2. Materials and Methods
New methods and protocols should be described in detail while well-established methods can be briefly described and approved Combustion with solid oxygen carriers (CLC) is the most suitable technology for CO2 capture. The main function of these systems is to separate the CO2 produced into a gas stream with a high CO2 concentration.
There is a variety of referenced literature, with the difference of TSO preparation methods, among them are: mechanical mixing [
3], coprecipitation [
4,
5], mediated synthesis of surfactant [
6,
7], granulation by freezing [
8,
9,
10] and impregnation [
11,
12]. The TSO particles used in these methods have a porous structure with a large surface area, facilitating the diffusion of reaction gases to metal oxide. But, any study in the preparation of large-scale TSO is novel to science [
10].
Given the high cost of producing synthetic TSOs, low-cost OTs, such as ilmenite, manganese, and iron ores, are sought. Like iron, manganese metal oxides are becoming increasingly important because they are cheap and non-toxic and have higher transport capacity compared to iron.
The highest oxidation state of Mn is MnO2, which decomposes at 500°C. However, at temperatures above 800 °C, only Mn3O4 is a stable material. For this reason, only the conversion between Mn3O4 and MnO is considered for its application in CLC. Mn2O3 can be used as an alternative to the CLOU process.
Recently, a large number of studies have been carried out on materials formed by the mixture of manganese oxides, which have shown good suitability to be used in the process [
13,
14,
15]; However, in this type of material, the decoupling of oxygen is highly conditioned by the conditions required for its previous oxidation. In addition, these solids have always been characterized by their low mechanical resistance, which has not allowed more than 4 hours of combustion with gases in a unit in continuous compound stirring. Evidently, this aspect has been an important conditioning factor in obtaining a manganese-based conveyor, not only for the coal removal process but for the process in general [
3]. Therefore, in this research, an oxygen transporter based on this oxide was developed that had improved characteristics that allowed to increase the combustion efficiency using thermogravimetry. For this, it was necessary to propose and develop the selection of the most suitable material for the prepared solid, based on the positive variables (oxygen transport capacity, mechanical resistance, and reaction speed) that have been reported with excellent results, as indicated in Equation (1).
Identifying solid compounds that have the ability to transfer oxygen in the CLC system is essential. Therefore, a compound must have a high conversion of fuel to CO
2 and H
2O. Jerndal et al., 2006 presented an extensive thermodynamic analysis of several systems considered CLC. It recorded oxides of Cu, Ni, Co, Fe, and Mn with favorable thermodynamics to react with CH
4, H
2, and CO, with equilibrium constant at the temperatures and pressures required in CLC [
16,
17].
2.1. Preparation of Materials
From the project “Combustion with CO2 capture by means of solid oxygen transporters (Chemical Looping Combustion)” with the aim of finding potential low-cost TSOs in the country, 16 natural minerals from different regions were characterized by X-ray fluorescence analysis (XRF), including OXMN009, from these data there is a resistance to rupture 5.7 and they were tested in a TGA using CH4, CO andH2 as fuel. To achieve the required particle size, the mill with blade attachment was used and the ground sample was passed through a sieve no.70 (Tyler series), which guaranteed a diameter between 100 and 300 μm.
The screening and sufficiency process of the size of the manganese-based oxygen transporter was carried out; then calcination was performed on the 0XMN009 solid conveyor, to increase its mechanical strength and obtain a modified manganese 0XMN009P; in the incipient impregnation method for Cu processing of the selected sample, adding a 5 M solution volume of copper nitrate trihydrate (Cu(NO3)2.3H2O) corresponding to the pore volume of the material, which allowed contact between the ore and the impregnant, then the copper nitrate was decomposed into copper oxide, the sample was calcined at a temperature of 550 °C and finally a second calcination was performed at 850 °C to stabilize the sample.
Available materials (original and modified solid oxygen carriers) were characterized by X-ray fluorescence (XRF) to quantify the concentration of manganese-based materials, followed by thermogravimetric analysis. SEM (Scanning Electron Microscopy) characterization and X-ray diffraction (XRD) were performed to identify the crystalline phases present in oxygen-carrying particles.
