Submitted:
22 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
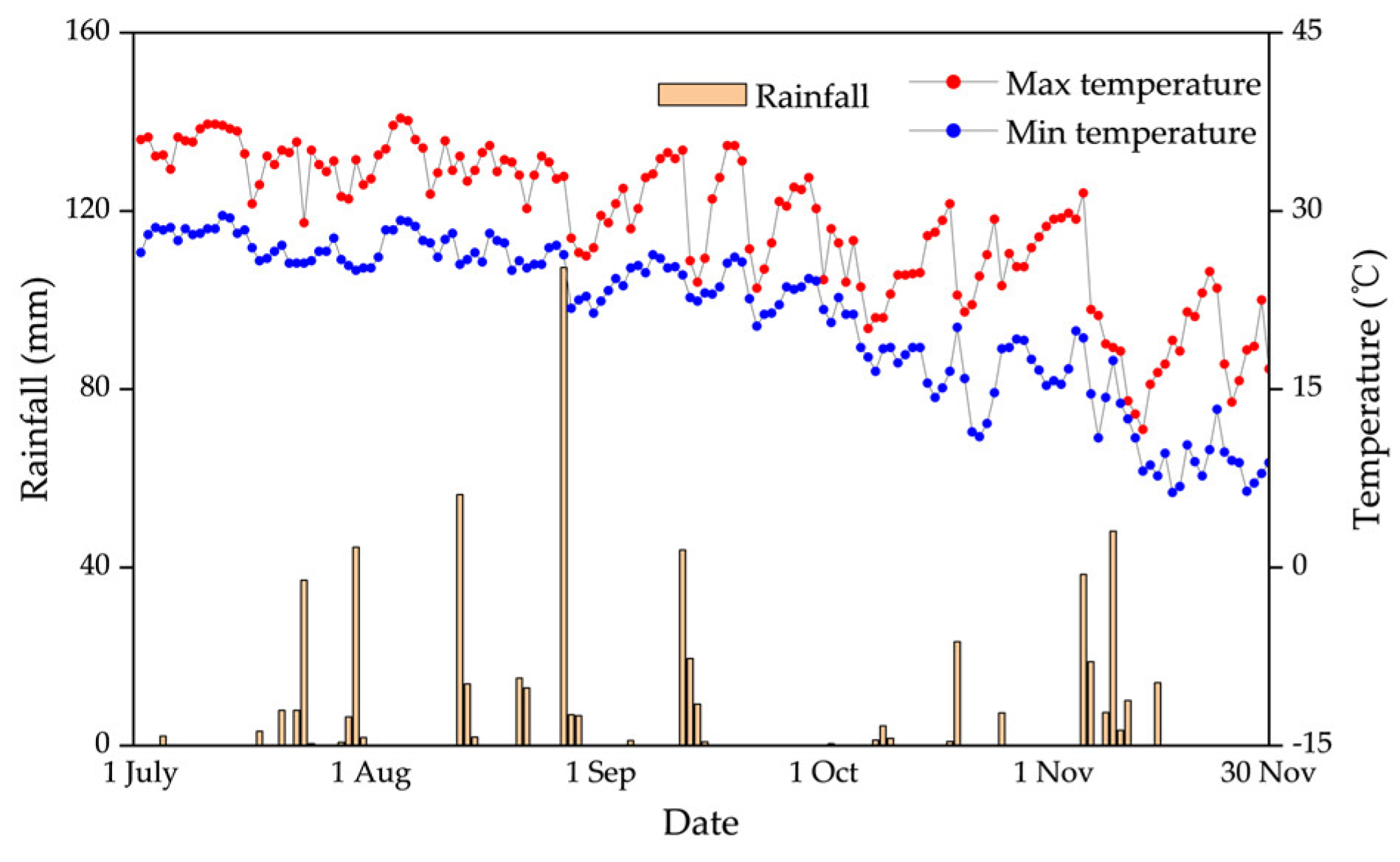
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Field Water Balance
2.3.2. Classification of Drought Stress Level
2.3.3. Rice Growth and Leaf Physiology
2.3.4. Yield and Its Composition
2.4. Statistical Analysis
3. Results
3.1. Four Levels of Continuous Drought Stress Process
3.2. Effects of Four Levels of Continuous Drought Stress on Water Consumption and Utilization
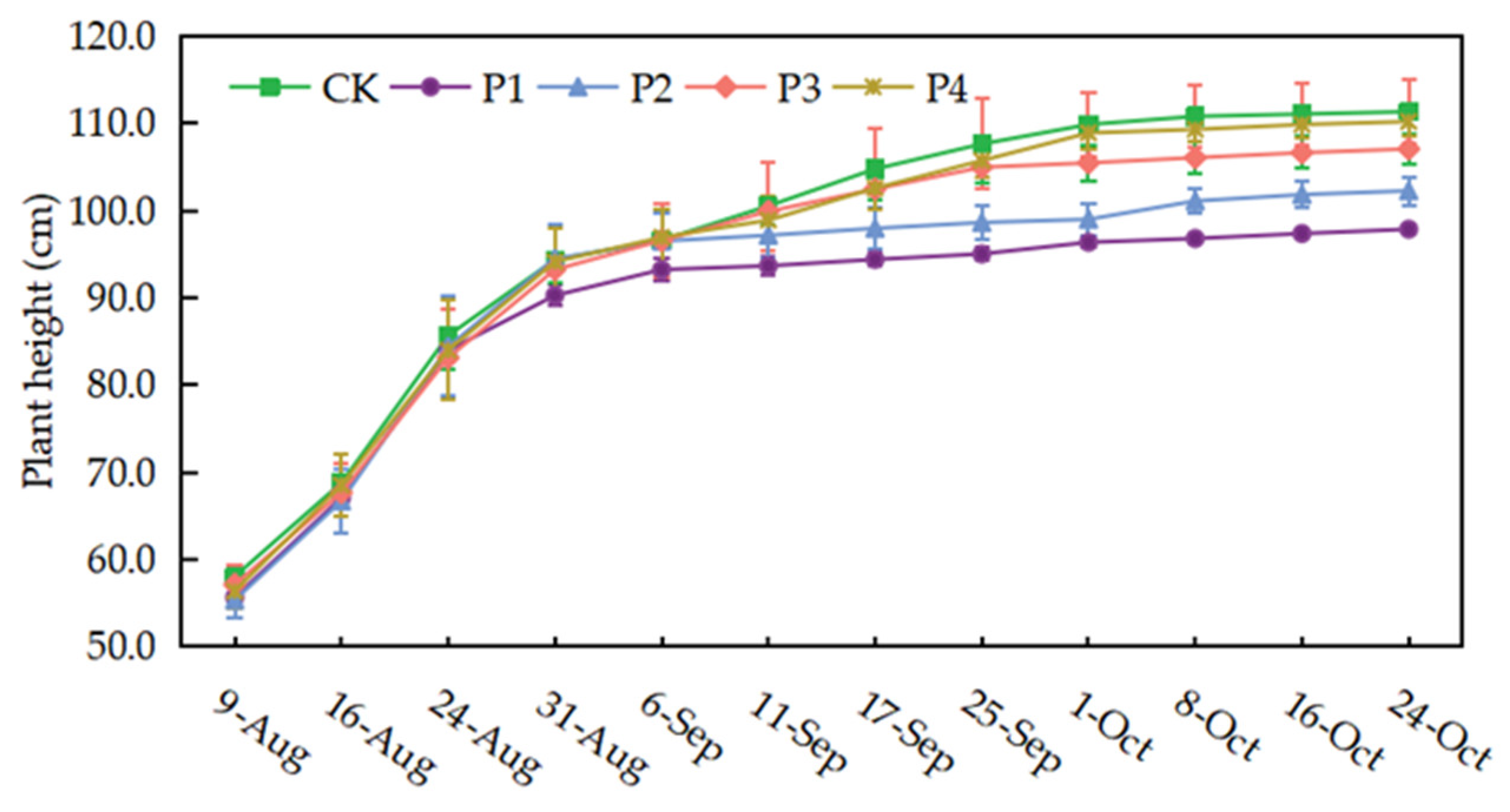
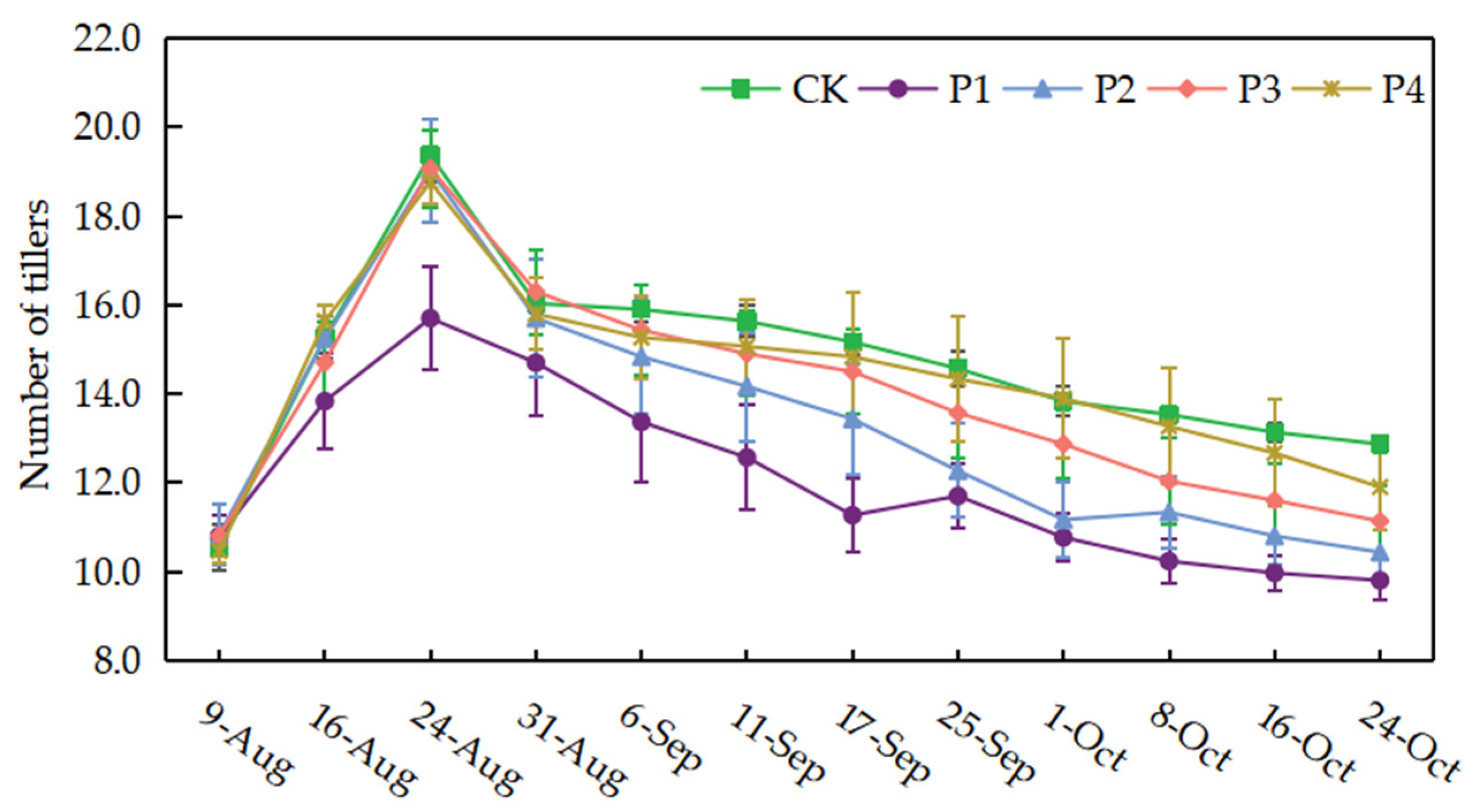
3.3. Effects of Four Levels of Continuous Drought Stress on Plant Height and Tillering
3.4. Effects of Four Levels of Continuous Drought Stress on Physiological Parameters
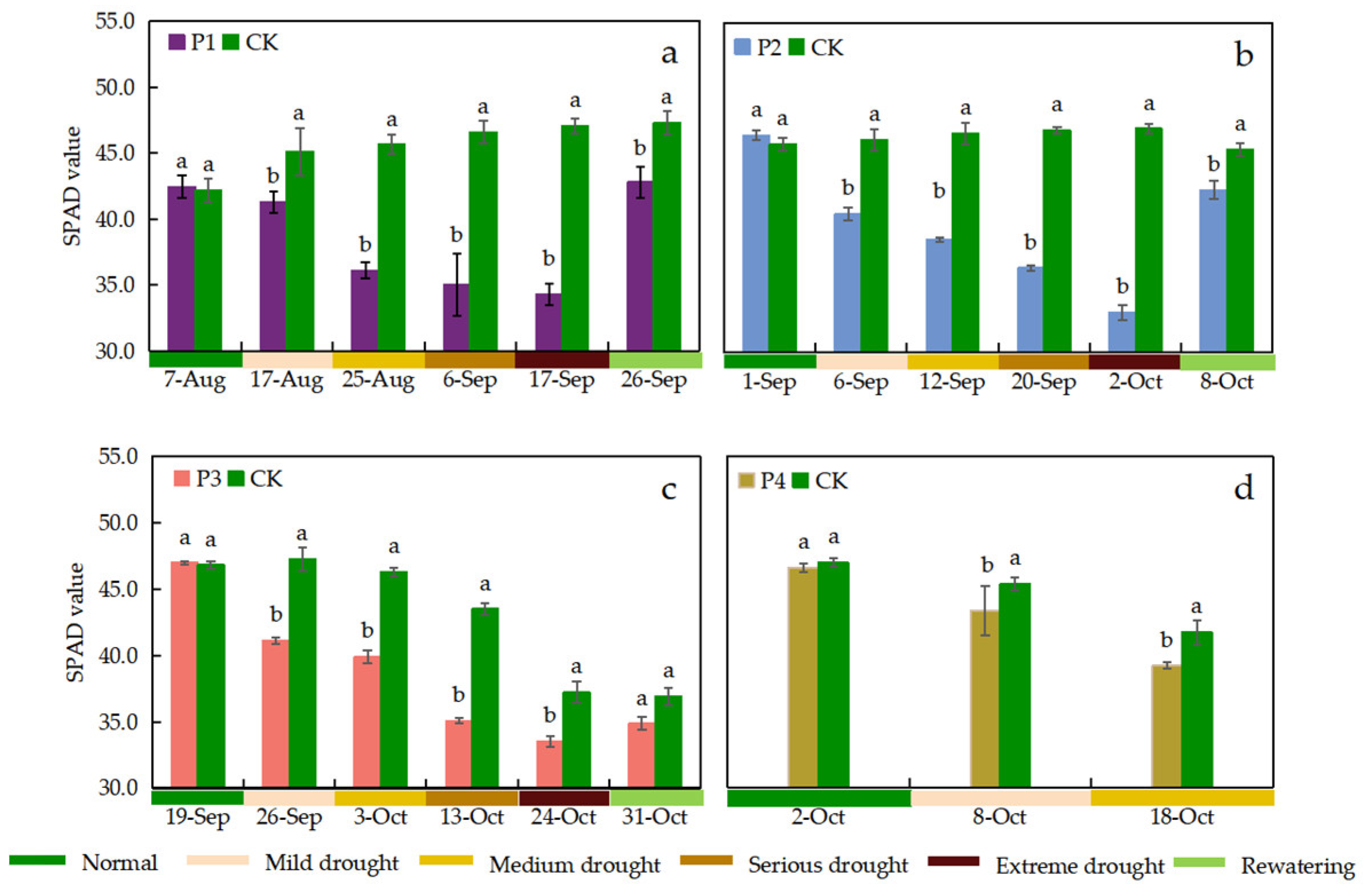
3.4.1. Relative Chlorophyll Content (SPAD Value)
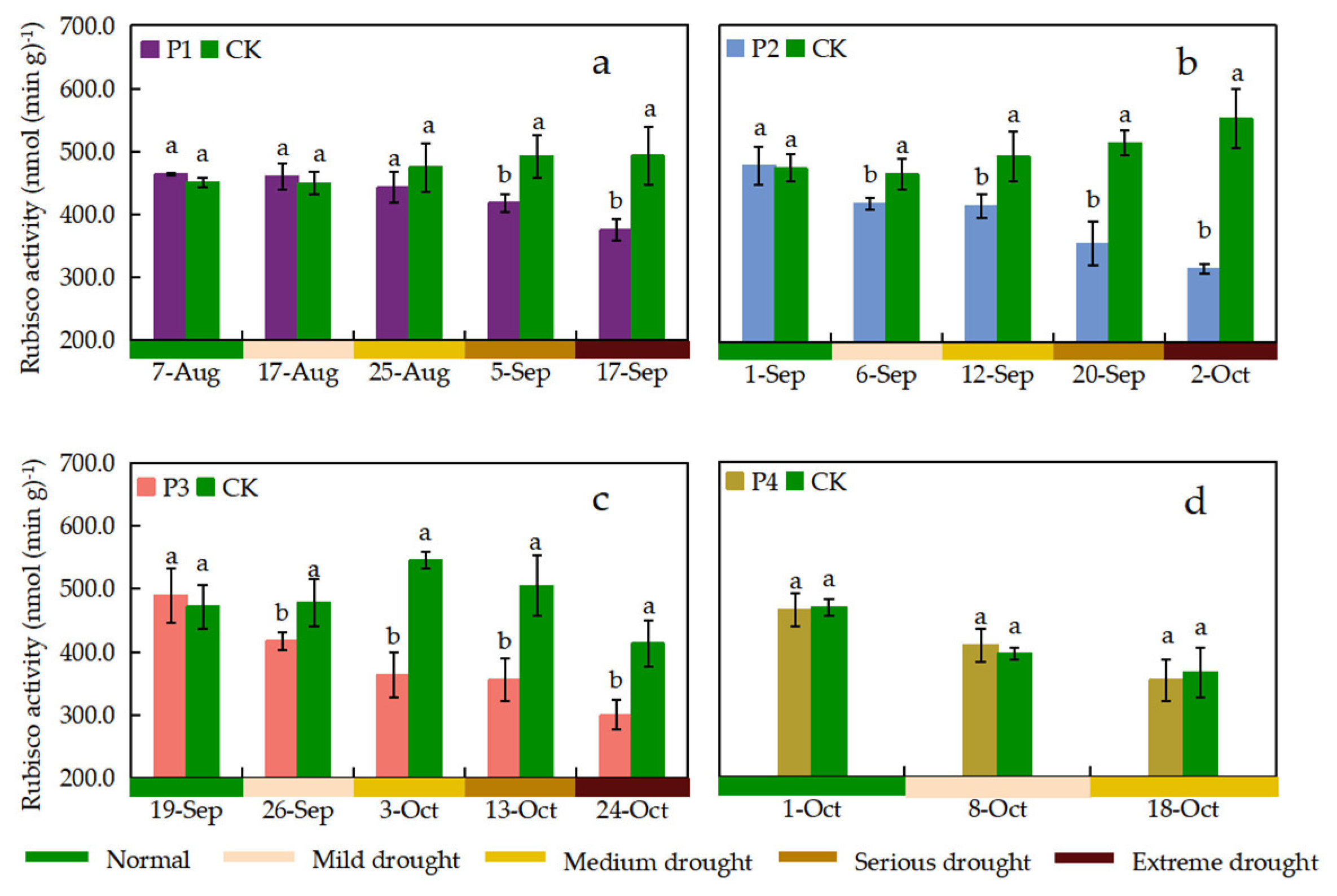
3.4.2. Activity of Rubisco
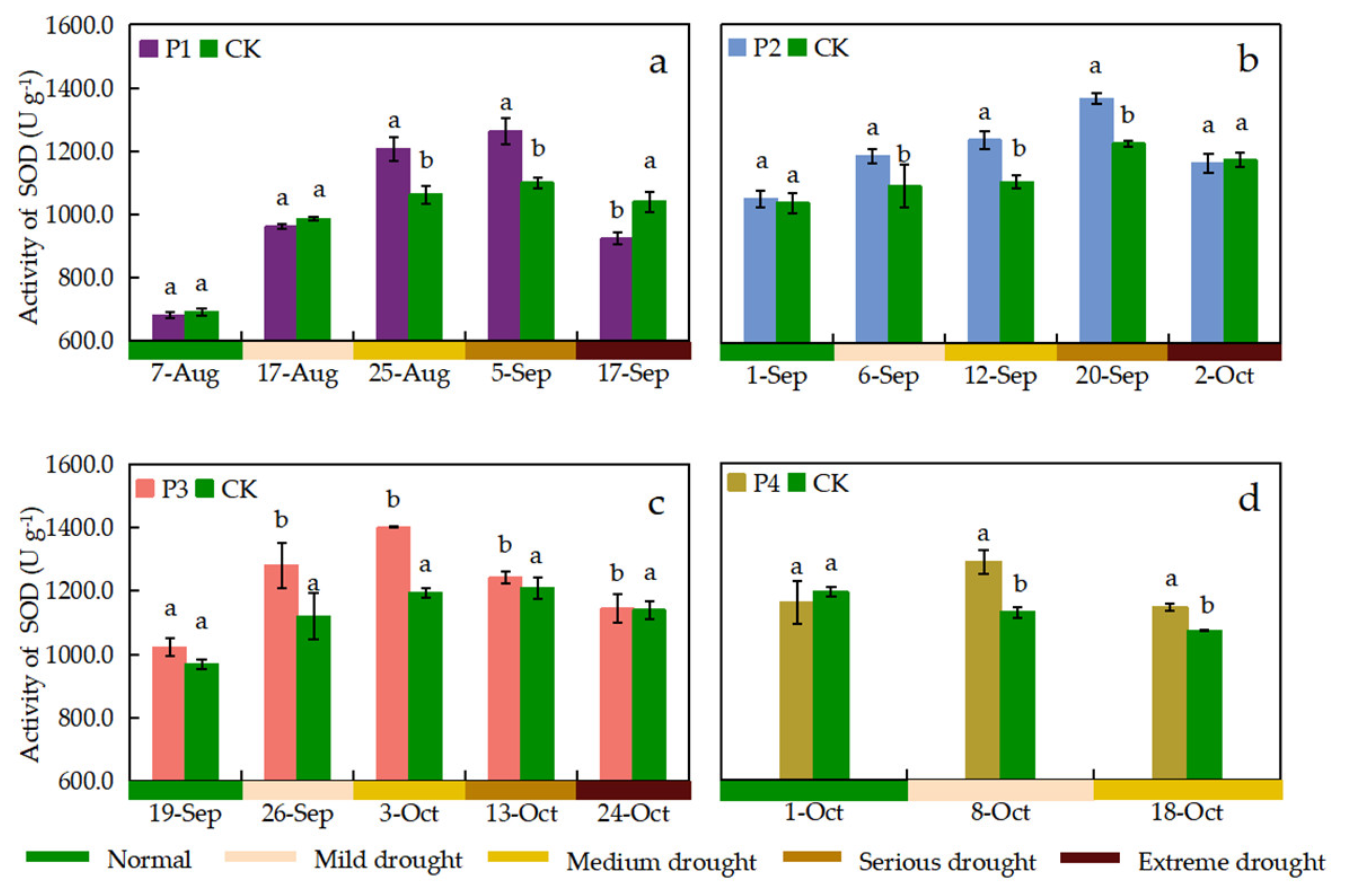
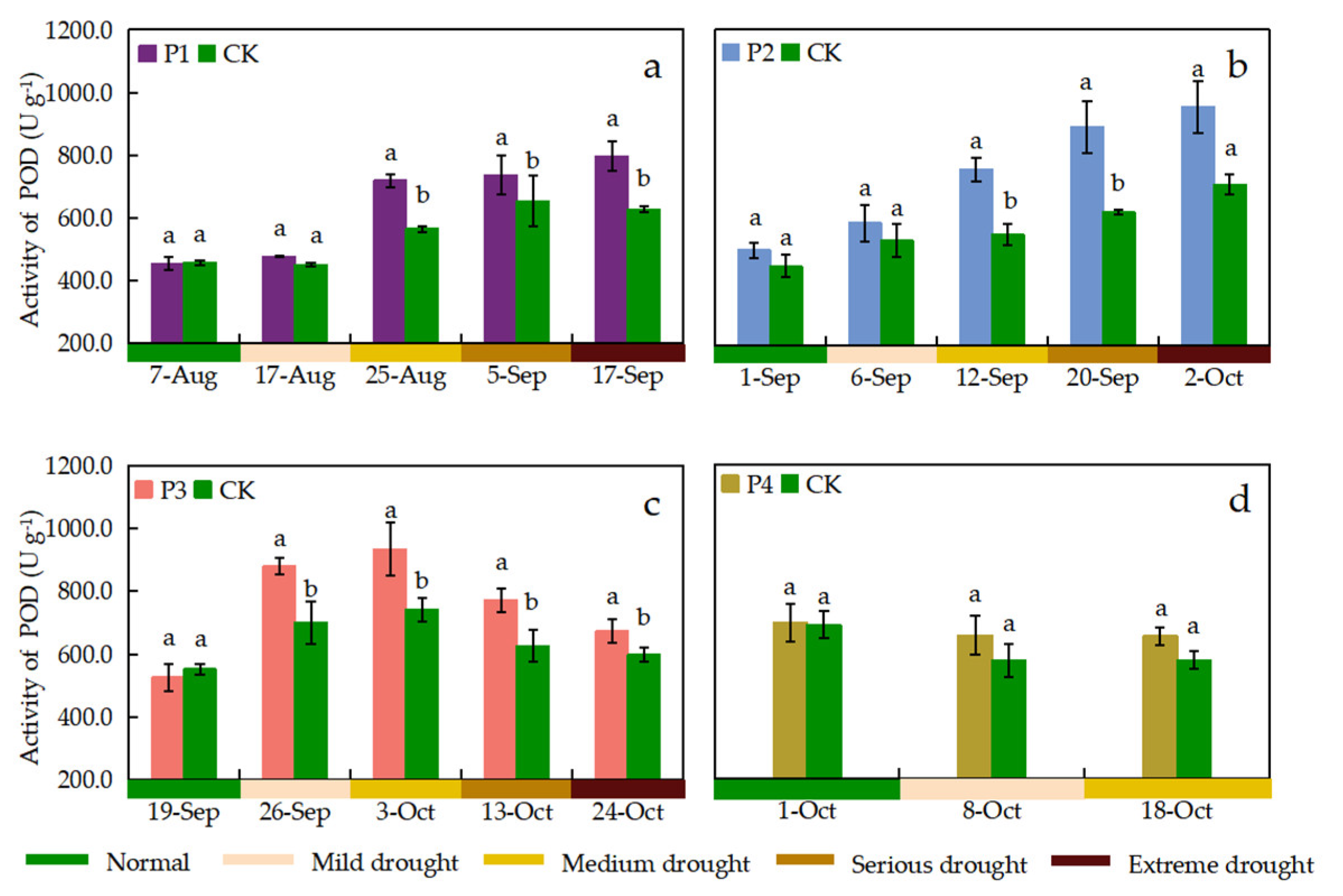
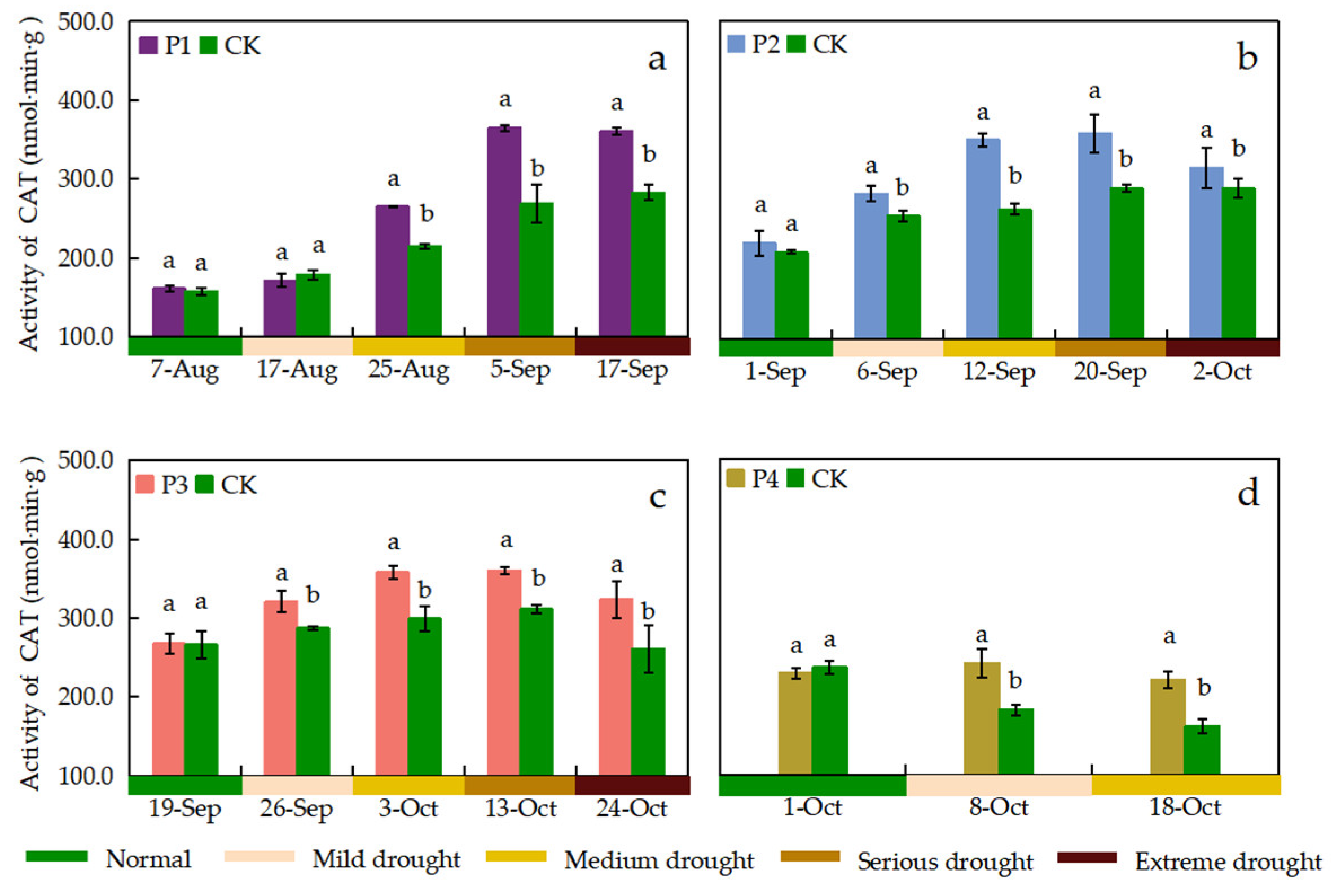
3.4.3. Activities of SOD, POD and CAT
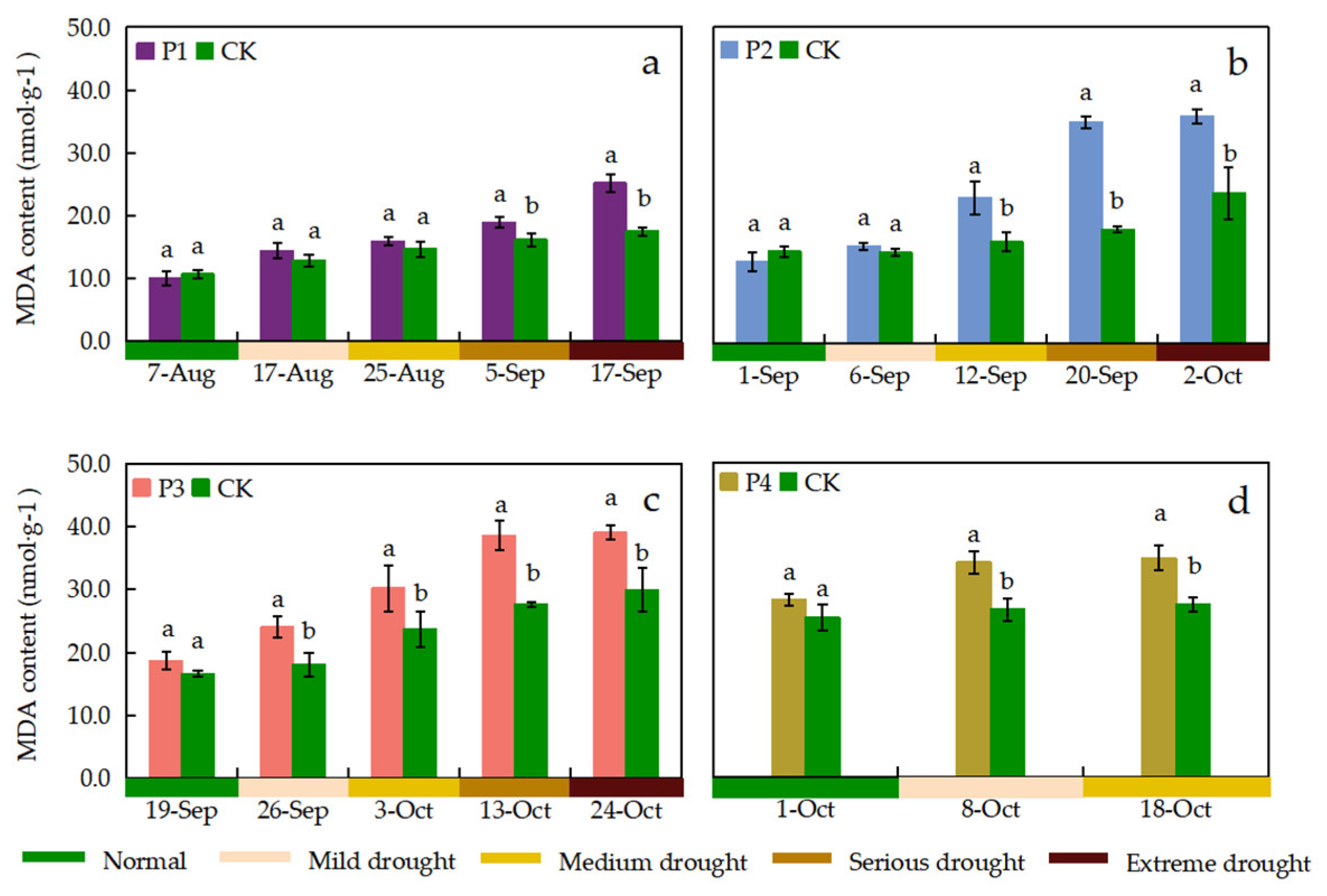
3.4.4. MDA Content
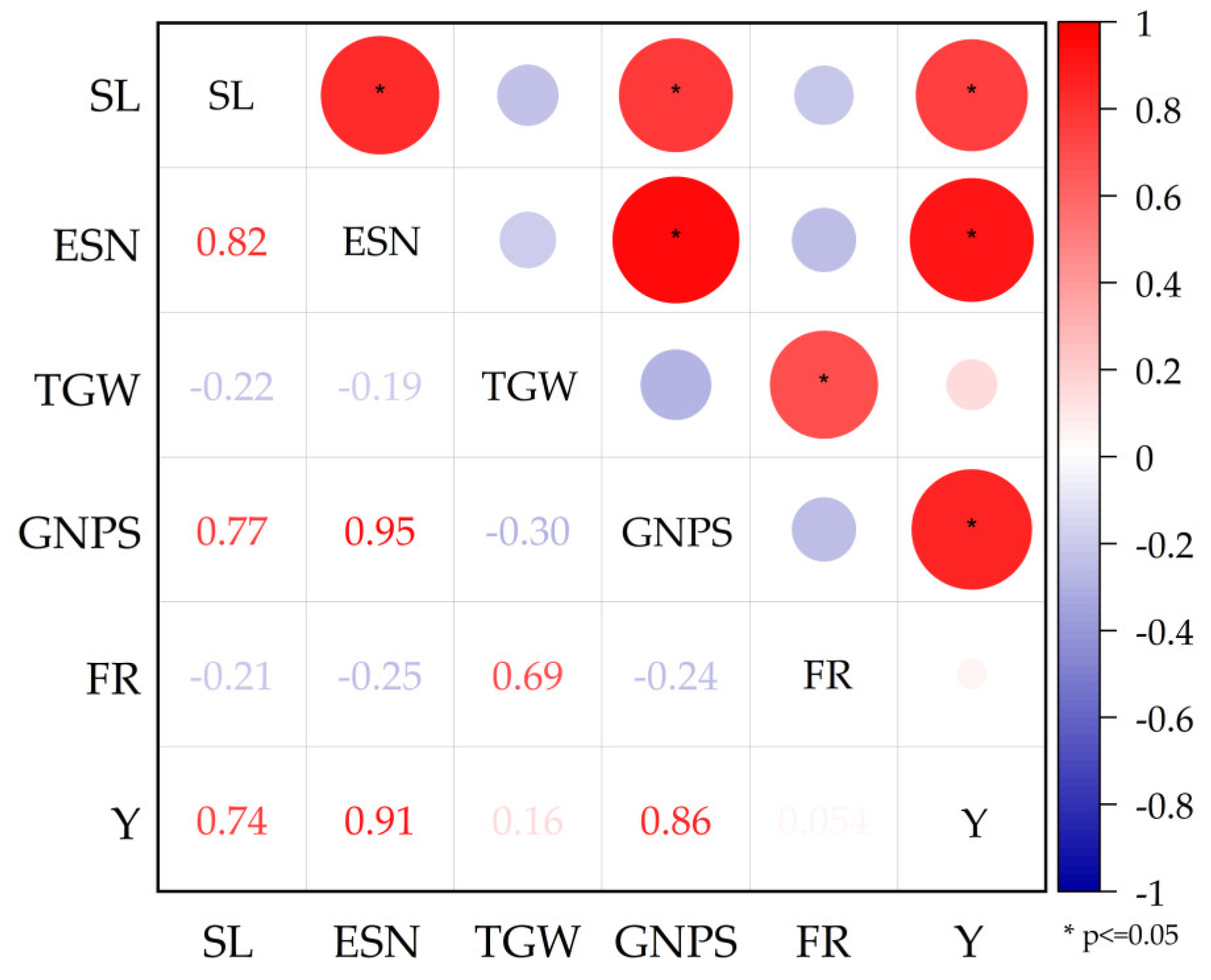
3.5. Effects of Four Levels of Continuous Drought Stress on Yield and Its Components
| Treatment | Spike length (cm) | Effective spike number (104 hm−2) | Thousand grain weight (g) | Grain number per spike | Fruiting rate (%) | Yield (kg·hm-2) | Yield reduction (%) |
|---|---|---|---|---|---|---|---|
| CK | 23.86±0.22a | 262.51±7.44a | 21.98±0.05a | 161.13±4.13a | 87.55±0.50b | 10778.36±363.24a | —— |
| P1 | 22.44±0.67b | 185.76±5.37b | 21.95±0.24a | 131.69±3.94c | 90.6±0.30a | 7154.97±155.93d | 33.62 |
| P2 | 22.85±0.34b | 196.76±4.34c | 21.56±0.60a | 134.31±7.11c | 84.56±0.77c | 7141.46±107.05d | 33.74 |
| P3 | 23.09±0.51ab | 225.97±12.37d | 20.43±0.09b | 149.62±0.77b | 83.52±0.28d | 7815.67±199.81c | 27.49 |
