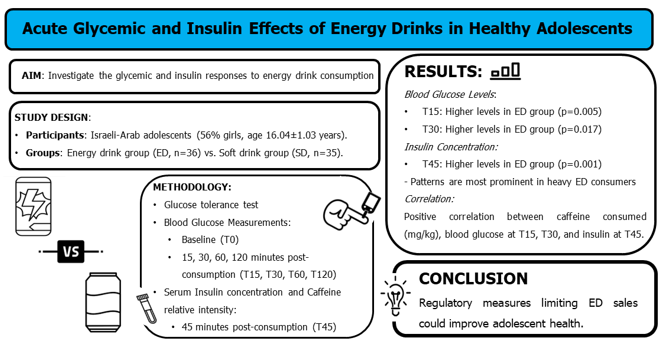
1. Introduction
Energy drinks (EDs) are caffeinated soft drinks advertised, especially to adolescents and young adults, as beverages intended to enhance energy, athletic performance, concentration, and alertness [
1,
2]. Despite these claimed benefits, ED consumption is regarded as a risk marker for various unhealthy lifestyle habits among adolescents, such as fast food consumption [
3] and alcohol and substance use [
4,
5].
Reports have indicated a growing trend in ED consumption among children and adolescents, with rates surging from 10% to as high as 50% within the past decade [
6]. A cross- sectional study focusing on ED consumption habits in the Middle East found that 70% of the 809 adolescent cohort aged 14-18 years reported ED consumption [
3]. In a 2011 survey conducted by the Israel Ministry of Health in the Israeli-Arab community, 42.3% of the 636 Arab adolescent (16-18 years) responders consumed EDs. More specifically, per-session consumption volumes of less than one can, one can, and more than one can were reported by 1.4%, 35.7%, and 5.2% of adolescents, respectively [
7]. The increased consumption of EDs by adolescents has been attributed to their ready availability, accessibility, and limited regulations in countries where they are commercialized [
6].
The primary metabolically detrimental ingredient in EDs appears to be caffeine. ED caffeine content ranges from a modest 50 mg to an alarming 505 mg per can or bottle, depending on the brand [
8]. High dosages of caffeine (e.g., 210-500 mg) can cause restlessness, nervousness, anxiety and depression [
9]. Moreover, caffeine has been proven to impair whole-body glucose disposal by 20-30% in adults [
10]. Caffeine ingestion of 1 mg/kg body weight is sufficient to impair glucose tolerance, with every additional milligram causing a 5.8% increase in the amount of insulin needed to dispose of circulating glucose [
11,
12,
13]. Moderate caffeine doses decreased insulin sensitivity in healthy volunteers and increased plasma catecholamines and systolic and diastolic blood pressure [
14]. However, ingestion of caffeine, together with sugar, taurine, and other ingredients typically included in EDs, may increase its impact on both the glycemic response and the cardiovascular system, compared to when consumed alone [
15,
16]. Consuming one can of ED a day may be sufficient to exceed the caffeine threshold in healthy adolescents and children. The severity of undesirable effects is dose-dependent, with a toxicity threshold of approximately 100 mg/day for adolescents and 2.5 mg/kg/day for children [
15,
17].
While numerous studies have assessed the association between ED consumption by adolescents and their subsequent psychological responses and lifestyle habits, very few have investigated the immediate physiological effects of ED consumption. More specifically, this study aimed to investigate the physiological responses of adolescents to ED consumption, with a specific focus on parameters such as glucose and insulin tolerance. Furthermore, it explores how the frequency of ED consumption may influence these responses.
2. Materials and Methods
2.1. Sample Size Calculation
The sample size was calculated with G*power software (Heinrich Heine University, Düsseldorf, Germany), based on MANOVA-repeated measurements, within-between interactions, with a medium effect size (f[V]=0.4), alpha=0.05, 80% power. A sample size of 73 subjects was needed. The final participant count was 71, a deviation that did not alter the calculated effect size.
2.2. Ethical Statement
The study was conducted according to the guidelines laid forth in the Declaration of Helsinki, and all procedures involving human participants were approved by the Ethics Committee at Saint Vincent de Paul Hospital (04-2020-svh; date of approval- February 2020) and registered at ClinicalTrials.gov (NCT04808128). The adolescents provided their consent to participate in the study, and written informed consent was obtained from their parents or legal guardians. Participation was voluntary.
2.3. Study Population
Healthy Israeli-Arab adolescents aged 14-18 in Northern Israel were recruited for this study. Adolescents who exhibited diabetes, hypoglycemic episodes, coronary heart disease, cardiac arrhythmia, secondary hypertension, structural heart lesions, hepatic or renal disorders, autonomic neuropathy, epilepsy, thyroid disorders, obstructive sleep apnea and migraines, caffeine intolerance, or food allergies were not eligible to participate in the study. Adolescents diagnosed with eating or mental disorders and prescribed psychiatric medications and adolescents who regularly use drugs, including smoking and alcohol consumption, were also excluded from the study. Weekly ED consumption frequency was classified as previously outlined [
18]. Participants were classified “infrequent” ED consumers if they consumed ED less than once per week, “frequent” ED consumers if they consumed ED between 1-3 times per week, and “heavy” ED consumers if they consumed ED at least four times per week.
