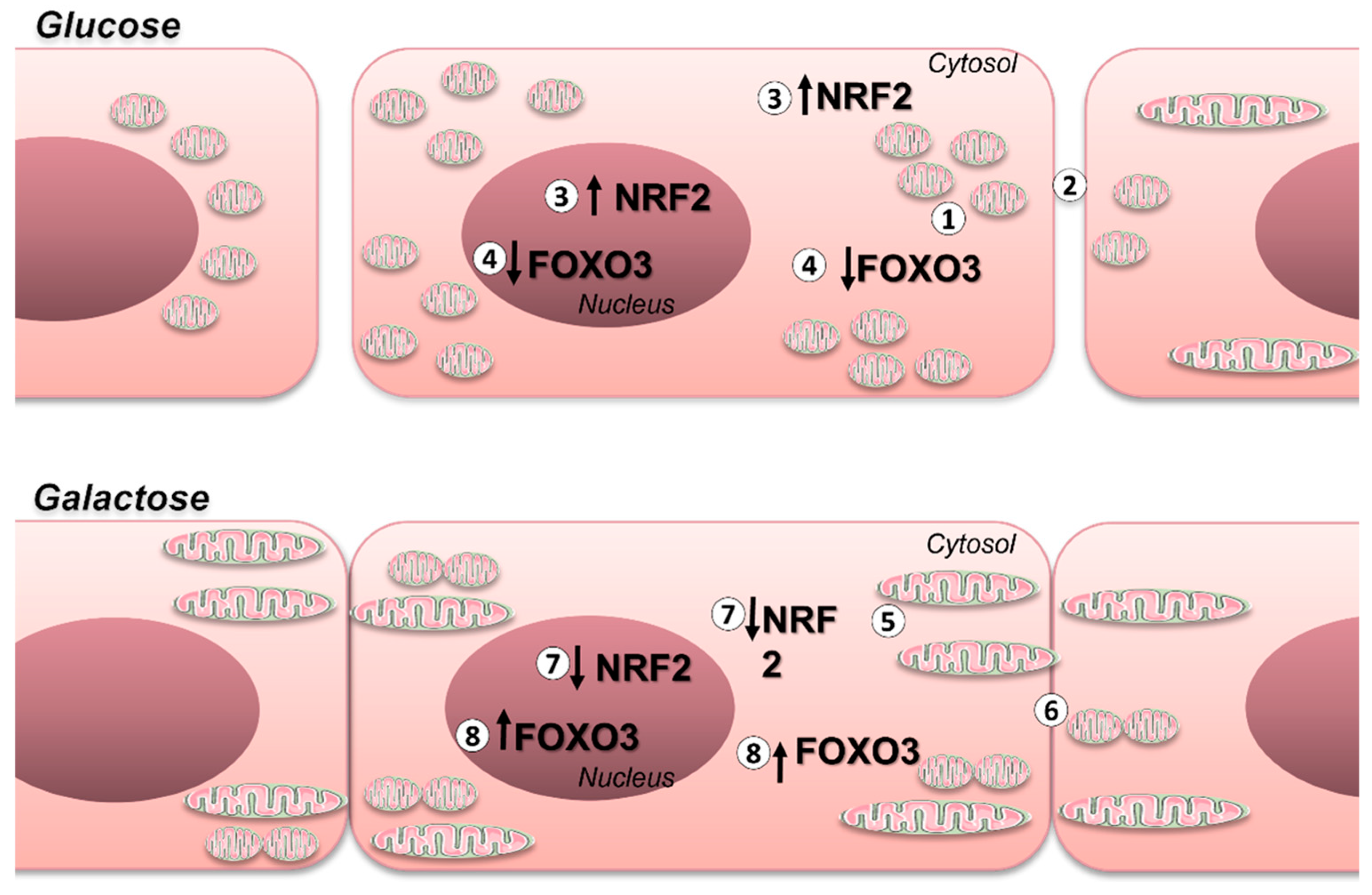
Therefore, in this study we aimed to evaluate the effects on mitochondrial dynamics and function elicited on endothelial cells by their exposure to a culture medium containing galactose instead of glucose. We found that these cells have greater mitochondrial oxidative respiration, enhanced mitochondrial network, and increased intercellular connectivity, which is a known important factor in the response of cells to apoptotic stimuli. In contrast, cells maintained in a glucose-containing medium display mitochondria fragmentation and decreased oxidative flux. In addition, cells maintained in glucose-containing medium have increased Nrf2 content and undergo time-dependent variations in nuclear vs cytosolic FoxO3 localization.
2. Materials and Methods
Cell culture and treatments—Bovine aortic endothelial cells (BAEC) were extracted from fresh bovine thoracic aorta as previously described by Peluffo and colleagues [
21]. BAEC were cultured in growth medium (DMEM) supplemented with 10% of fetal bovine serum (FBS; Gibco/Invitrogen, Waltham, MA, USA) containing 2 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 10 mM Hepes, 25 mM glucose, 44 mM NaHCO
3 and incubated at 37 °C in a humidified atmosphere of 5% CO
2. Cell’s suspensions were seeded in dish plates (100 × 20 mm), 96, 24 or 6—well plates at different cell densities, depending on the experimental procedure. Cells were cultured at confluences of 90% and then culture media was changed to: i) a glycolytic media, DMEM (25 mM glucose) without FBS for 3, 6, 12, 24 or 48 h, and ii) an oxidative media, glucose-free RPMI medium with 5 mM galactose and without FBS for 3, 6, 12, 24 or 48 h.
Oxygen consumption—Oxygen consumption rates (OCR) were measured using a Seahorse Bioscience system. To evaluate the mitochondrial oxygen consumption in BAEC, cells were plated in XF24 cell culture microplates (24—well plates at a cell density of 5 x 103 cells/well) and submitted to glycolytic (glucose) or oxidative (galactose) protocol for 3, 12 or 48 h. A calibration cartridge (Seahorse Bioscience, Agilent Technologies Spain, Las Rozas Madrid, Spain) was equilibrated overnight and then loaded with unbuffered cell culture media (port A), 0.6 μM oligomycin (port B), 0.3 μM FCCP (port C), and 0.1 μM rotenone plus 0.1 μM antimycin A (port D), all obtained from Sigma-Aldrich (Darmstadt, Germany). This allowed the determination of basal respiration, the maximal respiration reserve capacity, extra mitochondrial respiration. The experiments were run with the cells exposed to the same media conditions as while in culture but unbuffered. In all experiments, the protein concentration in each well was determined at the end of the measurements, using the Pierce BCA protein assay kit (Thermo Scientific, Waltham, MA, USA) after cell lysis in RIPA buffer (Sigma-Aldrich, Darmstadt, Germany) supplemented with protease inhibitor cocktail (Complete Mini; Roche Farma S.A., Madrid, Spain), and was used to calibrate the oxygen consumption data.
