Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
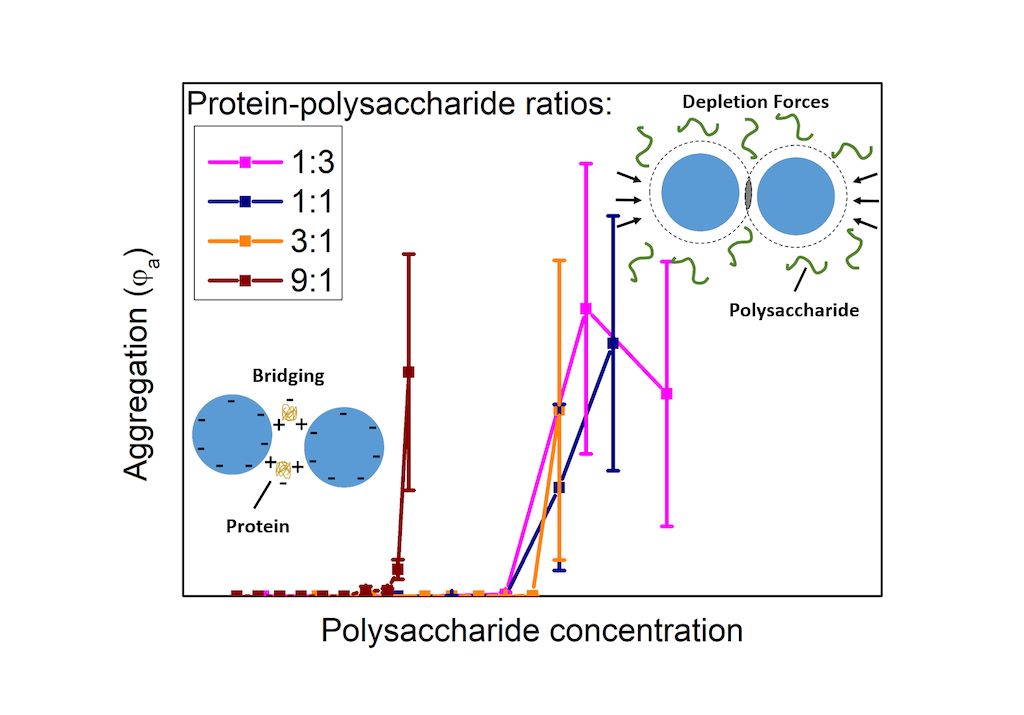
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material Characterization and Sample Preparation
2.2. FCS-Analysis
2.3. FCS-Setup
2.4. Correction for Viscosity Changes
3. Results and Discussion
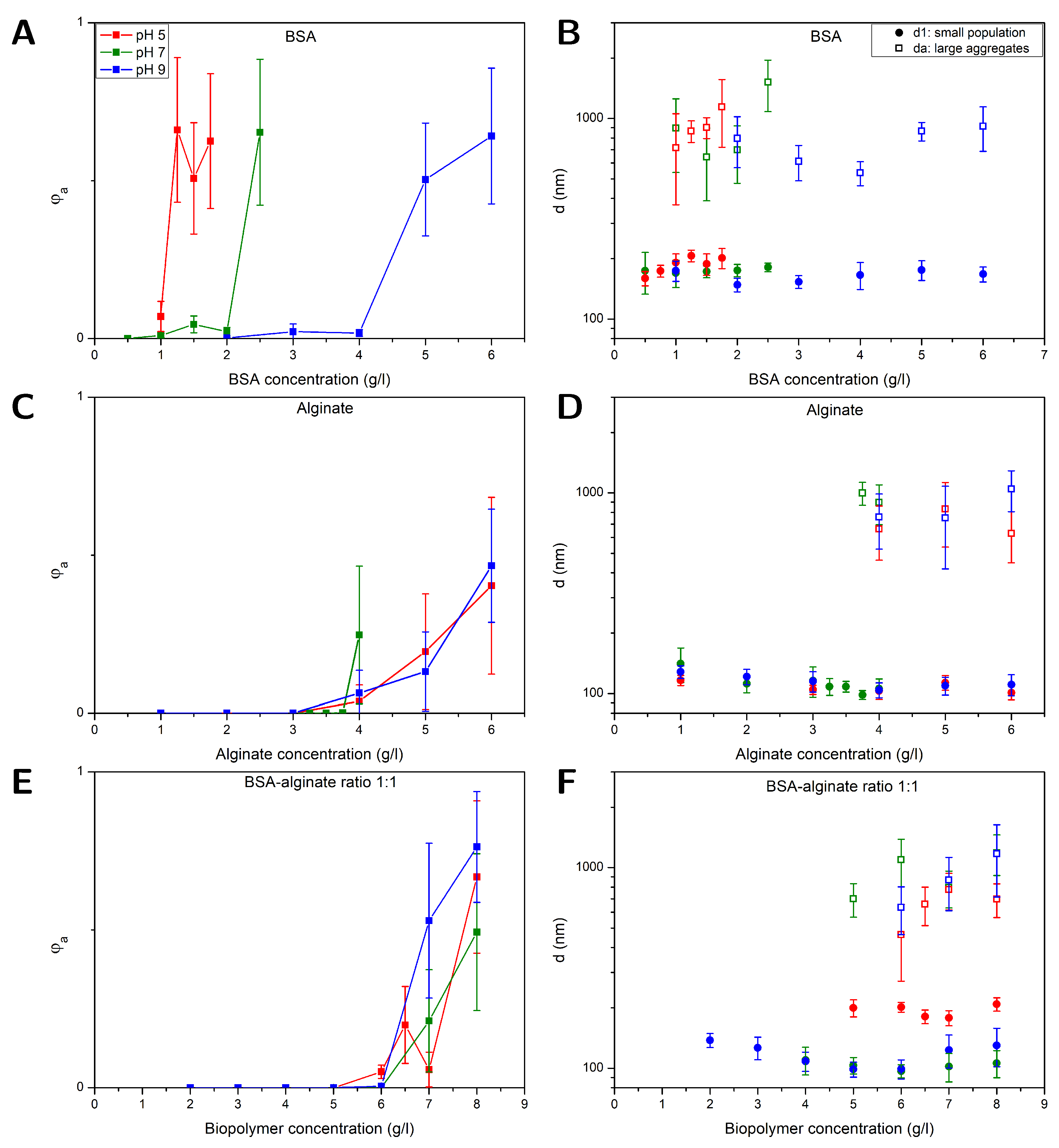
3.1. NP Aggregation in BSA Solutions
3.2. NP Aggregation in Alginate Solutions
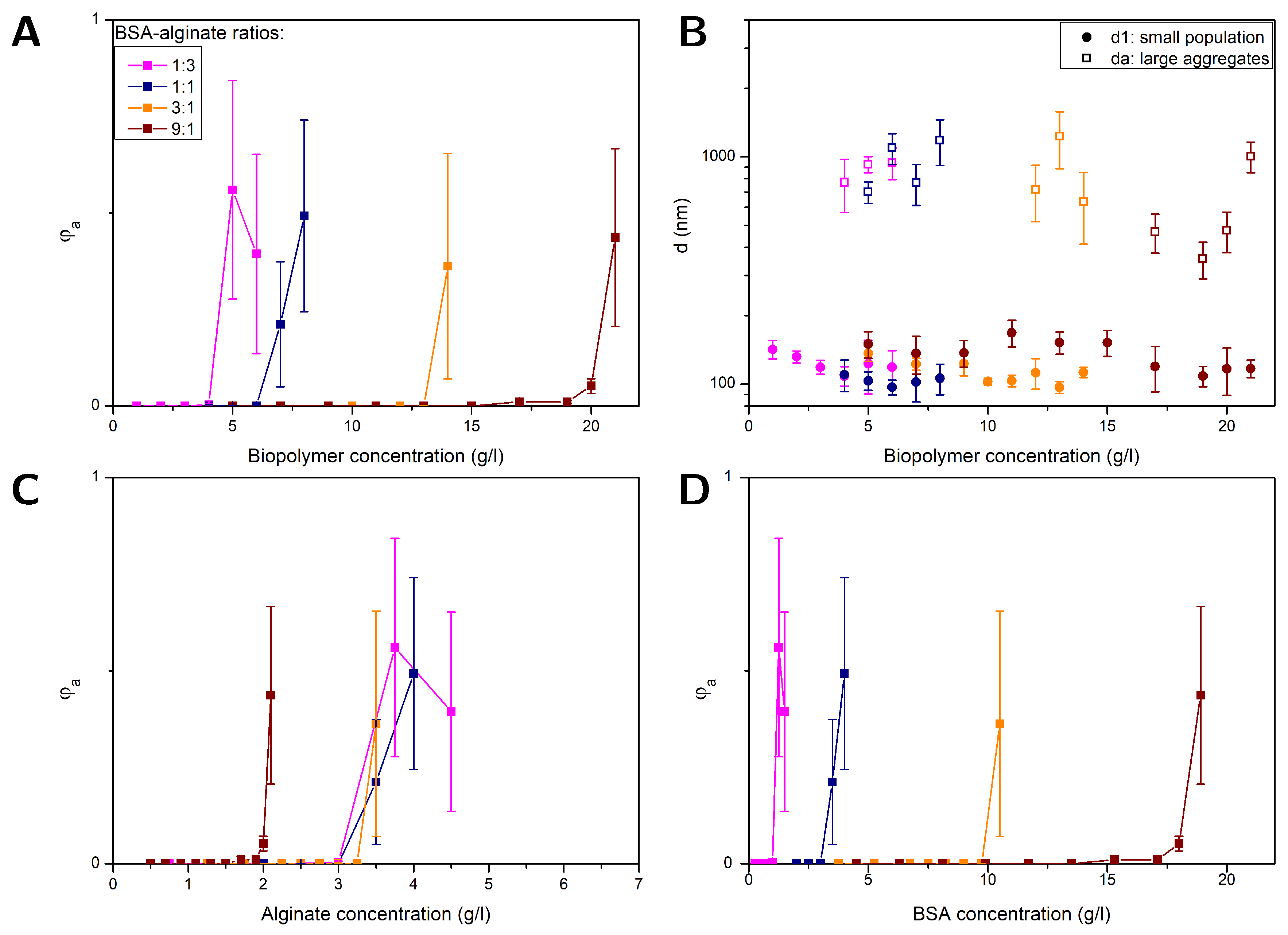
3.3. NP Aggregation in BSA-Alginate Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FCS | Fluorescence correlation spectroscopy |
| PS | Polystyrene |
| NP(s) | Nanoparticle(s) |
| BSA | Bovine serum albumin |
| EPS | Extracellular polymeric substances |
| NOM | Natural organic matter |
| HA | Humic acid |
| FA | Fulvic acid |
| TOC | Total organic carbon |
References
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Liu, Z.; Liu, S.; You, G.; Qu, H. et al. Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates. Int. J. Environ. Res. Public Health 2019, 16(23), 4639. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Wu, F.; Niu, L.; Tang, Z.; Liang, W. et al. Characterization, occurrence, environmental behaviors, and risks of nanoplastics in the aquatic environment: Current status and future perspectives. Fundamental Research 2021, 1(3), 317–328. [Google Scholar] [CrossRef]
- Deschênes, L.; Ells, T. Bacteria-nanoparticle interactions in the context of nanofouling. Adv. Colloid Interface Sci. 2020, 277, 102106. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Oriekhova, O.; Stoll, S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ. Sci. Nano 2018, 5, 792–799. [Google Scholar] [CrossRef]
- Walker, H.W.; Bob, M.M. Stability of particle flocs upon addition of natural organic matter under quiescent conditions. Water Res. 2001, 35(4), 875–882. [Google Scholar] [CrossRef]
- Ge, Z.; Lu, X. Impacts of extracellular polymeric substances on the behaviors of micro/nanoplastics in the water environment. Environ. Poll. 2023, 338, 122691. [Google Scholar] [CrossRef]
