Submitted:
20 June 2024
Posted:
20 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
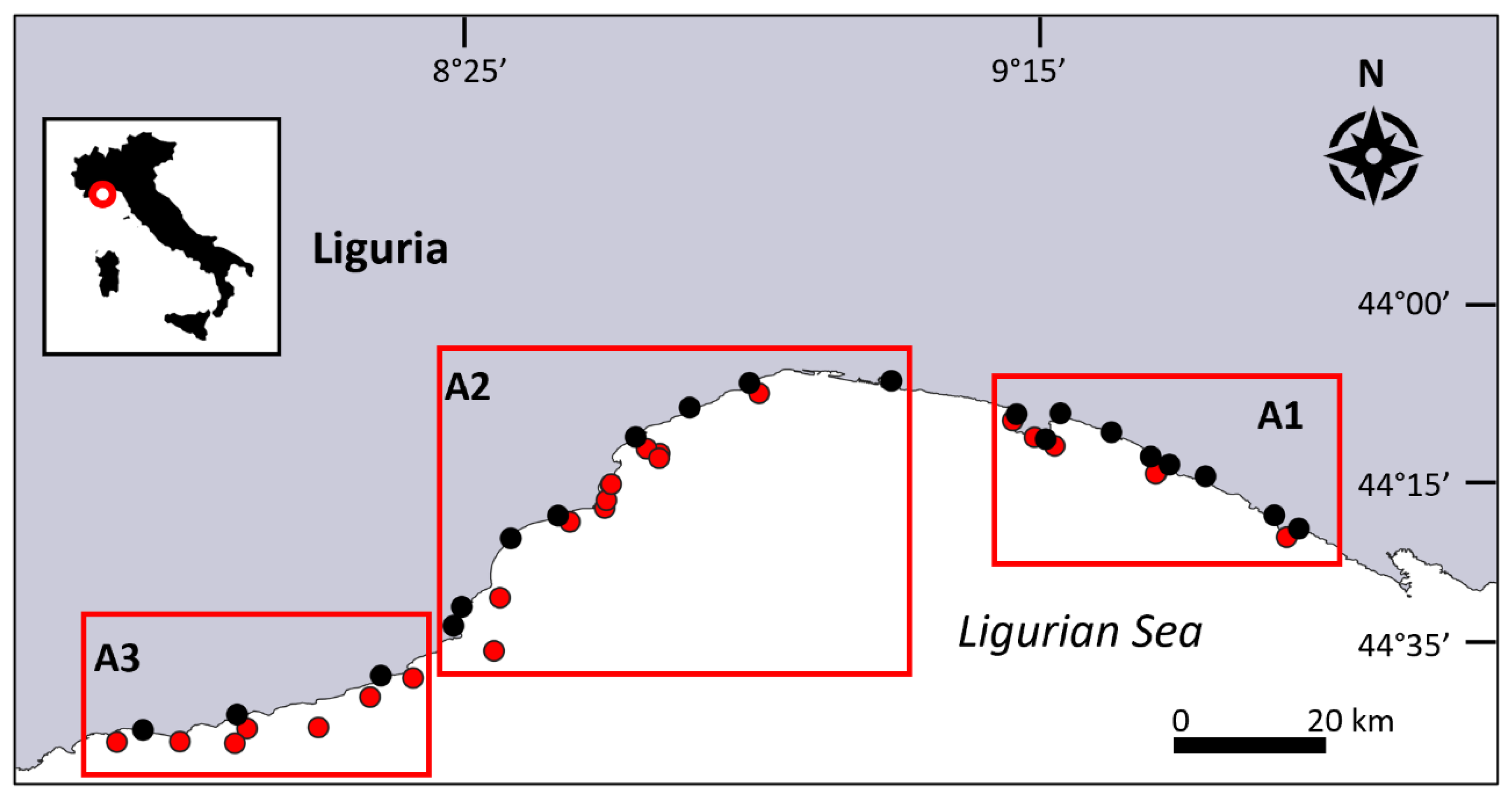
| Year | Site | Lat. (N) |
Long. (E) |
Transect ID |
Depth (m) |
N colony |
Density (col m-2) |
Epibiosis % |
Entanglement % |
Av. H ± SE (cm) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macroarea A1 | 2016 | Mesco Cape | 44.13108 | 9.63407 | PMMN_S2_T2 | 47 | 64 | 1.3 | 9.4 | 7.8 | 28.8 ± 2.3 | |
| 2019 | 66 | 0.5 | 15.9 | 4.5 | 20.3 ± 1.3 | |||||||
| 2022 | 66 | 0.8 | 9.1 | 3.0 | 23.6 ± 0.9 | |||||||
| 2015 | Manara Cape W | 44.243216 | 9.40207 | SLMO_S3_T1 | 59 | 82 | 0.9 | 20.7 | 3.7 | 38.4 ± 2.2 | ||
| 2019 | 107 | 4.1 | 40.0 | 77.6 | 35.5 ± 1.7 | |||||||
| 2019 | SLMO_S3_T3 | 41 | 198 | 2.0 | 73.0 | 21.2 | 30.2 ± 0.9 | |||||
| 2022 | 284 | 3.6 | 56.0 | 1.1 | 28.0 ± 1.1 | |||||||
| 2015 | SLMO_S3_T4 | 47 | 228 | 2.5 | 10.1 | 1.3 | 47.3 ± 2.2 | |||||
| 2019 | 95 | 1.4 | 89.4 | 46.3 | 35.0 ± 1.1 | |||||||
| 2022 | 94 | 1.0 | 39.4 | 3.2 | 29.0 ± 1.7 | |||||||
| 2015 | Manara Cape E | 44.24516 | 9.40409 | SLMO_S2_T1 | 55 | 119 | 1.6 | 16.0 | 0.8 | 34.3 ± 2.3 | ||
| 2019 | 212 | 2.6 | 76.0 | 22.2 | 35.3 ± 1.0 | |||||||
| 2015 | SLMO_S2_T2 | 64 | 97 | 1.0 | 9.3 | 4.1 | 32.2 ± 2.2 | |||||
| 2019 | 94 | 1.8 | 37.5 | 9.6 | 25.5 ± 1.1 | |||||||
| 2016 | Portofino Cape | 44.2923 | 9.22313 | AMPP_S1_T3 | 60 | 174 | 6.7 | 6.3 | 1.1 | 31.7 ± 1.2 | ||
| 2019 | 115 | 1.2 | 58.8 | 9.6 | 32.7 ± 1.6 | |||||||
| 2022 | 50 | 0.6 | 10.0 | - | 28.2 ± 1.3 | |||||||
| 2016 | Isuela Shoal | 44.33713 | 9.14897 | AMPP_S3_T2 | 33 | 838 | 10.6 | 24.6 | 2.7 | 24.2 ± 1.5 | ||
| 2019 | 833 | 8.3 | 36.0 | 1.6 | 29.2 ± 1.0 | |||||||
| 2022 | 649 | 6.6 | 8.8 | - | 30.8 ± 0.7 | |||||||
| 2016 | AMPP_S3_T3 | 52 | 332 | 3.8 | 15.1 | 6.9 | 24.9 ± 1.5 | |||||
| 2019 | 247 | 4.4 | 33.0 | 11.7 | 28.7 ± 1.5 | |||||||
| 2022 | 104 | 1.2 | 53.1 | 1.0 | 28.5 ± 0.9 | |||||||
| Macro-area A2 | 2015 | Arenzano-Varazze | 44.38512 | 8.6996 | NOAR_S1_T2 | 40 | 337 | 4.3 | 11.6 | 2.1 | 34.3 ± 1.3 | |
| 2018 | 411 | 5.1 | 60.3 | 42.8 | 33.2 ± 1.2 | |||||||
| 2021 | 315 | 3.6 | 79.0 | 31.0 | 21.0 ± 0.9 | |||||||
| 2015 | Vado Ligure | 44.2603 | 8.46638 | NOAR_S2_T1 | 48 | 277 | 3.4 | 12.3 | 9.0 | 34.6 ± 2.0 | ||
| 2018 | 207 | 4.0 | 28.5 | 50.7 | 31.1 ± 1.4 | |||||||
| 2015 | NOAR_S2_T2 | 63 | 184 | 1.5 | 12.5 | 14.2 | 30.0 ± 2.0 | |||||
| 2018 | 144 | 3.0 | 78.5 | 22.2 | 34.5 ± 1.2 | |||||||
| 2021 | 152 | 3.2 | 44.0 | 8.0 | 22.3 ± 1.0 | |||||||
| 2015 | NOAR_S2_T3 | 56 | 72 | 0.9 | 5.6 | 4.2 | 13.4 ± 1.5 | |||||
| 2018 | 81 | 1.5 | 66.7 | 76.5 | 25.3 ± 1.2 | |||||||
| 2016 | Savona A | 44.28739 | 8.50042 | SVCL_S3_T2 | 45 | 291 | 3.5 | 3.8 | 22.0 | 29.8 ± 1.6 | ||