MitoSOX Imaging—Mitochondrial superoxide was analyzed by labeling cells with MitoSOX Red (Molecular Probes, Carlsbad, CA, USA, essentially as described [
10]. Briefly, BAEC were grown in coverslips in 24—well culture (1 x 105 cells/well) plates and submitted to glycolytic (glucose) or oxidative (galactose) protocol for 24 h. After, BAEC were incubated with 3 μM MitoSOX Red for 10 min, fixed with paraformaldehyde and analyzed by fluorescence microscopy (Leica TCS SP5, Buffalo Grove, IL, USA).
Immunofluorescence (IF)—BAEC were grown on coverslips, 24-well culture (1 x 105 cells/well) plates and submitted to glycolytic (glucose) or oxidative (galactose) protocol for 3, 6, 12, 24 or 48 h. At the end of the incubation period, the cells were fixed with 3.7% formaldehyde, permeabilized with 0.1% Triton, and then incubated consecutively with a primary antibody for Tomm22 (1:200, HPA003037, MERCK, Darmstadt, Germany) for mitochondrial dynamics analyses, and Nrf2 (1:200, PA1-38312, Thermo Fisher, Waltham, MA, USA) or FOXO3 (1:100, #9467, Cell signaling) for redox-related transcription factors, followed by incubation with a secondary antibody (α-IgG rabbit ALEXA-488 conjugate, 1:2500). Secondary antibody controls are included in Supplementary
Figure 1. Cells were counterstained with DAPI, mounted and examined by confocal microscopy (Zeiss LSM 700, Obercochen, Germany), as previously described [
10]. Control IFs, incubated only with the secondary antibody, were used to control for the background signal that was subtracted from the analysis.
The total α-Tomm22 IF signal (Atlas Antibodies, HPA003037, Bromma, Sweden) was used for the evaluation of the cellular mitochondrial content. Mitochondrial fission was determined as the standard deviation of Tomm22 intensity signal across the cell. Mitochondrial subcellular distribution was also evaluated using Tomm22 signal determination. The perinuclear region signal was compared to the total cytosolic signal, and the asymmetry of the signal in the perinuclear region was also evaluated comparing the opposite max and min signals in opposite nuclear sides. The nuclei were identified by DAPI staining. This analytical procedure has already been reported [
22].
Gene expression analysis—Cultured cells were washed with PBS and total RNA was isolated using TrizolTM reagent (ThermoFisher Sci., Waltham, MA, USA) following the manufacturer’s instructions. cDNA was synthesized from total RNA preparations by reverse transcription of 1 μg of RNA using MMV reverse transcriptase (Promega Biotech Ibérica SL, Alcobendas (Madrid), Spain), in a final volume of 20 μ. The mixture was incubated at 37°C for 45 min and then cooled for 2 min at 4°C. The resulting cDNA was used as a template for subsequent qPCR. The primers used are listed below. Each 10 µl PCR reaction included 1 µl cDNA, 5 µl qPCRBIO SyGreen Mastermix (Cultek SL, Dutchcher Group, San Fernando de Henares, Madrid, Spain) and primers (0.3 µM). Samples were analyzed in triplicate on a Mastercycler® RealPlex2, Eppendorf Iberica SLU, San Sebastian de los Reyes, Madrid, Spain). 36B4 was used as loading control.
Acidic ribosomal protein 36B4 (36B4) -RPLP0- Gene ID: 286868
forward 5′-GCGACCTGGAGTCCAACTA-3′
reverse 5′-ATCTGCTGCATCTGCTTGG-3′
Cytochrome c (Cyt c) -CYC- Gene ID: 510767
forward 5′-GCCAATAAGAACAAAGGCATCA-3′
reverse 5′-GTTTTGTAATAAATAAGGCAGTGG-3′
Mitochondrial fission protein 1 (Fis1) -FIS1- Gene ID: 615565
forward 5′-GACATCCGTAAAGGCCTTGC-3′
reverse 5′-TCCATCTTTCTTCATGGCCT-3′
Mitochondrial dynamin like GTPase (Opa1) -OPA1- Gene ID: 524142
forward 5′-TGGAAAATGGTACGAGAGTCAG-3′
reverse 5′-ACTGCTGAAGGATTTCTTCC-3′
Peroxiredoxin 3 (Prx3)—PRDX3- Gene ID: 281998
forward 5′-TTCGGGCTTCGCTCATCCGA -3′
reverse 5′-ATGGTATGAGGAACTGGTGCT -3′
ATP synthase subunit β1 (Atp5bp)—ATP5BP-Gene ID: 327675
forward 5′-TGTACCACCTCTTCCTGAACA-3′
reverse 5′-AGTGCCTGCTGTGACTTCTC-3′
Superoxide dismutase 2 (Sod2)—SOD2- Gene ID: 281496
forward 5′-GGAACAACAGGTCTTATCCCCCT-3′
reverse 5′-TTACTTGCTGCAAGCCGTGTATC-3′
Mitofusin 2 (Mfn2)—MFN2- Gene ID: 534574
forward 5′-TGGCGCAAGACTACAAACTG-3′
reverse 5′-TCGTCCACCAACACAGAGAG-3′
Western blotting—Whole cell extracts were prepared as previously described [
22]. Protein concentration was evaluated by Lowry’s method using the RC/DC Protein Assay (Bio-Rad). A total of 18 μg of protein extract was loaded on 8/15% SDS-PAGE gels, which was then transferred to Immobilon-P membranes (Cytiva, Washington D. C., USA). Proteins of interest were identified by western blotting using the specific antibodies listed below, as described [
23]. The proteins were visualized using Clarity ECL Substrate (BioRad Spain, Albobendas, Madrid, Spain) and the image was captured with a chemiluminescence membrane’s reader (Nirco SL, Mostoles, Madrid, Spain). The films were then scanned and the protein bands were quantified using the ImageJ software. Original scanned blots are included in the Supplementary Material. Tubulin was used as a loading control.
α-OPA1 1:500 Sigma-Aldrich, Darmstadt, Germany Ref. HPA036926
α-Tomm22 1:2000 Atlas Antibodies, Bromma, Sweden Ref. HPA003037
α-PGC-1α 1:1000 Cayman Chemical, Ann Arbor, MI, USA Ref. 101707
α-Tubulin 1:1000 Sigma-Aldrich, Darmstadt, Germany Ref. 9026
Image analysis—Image J software was used for the analysis of areas, signals in an area and cross section signals from fluorescence and confocal microscopic images for Tomm22, Nrf2, FOXO3 and MitoSOX and western blot bands for α-Tubulin, α-Tomm22, α-OPA1.
