Submitted:
20 June 2024
Posted:
20 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
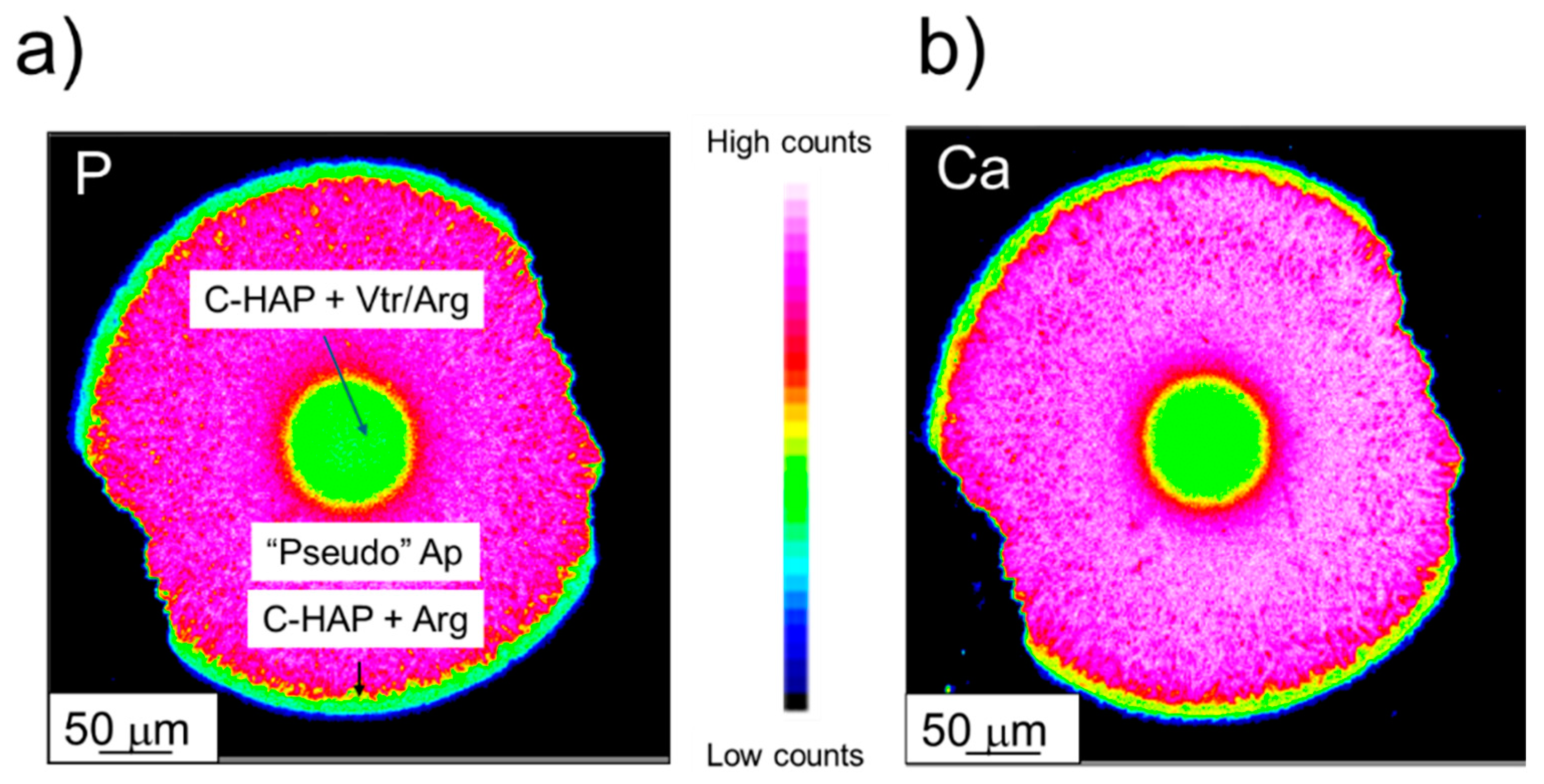
3. Results
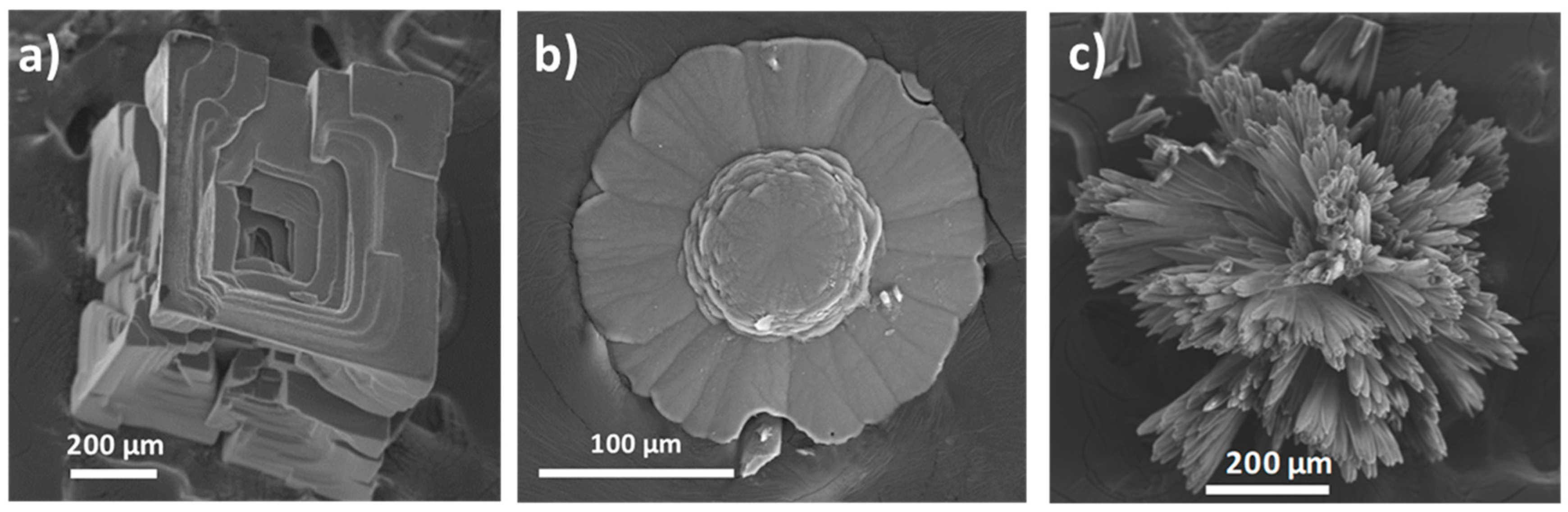
3.1. Solid Morphologies
3.1.1. Inert Silica Gel Growth Experiments
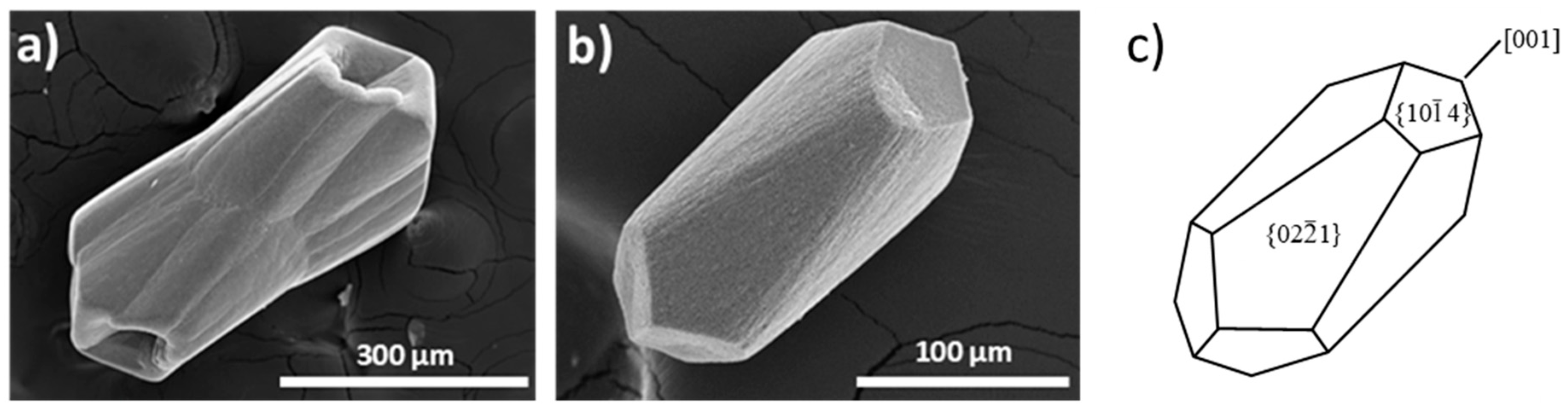
3.1.2. 50 ppm P-Bearing Silica Gel Growth Experiments
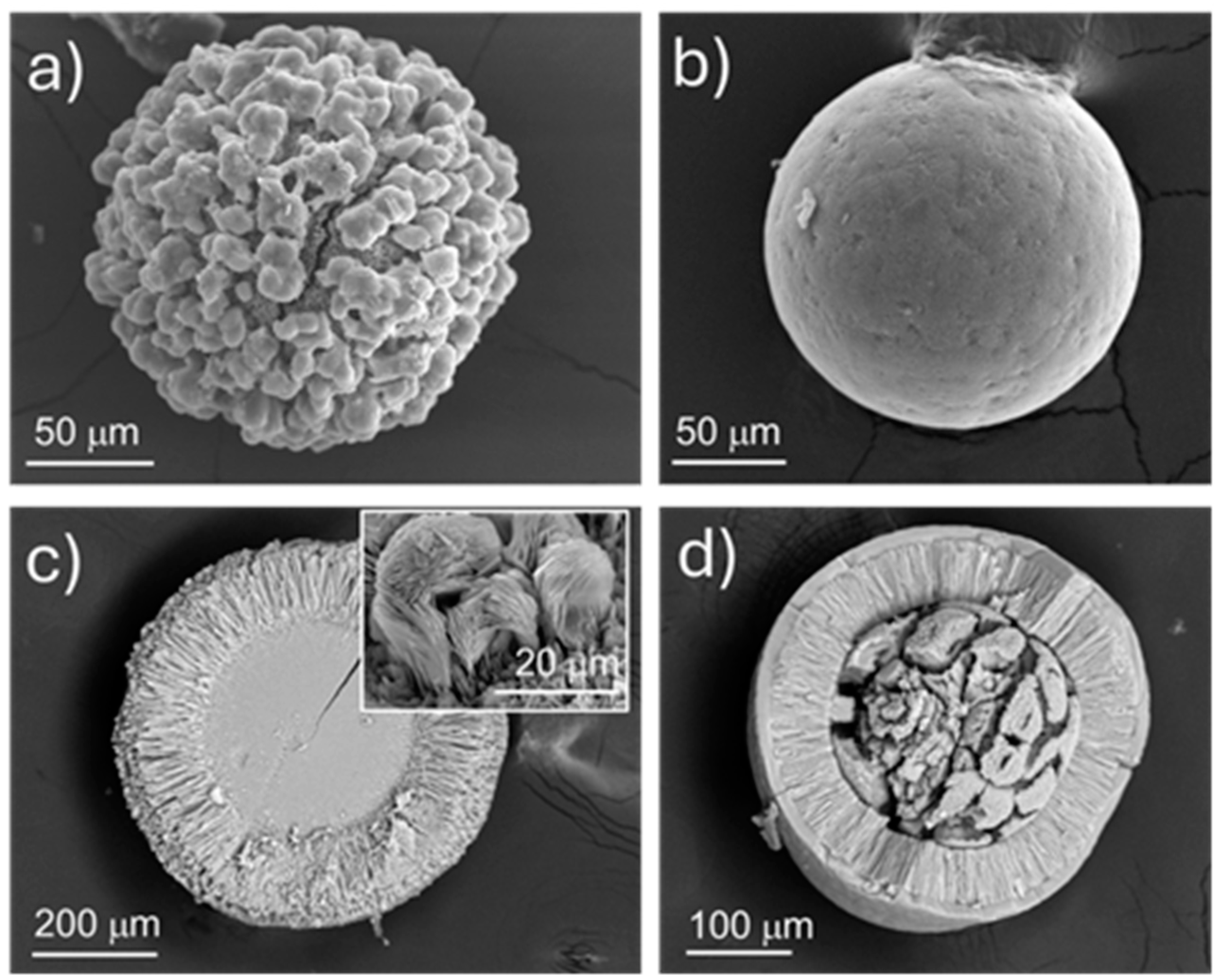
3.1.3. 500 ppm P-Bearing Silica Gel Growth Experiments