2.4. Study Design
This non-randomized, case-controlled, open-label study was conducted between March 2021 and August 2021. Eligible participants were asked to abstain from caffeine-containing beverages and medications and alcohol for 72 hours preceding the experiment. Additionally, they were instructed to avoid strenuous physical activity to prevent the depletion of muscle glycogen stores. Participants fasted overnight (with an allowance of water) before the experiment day.
At the study session, a glucose tolerance test was conducted. Participants were assigned to one of two study groups and administered either 250 ml of ‘XL’ brand energy drink containing 27.5 g carbohydrates (sugars) and 80 mg caffeine (ED arm), or 250 ml of an ‘exotic’-flavored soft drink containing 27.2 g carbohydrates (sugars), and 0 mg of caffeine (SD arm) (to full ingredient content see supplementary table 1). Each beverage was consumed alongside sucrose, resulting in a total intake of 45 g carbohydrates. This decision was based on a preliminary experiment demonstrating that carbohydrate quantities ranging from 40 g to 50 g were well-tolerated by adolescents and was ample to elicit a peak in glucose levels. According to the Helsinki Committee guidelines, ED was given only to volunteers who had previously consumed EDs. Participants who reported no previous ED consumption were assigned to the SD group. Those who confirmed ED consumption were randomly assigned to either the SD or ED group.
2.5. End Point Measurements
Blood glucose levels were measured at baseline and 15, 30, 60, and 120 minutes (min) after the consumption of the beverages (T0, T15, T30, T60, and T120, respectively). Blood samples were collected at T45 to measure serum insulin concentration and determine caffeine relative intensity. Moreover, the amount of caffeine consumed during the experiment in the ED group (80 mg) was divided by each participant’s weight to calculate the caffeine amount in milligrams per kilogram (mg/kg). This calculation was used to examine the correlation between these caffeine amounts and glucose and insulin parameters.
2.5.1. Blood Glucose
Blood glucose levels were measured with a ‘Freestyle Lite’ digital glucometer (Abbott Diabetes Care Inc, Alameda, California, United States), according to the manufacturer’s protocol. A blood drop was obtained from the finger of all participants at five time points. Samples were collected by a single researcher throughout the study.
2.5.2. Serum Insulin and Caffeine
Since the peak effect of caffeine is typically observed within 40-80 minutes of consumption [
15], venous blood samples were collected into a serum tube at T45. The tubes were spun down at 3,000g for 15 min, and the entire serum content was immediately frozen at -80 °C. The Architect c4000 clinical chemistry analyzer (Abbott Laboratories, Chicago, Illinois, United States) was used to determine serum insulin levels per the manufacturer’s guidelines. Untargeted metabolomics by liquid chromatography–mass spectrometry (LCMS) was used to determine the patterns and relative intensities of caffeine, as previously described [
19]. Since untargeted LCMS does not include internal standards (IS), the analysis relies on comparing the relative quantities of caffeine across all samples. This approach does not provide absolute quantification of caffeine. Instead, it focuses on contrasting it prevalence between the two groups. By evaluating caffeine’s patterns and relative intensities, untargeted LCMS can highlight significant differences or similarities within the datasets.
2.6. Measures
2.6.1. Socio-Demographic Data
Data regarding participant age, gender, religion, place of residence, and parents’ education were collected using the ‘Mabat Youth 2
nd National Health and Nutrition Survey of 7
th-12
th Grade Students 2015-2016’ self-report questionnaire developed by the Israeli Ministry of Health in Arabic [
20].
2.6.2. Anthropometrics
Participants’ weight, height, and waist circumference were measured between T60 and T120. The measurements were made according to the Israeli Ministry of Health protocol [
20]. Height was measured using a HR-001 portable height stadiometer (Tanita, Tokyo, Japan), with an accuracy of 0.1 cm. Body weight was measured with a BC-587 InnerScan digital weight (Tanita), with a minimal sensitivity of 0.1 kg and a maximum capacity of 200 kg. Weight circumference was measured with a standard measuring tape. The collected height and weight data were then used to calculate body mass index (BMI) and BMI Z-scores [
21]. Waist circumference and height data were also used to calculate waist-height ratio (WHtR), which has been proven to strongly predict metabolic complications in children and adolescents [
22].
2.7. Statistical Analysis
Data were processed and analyzed using SPSS software (version 25) (IBM Corp., Armonk, New York, United States). The Shapiro-Wilk test was used to assess the normality of sample distribution. A logarithmic transformation and the Mann-Whitney U test were applied when data was not normally distributed. When normality was confirmed, an independent t-test was used to determine differences in socio-demographic data between study arms and examine beverage type's influence on blood glucose levels and serum insulin levels. The Chi-squared test was employed to evaluate possible differences between categorical variables. Fisher’s exact test was used when the number of variables with less than five observations exceeded 20% of the total collected data. Spearman's rank correlation coefficient was used to assess the correlation between the amount of caffeine consumed (mg/kg) and blood glucose and serum insulin levels after adjustment for age, gender, and BMI Z-score. The analysis included a comparative assessment of results between the two study arms, both in general context and following stratification according to the weekly ED consumption frequency. A p-value < 0.05 was considered statistically significant and adapted to our one-tailed hypotheses when necessary. Graphs were prepared using Prism software (version 9.5.1) (GraphPad Software, Boston, Massachusetts, United States).