| 2019 | 158 | 3.9 | 51.0 | 21.5 | 28.7 ± 0.9 | |||||||
| 2022 | 314 | 4.5 | 7.6 | 9.2 | 25.0 ± 0.6 | |||||||
| 2016 | Savona B | 44.27878 | 8.52335 | SVCL_S2_T3 | 58 | 309 | 3.3 | 6.1 | 37.5 | 25.6 ± 1.7 | ||
| 2019 | 125 | 2.6 | 76.7 | 72.0 | 35.3 ± 1.7 | |||||||
| 2022 | 482 | 12.4 | 38.0 | 19.9 | 25.1 ± 0.8 | |||||||
| 2015 | Maledetti Shoal | 44.22381 | 8.43657 | NOAR_S4_T1 | 58 | 610 | 5.3 | 1.7 | 47.3 | 21.7 ± 1.0 | ||
| 2018 | 276 | 2.8 | 42.4 | 84.4 | 25.2 ± 1.0 | |||||||
| 2015 | NOAR_S4_T2 | 68 | 69 | 4.2 | 8.8 | 29.8 | 17.8 ± 1.5 | |||||
| 2018 | 179 | 2.3 | 7.8 | 97.2 | 27.8 ± 1.2 | |||||||
| 2021 | 89 | 2.1 | 28.2 | 12.8 | 12.5 ± 1.2 | |||||||
| 2018 | NOAR_S4_T4 | 56 | 271 | 3.2 | 42.1 | 87.8 | 29.8 ± 1.4 | |||||
| 2021 | 138 | 1.6 | 36.0 | 14.0 | 19.1 ± 0.7 | |||||||
| 2018 | NOAR_S4_T5 | 62 | 382 | 3.8 | 20.7 | 79.8 | 26.3 ± 1.3 | |||||
| 2021 | 352 | 4.2 | 30.0 | 58.0 | 21.5 ± 0.7 | |||||||
| 2018 | NOAR_S4_T7 | 53 | 68 | 0.7 | 10.3 | 36.8 | 15.8 ± 0.8 | |||||
| 2021 | 84 | 0.8 | 12.9 | 21.4 | 11.6 ± 0.5 | |||||||
| 2018 | NOAR_S4_T8 | 59 | 640 | 6.6 | 10.8 | 60.0 | 15.8 ± 0.4 | |||||
| 2021 | 421 | 4.2 | 53.0 | 25.0 | 12.5 ± 0.3 | |||||||
| 2017 | Finale Ligure | 44.15817 | 8.36462 | BONO_S1_T2 | 83 | 163 | 2.0 | 4.9 | 12.3 | 24.2 ± 1.2 | ||
| 2020 | 112 | 5.9 | 34.3 | 18.8 | 33.2 ± 3.4 | |||||||
| 2017 | BONO_S1_T3 | 77 | 405 | 5.1 | 3.6 | 35.3 | 32.9 ± 1.2 | |||||
| 2020 | 453 | 5.3 | 12.0 | 5.7 | 65.7 ± 2.6 | |||||||
| Macro-area A3 | 2017 | Albenga | 44.02396 | 8.24063 | ALGA_S3_T2 | 58 | 54 | 0.7 | 11.8 | 38.2 | 30.1 ± 1.5 | |
| 2020 | 72 | 1.2 | 70.8 | 1.4 | 35.4 ± 2.1 | |||||||
| 2015 | Diano Marina | 43.88217 | 8.08675 | SSDM_S1_T2 | 51 | 175 | 2.4 | 6.3 | 18.9 | 33.2 ± 1.5 | ||
| 2018 | 119 | 2.0 | 55.5 | 68.9 | 39.4 ± 1.2 | |||||||
| 2021 | 115 | 1.3 | 28.4 | 10.8 | 20.6 ± 1.0 | |||||||
| 2018 | Porto Maurizio | 43.84858 | 8.01065 | SSDM_S2_T3 | 36 | 76 | 1.2 | 1.3 | - | 37.8 ± 1.2 | ||
| 2021 | 156 | 3.1 | 21.0 | 11.0 | 21.5 ± 0.9 | |||||||
| 2017 | Sanremo E | 43.7929 | 7.79271 | SRSST_S1_T2 | 69 | 265 | 3.3 | 18.1 | 46.2 | 27.7 ± 0.9 | ||
| 2020 | 335 | 6.6 | 4.0 | 25.1 | 37.4 ± 1.3 | |||||||
| 2017 | SRSST_S1_T3 | 61 | 232 | 2.9 | 18.7 | 46.7 | 24.9 ± 1.2 | |||||
| 2020 | 135 | 3.5 | 7.0 | 26.7 | 31.7 ± 1.6 | |||||||
| 2017 | Sanremo W | 43.76695 | 7.77113 | BOSR_S3_T1 | 65 | 180 | 2.3 | 9.7 | 55.9 | 25.9 ± 1.1 | ||
| 2020 | 181 | 4.4 | 10.4 | 1.7 | 38.3 ± 2.1 | |||||||
| 2017 | BOSR_S3_T2 | 49 | 54 | 0.7 | 9.7 | 55.9 | 32.9 ± 1.5 | |||||
| 2020 | 77 | 0.9 | 33.3 | 15.6 | 50.5 ± 2.2 | |||||||
| 2016 | Bordighera E | 43.7699 | 7.67639 | CMBO_S1_T1 | 49 | 155 | 1.9 | 4.5 | 5.8 | 19.5 ± 1.6 | ||
| 2018 | 218 | 4.0 | 11.5 | 13.3 | 45.0 ± 1.2 | |||||||
