Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Clinical Parameters
2.3. DNA Extraction and 18S rRNA Gene Sequencing
2.4. Bioinformatics Analysis
2.5. Statistic Analysis
3. Results
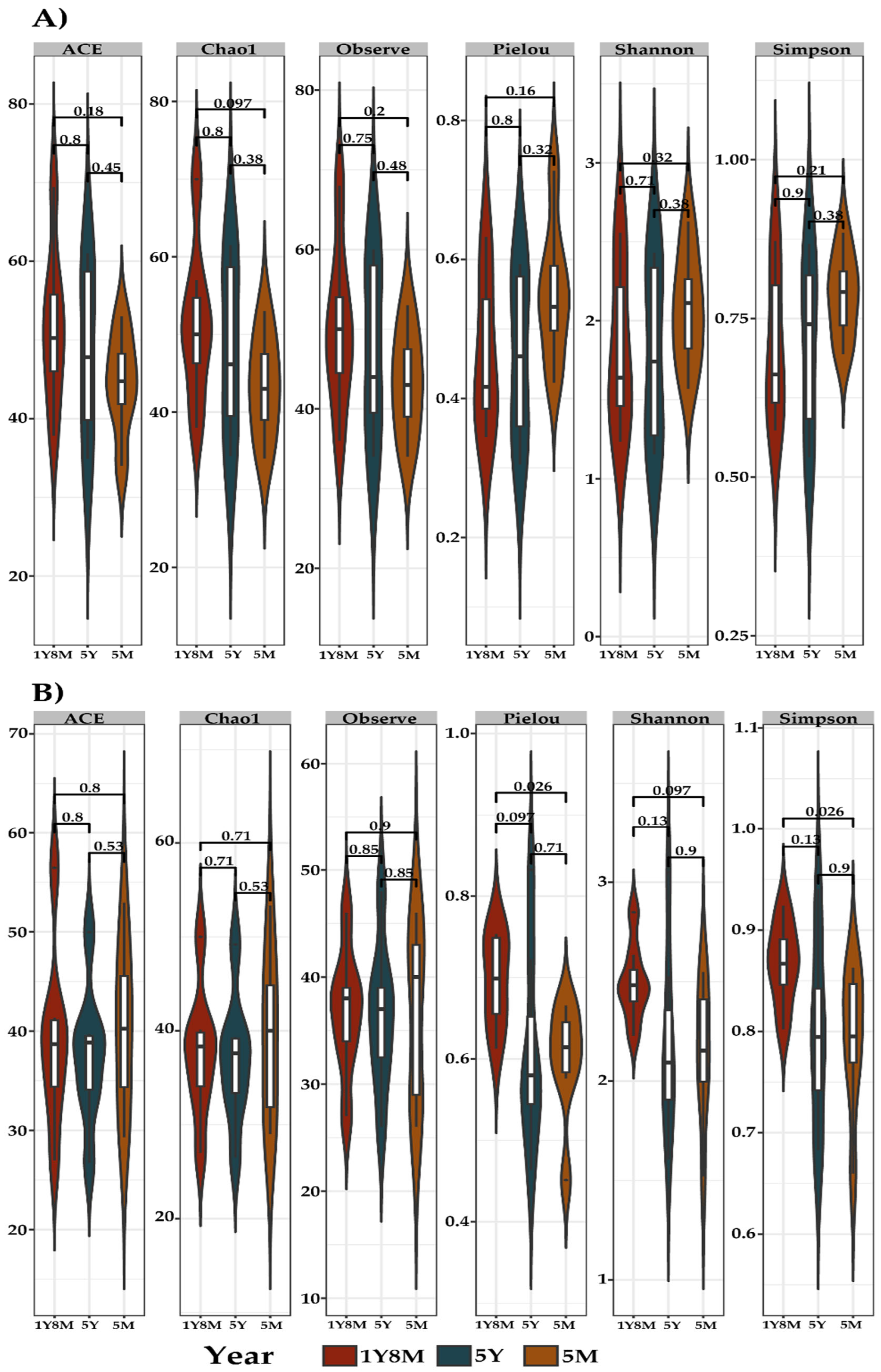
3.1. Impact of Age on the Diversity and Composition of Fungal and Protist Communities in the Bovine Microbiome
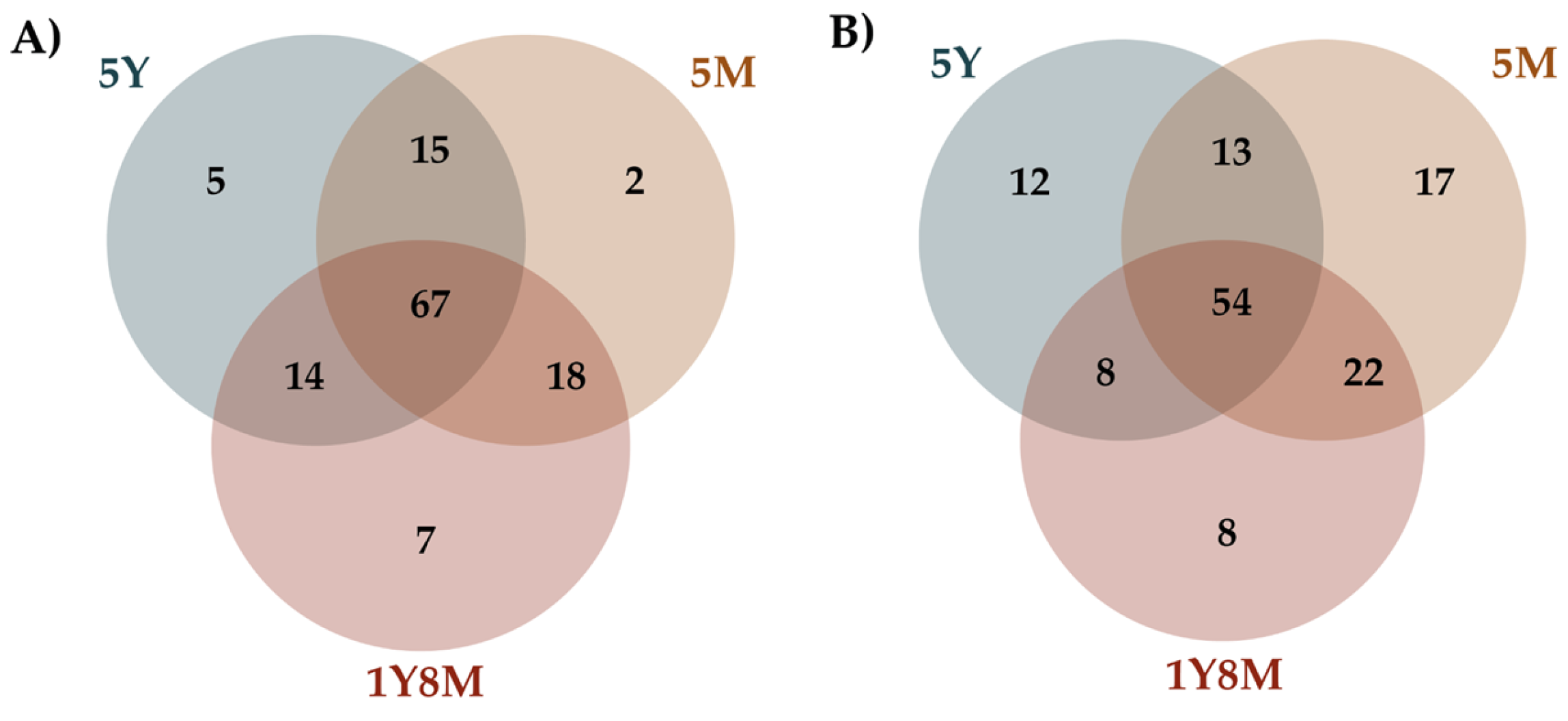
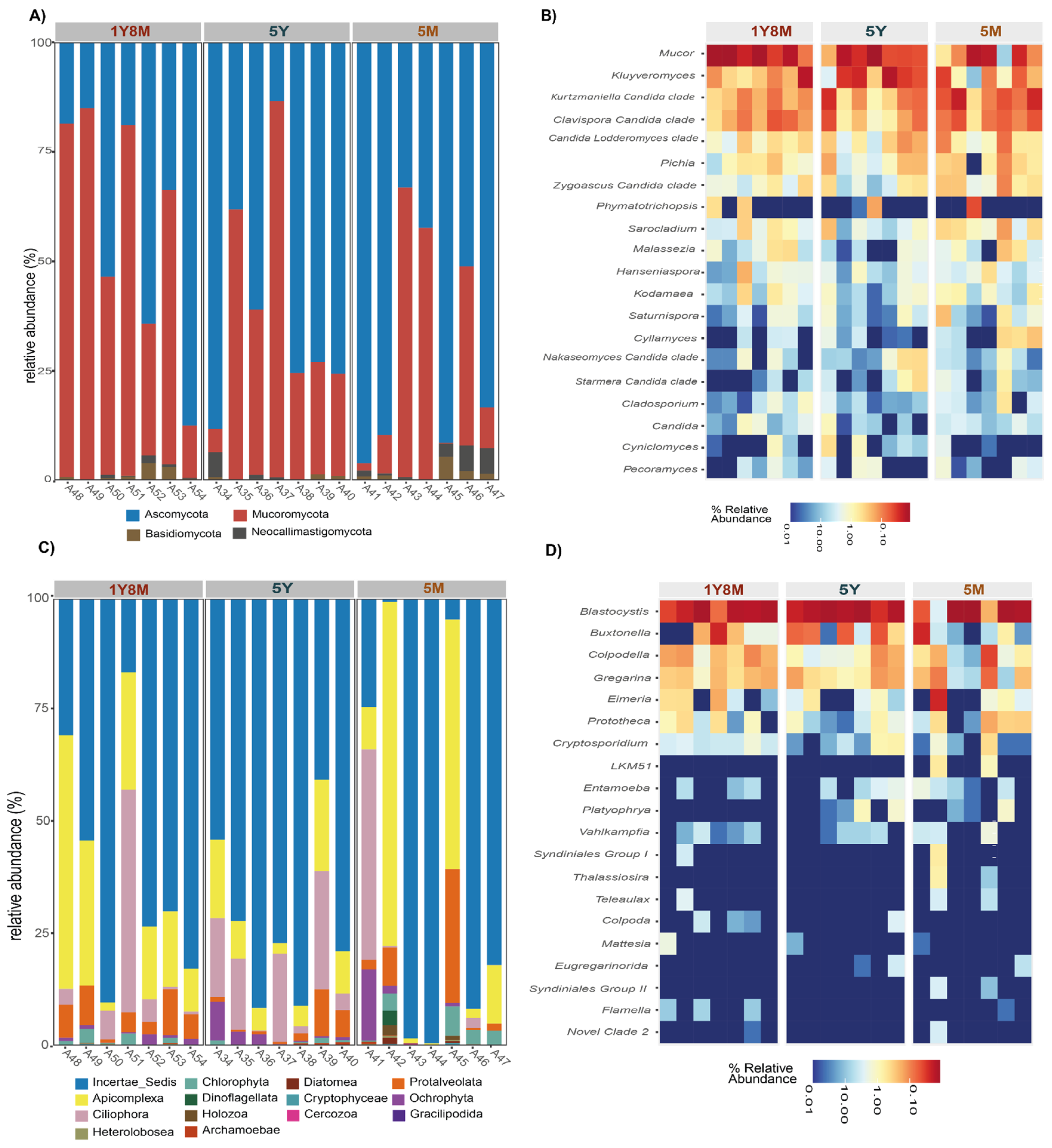
3.2. Taxonomic Composition of the Fungal and Protist Communities in the Gut Microbiota
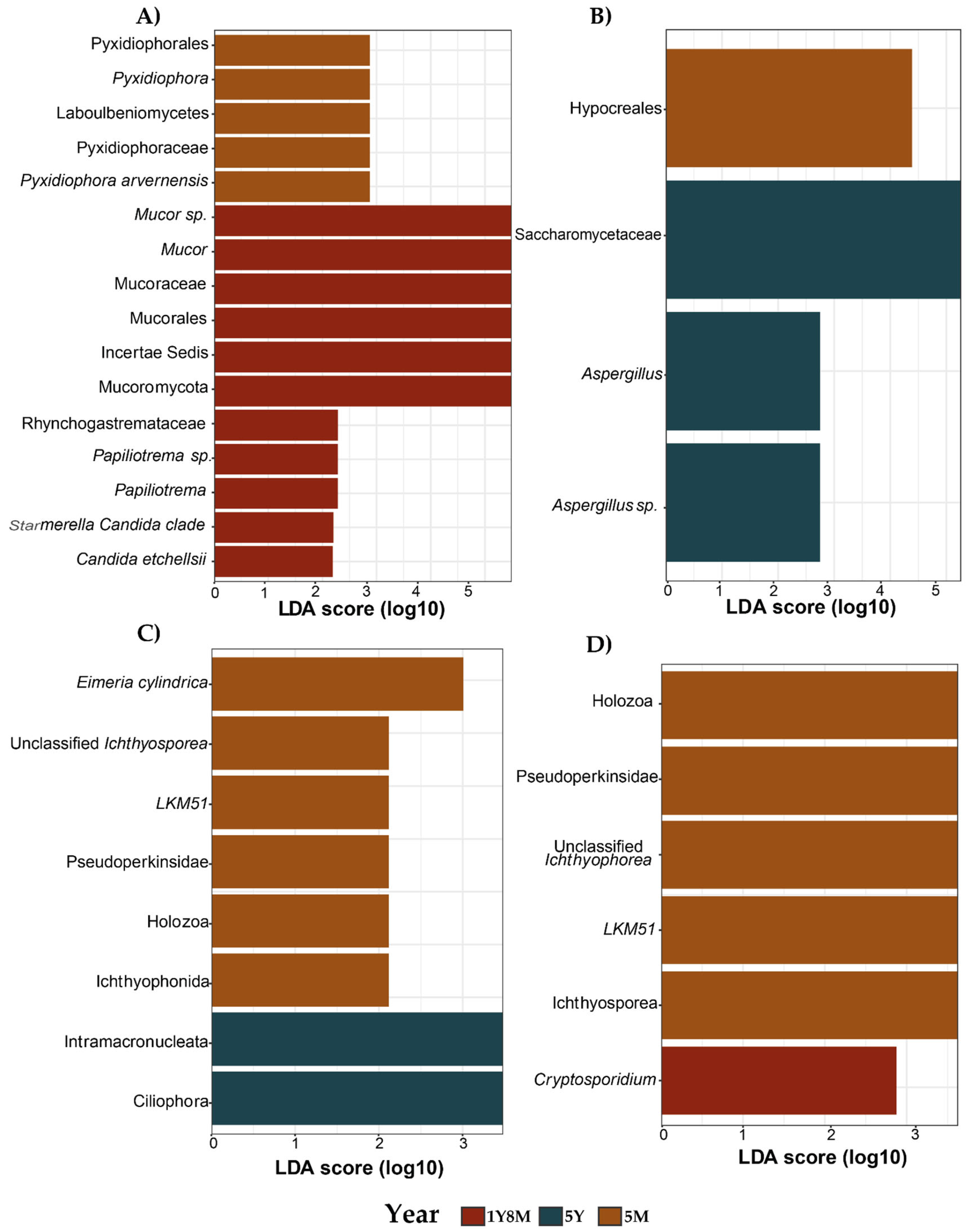
3.3. Biomarkers Identified Based on Age in Fungal and Protist
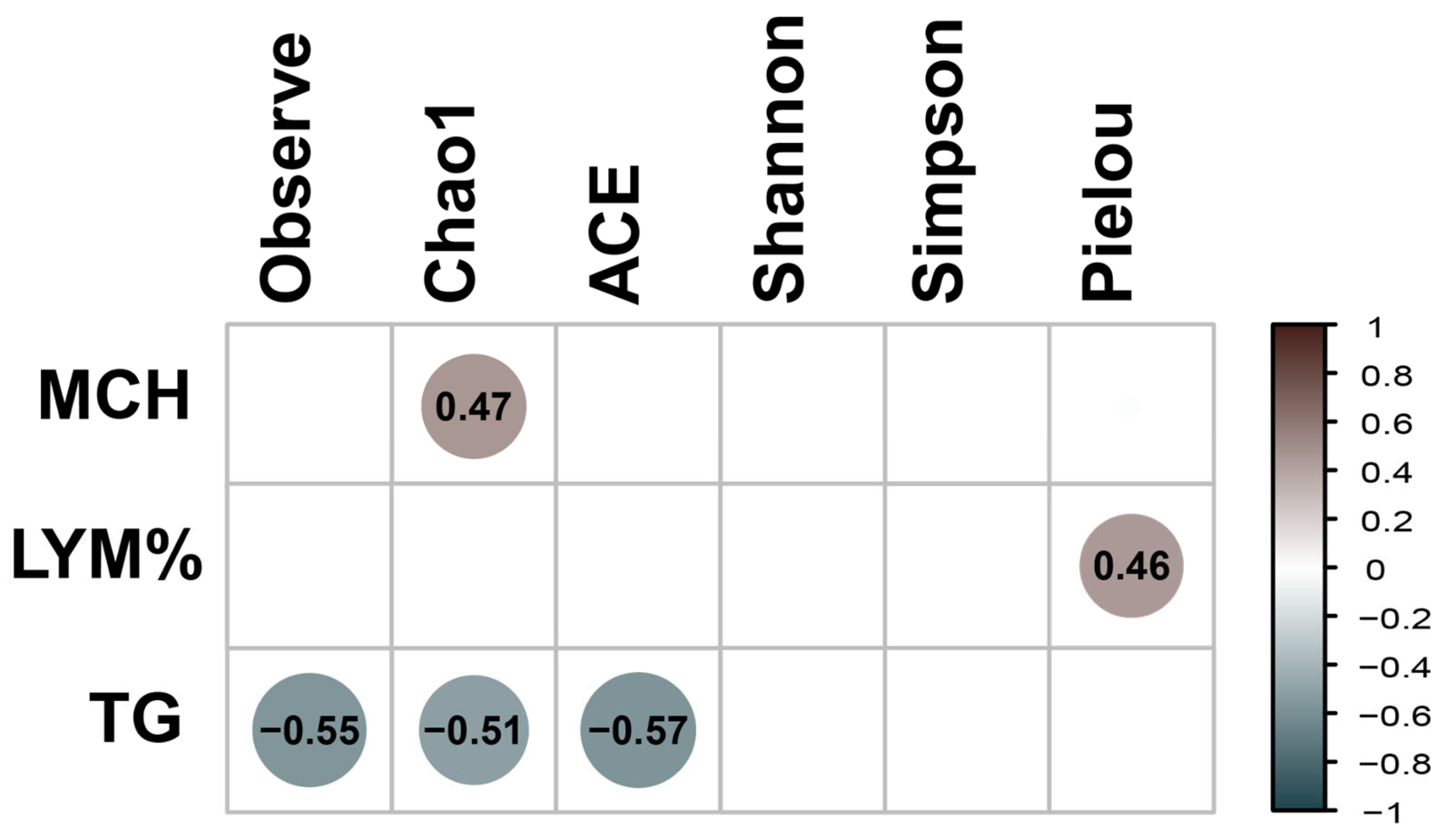
3.4. Correlation of Alpha and Beta Diversity of Fungi with Clinical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanjorjo, R.A.; Tseten, T.; Kang, M.-K.; Kwon, M.; Kim, S.-W. In Pursuit of Understanding the Rumen Microbiome. Fermentation 2023, 9, 114. [Google Scholar] [CrossRef]
- Pérez-Barbería, F.J. The Ruminant: Life History and Digestive Physiology of a Symbiotic Animal. In Sustainable and Environmentally Friendly Dairy Farms; García-Yuste, S., Ed.; Springer International Publishing: Cham, 2020; pp. 19–45. ISBN 978-3-030-46060-0. [Google Scholar]
- Negash, A. Gut Microbiota Ecology Role in Animal Nutrition and Health Performance. J. Clin. Microbiol. Antimicrob. 2022, 6, 1–14. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal Microbiota–Host Interaction and Its Effect on Nutrient Metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Noel, S.J.; Olijhoek, D.W.; Mclean, F.; Løvendahl, P.; Lund, P.; Højberg, O. Rumen and Fecal Microbial Community Structure of Holstein and Jersey Dairy Cows as Affected by Breed, Diet, and Residual Feed Intake. Animals 2019, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lyu, W.; Hong, Q.; Zhang, X.; Yang, H.; Xiao, Y. Gut Microbiota Influence Lipid Metabolism of Skeletal Muscle in Pigs. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ji, S.; Duan, C.; Tian, P.; Ju, S.; Yan, H.; Zhang, Y.; Liu, Y. Age-Related Changes in the Ruminal Microbiota and Their Relationship With Rumen Fermentation in Lambs. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhou, M.; Ma, T.; Bi, S.; Wang, W.; Zhang, Y.; Huang, X.; Guan, L.L.; Long, R. Survey of Rumen Microbiota of Domestic Grazing Yak during Different Growth Stages Revealed Novel Maturation Patterns of Four Key Microbial Groups and Their Dynamic Interactions. Anim. Microbiome 2020, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, Y.; Hu, M.; Hou, J.; Yang, L.; Wang, X.; Du, W.; Liu, J.; Xu, Q. Colonization and Development of the Gut Microbiome in Calves. J. Anim. Sci. Biotechnol. 2023, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen Microbial Community Composition Varies with Diet and Host, but a Core Microbiome Is Found across a Wide Geographical Range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Kyawt, Y.Y.; Aung, M.; Xu, Y.; Sun, Z.; Zhou, Y.; Zhu, W.; Padmakumar, V.; Tan, Z.; Cheng, Y. Dynamic Changes of Rumen Microbiota and Serum Metabolome Revealed Increases in Meat Quality and Growth Performances of Sheep Fed Bio-Fermented Rice Straw. J. Anim. Sci. Biotechnol. 