| P4 | 23.68±0.13a | 243.30±4.11d | 20.76±0.24b | 154.43±6.15ab | 85.35±0.90c | 8875.99±63.06b | 17.62 |
| F | 5.28* | 83.71** | 12.81** | 23.69** | 70.64** | 174.64** | —— |
4. Discission
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, M.; Yang, L.; Mao, X.; Li, J.; Li, L.; Wang, J.; Liu, H.; Zheng, H.; Li, Z.; et al. OsADR3 Increases Drought Stress Tolerance by Inducing Antioxidant Defense Mechanisms and Regulating OsGPX1 in Rice (Oryza Sativa L.). The Crop Journal 2021, 9, 1003–1017. [Google Scholar] [CrossRef]
- Rahman, K.U.; Hussain, A.; Ejaz, N.; Shang, S.; Balkhair, K.S.; Khan, K.U.J.; Khan, M.A.; Rehman, N.U. Analysis of Production and Economic Losses of Cash Crops under Variable Drought: A Case Study from Punjab Province of Pakistan. International Journal of Disaster Risk Reduction 2023, 85, 103507. [Google Scholar] [CrossRef]
- Dai, Y.; Liao, Z.; Lai, Z.; Bai, Z.; Zhang, F.; Li, Z.; Fan, J. Interactive Effects of Planting Pattern, Supplementary Irrigation and Planting Density on Grain Yield, Water-Nitrogen Use Efficiency and Economic Benefit of Winter Wheat in a Semi-Humid but Drought-Prone Region of Northwest China. Agricultural Water Management 2023, 287. [Google Scholar] [CrossRef]
- Yang, W.; Ren, S.; Zhang, X.; Gao, M.; Ye, S.; Qi, Y.; Zheng, Y.; Wang, J.; Zeng, L.; Li, Q.; et al. BENT UPPERMOST INTERNODE1 Encodes the Class II Formin FH5 Crucial for Actin Organization and Rice Development. The Plant Cell 2011, 23, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Palakolanu, S.R.; Chopra, P.; Rajurkar, A.B.; Gupta, R.; Iqbal, N.; Maheshwari, C. Improving Drought Tolerance in Rice: Ensuring Food Security through Multi-dimensional Approaches. Physiologia Plantarum 2021, 172, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Hassan, M.U.; Aamer, M.; Batool, M.; Sheng, F.; Ziming, W.; Huijie, L. A Critical Review on the Improvement of Drought Stress Tolerance in Rice (Oryza Sativa L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2020, 48, 1756–1788. [Google Scholar] [CrossRef]
