Hypotheses
Given the critical importance to survival of self-related interoceptive and exteroceptive information it is not surprising that substantial research shows major functional areas of the brain respond with greater saliency to self-related stimuli. Widespread networks, including vision and attention, motor, default mode (DMN), salience (SN) and central executive (CEN), are deployed to process them (Sui & Humphreys, 2017). Higher salience is attributed to, and memories are better retained of self-related items compared to those related to others (Alzueta et al., 2020; Sui et al., 2012; Żochowska et al., 2021). This is anatomically and functionally represented by a ventral-dorsal gradient in the medial prefrontal cortex (mPFC) and processing speeds (Alzeuta et al., 2019; D’Argembeau et al., 2008; Denny et al., 2102; Keyes & Brady, 2010). The mPFC, an element of the DMN, is key to our experience of ourselves, of ipseity (Davey et al., 2016; Schurz et al., 2014; Sui & Humphreys, 2017). Self-portraits are the product of interaction between three self-related images: one in the mirror, one being created and an internal representation. Based on this we hypothesised that an artist is more personally engaged when painting self-portraits than other subjects and therefore that mood states would be most evident in them and would differ stylistically from portraits of related and unrelated others.
Background
Rembrandt van Rijn (1606-1669) ‘Rembrandt’ was born in Leiden into a tolerant, ‘middle class’ family and enjoyed a comfortable and stable childhood. He received a thorough classical and religious education. His decision to become an artist rather than attend university was supported by his father who enrolled him as an apprentice with two well-known artists. Rembrandt was ‘discovered’ in 1629 by the secretary to Prince Frederick Hendrik Holland’s leader and a patron of the arts. Rembrandt rapidly became recognized as among the greatest painters of his day soon moving to Amsterdam and becoming commercially successful in a competitive marketplace. His mastery in conveying human emotion was recognised from the beginning of his career (Scallen 2004; Schwartz 2006; Schama, 1999). He painted more self-portraits than any artist before him, depicting himself in early adulthood, middle and old age.
1 More than one scholar has seen them as constituting an autobiography (Chapman, 1990; Clark 1978). He also painted numerous portraits on commission and of family and friends. Self-portraits and portraits represent nearly half of his painted work and were painted throughout his career providing the opportunity to examine changes in style longitudinally (van de Wetering 2017).
The self-portraits Rembrandt produced in his last decade are seen by many as the finest images of old age ever painted (Clark 2006; Covey 1991; McKee & Kauppinen 1987). It is primarily based on these, a few etchings, and a series of adverse life events, that some have concluded that Rembrandt suffered unipolar or bipolar depression (Schildkraut 2004, Schildkraut et al. 2007). The events include: the death of his father in 1630, of three children shortly after birth (1635, 1638 and 1640), of his mother (1640), of his wife (1642), his bankruptcy in middle age (1649-1658) and resulting reduced financial and social circumstances, the death of his mistress (1663) and the death of his only surviving son (1668). (Bikker, 2019; Bikker & Weber; 2014, Schama, 1999). Such life events, as well as old age itself, are risk factors and potential triggers, individually and cumulatively, of depression (Gathergood 2012; Keller et al., 2007; Kraaij et al., 2002).
2
It is important to see such events in their historical context. In 17th century Holland infant mortality was approximately 40% (Hogan & Kertzer, 1987) and the plague, which killed his wife, mistress and likely his son, was a common source of adult mortality (Rommes, 2015; Curtis, 2016). In this early stage of capitalism bankruptcy was already well catered for by specialised institutions. Bankruptcy among artists was not uncommon and the terms of Rembrandt’s were not unduly harsh allowing him to continue his work albeit under straitened circumstances. Following his bankruptcy and as he aged, Rembrandt’s social circle declined but he continued to be supported by his son and mistress, maintain friendships, and engage with patrons. He continued to work until his death (Crenshaw, 2006; Runia & de Witt, 2019; Strauss & van der Meulen, 1979).
Just as it is important to put the events of Rembrandt’s life in their historical context one must use modern criteria of diagnosis of physical and mental illness with caution as one is inevitably handicapped by the very different conditions in the past. Rembrandt lived in the pre-modern period in which concepts of mental and the prevalence of physical, illness were very different from our own (Mackenbach, 2021; Hodgkin, 2007; Foucault, 1961). It is important when attempting to describe the mental state of figures in the past to be conscious of the context in which they lived when applying modern insights (ter Borg & Trenité, 2012). Posthumous diagnosis is problematic even when there is considerable evidence of recent vintage (Voskuil, 2020; Frigg & Howard, 2011). It is all the more difficult when dealing with someone who died centuries ago.
Rembrandt’s life and work has been the subject of extensive study, beginning in his lifetime and extending to the present day (van de Wetering, 2005). There is, however, relatively little personal documentation. The few letters in his hand to survive were written to patrons on business matters. The remaining documents by or related to him are mainly fragmentary or related to financial and legal matters (Strauss & van der Meulen, 1979). There are contemporary descriptions of him, his studio and his working habits but we have little idea of what he thought or felt except in regard to the high value he put on his work. There are no contemporary accounts of Rembrandt suffering any physical or mental illness or cognitive decline. There are no indications of mental or physical illness in Rembrandt’s family. Despite the paucity of evidence the number and quality of his self-portraits have encouraged scholars to make diagnoses both physiological and psychological.
Somatic Illness
Rembrandt lived beyond the average span of his contemporaries (van Poppel et al., 2013) dying naturally of old age. A number of scholars have sought to identify somatic illnesses from examination of the self-portraits, particularly diseases associated with aging, vision and depression (Abastado & Chemla, 2007; Espinel, 1997). Physical illness is associated with depression as is aging (WHO 2022, 2017). Deterioration in vision, particularly macular degeneration, is also associated both with depression and aging (Eramudugolla et al., 2013). There is no evidence that Rembrandt wore spectacles, although they were widely available.
The extraordinary detail in Rembrandt’s depictions of himself in old age has encouraged meticulous examination of his facial features
3 resulting in conclusions that he suffered from a variety of diseases including: emporal arteritis, hyperthyroidism, rosacea and rhynophima, cataract, macular degradation, glaucoma, unilateral strabismus, presbyopia, xanthalasma, pinguecula, arcus senil and yellow-brown vision (Espinal, 1997; Marcus & Clarfield, 2002; Salvi et al., 2006). These analyses have been reviewed in detail by disease specialists and found unreliable (Friedman et al., 2007a, 2007b; Mondero et al., 2013; Wiser et al., 2016). The reliable evidence from such examination of the self-portraits is that Rembrandt suffered physiologically from no more than normal aging which may have included macular degeneration and presbyopia (Hage et al., 2019; Marcus & Clarfield, 2002; Trevor Roper, 1959). Given that he lived into old age Rembrandt was at risk of neurodegenerative disease however no symptoms were noticed by contemporaries one of whom described him 5 years before his death as “hale and hearty” and “‘in full use of his mind, memory and speech” (Schwartz, 2006).
Mental Illness
Many perceive sadness in Rembrandt’s self-portraits given the “unsparing” honesty with which he portrayed aging (Schama, 1999, 680). Scholarly, medical, psychiatric, and journalistic analyses of these and other images have concluded that at various points in his life Rembrandt suffered from depression. A review of an etching of St Jerome is said to demonstrate depression (Schildkraut, 2004) and together with another of an unidentified man to indicate manic depression (Schildkraut et al., 2007). It is suggested that these images and a decline in the number of etchings produced after his wife’s death, indicate a period of depressed mood. This, it is said, followed a period of high, ‘manic’ spending. Rembrandt spent freely following his marriage acquiring both an expensive house and a collection of art and rare objects for which he paid high prices. His mismanagement of financial matters during the 15 years following her death ultimately resulted in his bankruptcy (Crenshaw, 2006; Schwartz, 2006).
Elements of Depression
In Rembrandt’s day depression would have been described as melancholia. Indeed, it was fashionable for creative people to be seen as melancholic. Burton’s work on the subject (1621) was well known as was Durer’s etching ‘Melencolia’ which established the pose which Rembrandt used in several works (Schwartz, 2013). If Rembrandt noticeably suffered from melancholy he and his contemporaries would have been able to recognize and name it and he could certainly have depicted himself as such. Neither he nor they ever did so. Melancholic depression has been variously characterised as depression with particular psychotic and somatic features. DSM 5 now characterises it as major depressive disorder (MDD) accompanied by anhedonia, lack of responsiveness, psychomotor disturbance, cognitive impairment and vegetative disorders such as insomnia and weight loss (APA 2013; Parker et al., 2010). A useful succinct definition of depression is the disproportionate experience of sadness and bereavement which do not remit when the external cause dissipates (Belmaker & Agam, 2008). Unipolar depression is distinguished from bipolar depressive disorder (BPD) principally by the presence of manic symptoms in the latter (DSM 5). Manic periods are measured in weeks of duration and depressive ones in months (Tondo et al. 2017).
Depression and Creativity
Melancholia has been associated with creativity since antiquity (Akiskal & Akiskal, 2007; Dixon, 2013; Neihart, 1998; Sullivan, 2008,). Studies of artists appear to confirm a relationship between creativity and mood disorders (Ludwig, 1992) however, much of the evidence is anecdotal and the strength of the relationship varies between studies and research approaches (Slaby, 1992). A case study of 15 prominent Abstract Expressionist artists found that half suffered from depressive disorders during their lifetimes (Schildkraut et al., 1994). A larger study of writers found 80% suffered from affective mood disorder and 40% from BPD (Andreasen, 1987). A meta-analysis of the relationship between creativity and mood disorder found a stronger relationship with bipolar than unipolar depression (Taylor, 2017).
The strongest correlations with creativity are with mild to moderate forms of BPD and positive mood but not with unipolar depression or dysthymia (Baas et al., 2008, 2016; Greenwood et al., 2022; Kyaga et al., 2011; Murray & Johnson, 2010; Taylor, 2017). One study of non-clinical artists found an association between mild cyclothymia and creativity (Vellante et al., 2011). There appears to be an inverted U-shaped relationship between BPD symptom severity and creativity (Acar et al., 2018; Richards et al., 1988,). Milder symptoms, elevated mood, as opposed to mania or depression, are found in multiple studies of eminent creative people (Johnson et al., 2012). It is noteworthy that “unusually creative thinking” was listed in DSM-III as a symptom of hypomania (APA, 1980, p. 218).
Depressive episodes, as opposed to recurrent or persistent depression, are associated with the generation of creative ideas (Neiderland, 1989, 1973; Pollack, 1989). Lower levels of serotonin and norepinephrine, reported in depression, may encourage introspection (Heilman, 2016). Many creative people report that mind-wandering, an aspect of DMN functioning, facilitates creative problem solving (Baird et al., 2012). Low mood due to social rejection also seems associated with greater creativity (Akinola & Mendes, 2008) however persistent depression is not (Byron & Khazanchi, 2011; Silvia & Kimbrel, 2010) except, possibly, for writers (Kyaga et al., 2013). In summary milder BPD symptoms, elevated mood, and occasional mild depressive episodes appear positively associated with greater artistic creativity while persistent depression and mania are negatively associated.
Depression and Vision
Both anecdotally and experimentally, individuals with depression see the world as darker with ambient light diminishing as the disease progresses (Bubl et al. 2010, 2012; Friberg & Borrero, 2000). There appears to be a bi-directional relationship between feelings and perception with those feeling sad perceiving things as dim in comparison to those feeling happy seeing things as brighter (Zhang et al., 2016). In the early stage visual system depression is associated with reduced peripheral vision for which the magnocellular system is primarily responsible. This helps account for the bias which patients demonstrate toward focusing central vision on negative stimuli enhancing negative emotionality (de Zorzi et al., 2020). Later stage visual processing deficits include reduced facial emotion recognition including happiness, anger and fear and the deficiencies increase with disease severity. Non-clinical subjects and patients demonstrate greater attention to and respond more negatively to sad faces (Chang et al., 2010; Joormann & Gotlib, 2006; Krause et al.,. 2021; Nakamura et al., 2018; Xin et al., 2021; Zwick & Wolkenstein, 2017). BPD patients demonstrate similar deficits and are slow to recognise positive and quick to recognise negative, emotions compared with both unipolar patients and the healthy aged (Altamura et al., 2016; Ruihua et al., 2021; Schaefer et al., 2010).
There is compelling evidence of visual abnormalities in depression (Jung et al., 2020). Medicated and unmedicated, first episode and chronic MDD patients have reduced visual acuity, lower retinal contrast gain, lower contrast sensitivity, and altered surround suppression which correlate with disease severity (Bubl et al., 2009, 2010, 2012, 2015; Fam et al., 2013; Salmela et al., 2021; Schwitzer et al., 2017, 2015). BPD patients reveal similar deficits (Fernandes et al., 2019; O’Bryan et al., 2014). Investigations using optical coherence tomography (OTC) have found reductions in thickness of the retinal nerve fibre layer (RNFL), ganglion cell layer (GCL) and inner plexiform layer (IPL) and thicker choroid levels in MDD and BPD patients as compared to healthy controls (Lizano et al., 2019; Silverstein et al., 2020). Dysfunction in these layers is evidenced by decreases in a and b wave amplitudes which characterise electroretinogram (ERG) tests (Tan et al., 2020). Pattern electroretinogram (PERG) studies, which measure the activity of retinal ganglion cells, whose axons make up the optic nerve, find increases in P50 and N95 implicit time. (Schwitzer et al., 2022). ERG and PERG studies find rod and cone dysfunction in both medicated and medication naïve patients (Hébert et al., 2017). In mild cases there appears to be no significant difference between the retinas of patients and healthy controls but in more severe MDD and in BPD patients the differences are significant (Almonte, 2020; Demmin, 2020; Schönfeldt-Lecuona et al., 2018; Silverstein et al., 2020). A global study of retinal function in acute MDD patients found delayed, hypoactive central retinal signalling in photoreceptors and bipolar cells together with hyperactivity in the peripheral retina consistent with patient reports of dimness in their surroundings (Cosker et al., 2021).
Individuals with depression seeing the world as darker correlates with reduced contrast gain control evidenced by lower retinal PERG and EEG amplitudes and higher contrast-detection thresholds (Bubl et al., 2009, 2010, 2012, 2015; Schwitzer et al., 2015, 2017; Shoshina et al., 2021) related to altered mediation by dopamine and serotonin (Bubl et al., 2010; Fam et al., 2013; Friberg & Borrero, 2000). MDD patients have significantly reduced levels of dopamine which is critical to contrast gain control (Dunlop & Nemeroff, 2007). Dopamine hypofunction in the retina alters the behaviour of amacrine, horizontal, interplexiform and ganglia cells (Brandies &Yehuda, 2008). Rods are dopamine dependent and changes in dopamine levels affect cognitive vision processing (Vitay & Hamker, 2007). Patients show reduced amplitude in visual evoked potential (VEP) indicating that the dysfunction extends beyond the retina to the occipital cortex which is innervated by the mesocortical dopamine system (Bubl et al., 2015). Deficits in surround suppression also occur in the primary visual cortex (Salmela et al., 2021).
There is consistent evidence that colour perception is impaired in both MDD and BPD (Barrick et al., 2002; Fernandes et al. 2017). Compared to healthy controls depressed people are far more likely to select grey as representative of their mood, in contrast to yellow preferred by controls and associated with positive feeling. Patients also prefer desaturated colours and there is evidence of reduced colour in patients’ art (Carruthers et al., 2010; Wadeson, 1971; Wadeson & Carpenter, 1976). In melancholic depression there is both dimming of the environment and a reduced sense of colour related to retinal dopamine dysregulation (Hyett & Parker, 2013). Hypo-dopaminergic states in the retina are associated with poorer discrimination of red as well as blue color saturation (Kim et al., 2014). There is evidence from cocaine-dependent, tricyclic treated depression, Tourette’s, Parkinson’s and Huntington’s patients that low dopamine levels cause deficits in short-wave, blue-yellow cone function (Büttner et al., 1994; Lagerlöf, 1982; Melun et al., 2001; Pieri et al., 2000; Roy et al., 2003; Weil et al. 2016) as is the case in normal aging (Meng et al., 1999). This may be a result of altered communication between blue photoreceptors and horizontal cells and reflected in abnormal blue cone ERG measurements (Djamgoz et al., 1997; Roy et al., 2003).
Aging
The prevalence of depression in old age approaches 30% (Hu et al., 2022). In addition to the manifold physiological effects of aging, including neurodegenerative disease, there is both a longitudinal and a bi-directional association with depression and vision impairment (Virgili et al., 2022). Old age is often a period of social isolation and loneliness which are related to physiological and psychological deterioration. Loneliness is correlated with depression but while older people can experience social isolation, sparse or inadequate relationships, loneliness and feelings of loss, they are not directly related. Isolation alone does not necessarily imply loneliness or the likelihood of mood disorder (Coyle & Dugan, 2012; Domènech-Abella, et al., 2019). While many cognitive functions such as verbal and numerical abilities and general knowledge continue unimpaired into healthy old age others, including processing speed, reasoning, memory, and executive functions, often experience a decline, healthy individuals with higher education and occupational achievement develop a ‘cognitive reserve’ which assists in preserving cognitive capacity (Deary et al. 2009).
Aging and Creativity
There is evidence that, in contrast to general declines in cognitive function, reduced suppression of the DMN enhances creativity in older people in comparison with younger adults (Adnan et al., 2019; Spreng & Turner, 2019). In normal aging art creation is associated with increased functional connectivity within the DMN and with increased resilience (Bolwerk et al., 2014). Altered neurological architecture, cognitive reserve and increased connectivity may be reflected in the work of healthy aged artists who demonstrate continued vitality, increased mastery and an ability to convey life’s deeper meaning. This helps explain the frequency of masterpieces being painted in old age (Clark, 2006; Simonton, 1990). Exceptional artists often demonstrate enhanced creativity in old age (Freundlich & Shively, 2006). This contrasts with other fields of creativity such as mathematics and science in which creativity is highest in early to mid-adulthood (Jones & Weinberg, 2011).
Aging and Vision
Visual deficits due to neural degeneration are common in the elderly often the result of neural and macular degeneration, diabetic retinopathy, glaucoma and cataract (Eramudugolla et al., 2013; Jackson & Owsley, 2003; Rodda et al., 2011; Spear, 1993; Swenor & Ehrlich, 2021; Zhang et al., 2013). The effects include reduced spatial and temporal contrast sensitivity and resolution, slower visual processing, restricted visual field, reduced spatial acuity, presbyopia and reduced peripheral vision. Such changes appear related to thinning of the RNFL, loss of macular volume, reductions in thickness of the GCL, degeneration of the optic nerve, loss of volume in the IPL, inflammation, swelling of photoreceptors and microvascular abnormalities including increased choroid thickness (Chauhan et al., 2020; Trinh et al., 2021).
Aging is also accompanied by significant loss (≈30%) of rod density reducing perception of contrast, contrast sensitivity, and motion particularly at the periphery (Barbur & Rodriguez-Carmona, 2015). Deterioration of contrast perception begins with high spatial frequencies in middle age and then extends to all frequencies by age 60 (Yan et al., 2020). Deterioration in older healthy subjects is found in both the parvocellular and magnocellular pathways but predominately in the latter (Zhuang et al., 2021). Contour integration and surround suppression are also significantly reduced in the aged (Casco et al., 2011; McKendrick et al., 2018; Roudaia et al., 2008).
Macular degeneration, the most common age-related deficit, causes loss of central visual acuity due to macular thinning and deterioration of the retinal pigment epithelium. The latter, which includes cone loss, generates dysfunction in processing the tritan (blue-yellow) axis altering colour vision. Sensitivity to shortwave (blue-violet) colours is reduced and there is a preference for yellow backgrounds. Those who are socially excluded perceive their environment as darker and compensate by seeking brighter conditions (Pfundmair et al., 2019). Symptoms also include impaired vision in scotopic conditions, reduced contrast sensitivity at both low and normal levels of luminance and reduced motion perception, all of which are consistent with dysfunction in the magnocellular pathway and accelerated rod loss (Alizadeh-Ebadi et al., 2013; Erdinest et al., 2021; Faubert, 2002; Jackson & Owsley, 2003; Ou et al., 2021).