2024, 15, 34. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane Mitigation in Ruminants: From Microbe to the Farm Scale. animal 2010, 4, 351–365. [Google Scholar] [CrossRef]
- Lv, Q.-B.; Meng, J.-X.; Ma, H.; Liu, R.; Qin, Y.; Qin, Y.-F.; Geng, H.-L.; Ni, H.-B.; Zhang, X.-X. Description of Gut Mycobiota Composition and Diversity of Caprinae Animals. Microbiol. Spectr. 2023, 11, e0242422. [Google Scholar] [CrossRef]
- Chen, X.; An, M.; Zhang, W.; Li, K.; Kulyar, M.F.-E.-A.; Duan, K.; Zhou, H.; Wu, Y.; Wan, X.; Li, J.; et al. Integrated Bacteria-Fungi Diversity Analysis Reveals the Gut Microbial Changes in Buffalo With Mastitis. Front. Vet. Sci. 2022, 9, 918541. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Herrera, G.; Muñoz, M.; Vega, L.; Cruz-Saavedra, L.; García-Corredor, D.; Pulido-Medellín, M.; Bulla-Castañeda, D.M.; Giraldo, J.C.; Bernal, M.C.; et al. Describing the Intestinal Microbiota of Holstein Fasciola-Positive and -Negative Cattle from a Hyperendemic Area of Fascioliasis in Central Colombia. PLoS Negl. Trop. Dis. 2021, 15, e0009658. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Yang, M.; Wang, L.; Li, Z.; Yang, L.; Li, G.; Wen, T. Gut Fungal Community Composition Analysis of Myostatin Mutant Cattle Prepared by CRISPR/Cas9. Front. Vet. Sci. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2023, 12, 34. [Google Scholar] [CrossRef]