- Hornai, E.M.L.; Aycan, M.; Mitsui, T. The Promising B− Type Response Regulator Hst1 Gene Provides Multiple High Temperature and Drought Stress Tolerance in Rice. International Journal of Molecular Sciences 2024, 25, 2385. [Google Scholar] [CrossRef] [PubMed]
- Cheng-ai, W.; Bo-lun, W.; Wen-xiang, Z.; Lei, Z.; Xiu-zhe, Z.; Lian-wen, G.; Wen-ping, H. Effects of Drought Stress at Different Growth Stages on Grain Yield and Milling Quality of Rice. Chinese Journal of Rice Science 2007, 21, 643. [Google Scholar]
- Farooq, M.; Kobayashi, N.; Ito, O.; Wahid, A.; Serraj, R. Broader Leaves Result in Better Performance of Indica Rice under Drought Stress. Journal of plant physiology 2010, 167, 1066–1075. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, X.; Shao, C.; Qiu, C.; Chen, X.; Chen, J.; Xie, J.; Deng, G.; Peng, C. Effects of Drought Stress at Different Growth Stages on Yield and Water Use Efficiency of Double-Cropping Rice. Acta Agriculturae Jiangxi 2016, 28, 6–14. [Google Scholar]
- Nguyen, G.; Hailstones, D.; Wilkes, M.; Sutton, B. Drought-induced Oxidative Conditions in Rice Anthers Leading to a Programmed Cell Death and Pollen Abortion. Journal of Agronomy and Crop Science 2009, 195, 157–164. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Shi, W.; Yoshimoto, M.; Hasegawa, T.; Hincha, D.K.; Zuther, E.; Jagadish, S.K. Combined Drought and Heat Stress Impact during Flowering and Grain Filling in Contrasting Rice Cultivars Grown under Field Conditions. Field Crops Research 2018, 229, 66–77. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J. Carbohydrate, Hormone and Enzyme Regulations of Rice Grain Filling under Post-Anthesis Soil Drying. Environmental and experimental botany 2020, 178, 104165. [Google Scholar] [CrossRef]
