Submitted:
20 June 2024
Posted:
21 June 2024
You are already at the latest version
Abstract
Keywords:
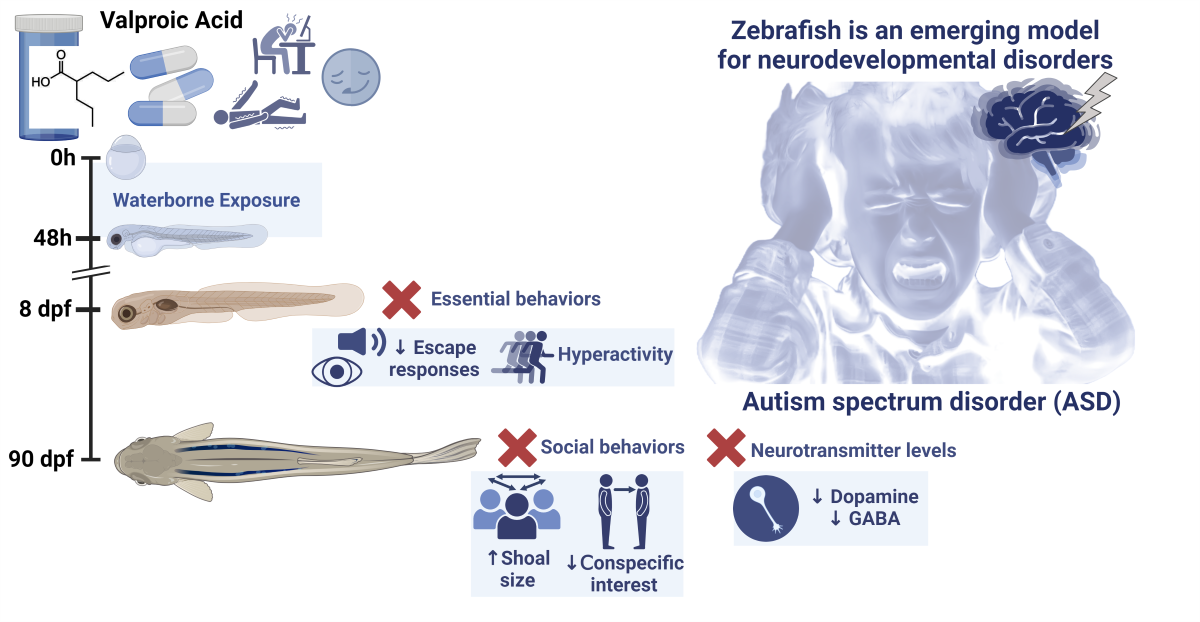
1. Introduction
2. Results
2.1. Systemic and Developmental Effects in Zebrafish larvae Exposed to VPA during Early Development
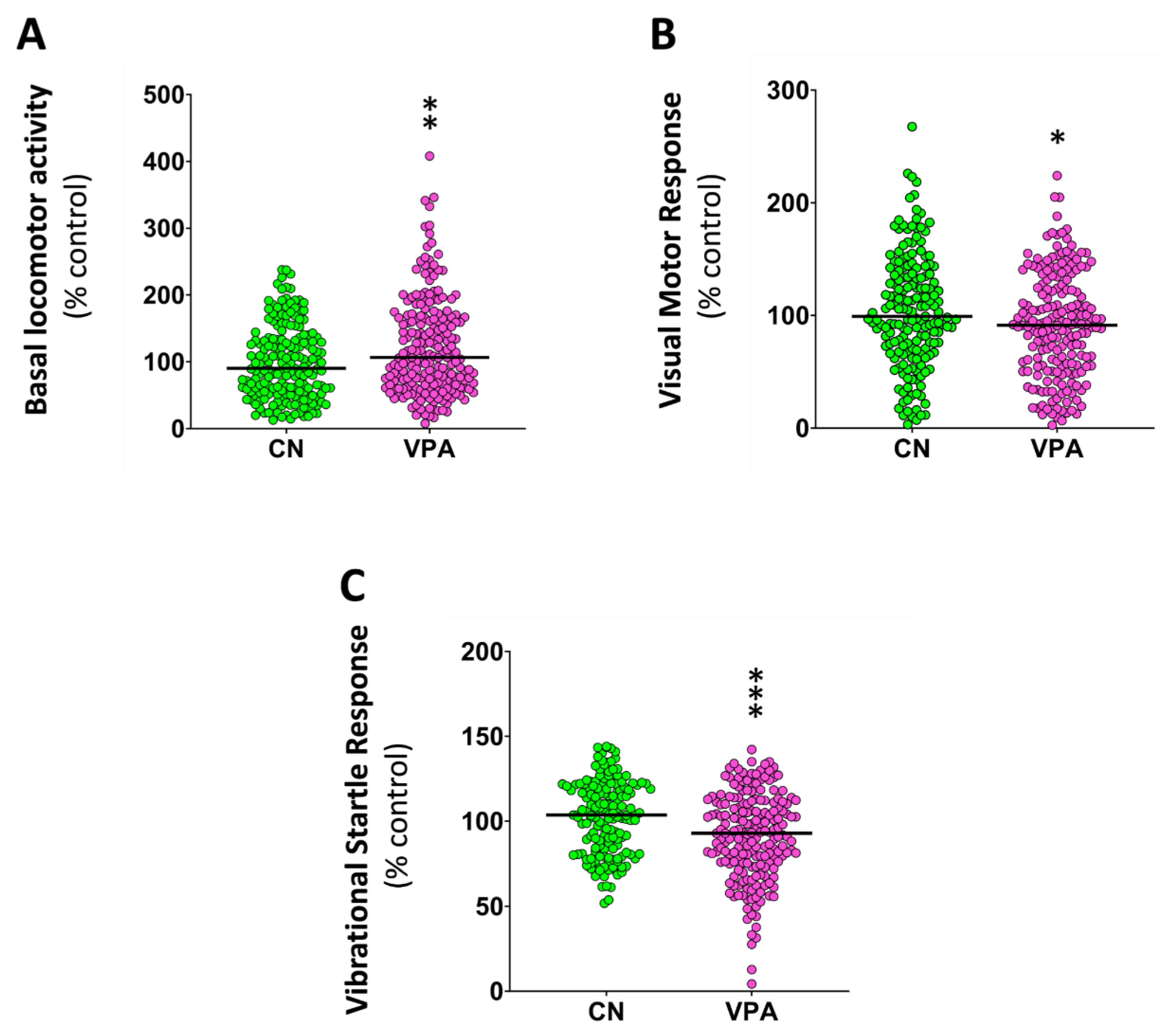
2.2. Neurobehavioral Effects in Zebrafish larvae Exposed to VPA during Early Development
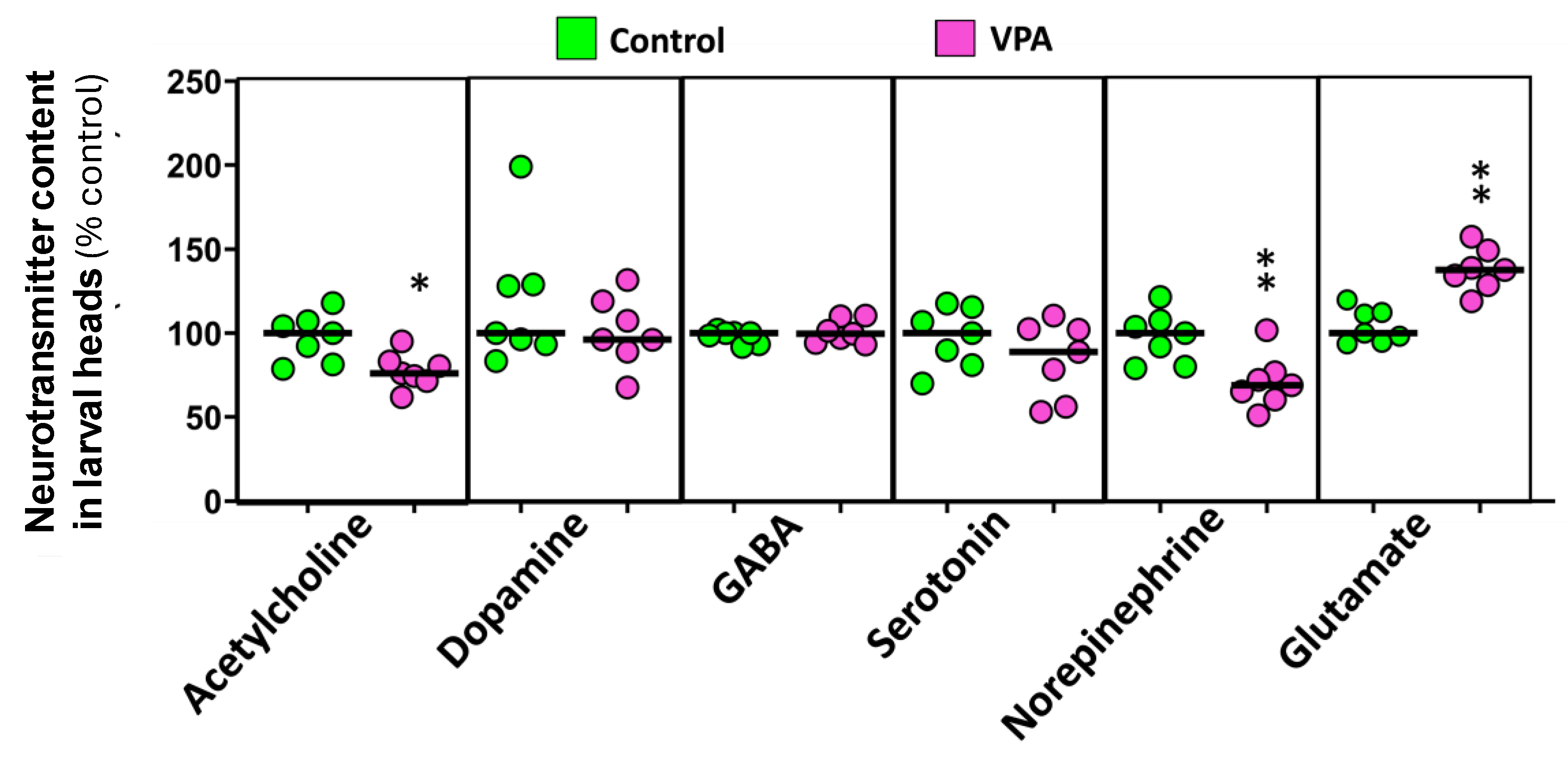
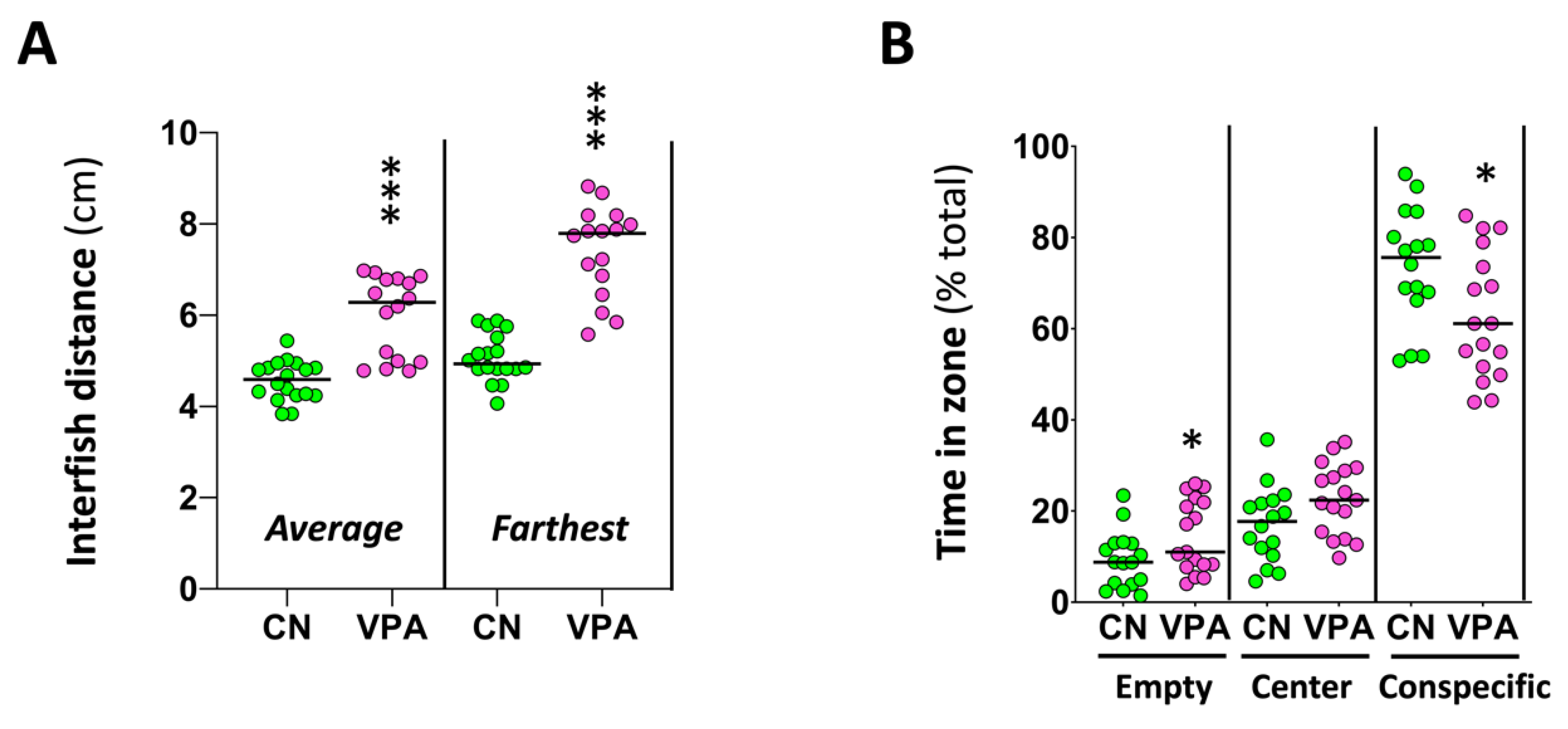
2.3. Neurobehavioral Effects in Adult Zebrafish Exposed to VPA during Early Development
3. Discussion
4. Materials and Methods
4.1. Zebrafish Housing and Husbandry
4.2. Experimental Procedure
4.3. Phenotypic Analysis
4.4. Neurobehavioral Assay in Larvae
4.5. Neurobehavioral Assays in Adults
4.6. Neurochemical Analysis by UHPLC-MS/MS
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Fernández, M.; Mollinedo-Gajate, I.; Peñagarikano, O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience 2018, 370, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Polšek, D.; Jagatic, T.; Cepanec, M.; Hof, P.R.; Šimić, G. RECENT DEVELOPMENTS IN NEUROPATHOLOGY OF AUTISM SPECTRUM DISORDERS. Transl. Neurosci. 2011, 2, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hettinger, J.A.; Liu, X.; Schwartz, C.E.; Michaelis, R.C.; Holden, J.J.A. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008, 147B, 628–636. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10. [Google Scholar] [CrossRef]
- Andres, C. Molecular genetics and animal models in autistic disorder. Brain Res. Bull. 2002, 57, 109–119. [Google Scholar] [CrossRef]