- Corsi, I.; Bergami, E.; Grassi, G. Behavior and Bio-Interactions of Anthropogenic Particles in Marine Environment for a More Realistic Ecological Risk Assessment. Front. Environ. Sci. 2020, 8, 60. [Google Scholar] [CrossRef]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene Nanoparticles Perturb Lipid Membranes. J. Phys. Chem. Lett. 2014, 5(1), 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ahimou, F.; Semmens, M.J.; Haugstad, G.; Novak, P.J. Effect of Protein, Polysaccharide, and Oxygen Concentration Profiles on Biofilm Cohesiveness. Appl. Environ. Microbiol. 2007, 73(9), 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Wozniak, D.J.L. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36(4), 893–916. [Google Scholar] [CrossRef] [PubMed]
- Moryl, M.; Kaleta, A.; Strzelecki, K.; Rozalska, S.; Rozalski, A. Effect of nutrient and stress factors on polysaccharides synthesis in Proteus mirabilis biofilm. Acta Biochim. Pol. 2014, 61(1), 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngso, J.; Pederson, J.S.; Arpanaei, A. et al. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef]
- Natan, M.; Banin, E. From Nano to Micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Zhou, J.; Tang, J.; Yang, C.; Chen, C. et al. Influence of environmental and biological macromolecules on aggregation kinetics of nanoplastics in aquatic systems. Water Res. 2020, 186, 116316. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef]
- Grassi, G.; Gabellieri, E.; Cioni, P.; Paccagnini, E.; Faleri, C.; Lupetti, P. et al. Interplay between extracellular polymeric substances (EPS) from a marine diatom and model nanoplastic through eco-corona formation. Sci. Total Environ. 2020, 725, 138457. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Fulaz, S.; Vitale, S.; Casey, E.; Quinn, L. Interactions between functionalised silica nanoparticles and Pseudomonas fluorescens biofilm matrix: A focus on the protein corona. PLoS ONE 2020, 15(7), e0236441. [Google Scholar] [CrossRef]
- Yu, S.; Shen, M.; Li, S.; Fu, Y.; Zhang, D.; Liu, H. et al. Aggregation kinetics of different surface-modified polystyrene nanoparticles in monovalent and divalent electrolytes. Environ. Pollut. 2019, 255, 113302. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.L.; Zhang, T.C.; Fu, Y. Effects of biofilm structure, microbial distributions and mass transport on biodegradation processes. Water Sci. Technol. 1995, 31(1), 143–152. [Google Scholar] [CrossRef]