- Rasheed, S.; Bashir, K.; Matsui, A.; Tanaka, M.; Seki, M. Transcriptomic Analysis of Soil-Grown Arabidopsis Thaliana Roots and Shoots in Response to a Drought Stress. Frontiers in plant science 2016, 7, 183475. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R. Impact of Drought on Photosynthesis: Molecular Perspective. Plant gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Evaristo de Deus, K.; Lanna, A.C.; Abreu, F.R.M.; Dias Silveira, R.D.; Jacinto Pereira, W.; Brondani, C.; Pereira Vianello, R. Molecular and Biochemical Characterization of Superoxide Dismutase (SOD) in Upland Rice under Drought. Australian Journal of Crop Science 2015, 9. [Google Scholar]
- Gusain, Y.S.; Singh, U.; Sharma, A. Bacterial Mediated Amelioration of Drought Stress in Drought Tolerant and Susceptible Cultivars of Rice (Oryza Sativa L.). African Journal of Biotechnology 2015, 14, 764–773. [Google Scholar]
- Farooq, M.; Wahid, A.; Lee, D.; Cheema, S.; Aziz, T. Drought Stress: Comparative Time Course Action of the Foliar Applied Glycinebetaine, Salicylic Acid, Nitrous Oxide, Brassinosteroids and Spermine in Improving Drought Resistance of Rice. Journal of Agronomy and Crop Science 2010, 196, 336–345. [Google Scholar] [CrossRef]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-Responsive Mechanisms in Rice Genotypes with Contrasting Drought Tolerance during Reproductive Stage. Journal of plant physiology 2012, 169, 336–344. [Google Scholar] [CrossRef]