Statistics—Statistical analysis and graphics were made using the GraphPad PRISM® software version 9 for Windows (GraphPad Software, San Diego, CA, USA). Normal (Gaussian) distribution was evaluated with the Shapiro-Wilk normality test. Significant differences among groups were evaluated by unpaired t-test or two-way analysis of variance (ANOVA), depending on the experimental design. Multiple comparisons were performed using Bonferroni’s post-hoc test. Results are expressed as mean ± SEM. p <0.05 was considered statistically significant. n ≥ 4 in all experiments. The number of independent experiments, for each particular experiment, is indicated in the corresponding figure legend.
3. Results
3.1. Effect of glucose- or galactose-containing medium in cellular respiration and mitochondrial superoxide radical (O2• -) levels in BAEC.
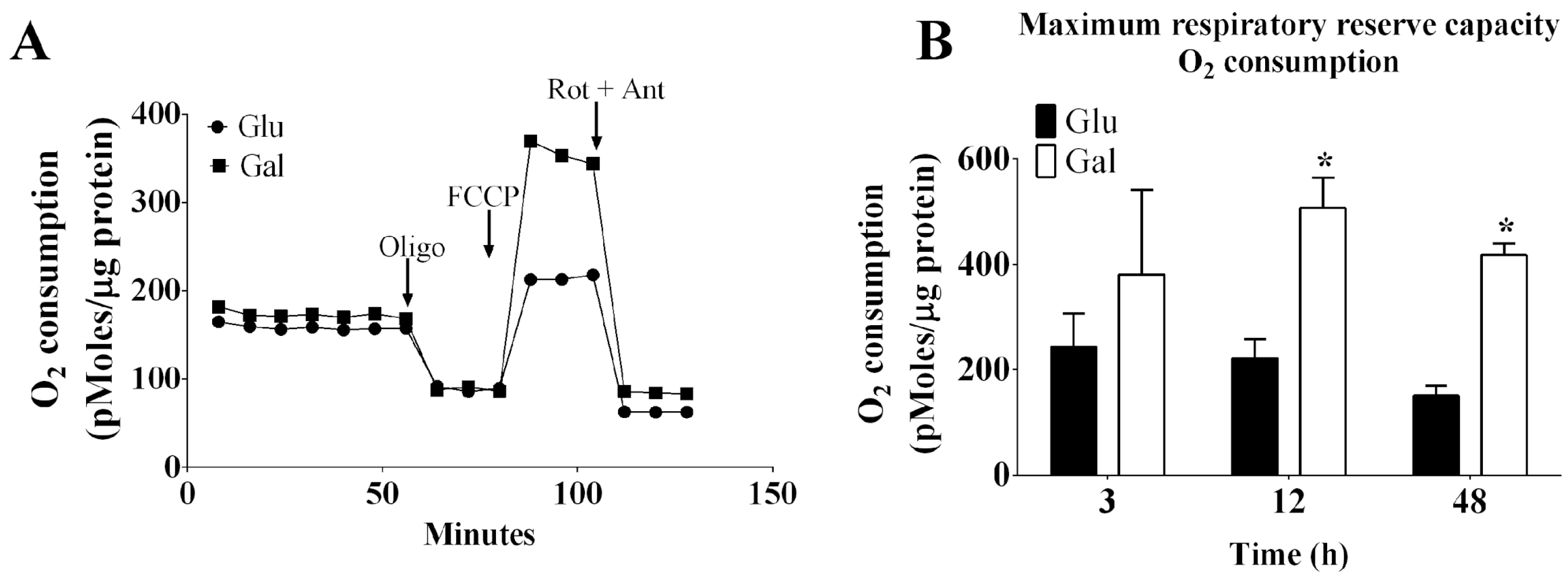
3.1.1. The mitochondrial oxygen consumption in BAEC conditioned in glucose or galactose medium is depicted in
Figure 1. Using a Seahorse system, the mitochondrial oxygen consumption in BAEC was evaluated. The BAEC are primary cell cultures, and cellular respiration can be affected by cell passage. In fact, cells with passage 4 (P4) displayed higher basal respiration rates, as well as a higher maximum respiratory reserve capacity, estimated following FCCP stimulation, than those in passage 5 (P5) or 8 (P8) (
Supplementary Figure S2). Therefore, it is very important to test the cellular respiration related pathways using early cell passages to obtain robust results in primary endothelial cell cultures. In subsequent experiments with BAEC, we used P4-P8 passages. In order to confirm the activation of oxidative phosphorylation in galactose containing medium, we compared mitochondrial oxygen consumption rates in BAEC conditioned in galactose or glucose cell culture media (in serum deprivation conditions) at 3, 12 or 48 h (
Figure 1A,B). In the absence of serum, the maximum respiratory reserve capacity of BAEC conditioned in culture medium containing galactose was found to be significantly higher when compared to that observed in cells conditioned in culture medium containing glucose after 12- 48h of incubation. Together these data suggest that galactose increased the maximal oxidative capacity of BAEC and its metabolic plasticity.
3.1.2. The mitochondria are major modulators of cellular reactive species (RS), thus, to test the impact of the medium containing glucose or galactose on mitochondrial RS, we incubated BAEC with MitoSOX Red, a reagent that labels mitochondrial superoxide. We did not detect differences in mitochondrial O
2•- level when BAEC were cultivated in medium containing glucose or galactose (
Supplementary Figure S2), suggesting that the redox balance was not significantly affected by medium manipulations.