METHODS
Rembrandt’s style and technique have been the subject of a decades long multi-disciplinary study devoted to authentication of his works known as the Rembrandt Project. It constitutes the most extensive examination of any artist’s oeuvre. The results are published in ‘A Corpus of Rembrandt’s Paintings’ (van de Wetering et al., 2005). Although a wide variety of scientific techniques were utilised computer-based analysis of stylistic elements was not among them not least as these techniques only recently became available. The former director of the Rembrandt Project revised the Corpus and attributed a total of 324 paintings to Rembrandt (van de Wetering, 2017). It is on this catalogue that the present study relies for both authenticity and chronology.
Forty-one paintings are self-portraits constituting some 12% of Rembrandt’s painted oeuvre. Eight have suffered physical deterioration. These are included as tests did not produce anomalous results. Seven images of Rembrandt in historical and biblical paintings are excluded. 116 portrait paintings were considered consisting of: 84 individual portraits of unrelated sitters, 11 group portraits of two or more unrelated individuals, 16 are of family members and 5 are of friends (‘related others’) (Manuth et al., 2019 (a); van de Wetering, 2017). One portrait of an unrelated individual was excluded due to poor condition. Character studies, known as ‘tronies’ were excluded as were images of models. Portraits so defined represent 35% of Rembrandt’s painted oeuvre. In total 156 paintings, 48% of Rembrandt’s painted work, are included. All are painted in oil on wood panel or canvas (Bomford et al., 1988). Works on paper, including self-portraits, are not considered except in regard to overall productivity.
All the images examined are high resolution digital images downloaded wherever possible directly from the institutions which own the original paintings. Where this was not possible high-resolution images available were obtained from open sources. Most institutions provided images in Tag Image File Format (TIF or TIFF) which preserves image quality regardless of copying. Some images were provided in Portable Network Graphics (PNG) format which is equivalent to TIFF in all relevant respects. The remaining images were obtained in Joint Photographic Experts Group (JPEG) format. The images had varying numbers of pixels which were not standardised as test results were found to be substantially the same after rounding. Images obtained in JPEG and PNG format were converted to TIFF using a publicly available on-line service (xconvert.com) to ensure both consistency and stability of the images during processing. Several images provided by institutions included picture frames or colour reference bars which were removed using Adobe Photoshop (adobe.com/uk). TIFF images were used to measure contrast and fractals and converted to JPEG to measure colour to meet software requirements.
Contrast
Contrast is the perception of differences in luminance, which is the intensity of light emitted from a source or reflected from an object. It is this which allows perception of edges and distinguishing an object from its background. Contrast is used to both help identify objects, primarily in the ventral (parvocellular) stream and to detect their location and motion, primarily via the dorsal (magnocellular) stream (Avidan et al., 2002; Gardner et al., 2005; Yang et al., 2019). Contrast is the primary signal from the retina to the visual cortex. Signalling is accomplished by contrast gain control, the adaptation of neurons to changes in luminance expressed by adjusting firing rates as contrast increases and decreases (Boynton, 2005). Retinal contrast is moderated by dopamine particularly in coupling amacrine and horizontal cells and tuning receptive fields (Djamgoz et al., 1997; Popova, 2020). In the cortex contrast signalling is impaired in disorders linked to dysfunction in GABA interneurons essential to lateral inhibition (Turkheimer et al., 2020).
Sensitivity to contrast is a measure of the degree of contrast necessary for someone to recognise an object. The minimal luminance contrast is the contrast threshold, and its reciprocal is contrast sensitivity. Spatial contrast, used to measure contrast sensitivity, refers to the light-dark transition of a border or an edge that delineates an object or pattern (Owsley, 2003). Contrast sensitivity can be measured at multiple spatial and temporal frequencies which can identify which visual pathways (ventral - higher frequency, or dorsal - lower frequency) are involved or impaired (Derrington & Lennie 1984; Pelli & Bex, 2013). Surround suppression, the reduction in perceived contrast of a stimulus caused by the presence of surrounding stimuli as compared to when it is viewed in isolation, can also be measured to detect abnormality in contrast processing (Pokorny, 2023; Salmela et al., 2021).
Artists consciously and unconsciously adjust contrast in their work in ways which exploit the visual system’s characteristics to create the appearance of depth and transparency (Cavanagh, 2005). The development of artistic techniques, including chiaroscuro used extensively by Rembrandt, can be tracked by detecting changes in brightness (Kim et al., 2014a). An effort to analyse the changes in chiaroscuro in the faces of several of Rembrandt’s self-portraits, although limited in scope, distinguished originals from copies and revealed that his use of this technique was similar in his early and late periods but quite different in middle age (Asmus & Parfenov, 2019).
To measure changes in levels of contrast an average measure of contrast was derived from each painting. In digital images the luminance of a pixel is derived from its red, green, and blue (RGB) wavelength components as 0.299R + 0.587G + 0.11. The images were analysed as a two-dimensional random process and the mean and autocorrelation function provided a value for the contrast of brightness in the portraits. The autocorrelation function quantifies the average relationship between data points in a time series and their previous data points using the Wiener–Kinchin theorem (Chatfield & Xing, 2019). Matrix transformation of luminance values was conducted using the 2-D Fourier Fast Transform and the average image contrast was characterized by the value at origin (Turkheimer et al., 2020). The calculations were performed using MATLAB (v. R2021a).
Colour
Colour perception and discrimination in healthy adults begins to deteriorate from age 50 and accelerates thereafter. The deterioration is general however loss of discrimination between colours on the yellow-blue axis is greater than red-green particularly in scotopic conditions. This results both from receptor and post-receptor dysfunction and yellowing and increasing density of the lens reducing light reaching the retina. The result in natural aging as well as related eye disease is to reduce shortwave, with a consequent increase in the proportion of long wave light, perceived (Trevor-Roper, 1959).
Several neurological conditions, in addition to depression and normal aging, are characterised by reduced levels of dopamine and low levels of blue cone response (Almonte et al., 2020; Colzato et al., 2015; Djamgoz et al., 1997; Roy et al., 2003). In Alzheimer’s disease and attention deficit hypoactivity disorder hypodopaminergic conditions are associated with reductions in tritan perception (Kim et al., 2014b; Polo et al., 2017; Tannock et al. 2006). This is consistent with shortwave cone dysfunction as these are responsible for processing blue colour and highly sensitive to dopamine dysregulation.
Examination of the use of colour in art using digital images and statistical techniques has yielded some insights. A large study of paintings from the 15th to the 20th century identified levels of colour contrast associated with particular artistic styles (Lee et al., 2017). A computational analysis of colour range was used to distinguish Renaissance from Baroque painters noting a decline from the earlier to the later period (Romero et al., 2018). Fractal colour analysis has been used to track both changes in colour and the use of chiaroscuro from the medieval to the modern era (Kim et al., 2014a). More recently it has been used to track the relationship of colour and complexity with emotional and physical pain (Turkheimer et al., 2022).
Here for each painting the colour median was measured in RGB and Cyan Magenta Yellow Black (CYMK) models. RGB, often known as additive colour mixing, is a method of encoding the three cone receptor wavelengths. In concept, each colour is made up of red, green, and blue light that shines with varying intensities. Hexadecimal coding allows integers ranging from 0 to 255 to be represented with only two digits. The CMYK colour model is a subtractive colour model used in colour printing. It subtracts or masks colours from the white backdrop of the paper as ink decreases reflected light. The RGB and CMYK average values were calculated using MATLAB (v. R2021a).
4
Fractals
Mandelbrot (1977/83) discovered that there is a random, chaotic element in the regularity of natural patterns which he called fractals. Fractals are repeating, power-log scaling, invariant spatial patterns found throughout nature from snowflakes, to mountain ranges. What characterizes these phenomena is statistical self-similarity of form over different magnifications. Fractals thus represent both the complexity and regularity found in nature. Viewed from a mathematical perspective, complexity is derived from chaos theory. This provides a means of quantifying the non-linear dynamics of natural systems. Fractals can be seen as emergent, non-linear, spatial and temporal patterns of both chaotic natural phenomena and of self-organising systems such as the brain, which can be measured empirically (Grosu et al., 2023; Turkheimer et al., 2015).
This form of regularity can be seen to follow a log power law of fractal dimension ‘D’ varying from 1 to 2 or 2 to 3 depending on whether 2 or 3 dimensions are being measured. This variable can be seen as a measure of spatial order-disorder (number of regularities) and simplicity- complexity (number of elements). D increases as the overall structure increases in fine spatial detail reflecting the relative complexity of the object (Mandelbrot, 1977/1983). A straight line has a D value of one and a completely filled two-dimensional space has a D value of 2. Increasing detail in the space is measured as increasing D value; essentially a ratio of filled, repeating structural detail to empty space. Fractals thus provide a statistically reliable method of quantifying visual complexity by measuring the relative amount of coarse and fine structure in any image including art images (Cutting & Garvin, 1987; Forsythe et al., 2011; Pentland, 1984; Spehar & Walker, 2016)
Fractals are a key aspect of modern understanding of health, both physical and mental. Numerous aspects of human physiology such as the bronchia of the lungs and the circulatory system, as well as the brain at multiple levels, demonstrate fractal structure (Di leva, 2016). Contrary to long-standing views, health is not characterized by linear, homeostasis and equilibrium but by non-linear, ’chaotic’ fluctuations related to points of attraction, ‘attractors’ and allostatic regulation (Varela et al., 2010; West, 2006). ‘Chaos’ in this sense is both inherent in and essential to mental and physical health and its loss a sign of distress (Bruzzo, 2007; Solé & Goodwin, 2000). Normal aging is characterized by declines in subcortical and cortical fractal values (Marzi et al., 2020; McDonough & Madan, 2021) and reduced dendritic arborisation (Goldberger et al., 2002). Higher fractal values in white matter correlate to greater cognitive fluidity in old age (Mustafa et al., 2012).
The self may be seen as a self-organising system demonstrating complexity and fractal dynamics. Self-complexity, possessing multiple traits and the ability to move among them, provides flexibility, adaptability and resilience to environmental changes moderating both physiological and psychological stress (Brown & Rafaeli, 2007; Linville, 1987). In contrast, lack of fluctuation, rigidity, is characterized by a lack of adaptability and psychopathology (Bruzzo, 2007). Rigidity in psychopathology has long been observed by leading clinical observers (Freud, 1896; Horney, 1942; James, 1890; Jung, 1916; Pincus et al., 2019). Deviations from fractal regulation are indicative of anxiety, depression, and BPD (de la Torre-Luque et al., 2016). Disrupted temporal fractal patterns of heart rate variability predict sub-clinical depression (Mandarano et al., 2020). In MDD patients deviant fractals are associated with inflexible and rigid and less spontaneously adaptive, rhythms and periods of hand motion and physical activity (Aybeck et al., 2012; Knapen et al., 2021). Non-linear fractal analysis of EEGs in MDD patients found higher levels of complexity compared with healthy controls consistent with neuroimaging studies revealing hyperactivity in frontal and parietal areas (Akar et al., 2015). BPD, in contrast, is characterized by pre-frontal grey matter reduction and hypoactivity and commensurate reduced fractal dimension compared with healthy controls (Squarcina et al., 2015).
Complexity and the Eye
There is considerable evidence that the eye and brain have evolved to perceive and prefer the statistical properties of natural scenes which are used to interpret and optimise sensory data (Graham & Redies, 2010; Nguyen & Spehar, 2021; Seriès & Seitz, 2013; Simoncelli & Olshausen, 2001; van Hateren & van der Schaaf, 1998). Features of the natural environment have fractal values in the mid-range of 1.3-1.5 and humans are attuned to these levels (Hagerhall et al., 2004; Heerwagen & Orians, 1993; Taylor & Sprott, 2008). EEG responses confirm that natural images with mid-range D values are appealing, reduce stress and stimulate calm attention: high alpha in the frontal lobes and high beta in the parietal area (Hagerhall et. al., 2008; Taylor, 2006; Taylor et al., 2011; Ulrich, 1984). Visual preference for mid-range fractals extends across categories (natural scenes, moving objects, mathematical patterns and paintings) and is associated with beauty in nature and in humans. It is already apparent in children and extends across cultural boundaries suggesting it is a trait (Draves et al.,. 2008; Forsythe et al., 2011; Friedenberg et al. 2022; Graham & Field, 2008; Robles et al., 2020; Spehar et al., 2003; Taylor & Sprott 2008).
Human eyes follow a search trajectory measured by saccades with a D value of 1.4-1.5 indicating a resonance between the visual system and natural scenes and objects (Franěk et al.,. 2019; Namazi et al., 2016). Nearly identical fractal patterns and patterns of activity are found throughout the visual system including in neurons of the retina, LNG and occipital cortex whose dendritic trees use fractal patterns in the range of 1.33-1.51 thus optimising efficiency in signal transmission (Palva et al., 2013; Smith et al.,. 2021; Werner, 2010). Retinal adaptation to contrast follows a fractal pattern (Greenlee et al., 1991).
These scale invariant, sparse, regularities of spatial, luminant and chromatic natural characteristics are also reflected in art (Graham & Field, 2007; Graham & Redies, 2010; Nascimento et al., 2007) and are an important element in observer preferences (Aks & Sprott, 1996). Artists unconsciously depict faces with fractal values in the mid- range (1.3-1.5) rather than as found in real faces (Redies et al., 2007a, 2007b). Departures from this norm, in either direction, are disturbing in painted and other images. Spatial frequencies in art significantly higher than those found in nature are associated with triggering epileptic seizure and migraine in susceptible individuals and aversion in healthy subjects (Fernandez & Wilkins, 2008). Mid-range fractal values are aesthetically appealing. Lower and significantly higher values are less appealing in both synthetic images and in art (Spehar et al. 2003; Taylor et al. 2005, 2006; Viengkham & Spehar, 2018) although there appears to be a preference for some natural images and abstract patterns with D values somewhat above 1.5 (Forsythe et al., 2011; Rawls et al., 2021).
Fractal analysis has illustrated changes in complexity which correspond to stylistically defined periods in artists’ careers (Lopes & Tenreiro Machado, 2019). Jackson Pollack was found to have an identifiable pattern of fractal development in particular periods. Complexity in his work increased over time from a D value close to 1 to 1.72 in his ‘drip’ paintings significantly above the preferred middle range of 1.3-1.5 (de la Calleja et al., 2016; Taylor, 1999; Taylor et al., 2011). This may account for early negative critical and public reactions (McElroy, 2010). His contemporary Mondrian moved in the opposite direction from early naturalistic paintings with a fractal dimension of 1.7 to abstract paintings consisting of lines of varying thickness with fractal values approaching 1 (Bountis et al., 2017; Taylor et al., 2005). Distinct patterns of fractals have been found in healthy aging painters (Monet, Chagall, Picasso) as compared with those who have suffered from degenerative disease (Dali, de Kooning, Morrisseau, Brooks) the former demonstrating increasing complexity with advancing age and the latter declining levels with the advance of disease (Forsythe et al., 2017).
Fractal Measurement
Two methods of fractal analysis were used in the present study: one ‘2’ and one ‘3’ dimensional. The former was obtained from ImageJ software (
http://rsbweb.nih.gov/ij/index.html) which uses “box counting” a widely used sampling and data collection process. The fundamental approach is converting the image to greyscale and placing a sequence of grids of decreasing calibre (boxes) over a picture methodically and recording data for each subsequent calibre (counting) creating grids of progressively larger sizes. FracLac generates the grey D value on the basis of the correlation between the variations in both average intensity and grid calibre due to the change in sampling process. The image type was set to 8-bit grayscale and the threshold adjusted with the “Default” algorithm which was chosen after tests using alternative binarizing functions for threshold segmentation produced outcomes within an error range of 0.03 to 0.05. Box sizes were set as 2,3,4,6,8,12,16,32 and 64 for counting and plotting. The range of D values is between 1 and 2 and the values are plotted with log(S) on the x-axis and log(N(s)) on the y-axis.
Considerable colour information is lost after conversion to greyscale which can affect the accuracy in roughness measurement. Every pixel of grayscale images has only one intensity channel; pixels of colour images have multiple intensity channels made up of red, green and blue (RGB). In order to reduce potential inaccuracy, a modified differential box counting approach (Nayak & Mishra, 2016) was used to determine fractal dimension values of RGB colour images. Combining the “probabilistic algorithm” method (Ivanovici & Richard, 2011) and the “box merging” method extending box counting, (Nikolaidis et al., 2011) the fractal dimension of RGB colour images was estimated using vectors in 5-dimensions (x, y, r, g, b). A greyscale image in this format generates 256 distinct shades of colour between 0 and 255. Applying this method to the 24-bit format for colour images resulted in individual RGB components calculated based on six possible combinations ranging from Ix,y (1) to Ix,y (6) which are averaged to improve accuracy of the roughness estimate. The range of D values using this method is between 2 and 3 which were plotted on the basis described above.
Results
If Rembrandt was depressed one would expect evidence of reduced contrast gain, contrast sensitivity, surround suppression, peripheral vision, and motion detection in paintings during Critical Years. His perception of brightness and colour in his environment would diminish and be reduced in his paintings. Alternatively, one might find heighted contrast used to compensate which might be evidenced by a shift in colour toward yellow. If he suffered from reduced visual acuity the complexity of his painting would decline. The same effects would be expected due to aging or degenerative brain disease.