- Song, Z.; Yang, X.; Chen, F.; Zhao, F.; Zhao, Y.; Ruan, L.; et al. Fate and transport of nanoplastics in complex natural aquifer media: Effect of particle size and surface functionalization. Sci. Total Environ. 2019, 669, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tallec, K.; Blard, O.; Gonzales-Fernandez, C.; Brotons, G.; Berchel, M.; Soudant, P.; et al. Surface functionalization determines behavior of nanoplastic solutions in model aquatic environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef]
- Mao, Y.; Li, H.; Huangfu, X.; Liu, Y.; He, Q. Nanoplastics display strong stability in aqueous environments: Insights from aggregation behaviour and theoretical calculations. Environ. Pollut. 2020, 258, 113760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ou, Q.; He, Q.; Wu, Z.; Ma, J.; Huangfu, X. Influence of dissolved black carbon on the aggregation and deposition of polystyrene nanoplastics: Comparison with dissolved humic acid. Water Res. 2021, 196, 117054. [Google Scholar] [CrossRef] [PubMed]
- Pradel, A.; Catrouillet, C.; Gigault, J. The environmental fate of nanoplastics: What we know and what we need to know about aggregation. NanoImpact 2023, 29, 100453. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Diniz, M.S. Recent Advances in the Aggregation Behavior of Nanoplastics in Aquatic Systems. Int. J. Mol. Sci 2023, 24, 13995. [Google Scholar] [CrossRef]
- Buffle, J.; Wilkinson, K.J.; Stoll, S.; Filella, M.; Zhang, J. A Generalized Description of Aquatic Colloidal Interactions: The Three-colloidal Component Approach. Environ. Sci. Technol. 1998, 32, 2887–2899. [Google Scholar] [CrossRef]
- Wheeler, K.E.; Chetwynd, A.J.; Fahy, K.M.; Hong, B.S.; Tochihuitl, J.A.; Foster, L.A. et al. Environmental dimensions of the protein corona. nature nanotechnol. 2021, 16, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.T.; Draget, K.I.; Skjåk-Bræk, G.; Smidsrød, O. Alginates. In Food polysaccharides and their application; Dekker: New York, United States, 1995; pp. 289–334. [Google Scholar]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 2015, 119, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, R.; Liu, Q.; Lin, Z.; Shi, Y.; Li, J.; et al. Engineering surface patterns on nanoparticles: new insights into nano-bio interactions. J. Mater. Chem. B 2022, 10, 2357–2383. [Google Scholar] [CrossRef]