3.2. Mitochondrial Dynamics of BAEC Conditioned in Glucose or Galactose Medium
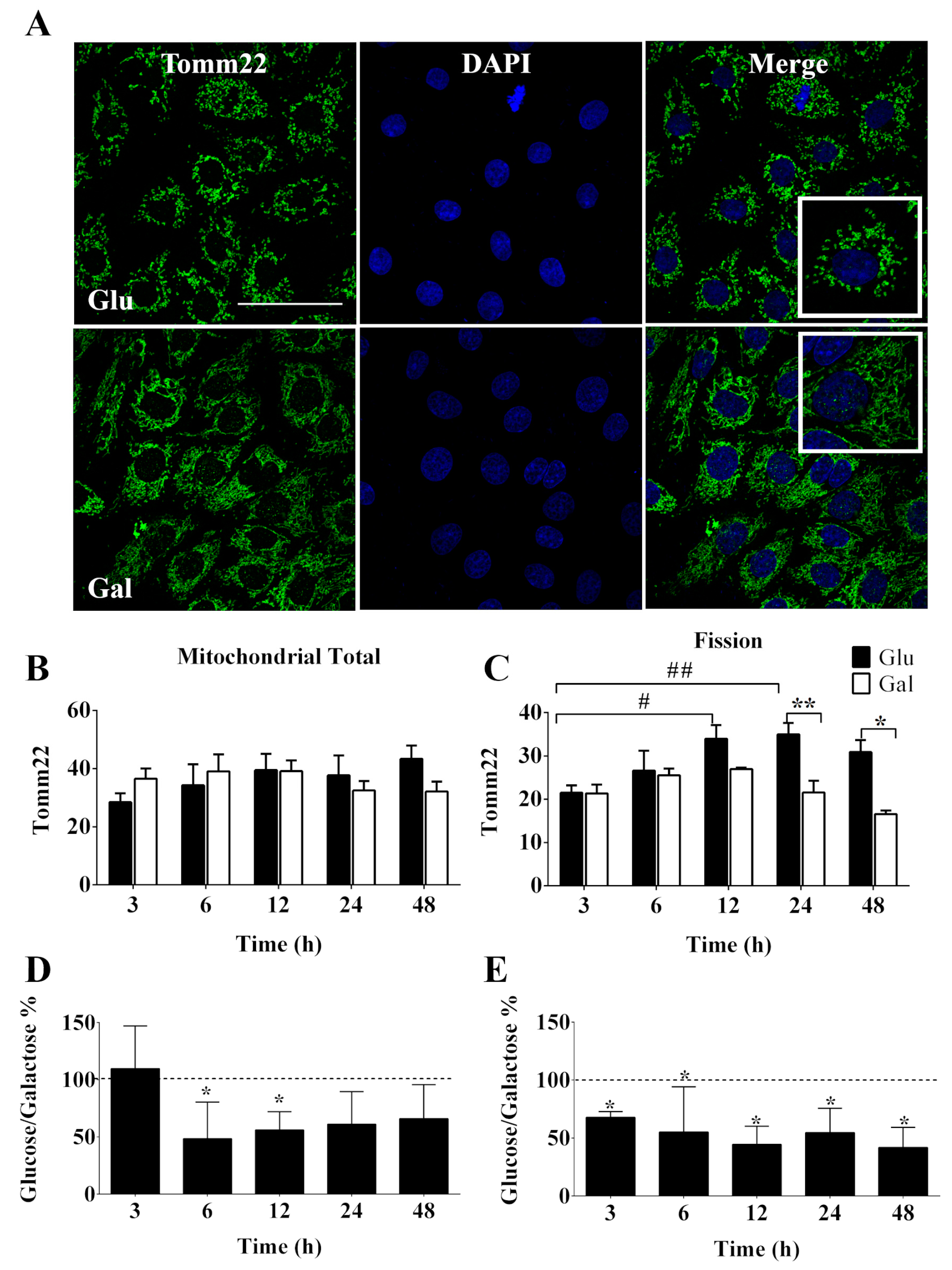
3.2.1 Mitochondria are plastic organelles that frequently change their morphology, volume, and intracellular distribution in response to fluctuations in metabolic demands, thus, the disruption of this balance results in mitochondrial dysfunction. Tomm22, is part of a protein translocase complex found in the outer mitochondrial membrane which is commonly used as a marker of mitochondrial volume, morphology, and intracellular movement by immunofluorescence. As shown in
Figure 2A,B, after 3, 6, 12, 24 or 48 h in a medium containing glucose or galactose in the absence of FBS, no change in mitochondrial total volume content was observed, suggesting that the observed increase in oxidative capacity in galactose media was not related to increases in mitochondrial mass. This observation was supported by Western blot analysis of Tomm22 cellular content at 24h, a similar result was also obtained for another mitochondrial protein OPA1, a marker of cristae density, and the ratio of the two was also preserved in the two media (
Supplementary Figure S4A). Furthermore, we did not detect significant gene expression changes in genes coding for mitochondrial proteins including components of the electron transport chain/ETC (
Cyt c, Atp5bp), antioxidants (
Sod2, Prx3) and regulator of mitochondrial dynamics (
Mnf2,
Opa1, Fis1) at 24 h, although a general tendency to higher values could be observed for cells in galactose (
Supplementary Figure S4B).
Nevertheless, we observed that mitochondria of BAEC in galactose were significantly more fused than those in glucose at 12 and 24h (
Figure 2C). It was also noted that the status of mitochondria fission in BAEC exposed to galactose was stable overtime (
Figure 2C), suggesting that serum deprivation induced mitochondrial fission only when cells were in glucose media. Changes in intracellular distribution of mitochondria were also evaluated, since mitochondrial function is also dependent on its subcellular localization by the determination perinuclear/total and left/right nuclear ratios, but no significant differences were identified in the cellular response to culture medium or time of conditioning (
Supplementary Figure S5A,B).
3.2.2 Next, we evaluated the intercellular coordination of the mitochondrial network since intercellular contacts provide metabolic coupling that allows coordinated cellular responses within tissues, that are of relevance in the response to stimuli (i.e., oxidative stress, inflammation, apoptosis). Therefore, we decided to evaluate the intercellular variability (standard deviation) of the immunofluorescence signal for Tomm22 as a surrogate marker for intercellular metabolic coupling. We observed that cells maintained in galactose medium displayed significantly lower intercellular variability for Tomm22 signal, than cells in glucose medium (
Figure 2D,E and
Supplementary Figure S5C,D). These results could be indicative of a more stable/extended intercellular contact surface that would result in a more coordinated intercellular response in galactose treated BAEC than in BAEC maintained in glucose medium.