- Varada, V.V.; Kumar, S.; Chhotaray, S.; Tyagi, A.K. Host-Specific Probiotics Feeding Influence Growth, Gut Microbiota, and Fecal Biomarkers in Buffalo Calves. AMB Express 2022, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Romanzin, A.; Degano, L.; Vicario, D.; Spanghero, M. Feeding Efficiency and Behavior of Young Simmental Bulls Selected for High Growth Capacity: Comparison of Bulls with High vs. Low Residual Feed Intake. Livest. Sci. 2021, 249, 104525. [Google Scholar] [CrossRef]
- Doyle, J.L.; Berry, D.P.; Veerkamp, R.F.; Carthy, T.R.; Evans, R.D.; Walsh, S.W.; Purfield, D.C. Genomic Regions Associated with Muscularity in Beef Cattle Differ in Five Contrasting Cattle Breeds. Genet. Sel. Evol. 2020, 52, 2. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Khan, S.; Amjadi, M.; Abdelnour, S.A.; Ohran, H.; Alanazi, K.M.; Abd El-Hack, M.E.; Taha, A.E.; Khan, R.; Gong, C.; et al. Genome-Wide Association Studies Reveal Novel Loci Associated with Carcass and Body Measures in Beef Cattle. Arch. Biochem. Biophys. 2020, 694, 108543. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Da Borso, F.; Rossi, A.; Taverna, M.; Bovolenta, S.; Piasentier, E.; Corazzin, M. Environmental Sustainability Assessment of Dairy Farms Rearing the Italian Simmental Dual-Purpose Breed. Animals 2020, 10, 296. [Google Scholar] [CrossRef]
- Falta, D.; Zapletalová, L.; Hanuš, O.; Kučera, J.; Večeřa, M.; Chládek, G.; Filipčík, R.; Kopec, T.; Lategan, F.S. The Interaction between the Milk Production, Milk Components with a Low Frequency of Analysis and Factors Affecting the Milk Composition in Dual-Purpose Simmental Cows. 2023.