For TSO’s XRF analysis, a PANalytical AXIOS max sequential wavelength dispersive X-ray fluorescence spectrometer (WDXRF) was used equipped with a rhodium tube with a maximum power of 4.0 KW. It uses a primary hybrid monochromator that produces a parallel beam, ideal for studying grazing samples (thin layers) to quantify elements present in the sample; useful for reading diffraction pattern information obtained with XRD.
For the XRD analysis of the TSOs, Panalytical Empyrean equipment with a cobalt X-ray source was used to perform a sweep between the 20° to 100° angles of the 2θθ range to identify the crystalline phases of the materials.
For the SEM analysis, a JEOL-JSM 6490 LV Scanning Electron Microscope was used, which uses electrons instead of light to form the image, to achieve this the device has a filament that generates a beam of electrons to illuminate the sample, and if the sample is not conductive, it must be covered with a thin layer of gold. The images produced by this method can be used to determine the morphology of TSO.
EDS analysis was used for elemental identification by chemical mapping using the same electron microscopy described above, which was useful in identifying Cu deposited in the immersion-treated TSO.
2.2. Thermogravimetric Balance Analysis
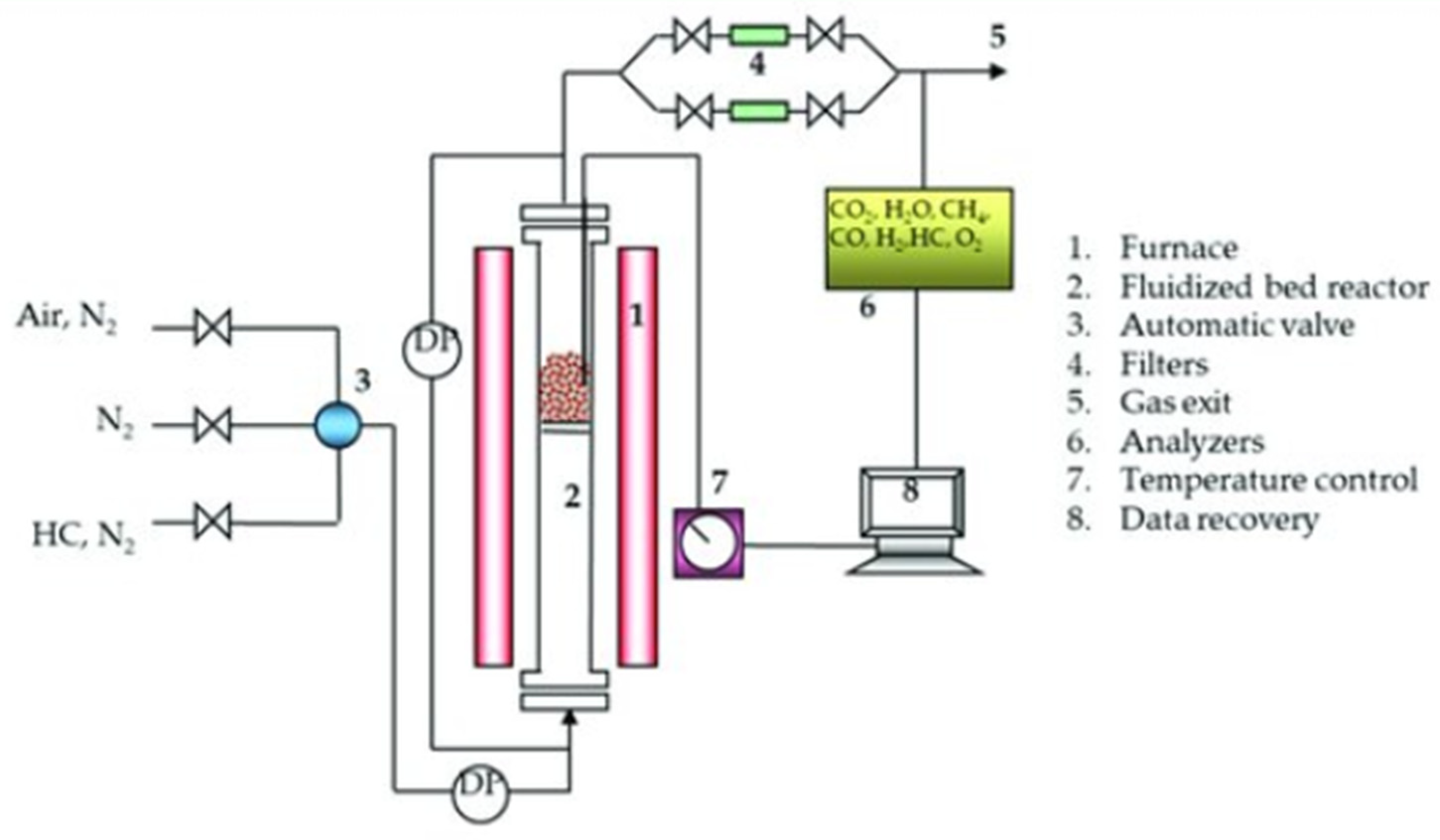
The assembly and commissioning of the thermogravimetric analysis (TGA) system for the CLC process was carried out. TGA analysis was performed on the solid oxygen (OXMN009) manganese transporting material with particle diameters D to determine: conversion at four different temperatures (between 650 and 950°C), using CH4, CO, and H2 as fuel, where the experimental design is indicated for three factors: CH4, CO and H2; therefore, the number of experiments was 24; In addition, the type of material (M1), temperature (Ti), the constant Ci fuel concentration.
TGA CI Electronics
The TGA Cl Electronics monitors the changes in mass that a sample undergoes when exposed to controlled atmospheres for a given period of time. This equipment has an accuracy of 0.1 μm and also offers the possibility of working at high temperatures and with atmospheres of different compositions.
The advantage offered by this equipment is the diversity of working atmospheres, which were CO, CH
4, and H
2, which were needed in the present study. The equipment is composed of a furnace, a data collection system called LabWeight, a reactor, a head that is where the most sensitive part of the equipment is located, the balance, with which the weight variations that the sample undergoes when exposed to the study conditions are determined, valves, meters, controllers, etc. A disbal system, temperature controller, and a bubbler allow the injection of steam into the reactor