- Kopac, T. A Protein corona, understanding the nanoparticle–protein interactions and future perspectives: A critical review. Int. J. Biol. Macromolecules 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Saptarshi, S.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Ries, J.; Schwille, P. Fluorescence correlation spectroscopy. Bioessays 2012, 34(5), 361–368. [Google Scholar] [CrossRef] [PubMed]
- Koynov, K.; Butt, H.J. Fluorescence correlation spectroscopy in colloid and interface science. Curr. Opin. Colloid Interface Sci. 2012, 17(6), 377–387. [Google Scholar] [CrossRef]
- PicoQuant, Practical manual for fluorescence microscopy techniques, Fluorescence correlation spectroscopy (FCS). Available online: https://www.picoquant.com/images/uploads/page/files/17319/5_fcs.pdf (accessed on 18 March 2024).
- Starchev, K.; Zhang, J.; Buffle, J. Applications of Fluorescence Correlation Spectroscopy— Particle Size Effect. J. Colloid Interface Sci. 1998, 203(1), 189–196. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, L.; Chen, B.; Ding, Y.; Ma, H.; Chen, D. Afterpulsing and its correction in fluorescence correlation spectroscopy experiments. Appl. Opt. 2003, 42(19), 4031–6. [Google Scholar] [CrossRef]
- Guckeisen, T.; Orghici, R.; Rathgeber, S. Probing the tendency for aggregation of nanoplastics in model extracellular biofilm substances with fluorescence correlation spectroscopy. Single Molecule Spectroscopy and Superresolution Imaging XVI. Proc. of SPIE 2023, 12386, 1238606. [Google Scholar]
- Beragoui, M.; Chadlia, A.; Khalfaoui, M.; Enciso, E.; Torralvo, M.J.; Duclaux, L. et al. Bovine serum albumin adsorption onto functionalized polystyrene lattices: A theoretical modeling approach and error analysis. Prog. Theor. Exp. Phys. 2015, 2015(3), 033J01. [Google Scholar]