- Vaulot, D.; Geisen, S.; Mahé, F.; Bass, D. Pr2-Primers: An 18S rRNA Primer Database for Protists. Mol. Ecol. Resour. 2022, 22, 168–179. [Google Scholar] [CrossRef]
- Mishra, P.; Tulsani, N.J.; Jakhesara, S.J.; Dafale, N.A.; Patil, N.V.; Purohit, H.J.; Koringa, P.G.; Joshi, C.G. Exploring the Eukaryotic Diversity in Rumen of Indian Camel (Camelus Dromedarius) Using 18S rRNA Amplicon Sequencing. Arch. Microbiol. 2020, 202, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Bukin, Y.S.; Mikhailov, I.S.; Petrova, D.P.; Galachyants, Y.P.; Zakharova, Y.R.; Likhoshway, Y.V. The Effect of Metabarcoding 18S rRNA Region Choice on Diversity of Microeukaryotes Including Phytoplankton. World J. Microbiol. Biotechnol. 2023, 39, 229. [Google Scholar] [CrossRef]
- Velásquez, A.R.; Burca, C.; Vargas, L. Effects of Salinity on Three Mandarin Cultivars Grafted on Two Different Rootstocks. Peruvian J. Agron. 2022, 6, 114–122. [Google Scholar] [CrossRef]
- Latimer, K.S. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology; John Wiley & Sons, 2011; ISBN 978-0-8138-2014-9.
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PloS One 2013, 8, e61217. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Core team, R. R A Language and Environment for Statistical Computing. Stat. Comput. 2020. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd, 2017; pp. 1–15 ISBN 978-1-118-44511-2.