Contrast
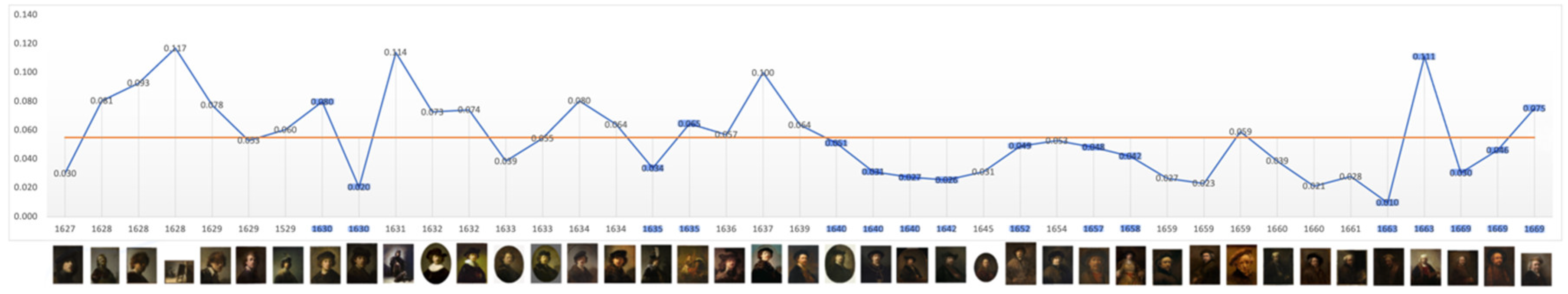
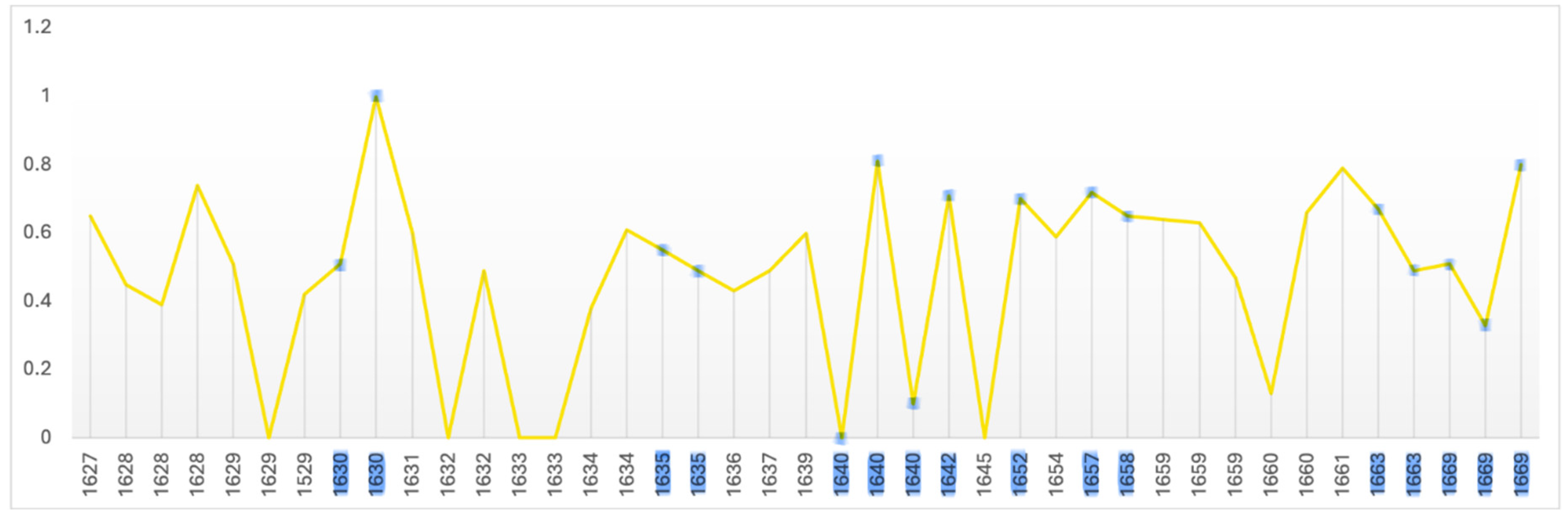
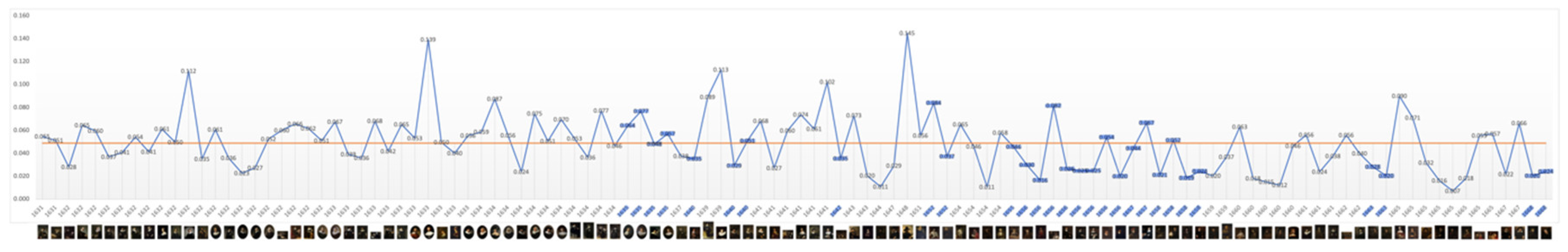
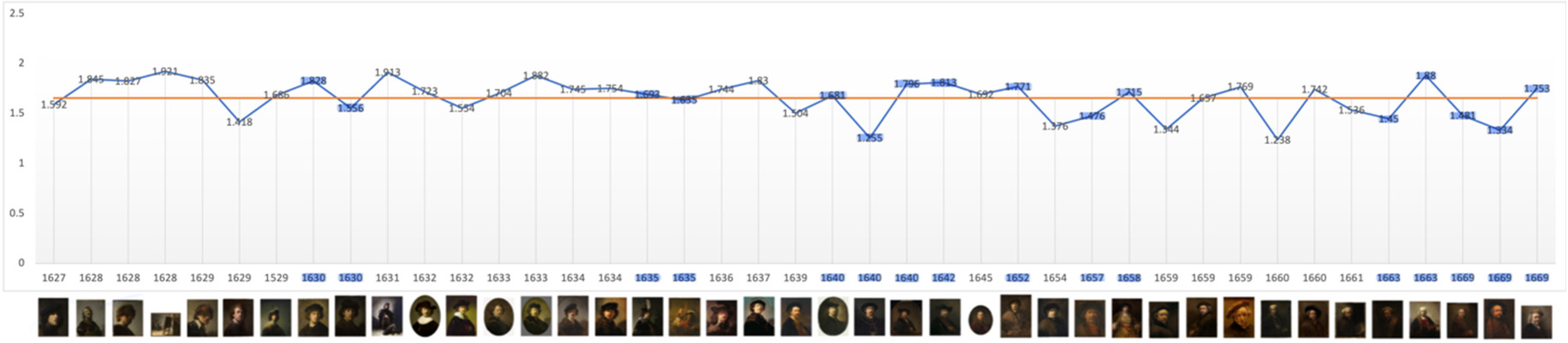
The level of average contrast, brightness, in Rembrandt’s self-portraits, varies in his early years but falls and remains below the mean in 1640, the year of his mother’s and a third infant’s death and two years before his wife’s death; it remains so for the following 23 years. It is noteworthy that it increases close to the mean during his bankruptcy and again in 1663, the year of his mistresses’ death (
Figure 1). This is consistent with a previous study of his use of chiaroscuro (Asmus & Parfenov, 2019). One could conclude from this that he went into dysthymic depression following the deaths of three infant children, his mother and during his wife’s illness, a depression lasting more than two decades which was alleviated on the death of his mistress. However plausible there is a notable absence of other symptoms of depression and contrasting evidence of continued creativity and productivity. The late rise in contrast is consistent with macular degeneration and a perceived shift towards yellow (Marcus & Clarfield, 2002; and see Colour below). The self-portrait on which he worked throughout his late period (1663-1669) has a markedly lighter background.
Contrast-Average Level – Self Portraits
Figure 1.
Mean: 0.055, SD: 0.027. Range: 0.009 – 0.117. Critical years: Mean: 0.047, SD: 0.026.
Figure 1.
Mean: 0.055, SD: 0.027. Range: 0.009 – 0.117. Critical years: Mean: 0.047, SD: 0.026.
Contrast-Average Level – Portraits
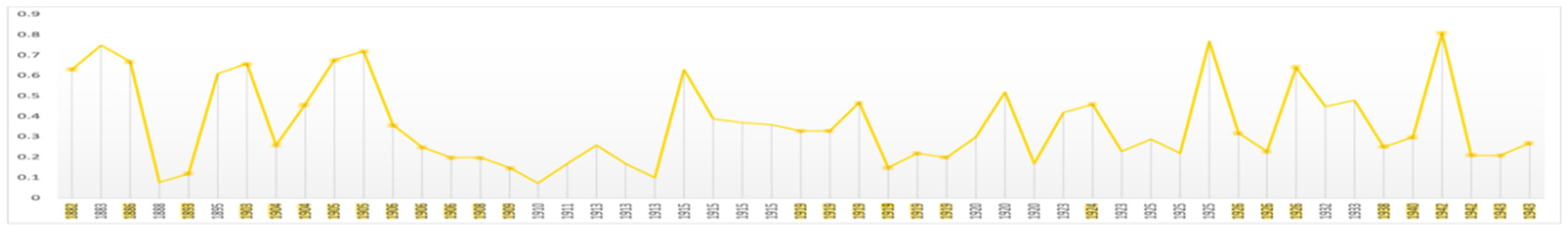
The mean levels of average contrast in his self-portraits (0.55) and portraits (0.53) are relatively stable but both are lower during Critical Years; 0.47 and 0.40 respectively (
Figure 1 and
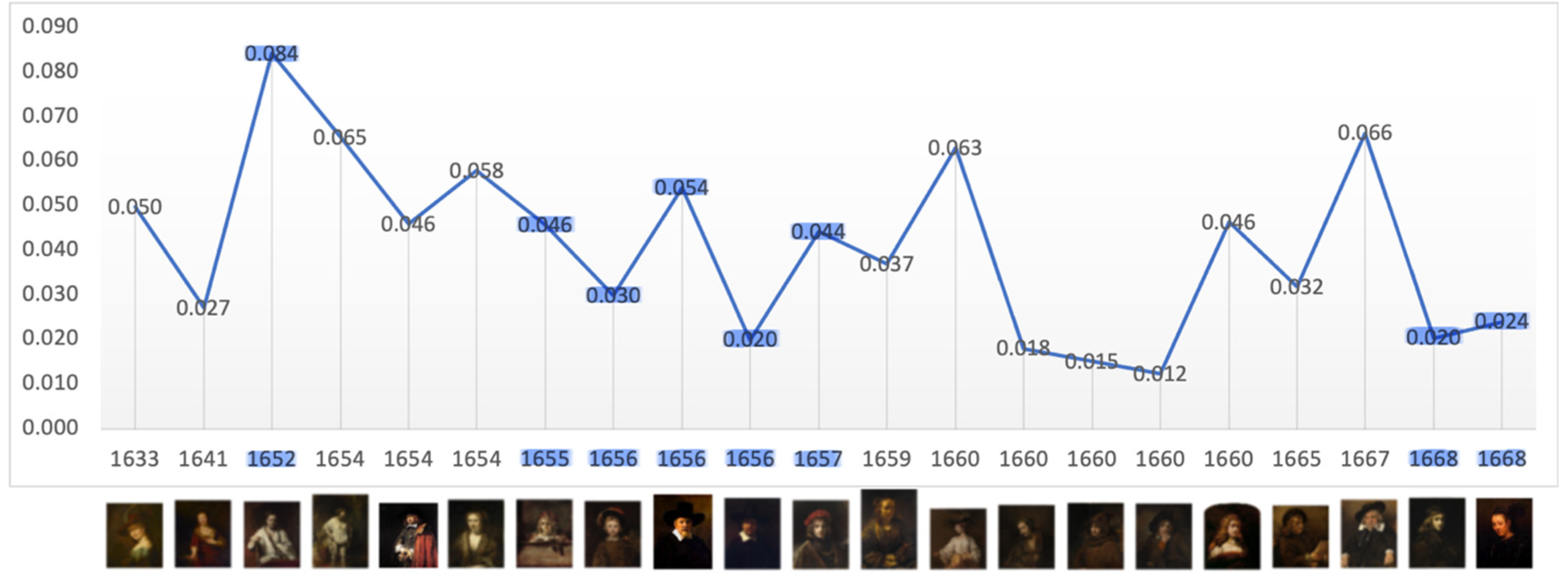
Figure 2). Thereafter contrast returns toward the mean. When the portraits are disaggregated the evidence is mixed. In Critical Years there is no meaningful decline in portraits of family and friends and in group portraits the mean level of contrast rises (
Figure 3 and
Figure 4). The level of contrast of individual portraits of unrelated sitters in Critical Years is much lower, 0.038, suggesting that in such periods he was particularly reluctant to paint them, but the number of such portraits painted increases as he sought to increase his income (see Productivity below and Supp.
Materials Figure S15). These varying results indicate some degree of low but transient mood. The explanation based both on connoisseurship and technical analysis, that he made deliberate changes of style in 1640 and again in 1663 is consistent with this (van de Wetering 2017).
Contrast – Average Level - Family and Friends
Figure 3.
Mean: 0.041, SD: 0.020, Range: 0.012 – 0.084. Critical years: Mean: 0.040; SD: 0.022.
Figure 3.
Mean: 0.041, SD: 0.020, Range: 0.012 – 0.084. Critical years: Mean: 0.040; SD: 0.022.
Contrast-Average Level - Group Portraits
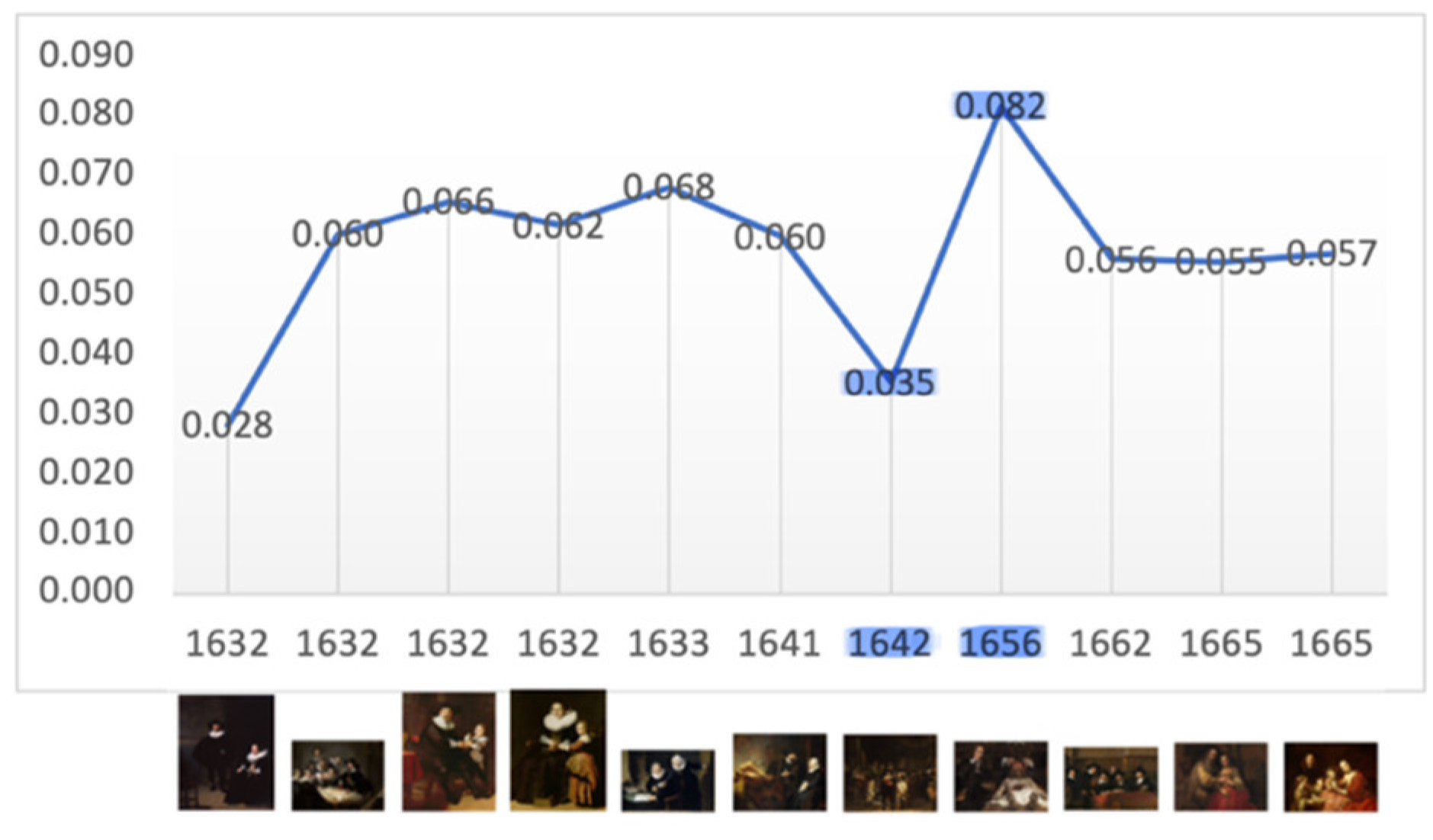
Figure 4.
Mean: 0.057, SD: 0.015. Range: 0.028 – 0.082. Critical years: Mean: 0.059; SD: 0.033.
Figure 4.
Mean: 0.057, SD: 0.015. Range: 0.028 – 0.082. Critical years: Mean: 0.059; SD: 0.033.
Colour
Primary colours were used sparingly by Rembrandt who virtually eliminated blue and green from his palette early in his career, before any adverse life event. Thereafter his palette was dominated by white, black, earth tones, brown, yellow and red. He continued to use smalt, a cobalt glass, to accelerate drying and thicken the texture of paint layers but not as a pigment. Unlike his other colours, which are stable, smalt fades to grey/brown over time thus analysis may therefore underestimate the amount of blue originally present (Bomford, 1988; van de Wetering, 1997).
Median Percentage of Colour- Self Portraits
Figure 5.
Blue. Mean: 26.78; SD: 12.62. Range: 2-56. Critical years: Mean: 26.94; SD: 8.96.
Figure 5.
Blue. Mean: 26.78; SD: 12.62. Range: 2-56. Critical years: Mean: 26.94; SD: 8.96.
Figure 6.
Yellow. Mean: 0.481; SD: 0.262. Range: 0 – 1. Critical years: Mean: 0.565; SD: 0.257.
Figure 6.
Yellow. Mean: 0.481; SD: 0.262. Range: 0 – 1. Critical years: Mean: 0.565; SD: 0.257.
Median Percentage of Colour- Portraits
Figure 7.
Blue. Mean: 21.99; SD: 9.63. Range: 2-56. Critical years: Mean: 22.79; SD: 9.15.
Figure 7.
Blue. Mean: 21.99; SD: 9.63. Range: 2-56. Critical years: Mean: 22.79; SD: 9.15.
Figure 8.
Yellow Mean: 0.564; SD: 0.315. Range: 0-1. Critical years: Mean: 0.639; SD: 0.281.
Figure 8.
Yellow Mean: 0.564; SD: 0.315. Range: 0-1. Critical years: Mean: 0.639; SD: 0.281.
When Rembrandt’s use of all colours is analysed there is a fall overall after his wife’s death and an overall rise during his bankruptcy followed by a surge in the year of his mistress’s death. There are comparable variations in the absence of adverse life events which together indicate a lack of correlation. When blue is isolated there also appears to be no meaningful difference in its use in Critical Years. His use of yellow however significantly increases in both self-portraits and portraits in Critical Years. This could indicate an effort to compensate for a sense of dimness or lack of colour in his environment in such years consistent with low mood. There is also an overall reduction in the use of blue beginning in 1657 and an increase in yellow beginning in 1660 which is consistent with age-related macular degeneration. This is consistent with those who observe a ‘yellow-brown’ shift in the last self-portraits three of which were painted and one finished in his last year (Marcus & Clarfield, 2002; van de Wetering, 2017).
Fractals
The two methods of fractal analysis both proved sensitive in detecting significant variations between and within the self-portraits and portraits.
Fractal Dimension: Self-Portraits (ImageJ)
The two-dimensional fractal D value of Rembrandt’s self-portraits has a wide range (1.238 – 1.921) with a mean value of 1.657 (
Figure 9). This is a relatively high level of complexity varying from within to above the median range associated with natural attractiveness and beauty but consistent with the appeal of his self-portraits as being complex but approachable. There appears to be a gradation downwards from that level to 1.601 when all portraits are considered, to group portraits, 1.595, to related others, 1.529 (Supp. Materials, Figs. S16-19). This is consistent with the saliency gradient apparent in the mPFC. It is also consistent with a more informal style in painting of related others detected by art historians (Runia and de Witt, 2019; van de Wetering, 2017). The mean D value of individual portraits of unrelated people of 1.621 is remarkably close to ‘Phi’ the Golden Ratio of 1.618, often called the ‘Golden Mean’. which has long been associated with mathematical, natural, and artistic beauty (Pacioli (1509); Livio, 2002; Supp. Materials,
Figure S19). This suggests fluctuation around an attractor indicative of intuitive understanding of what appeals to the observer.