- Keil-Stietz, K.; Lein, P.J. Gene×environment interactions in autism spectrum disorders. Curr. Top. Dev. Biol. 2023, 152, 221–284. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; N. Kizlyk, M.; V. Kysil, E.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish models of autism spectrum disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef]
- Ranger, P.; Ellenbroek, B.A. Perinatal Influences of Valproate on Brain and Behaviour: An Animal Model for Autism. Curr. Top. Behav. Neurosci. 2016, 29, 363–386. [Google Scholar] [CrossRef]
- Roullet, F.I.; Crawley, J.N. Mouse models of autism: testing hypotheses about molecular mechanisms. Curr. Top. Behav. Neurosci. 2011, 7, 187–212. [Google Scholar] [CrossRef]
- Topál, J.; Román, V.; Turcsán, B. The dog (Canis familiaris) as a translational model of autism: It is high time we move from promise to reality. Wiley Interdiscip. Rev. Cogn. Sci. 2019, 10. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; De Paula Cognato, G.; Bonan, C.D. Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): A developmental behavior analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.H.; Cho, H.J.; Han, E.; Hong, T.I.; Ariyasiri, K.; Choi, J.H.; Hwang, K.S.; Jeong, Y.M.; Yang, S.Y.; Yu, K.; et al. Zebrafish knockout of Down syndrome gene, DYRK1A, shows social impairments relevant to autism. Mol. Autism 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Li, C.Y.; Hu, C.C.; Wang, Y.; Lin, J.; Jiang, Y.H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dufour-Rainfray, D.; Vourc’h, P.; Tourlet, S.; Guilloteau, D.; Chalon, S.; Andres, C.R. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci. Biobehav. Rev. 2011, 35, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lei, L.; Tian, L.; Hou, F.; Roper, C.; Ge, X.; Zhao, Y.; Chen, Y.; Dong, Q.; Tanguay, R.L.; et al. Developmental and behavioral alterations in zebrafish embryonically exposed to valproic acid (VPA): An aquatic model for autism. Neurotoxicol. Teratol. 2018, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval zebrafish model for studying the effects of valproic acid on neurodevelopment: An approach towards modeling autism. J. Pharmacol. Toxicol. Methods 2019, 95, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Turner, N.; Hu, B. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet. Genomics 2013, 23, 236–241. [Google Scholar] [CrossRef]
- Terbach, N.; Williams, R.S.B. Structure-function studies for the panacea, valproic acid. Biochem. Soc. Trans. 2009, 37, 1126–1132. [Google Scholar] [CrossRef]
- Bialer, M.; Yagen, B. Valproic acid: Second generation. Neurotherapeutics 2007, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; King, J.; Cunningham, M.; Stephan, M.; Kerr, B.; Hersh, J.H. Fetal valproate syndrome and autism: additional evidence of an association. Dev. Med. Child Neurol. 2001, 43, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Hamidpour, S.K.; Abedi, M.; Shojaei, H.; Tavirani, M.R.; Namazi, N.; Larijani, B.; Arjmand, B. Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective. Front. psychiatry 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, J.; Kim, K.C.; Shin, C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Marquart, G.D.; Horstick, E.J.; Tabor, K.M.; Pajevic, S.; Burgess, H.A. Morphometric analysis and neuroanatomical mapping of the zebrafish