Figure 2.
El método utilizado para el procedimiento de análisis termogravimétrico consistió en agregar 50 mg ± 1 mg de TSO a la canasta, que luego se colocó en un extremo de la TGA y se calentó a la temperatura de trabajo en una atmósfera de aire. Una vez que se alcanza la temperatura de funcionamiento, el TSO mantiene una masa constante y comienza el análisis. El TSO pasa por ciclos de reducción y oxidación, alternando líneas de gas, manteniendo un caudal volumétrico constante de 25 LN/h. El registro obtenido en la termobalanza es: el cambio de peso o pérdida de masa, y el tiempo de la reacción de oxidación o reducción del TO; luego de la purga de N2 para garantizar un ambiente inerte después de la reducción; dando lugar a la regeneración completa de TO por ganancia de peso en la etapa de oxidación—reducción
3. Results
Among Mn minerals, OXMN09 had the lowest reactivity with CH4 in TGA, however, in the literature Mattisson, Johansson, & Lyngfelt, (2006) reported that Mn-based TSO had low reactivity with CH4 and high reactivity with H2 and CO. Therefore, the material of the present study OXMN009 was brought to a particle size between 100 and 300 µm.
Orrego (2017) investigated the effect of the initial impregnation of Cu in Fe and Mn TSO, the study variables were the type of fuel (CH4 and H2) and percentage of Cu impregnated (1.5%, 3.5%, and 5%), found that using H2 as reducing gas and Cu impregnation percentage between 3.5% and 5%, the improvement in TSO reactivity was more pronounced. For the above reasons, and considering that the detection limit of the XRD equipment used to identify the crystalline phases in the material is 4%, it was decided to impregnate the TSO for this study with 5% Cu.
To obtain OXMN009 with 5 wt.% Cu, the impregnation procedure was carried out, as indicated above, resulting in that, although multiple calculations were required, the particle size remained constant in the TSO, indicating that There was no significant agglomeration of particles when subjected to temperatures up to 1050 °C in an oxidizing atmosphere.
Through the XRF analysis, the characterization of the OXMN009 and OXMN009P materials (93.15% and 89.32%) is reflected, as indicated in
Table 1, it was carried out to determine the presence of Mn elements associated with low-cost TO, so the presence of these elements in high concentrations indicates the potential of the mineral as TO [
18,
19,
20,
21].