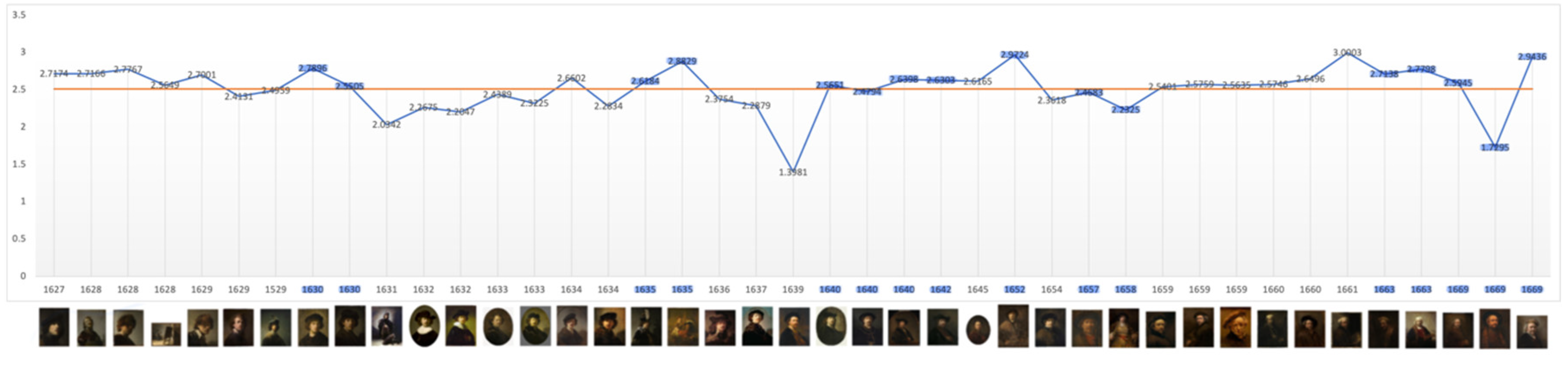
Fractal Dimension (RGB): Self-Portraits
When colour is adjusted for the fractal mean value of the self-portraits and all categories of portraits varies only marginally from 2.5 (Range: 2.514 - 2.571): Self-portraits: 2.515, Portraits: 2.52, Groups: 2.514, Family and Friends: 2.571, Individual Unrelated Portraits: 2.509 (
Figure 10 and see Supp. Materials: Figs. 20-23). A D level of 2.5 in this format is the equivalent in two-dimensions of 1.5. This is at the ‘cusp,’ of the widely recognised mid-range of natural and aesthetic preference; the point at which arousal, attention and beauty reach optimal levels before preference declines (Forsythe, 2011; Taylor et al., 2011; Wang & Ogawa, 2015).
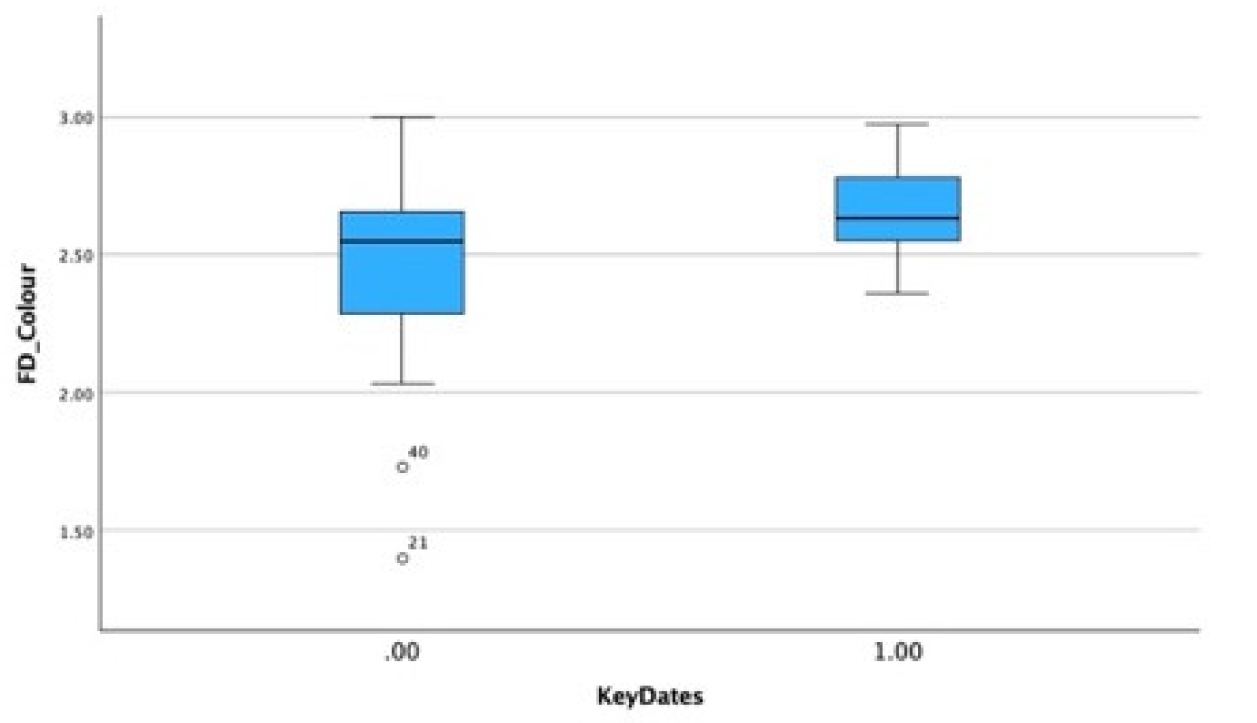
In Critical Years all categories, except related individuals, the D value rises: Self-portraits: 2.599, Portraits: 2.54, Groups: 2.545, Portraits of Unrelated Individuals: 2.529, suggesting greater complexity. There is also a statistically significant increase in the colour adjusted values in self-portraits produced near critical dates (Mean FD = 2.650 Std = 0.174) compared with those produced during non-critical periods (Mean FD = 2.453 Std = 0.338) and Wilcoxon test p=0.05 (
Figure 11 and
Figure 12).
5 This difference is consistent with EEG findings showing moderate increases in fractal dimension, complexity, in both beta and gamma wave analysis of frontal and parietal areas in MDD (Ahmadlou et al., 2012, Akar et al., 2015). Consistent with the 2D analysis there is no correlation with age so the effect was temporary. There is no appreciable change in D levels in old age using either method which seems to exclude neurological disease.
The higher D values in Critical Years and during Critical Periods indicate an increase in larger spatial frequencies as compared with lower spatial frequencies. This may be due to changes in the backgrounds in the paintings. Visual comparison of self-portraits in critical and non-critical periods does not reveal obvious differences. This may be an effect of the wide variety of materials and techniques Rembrandt used in backgrounds some of which are still being discovered (Bomford, 1988; Gonzalez et al., 2023; van de Wetering, 1997). Many of Rembrandt’s effects are the result of alterations of the surface by brushstroke, paintbrush tip, palette knife and other three dimensional techniques not measured here.
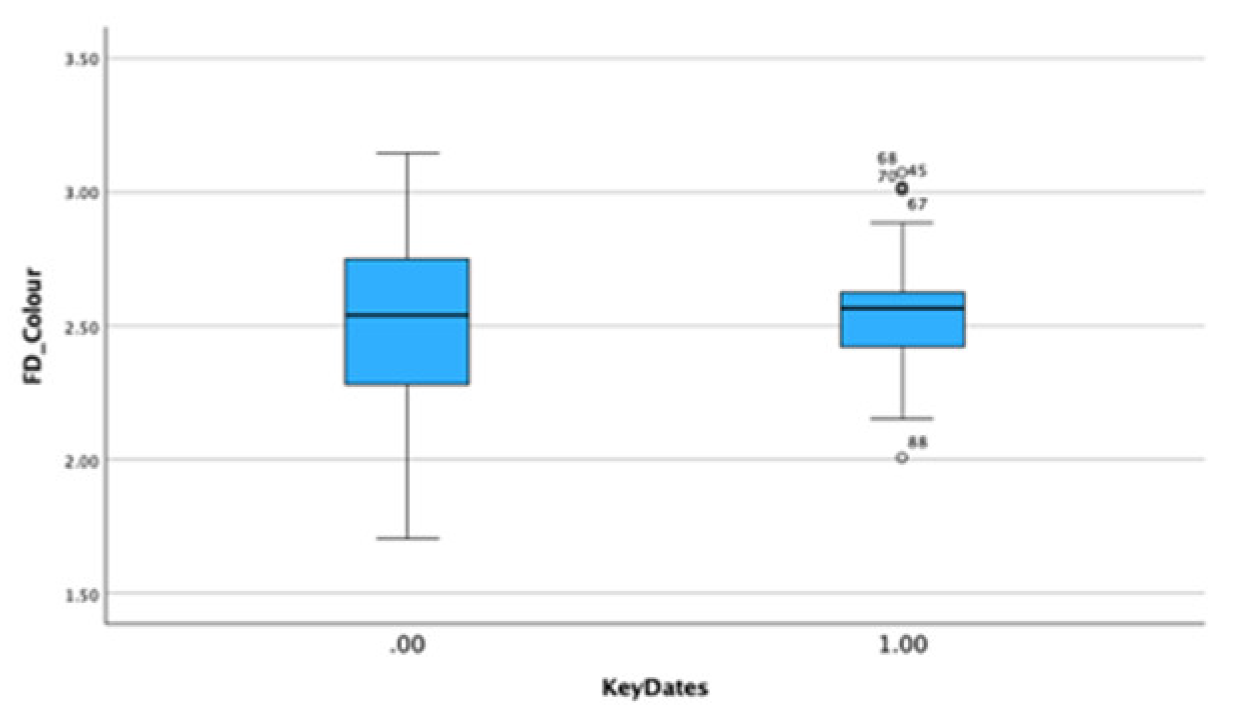
There is no significant difference in the portraits between non-critical periods (mean FD = 2.501 Std=0.332) and those on or near critical dates (mean FD = 2.566 Std = 0.245). This suggests that self-portraits are more likely to reveal inner states consistent with experimental evidence that self-images are of greater saliency than images of others. Both methods find the mean value in portraits of family and friends is markedly lower than in other portraits suggesting a less complex more informal style consistent with connoisseurship and a saliency gradient between self-portraits, related and unrelated portraits.
Rembrandt Self-Portraits: Fractals (RGB) Rembrandt Portraits Fractals (RGB)
Productivity
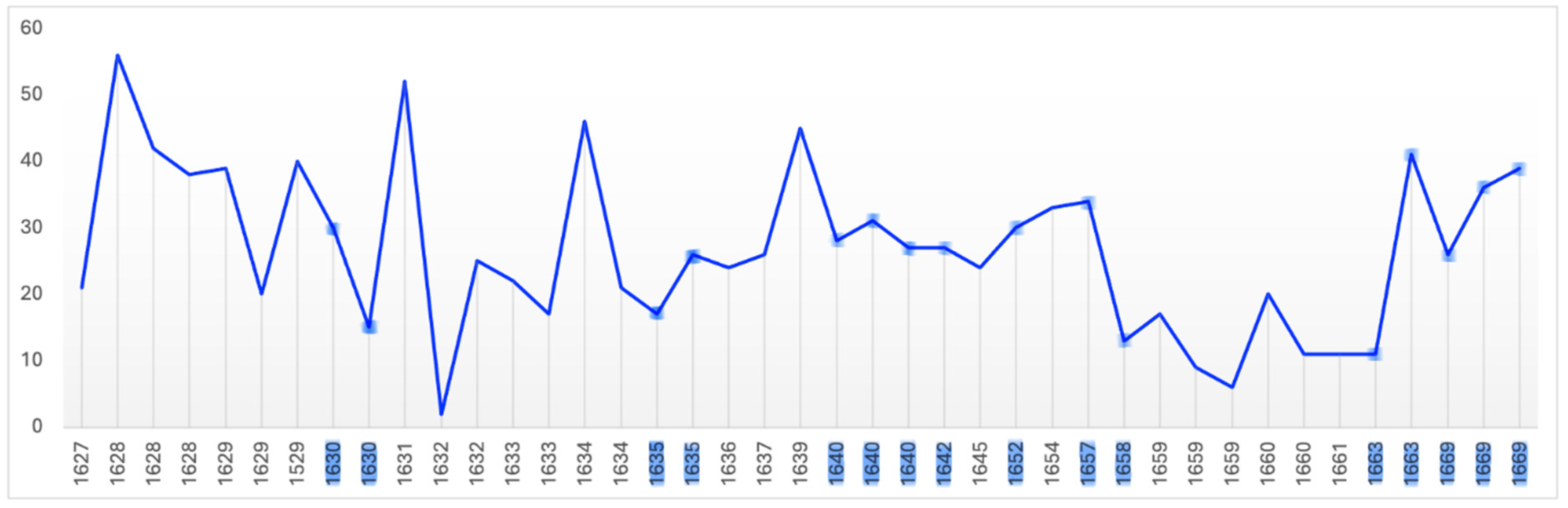
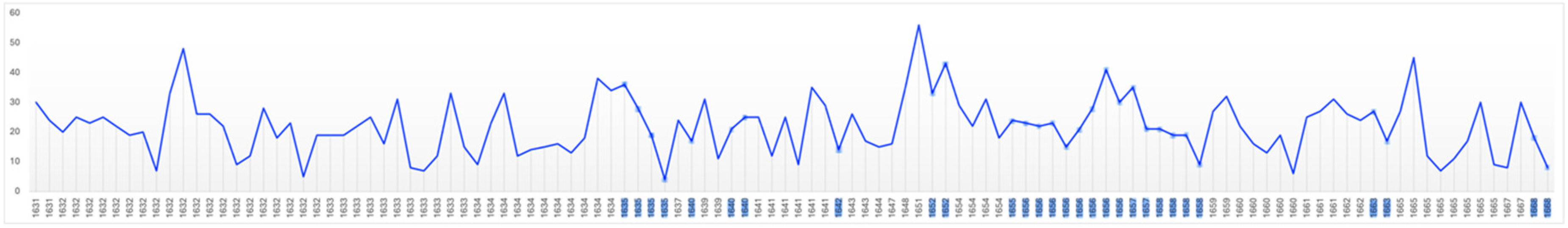
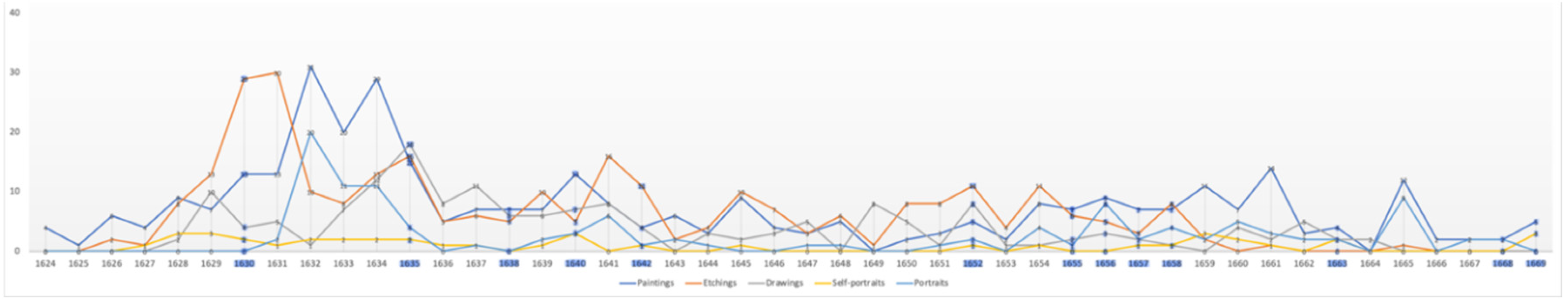
There is evidence that productivity declines with old age (Simonton, 1990). Productivity in artists can be measured both quantitatively and qualitatively. Rembrandt’s annual productivity measured in terms of number of paintings, etchings, drawings, self-portraits and portraits is illustrated in
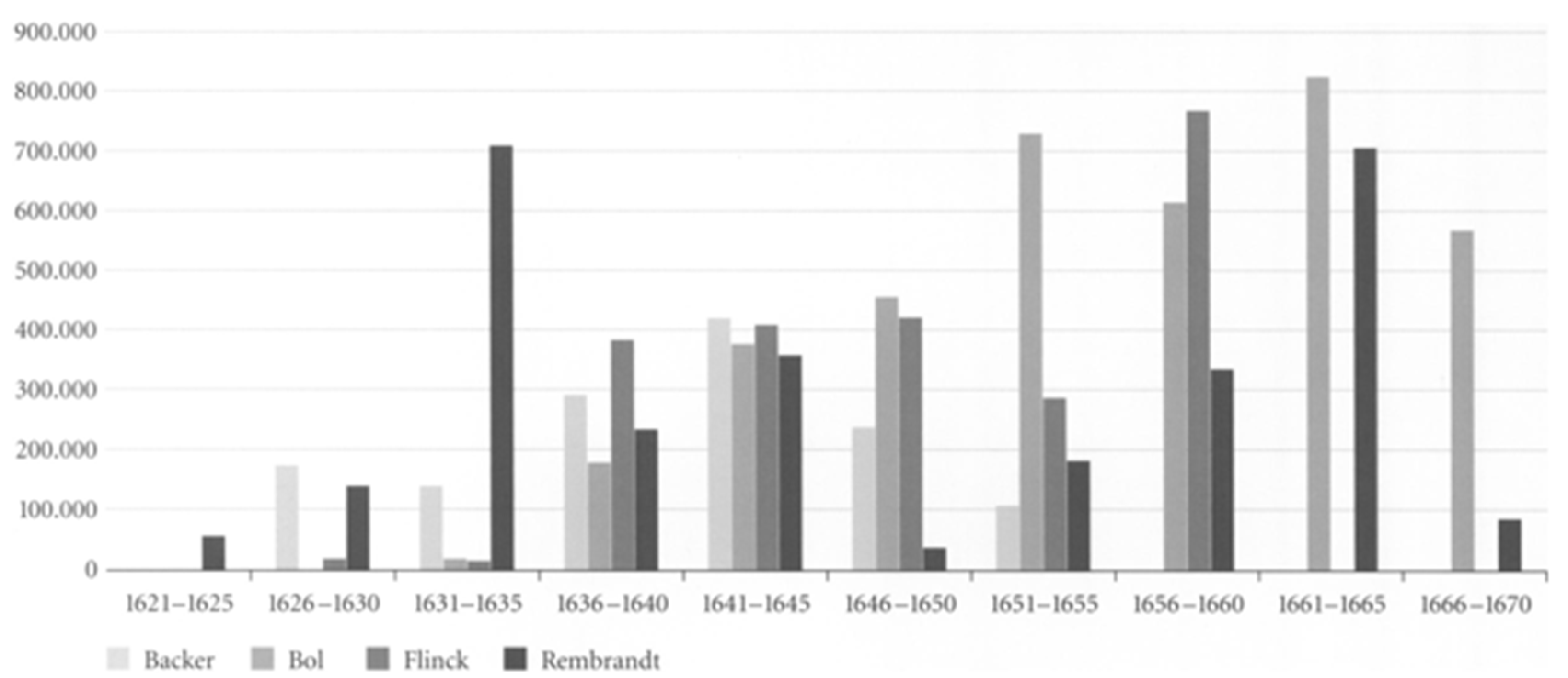
Figure 13. Others have measured his productivity in terms of surface area painted (
Figure 14).
Average Annual Production – Paintings, Etchings, Drawings, Self-portraits & Portraits
Average Annual Portraits: 2.5; Self-Portraits: 0.9. Critical Year Annual Averages: Portraits: 2.0., Self-portraits: 0.8.
Total Painted Surface Area
It is evident that Rembrandt’s productivity, whether measured in number of paintings, etchings or drawings, individually, in combination, or in surface area painted, fluctuated considerably during his life. After his wife’s death the number of his paintings rose, the number etchings and drawings fell then all three returned to their average levels until the beginning of his financial difficulties in 1649 when all fell in number, perhaps contributing to those problems. From that point on until 1653 the number of paintings and etchings, from which, unlike
drawings, he derived significant income, rose (Crenshaw, 2006; Schwartz, 2006; Slive, 2009). While both fell in 1653 they thereafter continued to rise in number through and beyond his bankruptcy. In short, neither his wife’s death nor his bankruptcy reduced his productivity as measured by number of paintings and etchings or by area painted which would be expected in depression; instead it increased.
During the five year period (1641-1645) which includes the last year of his wife’s illness, her death and the three years following, the area he painted increased over the previous 5 year period by two-thirds; from 225,000 to 375,000 square centimetres. By the same measure his productivity had dropped sharply in the previous five years (1636-1640) in which he lost three children and his mother which might suggest, somewhat improbably, that he was more affected by their deaths than that of his wife. A more plausible explanation is that it marks the period following his leaving the workshop of Hendrick Uylenburgh which provided a steady stream of commissioned portraits which established Rembrandt’s reputation. It is likely that beginning to work on his own initially generated fewer commissions (Manuth et al.,. 2019a; Schwartz, 2006).