| 2021 | 556 | 8.4 | 7.0 | 3.4 | 21.7 ± 1.0 | |||||||
| 2016 | CMBO_S1_T2 | 60 | 179 | 1.9 | 8.4 | 13.4 | 26.8 ± 2.1 | |||||
| 2018 | 180 | 3.8 | 13.3 | 31.7 | 34.9 ± 1.6 | |||||||
| 2021 | 823 | 13.1 | 49.0 | 6.0 | 24.8 ± 0.9 | |||||||
| 2016 | CMBO_S1_T3 | 66 | 400 | 5.0 | 2.8 | 11.3 | 22.5 ± 1.6 | |||||
| 2018 | 517 | 8.6 | 7.4 | 44.7 | 25.9 ± 1.2 | |||||||
| 2021 | 407 | 5.1 | 0.0 | 0.0 | 13.6 ± 1.1 | |||||||
| 2016 | Mortola Cape | 43.76952 | 7.56353 | CMBO_S3_T2 | 35 | 302 | 3.8 | 29.1 | 3.3 | 28.1 ± 2.1 | ||
| 2020 | 243 | 4.5 | 39.3 | 7.4 | 43.1 ± 1.9 | |||||||
2.2. ROV Footage Analysis
2.3. Statistical Analysis
3. Results
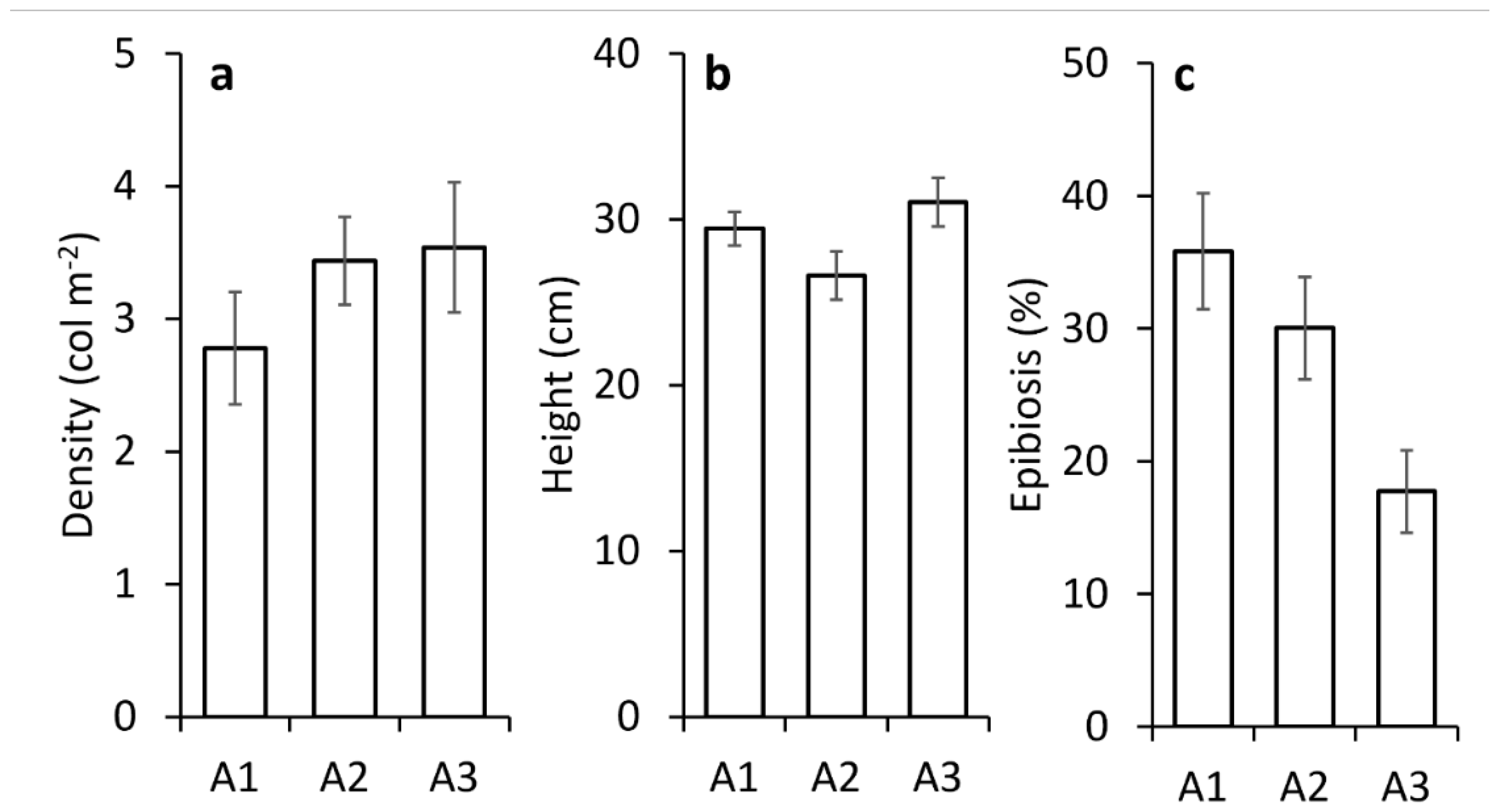
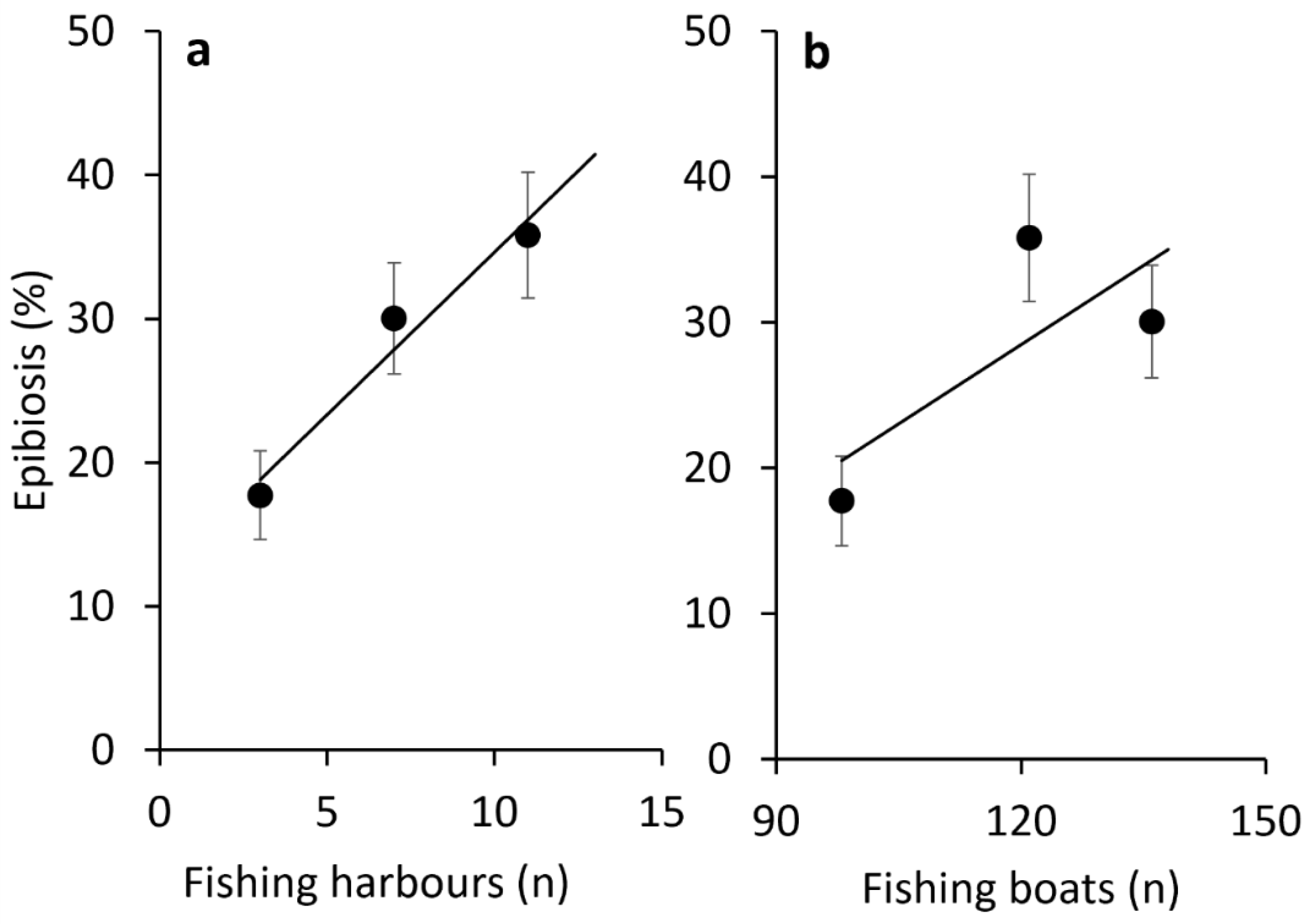
3.1. Health Status of Paramuricea clavata Forests in the Ligurian Sea
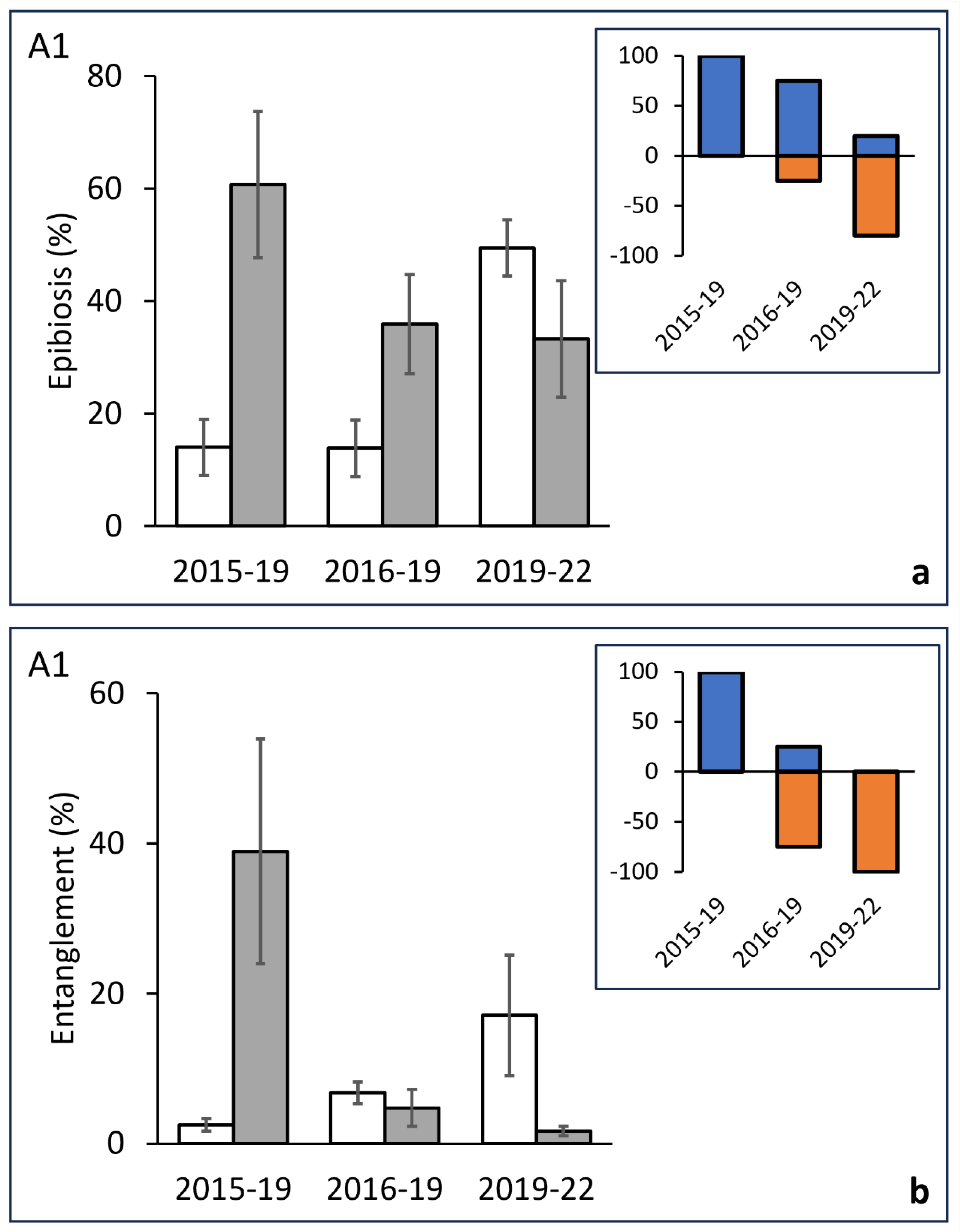
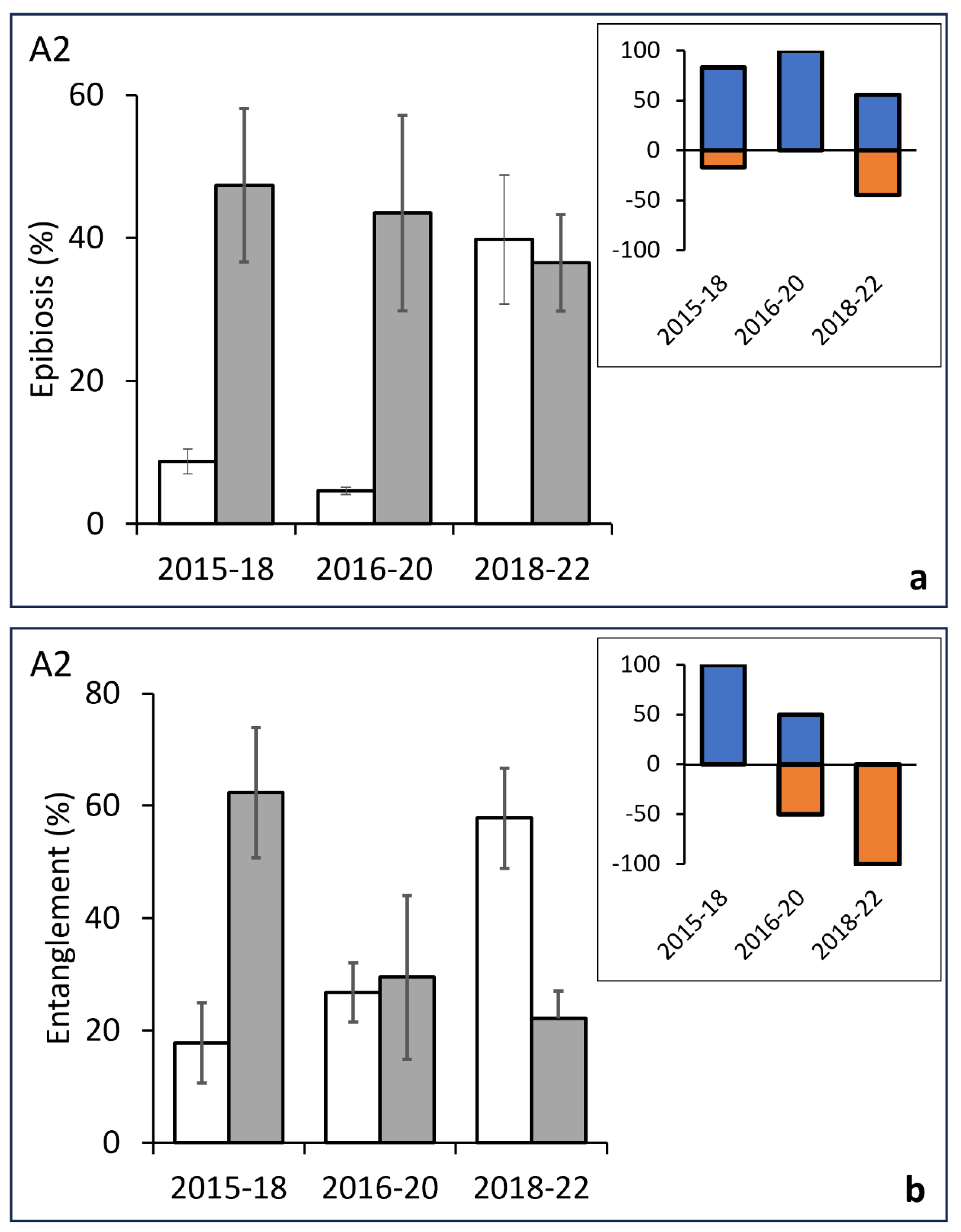
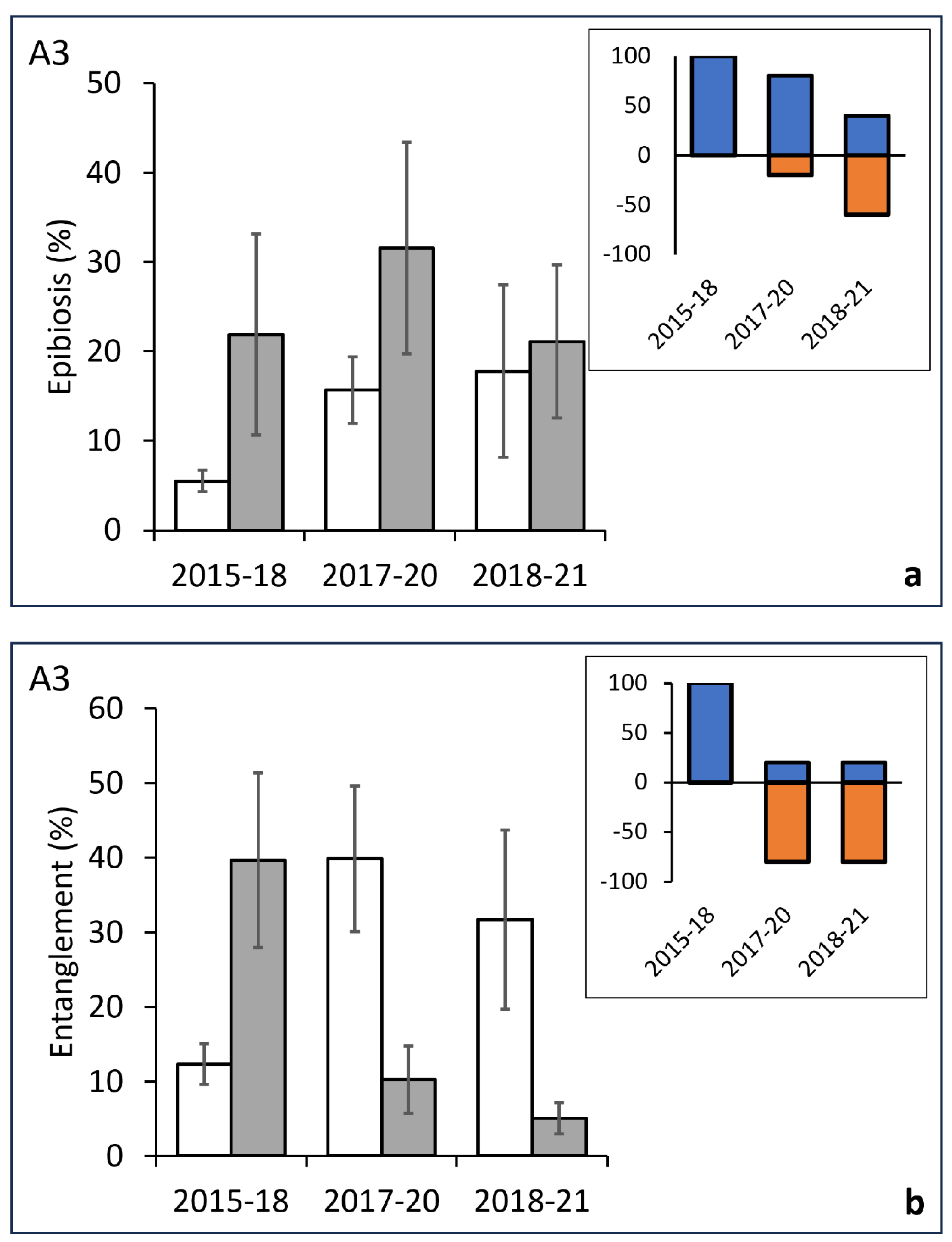
3.2. Temporal Analysis of Epibiosis and Entanglement
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal forests in deep coastal bottoms and continental shelf of the Mediterranean Sea. In Marine Animal Forests: the ecology of benthic biodiversity hotspots; 2017; pp. 207–233. [Google Scholar]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. An overview of the animal forests of the world. In Marine Animal Forests: the ecology of benthic biodiversity hotspots; 2017; pp. 1–28. [Google Scholar]
- Piazzi, L.; Atzori, F.; Cadoni, N.; Cinti, M.F.; Frau, F.; Pansini, A.; Pinna, F.; Stipcich, P.; Ceccherelli, G. Animal Forest mortality: Following the consequences of a gorgonian coral loss on a Mediterranean Coralligenous assemblage. Diversity 2021, 13, 133. [Google Scholar] [CrossRef]
- Linares, C.; Doak, D.F. Forecasting the combined effects of disparate disturbances on the persistence of long-lived gorgonians: a case study of Paramuricea clavata. Mar Ecol Prog Ser 2020, 402, 59–68. [Google Scholar] [CrossRef]
- Cerrano, C.; Milanese, M.; Ponti, M. Diving for science-science for diving: volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquat Conserv Mar Freshw Ecos 2017, 27, 303–323. [Google Scholar] [CrossRef]
- Betti, F.; Bavestrello, G.; Bo, M.; Ravanetti, G.; Enrichetti, F.; Coppari, M.; Cappanera, V.; Venturini, S.; Cattaneo-Vietti, R. Evidences of fishing impact on the coastal gorgonian forests inside the Portofino MPA (NW Mediterranean Sea). Ocean Coast Manag 2020, 187, 105105. [Google Scholar] [CrossRef]
- Otero, M.M.; Numa, C.; Bo, M.; Orejas, C.; Garrabou, J.; Cerrano, C.; et al. Overview of the Conservation Status of Mediterranean Anthozoans; IUCN: Málaga, Spain, 2017; pp. 1–73. ISBN 978-2-8317-1845-3. http://hdl.handle.net/10261/150115. [CrossRef]