Samples OXMN009 and OXMN009P have a high Mn content, which makes Mn oxide the main active phase in these materials; the Si content is low, so the presence of mixed oxides of Mn and Si as the active phase is negligible. The presence of a variety of metals in low proportions in XRF analysis is a common characteristic of natural minerals, so to facilitate subsequent calculations, only active phases that can form with most elements are considered.
The results reported in
Table 2 correspond to the crystalline phases detected in the diffractograms obtained. The large number of low-intensity peaks found and the overlap of peaks at closed 2
θθ angles, which usually occurs in natural minerals, indicate that the crystalline phases reported in
Table 2 are not all they are likely that there are also unidentified phases. XRD analysis detected thermite (CuO) in the Cu-treated TSO, consistent with an initial impregnation process performed in which the sample was calcined at 850 °C in an oxidizing atmosphere to decompose the added copper nitrate and form copper oxide. copper.
Table 2.
Reduction and oxidation reactions of the active phases found in TSO.
Table 2.
Reduction and oxidation reactions of the active phases found in TSO.
| Active phase |
Stage |
Reagent |
Reaction |
| |
Reduction |
CO |
CuO + CO → Cu + CO2
|
| CuO |
H2
|
CuO + H2 → Cu + H2O |
| |
Oxidation |
CH4
|
CuO + CH4 → Cu + CO2 + H2O |
| |
Reduction |
CO |
Mn3O4 + CO → 3MnO + CO2
|
| Mn3O4
|
H2
|
Mn3O4 + H2 → 3MnO + H2O
|
| |
Oxidation |
CH4
|
Mn3O4 + CH4 → Mn3O4 + CO2 + H2O |
Table 3.
X-RAY Disfraction.
Table 3.
X-RAY Disfraction.
| CRYSTALLINE PHASE |
|
|
|
| Name |
Formula |
OXMN009 |
OXMN009P |
Hausmanite
Bixbite
Quartz
Theronite
Hematite
Chromite
Pseudobrookite
|
Mn3O4
MnO3
SiO2
CuO
Fe2O3
FeCr2O4
Fe2TiO5
|
X
X |
X
X
X |
In samples OXMN009 and OXMN009P, two oxidation states of manganese oxide were detected: chertolite (Mn
2O
3) and bixbyite (Mn
3O
4). [18,27], studied the reduction and oxidation behavior of Mn oxides and found that once Mn
2O
3 was reduced to Mn
3O
4, its reoxidation was not detectable at high temperatures (800 °C), because on the other hand on the other hand, they found that Mn
3O
4 can be reduced to MnO and is easily reoxidized to Mn
3O
4 in air. Therefore, only the Mn
3O
4/MnO system in CLC is considered [
15].
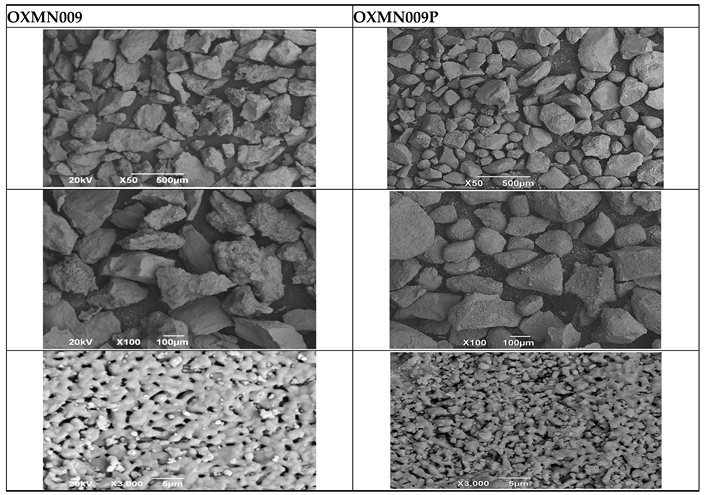
SEM microscopy of TSO was taken in its native state and processed at magnifications of 50, 100, and 3000 to understand the morphology, size distribution, and possible differences caused by Cu treatment. SEM microscopy of samples OXMN009 and OXMN009P are indicated in
Table 4. No difference was observed between the two solids at 50x and 100x magnification. Both have irregular size distribution, flat particle shape, and rough surface; At 3000x magnification, a large number of pores can be seen on the surface of sample OXMN009, but in sample OXMN009P these pores appear to be occupied, possibly by impregnated CuO particles.