Between 1646 and 1649 Rembrandt’s productivity measured by number and area painted and number of etchings fell below their long term average as he concentrated on more prestigious and costly history paintings contributing to his financial distress when they failed to sell (Bok & van der Molen, 2009; Crenshaw, 2006; Schwartz, 2006). In 1649 he failed to make a mortgage payment which was the principal event leading to bankruptcy, but from that point on his productivity in number of paintings and etchings and surface area painted began to increase in an effort to meet his obligations. Biographers attribute the initial decline to his decision to cease painting portraits on commission except when in need of money (Bok & van der Molen, 2009) which is consistent with the increasing number he painted in the middle of the bankruptcy period (1654-1658).
From a low point in 1650 his output, measured by surface area, increased until it reached its long-term average of 320,000 square centimetres in 1660; that is, it increased significantly during the period of his greatest financial stress. In the five year period (1656-1660), which includes the bankruptcy’s culmination including the sale of his house and art collection and move to modest rented accommodation, his productivity began to rise suggesting a determination to recover financially. In the following five year period (1661-65) Rembrandt’s productivity reached a peak of 700,000 square centimetres equalling the level reached during his employment by Uylenburgh in the first half of the 1630s (Bikker et al., 2014; Bok & van der Molen, 2009). Thus there is an inverse correlation between adverse life events and painted productivity; his productivity rising during his wife’s illness, death and its aftermath and during and in the aftermath of his bankruptcy. Rembrandt’s productivity, measured by surface area painted declined in the final fours of his life which may be a function of age, reduced vitality, and/or depression, however, measured by number of paintings it began to rise in his last two years and he left three completed and 26 unfinished paintings at his death (Crenshaw, 2006; Strauss & van der Meulen, 1979). It appears that adverse events, including old age, stimulated Rembrandt to work rather than despair.
Conclusions drawn as to productivity from quantitative measures must, however, be qualified as we do not know how many paintings and drawings have been lost nor how many prints were made (Slive, 2009). For example, in 1641, when the number of his paintings appears to decline after his wife’s death, we know that he painted an Ovidian series of paintings now lost. We know neither how many or what size they were (Crenshaw, 2006). If included the numerical decline in that year might be reversed and the area painted increase further. Paintings also continue to be discovered.
6 Thus, it is likely that current quantitative data underestimate his productivity.
Quantitative measures, while measuring sheer activity, do not reflect qualitative change. In the year after his wife’s death he completed ‘The Night Watch’ perhaps his greatest and largest masterpiece and a self-portrait which depicts him in a confident pose modelled on Titian. In the year he lost his house and possessions he painted his only three-quarter length self-portrait in the posture of a prince and master of his profession. In the year following he portrays himself in the self-assured pose of a Renaissance courtier in the manner of Raphael. His self-portraits, at these two critical moments, his wife’s death and his bankruptcy, events which may well have triggered depression in others, reveal resilience and self-confidence. His ability to overcome such setbacks may be explained by the number of protective factors which his life afforded him. There is no family history of depression or mental illness and his life experience included family cohesion, a stable home, good education and health, supportive friendships and social networks, high self-esteem, a sense of efficacy and of purpose, resilience and religious belief (Askeland et al., 2020; Blazer, 2003; Breton et al., 2015; Burnette et al., 2017; Worrall et al., 2020). Also notable is the absence of any reported childhood adverse experience. (Chapman et al., 2004; Kim et al., 2022; Ye et al., 2023).
In his last four years he painted several of his finest portraits, including his only equestrian one, and several masterpieces. The number of self-portraits notably increased four being completed in the last year of his life. This is consistent with an increasing concern with his inner life and his legacy. In them he conveys life’s experience as no one has ever done before or since. There is a broad consensus among art historians and artists that Rembrandt’s late style was his finest. Thus, his productivity, measured qualitatively, can be seen to have increased in old age (Bikker et al., 2014).
Discussion
Informed commentators on Rembrandt’s self-portraits caution against projecting back modern ideas of self-expression onto 17th century painters who, before the ages of the Enlightenment and Romanticism, were more likely to be motivated to paint their images to learn the art of expression or to advertise their name and skill. Indeed, the term ‘self-portrait’ did not exist at the time (van de Wetering, 1999). There is reason, however, to argue that Reformation Holland was a crucible of the modern view of the individual and the increasing status of artists (Chapman, 1990). In any event none of the various purposes which the self-portraits served precludes the expression of one’s state of mind. Rembrandt’s extraordinary capacity to depict facial emotion was noticed early in his career and has been appreciated ever since. It would be astonishing if he did not convey something of his feelings in his self-portraits. Few scholars doubt that the corpus of Rembrandt’s self-portraits, painted, etched, and drawn, was not only the first, but remains the most comprehensive, example in Western art of self-study if not autobiography (Chapman, 1990; Clark, 1978).
The etchings on which a diagnosis of unipolar and then bipolar depression were based (Schildkraut, 2004; Schildkraut et al. 2007) may be of melancholy subjects but they are not images of Rembrandt. Whenever Rembrandt included his self-image in any work, he ensured that he was notably recognisable and never did so in a melancholy pose. It is understandable that his last self-portraits are associated with the cumulative effects of the adverse events of his life. To some they convey a sense of melancholy; this may be attributed to him or to the observers’ own reaction to his unsparing depiction of old age. The analysis here suggests the latter is more likely.
The results show a reduction in average contrast in his self-portraits that began before his wife’s death and lasted for more than two decades thereafter then increasing once more in old age. This is consistent with finding a change during this ‘middle’ period in his use of chiaroscuro, in contrast to his early and late periods, (Asmus & Parfenov, 2019). The duration of this style could indicate dysthymic depression, however the evidence of contrast from the portraits is inconsistent with an overall darkening of perception. In group portraits brightness increases in Critical Years and in related portraits, apparently of higher salience, it does not alter meaningfully. Individual unrelated portraits, like self-portraits, do show a decline in brightness in Critical Years which may be best explained as transient low mood in the latter and a reluctance to paint, out of financial necessity, in the former. There are no other indicators of magnocellular deficits such as in perceiving motion or peripheral vision as his group portraits, like ‘The Night Watch’ demonstrate.
Rembrandt’s use of colour, his palette, is known to have changed, removing blue and green, before any adverse events. There is an overall decrease in the use of colour after his wife’s death, an increase during his bankruptcy and then an increase in the year of his mistress’s death. There are comparable variations in the absence of adverse life events. There appears to be no general relationship between colour and adversity. The colour variations are also short, with sudden rises and falls, inconsistent with observations that the world overall has less colour in depression nor is there evidence of unsaturated colours. There does appear to be a shift towards yellow and higher average contrast in the late self-portraits and portraits consistent with connoisseurship and age-related macular degeneration. There is, however, no apparent loss of visual acuity or blurred vision attested to by the extraordinary detail in his late self-portraits which led to various diagnoses.
It is important, however, in considering the influence of eye disease on artists not to fall into the ‘El Greco fallacy’ and confuse a chosen style with evidence of visual deficiency. Some concluded that El Greco’s figures were elongated because of astigmatism. Examination of his canvases not only showed that he initially drew the figures to scale and then elongated them when he painted, but experiment demonstrated that if astigmatism caused him to perceive his sitters as elongated, then the same astigmatism would self-correct the image when transferred onto canvas (Anstis, 2002; Firestone & Scholl, 2014). A similar error was made in a study of Rembrandt’s self-portraits which concluded from examination of the direction of gaze in each eye that he had strabismus (Livingstone & Conway, 2004). The authors overlooked the natural result of painting himself looking sideways at a mirror (Mondero et al., 2013; Schwartz, 2006). It is also to be borne in mind that Rembrandt painted using Northern hemisphere daylight, firelight and candlelight; his environment was naturally dimmer than our own.
Depression is also characterised by a negative bias in interpreting facial emotions and a pre-occupation with sadness. It is noteworthy that fully half of Rembrandt’s paintings depict elderly people and his interest in them began early in his career (Covey, 1991; McKee & Kaupinen, 1987; Muller, 1968). This could reflect a pre-occupation with sadness or death. However, Rembrandt’s paintings of elderly subjects illustrate a rich inner life and experience and many are rendered with great tenderness (Muller, 1968; Rosenberg, 1964). He rarely painted images of people dying: three of the crucifixion on commission and one of Lazarus. He frequently used images of himself in etchings and drawings to illustrate various moods including anger, joy, surprise, introspection and pain, only one can be said to illustrate sadness (Hinterding et al., 2000; Schatborn & Hinterding, 2019). It is noteworthy that his first painted self-portrait and one of his last depict him laughing (1628, 1663).
There is no evidence of deficits in perception of facial emotion. Rembrandt’s portraits are notable for their vitality exploring a wide range of human emotion and personality including youthful joyfulness, couples’ loving relationships, the confidence of public figures and the spirituality of old age. His capacity to reveal the inner lives of those he painted using a wide variety of detailed facial expression remained unaffected throughout his life. His final works include profound images of love and tenderness: the Jewish Bride, the Prodigal Son and portraits of his son. While the late self-portraits depict him as old they are not negative images. In one he paints himself as Xeuxis, the greatest painter of antiquity, said to have died laughing (1663). In others he depicts himself as St Paul (1663) and at an easel with Giotto’s perfect circles in the background (1663-69). These suggest a well-balanced man who has found spiritual peace and fulfilment of his ambition of artistic mastery.
The fractal dimension of his self-portraits increases in Critical Years and in Critical Periods in comparison with portraits consistent with increased EEG measurements in depression. Nevertheless, these D values remain with the range maintained throughout his career indicating transient changes in mood and militate against a diagnosis of persistent unipolar or bipolar depression. The D values of the self-portraits fluctuate around a lifetime mean of 1.65 (SD = 0.184) measured in two dimensions and around 2.5 (SD = 0.308) when colour is considered. In the portraits of unrelated individuals, they fluctuate around 1.621 (SD = 0.166) approximating the Golden Mean. These values are consistent with not only a stable, healthy mind but one intuitively attuned to levels associated with beauty and observer preference and one notably aware of his own complexity.
Artists with Alzheimer’s. Parkinson’s and Lewy Body disease show significant declines in the fractal dimension as disease progresses indicating reduced complexity, less detail and greater abstraction (van Buren et al. 2013). This analysis shows no such decline in Rembrandt’s self-portraits or portraits. The fractal levels in old age are consistent with those of early and mid-adulthood which appears to exclude neurogenerative disease or cognitive change due to aging. There is no evidence of the other cognitive or motor symptoms of degenerative disease. This suggests that he did not suffer from cognitive decline and had considerable cognitive reserve (Stern, 2012). He continued to paint until his death leaving behind both completed and unfinished works (Strauss & van der Meulen, 1979; van Heel, & de Barr 1987).
His creativity was not only undimmed but enhanced in old age. He painted several of his greatest masterpieces, including portraits in his last years (Clark 2006, 1978; Marcus & Clarfield, 2002). His late creativity may have been a compensatory reaction to the losses he had suffered (Neiderland, 1989, 1973; Pollack, 1989) but the paintings of this period show a master at the height of his power and using techniques which have been admired ever since by painters and connoisseurs alike (Bikker et al., 2014; Chapman, 1990; Clark, 1978; White & Buvelot, 1999). His last self-portraits are remarkable technically and stylistically. Scientific examination of their materials reveal that Rembrandt continued to innovate until the end of his life (Bomford et al., 1988; van de Wettering, 1997, 2005, 2017; van Loon et al., 2020). His continued experimentation in technique and in many scholars’ view, continued growth as an artist, are inconsistent with depression, at least as clinically defined, and with the cognitive decline due to age or neurodegenerative disease.
The evidence of productivity is also inconsistent with depression. His productivity measured by number of paintings and area painted increased following his wife’s death. A decline in productivity between 1646-1650 began four years after her death and in a period of no other adverse events. It followed a decision not to paint portraits to paint more prestigious and costly history paintings; a decision which was reversed by financial pressures his productivity rising during and following his bankruptcy; a rational and flexible response to events (Schwartz, 2006; Crenshaw, 2006; Schama, 2009). In the aftermath of bankruptcy, in reduced circumstances, his productivity returned to the highest level in his career (Bok & van der Molen, 2009).
The conclusion that Rembrandt suffered from BPD is based on the subject of two etchings, the decline in the number of etchings following the death of infant children and his wife and his previous apparently extravagant use of his recently acquired wealth (Schildkraut et al., 2007). He was seen by his wife’s relatives and by observers at art auctions, as reckless with money and why he did not pay off his debts, particularly his large mortgage, when he had the means to do so remains perplexing (Schwartz, 2006; Crenshaw, 2006). Spending sprees are characteristic signs of mania and there is an association between mild symptoms of BPD, positive mood, and creativity. Rembrandt had elevated moments one of which he painted as a self-portrait with his wife (1635). Mania can manifest as intense work in short periods; heightened creativity was once a symptom of hypomania. However, the intervals, measured in years rather than weeks or months, of his increases in productivity and his expenditure on art, costumes and rare objects are inconsistent with a diagnosis of BPD.
Looked at in isolation, the period shortly before and after his wife’s death this might suggest mania followed by depression characteristic of BPD. It can also be regarded as elevated mood associated with healthy creative people or those who become wealthy for the first time followed by normal grief. It is noteworthy that the objects of his expenditure were on a house large enough to accommodate a family, a studio with up to 50 apprentices and art dealing and on artworks and objects related to his work (van de Wetering, 2017). There is no suggestion of abuse of alcohol, promiscuity, or other reckless behaviour characteristic of BPD. Mania would also not be characterized by the steady growth in productivity seen from 1650-1665. The deaths were not unusual for a man of his time and place although such experiences can have a cumulative effect (Gathergood, 2012; Keller et al., 2007). Nevertheless, after his wife’s death he soon established intimate relations first with his son’s nurse and then an enduring relationship with his housekeeper. There is no indication of dysfunction in any domain.
Rembrandt’s character has been described as eccentric, temperamental, disputatious even vindictive (Bikker, 2019; Schama, 1999). There is also agreement that he was a very hard-working, sober and dedicated artist capable of managing a large studio, dealing in art (his own and others’) and continuously developing his skills (Manuth et al., 2019; Schama, 1999; Schwartz, 2006, 2019; van de Wetering, 1997). Judging by the images he made of her he loved and felt the loss of his wife who he twice painted posthumously. However, there is an important distinction between healthy grieving (mourning) and pathological grief (melancholy) (Freud, 1917; Jansson, 2020). The evidence here suggests normal grieving and occasional periods of low mood due to adversity. After his wife’s death, while apparently grieving her loss, he was never without an intimate companion until his final years. He also remained deeply attached to his son, his productivity increased and his engagement with students, friends, patrons and the art market never waned.
Some have suggested that his refusal to follow changing artistic fashion later in life lead to less demand for his work and thus contributed to low mood. In fact, he remained in demand both in Holland and elsewhere (Runia & De Witt, 2019). In 1661 he painted portraits commissioned by prominent Dutch patrons. In 1661-3 he completed two major commissions for an Italian patron and in 1666-67 he negotiated with another for a triptych (Dirkx, 2013). Cosimo de Medici visited him in 1667 and apparently purchased the self-portrait now in the Uffizi. Thus, although living in straightened circumstances he remained in demand and continued to cultivate the market for his art (Slive, 1953). There is no evidence of withdrawal or of becoming dysfunctional in any sphere of life (Bikker, 2019; Crenshaw, 2006; Manuth & de Winkel, 2019; Schwartz, 2019). There is, in short, no evidence of mania nor of disproportionate experience of sadness or bereavement and when he experienced such feelings they remitted when the cause dissipated and resulted in enhanced productivity and creativity.
Conclusion
Empirical analysis of contrast, colour, fractal dimension and productivity, quantitative and qualitative, in Rembrandt’s self-portraits and portraits do not support diagnoses of unipolar or of bipolar depression as clinically defined nor of neurodegenerative disease or cognitive deterioration due to aging. He does appear to have experienced periods of low mood which can be characterised as normal grieving and response to adversity but the periods were short, did not affect him in any functional domain and resulted in increased creativity and productivity. The duration of the mid-career change in contrast, the absence of significant change in use of colour, the short duration of increases in fractal dimension, the long-term increase in productivity, quantitative and qualitative, and the absence of other symptoms of MDD or BPD including anhedonia, cognitive impairment, psychomotor disturbance, loss of energy, low self-esteem and functional impairment, all weigh against such diagnoses. The evidence is that Rembrandt remained healthy, active, creative, and productive throughout his life (Bikker, 2019; Schama, 1999; van de Wetering, 1997).
There is evidence of age-related macular degeneration but limited in its effects to a shift on the tritan spectrum; otherwise, he appears to have aged normally with vision otherwise unimpaired. There is also evidence that supports connoisseurs’ detection of a less formal style when he painted portraits of his family and friends (Runia & de Witt, 2019; van de Wetering, 2017). The levels of fractal dimension are markedly lower than the values found in the self-portraits and portraits of unrelated people. There is also evidence of a gradient in salience and complexity higher in self-portraits, somewhat lower in portraits of related others and lowest in portraits of unrelated others consistent with experimental evidence. More broadly analysis confirms that aesthetic preference is closely aligned with mid-level fractal dimension and the Golden Mean.
Limitations
This study is based on digital images of paintings not the paintings themselves. In addition the images were not generated on a consistent basis although there is reason to believe that statistically this may provide reliable results. Only three elements of style were examined and colour analysis is limited by the inability of devices to perceive colour as the eye does. While the sample examined is large it does not include history, landscape or genre paintings nor any works on paper. Stylistic examination of these works might yield different results and would be a useful extension of research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Acknowledgements
This work represents independent research partly funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Notes
| 1 |
|
| 2 |
The years in which these events occurred are referred to here as Critical Years and highlighted in the figures below. |
| 3 |
These include: temporal veins, periorbital structures, brow, inter-pupillary distance, wrinkles, the droop of his eyelids, nasolabial folds, marionette lines, submalar hollows, jowl formation and accumulation of neck fat. |
| 4 |
For a detailed explanation of colour extraction using MatLab (see: Duth & Deepa, 2018). |
| 5 |
The data are not normally distributed so a non-parametric test was used.. |
| 6 |
|
References
- Abastado, P. & Chemla, D. (2007). Rembrandt’s doctors. J Med Ethics; Medical Humanities; 33:35–37. [CrossRef]
- Acar S. Chen, X. & Cayirdag, N. (2018). Schizophrenia and creativity: A meta-analytic review. Schizophr Res. May;195:23-31. [CrossRef]
- Adnan, A., Beaty, R., Lam, J., Spreng, R. N., & Turner, G. R. (2019). Intrinsic default-executive coupling of the creative aging brain. Social cognitive and affective neuroscience, 14(3), 291–303. [CrossRef]
- Ahmadlou, M., Adeli, H., & Adeli, A. (2012). Fractality analysis of frontal brain in major depressive disorder. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 85(2), 206–211. [CrossRef]
- Akar, S. A., Kara, S., Agambayev, S., & Bilgic, V. (2015). Nonlinear analysis of EEG in major depression with fractal dimensions. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2015, 7410–7413. [CrossRef]
- Akiskal, H.S. & Akiskal, K.K. (2007). In search of Aristotle: temperament, human nature, melancholia, creativity and eminence. J Affect Disord. Jun;100(1-3):1-6. [CrossRef]
- Akinola, M., & Mendes, W. B. (2008). The dark side of creativity: biological vulnerability and negative emotions lead to greater artistic creativity. Personality & social psychology bulletin, 34(12), 1677–1686. [CrossRef]
- Aks, D. J., & Sprott, J. C. (1996). Quantifying aesthetic preference for chaotic patterns. Empirical Studies of the Arts, 14(1), 1–16. [CrossRef]
- Alizadeh-Ebadi, M., Markowitz, S. N., & Shima, N. (2013). Background chromatic contrast preference in cases with age-related macular degeneration. Journal of Optometry, 6(2), 80–84. [CrossRef]
- Almonte, M.T, Capellàn, P., Yap, T.E., Cordeiro, M.F. (2020). Retinal correlates of psychiatric disorders. Ther Adv Chronic Dis. Mar 9;11:2040622320905215. [CrossRef]
- Altamura, M., Padalino, F. A., Stella, E., Balzotti, A., Bellomo, A., Palumbo, R., Di Domenico, A., Mammarella, N., & Fairfield, B. (2016). Facial Emotion Recognition in Bipolar Disorder and Healthy Aging. The Journal of nervous and mental disease, 204(3), 188–193. [CrossRef]
- Alzueta, E., Melcón, M., Jensen, O., & Capilla, A. (2020). The ‘Narcissus Effect’: Top-down alpha-beta band modulation of face-related brain areas during self-face processing. NeuroImage, 213, Article 116754. [CrossRef]
- Alzueta, E., Melcón, M., Poch, C., & Capilla, A. (2019). Is your own face more than a highly familiar face?. Biological psychology, 142, 100–107. [CrossRef]
- American Psychiatric Association. (1980). Diagnostic and Statistical Manual of Mental Disorders (3rd ed), American Psychiatric Publishing, Washington, D.C.