- Jiang, H.; Chen, W.; Su, L.; Huang, M.; Lin, L.; Su, Q.; Li, G.; Ahmad, H.I.; Li, L.; Zhang, X.; et al. Impact of Host Intraspecies Genetic Variation, Diet, and Age on Bacterial and Fungal Intestinal Microbiota in Tigers. MicrobiologyOpen 2020, 9, e1050. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yang, Y.; Qin, S.; Lv, S.; Jin, T.; Li, K.; Han, Z.; Li, Y. Microbiome Analysis Reveals Gut Microbiota Alteration of Early-Weaned Yimeng Black Goats with the Effect of Milk Replacer and Age. Microb. Cell Factories 2021, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.; Fu, Y.; Zhong, H.; Gou, W.; Jiang, Z.; Liang, Y.; Miao, Z.; Xu, J.-J.; Huynh, T.; Wahlqvist, M.L.; et al. Mapping the Human Gut Mycobiome in Middle-Aged and Elderly Adults: Multiomics Insights and Implications for Host Metabolic Health. Gut 2022, 71, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Al Bataineh, M.T.; Alzaatreh, A.; Hajjo, R.; Banimfreg, B.H.; Dash, N.R. Compositional Changes in Human Gut Microbiota Reveal a Putative Role of Intestinal Mycobiota in Metabolic and Biological Decline during Aging. Nutr. Healthy Aging 2021, 6, 269–283. [Google Scholar] [CrossRef]
- Sun, B.; Gu, Z.; Wang, X.; Huffman, M.A.; Garber, P.A.; Sheeran, L.K.; Zhang, D.; Zhu, Y.; Xia, D.-P.; Li, J. Season, Age, and Sex Affect the Fecal Mycobiota of Free-Ranging Tibetan Macaques (Macaca Thibetana). Am. J. Primatol. 2018, 80, e22880. [Google Scholar] [CrossRef] [PubMed]
- Kapitan, M.; Niemiec, M.J.; Steimle, A.; Frick, J.S.; Jacobsen, I.D. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. In Fungal Physiology and Immunopathogenesis; Rodrigues, M.L., Ed.; Springer International Publishing: Cham, 2019; pp. 265–301. ISBN 978-3-030-30237-5. [Google Scholar]
- Bożena, D.-K.; Iwona, D.; Ilona, K. The Mycobiome – a Friendly Cross-Talk between Fungal Colonizers and Their Host. Ann. Parasitol. 2016, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Leonardi, I. Fungal Dysbiosis: Immunity and Interactions at Mucosal Barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The Gut Mycobiota: Insights into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Weis, S.; Meisner, A.; Schwiertz, A.; Unger, M.M.; Becker, A.; Faßbender, K.; Schnell, S.; Schäfer, K.-H.; Egert, M. Association between Parkinson’s Disease and the Faecal Eukaryotic Microbiota. Npj Park. Dis. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- Parfrey, L.W.; Walters, W.A.; Lauber, C.L.; Clemente, J.C.; Berg-Lyons, D.; Teiling, C.; Kodira, C.; Mohiuddin, M.; Brunelle, J.; Driscoll, M.; et al. Communities of Microbial Eukaryotes in the Mammalian Gut within the Context of Environmental Eukaryotic Diversity. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Sanchez, D.; Zapata, C.; Romero, Y.; Flores-Huarco, N.H.; Oros, O.; Alvarado, W.; Quilcate, C.; Guevara-Alvarado, H.M.; Estrada, R.; Coila, P. Parasitism-Induced Changes in Microbial Eukaryotes of Peruvian Alpaca Gastrointestinal Tract. Life 2024, 14, 187. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; He, Y.; Kulyar, M.F.-A.; Iqbal, M.; Li, K.; Liu, J. Longitudinal Characterization of the Gut Bacterial and Fungal Communities in Yaks. J. Fungi 2021, 7, 559. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Guo, M.; Liu, G.; Yang, Y.; Wang, X.; Chen, Y.; Zhang, E. Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization. Animals 2019, 9, 1028. [Google Scholar] [CrossRef]
- Geng, X.; Liu, Y.; Xu, W.; Li, G.; Xue, B.; Feng, Y.; Tang, S.; Wei, W.; Yuan, H. Eukaryotes May Play an Important Ecological Role in the Gut Microbiome of Graves’ Disease. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef]
- Mann, A.E.; Mazel, F.; Lemay, M.A.; Morien, E.; Billy, V.; Kowalewski, M.; Di Fiore, A.; Link, A.; Goldberg, T.L.; Tecot, S.; et al. Biodiversity of Protists and Nematodes in the Wild Nonhuman Primate Gut. ISME J. 2020, 14, 609–622. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, K.; Yang, M.; Li, X.; Chen, Y.; Li, J.; Xu, H.; Dhakal, P.; Zhang, L. Metagenomic Analysis Reveals the Relationship between Intestinal Protozoan Parasites and the Intestinal Microecological Balance in Calves. Parasit. Vectors 2023, 16, 257. [Google Scholar] [CrossRef]