The EDS analysis was performed on the TSOs in their raw and processed states. To create a representative distribution of the elements on the surface of the samples, 9 points were chosen for the detection of dispersive energy in the microscopies at 100x magnification of each of the TSOs (see
Table 4). For each solid oxygen carrier,
Table 5 lists the elements that were found in their maximum and minimum concentrations.
Finding high Mn contents in OXMN009 and OXMN009P, the elemental composition results in
Table 5, support the findings in FRX (see
Table 1) and DRX (see
Table 2). Cu was discovered in the processed TSO at each elemental detection point, indicating that the element is well distributed within the TSO particles. It should also be noted that the EDS results were only used to determine the presence of the impregnated phase and its distribution in the TSO particles because they do not represent the total elemental composition of the samples.
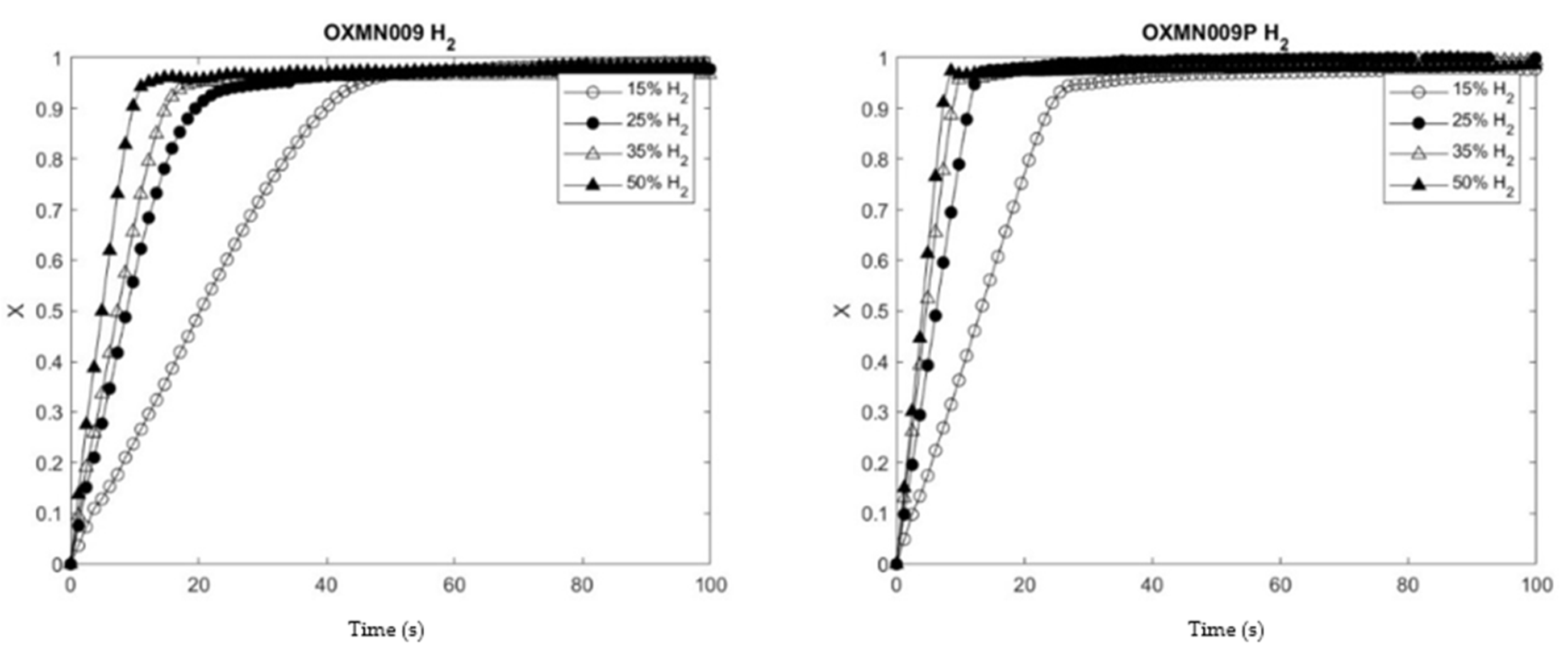
The TSO OXMN009 and 0XMN009P, under study, were analyzed in TGA, which allowed the determination of the oxygen transport capacities, reaction rate indices, and kinetic parameters for the materials with the best behavior.