3. Results
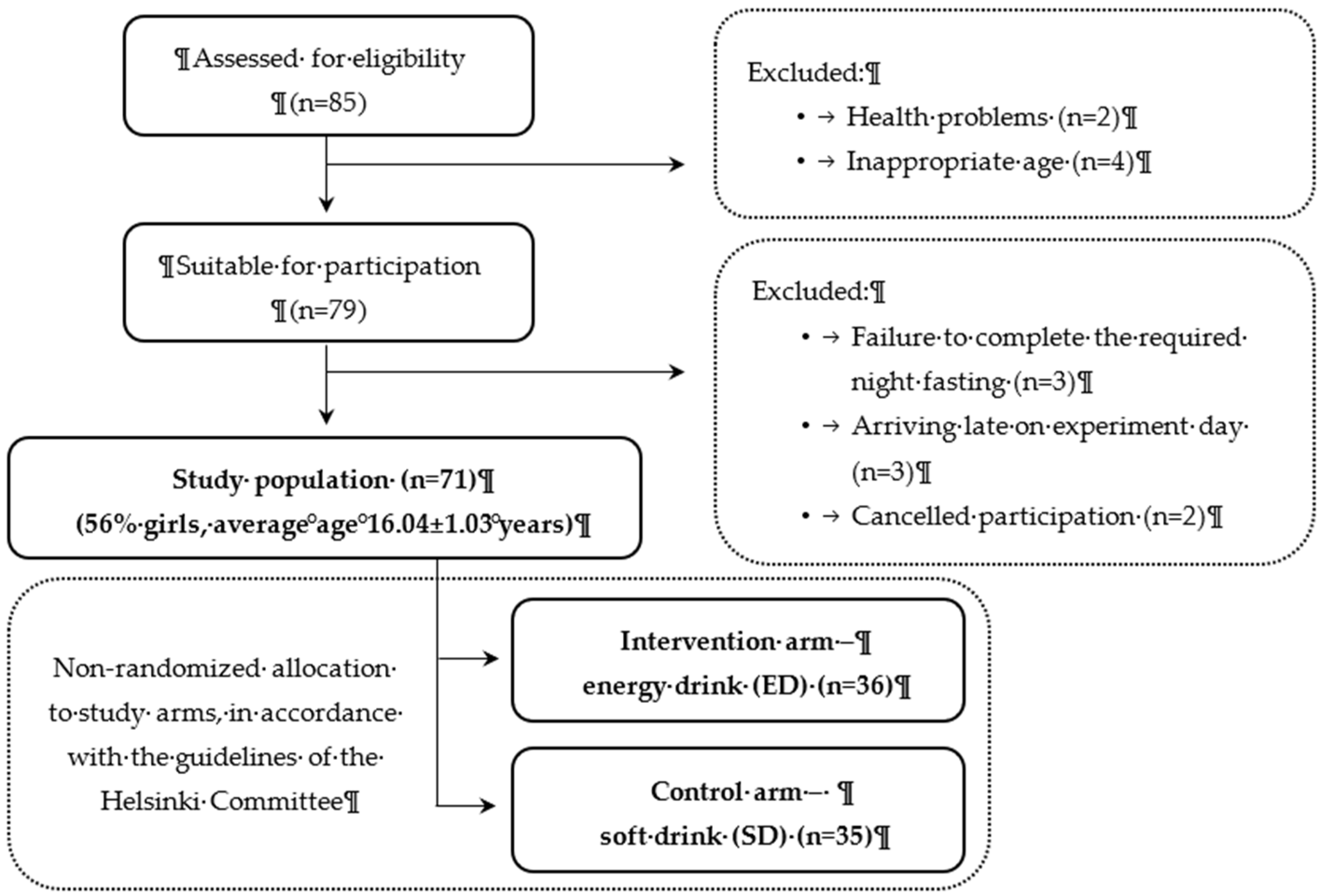
A total of 85 participants were initially assessed for eligibility, and exclusion criteria led to the removal of two individuals due to health problems and four based on age inappropriateness. Additionally, eight participants were excluded for specific reasons: failure to complete the required night fasting (n=3), arriving late on experiment day (n=3), and cancellation of participation (n=2). This left a final cohort of 71 participants (56% girls) for the main analysis (see Fig. 1 for the CONSORT flow chart).
Figure 1.
CONSORT flow chart for participant enrolment.
Figure 1.
CONSORT flow chart for participant enrolment.
3.1. Participant Demographic and Anthropometric Characteristics
Most participants were females (56%), with a mean age of 16.04±1.03 years, and identified themselves as Muslim (90%), living in rural areas (89%). Thirty-nine percent of the participants' mothers completed 12 years of formal education, whereas only 27% of the fathers achieved the same educational level. The study population was primarily of average weight (BMI 22.48±4.21 and BMI Z-score=0.25±1.09) and had a normal WHtR (0.45±0.06). No significant differences in the demographic and anthropometric characteristics were observed between the two study arms (
Table 1).
The distribution of participants across the three frequencies (infrequent, frequent, or heavy) in both study arms did not show statistically significant differences (p=0.169), as presented in
Table 1. Most participants described themselves as heavy (n=28, 39%) or frequent (n=27, 38%) ED consumers, drinking ED at least four times per week or drinking ED between 1-3 times per week, respectively (
Table 2). Only 16 (23%) participants were infrequent ED consumers, drinking ED less than once per week. Of note, no statistically significant differences existed between the demographics or anthropometric characteristics of the ED consumption frequency subcohorts of the SD vs. ED arms. (
Table 2).