- Martin, Y.; Bonnefont, J.L.; Chancerelle, L. Gorgonians mass mortality during the 1999 late summer in French Mediterranean coastal waters: the bacterial hypothesis. Water Res 2002, 36, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Lamberti, C.V.; Sonni, C.; Pellegrini, D. Mucilage impact on gorgonians in the Tyrrhenian sea. STOTEN 2005, 353, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Schiaparelli, S.; Castellano, M.; Povero, P.; Sartoni, G.; Cattaneo-Vietti, R. A benthic mucilage event in North-Western Mediter- ranean Sea and its possible relationships with the summer 2003 European heatwave: Short term effects on littoral rocky assemblages. Mar Ecol 2007, 28, 341–353. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob Chang Biol 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Vezzulli, L.; Previati, M.; Pruzzo, C.; Marchese, A.; Bourne, D.G.; Cerrano, C. VibrioSea Consortium. Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environ Microbiol 2010, 12, 2007–2019. [Google Scholar] [CrossRef]
- Vezzulli, L.; Pezzati, E.; Huete-Stauffer, C.; Pruzzo, C.; Cerrano, C. 16SrDNA pyrosequencing of the Mediterranean gorgonian Paramuricea clavata reveals a link among alterations in bacterial holobiont members, anthropogenic influence and disease outbreaks. PLoS One 2013, 8, e67745. [Google Scholar] [CrossRef]
- Piazzi, L.; Atzori, F.; Cadoni, N.; Cinti, M.F.; Frau, F.; Ceccherelli, G. Benthic mucilage blooms threaten coralligenous reefs. Mar Environ Res 2018, 140, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Verdura, J.; Linares, C.; Ballesteros, E.; Coma, R.; Uriz, M.J.; Bensoussan, N.; Cebrian, E. Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species. Sci Rep 2019, 9, 5911. [Google Scholar] [CrossRef] [PubMed]
- Ceccherelli, G.; Pinna, F.; Pansini, A.; Piazzi, L.; La Manna, G. The constraint of ignoring the subtidal water climatology in evaluating the changes of coralligenous reefs due to heating events. Sci Rep 2020, 10, 17332. [Google Scholar] [CrossRef] [PubMed]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.G.; Schiaparelli, S.; Siccardi, A.; Sponga, F. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol Let 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Garrabou, J.; Perez, T.; Sartoretto, S.; Harmelin, J.G. Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar Ecol Progr Ser 2001, 217, 263–272. [Google Scholar] [CrossRef]
- Cebrian, E.; Uriz, M.J.; Garrabou, J.; Ballesteros, E. Sponge mass mortalities in a warming Mediterranean Sea: are cyanobacteria-harboring species worse off? PLoS One 2011, 6, e20211. [Google Scholar] [CrossRef]