- Yoon, J.Y.; Kim, J.H.; Kim, W.S. The relationship of interaction forces in the protein adsorption onto polymeric microspheres. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 413–419. [Google Scholar] [CrossRef]
- Norde, W.; Anusiem, A.C.I. Adsorption, desorption and re-adsorption of proteins on solid surfaces. Coll. Surf. 1992, 66, 73–80. [Google Scholar] [CrossRef]
- Zsom, R.L.J. ; Dependence of preferential bovine serum albumin oligomer adsorption on the surface properties of monodisperse polystyrene latices. J. Colloid Interface Sci. 1986, 111(2), 434–445. [Google Scholar] [CrossRef]
- Wangkam, T.; Yodmongkol, S.; Disrattakit, J.; Sutapun, B.; Amarit, R.; Somboonkaew, A. et al. Adsorption of bovine serum albumin (BSA) on polystyrene (PS) and its acid copolymer. Curr. Appl. Phys. 2012, 12(1), 44–52. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Dulm, P. V.; Norde, W.; Lyklema, J. Ion participation in protein adsorption at solid surfaces. J. Colloid Interface Sci. 1981, 82(1), 77–82. [Google Scholar] [CrossRef]
- Elgersma, A.V.; Zsom, R.L.J.; Norde, W.; Lyklema, J. The adsorption of bovine serum albumin on positively and negatively charged polystyrene latices. J. Colloid Interface Sci. 1990, 138(1), 145–156. [Google Scholar] [CrossRef]
- Grant, M.L. ; Nonuniform Charge Effects in Protein-Protein Interactions. J. Phys. Chem. B 2001, 105(14), 2858–2863. [Google Scholar] [CrossRef]
- Kubiak-Ossowska, K.; Jachimska, B.; Mulheran, P. How Negatively Charged Proteins Adsorb to Negatively Charged Surfaces: A Molecular Dynamics Study of BSA Adsorption on Silica. J. Phys. Chem. B 2016, 120, 10463–10468. [Google Scholar] [CrossRef]
- Meseth, U.; Wohland, T.; Rigler, R.; Vogelc, H. Resolution of Fluorescence Correlation Measurements. Biophys. J. 1999, 76, 1619–1631. [Google Scholar] [CrossRef]
- Chen, K.; Xu, Y.; Rana, S.; Miranda, O.R.; Dubin, P.L.; Rotello, V.M. et al. Electrostatic Selectivity in Protein-Nanoparticle Interactions. Biomacromolecules 2011, 12, 2552–2561. [Google Scholar] [CrossRef]
- Guckeisen, T.; Hosseinpour, S.; Peukert, W. Isoelectric Points of Proteins at the Air/Liquid Interface and in Solution. Langmuir 2019, 35, 5004–5012. [Google Scholar] [CrossRef]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F. et al. Measurements and Theoretical Interpretation of Points of Zero Charge/Potential of BSA Protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef]