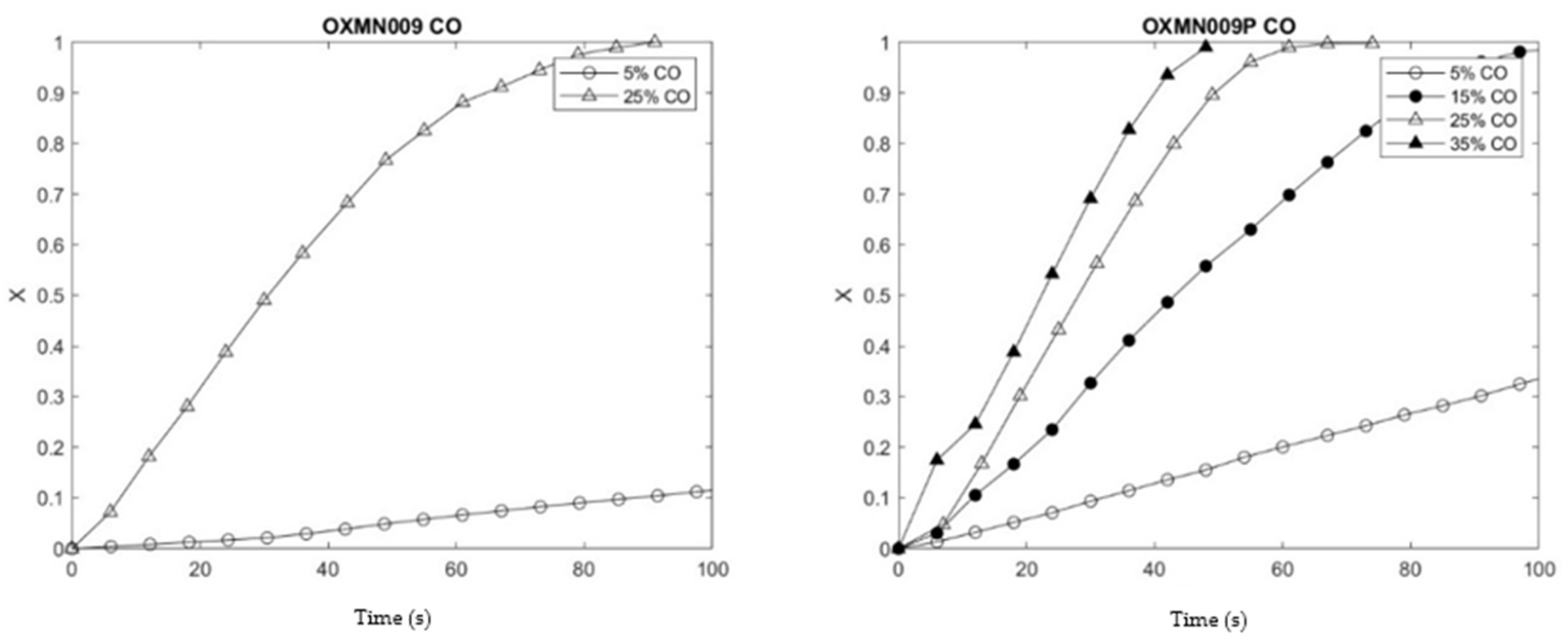
To know the effect of copper treatment on the studied TSO, the reduction and oxidation conversion curves of the native and treated TSO obtained under the same operating conditions in TGA were compared. The operating temperature of 950 °C was chosen for these preliminary tests to show agglomeration problems, which did not occur in any of the samples studied. The analysis of the samples in an H2 atmosphere gave positive results in terms of reactivity, in addition, the samples treated with Cu showed higher reduction and oxidation conversions at 30 seconds. On the other hand, the analysis in a CO atmosphere showed similar results in OXMN009P for the reduction of Mn TSO and greater oxidation conversion at 30 seconds. However, this result is insufficient, since the impregnation of Cu reactivity would have been improved TSO with CO as fuel.