- Cebrian, E.; Linares, C.; Marschal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol Inv 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Ledoux, J.B.; Linares, C.; Bensoussan, N.; López-Sendino, P.; et al. Collaborative database to track mass mortality events in the Mediterranean Sea. Front Mar Sci 2019, 6, 478167. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob Chang Biol 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Cocito, S.; Sgorbini, S. Long-term trend in substratum occupation by a clonal, carbonate bryozoan in a temperate rocky reef in times of thermal anomalies. Mar Biol 2014, 161, 17–27. [Google Scholar] [CrossRef]
- Linares, C.; Coma, R.; Diaz, D.; Zabala, M.; Hereu, B.; Dantart, L. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Mar Ecol Prog Ser 2005, 305, 127–137. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G. Medium-term effects of die-off of rocky benthos in the Ligurian Sea. What can we learn from gorgonians? Chem Ecol 2008, 24, 73–82. [Google Scholar] [CrossRef]
- Huete-Stauffer, C.; Vielmini, I.; Palma, M.; Navone, A.; Panzalis, P.; Vezzulli, L.; Misic, C.; Cerrano, C. Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to a mass mortality event. Mar Ecol 2011, 32, 107–116. [Google Scholar] [CrossRef]
- Teixidó, N.; Casas, E.; Cebrian, E.; Linares, C.; Garrabou, J. Impacts on coralligenous outcrop biodiversity of a dramatic coastal storm. PLoS One 2013, 8, e53742. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cerrano, C.; Zanzi, D.; Cattaneo-Vietti, R. Damage by fishing activities to the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat Conserv Mar Freshw Ecosyst 1997, 7, 253–262. [Google Scholar] [CrossRef]
- Coma, R.; Pola, E.; Ribes, M.; Zabala, M. Long-term assessment of temperate octocoral mortality patterns, protected vs. unprotected areas. Ecol Appl 2004, 14, 1466–1478. [Google Scholar] [CrossRef]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol Conserv 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; Canese, S. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar Pollut 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Angiolillo, M.; Fortibuoni, T. Impacts of marine litter on Mediterranean reef systems: From shallow to deep waters. Front Mar Sci 2020, 7, 581966. [Google Scholar] [CrossRef]
- Sini, M.; Kipson, S.; Linares, C.; Koutsoubas, D.; Garrabou, J. The yellow gorgonian Eunicella cavolini: Demography and disturbance levels across the Mediterranean Sea. PLoS One 2015, 10, e0126253. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bo, M.; Morri, C.; Montefalcone, M.; Toma, M.; Bavestrello, G.; Tunesi, L.; Canese, S.; Giusti, M.; Salvati, E.; Bertolotto, R.M.; Bianchi, C.N. Assessing the environmental status of temperate mesophotic reefs: A new, integrated methodological approach. Ecol Ind 2019, 102, 218–229. [Google Scholar] [CrossRef]