- Song, J.-K.; Yin, Y.-L.; Yuan, Y.-J.; Tang, H.; Ren, G.-J.; Zhang, H.-J.; Li, Z.-X.; Zhang, Y.-M.; Zhao, G.-H. First Genotyping of Blastocystis Sp. in Dairy, Meat, and Cashmere Goats in Northwestern China. Acta Trop. 2017, 176, 277–282. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, Y.; Wang, J.; Lai, P.; Li, Y.; Song, J.; Qi, M.; Zhao, G. Molecular Characterization of Blastocystis Sp. in Camelus Bactrianus in Northwestern China. Animals 2021, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogórek, K.; Korycińska, J. Blastocystis: How Do Specific Diets and Human Gut Microbiota Affect Its Development and Pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity Changes the Human Gut Mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Borges, F.M.; de Paula, T.O.; Sarmiento, M.R.A.; de Oliveira, M.G.; Pereira, M.L.M.; Toledo, I.V.; Nascimento, T.C.; Ferreira-Machado, A.B.; Silva, V.L.; Diniz, C.G. Fungal Diversity of Human Gut Microbiota Among Eutrophic, Overweight, and Obese Individuals Based on Aerobic Culture-Dependent Approach. Curr. Microbiol. 2018, 75, 726–735. [Google Scholar] [CrossRef]
- Alonso, R.; Pisa, D.; Fernández-Fernández, A.M.; Carrasco, L. Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhang, W.; Kulyar, M.F.-A.; Ullah, K.; Han, Z.; Qin, J.; Bi, C.; Wang, Y.; Li, K. Comparative Analysis of Gut Microbiota in Healthy and Diarrheic Yaks. Microb. Cell Factories 2022, 21, 111. [Google Scholar] [CrossRef]
- Rajamanikam, A.; Isa, M.N.M.; Samudi, C.; Devaraj, S.; Govind, S.K. Gut Bacteria Influence Blastocystis Sp. Phenotypes and May Trigger Pathogenicity. PLoS Negl. Trop. Dis. 2023, 17, e0011170. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New Insights into the Interactions between Blastocystis, the Gut Microbiota, and Host Immunity. PLOS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Arowolo, F.; Pierre, J.F.; Blaser, M.; Shanmuganayagam, D. Longitudinal Effects of Dietary Oxidized Lipids on the Gut Microbiome and Mycobiome in Pigs. FASEB J. 2020, 34, 1–1. [Google Scholar] [CrossRef]
- Summers, K.L.; Arfken, A.M. The Gut Mycobiome and Animal Health. In Gut Microbiota, Immunity, and Health in Production Animals; Kogut, M.H., Zhang, G., Eds.; Springer International Publishing: Cham, 2022; pp. 85–125. ISBN 978-3-030-90303-9. [Google Scholar]
- Keomoungkhoun, B.; Arjentinia, I.P.G.Y.; Sangmaneedet, S.; Taweenan, W. First Report on the Molecular Prevalence and Associated Risk Factors of Eimeria Spp. in Dairy Cattle in Khon Kaen, Thailand. Vet. World 2023, 16, 1489–1495. [Google Scholar] [CrossRef]
- Lucas, A.S.; Swecker, W.S.; Lindsay, D.S.; Scaglia, G.; Neel, J.P.S.; Elvinger, F.C.; Zajac, A.M. A Study of the Level and Dynamics of Eimeria Populations in Naturally Infected, Grazing Beef Cattle at Various Stages of Production in the Mid-Atlantic USA. Vet. Parasitol. 2014, 202, 201–206. [Google Scholar] [CrossRef]
- Hastutiek, P.; Lastuti, N.D.R.; Suwanti, L.T.; Sunarso, A.; Kurniawati, D.A.; Yudhana, A. Occurrence and Biodiversity of Eimeria Spp. (Apicomplexa: Eimeriidae) in Madura Cattle Reared on Kamal Subdistrict, Madura Island, Indonesia. Vet. World 2022, 15, 2084–2088. [Google Scholar] [CrossRef]
- Aboelsoued, D.; Abdel Megeed, K.N. Diagnosis and Control of Cryptosporidiosis in Farm Animals. J. Parasit. Dis. 2022, 46, 1133–1146. [Google Scholar] [CrossRef]
- Papanikolopoulou, V.; Baroudi, D.; Guo, Y.; Wang, Y.; Papadopoulos, E.; Lafi, S.Q.; Abd El-Tawab, M.M.; Diakou, A.; Giadinis, N.D.; Feng, Y.; et al. Genotypes and Subtypes of Cryptosporidium Spp. in Diarrheic Lambs and Goat Kids in Northern Greece. Parasitol. Int. 2018, 67, 472–475. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Pane, S.; Putignani, L. Cryptosporidium: Still Open Scenarios. Pathogens 2022, 11, 515. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Karanis, P. An Overview of Methods/Techniques for the Detection of Cryptosporidium in Food Samples. Parasitol. Res. 2018, 117, 629–653. [Google Scholar] [CrossRef]
- Ryan, U.; Hijjawi, N.; Xiao, L. Foodborne Cryptosporidiosis. Int. J. Parasitol. 2018, 48, 1–12. [Google Scholar] [CrossRef]
- Chacón, M.R.; Lozano-Bartolomé, J.; Portero-Otín, M.; Rodríguez, M.M.; Xifra, G.; Puig, J.; Blasco, G.; Ricart, W.; Chaves, F.J.; Fernández-Real, J.M. The Gut Mycobiome Composition Is Linked to Carotid Atherosclerosis. Benef. Microbes 2018, 9, 185–198. [Google Scholar] [CrossRef]
- Ahmad, H.F.; Castro Mejia, J.L.; Krych, L.; Khakimov, B.; Kot, W.; Bechshøft, R.L.; Reitelseder, S.; Højfeldt, G.W.; Engelsen, S.B.; Holm, L.; et al. Gut Mycobiome Dysbiosis Is Linked to Hypertriglyceridemia Among Home Dwelling Elderly Dane 2020.