- Zhu, P.; Jia, X.; Zhao, C.; Shao, M. Long-Term Soil Moisture Evolution and Its Driving Factors across China’s Agroecosystems. Agricultural Water Management 2022, 269, 107735. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Liu, Q.; Wang, Q.; Lin, A.; Luo, J.; Du, Y.; Lin, Y.-W.; Wei, H. In Vitro Measurement of Superoxide Dismutase-like Nanozyme Activity: A Comparative Study. Analyst 2021, 146, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.A.M.; Sankaranarayanan, A.; Senthilkumar, M. Plant-Microbe Interactions; Springer, 2021; ISBN 1-07-161079-1. [Google Scholar]
- Sinha, A.K. Colorimetric Assay of Catalase. Analytical biochemistry 1972, 47, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Sakoda, K.; Fukayama, H.; Kondo, E.; Suzuki, Y.; Makino, A.; Terashima, I.; Yamori, W. Overexpression of Both Rubisco and Rubisco Activase Rescues Rice Photosynthesis and Biomass under Heat Stress. Plant, Cell & Environment 2021, 44, 2308–2320. [Google Scholar]
- Fageria, N. Yield Physiology of Rice. Journal of plant nutrition 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant Growth under Drought Stress: Significance of Mineral Nutrients. Water stress and crop plants: a sustainable approach 2016, 2, 649–668. [Google Scholar]
- Prasad, P.; Staggenborg, S.; Ristic, Z. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. Response of crops to limited water: Understanding and modeling water stress effects on plant growth processes 2008, 1, 301–355. [Google Scholar]
- Cai, T.; Xu, H.; Peng, D.; Yin, Y.; Yang, W.; Ni, Y.; Chen, X.; Xu, C.; Yang, D.; Cui, Z.; et al. Exogenous Hormonal Application Improves Grain Yield of Wheat by Optimizing Tiller Productivity. Field Crops Research 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant Drought Stress: Effects, Mechanisms and Management. Sustainable agriculture 2009, 153–188. [Google Scholar]