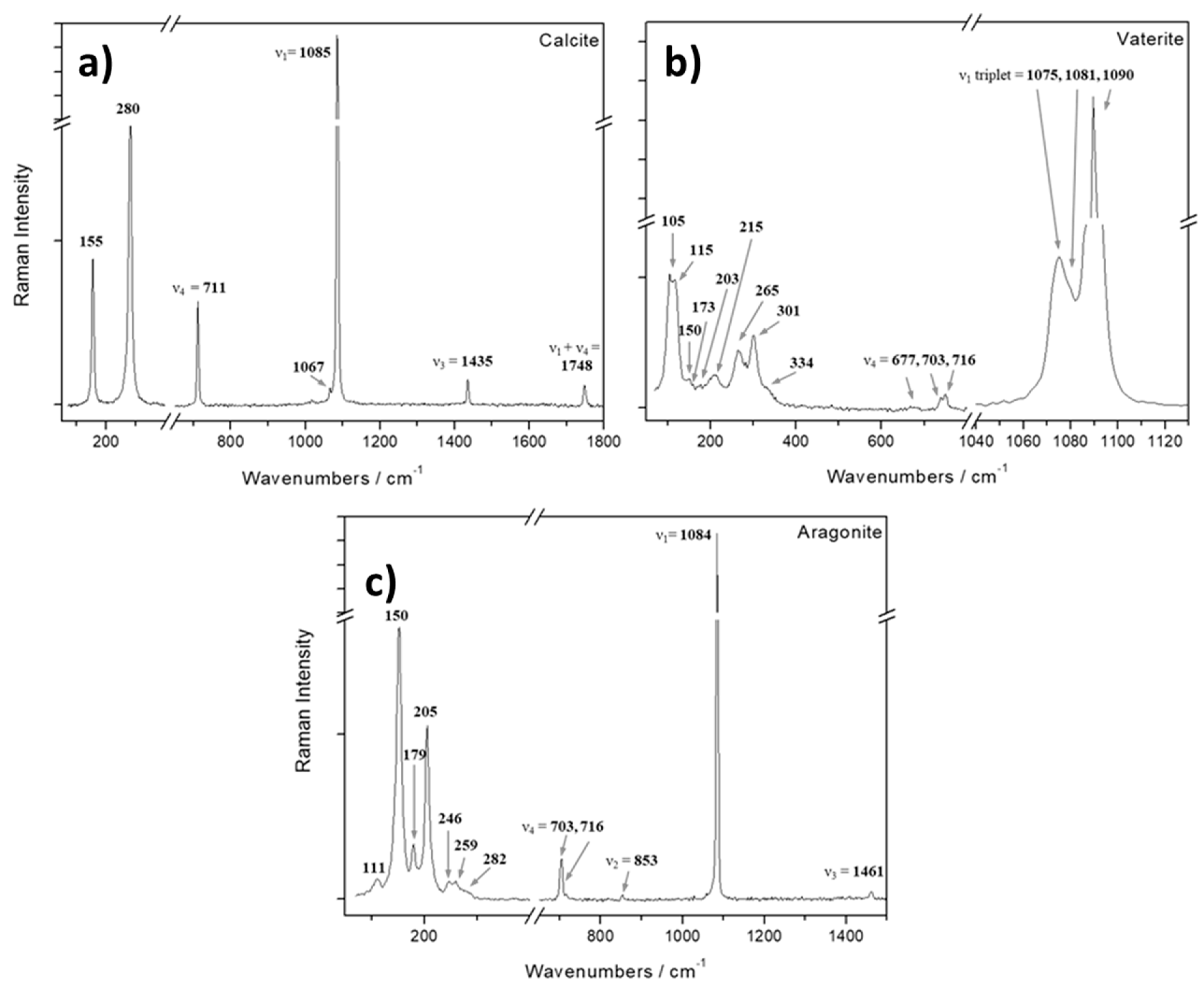
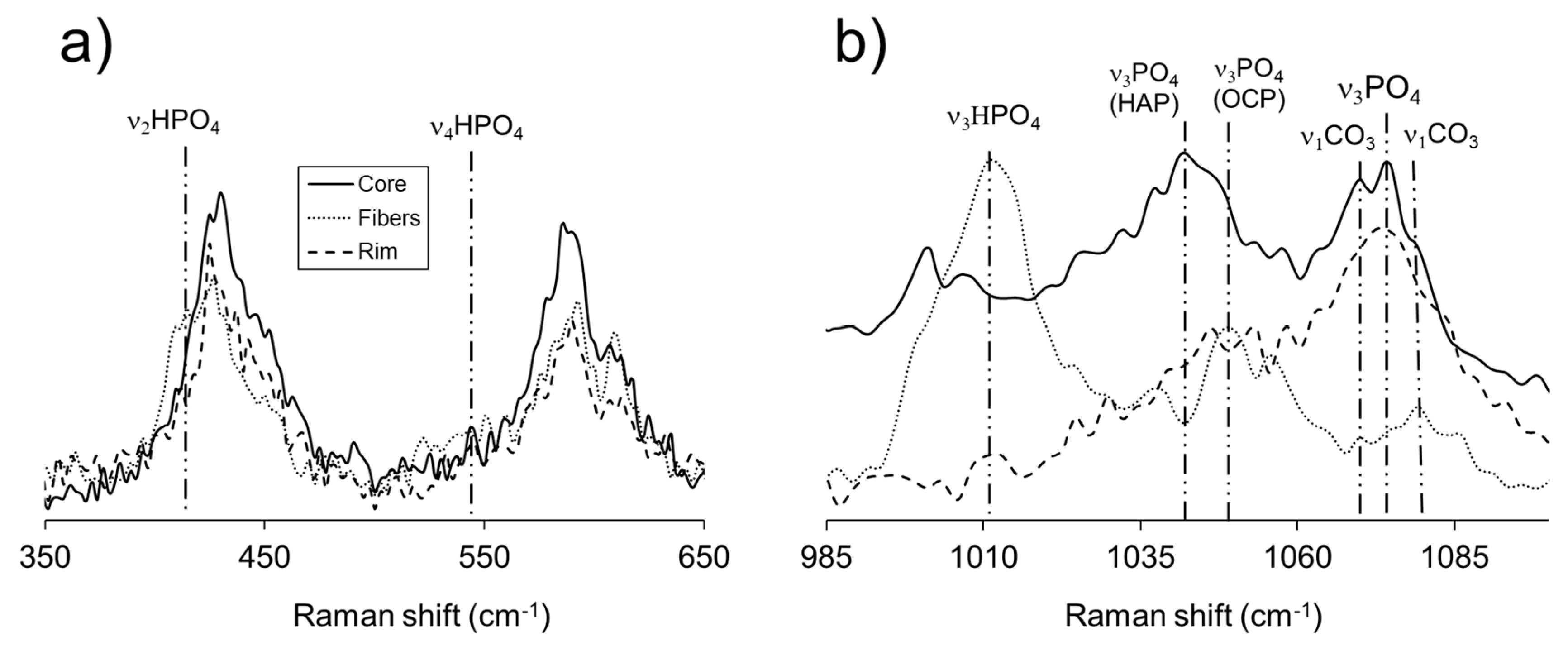
3.2. Raman Spectroscopy
3.2.1. Inert Silica Gel Growth Experiments
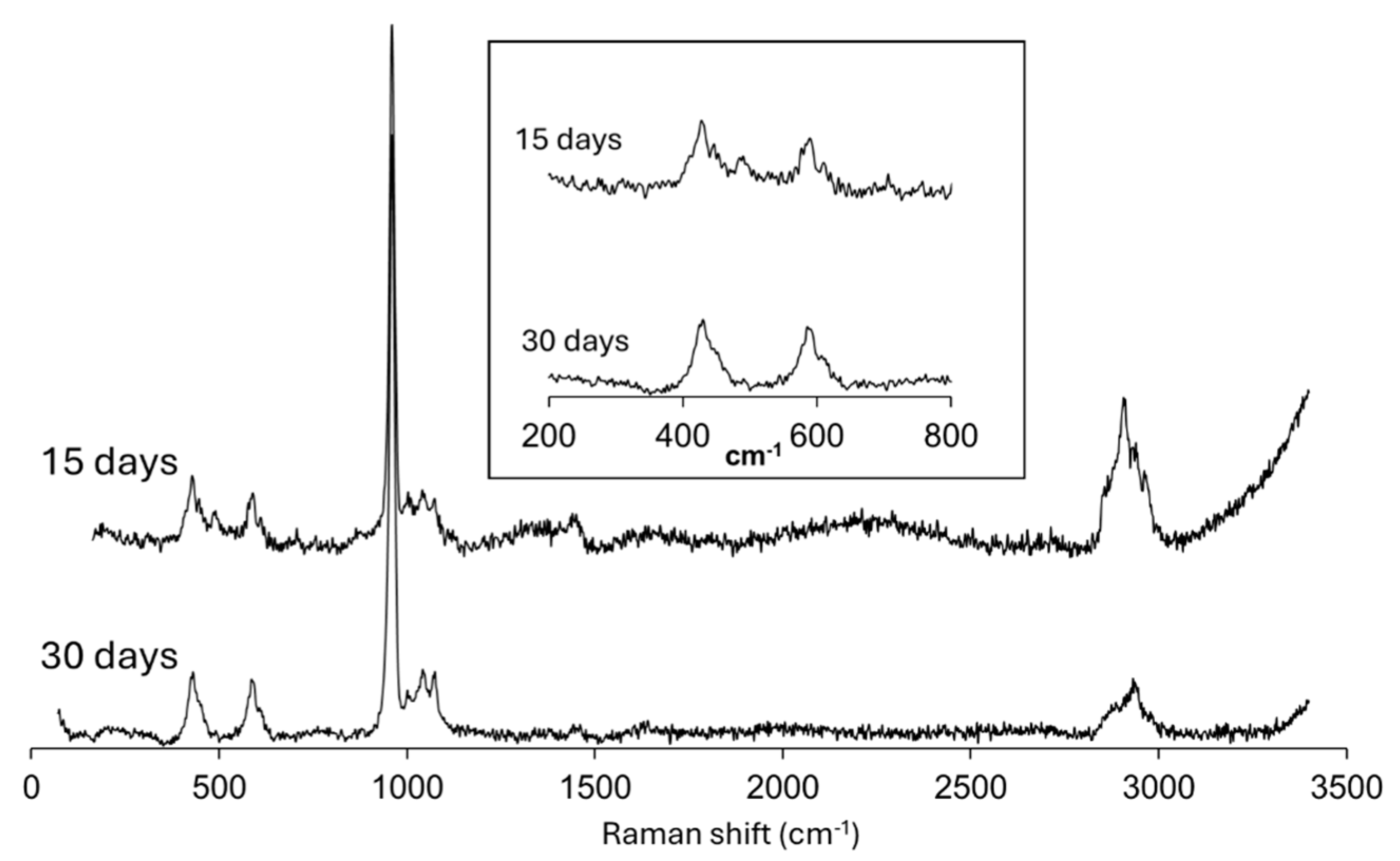
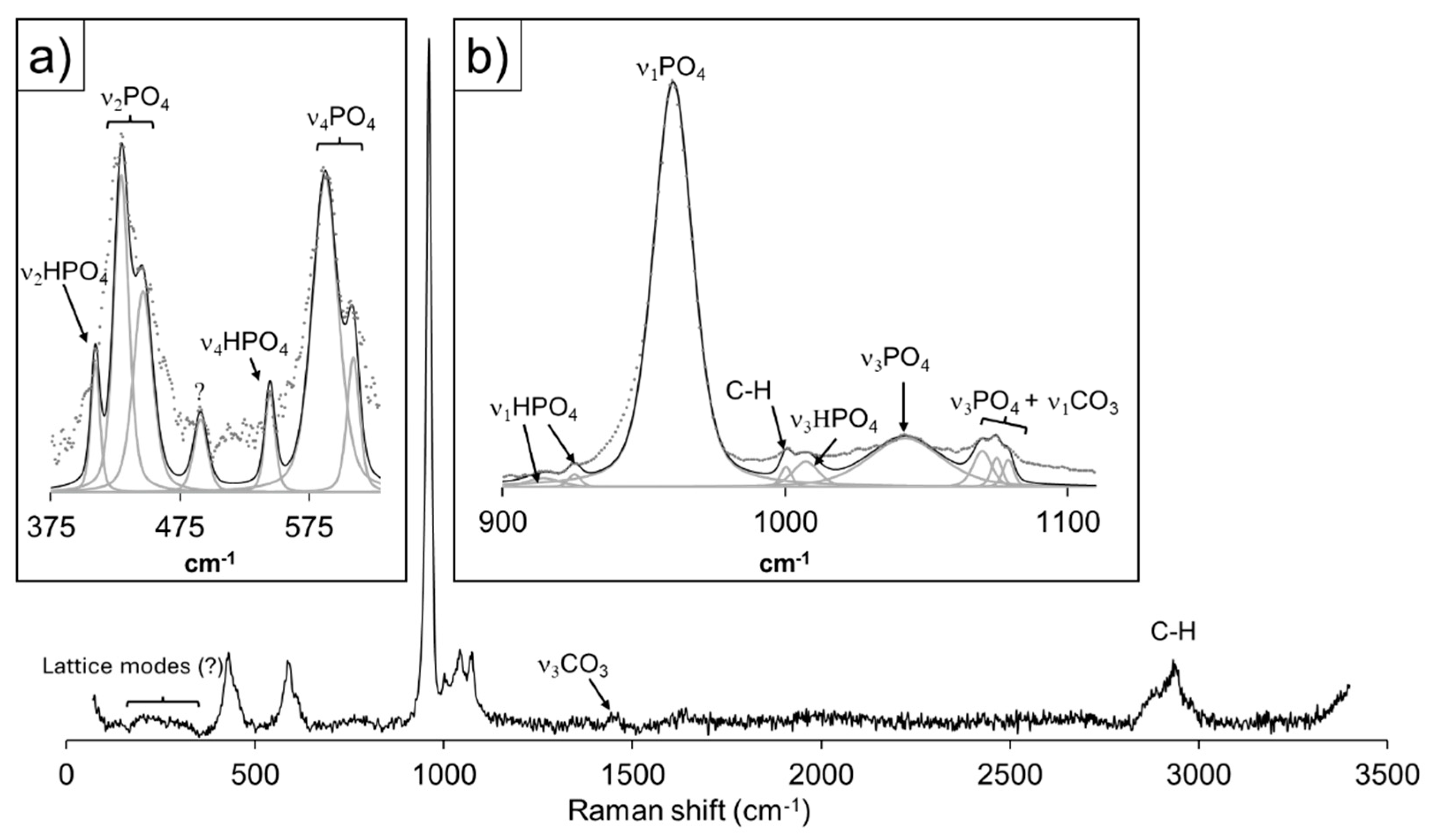
3.2.1. 50 ppm P-Bearing Silica Gel Growth Experiments
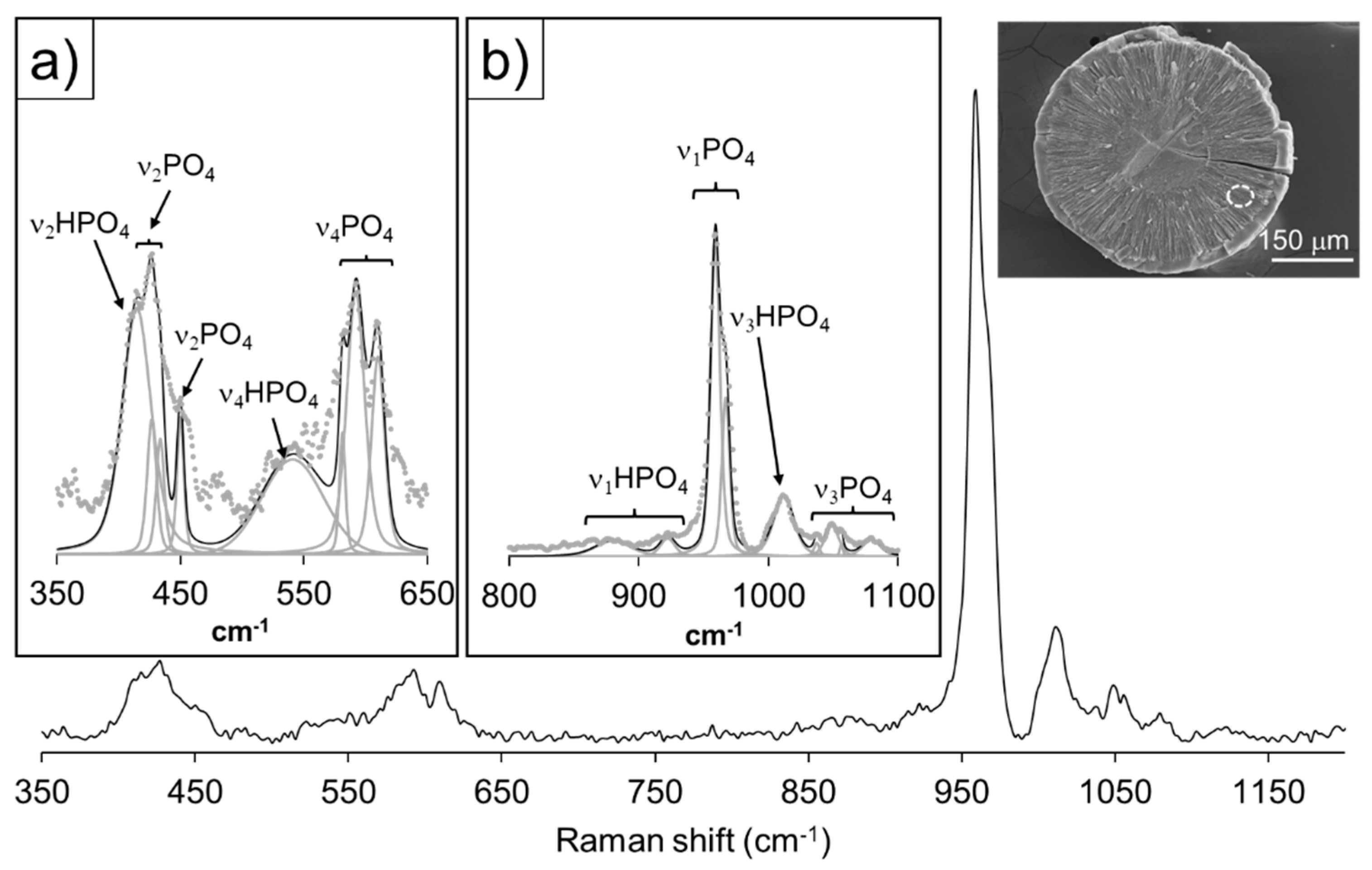
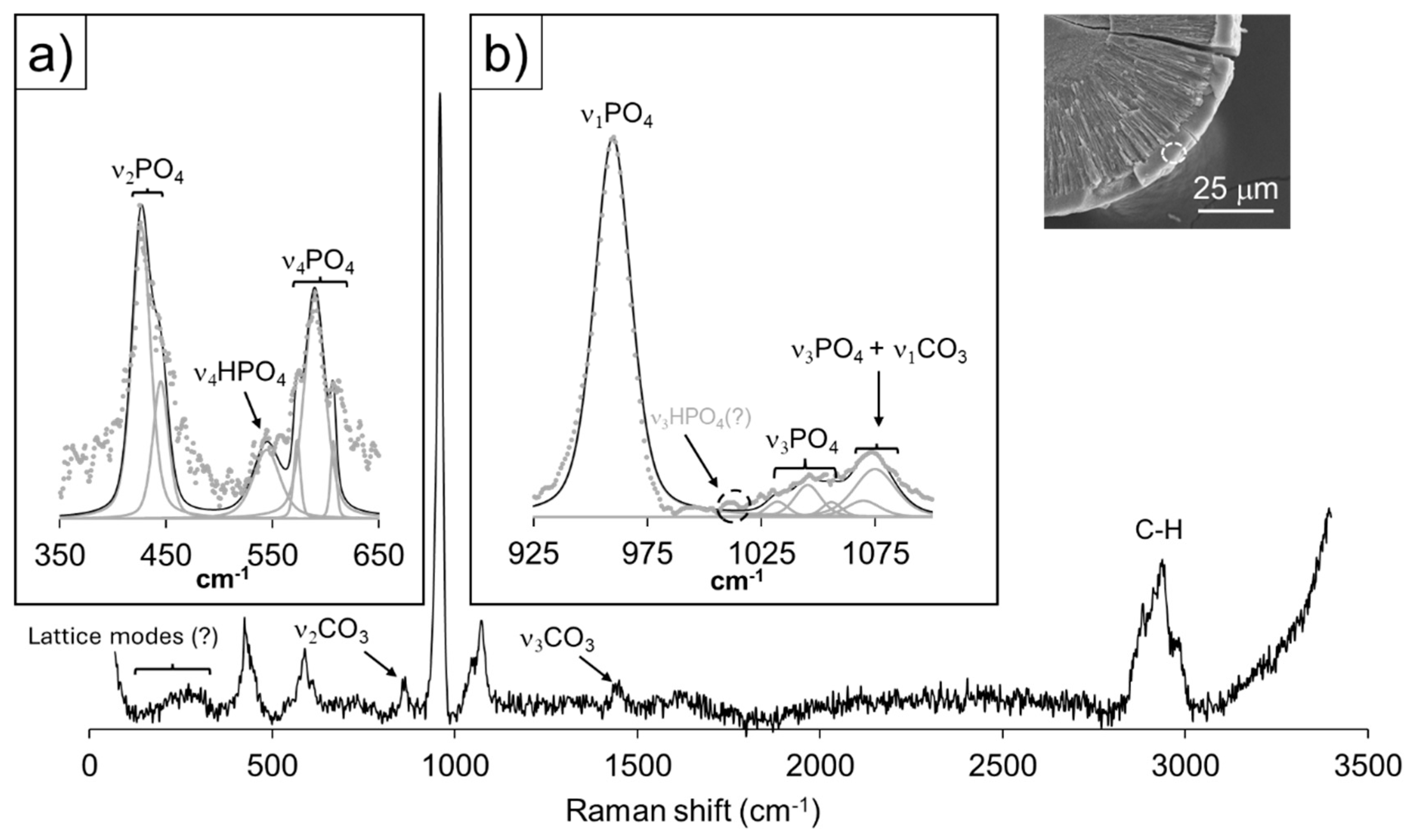
3.2.2. 500 ppm P-Bearing Silica Gel Growth Experiments