3.2. Glycemic Control
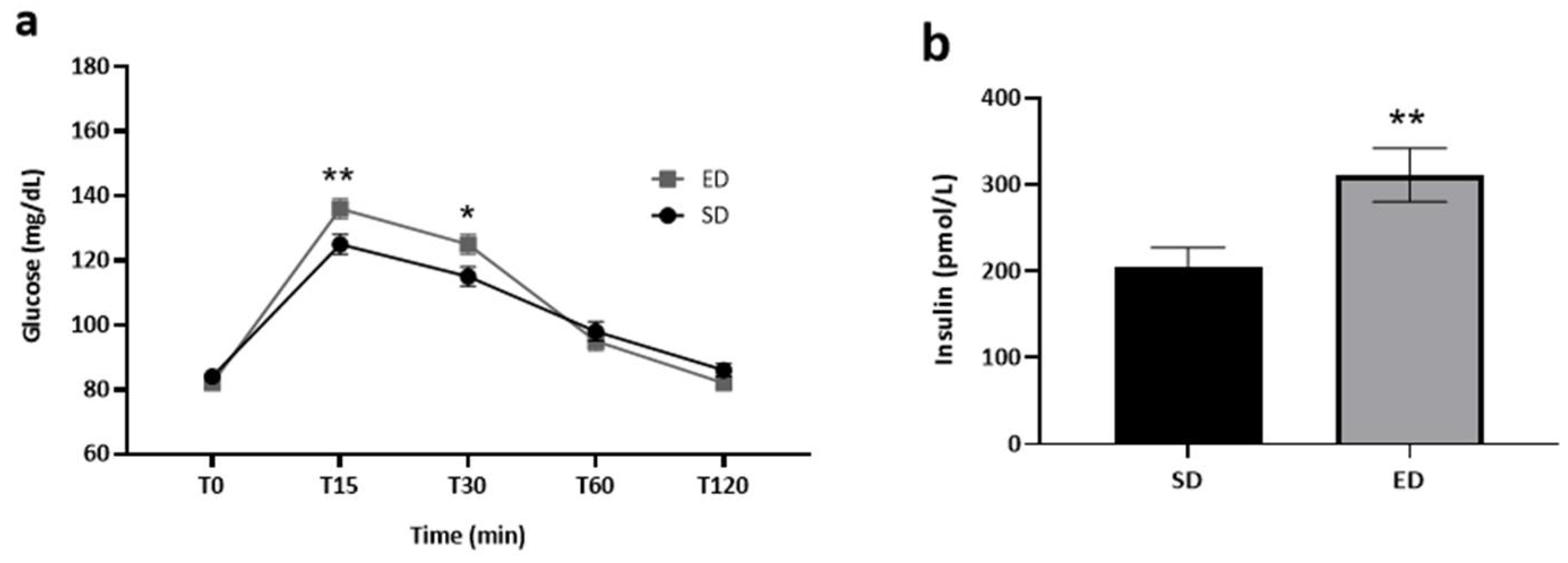
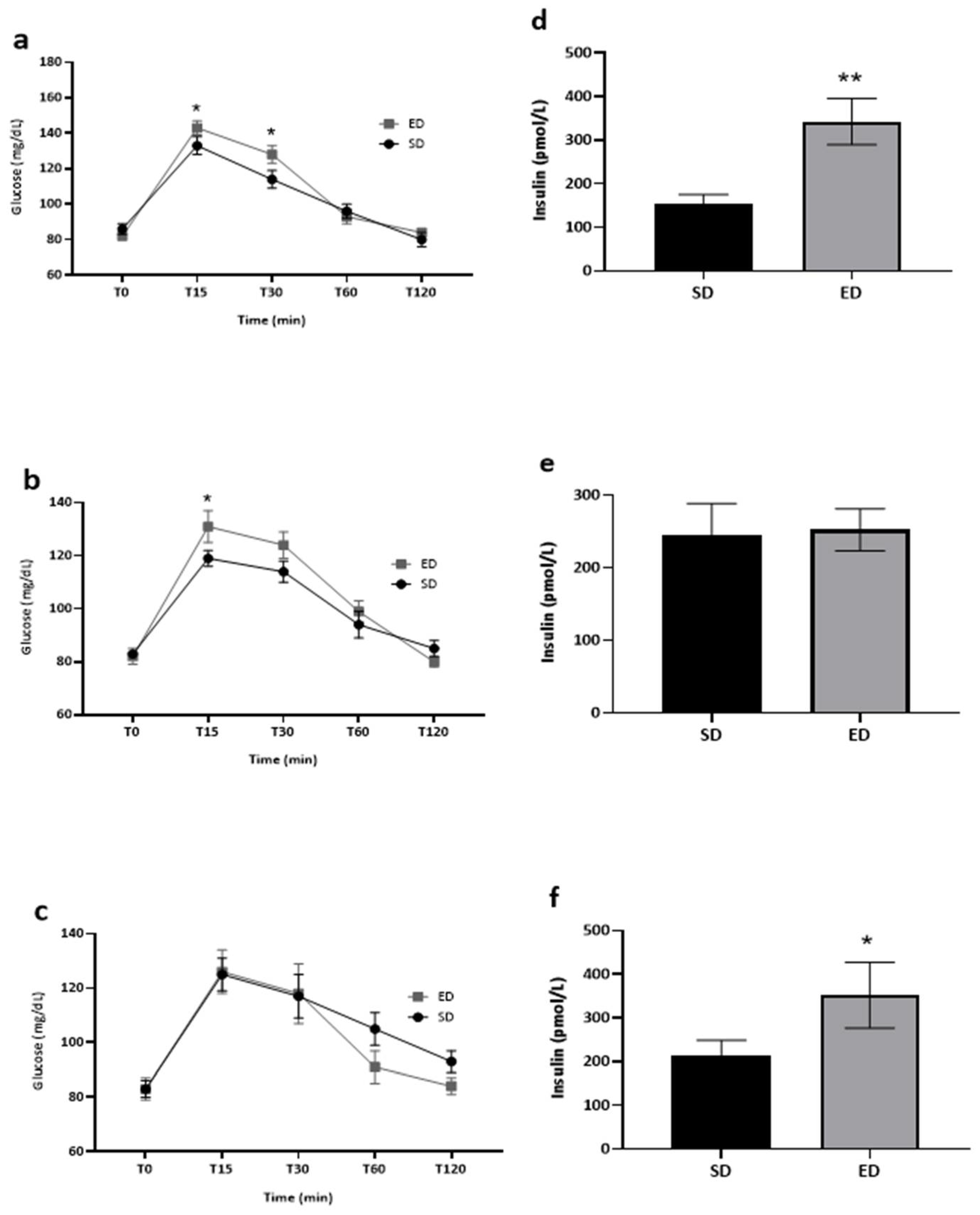
The influence of beverage type on blood glucose and serum insulin levels within each group is depicted in
Figure 2. It is worth noting that blood glucose data were accessible for all the study participants (n=71). In contrast, serum insulin data were available for 66 participants, as five participants chose not to provide venous blood samples. At T0, normal fasting glucose levels were measured, with no significant difference between the two groups (ED: 82±2 mg/dL; SD: 84±2 mg/dL) (p=
0.169). By T15, the mean blood glucose concentration was significantly higher (t[69]=2.643, p=0.005) in the ED group as compared to the SD group (136±3 mg/dL vs. 125±3 mg/dL, respectively). Thereafter, glucose concentrations decreased in both groups but were still significantly higher (t[69]=2.163, p=0.017) in the ED group (125±3 mg/dL) compared to the SD group (115±3 mg/dL) at T30. At T60 and T120, glucose concentrations were lower in the ED as compared to the SD group, although differences were not significant (p=0.242 and p=0.061, respectively; Fig. 2a). Significantly higher mean insulin concentration was measured (t[64]=2.794, p=0.001) in the ED compared to the SD group at T45 (310.7±30.9 pmol/L vs. 205.0±21.8 pmol/L, respectively; Fig. 2b).
Among heavy ED consumers, significantly higher levels of blood glucose were measured at both T15 (
t[
26]=1.799,
p=0.042) and T30 (
t[
26]=2.002,
p=0.028) among those administered ED as compared to their SD counterparts (Fig. 3a). Blood glucose levels of frequent ED consumers were significantly higher at T15 (
t[
25]=1.810,