- Tsounis, G.; Martinez, L.; Bramanti, L.; Viladrich, N.; Gili, J.M.; Martinez, A.; Rossi, S. Anthropogenic effects on reproductive effort and allocation of energy reserves in the Mediterranean octocoral Paramuricea clavata. Mar Ecol Prog Ser 2012, 449, 161–172. [Google Scholar] [CrossRef]
- Kipson, S.; Linares, C.; Cˇižmek, H.; Cebrián, E.; Ballesteros, E.; Bakran-Petricioli, T.; Garrabou, J. Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar Ecol 2015, 36, 982–993. [Google Scholar] [CrossRef]
- Canessa, M.; Amedeo, I.; Bavestrello, G.; Panzalis, P.; Trainito, E. The diversity, structure, and development of the epibiont community of Paramuricea clavata (Risso, 1826) (Cnidaria, Anthozoa). Water 2023, 15, 2664. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Cappanera, V.; Mariotti, M.; Massa, F.; Merotto, L.; Povero, P.; Rigo, I.; Toma, M.; Tunesi, L.; Vassallo, P.; Venturini, S.; Bo, M. High megabenthic complexity and vulnerability of a mesophotic rocky shoal support its inclusion in a Mediterranean MPA. Diversity 2023, 15, 933. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Azzini, F.; Calcinai, B.; Castellano, L.; Muti, C.; Valisano, L.; Zega, G.; Bavestrello, G. Gorgonian population recovery after a mass mortality event. Aquat Conserv Mar Freshw Ecos 2005, 15, 147–157. [Google Scholar] [CrossRef]
- Fava, F.; Bavestrello, G.; Valisano, L.; Cerrano, C. Survival, growth and regeneration in explants of four temperate gorgonian species in the Mediterranean Sea. Ital J Zool 2010, 77, 44–52. [Google Scholar] [CrossRef]
- Gómez-Gras, D.; Linares, C.; Dornelas, M.; Madin, J.S.; Brambilla, V.; Ledoux, J.B.; et al. Climate change transforms the functional identity of Mediterranean coralligenous assemblages. Ecol Lett 2021, 24, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Iborra, L.; Leduc, M.; Fullgrabe, L.; Cuny, P.; Gobert, S. Temporal trends of two iconic Mediterranean gorgonians (Paramuricea clavata and Eunicella cavolini) in the climate change context. J Sea Res 2022, 186, 102241. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS One 2019, 14, e0223949. [Google Scholar] [CrossRef]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Canese, S.; Bo, M. Assessment and distribution of seafloor litter on the deep Ligurian continental shelf and shelf break (NW Mediterranean Sea). Mar Pollut 2020, 151, 110872. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Estaque, T.; Richaume, J.; Bianchimani, O.; Schull, Q.; Mérigot, B.; Bensoussan, N.; et al. Marine heatwaves on the rise: One of the strongest ever observed mass mortality event in temperate gorgonians. Glob Chang Biol 2023, 29, 6159–6162. [Google Scholar] [CrossRef] [PubMed]