4. Discussion
4.1. The Effect of Phosphate Ions on the Growth of Calcium Carbonate
4.2. Growth of Ca-Phosphates in the Presence of Carbonate Ions
4.2.1. Phase Identification
4.2.1. Reaction Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ridgwell, A.; Zeebe, R.E. The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. SC, Lett. 2005, 234(3-4), 299–315. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates. Developments in Sedimentology; Elsevier: Amsterdam. The Netherlands, 1990; p. 446. [Google Scholar]
- Hua, B.; Deng, B.L.; Thorton, E.C.; Yang, J.; Amonette, J.E. Incorporation of chromate into calcium carbonate structure during coprecipitation. Water, Air, Soil Pollut. 2007, 179, 381–390. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Elzinga, E.J; Lee, Y.J.; Reeder, R.J. Coprecipitation of chromate with calcite: Batch experiments and X-ray absorption spectroscopy. Geochim. Cosmochim. Acta. 2007, 71, 1480–1493. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Fernández-González, A.; Prieto, M. The role of sulfate groups in controlling CaCO3 polymorphism. Geochim. Cosmochim. Acta. 2010, 74, 6064–6076. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Pina, C.M.; Astilleros, J.M.; Sánchez-Pastor, N. The carbonatation of Gypsum: Pathways and pseudomorph formation. Am. Mineral. 2009, 94, 1223–1234. [Google Scholar] [CrossRef]
- Parker, S.C.; Titiloye, J.O.; Watson G., W. Molecular modelling of carbonate minerals: studies of growth and morphology. Phil. Trans. R. Soc. Lond. A. 1993, 344, 37–44. [Google Scholar] [CrossRef]
- Sangwal, K. Additives and Crystallization Processes: From Fundamentals to Applications. Wiley-Blackwell: Chichester, West Sussex, England, 2007, pp. 468.