3.3. Effect of Glucose- or Galactose-Containing Medium on Translocation and Expression of Nrf2
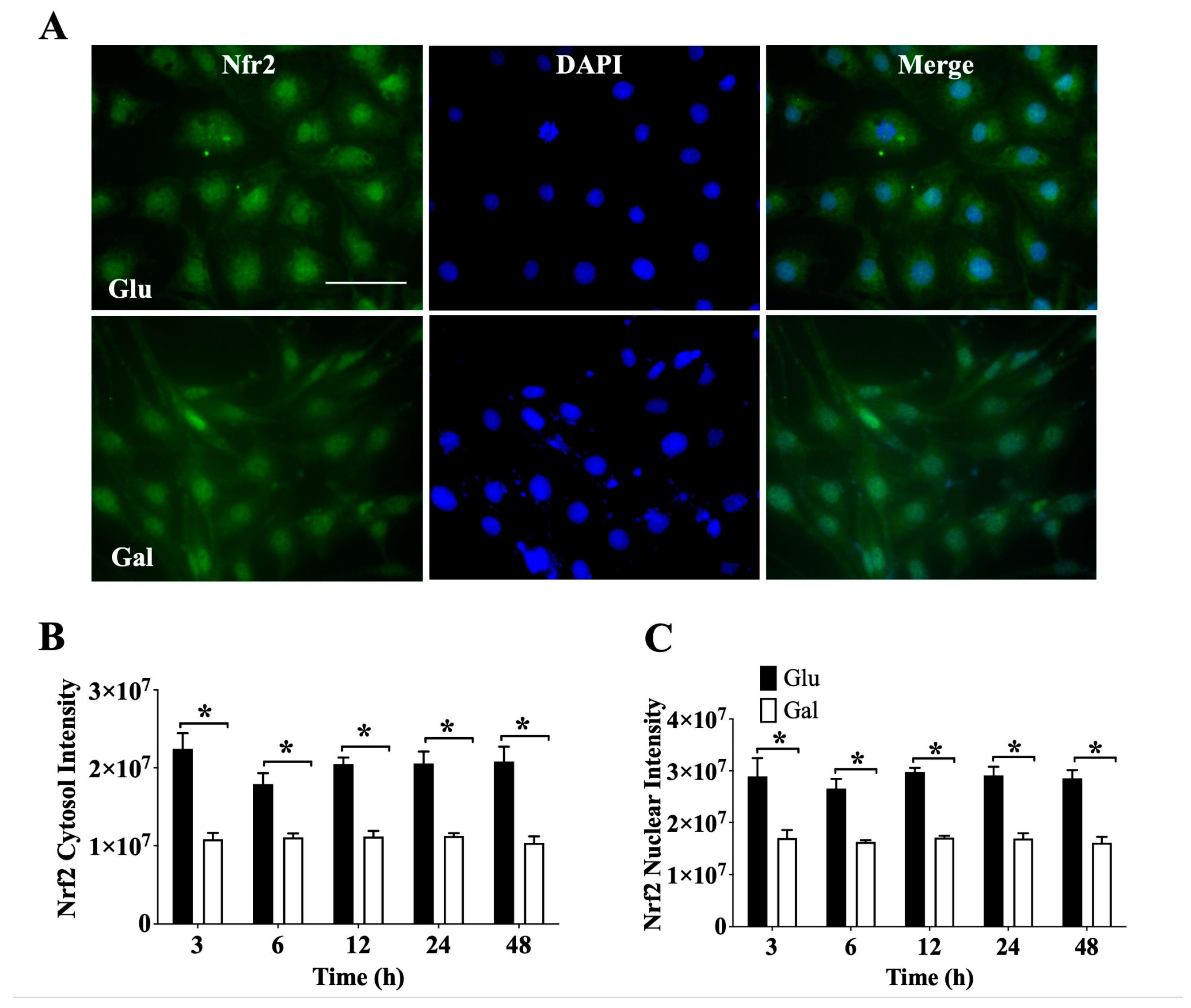
3.3.1 In order to evaluate how substrate utilization impacted on the endothelial cell capacity to respond to oxidative stress stimuli, we evaluated the basal levels and subcellular localization of the main redox sensitive transcription factors, Nrf2 and FOXO3, that translocate to the nuclei following oxidative stimulation. We first used immunofluorescence (IF) to investigate Nrf2 levels and subcellular localization (
Figure 3A). Evaluating the signal intensity of cytosolic (
Figure 3B) and nuclear (
Figure 3C) Nrf2, we observed a significantly higher total level of Nrf2 in cells maintained in medium containing glucose than in galactose at all times tested, and this difference was observed both in the cytosol and the nucleus. Nevertheless, when the Nrf2 nuclear/ cytosolic ratio was evaluated, it was noted that at 48 h BAEC in galactose had a higher ratio than BAEC in glucose (
Supplementary Figure S6A). These results suggest that to maintain ROS homeostasis, BAEC in glucose need higher levels of Nrf2, both in the nucleus and in the cytosol, than BAEC maintained in galactose medium.
3.4. Effect of Glucose- or Galactose-Containing Medium on Translocation and Expression of FOXO3 Transcription Factor
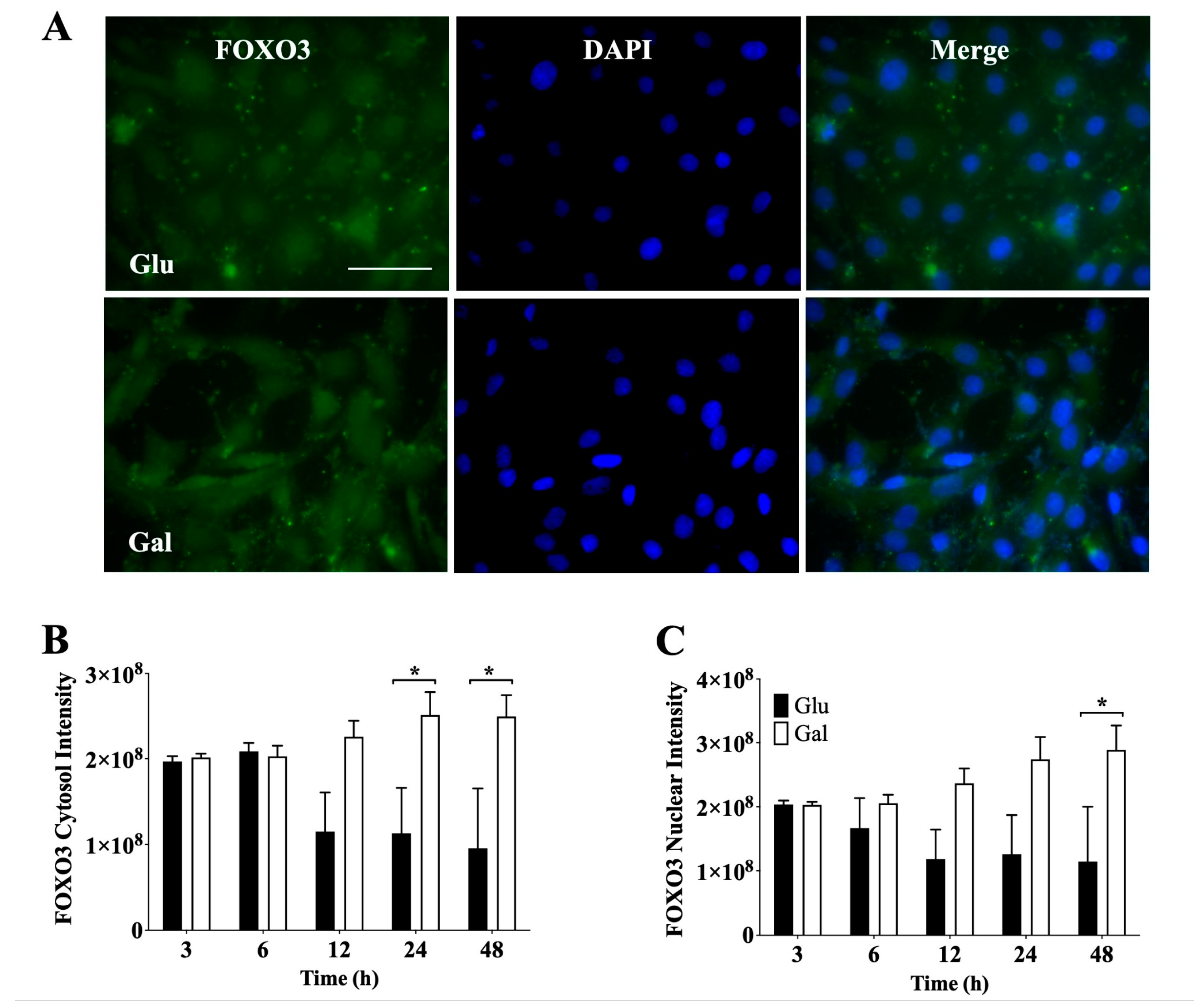
3.4.1 Next, we evaluated by IF the signal intensity of cytosolic (
Figure 4B) and nuclear (
Figure 4C) FOXO3, as shown in
Figure 4. In contrast with Nrf2 data we observed a higher total level of cytosolic FOXO3 in BAEC maintained in galactose medium at 24h and 48h of incubation. Consistently, the levels of nuclear FoxO3 were significantly higher in BAEC maintained galactose containing medium at 48h of incubation. Furthermore, it was noted that BAEC in glucose showed a significant time-dependent reduction in FOXO3. However, no significant difference in the nuclear/cytosol ratio was observed between glucose and galactose media BAEC. (
Supplementary Figure S6B). These results suggest that galactose treated cells may be more dependent on FOXO3 to maintain redox homeostasis than glucose treated cells.
4. Discussion
The results presented in this study show that primary endothelial cells effectively modulate their metabolism in response to cell culture conditions, which significantly impacts on the mechanisms that regulate cellular REDOX homeostasis. Therefore, the use of culture media containing galactose instead of glucose is pertinent for the study of endothelial physiological processes involving mitochondrial function and or REDOX control such as angiogenesis, NO production, response to blood flow, interaction with immune cells, intercellular communication and permeability. Additionally, we noted that cells in galactose exhibited higher oxidative capacity, more fussed mitochondria, and seem to be more metabolically coupled through intercellular communication than BAEC in glucose media. Furthermore, we observed that cells in glucose have higher levels of Nrf2 while those in galactose have higher levels of FOXO3, suggesting that the mechanisms involved in RS control are significantly altered by the culture media.
While there is widespread evidence showcasing the functional relevance of mitochondria in endothelial physiology and REDOX homeostasis, this aspect is often overlooked [
24]. Due to the so called “Cabtree effect”, well-documented in the literature for different cell types, BAEC in glucose media under serum deprivation conditions exhibited lower maximal OCRs than cells maintained in galactose-containing media, suggesting an enhanced metabolic plasticity. Similarly, increased oxidative metabolism and decreased anaerobic metabolism can be observed in muscle cells incubated with galactose instead of glucose [
1,