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, American Psychiatric Publishing, Washington, D.C.
- Andreasen, N. C. (1987). Creativity and mental illness: prevalence rates in writers and their first-degree relatives. Am J Psychiatry. Oct;144(10):1288-92. [CrossRef]
- Anstis, S. (2002). Was El Greco Astigmatic? Leonardo, 35, 208-208.
- Askeland, K. G., Bøe, T., Breivik, K., La Greca, A. M., Sivertsen, B., & Hysing, M. (2020). Life events and adolescent depressive symptoms: Protective factors associated with resilience. PloS one, 15(6), e0234109. [CrossRef]
- Asmus, J. & Parfenov, V. (2019). Characterization of Rembrandt self-portraits through digital-chiaroscuro statistics, Journal of Cultural Heritage, Volume 38, Pages 167-173, ISSN 1296-2074. [CrossRef]
- Avidan, G., Harel, M., Hendler, T., Ben-Bashat, D., Zohary, E., & Malach, R. (2002). Contrast sensitivity in human visual areas and its relationship to object recognition. Journal of neurophysiology, 87(6), 3102–3116. [CrossRef]
- Aybek, S., Ionescu, A., Berney, A., Chocron, O.,, Aminian K. & Vingerhoets, F.J. (2012). Fractal temporal organisation of motricity is altered in major depression. Psychiatry Res. Dec 30;200(2-3):288-93. [CrossRef]
- Baas, M., De Dreu, C. K. W., & Nijstad, B. A. (2008). A meta-analysis of 25 years of mood-creativity research: Hedonic tone, activation, or regulatory focus? Psychological Bulletin, 134(6), 779-806 806. [CrossRef]
- Baas. M., Nijstad, B.A,, Boot, N.C. & De Dreu, C.K.W. (2016). Mad genius revisited: Vulnerability to psychopathology, biobehavioral approach-avoidance, and creativity. Psychol Bull. Jun;142(6):668-692. [CrossRef]
- Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W., Franklin, M. S., & Schooler, J. W. (2012). Inspired by distraction: mind wandering facilitates creative incubation. Psychological science, 23(10), 1117–1122. [CrossRef]
- Barbur, J.L. & Rodriguez-Carmona, M. (2015). Color vision changes in normal aging in Handbook of Color Psychology (eds. Elliot, A. J., Fairchild, M. D., & Franklin, A.) 180–196. Cambridge University Press.
- Barrick, C. B., Taylor, D., & Correa, E. I. (2002). Color sensitivity and mood disorders: biology or metaphor?. Journal of affective disorders, 68(1), 67–71. [CrossRef]
- Belmaker, R. H., & Agam, G. (2008). Major depressive disorder. The New England journal of medicine, 358(1), 55–68. [CrossRef]
- Bikker, J. (2019). Rembrandt Biography of a Rebel. Amsterdam: Rijksmuseum. ISBN 978-94-6208-475-9.
- Bikker, J., Weber, G. J. M., Wieseman, M.E., & Hinterding, E. (2014). Rembrandt, The Late Works. London: National Gallery Publications Ltd. ISBN 978-1-85709-557-9.
- Blazer, D. G. (2003). Depression in late life: Review and commentary. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 58(3), M249–M265.
- Bok, M., & van der Molen, T. (2009). Productivity Levels of Rembrandt and His Main Competitors in the Amsterdam Art Market. Jahrbuch der Berliner Museen January, 51:61-68.
- Bolwerk, A., Mack-Andrick, J., Lang, F. R., Dörfler, A., & Maihöfner, C. (2014). How art changes your brain: differential effects of visual art production and cognitive art evaluation on functional brain connectivity. PloS one, 9(7), e101035. [CrossRef]
- Bomford, D., Brown, C. & Roy, A. (1988). Art in the Making: Rembrandt. National Gallery Publications Ltd.
- Bountis, T., Fokas, A.S., & Psarakis, E.Z. (2017). Fractal analysis of tree paintings by Piet Mondrian (1872-1944). Int. J. Arts Technol., 10, 27-42.
- Boynton G. M. (2005). Contrast gain in the brain. Neuron, 47(4), 476–477. [CrossRef]
- Brandies, R., & Yehuda, S. (2008). The possible role of retinal dopaminergic system in visual performance. Neuroscience and biobehavioral reviews, 32(4), 611–656. [CrossRef]
- Breton, J. J., Labelle, R., Berthiaume, C., Royer, C., St-Georges, M., Ricard, D., Abadie, P., Gérardin, P., Cohen, D., & Guilé, J. M. (2015). Protective factors against depression and suicidal behaviour in adolescence. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 60(2 Suppl 1), S5–S15.
- Brown, G., & Rafaeli, E. (2007). Components of Self-Complexity as Buffers for Depressed Mood. Journal of Cognitive Psychotherapy. 21. 310-333. 10.1891/088983907782638761.
- Bruzzo, A. (2007). The chaotic nature of Self. Dialegesthai. Rivista telematica di filosofia, vol. 9: https://mondodomani.org/dialegesthai/>, ISSN 1128-5478.
- Bubl, E., Tebartz, Van Elst L., Gondan, M., Ebert, D. & Greenlee, M.W. (2009). Vision in depressive disorder. World J Biol Psychiatry.;10(4 Pt 2):377-84. [CrossRef]
- Bubl, E., Kern, E., Ebert, D., Bach, M. & Tebartz van Elst, L. (2010). Seeing gray when feeling blue? Depression can be measured in the eye of the diseased. Biol Psychiatry. Jul 15;68(2):205-8. [CrossRef]
- Bubl, E., Ebert, D., Kern, E., van Elst, L. T., & Bach, M. (2012). Effect of antidepressive therapy on retinal contrast processing in depressive disorder. The British journal of psychiatry : the journal of mental science, 201, 151–158. [CrossRef]
- Bubl, E., Kern, E., Ebert, D., Riedel, A., Tebartz van Elst, L., & Bach, M. (2015). Retinal dysfunction of contrast processing in major depression also apparent in cortical activity. European archives of psychiatry and clinical neuroscience, 265(4), 343–350. [CrossRef]
- Burnette, C. E., Roh, S., Lee, K. H., Lee, Y. S., Newland, L. A., & Jun, J. S. (2017). A Comparison of Risk and Protective Factors Related to Depressive Symptoms among American Indian and Caucasian Older Adults. Health & social work, 42(1), e15–e23. [CrossRef]
- Burton, R. (1621). The Anatomy of Melancholy. Penguin 2023. ISBN: 9780141192284.
- Büttner, T., Kuhn, W., Patzold, T., & Przuntek, H. (1994). L-Dopa improves colour vision in Parkinson’s disease. Journal of neural transmission. Parkinson’s disease and dementia section, 7(1), 13–19. [CrossRef]
- Byron, K., & Khazanchi, S. (2011). A meta-analytic investigation of the relationship of state and trait anxiety to performance on figural and verbal creative tasks. Personality & social psychology bulletin, 37(2), 269–283. [CrossRef]
- Carruthers, H. R., Morris, J., Tarrier, N., & Whorwell, P. J. (2010). The Manchester Color Wheel: development of a novel way of identifying color choice and its validation in healthy, anxious and depressed individuals. BMC medical research methodology, 10, 12. [CrossRef]
- Casco, C., Robol, V., Barollo, M., & Cansino, S. (2011). Effects of aging on visual contour integration and segmentation. Investigative ophthalmology & visual science, 52(7), 3955–3961. [CrossRef]
- Cavanagh, P. (2005). The artist as neuroscientist. Nature 434, 301–307. [CrossRef]
- Chang, Y., Xu, J., Shi, N., Zhang, B., & Zhao, L. (2010). Dysfunction of processing task-irrelevant emotional faces in major depressive disorder patients revealed by expression-related visual MMN. Neuroscience letters, 472(1), 33–37. [CrossRef]
- Chapman, D. P., Whitfield, C. L., Felitti, V. J., Dube, S. R., Edwards, V. J., & Anda, R. F. (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225.
- Chapman, H. P. (1990). Rembrandt’s Self-Portraits. Princeton University Press. ISBN 0-691-04061-3.
- Chatfield, C. and Xing, H. (2019). The Analysis of Time Series: An Introduction with R. 7th edn. Chapman and Hall/CRC. [CrossRef]
- Chauhan, B. C., Vianna, J. R., Sharpe, G. P., Demirel, S., Girkin, C. A., Mardin, C. Y., Scheuerle, A. F., & Burgoyne, C. F. (2020). Differential Effects of Aging in the Macular Retinal Layers, Neuroretinal Rim, and Peripapillary Retinal Nerve Fiber Layer. Ophthalmology, 127(2), 177–185. [CrossRef]
- Clark, K. (1978). An Introduction to Rembrandt. John Murray, London. ISBN 0-7195-3431-3.
- Clark, K.; The Artist Grows Old. (2006) Daedalus ; 135 (1): 77–90. [CrossRef]
- Colzato, L. S., Sellaro, R., Hulka, L. M., Quednow, B. B., & Hommel, B. (2014). Cognitive control predicted by color vision, and vice versa. Neuropsychologia, 62, 55–59. [CrossRef]
- Cosker, E., Moulard, M., Baumann, C., Luc, A., Angioi-Duprez, K., Laprévote, V., Schwan, R., & Schwitzer, T. (2021). Complete evaluation of retinal function in Major Depressive Disorder: From central slowdown to hyperactive periphery. Journal of affective disorders, 295, 453–462. [CrossRef]
- Covey, H. C. (1991). Images of older people in Western art and society. Praeger. ISBN 0-275-93435-7.
- Coyle, C. E., & Dugan, E. (2012). Social Isolation, Loneliness and Health Among Older Adults. Journal of Aging and Health, Dec. 24(8), 1346–1363. [CrossRef]
- Crenshaw, P. (2006/2014). Rembrandt’s Bankruptcy. Cambridge University Press. ISBN 978-0-521-85825-0.
- Curtis, Daniel. (2016). Was Plague an Exclusively Urban Phenomenon? Plague Mortality in the Seventeenth-Century Low Countries. Journal of Interdisciplinary History. 47. 139-170. 10.1162/JINH_a_00975.
- Cutting, J. E., & Garvin, J. J. (1987). Fractal curves and complexity. Perception & Psychophysics, 42(4), 365–370. [CrossRef]
- D’Argembeau, A., Feyers, D., Majerus, S., Collette, F., Van der Linden, M., Maquet, P., & Salmon, E. (2008). Self-reflection across time: cortical midline structures differentiate between present and past selves. Social cognitive and affective neuroscience, 3(3), 244–252. [CrossRef]
- Davey, C. G., Pujol, J., & Harrison, B. J. (2016). Mapping the self in the brain’s default mode network. NeuroImage, 132, 390–397. [CrossRef]
- Deary, I.J., Corley, J., Gow, A.J., Harris, S.E., Houlihan, L.M., Marioni, R.E., Penke, L., Rafnsson, S.B., Starr, J.M. (2009). Age-associated cognitive decline, British Medical Bulletin, Volume 92, Issue 1, December, Pages 135–152. [CrossRef]
- de la Calleja, E.M., Cervantes, F. and de la Calleja, J. (2016). Order-fractal transitions in abstract paintings, Annals of Physics,Volume 371, Pages 313-322, ISSN 0003-4916. [CrossRef]
- de la Torre-Luque A, Bornas X, Balle M, Fiol-Veny A. (2016). Complexity and nonlinear biomarkers in emotional disorders: A meta-analytic study. Neurosci Biobehav Rev. Sep;68:410-422. [CrossRef]
- Demmin, D. L., Netser, R., Roché, M. W., Thompson, J. L., & Silverstein, S. M. (2020). People with current major depression resemble healthy controls on flash Electroretinogram indices associated with impairment in people with stabilized schizophrenia. Schizophrenia research, 219, 69–76. [CrossRef]
- Denny, B.T., Kober, H., Wager, T.D., & Ochsner, K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. Aug;24(8):1742-52. [CrossRef]
- Derrington, A. M., & Lennie, P. (1984). Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. The Journal of physiology, 357, 219–240. [CrossRef]
- De Zorzi, L., Robin, M. S., Honoré, J., Bubrovszky, M., Vaiva, G., & Sequeira, H. (2020). Depression restricts visual capture and promotes the perception of negative information. Biological psychology, 154, 107923. [CrossRef]
- Di leva, A. (2016). The Fractal Geometry of the Brain. Springer-Verlag ISBN: 9781493981489.
- Dirkx, M. (2013). Rembrandt’s Genoese altarpiece. Rembrandt’s Room. https://arthistoriesroom.wordpress.com/2013/08/24/rembrandts-genoese-altarpiece/.
- Dixon, L.S. (2013). The dark side of genius : the melancholic persona in art, ca. 1500-1700. Pennsylvania State University Press. ISBN: 978-0-271-05935-8.
- Djamgoz, M. B., Hankins, M. W., Hirano, J., & Archer, S. N. (1997). Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision research, 37(24), 3509–3529. [CrossRef]
- Domènech-Abella, J., Mundó, J., Haro, J.M., Rubio-Valera, M. (2019). Anxiety, depression, loneliness and social network in the elderly: Longitudinal associations from The Irish Longitudinal Study on Ageing (TILDA), Journal of Affective Disorders,Volume 246, Pages 82-88, ISSN 0165-0327. [CrossRef]
- Draves, S., Abraham, R.H., Viotti, P., Abraham, F.D., & Sprott, J.C. (2008). The Aesthetics and Fractal Dimension of Electric Sheep. Int. J. Bifurc. Chaos, 18, 1243-1248.
- Dunlop, B. W., & Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Archives of general psychiatry, 64(3), 327–337. [CrossRef]
- Duth, P.S., & Deepa, M. (2018). Color detection in RGB-modeled images using MAT LAB. International journal of engineering and technology, 7, 29.
- Eramudugolla, R., Wood, J., & Anstey, K. J. (2013). Co-morbidity of depression and anxiety in common age-related eye diseases: a population-based study of 662 adults. Frontiers in aging neuroscience, 5, 56. [CrossRef]
- Erdinest, N., London, N., Lavy, I., Morad, Y., & Levinger, N. (2021). Vision through Healthy Aging Eyes. Vision, 5(4), 46. [CrossRef]
- Espinel C. H. (1997). A medical evaluation of Rembrandt. His self-portrait: ageing, disease, and the language of the skin. Lancet, 350(9094), 1835–1837. [CrossRef]
- Fam. J., Rush, A.J., Haaland, B., Barbier, S., & Luu, C. (2013). Visual contrast sensitivity in major depressive disorder. J Psychosom Res. Jul;75(1):83-6. [CrossRef]
- Faubert J. (2002). Visual perception and aging. Can J Exp Psychol. Sep;56(3):164-76. [CrossRef]
- Fernandes, T. M. P., Andrade, S. M., de Andrade, M. J. O., Nogueira, R. M. T. B. L., & Santos, N. A. (2017). Colour discrimination thresholds in type 1 Bipolar Disorder: a pilot study. Scientific reports, 7(1), 16405. [CrossRef]
- Fernandes, T. P., Silverstein, S. M., Almeida, N. L., & Santos, N. A. (2019). Visual impairments in type 1 bipolar disorder. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry, 20(10), 790–798. [CrossRef]
- Fernandez, D., & Wilkins, A. J. (2008). Uncomfortable images in art and nature. Perception, 37(7), 1098–1113. [CrossRef]
- Firestone, C., & Scholl, B. J. (2014). “Top-Down” Effects Where None Should Be Found: The El Greco Fallacy in Perception Research. Psychological Science, 25(1), 38–46. http://www.jstor.org/stable/24539465.
- Forsythe, A., Williams, T., & Reilly, R. G. (2017). What paint can tell us: A fractal analysis of neurological changes in seven artists. Neuropsychology, 31(1), 1–10. [CrossRef]
- Forsythe, A., Nadal, M., Sheehy, N., Cela-Conde, C. J., & Sawey, M. (2011). Predicting beauty: fractal dimension and visual complexity in art. British journal of psychology (London, England : 1953), 102(1), 49–70. [CrossRef]
- Foucault, M. (1961/2001). Madness and Civilization: A History of Insanity in the Age of Reason. Routledge. ISBN-10067972110X.
- Franěk,M., Petružálek, J., & Šefara, D. (2019). Eye movements in viewing urban images and natural images in diverse vegetation periods, Urban Forestry & Urban Greening, Volume 46, 126477, ISSN 1618 8667. [CrossRef]
- Freud, S. (1896). Further Remarks on the Neuro-Psychoses of Defence. The Standard Edition of the Complete Psychological Works of Sigmund Freud 3:157-185.
- Freud, S. (1917). Mourning and Melancholia. The Standard Edition of the Complete Psychological Works of Sigmund Freud 14:237-258.
- Freundlich, A. L., & Shively, J. A. (2006). Creativity and the exceptional aging artist. Clinical interventions in aging, 1(2), 197–200. [CrossRef]
- Friberg, T.R., Borrero G. (2000). Diminished perception of ambient light: a symptom of clinical depression? J Affect Disord. Dec;61(1-2):113-8. [CrossRef]
- Friedenberg, J., Martin, P., Uy, N., & Kvapil, M. (2022). Judged Beauty of Fractal Symmetries. Empirical Studies of the Arts, 40(1), 100–120. [CrossRef]
- Friedman, T., Lurie, D., & Westreich, M. ( 2007a). Rembrandt’s sentinel vein, Aesthetic Surgery Journal, Volume 27, Issue 1, Pages 105-107,ISSN 1090-820X. [CrossRef]
- Friedman, T., Westreich, M., Lurie, D. J., & Golik, A. (2007). Rembrandt--aging and sickness: a combined look by plastic surgeons, an art researcher and an internal medicine specialist. The Israel Medical Association journal : IMAJ, 9(2), 67–71.