- Moonmoon, S.; Islam, M.T. Effect of Drought Stress at Different Growth Stages on Yield and Yield Components of Six Rice (Oryza Sativa L.) Genotypes. Fundamental and Applied Agriculture 2017, 2, 285–289. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynthesis Research 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Dai, Y.; Fan, J.; Liao, Z.; Zhang, C.; Yu, J.; Feng, H.; Zhang, F.; Li, Z. Supplemental Irrigation and Modified Plant Density Improved Photosynthesis, Grain Yield and Water Productivity of Winter Wheat under Ridge-Furrow Mulching. Agricultural Water Management 2022, 274, 107985. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza Sativa L.) with Reduced Chlorophyll Content Exhibit Higher Photosynthetic Rate and Efficiency, Improved Canopy Light Distribution, and Greater Yields than Normally Pigmented Plants. Field Crops Research 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Kyparissis, A.; Petropoulou, Y.; Manetas, Y. Summer Survival of Leaves in a Soft-Leaved Shrub (Phlomis Fruticosa L., Labiatae) under Mediterranean Field Conditions: Avoidance of Photoinhibitory Damage through Decreased Chlorophyll Contents. Journal of Experimental Botany 1995, 46, 1825–1831. [Google Scholar] [CrossRef]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different Mechanisms of Adaptation to Cyclic Water Stress in Two South Australian Bread Wheat Cultivars. Journal of experimental botany 2008, 59, 3327–3346. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, H.; Zhou, L.; Xu, Z.; Zhou, G. Tracking Chlorophyll Fluorescence as an Indicator of Drought and Rewatering across the Entire Leaf Lifespan in a Maize Field. Agricultural Water Management 2019, 211, 190–201. [Google Scholar] [CrossRef]