p=0.041) in the ED group compared to the parallel SD group (Fig. 3b). In contrast, no influence of beverage type on blood glucose levels was noted throughout the entire experiment among infrequent ED consumers (Fig. 3c). Heavy ED consumers who consumed ED as part of the intervention expressed significantly higher levels of insulin when compared to their fellow SD consumers (Fig. 3d:
t[
26]=3.289,
p=0.001; 342.7±53.1 pmol/L vs. 152.2±23.1 pmol/L, respectively). In contrast, there were no differences in serum insulin levels between frequent consumers in the ED vs. SD group (
p=0.447; Fig. 3e). However, similar trends to heavy ED consumers were noted among infrequent consumers (Fig. 3f:
t[
11]=1.904,
p=0.042; 351.5±75.2 pmol/L vs. 212.1±36.4 pmol/L, respectively).
Moderate and significant correlations between the amount of caffeine consumed (mg/kg) and blood glucose concentration at T15 and T30 were observed (
Table 3; r
s[66]=0.37,
p=0.002 and r
s[66]=0.31,
p=0.010, respectively). Similarly, a parallel relationship was noted between caffeine amount (mg/kg) and insulin concentration at T45 (
Table 3; r
s[61]=0.41,
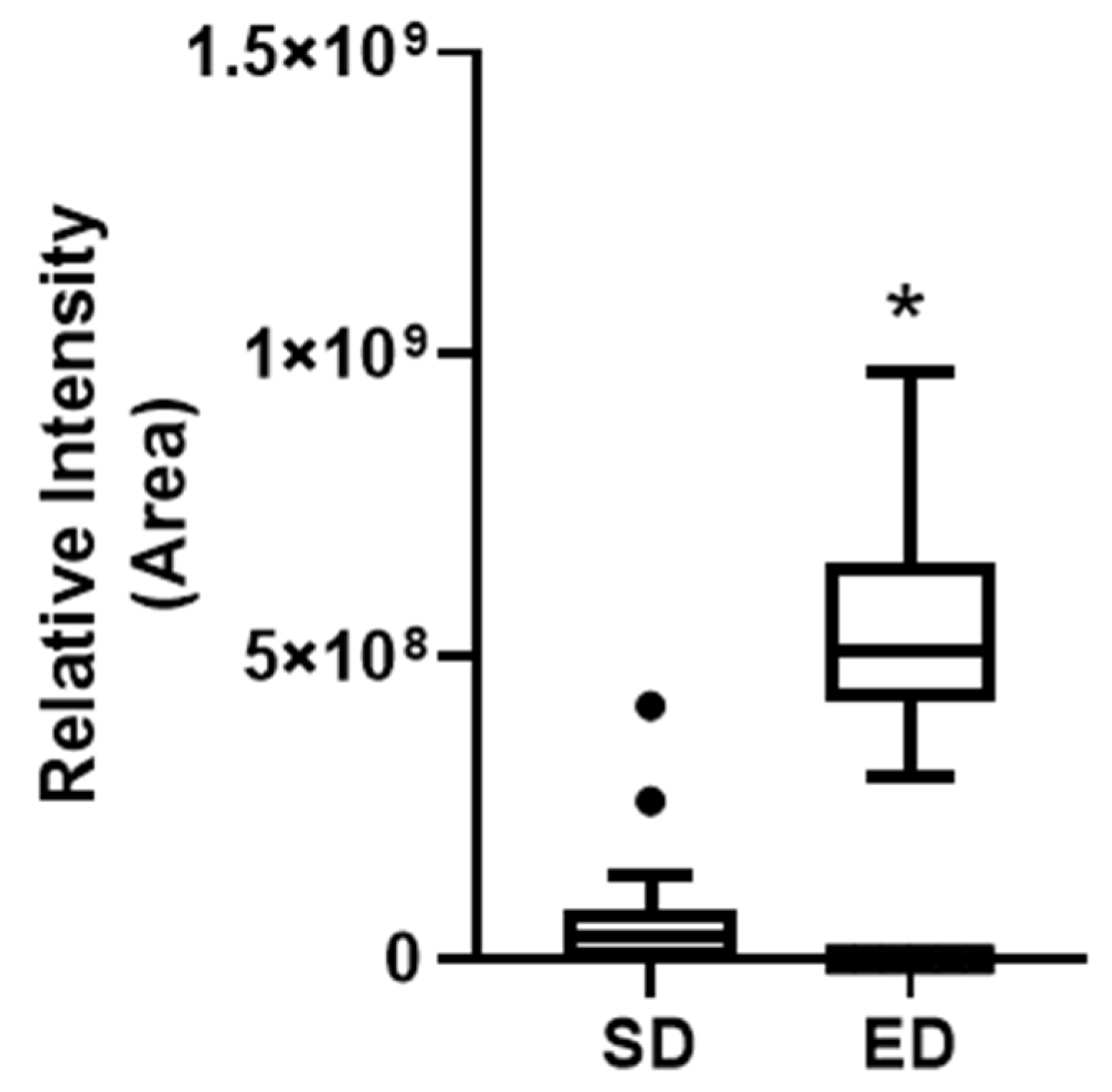
p=0.001). Notably, at T45, a significant disparity in serum caffeine levels emerged between the ED and SD groups (Fig.4, and
Table 4), suggesting a peak in caffeine levels coinciding with the assessment of insulin levels. Such findings reinforce the correlations presented in
Table 3.
Table 3.
Correlation between the amount of caffeine consumed (mg/kg) and.blood glucose and serum insulin levels.
Table 3.
Correlation between the amount of caffeine consumed (mg/kg) and.blood glucose and serum insulin levels.
|
|
rs |
df |
p |
| Glucose |
T0 |
-0.02 |
66 |
0.906 |
| T15 |
0.37 |
66 |
0.002** |
| T30 |
0.31 |
66 |
0.010* |
| T60 |
-0.08 |
66 |
0.503 |
| T120 |
-0.19 |
66 |
0.122 |
| Insulin |
T45 |
0.41 |