Table 6 shows the Reaction rate indices (
RI) obtained for the preliminary analyses carried out on each sample, which were calculated according to Equation (1), facilitating the comparison of the reaction rate between different TSOs; indicated in Equation 1, in the conversion delta between 0 and 0.15 when the reaction is faster and the
Pr was set at 0.15 atm which is commonly used in the literature [
8,
13]. OXMN009P has a higher
RI than OXMN009, and both materials present
RI in the range reported in the literature for other Mn materials (see
Table 6).
The activation energies (
Ea) obtained for OXMN009 and OXMN009P using CO, H
2, and CH
4 as reactive gases are between 5 and 18.7 kJ/mol, a similar range to that found in the literature for Mn-based TSO (10, 2 to 19.5 kJ/mol)[
22] and lower than that reported for synthetic TSO of Fe, Cu, Ni, and Mn (14 to 33 kJ/mol) [
5] and other materials of natural origin such as activated Ilmenite (25.5 to 80.7 kJ/mol) [
23]. The
Ea reported for OXMN009P are lower than those of OXMN009 in the reduction reactions, indicating that there is less dependence of the reaction on temperature and therefore a lower energy is necessary to trigger the reaction Levenspiel, (1993); However, the oxidation a lower
Ea was obtained in OXMN009.
The kinetic parameters (k0) found for OXMN009 and OXMN009P are of a similar order of magnitude in all the reactions studied and are close to that reported in the literature Zafar, (2007) for the oxidation of MnO to Mn3O4 (1.04 m3 mol 0.65 s−1).
Figure 3 shows that the experimental data fit linearly to the grain SCM model for both OXMN009 and OXMN009P for all gases studied.
The calculated reaction orders of OXMN009 and OXMN009P with CH
4 (0.7; 0.7), H
2 (1.11; 1.09), CO (1.5; 1.0) respectively as shown in
Table 8, are within the ranges reported for synthetic Mn with CH
4 (1.0) and O
2 (0.65), and within the ranges reported for synthetic Cu-based minerals with CH
4 (0.4), H
2 (0.6), CO (0.8) and O
2 (1.0); Fe with CH
4 (1.3), H