- Previati, M.; Scinto, A.; Cerrano, C.; Osinga, R. Oxygen consumption in Mediterranean octocorals under different temperatures. J Experim Mar Biol Ecol 2010, 390, 39–48. [Google Scholar] [CrossRef]
- Ezzat, L.; Merle, P.L.; Furla, P.; Buttler, A.; Ferrier-Pages, C. The response of the Mediterranean gorgonian Eunicella singularis to thermal stress is independent of its nutritional regime. PLoS One 2013, 8, e64370. [Google Scholar] [CrossRef]
- Rodolfo-Metalpa, R.; Bianchi, C.N.; Peirano, A.; Morri, C. Tissue necrosis and mortality of the temperate coral Cladocora caespitosa. Ital J Zool 2005, 72, 271–276. [Google Scholar] [CrossRef]
- Cupido, R.; Cocito, S.; Barsanti, M.; Sgorbini, S.; Peirano, A.; Santangelo, G. Unexpected long-term population dynamics in a canopy-forming gorgonian coral following mass mortality. Mar Ecol Progr Ser 2009, 394, 195–200. [Google Scholar] [CrossRef]
- Rivetti, I.; Fraschetti, S.; Lionello, P.; Zambianchi, E.; Boero, F. Global warming and mass mortalities of benthic invertebrates in the Mediterranean Sea. PLoS One 2014, 9, e115655. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bava, S.; Bavestrello, G.; Betti, F.; Lanteri, L.; Bo, M. Artisanal fishing impact on deep coralligenous animal forests: A Mediterranean case study of marine vulnerability. Ocean Coastal Manag 2019, 177, 112–126. [Google Scholar] [CrossRef]
- Russo, E.; Anelli Monti, M.; Toninato, G.; Silvestri, C.; Raffaetà, A.; Pranovi, F. Lockdown: how the COVID-19 pandemic affected the fishing activities in the adriatic sea (Central Mediterranean Sea). Front Mar Sci 2021, 8, 685808. [Google Scholar] [CrossRef]
- Giannakis, E.; Hadjioannou, L.; Jimenez, C.; Papageorgiou, M.; Karonias, A.; Petrou, A. Economic consequences of coronavirus disease (COVID-19) on fisheries in the eastern Mediterranean (Cyprus). Sustainability 2020, 12, 9406. [Google Scholar] [CrossRef]
- Coll, M.; Ortega-Cerdà, M.; Mascarell-Rocher, Y. Ecological and economic effects of COVID-19 in marine fisheries from the Northwestern Mediterranean Sea. Biol Conserv 2021, 255, 108997. [Google Scholar] [CrossRef] [PubMed]
- Dapueto, G.; Massa, F.; Costa, S.; Cimoli, L.; Olivari, E.; Chiantore, M.; Federici, B.; Povero, P. A spatial multi-criteria evaluation for site selection of offshore marine fish farm in the Ligurian Sea, Italy. Ocean Coast Manag 2015, 116, 64–77. [Google Scholar] [CrossRef]
- FAO. International Guidelines for the Management of Deep-Sea Fisheries in the High Seas. Rome, 2009; 73p.

| df | SS | MS | Pseudo-F | P (perm) | Pair-wises | T | P (perm) | |
|---|---|---|---|---|---|---|---|---|