- Mao, Y.; Cates, M.E.; Lekkerkerker, H.N.W. Depletion force in colloidal systems. Physica A 1995, 222, 10–24. [Google Scholar] [CrossRef]
- Smith, N.J.; Williams, P.A. Depletion Flocculation of Polystyrene Latices by Water-soluble Polymers. J. Chem. Soc., Faraday Trans. 1995; 91, 1483–1489. [Google Scholar]
- Sharma, A.; Tan, S.N.; Walz, J.Y. Effect of Nonadsorbing Polyelectrolytes on Colloidal Interactions in Aqueous Mixtures. J. Colloid Interface Sci. 1997, 191, 236–246. [Google Scholar] [CrossRef]
- Bondy, C. ; The creaming of rubber latex. Trans. Faraday Soc. 1939, 35, 1093–1108. [Google Scholar] [CrossRef]
- Zhao, Y.; Carvajal, M.T.; Harris, M.T. Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. J. Coll. Int. Sci. 2009, 332(2), 1345–353. [Google Scholar] [CrossRef]
- Sabet, S.; Seal, C.K.; Swedlund, P.J.; McGillivray, D.J. Depositing alginate on the surface of bilayer emulsions. Food Hydrocoll. 2020, 100, 105385. [Google Scholar] [CrossRef]
- Garcia, A.G.; Nagelkerke, M.; Tuinier, R.; Vis, M. Polymer-mediated colloidal stability: on the transition between adsorption and depletion. Adv. Colloid Interface Sci. 2020, 275, 102077. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Dobson, M.D.; Vendruscolo, M. Characterization of the nucleation barriers for protein aggregation and amyloid formation. HFSP J. 2007, 1(2), 137–146. [Google Scholar] [CrossRef]
- McSwain, B.S.; Irvine, R.L.; Hausner, M.; Wilderer, P.A. Composition and Distribution of Extracellular Polymeric Substances in Aerobic Flocs and Granular Sludge. Appl. Environ. Microbiol. 2005, 71(2), 1051–1057. [Google Scholar] [CrossRef]
| Fluospheres | R-PGCX25 | R-PGX25 | ||
|---|---|---|---|---|
| (COOH) | (COOH) | (plain) | ||
| Diameter supplier | 109 nm | 100 nm | 100 nm | |
| Hydrodynamic diameter (FCS) | reference | 105±6 nm | 97±7 nm | |
| Hydrodynamic diameter (DLS) | 111±2 nm | 105±2 nm | 124±2 nm | |
| Polydispersity Index (DLS) | 0.038 | 0.031 | 0.073 | |
| Zeta potential (DLS) | -52±4 mV | -51±8 mV | -28±4 mV | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