25]. Cells grown in galactose-containing medium can double the rate of oxygen consumption and, consequently, enhance the susceptibility to mitochondrial toxins compared to cells grown in glucose-containing medium [
2]. Similar observations have been found when cells are grown in low-glucose media, and this concept is being currently investigated to study the susceptibility of cancer cells to mitochondrial toxins, such as metformin, and its therapeutic applications [
26].
We assessed the metabolic plasticity of BAEC in vitro and found that those cultured in galactose-containing medium exhibited greater oxidative capacity and plasticity compared to cells in glucose-containing medium. Although there was no change in mitochondrial volume over time or with different energy substrates, BAEC in galactose-containing media showed a net increase in oxidative capacity per mitochondrion. This finding is further supported by the observation that BAEC in glucose-containing medium displayed more mitochondrial fragmentation (fission), while those in galactose-containing medium maintained a higher mitochondrial fusion index. This suggests that changes in respiratory capacity were linked to the fission/fusion state and possibly a higher ETC content per mitochondrion rather than effects mediated by mitochondrial biogenesis or mitophagy.
Previous studies have associated increased fragmentation and decreased mitochondrial fusion with reduced mitochondria membrane potential and increased superoxide production, possibly due to a reduced supercomplex formation potentially leading to apoptosis, as noted in endothelial cells maintained in hyperglycemic media [
7,
27,
28,
29]. However, under basal conditions, BAEC treated with glucose and galactose showed similar levels of superoxide, suggesting the maintenance of redox balance in both conditions. Our data suggest that BAEC have the capacity to rapidly adjust to changing energy demands through changes in mitochondrial dynamics and possibly ETC content, indicating a metabolic plasticity modulated by simple changes in culture media. Furthermore, cells maintained in glucose-containing medium when deprived of FBS can quickly induce apoptosis while cells maintained in galactose-containing medium are more resistant to growth factor deprivation, making the former conditions more suitable for testing responses requiring growth factor deprivation. Additionally, analysis of intercellular mitochondrial variability revealed that cells in glucose-containing media exhibited reduced intercellular communication, potentially due to the smaller cell-cell contact zone, affecting data variability. Conversely, in galactose-containing, intercellular metabolic coupling appeared greater, suggesting an enhanced ability to coordinate responses to external stimuli such as oxidative stress. Importantly, previous studies have linked mitochondrial activity in endothelial cells to improved and more stable cellular contacts, facilitating the formation of stable vessel structures and coordination during cell migration [
9,
10].
There is a well-documented interplay between metabolic and redox regulatory pathways. Thus, we investigated the impact of cell culture on the two main REDOX transcriptional regulators in BAEC, Nrf2 and FOXO1/3. Nrf2 is known to activate cellular antioxidant responses, promoting the synthesis of crucial enzymes during oxidative processes [
30,
31]. We observed that the nuclear and cytosolic content of Nrf2 in endothelial cells maintained in glucose-containing medium was higher compared to those under galactose, suggesting that Nrf2 may play a predominant role in the control of REDOX homeostasis in BAEC cultured in glucose conditions [
32]. To our knowledge, this study represents the first investigation into the involvement of Nrf2 in glycolytic environment in the absence of FBS in endothelial cells.