- Chernov, A.A. Growth of copolymer chains and mixed crystals trial-and-error statistics. Soviet Physics Uspekhi. 1970, 13, 101–128. [Google Scholar] [CrossRef]
- Schosseler, P.M.; Wehrli, B.; Schweiger, A. Uptake of Cu2+ by the calcium carbonates vaterite and calcite as studied by continuous wave (cw) and pulse electron paramagnetic resonance. Geochim. Cosmochim. Acta. 1999, 63, 1955–1967. [Google Scholar] [CrossRef]
- Prieto, M.; Astilleros, J.; Fernández-Díaz, L. Environmental remediation by crystallization of solid solutions. Elements, 2013, 9, 195–201. [Google Scholar] [CrossRef]
- Astilleros, J.M.; Fernández-Díaz, L.; Putnis, A. The role of magnesium in the growth of calcite: An AFM study. Chem. Geol. 2010, 271, 52–58. [Google Scholar] [CrossRef]
- Crocket, H.; Winchester, J.W. Coprecipitation of zinc with calcium carbonate. Geochim. Cosmochim. Acta. 1966, 30, 1093–1109. [Google Scholar] [CrossRef]
- Reeder, R.J.; Lamble, G.M.; Northrup, P.A. XAFS study of the coordination and local relaxation around Co2+, Zn2+, Pb2+, and Ba2+ trace elements in calcite. Am. Mineral. 1999, 84, 1049–1060. [Google Scholar] [CrossRef]
- Sánchez-Pastor, N.; Gigler, A.; Cruz, J.; Park, S.; Jordan, G.; Fernández-Díaz, L. Growth of calcium carbonate in the presence of Cr (VI). Cryst. Growth Des. 2011, 11, 3081–3089. [Google Scholar] [CrossRef]
- Fernández-González, Á.; Fernández-Díaz, L. Growth of calcium carbonate in the presence of Se (VI) in silica hydrogel. Am. Mineral. 2013, 98, 1824–1833. [Google Scholar] [CrossRef]
- Kohn, M.J.; Cerling, T.E. Stable isotope composition of Biological Apatite. Rev. Min. Geochem. 2002, 48, 455–488. [Google Scholar] [CrossRef]
- Smith, S.C.; Douglas, M.; Moore, D.A.; Kukkadapu, R.V.; Arey, B.W. Uranium extraction from laboratory-synthesized, uranium-doped hydrous ferric oxides. Environ. Sci. Technol. 2009, 43, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M. The global phosphorous cycle: past, present, future. Elements. 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Rouff, A.A.; Reeder, R.J. The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: an x-ray absorption spectroscopy study. Geochim. Cosmochim. Acta. 2006, 70, 2715–2725. [Google Scholar] [CrossRef]
- Kuczumow, A.; Blicharski, T.; Gorzelak, M.; Kosinski, J.; Lasota, A.; Gagala, J.; Nowak, J.; Jarzebski, M.; Jablonski, M. Measurements of energetic states resulting from ion exchanges in the isomorphic crystals of apatites and bioapatites. Molecules 2022, 27, 8913. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and chemistry of the apatites and other calcium orthophosphates., 1st ed.; Elsevier Science BV: Amsterdam, The Netherlands, 1994; p. 404. [Google Scholar]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Cyrstallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nat. 1962, 196, 1050–1054. [Google Scholar] [CrossRef]
- Rey, C.; Hina, A.; Tofighi, A.; Glimcher, M.J. Maturation of poorly crystalline apatites: Chemical and structural aspects in vivo and in vitro. Cells Mater. 1995, 5, 345–356. [Google Scholar] [CrossRef]
- Elliott, J.C. Calcium Phosphate Biominerals. Rev. Min. Geochem. 2002, 48, 427–453. [Google Scholar] [CrossRef]
- Tung, M.S.; Brown, W.E. An intermediate state in hydrolysis of amorphous calcium phosphate. Calcif Tissue Int. 1983, 35, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Montel, G.; Bonel, G.; Heughebaert, J.C.; Trombe, J.C.; Rey, C. New concepts in the composition, crystallization and growth of the mineral component of calcified tissues. J. Cryst. Growth. 1981, 53, 74–99. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, A.R.; Posner, A.S. Crystal structure of hydroxyapatite. Nat. 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium phosphate as a Precursor in Biomineral Formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef]
- Fernández, M.E.; Zorrilla-Cangas, C.; García-García, R.; Asensio, J.A.; Reyes-Gasga, J. New model for the hydroxyapatite-octacalcium phosphate interface. Acta Crystall. Sec. B: Struct. Sci. 2003, B59, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Grüner, D.; Worch, H.; Pompe, W.; Liche, H.; El Khassawna, T.; Heiss, C.; Wenisch, S.; Kniep, R. First evidence of octacalcium phosphate at osteocalcin nanocomplex as skeletal bone component directing collagen triple-helix nanofibril mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef]
- Miake, Y.; Shimoda, S.; Fukae, M.; Aoba, T. Epitaxial overgrowth of apatite crystals on the thin-ribbon precursor at early stages of porcine enamel mineralization. Calcif. Tissue Int. 1993, 53, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rooji, J.F.; Nancollas, G.H. The formation and remineralization of artificial white spots lesions: a constant composition approach. J. De. Res. 1984, 63, 864–867. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; Wood, G.J.; Barry, J.C.; Featherstone, J.D.B. The structure of (100) defects in carbonates apatite crystallites: a high-resolution electron microscope study. Ultramicroscopy 1986, 19, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Moriwaki, Y. Lengthwise and oriented growth of octacalcium phosphate crystal in polyacryalamide gel in a model system of tooth enamel apatite formation. J. Cryst. Growth 1998, 194, 125–132. [Google Scholar] [CrossRef]
- Falini, G.; Gazzano, M.; Ripamonti, A. Control of the architectural assembly of octacalcium phosphate crystals in denatured collagenous matrices. J. Mat. Chem. 2000, 10, 535–538. [Google Scholar] [CrossRef]
- Grassmann, O.; Löbmann, P. Morphogenetic control of calcite crystal growth in sulfonic acid based hidrogels. Chem. Eur. J. 2003, 9, 1310–1316. [Google Scholar] [CrossRef]
- Grassmann, O.; Löbmann, P. Biomimetic nucleation and growth of CaCO3 in hydrogels incorporating carboxylate groups. Biomater. 2004, 25, 277–282. [Google Scholar] [CrossRef]
- Helbig, U. Growth of calcium carbonate in polyacrylamide hydrogel: investigation of the influence of polymer content. J. Cryst. Growth. 2008, 310, 2863–2870. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Putnis, A.; Prieto, M.; Putnis, C.V. The role of magnesium in the crystallization of calcite and aragonite in a porous medium. J. Sedimentary Res. 1996, 66, 482–491. [Google Scholar] [CrossRef]
- Prieto, M.; Putnis, A.; Fernández-Díaz, L. Crystallization of solid solutions from aqueous solutions in a porous medium: zoning in (Ba, Sr), SO4. Geol. Mag. 1993, 130, 289–299. [Google Scholar] [CrossRef]
- Prieto, M.; Putnis, A.; Fernández-Díaz, L.; López-Andrés, S. Metastability in diffusing-reacting systems. J. Cryst. Growth, 1994, 142, 225–235. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: a general-purpose peak fitting program. J.Appl. Cryst. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- KrystalShaper V1.5.0 © JCrystalSoft, 2018. Available online: http://www.jcrystal.com/products/krystalshaper/ (Accessed 29 Sept 2019).