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The Mycobiota of the Human Body: A Spark Can Start a Prairie Fire. Gut Microbes 2020, 11, 655–679. [Google Scholar] [CrossRef]
- Lv, L.; Gu, S.; Jiang, H.; Yan, R.; Chen, Y.; Chen, Y.; Luo, R.; Huang, C.; Lu, H.; Zheng, B.; et al. Gut Mycobiota Alterations in Patients with COVID-19 and H1N1 Infections and Their Associations with Clinical Features. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Delavy, M.; Sertour, N.; Patin, E.; Le Chatelier, E.; Cole, N.; Dubois, F.; Xie, Z.; Saint-André, V.; Manichanh, C.; Walker, A.W.; et al. Unveiling Candida Albicans Intestinal Carriage in Healthy Volunteers: The Role of Micro- and Mycobiota, Diet, Host Genetics and Immune Response. Gut Microbes 2023, 15, 2287618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhan, H.; Xu, W.; Yan, S.; Ng, S.C. The Role of Gut Mycobiome in Health and Diseases. Ther. Adv. Gastroenterol. 2021, 14, 17562848211047130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
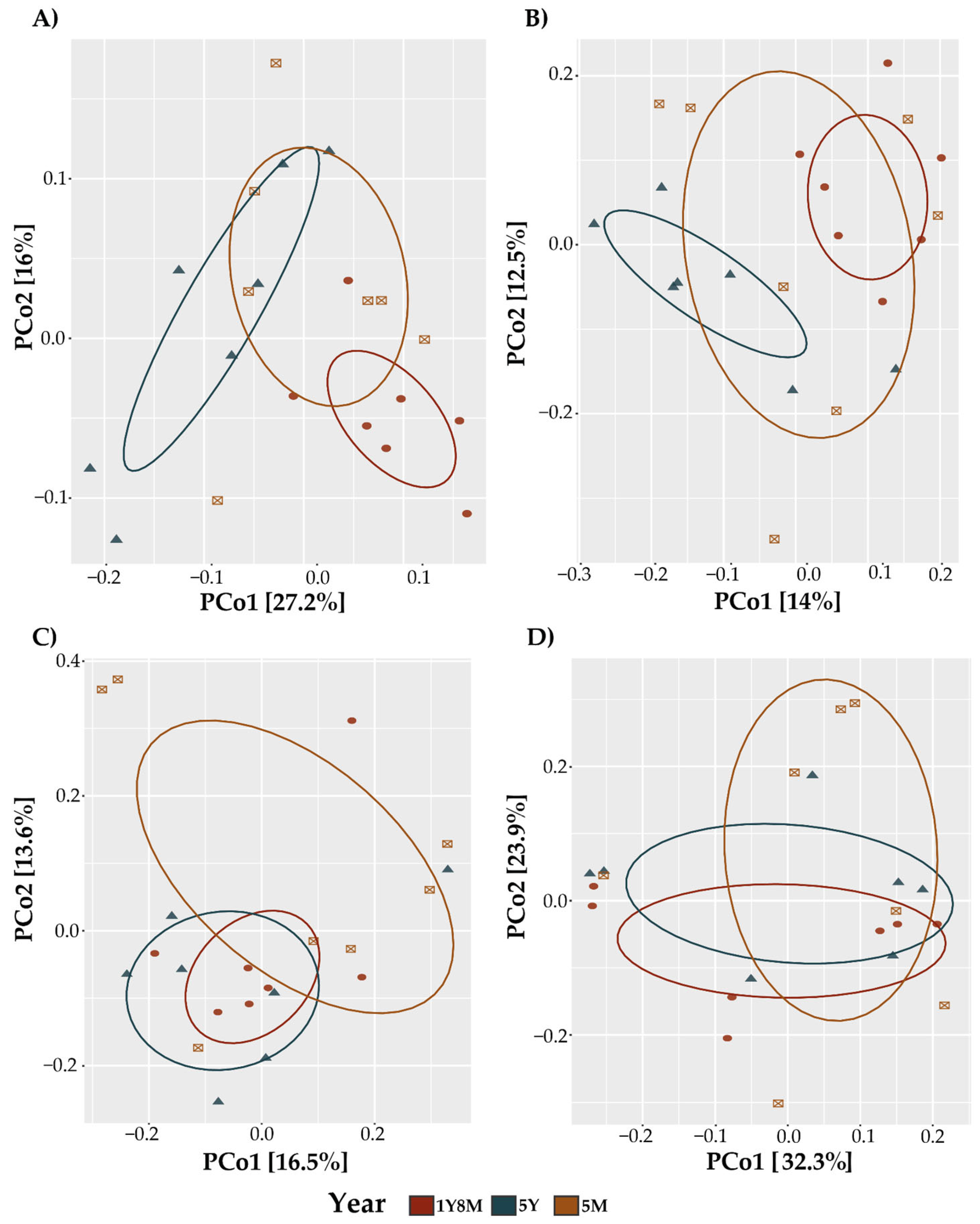
| Items | Df | SumOfSqs | R2 | F | Pr (>F) |
|---|---|---|---|---|---|
| Jaccard | |||||
| Year | 2 | 0.397308 | 0.130516 | 1.472821 | 0.015* |
| Sex | 1 | 0.197202 | 0.064781 | 1.462057 | 0.055 |
| Year:Sex | 2 | 0.426412 | 0.140076 | 1.580706 | 0.007** |
| Residual | 15 | 2.023202 | 0.664625 | ||
| Total | 20 | 3.044126 | 1 | ||
| Unweighted unifrac | |||||
| Year | 2 | 0.16684 | 0.20852 | 2.7976 | 0.002** |
| Sex | 1 | 0.04198 | 0.05247 | 1.4079 | 0.168 |
| Year:Sex | 2 | 0.14402 | 0.18 | 2.4149 | 0.002** |
| Residual | 15 | 0.44728 | 0.55901 | ||
| Total | 20 | 0.80012 | 1 | ||
| Jaccard | Unweighted Unifrac | |||||||
| Mantel test | Partial Mantel test | Mantel test | Partial Mantel test | |||||
| Variables | r | p | r | p | r | p | r | p |
| MCV | 0.196815555 | 0.03* | 0.209922522 | 0.014* | 0.137675537 | 0.07 | 0.104071883 | 0.124 |
| MCH | 0.177721795 | 0.022* | 0.192394859 | 0.01* | 0.149206478 | 0.045* | 0.114890751 | 0.082 |
| LYM% | 0.192477483 | 0.064 | 0.194205056 | 0.068 | 0.195319744 | 0.048* | 0.178444189 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