FOXO3′s role in redox control is intricately linked to metabolism and vascular cell physiology [
33]. It has been established that endothelial cells cultured in high-glucose media (standard medium containing approximately 25 mM) exhibited elevated RS generation, Akt inhibition, and therefore high FOXO3 activation, correlating with cellular apoptosis [
34]. In tumor cells, which exhibit highly active glycolytic metabolism, elevated nuclear levels of FOXO3 were correlated with apoptosis and reduced cell survival [
35,
36,
37]. However, FOXO3a activity can also promote cell survival when activated under conditions favoring oxidative phosphorylation, especially through the induction of antioxidant genes [
11]. Our findings indicated that after 12h of incubation, BAEC maintained in glucose-containing media exhibited reduced total FOXO3 content, whereas those in galactose-containing media, maintained constant FOXO3 levels. This suggests that FOXO3 levels are better preserved under galactose conditions, and underscores a potential predominant role of FOXO3 in REDOX control.
Endothelial cells are particularly vulnerable to oxidative stress due to their direct exposure to the vascular environment and frequent contact with RS. If not detoxified, RS can lead to endothelial dysfunction. Therefore, the modulation of transcription factors related to antioxidant defenses, such as Nrf2 and FOXO3, is crucial for preserving endothelium integrity. However, it is essential to exercise caution when studying the activation of these transcription factors and the mitochondrial function, considering the experimental protocol used. Experimental protocols that maintain mild levels of transcription factors like Nrf2 and FOXO3, as well as preserving mitochondrial modulation and function, are essential for accurately elucidating the mechanism of action of certain drugs. Depending on the energy substrate used, the mechanism of action of these molecules can be altered potentially leading to erroneous conclusions.
In this study, we conclude that experimental protocols using primary endothelial cells maintained at standard glucose concentrations and without FBS exhibited increased mitochondrial fission and reduced respiratory reserve capacity, indicative of glycolytic metabolism activation. Such protocols yield greater intercellular variability, likely stemming from a smaller mitochondrial cell network, potentially compromising cell-cell contact and communication. Conversely, BAEC cultured in a galactose-containing medium, exhibited enhanced connectivity within the mitochondrial network and increased respiratory reserve capacity. Larger mitochondrial networks may augment cell-cell contact surface, thereby potentially reducing intercellular variability.
The significance of these alterations on REDOX control is underscored by the observation that, BAECs cultured in media with standard glucose concentrations exhibited elevated levels of Nrf2 and decreased levels of FOXO3 compared to BAEC incubated in galactose (see
Figure 5), consistent with prior findings elucidating the relationship between oxidative metabolism and REDOX regulatory networks.
5. Study Limitations
This study was conducted solely on a single cell type and species; thus, the generalizability of the findings to other cell types and species remains to be established.
The analytical procedure employed for assessing mitochondrial dynamics does not include high-resolution TEM analysis, and the derived conclusions would require further validation, including the analysis of proteins involved in the regulation of mitochondrial dynamics.
Since we did not assess the antibodies used in immunofluorescence on knock-out cells, some signals may stem from non-specific staining of the samples.
Further testing would be required to determine if the observed nuclear localization changes do actually impact on Nrf2 and FOXO3A transcriptional activity.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Effect of cell passage on mitochondrial respiration in BAEC; Figure S2: Effect of glucose- or galactose-containing medium on mitochondrial O2- production in BAEC; Figure S3: Effect of glucose- or galactose-containing medium on mitochondrial dynamics and intercellular variability in BAEC; Figure S4: Effect of glucose- or galactose-containing medium on Nrf2 or FOXO3 translocation in BAEC; Data in Brief: Supporting data files.
Author Contributions
Conceptualization, M.M., A.F.B, and R.R.; methodology, M.M.; validation, M.M. and A.F.B; formal analysis, L.S.G, M.M. and A.F.B; investigation, L.S.G and L.D.; resources, M.M. and A.F.B; data curation, L.S.G. and M.M; writing—original draft preparation, L.S.G.; writing—review and editing, M.M., A.F.B, and R.R.; supervision, M.M. and A.F.B; project administration, M.M; funding acquisition, M.M. and A.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIN/AEI, grant numbers RTI2018-093864-B-I00 and PID2021-122765OB-I00, the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement 721236-TREATMENT and the Brazilian institutions: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF grants 00193-00000884/2021-89 and 00193-00002348/2022-07) and Instituto Nacional de Ciência e Tecnologia e Neuro-ImunoModulação (INCT-NIM grant 485489/2014-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data is included as Supplementary File “Data in Brief”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aguer, C.; Gambarotta, D.; Mailloux, R.J.; Moffat, C.; Dent, R.; McPherson, R.; Harper, M.E. Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree Effect: Replacing Media Glucose with Galactose Increases Susceptibility of HepG2 Cells to Mitochondrial Toxicants. Toxicological Sciences 2007, 97, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Orlicka-Płocka, M.; Gurda-Wozna, D.; Fedoruk-Wyszomirska, A.; Wyszko, E. Circumventing the Crabtree Effect: Forcing Oxidative Phosphorylation (OXPHOS) via Galactose Medium Increases Sensitivity of HepG2 Cells to the Purine Derivative Kinetin Riboside. Apoptosis 2020, 25, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Altamirano, L.; Paudel, S.; Welker, L.; Konkle, M.E.; Chakraborty, N.; Menze, M.A. Modulation of Cellular Energetics by Galactose and Pioglitazone. Cell Tissue Res 2017, 369, 641–646. [Google Scholar] [CrossRef]
- Monsalve, M.; Prieto, I.; de Bem, A.F.; Olmos, Y. Methodological approach for the evaluation of FOXO as a positive regulator of antioxidant genes. Methods in Molecular Biology 2019, 1890, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Caja, S.; Enríquez, JA. Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biol 2017, 12, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Valle, I.; Álvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 2005, 66, 562–573. [Google Scholar] [CrossRef] [PubMed]