- Fernández-Díaz, L.; Astilleros, J.M.; Pina, C.M. The morphology of calcite crystals grown in a porous medium doped with divalent cations. Chem. Geol. 2006, 225, 314–321. [Google Scholar] [CrossRef]
- Krishnamurti, D. The Raman spectrum of calcite and its interpretation. Proc. Indian. Acad. Sci. Section. A. 1957, 46, 183–202. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman study on certain carbonates. Analele Stiintifice ale Universitatii “Al. I. Cuza” - Iasi, 2009, 55, 97–112. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spect. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Gauldie, R.W.; Sharma, S.K.; Volk, E. Micro-raman spectral study of vaterite and aragonite otoliths of the coho salmon, Oncorhynchus kisutch. Comp. Biochem. Physiol. Part A: Physiol. 1997, 118, 753–757. [Google Scholar] [CrossRef]
- Couture, J.P.M. Anisotropy of the Raman effect in cubic crystals; theoretical study. C. R. Hebd. Seances. Acad. Sci. 1947, 224, 902–904. [Google Scholar]
- Urmos, J.; Sharma, S.K.; Mackenzie, F.T. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaître, J.; Leroy, G. Raman microspectrometry studies of brushite cement: in vivo evolution in a sheep model. Bone 1999, 25 (Suppl.), 81S–84S. [Google Scholar] [CrossRef]
- de Aza, P.N.; Guitian, F.; Santos, C.; de Aza, S.; Cusco, R.; Artus, L. Vibrational investigation of calcium phosphate compounds. 2. Comparison between hydroxyapatite and -tricalcium phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Sauer, G.R.; Zunic, W.B.; During, J.R.; Wuthier, R.E. Fourier transform Raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Arends, J. Raman spectra of human dental calculus. J. Dental Res. 1993, 72, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.C.; Bartlett, M.L.; Young, R.A. Compositional analysis of apatites with laser-Raman spectroscopy: (OH, F, Cl) apatites. Arch. Oral Biol. 1974, 19, 995–1006. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman studies of rock-forming minerals. Part II. Minerals containing MO3, MO4, and MO6 groups. J. Chem. Soc. (A) 1970, 286–291. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate assignment and calibration in the Raman spectrum of apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Titiloye J., O.; Parker S., C.; Mann S., J. Atomistic simulation of calcite surfaces and the influence of growth additives on their morphology. J. Cryst. Growth. 1993, 131, 533–545. [Google Scholar] [CrossRef]
- Davis, K.J.; Dove, P.M.; Wasylenki, L.E.; De Yoreo, J.J. Morphological consequences of differential Mg2+ incorporation at structurally distinct steps on calcite. Am. Mineral. 2004, 89, 714–720. [Google Scholar] [CrossRef]
- Mayorga, I.C; Astilleros, J.M.; Fernández-Díaz, L. Precipitation of CaCO3 Polymorphs from Aqueous Solutions: The Role of pH and Sulphate Groups. Minerals, 2019, 9, 178. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Veis, A.; Dorvee, J.R. Biomineralization Mechanisms: A New Paradigm for Crystal Nucleation in Organic Matrices. Calcif. Tissue Int. 2013, 93, 307–315. [Google Scholar] [CrossRef]
- Bachra, B.N; Trautz, O.R; Simon, S.L. Precipitation of calcium carbonates and phosphates. I. Spontaneous precipitation of calcium carbonates and phosphates under physiological conditions. Arch. Biochem. Biophys. 1963, 103, 124–138. [Google Scholar] [CrossRef]
- Rey, C.C.; Combes, C.; Drouet, C. Synthesis and physical chemical characterizations of octacalcium phosphate-based biomaterials for hard-tissue regeneration. In: Octacalcium Phosphate Biomaterials: Understanding of Bioactive Properties and Application. 1st ed.; Suzuki, O., Insley, G., Eds.; Woodhead Publishing Series in Biomaterials. Elsevier, 2019, pp. 177–212. [CrossRef]
- Havlin, J.L.; Schlegel, A.J. Review of phosphite as a plant nutrient and fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Wolf-Gladrow, D.A. CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier, Amsterdam. 2001, 346 pp.
- Legros, R.; Balmain, N.; Bonel, G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue. Int., 1987, 41, 137–144. [Google Scholar] [CrossRef]
- Kuczumow, A.; Gorzelak, M.; Kosiński, J.; Lasota, A.; Blicharski, T.; Gągała, J.; Nowak, J.; Jarzębski, M.; Jabłoński, M. Hierarchy of Bioapatites. Int. J. Mol. Sci. 2022, 23, 9537. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem Rev. 2008, 108, 4694–715. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced fourier transform infrared spectroscopy study. Calcif Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef]
- Pinto A., J.; Jiménez, A.; Prieto, M. Interaction of phosphate-bearing solutions with gypsum: Epitaxy and induced twinning of brushite (CaHPO4·2H2O) on the gypsum cleavage surface. Am. Mineral. 2009, 94, 313–322. [Google Scholar] [CrossRef]

| Mixture | Reagents | GEL | Tw1 | Morphologies | |
|---|---|---|---|---|---|
| [CaCl2] | [Na2CO3] | [Na2PO4] | |||
| I | 0.5 M | 0.5 M | - | 216 h | Rhombohedral Flower-like Radial |
| II | 0.5 M | 0.5 M | 50 ppm | 216 h | Elongated |
| III | 0.5 M | 0.5 M | 500 ppm | 168 h 360 h |
Spheres Elongated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