- Brito, G.G. de; Sofiatti, V.; Lima, M.M. de A.; Carvalho, L.P. de; Silva Filho, J.L. da Physiological Traits for Drought Phenotyping in Cotton. Acta Scientiarum. Agronomy 2011, 33, 117–125. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A Study on Soybean Responses to Drought Stress and Rehydration. Saudi Journal of Biological Sciences 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Salvucci, M.E. The Regulatory Properties of Rubisco Activase Differ among Species and Affect Photosynthetic Induction during Light Transitions. Plant physiology 2013, 161, 1645–1655. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 Availability and Inactivation of Rubisco Limit Photosynthesis in Cotton Plants under Heat and Drought Stress in the Field. Environmental and Experimental Botany 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. African journal of agricultural research 2011, 6, 2026–2032. [Google Scholar]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and Function of Ascorbate Peroxidase Isoenzymes. Journal of experimental botany 2002, 53, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, F.; Chen, G.; Wang, J.; Huang, F.; Cai, T.; Zhang, P.; Jia, Z. Can Deep Fertilizer Application Enhance Maize Productivity by Delaying Leaf Senescence and Decreasing Nitrate Residue Levels? Field Crops Research 2022, 277, 108417. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M. Drought-Stress-Induced Changes in Activities of Superoxide Dismutase, Catalase, and Peroxidase in Wheat Species. Plant and cell physiology 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential Responses of Lipid Peroxidation and Antioxidants in the Leaves of Drought-Tolerant P. Acutifolius Gray and Drought-Sensitive P. Vulgaris L. Subjected to Polyethylene Glycol Mediated Water Stress. Plant science 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Okami, M.; Kato, Y.; Kobayashi, N.; Yamagishi, J. Morphological Traits Associated with Vegetative Growth of Rice (Oryza Sativa L.) during the Recovery Phase after Early-Season Drought. European Journal of Agronomy 2015, 64, 58–66. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Min, Z.; Zou, D.; Liu, H.; Wang, J.; Zheng, H.; Jia, Y.; Yang, L.; Xin, W. Response of Rice with Overlapping Growth Stages to Water Stress by Assimilates Accumulation and Transport and Starch Synthesis of Superior and Inferior Grains. International Journal of Molecular Sciences 2022, 23, 11157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Venuprasad, R.; Lafitte, H.R.; Atlin, G.N. Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crop Science 2007, 47, 285–293. [Google Scholar] [CrossRef]
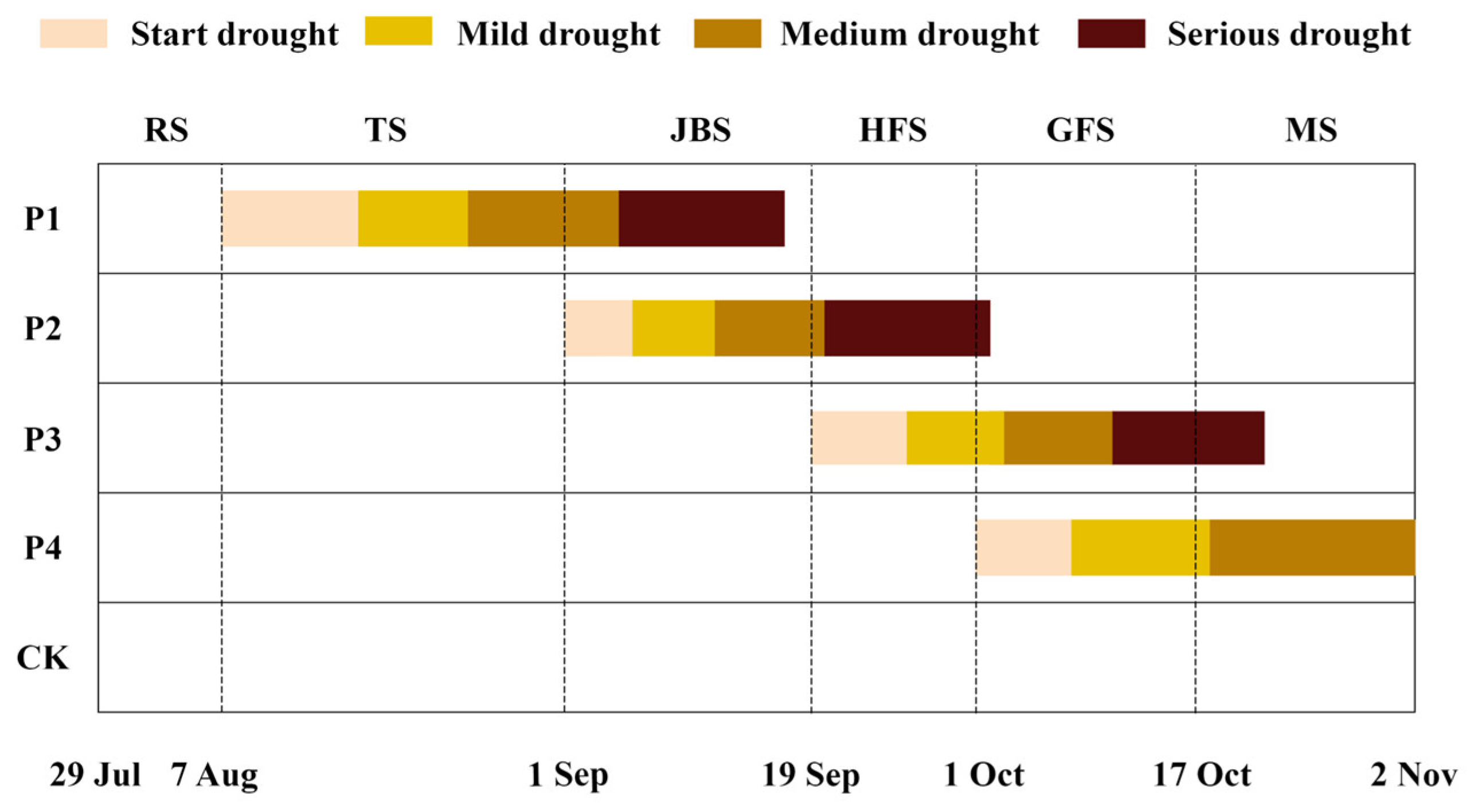
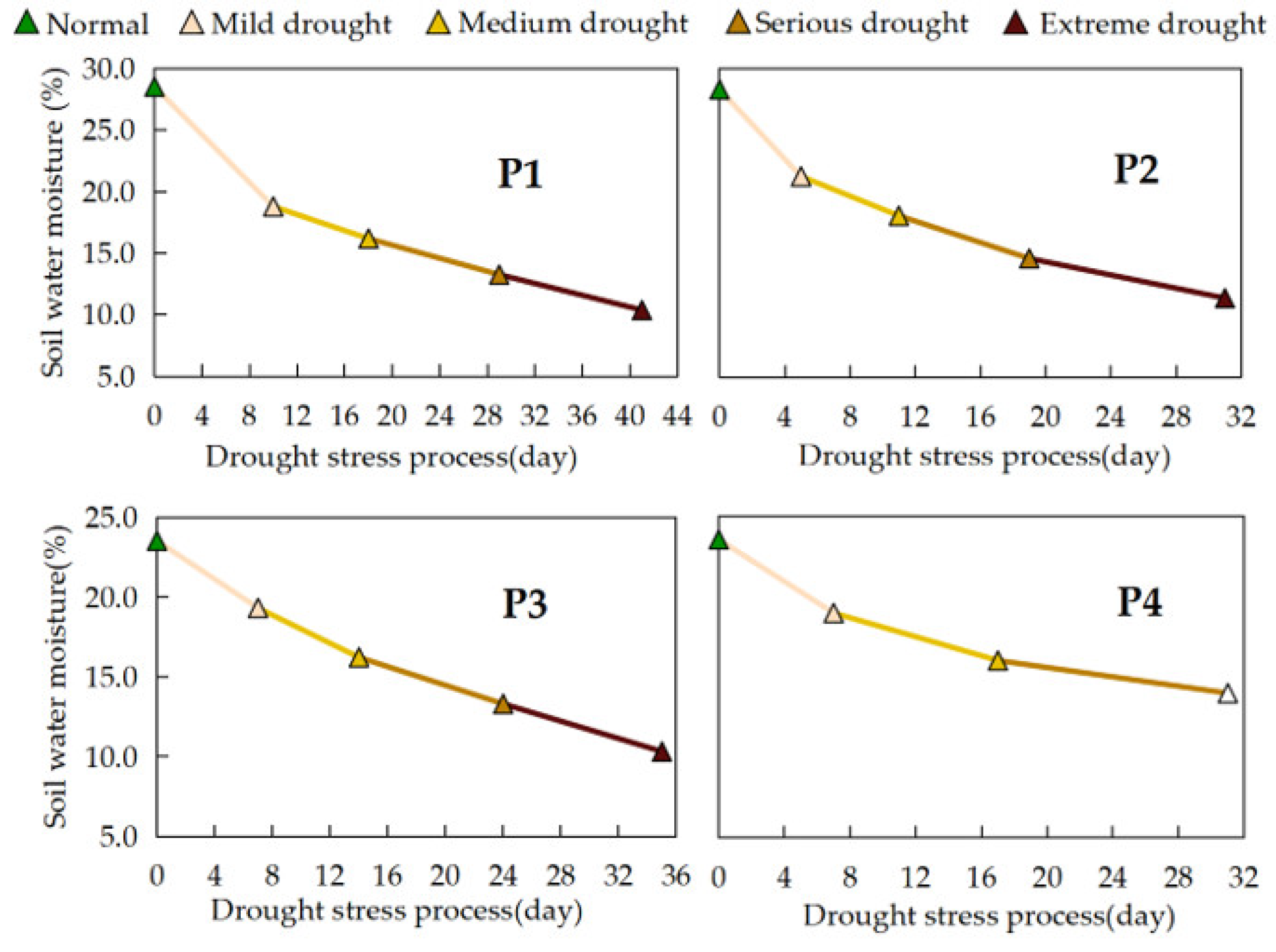
| Treatments | Drought stress at different growth stages | ||||
|---|---|---|---|---|---|
| TS | JBS | HFS | GFS | MS | |
| CK | Normal | Normal | Normal | Normal | Normal |
| P1 | Drought stress | Rewatering after extreme drought stress | |||
| P2 | Normal | Drought stress | Rewatering after extreme drought stress | ||
| P3 | Normal | Normal | Drought stress | Rewatering after extreme drought stress | |
| P4 | Normal | Normal | Normal | Drought stress | Persistent to extreme drought |
| Growth stage | RS | ETS | LTS | JBS | HFS | GFS | MS |
|---|---|---|---|---|---|---|---|
| Water layer control standards | 0-20-40 | 0-20-50 Drying for 3 days |
0-20-50 | 0-20-50 Drying for 3 days |
0-20-50 Drying for 3 days |
0-20-50 Drying for 3 days |
0-20-30 |
| Late paddy sunning | Late drying |
| Treatment | Water consumption (W, mm) | Water use efficiency (WUE, kg m–3) |
|---|---|---|
| CK | 643.91±7.70a | 1.673±0.042b |
| P1 | 499.83±9.97c | 1.430±0.053c |
| P2 | 552.30±2.15b | 1.293±0.021d |
| P3 | 548.32±5.34b | 1.427±0.025c |
| P4 | 504.24±4.29c | 1.760±0.010a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