61 |
0.001** |
Figure 4.
Indicate caffeine relative intensity as represented by the "Area" values between energy drink (ED) and soft drink (SD) groups after using untargeted metabolomics. Given the non-normal distribution of the data, the Mann-Whitney U test was used to compare the two groups. Results are presented as the median with Tukey’s whiskers. N=33 in each group. *p=9.03X10-7.
Figure 4.
Indicate caffeine relative intensity as represented by the "Area" values between energy drink (ED) and soft drink (SD) groups after using untargeted metabolomics. Given the non-normal distribution of the data, the Mann-Whitney U test was used to compare the two groups. Results are presented as the median with Tukey’s whiskers. N=33 in each group. *p=9.03X10-7.
Table 4.
Caffeine relative intensity as represented by the "Area" values between energy drink and soft drink groups.
Table 4.
Caffeine relative intensity as represented by the "Area" values between energy drink and soft drink groups.
| |
25th Percentile |
Median |
75th Percentile |
Mann-Whitney U p-value |
n |
| ED |
4.31E+08 |
5.10E+08 |
6.33E+08 |
9.03E-07 |
33 |
| SD |
1.16E+06 |
3.55E+07 |
8.03E+07 |
33 |
4. Discussion
Past studies have recorded a spectrum of adverse outcomes associated with energy drink (ED) consumption, encompassing conditions such as hypertension, liver damage, renal failure, confusion, seizures, depression, psychotic states, anxiety, restlessness, hyperglycemia, diabetes, and obesity [
23]. However, there is a notable lack of studies examining the physiological impact of ED on adolescents, particularly within the context of glycemic control. The present study provides novel insights into this area, particularly emphasizing the potential mediating role of ED consumption frequency.