2 (0.8), CO (1.0) and O
2 (1.0); Nor with CH
4 (0.4), H
2 (0.6), CO (0.8) and O
2 (1.0) [
24]. In general, the reaction order also depends on the fuel used. Regarding CH
4, the reaction order obtained presents the same order of magnitude based on what was previously reported by Zafar and collaborators [
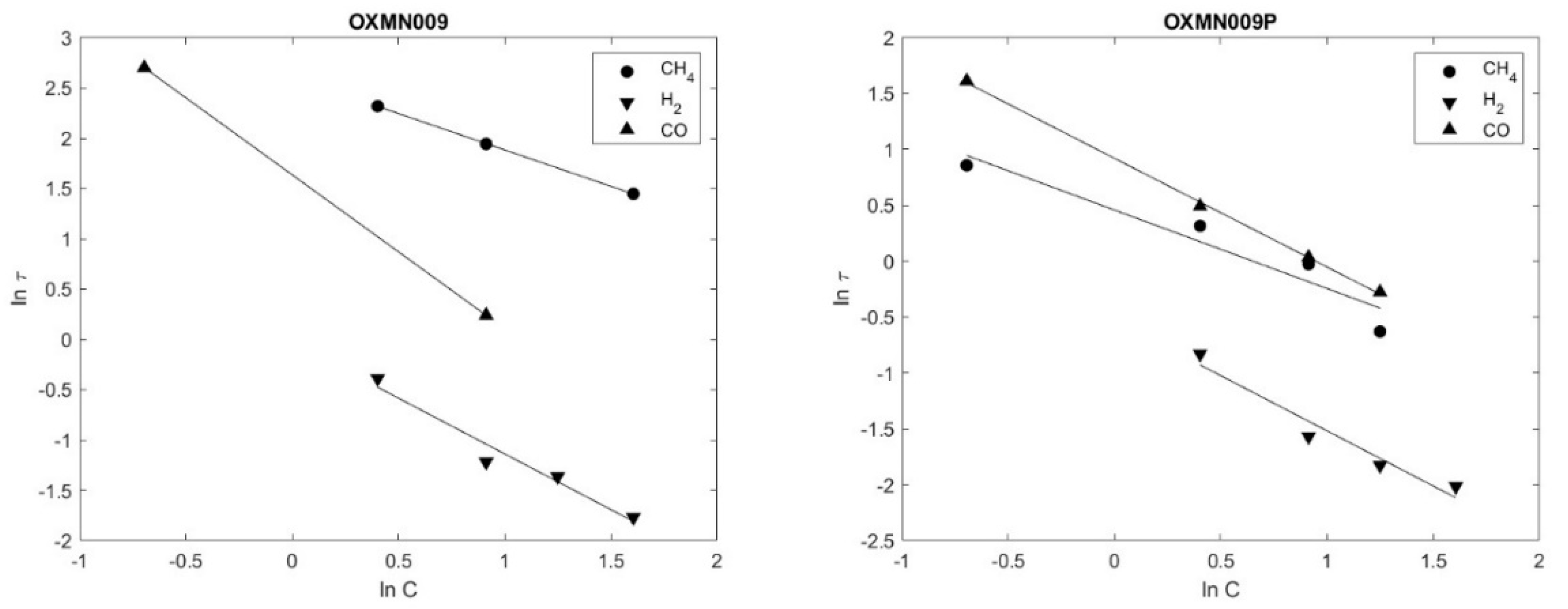
21]. The results indicate that the reaction rate for the transporters studied follows first-order kinetics on average. The complete conversion times
τ found are shown in
Table 8. The correlation coefficients obtained from the linear regression X vs. Time are in the range of 0.9594 to 0.9999, reflecting that the conversion varies linearly with time and fits the SCM model proposed following what was stated by Arango, (2016) [
15].
Table 7.
Reaction Order.
| Material |
Reactive |
n |
r2
|
| OXMN009 |
H2
|
1,1 |
0,9574 |
| CH4
|
0,7 |
0,9999 |
| CO |
1,5 |
0,9999 |
| OXMN009P |
H2
|
1,0 |
0,9472 |
| CH4
|
0,7 |
0,9174 |
| CO |
1,0 |
0,9990 |
Table 8.
Kinetics of materials 0XMN009 and OXMN009P.
Table 8.
Kinetics of materials 0XMN009 and OXMN009P.
| Sample |
Fuel |
Ea (kJ/mol) |
Ko
m/s(mol/m3)^n
|
Order
(n) |
Temperature °C |
Fuel concentration% (v/v) |
Oxygen concentration% (v/v) |
| OXMN009 |
CH4
|
59,38 |
2,26E+00 |
0,7 |
650–950 |
15–30 |
21 |
| H2
|
18,74 |
5,29E-01 |
1,11 |
650–950 |
15–30 |
21 |
| CO |
23,97 |
1,38E-01 |
1,53 |
650–900 |
15–30 |
21 |
| OXMN009P |
CH4
|
40,49 |
1,44E+00 |
0,7 |
650–950 |
15–30 |
21 |
| H2
|
12,09 |
4,61E-01 |
0,99 |
650–950 |
15–30 |
21 |
| CO |
11,07 |
7,96E-02 |
0,97 |
650–950 |
15–30 |
21 |
Figure 4.
Comparison OXMN009 y OXMN009P. Red: 25%CO y 75% N2; Ox:100% Air; T: 950 °C.
Figure 4.
Comparison OXMN009 y OXMN009P. Red: 25%CO y 75% N2; Ox:100% Air; T: 950 °C.
Figure 5.
Ln(τ) Vs Ln(C) OXMN009; OXMN009P (Reductor gas: H2, CO y CH4 entre 5 %–50%, balance con N2. Air 100 %. T = 950°C.
Figure 5.
Ln(τ) Vs Ln(C) OXMN009; OXMN009P (Reductor gas: H2, CO y CH4 entre 5 %–50%, balance con N2. Air 100 %. T = 950°C.