- Frigg, R. and Howard, C. (2011). Fact and Fiction in the Neuropsychology of Art in The Aesthetic Mind: Philosophy and Psychology Schellekens, E and Goldie, P (eds), Oxford University. [CrossRef]
- Gardner, J. L., Sun, P., Waggoner, R. A., Ueno, K., Tanaka, K., & Cheng, K. (2005). Contrast adaptation and representation in human early visual cortex. Neuron, 47(4), 607–620. [CrossRef]
- Gathergood, J. (2012). Debt And Depression: Causal Links And Social Norm Effects. The Economic Journal, 122 (September), 1094–1114. [CrossRef]
- Goldberger, A. L., Amaral, L. A., Hausdorff, J. M., Ivanov, P. C.h, Peng, C. K., & Stanley, H. E. (2002). Fractal dynamics in physiology: alterations with disease and aging. Proceedings of the National Academy of Sciences of the United States of America, 99 Suppl 1(Suppl 1), 2466–2472. [CrossRef]
- Gonzalez, V., Fazlic, I., Cotte, M. Vanmeert, F., Gestels, A., De Meyer, S., Broers, F., Hermans, J., van Loon, A., Janssens, K., Noble, P., Keune, K. (2023). Lead(II) Formate in Rembrandt’s Night Watch: Detection and Distribution from the Macro- to the Micro-scale. Angew. Chem. Int. Ed.;. [CrossRef]
- Graham, D. J., & Redies, C. (2010). Statistical regularities in art: Relations with visual coding and perception. Vision research, 50(16), 1503–1509. [CrossRef]
- Graham, D. J., & Field, D. J. (2008). Variations in intensity statistics for representational and abstract art, and for art from the Eastern and Western hemispheres. Perception, 37(9), 1341–1352. [CrossRef]
- Greenlee MW, Georgeson MA, Magnussen S, Harris JP. (1991). The time course of adaptation to spatial contrast. Vision Res.;31(2):223-36. [CrossRef]
- Greenwood, T. A., Chow, L. J., Gur, R. C., & Kelsoe, J. R. (2022). Bipolar spectrum traits and the space between Madness and Genius: The Muse is in the Dose. Journal of psychiatric research, 153, 149–158. [CrossRef]
- Grosu, G.F., Hopp, A.V., Moca, V.V., Bârzan, H., Ciuparu, A. Ercsey-Ravasz, M., Winkel, M., Linde, H., & Mureșan, R.C. (2023). The fractal brain: scale-invariance in structure and dynamics, Cerebral Cortex, Volume 33, Issue 8, 15 April, Pages 4574–4605. [CrossRef]
- Hage, J. J., Lange, J., & Karim, R. B. (2019). Rembrandt’s Aging Face in Plastic Surgical Perspective. Annals of plastic surgery, 83(2), 123–131. [CrossRef]
- Hagerhall, C.M., Purcell, T., Taylor, R. (2004). Fractal dimension of landscape silhouette outlines as a predictor of landscape preference, Journal of Environmental Psychology, Volume 24, Issue 2, Pages 247-255, ISSN 0272-4944. [CrossRef]
- Hébert, M., Mérette, C., Paccalet, T., Gagné, A-M.,& Maziade, M. (2017). Electroretinographic anomalies in medicated and drug free patients with major depression: Tagging the developmental roots of major psychiatric disorders, Progress in Neuro-Psychopharmacology and Biological Psychiatry,Volume 75, Pages 10-15, ISSN 0278-5846. [CrossRef]
- Heerwagen, J. H., & Orians, G. H. (1993). Humans, habitats, and aesthetics. In S. R. Kellert & E. O. Wilson (Eds.), The biophilia hypothesis (pp. 138–172). Covelo, CA: Island Press.
- Heilman K. M. (2016). Possible Brain Mechanisms of Creativity. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists, 31(4), 285–296. [CrossRef]
- Hinterding, E., Luiijten, G., & Royalton-Kirsch, M. (2000). Rembrandt, The Printmaker. British Museum. ISBN 0-7141-2625-X.
- Hodgkin, K. (2007). Without Sense and Understanding: Concepts of Madness in Early Modern Thought. In: Madness in Seventeenth-Century Autobiography. Early Modern History: Society and Culture. Palgrave Macmillan, London. [CrossRef]
- Hogan, D. P., & Kertzer, D. I. (1987). The social bases of declining infant mortality: lessons from a nineteenth-century Italian town. European journal of population = Revue europeenne de demographie, 2(3-4), 361–385. [CrossRef]
- Horney, K. (1942). Self-analysis. New York: W. W. Norton. ISBN-100393311651.
- Hu, T., Zhao, X., Wu, M., Li, Z., Luo, L., Yang, C., & Yang, F. (2022). Prevalence of depression in older adults: A systematic review and meta-analysis. Psychiatry Res. May;311:114511. [CrossRef]
- Hyett, M., & Parker, G. (2013). Loss of light in the eyes: a window to melancholia. Medical hypotheses, 81(2), 186–191. [CrossRef]
- Ivanovici, M. & Richard, N. (2011). ‘Fractal Dimension of Color Fractal Images’, IEEE Transactions on Image Processing, 20(1), pp. 227–235. [CrossRef]
- Jackson, G.R. & Owsley, C. (2003). Visual dysfunction, neurodegenerative diseases, and aging Neurol Clin N Am 21 709–728. [CrossRef]
- James, W. (1890). The principles of psychology (Vol 1). New York: Holt. ISBN-101543183182.
- Jansson, A. (2020). Melancholia and Depression. Oxford Research Encyclopedia of Psychology . [CrossRef]
- Johnson, S. L., Murray, G., Fredrickson, B., Youngstrom, E. A., Hinshaw, S., Bass, J. M., Deckersbach, T., Schooler, J., & Salloum, I. (2012). Creativity and bipolar disorder: touched by fire or burning with questions?. Clinical psychology review, 32(1), 1–12. [CrossRef]
- Jones, B. F. and Weinberg, B.A. (2011). Age dynamics in scientific creativity. Proceedings of the National Academy of Sciences November 108(47):18910. [CrossRef]
- Joormann, J., & Gotlib, I. H. (2006). Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of abnormal psychology, 115(4), 705–714. [CrossRef]
- Jung, C. G. (1916). Collected papers on analytical psychology. Balliere, Tindall and Cox. ISBN-100343867567.
- Jung, K. I., Hong, S. Y., Shin, D. Y., Lee, N. Y., Kim, T. S., & Park, C. K. (2020). Attenuated Visual Function in Patients with Major Depressive Disorder. Journal of clinical medicine, 9(6), 1951. [CrossRef]
- Keller, M.C., Neale, M.C., & Kendler, K.S. (2007). Association of Different Adverse Life Events With Distinct Patterns of Depressive Symptoms. American Journal of Psychiatry. [CrossRef]
- Keyes, H., & Brady, N. (2010). Self-face recognition is characterized by “bilateral gain” and by faster, more accurate performance which persists when faces are inverted. Quarterly journal of experimental psychology (2006), 63(5), 840–847. [CrossRef]
- Kim, D., Son, S. W., & Jeong, H. (2014a). Large-scale quantitative analysis of painting arts. Scientific reports, 4, 7370. [CrossRef]
- Kim, S., Al-Haj, M., Chen, S., Fuller, S., Jain, U., Carrasco, M., & Tannock, R. (2014b). Colour vision in ADHD: part 1--testing the retinal dopaminergic hypothesis. Behavioral and brain functions : BBF, 10, 38. [CrossRef]
- Kim, Y., Lee, H., & Park, A. (2022). Patterns of adverse childhood experiences and depressive symptoms: self-esteem as a mediating mechanism. Social psychiatry and psychiatric epidemiology, 57(2), 331–341. [CrossRef]
- Knapen, S.E., Li, P., Riemersma-van der Lek, R.F., Verkooijen, S., Boks, M.P.M., Schoevers, R.A., Scheer, F.A.J.L., Hu, K. (2021). Fractal biomarker of activity in patients with bipolar disorder. Psychol Med. Jul;51(9):1562-1569. [CrossRef]
- Kraaij, V., Arensman, E., & Spinhoven, P. (2002). Negative life events and depression in elderly persons: a meta-analysis. The journals of gerontology. Series B, Psychological sciences and social sciences, 57(1), P87–P94. [CrossRef]
- Krause, F.C., Linardatos, E., Fresco, D.M., & Moore, M.T. (2021). Facial emotion recognition in major depressive disorder: A meta-analytic review, Journal of Affective Disorders, Volume 293, Pages 320-328, ISSN 0165-0327. [CrossRef]
- Kyaga, S., Lichtenstein, P., Boman, M., Hultman, C., Långström, N., & Landén, M. (2011). Creativity and mental disorder: Family study of 300 000 people with severe mental disorder. British Journal of Psychiatry, 199(5), 373-379. [CrossRef]
- Kyaga, S., Landén, M., Boman, M., Hultman, C.M., Långström, N., & Lichtenstein, P. (2013). Mental illness, suicide and creativity: 40-year prospective total population study. J Psychiatr Res. Jan;47(1):83-90. [CrossRef]
- Lagerlöf, O. (1982). Tricyclic psychopharmaca and colour vision. Documenta Ophthalmologica Proceedings Series, 33, 487–491.
- Lee, B., Kim, D., Jeong, H., Sun, S., & Park, J. (2017). Understanding the Historic Emergence of Diversity in Painting via Color Contrast. [CrossRef]
- Linville, P.W. (1987). Self-complexity as a cognitive buffer against stress-related illness and depression. Journal of personality and social psychology, 52 4, 663-76.
- Livingstone, M. S., & Conway, B. R. (2004). Was Rembrandt stereoblind?. The New England journal of medicine, 351(12), 1264–1265. [CrossRef]
- Livio, M. (2002) The Golden Ratio The Story of Phi, the World’s Most Astonishing Number. Broadway Books. ISBN-100767908163.
- Lizano, P., Lutz, O., Ling, G., Lee, A. M., Eum, S., Bishop, J. R., Kelly, S., Pasternak, O., Clementz, B., Pearlson, G., Sweeney, J. A., Gershon, E., Tamminga, C., & Keshavan, M. (2019). Association of Choroid Plexus Enlargement With Cognitive, Inflammatory, and Structural Phenotypes Across the Psychosis Spectrum. The American journal of psychiatry, 176(7), 564–572. [CrossRef]
- Lopes, A.M., & Tenreiro Machado, J.A. (2019). Complexity Analysis of Escher’s Art. Entropy, May 31;21(6):553. [CrossRef]
- Ludwig, A.M. (1992). Creative achievement and psychopathology: comparison among professions. Am J Psychother. Jul;46(3):330-56. Erratum in: Am J Psychother 1993 Winter;47(1):160. [CrossRef]
- Mackenbach J. P. (2021). The rise and fall of diseases: reflections on the history of population health in Europe since ca. 1700. European journal of epidemiology, 36(12), 1199–1205. [CrossRef]
- Mandarano P, Ossola P, Castiglioni P, Faini A, Marazzi P, Carsillo M, Rozzi S, & Lazzeroni D. (2022). Heart Rate Fractality Disruption as a Footprint of Subthreshold Depressive Symptoms in a Healthy Population. Clin Neuropsychiatry. Jun;19(3):163-173. [CrossRef]
- Mandelbrot, B.B. (1977/83). The Fractal Geometry of Nature W.H. Freeman & Co. . ISBN-100716711869.
- Manuth, V., & de Winkel, M. (2019). Rembrandt’s Self Portraits. Taschen. ISBN-103836577003.
- Manuth, V., de Winkel, M. & van Leeuwen, R. (2019). Rembrandt. The Complete Paintings.Taschen. ISBN-10-3836526328.
- Marcus, E. L., & Clarfield, A. M. (2002). Rembrandt’s late self-portraits: psychological and medical aspects. International journal of aging & human development, 55(1), 25–49. [CrossRef]
- Marzi, C., Giannelli, M., Tessa, C., Mascalchi, M., & Diciotti, S. (2020). Toward a more reliable characterization of fractal properties of the cerebral cortex of healthy subjects during the lifespan. Scientific reports, 10(1), 16957. [CrossRef]
- McDonough, I.M., & Madan, C.R. (2021). Structural complexity is negatively associated with brain activity: a novel multimodal test of compensation theories of aging. Neurobiol Aging. Feb;98:185-196. [CrossRef]
- McElroy, S. (2010). If It’s So Easy, Why Don’t You Try It. New York Times Dec. 3.
- https://www.nytimes.com/2010/12/05/nyregion/05spotli.html.
- McKee, P., & Kauppinen, H. (1987). The art of aging. A celebration of old age in Western art. Human Sciences Press. ISBN-100898853044.
- McKendrick, A. M., Chan, Y. M., & Nguyen, B. N. (2018). Spatial vision in older adults: perceptual changes and neural bases. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists), 38(4), 363–375. [CrossRef]
- Melun, J. P., Morin, L. M., Muise, J. G., & DesRosiers, M. (2001). Color vision deficiencies in Gilles de la Tourette syndrome. Journal of the neurological sciences, 186(1-2), 107–110. [CrossRef]
- Meng, S. Z., Ozawa, Y., Itoh, M., & Takashima, S. (1999). Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain research, 843(1-2), 136–144. [CrossRef]
- Mondero, N. E., Crotty, R. J., & West, R. W. (2013). Was Rembrandt strabismic?. Optometry and vision science : official publication of the American Academy of Optometry, 90(9), 970–979. [CrossRef]
- Muller, J. E. (1968). Rembrandt. Thames and Hudson. ASIN: B000GR7QMW.
- Murray, G., & Johnson, S. L. (2010). The clinical significance of creativity in bipolar disorder. Clinical psychology review, 30(6), 721–732. [CrossRef]
- Mustafa, N., Ahearn, T.S., Waiter, G.D., Murray, A.D., Whalley, L.J., & Staff, R.T. (2012). Brain structural complexity and life course cognitive change. Neuroimage. Jul 2;61(3):694-701. [CrossRef]
- Nakamura, A., Takizawa, R., & Shimoyama, H. (2018). Increased sensitivity to sad faces in depressive symptomatology: A longitudinal study. Journal of affective disorders, 240, 99–104. [CrossRef]
- Namazi, H., Kulish, V. & Akrami, A. (2016). The analysis of the influence of fractal structure of stimuli on fractal dynamics in fixational eye movements and EEG signal. Sci Rep 6, 26639 . [CrossRef]
- Nascimento, S. M., Linhares, J. M., Montagner, C., João, C. A., Amano, K., Alfaro, C., & Bailão, A. (2017). The colors of paintings and viewers’ preferences. Vision research, 130, 76–84. [CrossRef]
- Nayak, S.R. & Mishra, J. (2016). An improved method to estimate the fractal dimension of colour images. Perspectives in Science, 8, pp. 412–416. [CrossRef]
- Neihart, M. (1998). Creativity, the Arts, and Madness Roeper Review, Volume 21, pp. 47-50.
- Neiderland, W. G. (1973). Psychoanalytic concepts of creativity and aging. Psychoanalytic approaches to creativity. Journal of Geriatric Psychiatry, 6, 160–168.
- Neiderland, W. G. (1989). Trauma, loss, restoration and creativity. In D. R. Dietrich & P. C. Shabad (Eds.), The problem of loss and memory: Psychoanalytic perspectives. Madison, CT: International Universities Press.
- Nguyen, L. Y., & Spehar, B. (2021). Visual adaptation to natural scene statistics and visual preference. Vision research, 180, 87–95. [CrossRef]
- Nikolaidis, N.S., Nikolaidis, I.N., & Tsouros, C.C. (2011). A Variation of the Box-Counting Algorithm Applied to Colour Images. ArXiv, abs/1107.2336.
- O’Bryan, R. A., Brenner, C. A., Hetrick, W. P., & O’Donnell, B. F. (2014). Disturbances of visual motion perception in bipolar disorder. Bipolar disorders, 16(4), 354–365. [CrossRef]
- Ou, W. C., Lesmes, L. A., Christie, A. H., Denlar, R. A., & Csaky, K. G. (2021). Normal- and Low-Luminance Automated Quantitative Contrast Sensitivity Assessment in Eyes With Age-Related Macular Degeneration. American journal of ophthalmology, 226, 148–155. [CrossRef]
- Owsley C. (2003). Contrast sensitivity. Ophthalmology clinics of North America, 16(2), 171–177. [CrossRef]
- Pacioli, L. (1509) De divina proportione.in Livio, M. (2002) The Golden Ratio The Story of Phi, the World’s Most Astonishing Number. Broadway Books. ISBN-100767908155.
- Palva, J. M., Zhigalov, A., Hirvonen, J., Korhonen, O., Linkenkaer-Hansen, K., & Palva, S. (2013). Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proceedings of the National Academy of Sciences of the United States of America, 110(9), 3585–3590. [CrossRef]
- Parker, G., Fink, M., Shorter, E., Taylor, M. A., Akiskal, H., Berrios, G., Bolwig, T., Brown, W. A., Carroll, B., Healy, D., Klein, D. F., Koukopoulos, A., Michels, R., Paris, J., Rubin, R. T., Spitzer, R., & Swartz, C. (2010). Issues for DSM-5: whither melancholia? The case for its classification as a distinct mood disorder. The American journal of psychiatry, 167(7), 745–747. [CrossRef]
- Pelli, D. G., & Bex, P. (2013). Measuring contrast sensitivity. Vision research, 90, 10–14. [CrossRef]
- Pentland, A. (1984). Fractal-Based Description of Natural Scenes. IEEE Transactions on Pattern Analysis and Machine Intelligence, PAMI-6, 661-674.1984. [CrossRef]
- Pfundmair, M., Danböck, S.K., & Agthe, M. (2019). Out of the dark, into the light: The impact of social exclusion on judgments of darkness and brightness. Acta psychologica, 199, 102901. [CrossRef]
- Pieri, V., Diederich, N. J., Raman, R., & Goetz, C. G. (2000). Decreased color discrimination and contrast sensitivity in Parkinson’s disease. Journal of the neurological sciences, 172(1), 7–11. [CrossRef]
- Piguet, O., Hornberger, M., Mioshi, E., & Hodges, J. R. (2011). Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. The Lancet. Neurology, 10(2), 162–172. [CrossRef]
- Pincus, D., Cadsky, O., Berardi, V., Asuncion, C. M., & Wann, K. (2019). Fractal Self-Structure and Psychological Resilience. Nonlinear dynamics, psychology, and life sciences, 23(1), 57–78.
- Pokorny, V. J., Schallmo, M. P., Sponheim, S. R., & Olman, C. A. (2023). Weakened untuned gain control is associated with schizophrenia while atypical orientation-tuned suppression depends on visual acuity. Journal of vision, 23(2), 2. [CrossRef]
- Pollock, G. H. (1989). The mourning process, the creative process and the creation. In Dietrich, D.R., & Shabad, P.C. (eds.), The problem of loss and memory: Psychoanalytic perspectives. International Universities Press. ISBN-100823643492.
- Polo, V., Rodrigo, M. J., Garcia-Martin, E., Otin, S., Larrosa, J. M., Fuertes, M. I., Bambo, M. P., Pablo, L. E., & Satue, M. (2017). Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease. Eye, 31(7), 1034–1041. [CrossRef]
- Popova E. (2020). Role of Dopamine in Retinal Function. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. https://www.ncbi.nlm.nih.gov/books/NBK561740/.
- Rawls, E., White, R., Kane, S., Stevens, C. E., Jr, & Zabelina, D. L. (2021). Parametric Cortical Representations of Complexity and Preference for Artistic and Computer-Generated Fractal Patterns Revealed by Single-Trial EEG Power Spectral Analysis. NeuroImage, 236, 118092. [CrossRef]
- Redies, C., Hänisch, J., Blickhan, M., Denzler, J. (2007a). Artists portray human faces with the Fourier statistics of complex natural scenes. Network. Sep;18(3):235-48. [CrossRef]
- Redies, C., Hasenstein, J., Denzler, J. (2007b). Fractal-like image statistics in visual art: similarity to natural scenes. Spat Vis.;21(1-2):137-48. [CrossRef]
- Richards, R., Kinney, D. K., Lunde, I., Benet, M., & Merzel, A.P.C. (1988). Creativity in manic-depressives, cyclothymes, their normal relatives, and control subjects. Journal of Abnormal Psychology, 97(3), 281–288. [CrossRef]
- Robles, K.E., Liaw, N.A., Taylor, R.P., Robles, K.E., Liaw, N.A., Taylor, R.P., Baldwin, D.A., & Sereno, M. (2020). A shared fractal aesthetic across development. Humanit Soc Sci Commun 7, 158 . [CrossRef]
- Rodda, J., Walker, Z., & Carter, J. (2011). Depression in older adults. BMJ; 343. [CrossRef]
- Romero, J, Gómez-Robledo, L, Nieves, J. (2018). Computational color analysis of paintings for different artists of the XVI and XVII centuries. Color Res Appl. ; 43: 296– 303. [CrossRef]
- Rommes, R. (2015) Plague in Northwestern Europe. The Dutch Experience, 1350-1670. Popolazione e Storia Vol. 16. No 16. https://popolazioneestoria.it/article/view/705.
- Rosenberg, J. (1964). Rembrandt: Life and work. Phaidon. ASIN: B0000CMAAG.