The current study provided evidence of the harmful effects of ED consumption on glucose levels at both T15 and T30 post-consumption. These findings were further substantiated by concurrent alteration in insulin levels. A single can of ED containing 80 mg caffeine elicited significant alteration in glucose and insulin levels. These patterns were particularly evident in heavy ED consumers. Furthermore, our study revealed a moderate yet noteworthy direct correlation between caffeine amount (mg/kg) and glucose and insulin levels. To date, no other studies have explored the influence of ED on glycemic control in adolescents. One study found impaired glucoregulation, characterized by hyperinsulinemia, in healthy adolescents after consuming energy shots [
24]. Yet, it must be emphasized that EDs and energy shots are distinctly different. EDs are typically available as pre-packaged and ready-to-consume beverages [
25,
26]. Conversely, energy shots are distributed in smaller volumes and classified and advertised as dietary supplements [
27]. Additionally, energy shots often include a higher concentration of caffeine in comparison to EDs [
28]. The present results also agreed with Beaudoin et al.'s findings that caffeine ingestion impairs insulin sensitivity dose-dependently in healthy men and women [
13].
Infrequent and heavy ED consumers who consumed ED displayed significantly higher insulin levels compared to those who consumed SD. However, this trend was not evident among frequent ED consumers. Furthermore, no significant differences in blood glucose concentrations were observed following ED versus SD consumption by infrequent ED consumers. This suggests that occasional ED consumption in adolescents who are not habitual consumers may result in immediate impairment of insulin sensitivity, leading to enhanced insulin release. In contrast, besides the apparent elevation of insulin concentrations among heavy consumers in the ED group compared to the SD group, significantly higher blood glucose concentrations were observed at T15 and T30 in the ED group, highlighting a substantial and persistent impact on insulin sensitivity. These findings were supported by the metabolomics profiling of caffeine, which measured significantly higher levels in the ED group than in the SD group, underscoring caffeine's potential role in compromising insulin sensitivity [
29,
30]. These findings align with the results reported by Olateju et al., who demonstrated a more pronounced increase in glucose levels with caffeine-enhanced carbohydrate EDs compared to caffeine-free equivalent, indicating a possible impairment in insulin action [
31]. Taken together, regular consumption of EDs by adolescents, particularly among heavy consumers, may result in prolonged impairment of insulin sensitivity, ultimately leading to elevated blood glucose levels.
4.1. Strengths and Limitations
One of the strengths of this study is that, to the best of our knowledge, it is the first to address the acute glycemic and insulin effects of EDs in healthy adolescents. Most previous research has focused on healthy adults or adults with diabetes. Moreover, the study’s specific focus on Israeli-Arab adolescents highlights a group that has not received much attention in prior research. Another strength of this study was its examination of the interplay between acute ED consumption and ED consumption frequency. Moreover, the study included 71 adolescents, a considerably larger population than several prior studies in ED intervention research. Additionally, the study utilized one of the two most available and purchased brands of EDs in the Northern Israel region, both of which contain similar caffeine and ingredient composition. This choice suggests the generalizability of the presented results. The study was limited by homogeneity since it exclusively monitored adolescents from the Arab community living in Northern Israel, which may restrict the generalizability of the findings. The study was neither blinded nor randomized, as per the request of the Helsinki committee, to avoid administering ED to adolescents who had never previously consumed them. While this approach may have introduced potential bias, statistical analysis was conducted by a third party with only access to the participant’s serial numbers. Future studies that include more diverse populations will enhance our understanding of how ED consumption affects physiological parameters among adolescents.
5. Conclusions
This study addressed the relatively understudied topic of ED consumption and its physiological effects, particularly on a critical aspect of glycemic control. Infrequent and heavy ED consumers experienced marked changes in insulin levels, while frequent consumers displayed changes solely in glucose levels. The results underscore the importance of considering ED consumption frequency, as it proved to be a significant mediating factor in consumer responses. The presented findings fill a significant gap in the research landscape, particularly concerning Israeli-Arab adolescents, and will support the development of health and educational interventions for this age group. They may also promote regulations limiting the sale of ED to children and adolescents, thereby potentially improving the health of this age group. Long-term follow-up studies will be needed to further understand the long-term effects of ED consumption on adolescents.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: LN, BM, NE, Methodology: LN, GN, BM, OH, MB, EN, HH, and FM, Formal analysis and investigation: LN, GN and RS, Resources: LN and OH, Supervision: LN, BM and OH Writing - original draft preparation: LN and GN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines laid forth in the Declaration of Helsinki, and all procedures involving human participants were approved by the Ethics Committee at Saint Vincent de Paul Hospital (04-2020-svh; date of approval- February 2020) and registered at ClinicalTrials.gov (NCT04808128). .
Informed Consent Statement
The adolescents provided their consent to participate in the study, and written informed consent was obtained from their parents or legal guardians.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to containing information that could compromise the privacy of research participants, but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Saint Vincent de Paul Hospital staff, parents and adolescents whose support and collaboration made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marinoni M, Parpinel M, Gasparini A, et al. Psychological and socio-educational correlates of energy drink consumption in children and adolescents: a systematic review. Eur J Pediatr 2022; 181: 889–901. [CrossRef]
- Shah SA, Chu BW, Lacey CS, et al. Impact of Acute Energy Drink Consumption on Blood Pressure Parameters: A Meta-analysis. Ann Pharmacother 2016; 50: 808–815. [CrossRef]
- Almulla AA, Faris MA-IE. Energy drinks consumption is associated with reduced sleep duration and increased energy-dense fast foods consumption among school students: A cross-sectional study. Asia Pac J Public Health 2020; 32: 266–273. [CrossRef]
- Brunborg GS, Raninen J, Burdzovic Andreas J. Energy drinks and alcohol use among adolescents: A longitudinal study. Drug Alcohol Depend 2022; 241: 109666. [CrossRef]
- Arria AM, Caldeira KM, Kasperski SJ, et al. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. J Addict Med 2010; 4: 74–80. [CrossRef]
- Silva-Maldonado P, Arias-Rico J, Romero-Palencia A, et al. Consumption Patterns of Energy Drinks in Adolescents and Their Effects on Behavior and Mental Health: A Systematic Review. J Psychosoc Nurs Ment Health Serv 2022; 60: 41–47. [CrossRef]
- The Israeli Ministry of Health. The Israeli Ministry of Health- Energy drinks Arab children 2011 survey. English. https://www.health.gov.il/English/Pages/HomePage.aspx (2011, accessed 3 September 2021).