brain. Methods 2018, 150, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.P.; Zhou, F.; Sai, L.Y.; Chen, H.; Lin, S.L.; Schachner, M. Duloxetine ameliorates valproic acid-induced hyperactivity, anxiety-like behavior, and social interaction deficits in zebrafish. Autism Res. 2022, 15, 27–41. [Google Scholar] [CrossRef]
- Terbach, N.; Shah, R.; Kelemen, R.; Klein, P.S.; Gordienko, D.; Brown, N.A.; Wilkinson, C.J.; Williams, R.S.B. Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid using the simple biomedical model Dictyostelium. J. Cell Sci. 2011, 124, 2267–2276. [Google Scholar] [CrossRef]
- Zellner, D.; Padnos, B.; Hunter, D.L.; MacPhail, R.C.; Padilla, S. Rearing conditions differentially affect the locomotor behavior of larval zebrafish, but not their response to valproate-induced developmental neurotoxicity. Neurotoxicol. Teratol. 2011, 33, 674–679. [Google Scholar] [CrossRef]
- Bai, W.J.; Luo, X.G.; Jin, B.H.; Zhu, K.S.; Guo, W.Y.; Zhu, X.Q.; Qin, X.; Yang, Z.X.; Zhao, J.J.; Chen, S.R.; et al. Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice. Zool. Res. 2022, 43, 851. [Google Scholar] [CrossRef]
- Maltezos, S.; Horder, J.; Coghlan, S.; Skirrow, C.; O’Gorman, R.; Lavender, T.J.; Mendez, M.A.; Mehta, M.; Daly, E.; Xenitidis, K.; et al. Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Transl. Psychiatry 2014 43 2014, 4, e373–e373. [Google Scholar] [CrossRef]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Glennon, J.C.; Franke, B.; Buitelaar, J.K.; Faraone, S.; Asherson, P.; Banaschewski, T.; P Ebstein, R.; et al. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: association to overlapping traits in ADHD and autism. Transl. Psychiatry 2017 71 2017, 7, e999–e999. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, A.; Shi, M.Y.; Yan, Z. Disrupted Glutamatergic Transmission in Prefrontal Cortex Contributes to Behavioral Abnormality in an Animal Model of ADHD. Neuropsychopharmacol. 2017 4210 2017, 42, 2096–2104. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Karbhari, N.; Brooks, R.A.J.; De La Rocha, A.J.; Janardhan, S.; Levin, E.D. Persistent behavioral effects following early life exposure to retinoic acid or valproic acid in zebrafish. Neurotoxicology 2016, 52, 23–33. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, Vol. 23, Page 3861 2022, 23, 3861. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 2014, 121, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. Contributions of Matrix Metalloproteinases to Neural Plasticity, Habituation, Associative Learning and Drug Addiction. Neural Plast. 2009, 2009, 579382. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Bedrossiantz, J.; Prats, E.; Garcia, X.R.; Gómez-Canela, C.; Piña, B.; Raldúa, D. Deciphering the mode of action of pollutants impairing the fish larvae escape response with the vibrational startle response assay. Sci. Total Environ. 2019, 672, 121–128. [Google Scholar] [CrossRef]
- Mora-Zamorano, F.X.; Svoboda, K.R.; Carvan, M.J. The nicotine-evoked locomotor response: A behavioral paradigm for toxicity screening in zebrafish (Danio rerio) embryos and eleutheroembryos exposed to methylmercury. PLoS One 2016, 11, 1–15. [Google Scholar] [CrossRef]
- Kalueff, A. V. The rights and wrongs of zebrafish: Behavioral phenotyping of zebrafish; 2017; ISBN 9783319337746.