- Roudaia, E., Bennett, P. J., & Sekuler, A. B. (2008). The effect of aging on contour integration. Vision research, 48(28), 2767–2774. [CrossRef]
- Roy, A., Roy, M., Berman, J., & Gonzalez, B. (2003). Blue cone electroretinogram amplitudes are related to dopamine function in cocaine-dependent patients. Psychiatry research, 117(2), 191–195. [CrossRef]
- Ruihua, M., Meng, Z., Nan, C., Panqi, L., Hua, G., Sijia, L., Jing, S., Ke, Z., Yunlong, T., Shuping, T., Fude, Y., Li, T., & Zhiren, W. (2021). Differences in Facial Expression Recognition Between Unipolar and Bipolar Depression. Frontiers in psychology, 12, 619368. [CrossRef]
- Runia, E, & De Witt, D. (eds) (2019). Rembrandt’s Social Network: Family, Friends and Acquaintances. WBOOKS. ISBN 978-94-625-8325-2.
- Salmela, V., Socada, L., Söderholm, J., Heikkilä, R., Lahti, J., Ekelund, J., & Isometsä, E. (2021). Reduced visual contrast suppression during major depressive episodes. Journal of psychiatry & neuroscience : JPN, 46(2), E222–E231. [CrossRef]
- Salvi, S. M., Akhtar, S., & Currie, Z. (2006). Ageing changes in the eye. Postgraduate medical journal, 82(971), 581–587. [CrossRef]
- Scallen, C. B. (2004). Rembrandt, Reputation, and the Practice of Connoisseurship. Amsterdam University Press. ISBN: 9053566252. http://www.jstor.org/stable/j.ctt46mx9g.
- Schama, S. (1999). Rembrandt’s Eyes. Alfred A. Knopf, Random House. ISBN-100141979534.
- Schaefer, K. L., Baumann, J., Rich, B. A., Luckenbaugh, D. A., & Zarate, C. A., Jr (2010). Perception of facial emotion in adults with bipolar or unipolar depression and controls. Journal of psychiatric research, 44(16), 1229–1235. [CrossRef]
- Schatborn, P. & Hinterding, E. (2019). Rembrandt, The Complete Drawings and Etchings. Taschen. ISBN-103836575442.
- Schildkraut, J.J., Hirshfeld, A.J., & Murphy, J.M. (1994). Mind and mood in modern art, II: Depressive disorders, spirituality, and early deaths in the abstract expressionist artists of the New York School. Am J Psychiatry. Apr;151(4):482-8. [CrossRef] [PubMed]
- Schildkraut J. J. (2004). Saint Jerome in a dark chamber: Rembrandt’s metaphoric portrayal of the depressed mind. The American journal of psychiatry, 161(1), 26–27. [CrossRef]
- Schildkraut, J. J., Cohn, M. B., & Hawkins, H. (2007). Then melancholy, now depression: a modern interpretation of Rembrandt’s mental state in midlife. The Journal of nervous and mental disease, 195(1), 3–9. [CrossRef]
- Schönfeldt-Lecuona, C., Schmidt, A., Kregel, T., Kassubek, J., Dreyhaupt, J., Freudenmann, R. W., Connemann, B. J., Pinkhardt, E. H., & Gahr, M. (2018). Retinal changes in patients with major depressive disorder - A controlled optical coherence tomography study. Journal of affective disorders, 227, 665–671. [CrossRef]
- Schurz, M., Radua, J., Aichhorn, M., Richlan, F., & Perner, J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and biobehavioral reviews, 42, 9–34. [CrossRef]
- Schwartz, G. (2013). Rembrandt’s Durer. Old Master Catalogue. Christopher-Clark Gallery. https://www.garyschwartzarthistorian.nl/wp-content/uploads/2013/04/2013-oldmastercatalog_schwartz-essay-extract_web.pdf.
- Schwartz, G. (2006). Rembrandt’s Universe, His art, his life, his world. Thames & Hudson. ISBN-100500093865.
- Schwitzer T, Lavoie J, Giersch A, Schwan R, & Laprevote V. (2015). The emerging field of retinal electrophysiological measurements in psychiatric research: A review of the findings and the perspectives in major depressive disorder. J Psychiatr Res. Nov;70:113-20. [CrossRef]
- Schwitzer, T., Schwan, R., Bubl, E., Lalanne, L., Angioi-Duprez, K., & Laprevote, V. (2017). Looking into the brain through the retinal ganglion cells in psychiatric disorders: A review of evidences. Progress in neuro-psychopharmacology & biological psychiatry, 76, 155–162. [CrossRef]
- Schwitzer, T., Le Cam, S., Cosker, E., Vinsard, H., Leguay, A., Angioi-Duprez, K., Laprevote, V., Ranta, R., Schwan, R., & Dorr, V. L. (2022). Retinal electroretinogram features can detect depression state and treatment response in adults: A machine learning approach. Journal of affective disorders, 306, 208–214. [CrossRef]
- Seriès, P. & Seitz, A.R. (2013). Learning what to expect (in visual perception). Front Hum Neurosci. Oct 24;7:668. [CrossRef]
- Shoshina, I.I., Mukhitova, Y.V., Tregubenko, I. A., Pronin, S.V., & Isaeva, E. R. (2021). Contrast Sensitivity of the Visual System and Cognitive Functions in Schizophrenia and Depression. Hum Physiol 47, 516–527. [CrossRef]
- Silverstein, S.M., Demmin, D.L., Schallek, J.B., & Fradkin, S.I. (2020). Measures of Retinal Structure and Function as Biomarkers in Neurology and Psychiatry, Biomarkers in Neuropsychiatry,Volume 2, 100018, ISSN 2666-1446. [CrossRef]
- Silvia, P. J., & Kimbrel, N. A. (2010). A dimensional analysis of creativity and mental illness: Do anxiety and depression symptoms predict creative cognition, creative accomplishments, and creative self-concepts? Psychology of Aesthetics, Creativity, and the Arts, 4(1), 2–10. [CrossRef]
- Simoncelli, E. P., & Olshausen, B. A. (2001). Natural image statistics and neural representation. Annual review of neuroscience, 24, 1193–1216. [CrossRef]
- Simonton, D.K. (1990). Creativity in the later years: optimistic prospects for achievement. Gerontologist. Oct;30(5):626-31. [CrossRef]
- Slaby A. E. (1992). Creativity, depression and suicide. Suicide & life-threatening behavior, 22(2), 157–166.
- Slive, S. (1953). Rembrandt and his Contemporary Critics. Journal of the History of Ideas, 14(2), 203–220. [CrossRef]
- Slive, S. (2009). The Drawings of Rembrandt, A New Study. Thames and Hudson. ISBN-100500238677.
- Smith, J. H., Rowland, C., Harland, B., Moslehi, S., Montgomery, R. D., Schobert, K., Watterson, W. J., Dalrymple-Alford, J., & Taylor, R. P. (2021). How neurons exploit fractal geometry to optimize their network connectivity. Scientific reports, 11(1), 2332. [CrossRef]
- Solé R., & Goodwin B. (2000). Signs of Life. How Complexity Pervades Biology, Basic Books. ISBN-100465019285.
- Spear P. D. (1993). Neural bases of visual deficits during aging. Vision research, 33(18), 2589–2609. [CrossRef]
- Spehar, B., Clifford, C.W.G., Newell, B.R., & Taylor, R.P. (2003). Universal aesthetic of fractals,.
-
Computers & Graphics, Volume 27, Issue 5, Pages 813-820, ISSN 0097-8493. [CrossRef]
- Spehar, B., Walker, N., & Taylor, R. P. (2016). Taxonomy of Individual Variations in Aesthetic Responses to Fractal Patterns. Frontiers in human neuroscience, 10, 350. [CrossRef]
- Spreng, R. N., & Turner, G. R. (2019). The Shifting Architecture of Cognition and Brain Function in Older Adulthood. Perspectives on Psychological Science, 14(4), 523–542. [CrossRef]
- Squarcina, L., De Luca, A., Bellani, M., Brambilla, P., Turkheimer, F. E., & Bertoldo, A. (2015). Fractal analysis of MRI data for the characterization of patients with schizophrenia and bipolar disorder. Physics in medicine and biology, 60(4), 1697–1716. [CrossRef]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. Nov;11(11):1006-12. [CrossRef]
- Strauss, W. L. & van der Meullen (1979). The Rembrandt Documents. Abaris Books, Inc. ISBN: 9780913870686.
- Sui, J., & Humphreys, G. W. (2017). The ubiquitous self: what the properties of self-bias tell us about the self. Annals of the New York Academy of Sciences, 1396(1), 222–235. [CrossRef]
- Sui, J., He, X., & Humphreys, G. W. (2012). Perceptual effects of social salience: evidence from self-prioritization effects on perceptual matching. Journal of experimental psychology. Human perception and performance, 38(5), 1105–1117. [CrossRef]
- Sullivan, E. (2008). Melancholy, medicine, and the arts. Lancet, 372(9642), 884–885. [CrossRef]
- Swenor, B.K., & Ehrlich, J.R. (2021). Ageing and vision loss: looking to the future. Lancet Glob Health. Apr;9(4):e385-e386. [CrossRef]
- Tan, A., Schwitzer, T., Conart, J.-B., & Angioi-Duprez, K. (2020). Study of retinal structure and function in patients with major depressive disorder, bipolar disorder or schizophrenia: A review of the literature, Journal Français d’Ophtalmologie, Volume 43, Issue 5,Pages e157-e166, ISSN 0181-5512. [CrossRef]
- Tannock, R., Banaschewski, T., & Gold, D. (2006). Color naming deficits and attention-deficit hyperactivity disorder: a retinal dopaminergic hypothesis. Behavioral and brain functions : BBF, 2, 4. [CrossRef]
- Taylor, C. L. (2017). Creativity and Mood Disorder: A Systematic Review and Meta-Analysis. Perspectives on Psychological Science, 12(6), 1040–1076.. [CrossRef]
- Taylor, R.P., Micolich, A.P., & Jonas, D. (1999). Fractal analysis of Pollock’s drip paintings, Nature, 399(6735), pp. 422–422. [CrossRef]
- Taylor, R., Newell, B., & Spehar, B. (2005). Fractals: A Resonance between Art and Nature, Mathematics and Culture II, ISBN : 978-3-540-21368-0.
- Taylor, R. P. (2006). Reduction of Physiological Stress Using Fractal Art and Architecture. Leonardo, Volume 39, Number 3, June, pp. 245-251.
- Taylor, R. P., & Sprott, J. C. (2008). Biophilic fractals and the visual journey of organic screen-savers. Nonlinear Dynamics, Psychology, and Life Sciences, 12(1), 117–129.
- Taylor, R., Branka, S., Hagerhall, C., & Van Donkelaar, P. (2011). Perceptual and Physiological Responses to Jackson Pollock’s Fractals. Frontiers in Human Neuroscience Vol. 5. ISSN 1662-5161. [CrossRef]
- ter Borg, M., & Trenité, D. K. (2012). The cultural context of diagnosis: the case of Vincent van Gogh. Epilepsy & behavior : E&B, 25(3), 431–439. [CrossRef]
- Tondo, L., Vázquez, G. H., & Baldessarini, R. J. (2017). Depression and Mania in Bipolar Disorder. Current neuropharmacology, 15(3), 353–358. [CrossRef]
- Trevor-Roper, P. D. (1959). The influence of eye disease on pictorial art. Proceedings of the Royal Society of Medicine, 52(9), 721–744.
- Trinh, M., Khou, V., Zangerl, B., Kalloniatis, M., & Nivison-Smith, L. (2021). Modelling normal age-related changes in individual retinal layers using location-specific OCT analysis. Scientific reports, 11(1), 558. [CrossRef]
- Turkheimer, F. E., Leech, R., Expert, P., Lord, L. D., & Vernon, A. C. (2015). The brain’s code and its canonical computational motifs. From sensory cortex to the default mode network: A multi-scale model of brain function in health and disease. Neuroscience and biobehavioral reviews, 55, 211–222. [CrossRef]
- Turkheimer, F. E., Fagerholm, E. D., Vignando, M., Dafflon, J., Da Costa, P. F., Dazzan, P., & Leech, R. (2020). A GABA Interneuron Deficit Model of the Art of Vincent van Gogh. Frontiers in psychiatry, 11, 685. [CrossRef]
- Turkheimer, F. E., Liu, J., Fagerholm, E. D., Dazzan, P., Loggia, M. L., & Bettelheim, E. (2022). The art of pain: A quantitative color analysis of the self-portraits of Frida Kahlo. Frontiers in human neuroscience, 16, 1000656. [CrossRef]
- Ulrich R. S. (1984). View through a window may influence recovery from surgery. Science (New York, N.Y.), 224(4647), 420–421. [CrossRef]
- van Buren, B., Bromberger, B., Potts, D., Miller, B., & Chatterjee, A. (2013). Changes in painting styles of two artists with Alzheimer’s disease. Psychology of Aesthetics, Creativity, and the Arts, 7(1), 89–94. [CrossRef]
- Van de Wetering, E. (1997/2018). Rembrandt, The Painter at Work. Amsterdam University Press. ISBN-10-9780520258846.
- Van de Wetering, E. (1999). The multiple functions of Rembrandt’s self-portraits. In White, C., & Buvelot, Q. (eds.), Rembrandt by himself (pp. 8–37). National Gallery Publications. ISBN-100300077890.
- Van de Wetering, E. (2005). A Corpus of Rembrandt Paintings Vol. IV. Stichting Foundation Rembrandt Research Project, Springer. ISBN-109401791732.
- Van de Wetering, E. (2017). Rembrandt’s Paintings Revisited, A complete survey (reprint of A Corpus of Rembrandt Paintings Vol VI), Springer. ISBN-109789402410273.
- van Hateren, J. H., & van der Schaaf, A. (1998). Independent component filters of natural images compared with simple cells in primary visual cortex. Proceedings. Biological sciences, 265(1394), 359–366. [CrossRef]
- van Heel, D., & de Barr, D.J.M. (1987). Dossier Rembrandt / The Rembrandt Papers. Museum Het Rembrandthuis Pages: 86–88. ISBN 10: 2810419639.
- van Loon, A., Noble, P., de Man, M., Alfeld, T., Callewaert, G., Van der Snickt, K., Janssens & J. Dik, (2020). The role of smalt in complex pigment mixtures in Rembrandt’s Homer 1663: combining MA-XRF imaging, microanalysis, paint reconstructions and OCT. Herit Sci 8, 90. [CrossRef]
- van Poppel, F., van de Kaa, D.J., & Bijwaard, G.E. (2013). Life expectancy of artists in the Low Countries from the fifteenth to the twentieth century, Population Studies, 67:3, 275-292. [CrossRef]
- Varela, M., Ruiz-Esteban, R., & Mestre de Juan, M. J. (2010). Chaos, fractals, and our concept of disease. Perspectives in biology and medicine, 53(4), 584–595. [CrossRef]
- Vellante, M., Zucca, G., Preti, A., Sisti, D., Rocchi, M. B., Akiskal, K. K., & Akiskal, H. S. (2011). Creativity and affective temperaments in non-clinical professional artists: an empirical psychometric investigation. Journal of affective disorders, 135(1-3), 28–36. [CrossRef]
- Viengkham C, & Spehar B. (2018) Preference for Fractal-Scaling Properties Across Synthetic Noise Images and Artworks. Front Psychol. Aug 29;9:1439. [CrossRef]
- Virgili, G., Parravano, M., Petri, D., Maurutto, E., Menchini, F., Lanzetta, P., Varano, M., Mariotti, S. P., Cherubini, A., & Lucenteforte, E. (2022). The Association between Vision Impairment and Depression: A Systematic Review of Population-Based Studies. Journal of clinical medicine, 11(9), 2412. [CrossRef]
- Vitay J., & Hamker, F.H. (2007). On the Role of Dopamine in Cognitive Vision. In: Paletta L., Rome E. (eds) Attention in Cognitive Systems. Theories and Systems from an Interdisciplinary Viewpoint. LNAI 4840, pp. 352–366 WAPCV. Lecture Notes in Computer Science, vol 4840. Springer.
- Voskuil P. (2020). Vincent van Gogh and his illness. A reflection on a posthumous diagnostic exercise. Epilepsy & behavior : E&B, 111, 107258. [CrossRef]
- Wadeson, H., & Carpenter, W. T., Jr (1976). A comparative study of art expression of schizophrenic, unipolar depressive, and bipolar manic-depressive patients. The Journal of nervous and mental disease, 162(5), 334–344. [CrossRef]
- Wadeson, H. (1971). Characteristics of art expression in depression. J Nerv Ment Dis. Sep;153(3):197-204. [CrossRef] [PubMed]
- Wang, J.H. & Ogawa, S.. (2015). Fractal Analysis Of Colors And Shapes For Natural And Urbanscapes URBANSCAPES. ISPRS - International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences. XL-7/W3. 1431-1438. 10.5194/isprsarchives-XL-7-W3-1431-2015.
- Weil, R.S., Schrag, A.E., Warren, J.D., Crutch, S.J., Lees, A.J., & Morris, H.R. (2016). Visual dysfunction in Parkinson’s disease. Brain. Nov 1;139(11):2827-2843. [CrossRef]
- Werner, G. (2010). Fractals in the nervous system: conceptual implications for theoretical neuroscience. Frontiers in Physiology. Vol 1. https://www.frontiersin.org/articles/10.3389/fphys.2010.00015. ISSN 1664-042X. [CrossRef]
- West, B.J., (2006). Where Medicine Went Wrong: Rediscovering the Path to Complexity. World Scientific Publishing. ISBN-109812568832.
- White, C., & Buvelot, Q. (eds) (1999). Rembrandt by Himself. National Gallery Publications Ltd. ISBN-10-0300077890.
- Wiser, I., Parnass, A. J., Rachmiel, R., Westreich, M., & Friedman, T. (2016). Rembrandt’s Ocular Pathologies. Ophthalmic plastic and reconstructive surgery, 32(4), 305–309. [CrossRef]
- World Health Organization (2022). https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 285. World Health Organization (2017). https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults.
- Worrall, C., Jongenelis, M.L., McEvoy, P.M., Ben, J., Newton, R.U., & Pettigrew, S. (2020). An Exploratory Study of the Relative Effects of Various Protective Factors on Depressive Symptoms Among Older People. Frontiers in Public Health Vol. 8. ISSN=2296-256. DOI=10.3389/fpubh.2020.579304. https://www.frontiersin.org/articles/10.3389/fpubh.2020.579304.
- Xin, W., Yu, R., & Zhao, L. (2021). Event-related-potential based evidence of cognitive dysfunction of processing emotional faces in major depressive disorder patients. Neuroscience letters, 742, 135545. [CrossRef]
- Yan, F. F., Hou, F., Lu, H., Yang, J., Chen, L., Wu, Y., Chen, G., & Huang, C. B. (2020). Aging affects gain and internal noise in the visual system. Scientific reports, 10(1), 6768. [CrossRef]
- Yang, Y., Wang, Y., Zhang, C., Zhu, J., & Yu, Y. (2019). Neuroanatomical substrates underlying contrast sensitivity. Quantitative imaging in medicine and surgery, 9(3), 503–509. [CrossRef]
- Ye, Z., Wei, X., Zhang, J., Li, H., & Cao, J. (2023). The impact of adverse childhood experiences on depression: the role of insecure attachment styles and emotion dysregulation strategies. Current psychology, 1–11. [CrossRef]
- Zhang, X., Zuo, B., Erskine, K., & Hu, T. (2016). Feeling light or dark? Emotions affect perception of brightness. Journal of Environmental Psychology, 47, 107–111. [CrossRef]
- Zhang, X., Bullard, K. M., Cotch, M. F., Wilson, M. R., Rovner, B. W., McGwin, G., Jr, Owsley, C., Barker, L., Crews, J. E., & Saaddine, J. B. (2013). Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005-2008. JAMA ophthalmology, 131(5), 573–581. [CrossRef]
- Zhuang, X., Tran, T., Jin, D., Philip, R., & Wu, C. (2021). Aging effects on contrast sensitivity in visual pathways: A pilot study on flicker adaptation. PloS one, 16(12), e0261927. [CrossRef]
- Żochowska, A., Nowicka, M. M., Wójcik, M. J., & Nowicka, A. (2021). Self-face and emotional faces-are they alike?. Social cognitive and affective neuroscience, 16(6), 593–607. [CrossRef]
- Zwick, J. C., & Wolkenstein, L. (2017). Facial emotion recognition, theory of mind and the role of facial mimicry in depression. Journal of affective disorders, 210, 90–99. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).