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks--a growing problem. Drug Alcohol Depend 2009; 99: 1–10. [CrossRef]
- Kaur S, Christian H, Cooper MN, et al. Consumption of energy drinks is associated with depression, anxiety, and stress in young adult males: Evidence from a longitudinal cohort study. Depress Anxiety 2020; 37: 1089–1098. [CrossRef]
- Graham TE, Sathasivam P, Rowland M, et al. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can J Physiol Pharmacol 2001; 79: 559–65.
- Greer F, Hudson R, Ross R, et al. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes 2001; 50: 2349–2354. [CrossRef]
- Robertson TM, Clifford MN, Penson S, et al. A single serving of caffeinated coffee impairs postprandial glucose metabolism in overweight men. Br J Nutr 2015; 114: 1218–25. [CrossRef]
- Beaudoin M-S, Allen B, Mazzetti G, et al. Caffeine ingestion impairs insulin sensitivity in a dose-dependent manner in both men and women. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 2013; 38: 140–147. [CrossRef]
- Keijzers GB, De Galan BE, Tack CJ, et al. Caffeine can decrease insulin sensitivity in humans. Diabetes Care 2002; 25: 364–9. [CrossRef]
- Soós R, Gyebrovszki Á, Tóth Á, et al. Effects of caffeine and caffeinated beverages in children, adolescents and young adults: Short review. Int J Environ Res Public Health 2021; 18: 12389. [CrossRef]
- González-Domínguez R, Mateos RM, Lechuga-Sancho AM, et al. Synergic effects of sugar and caffeine on insulin-mediated metabolomic alterations after an acute consumption of soft drinks. Electrophoresis 2017; 38: 2313–2322. [CrossRef]
- Seifert SM, Schaechter JL, Hershorin ER, et al. Health Effects of Energy Drinks on Children, Adolescents, and Young Adults. Pediatrics 2011; 127: 511–528. [CrossRef]
- Degirmenci N, Fossum IN, Strand TA, et al. Consumption of energy drinks among adolescents in Norway: a cross-sectional study. BMC Public Health 2018; 18: 1391. [CrossRef]
- Pinto Y, Frishman S, Turjeman S, et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 2023; 72: 918–928. [CrossRef]
- The Israeli Ministry of Health. Mabat_youth_2015-2016 - The Israeli Ministry of Health. Health surveys (Mabat). https://www.health.gov.il/publicationsfiles/mabat_kids2_11_2015-2016-eng.pdf (2017, accessed 4 September 2021).
- Clinic Tools. BMI calculator. https://my.pbrc.edu/Clinic/Tools/BMI/ (2021).
- Sharma AK, Metzger DL, Daymont C, et al. LMS tables for waist-circumference and waist-height ratio z-scores in children aged 5–19 y in NHANES III: Association with cardio-metabolic risks. Pediatr Res 2015; 78: 723–729. [CrossRef]
- Mansour B, Amarah W, Nasralla E, et al. Energy drinks in children and adolescents: demographic data and immediate effects. Eur J Pediatr 2019; 178: 649–656. [CrossRef]
- Shearer J, Reimer RA, Hittel DS, et al. Caffeine-containing energy shots cause acute impaired glucoregulation in adolescents. Nutrients 2020; 12: 3850. [CrossRef]
- Jagim AR, Harty PS, Tinsley GM, et al. International society of sports nutrition position stand: energy drinks and energy shots. J Int Soc Sports Nutr 2023; 20: 2171314. [CrossRef]
- Jagim AR, Harty PS, Barakat AR, et al. Prevalence and Amounts of Common Ingredients Found in Energy Drinks and Shots. Nutrients 2022; 14: 314. [CrossRef]
- Chatterjee A, Abraham J. A Comprehensive Study on Sports and Energy Drinks. In: Sports and Energy Drinks. Elsevier, pp. 515–537.
- Committee on Nutrition and the Council on Sports Medicine and Fitness. Sports drinks and energy drinks for children and adolescents: are they appropriate? Pediatrics 2011; 127: 1182–1189. [CrossRef]
- Shi X, Xue W, Liang S, et al. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: a systematic review and meta-analysis. Nutr J 2016; 15: 103. [CrossRef]
- Cherniack EP, Buslach N, Lee HF. The Potential Effects of Caffeinated Beverages on Insulin Sensitivity. J Am Coll Nutr 2018; 37: 161–167. [CrossRef]
- Olateju T, Begley J, Green DJ, et al. Physiological and glycemic responses following acute ingestion of a popular functional drink in patients with type 1 diabetes. Can J Diabetes 2015; 39: 78–82. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).