- Abbas, K.; Saputra, F.; Suryanto, M.E.; Lai, Y.H.; Huang, J.C.; Yu, W.H.; Chen, K.H.C.; Lin, Y.T.; Hsiao, C. Der Evaluation of effects of ractopamine on cardiovascular, respiratory, and locomotory physiology in animal model zebrafish larvae. Cells 2021, 10. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7. [Google Scholar] [CrossRef]
- McCutcheon, V.; Park, E.; Liu, E.; Wang, Y.; Wen, X.Y.; Baker, A.J. A Model of Excitotoxic Brain Injury in Larval Zebrafish: Potential Application for High-Throughput Drug Evaluation to Treat Traumatic Brain Injury. Zebrafish 2016, 13, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.; Freeman, J. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of Brain Microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.Y.; Liu, F.C. Pathophysiological Studies of Monoaminergic Neurotransmission Systems in Valproic Acid-Induced Model of Autism Spectrum Disorder. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Ali, E.H.A.; Elgoly, A.H.M. Combined prenatal and postnatal butyl paraben exposure produces autism-like symptoms in offspring: Comparison with valproic acid autistic model. Pharmacol. Biochem. Behav. 2013, 111, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pavǎl, D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Nguyen, M.; Roth, A.; Kyzar, E.J.; Poudel, M.K.; Wong, K.; Stewart, A.M.; Kalueff, A. V. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD). Neurochem. Int. 2014, 66, 15–26. [Google Scholar] [CrossRef]
- Schür, R.R.; Draisma, L.W.R.; Wijnen, J.P.; Boks, M.P.; Koevoets, M.G.J.C.; Joëls, M.; Klomp, D.W.; Kahn, R.S.; Vinkers, C.H. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of 1H-MRS studies. Hum. Brain Mapp. 2016, 37, 3337–3352. [Google Scholar] [CrossRef]
- Purkayastha, P.; Malapati, A.; Yogeeswari, P.; Sriram, D. A Review on GABA/Glutamate Pathway for Therapeutic Intervention of ASD and ADHD. Curr. Med. Chem. 2015, 22, 1850–1859. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Ricarte, M.; Prats, E.; Montemurro, N.; Bedrossiantz, J.; Bellot, M.; Gómez-Canela, C.; Raldúa, D. Environmental concentrations of tire rubber-derived 6PPD-quinone alter CNS function in zebrafish larvae. Sci. Total Environ. 2023, 896, 165240. [Google Scholar] [CrossRef] [PubMed]
- Bellot, M.; Manen, L.; Prats, E.; Bedrossiantz, J.; Barata, C.; Gómez-Canela, C.; Antolin, A.A.; Raldúa, D. Short-term exposure to environmental levels of nicotine and cotinine impairs visual motor response in zebrafish larvae through a similar mode of action: Exploring the potential role of zebrafish α7 nAChR. Sci. Total Environ. 2024, 912, 169301. [Google Scholar] [CrossRef] [PubMed]
- Bedrossiantz, J.; Prats, E.; Raldúa, D. Neurotoxicity Assessment in Adult Danio rerio using a Battery of Behavioral Tests in a Single Tank. J. Vis. Exp. 2023, 2023. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Tornero-Cañadas, D.; Prats, E.; Piña, B.; Tauler, R.; Raldúa, D. Comprehensive characterization of neurochemicals in three zebrafish chemical models of human acute organophosphorus poisoning using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 1735–1748. [Google Scholar] [CrossRef]
- Prats, E.; Gómez-Canela, C.; Ben-Lulu, S.; Ziv, T.; Padrós, F.; Tornero, D.; Garcia-Reyero, N.; Tauler, R.; Admon, A.; Raldúa, D. Modelling acrylamide acute neurotoxicity in zebrafish larvae. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