| Density | ||||||||
| Macro-area | 2 | 5201.1 | 2600.5 | 2.716 | 0.3686 | |||
| Res | 107 | 1.0245E+05 | 957.5 | |||||
| Total | ||||||||
| Height | Height | |||||||
| Macro-area | 2 | 1360.9 | 680.44 | 35.551 | 0.0267 | A1 vs A2 | 20.462 | 0.0357 |
| Res | 107 | 20480 | 191.4 | A1 vs A3 | 0.70001 | 0.5049 | ||
| Total | 109 | 21840 | A2 vs A3 | 22.188 | 0.0249 | |||
| Epibiosis | Epibiosis | |||||||
| Macro-area | 2 | 11824 | 5912.2 | 38.355 | 0.006 | A1 vs A2 | 12.312 | 0.1907 |
| Res | 107 | 1.65E+09 | 1541.4 | A1 vs A3 | 2.721 | 0.0012 | ||
| Total | 109 | 1.77E+09 | A2 vs A3 | 16.747 | 0.0545 | |||
| Entanglement | Entanglement | |||||||
| Macro-area | 2 | 25589 | 12795 | 68.677 | 0.0001 | A1 vs A2 | 37.941 | 0.0001 |
| Res | 107 | 1.99E+09 | 1863 | A1 vs A3 | 19.837 | 0.0119 | ||
| Total | 109 | 2.25E+09 | A2 vs A3 | 16.131 | 0.0553 |
| df | SS | MS | Pseudo-F | P (perm) | Pair-wises | t | P (perm) | Unique perms | P (MC) | |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | ||||||||||
| Epibiosis | ||||||||||
| Before/After | 5 | 11612 | 2322.3 | 29.303 | 0.0228 | 2015/19 | 40.632 | 0.027 | 35 | 0.0024 |
| Res | 20 | 15850 | 792.52 | 2016/19 | 20.984 | 0.0532 | 35 | 0.0529 | ||
| Total | 25 | 27462 | 2019/22 | 0.79759 | 0.502 | 126 | 0.4923 | |||
| Entanglement | ||||||||||
| Before/After | 5 | 18518 | 3703.6 | 21.411 | 0.0244 | 2015/19 | 27.952 | 0.0259 | 35 | 0.006 |
| Res | 20 | 34595 | 1729.7 | 2016/19 | 0.4955 | 0.7255 | 35 | 0.7313 | ||
| Total | 25 | 53113 | 2019/22 | 14.721 | 0.0794 | 126 | 0.1193 | |||
| A2 | ||||||||||
| Epibiosis | ||||||||||
| Before/After | 5 | 23565 | 4713 | 46.103 | 0.001 | 2015/18 | 27.982 | 0.0135 | 462 | 0.0043 |
| Res | 32 | 32713 | 1022.3 | 2016/20 | 38.243 | 0.0287 | 35 | 0.0029 | ||
| Total | 37 | 56278 | 2018/22 | 0.516 | 0.709 | 8170 | 0.7162 | |||
| Entanglement | ||||||||||
| Before/After | 5 | 15630 | 3125.9 | 32.804 | 0.0031 | 2015/18 | 2.362 | 0.0203 | 461 | 0.0157 |
| Res | 32 | 30493 | 952.92 | 2016/20 | 0.59803 | 0.8289 | 35 | 0.6893 | ||
| Total | 37 | 46123 | 2018/22 | 30.371 | 0.0046 | 8150 | 0.0043 | |||
| A3 | ||||||||||
| Epibiosis | ||||||||||
| Before/After | 5 | 9829.4 | 1965.9 | 12.736 | 0.2349 | 2015/18 | 18.318 | 0.0536 | 35 | 0.0779 |
| Res | 22 | 33959 | 1543.6 | 2016/20 | 11.444 | 0.2483 | 91 | 0.2815 | ||
| Total | 27 | 43788 | 2018/21 | 0.83113 | 0.6803 | 126 | 0.5607 | |||
| Entanglement | ||||||||||
| Before/After | 5 | 17345 | 3469 | 19.534 | 0.0293 | 2015/18 | 21.685 | 0.0855 | 35 | 0.0435 |
| Res | 22 | 39068 | 1775.8 | 2016/20 | 15.939 | 0.1094 | 91 | 0.1148 | ||
| Total | 27 | 56413 | 2018/21 | 15.329 | 0.0403 | 91 | 0.0872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