- García-Quintans, N.; Sánchez-Ramos, C.; Prieto, I.; Tierrez, A.; Arza, E.; Alfranca, A.; Redondo, J.M.; Monsalve, M. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 2016, 19, 217–228. [Google Scholar] [CrossRef] [PubMed]
- García-Quintans, N.; Prieto, I.; Sánchez-Ramos, C.; Luque, A.; Arza, E.; Olmos, Y.; Monsalve, M. Regulation of endothelial dynamics by PGC-1α relies on ROS control of VEGF-A signaling. Free Radic Biol Med 2016, 93, 41–51. [Google Scholar] [CrossRef]
- Borniquel, S.; Garcia-Quintans, N.; Valle, I.; Olmos, Y.; Wild, B.; Martinez-Granero, F.; Soria, E.; Lamas, S.; Monsalve, M. Inactivation of Foxo3a and Subsequent Downregulation of PGC-1 Mediate Nitric Oxide-Induced Endothelial Cell Migration. Mol Cell Biol 2010, 30, 4035–4044. [Google Scholar] [CrossRef]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal. 2013, 19, 1507–21. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Patel, J.; Khacho, M. Linking Mitochondrial Dynamics, Cristae Remodeling and Supercomplex Formation: How Mitochondrial Structure Can Regulate Bioenergetics. Mitochondrion 2019, 49, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Vorobjev, I.A.; Popkov, V.A.; Babenko, V.A.; Zorova, L.D.; Pevzner, I.B.; Silachev, D.N.; Zorov, S.D.; Andrianova, N. V.; Plotnikov, E.Y. Lessons from the Discovery of Mitochondrial Fragmentation (Fission): A Review and Update. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int J Mol Sci 2021, 22, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Hyde, B.; Shirihai, O.S. Mitochondrial Fusion, Fission and Autophagy as a Quality Control Axis: The Bioenergetic View. Biochim Biophys Acta Bioenerg 2008, 1777, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Jendrach, M.; Mai, S.; Pohl, S.; Vöth, M.; Bereiter-Hahn, J. Short- and Long-Term Alterations of Mitochondrial Morphology, Dynamics and MtDNA after Transient Oxidative Stress. Mitochondrion 2008, 8, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Paltauf-Doburzynska, J.; Malli, R.; Graier, W.F. Hyperglycemic Conditions Affect Shape and Ca2+ Homeostasis of Mitochondria in Endothelial Cells. J Cardiovasc Pharmacol 2004, 44, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Giedt, R.J.; Yang, C.; Zweier, J.L.; Matzavinos, A.; Alevriadou, B.R. Mitochondrial Fission in Endothelial Cells after Simulated Ischemia/Reperfusion: Role of Nitric Oxide and Reactive Oxygen Species. Free Radic Biol Med 2012, 52, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Gonzalez-Lafuente, L.; Pérez-Liébana, I.; Buendia, I.; López-Bernardo, E.; Sánchez-Ramos, C.; Prieto, I.; Cuadrado, A.; Satrustegui, J.; Cadenas, S.; et al. Heme-Oxygenase i and PCG-1α Regulate Mitochondrial Biogenesis via Microglial Activation of Alpha7 Nicotinic Acetylcholine Receptors Using PNU282987. Antioxid Redox Signal 2017, 27, 93–105. [Google Scholar] [CrossRef]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Peluffo, G.; Calcerrada, P.; Piacenza, L.; Pizzano, N.; Radi, R. Superoxide-Mediated Inactivation of Nitric Oxide and Peroxynitrite Formation by Tobacco Smoke in Vascular Endothelium: Studies in Cultured Cells and Smokers. Am J Physiol Heart Circ Physiol 2009, 296. [Google Scholar] [CrossRef]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-Arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int J Mol Sci. 2021, 22, 3903. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, M.; Wu, Z.; Adelmant, G.; Puigserver, P.; Fan, M.; Spiegelman, B.M. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol Cell. 2000, 6, 307–16. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Dott, W.; Mistry, P.; Wright, J.; Cain, K.; Herbert, K.E. Modulation of Mitochondrial Bioenergetics in a Skeletal Muscle Cell Line Model of Mitochondrial Toxicity. Redox Biol 2014, 2, 224–233. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chan, D.K.; Haugrud, A.B.; Miskimins, W.K. Mechanisms by Which Low Glucose Enhances the Cytotoxicity of Metformin to Cancer Cells Both in Vitro and in Vivo. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Abuarab, N.; Munsey, T.S.; Jiang, L.H.; Li, J.; Sivaprasadarao, A. High Glucose-Induced ROS Activates TRPM2 to Trigger Lysosomal Membrane Permeabilization and Zn2+-Mediated Mitochondrial Fission. Sci Signal 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Long, J.; Wang, J.; Haudek, S.B.; Overbeek, P.; Chang, B.H.J.; Schumacker, P.T.; Danesh, F.R. Mitochondrial Fission Triggered by Hyperglycemia Is Mediated by ROCK1 Activation in Podocytes and Endothelial Cells. Cell Metab 2012, 15, 186–200. [Google Scholar] [CrossRef]
- Zeng, Y.; Pan, Q.; Wang, X.; Li, D.; Lin, Y.; Man, F.; Xiao, F.; Guo, L. Impaired Mitochondrial Fusion and Oxidative Phosphorylation Triggered by High Glucose Is Mediated by Tom22 in Endothelial Cells. Oxid Med Cell Longev 2019, 2019. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription Factor Nrf2 Coordinately Regulates a Group of Oxidative Stress-Inducible Genes in Macrophages. Journal of Biological Chemistry 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Afzal-Ahmed, I.; Mann, G.E.; Shennan, A.H.; Poston, L.; Naftalin, R.J. Preeclampsia Inactivates Glucose-6-Phosphate Dehydrogenase and Impairs the Redox Status of Erythrocytes and Fetal Endothelial Cells. Free Radic Biol Med 2007, 42, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, M.; Olmos, Y. The Complex Biology of FOXO. Curr Drug Targets 2011, 12, 1322–1350. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ma, J.; Gao, X.; Tian, P.; Li, W.; Zhang, L. High Glucose Induced Oxidative Stress and Apoptosis in Cardiac Microvascular Endothelial Cells Are Regulated by FoxO3a. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gomes, A.R.; Monteiro, L.J.; Wong, S.Y.; Wu, L.H.; Ng, T.T.; Karadedou, C.T.; Millour, J.; Ip, Y.C.; Cheung, Y.N.; et al. Constitutively Nuclear FOXO3a Localization Predicts Poor Survival and Promotes Akt Phosphorylation in Breast Cancer. PLoS One 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-Evaluating the Role of FOXOs in Cancer. Semin Cancer Biol 2018, 50, 90–100. [Google Scholar] [CrossRef]
- Tenbaum, S.P.; Ordóñez-Morán, P.; Puig, I.; Chicote, I.; Arqués, O.; Landolfi, S.; Fernández, Y.; Herance, J.R.; Gispert, J.D.; Mendizabal, L.; et al. β-Catenin Confers Resistance to PI3K and AKT Inhibitors and Subverts FOXO3a to Promote Metastasis in Colon Cancer. Nat Med 2012, 18, 892–901. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).