Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Hypothesis
Background
Biography
Somatic Illness
Mental Illness
Creativity
Bipolar Disorder
Schizophrenia
Positive Symptoms
Negative Symptoms
Recognition of Self and Others
Anxiety
Substance Abuse
Schizoaffective Disorder
Schizophrenia and Vision
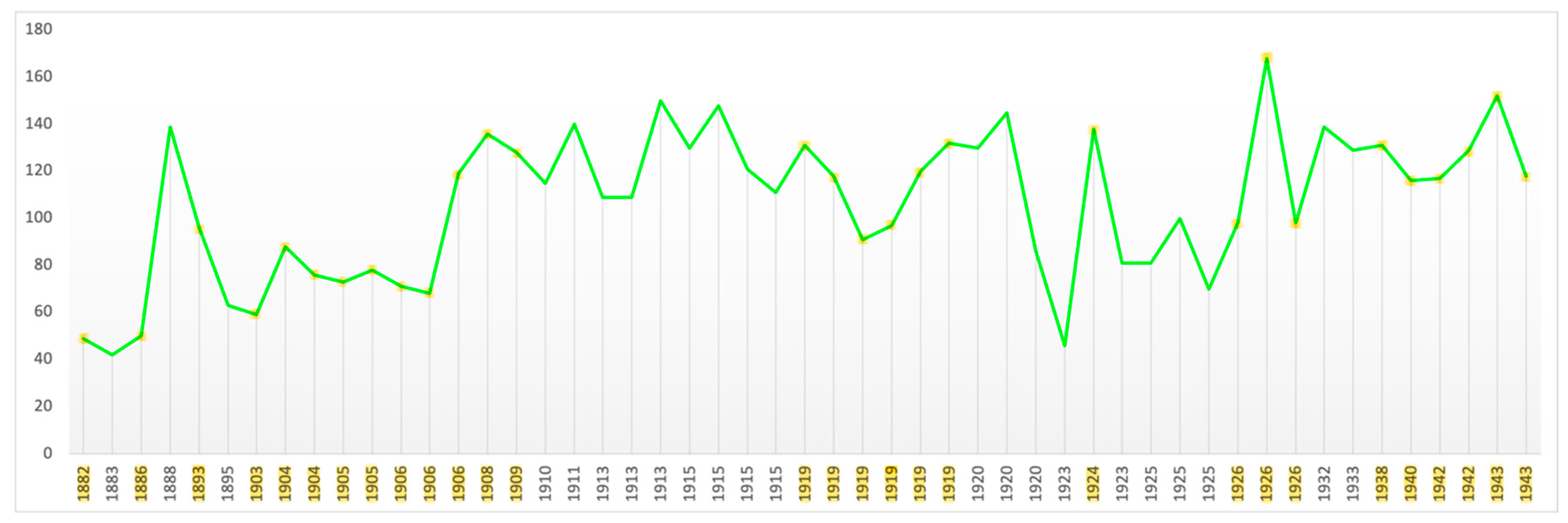
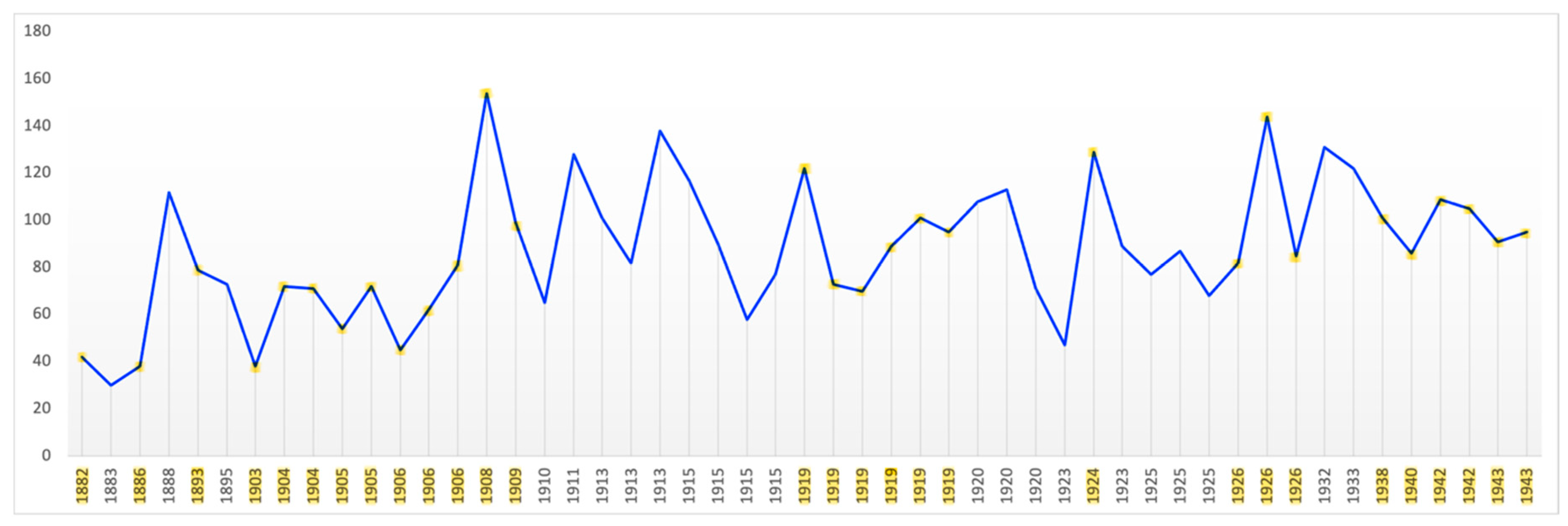
Contrast
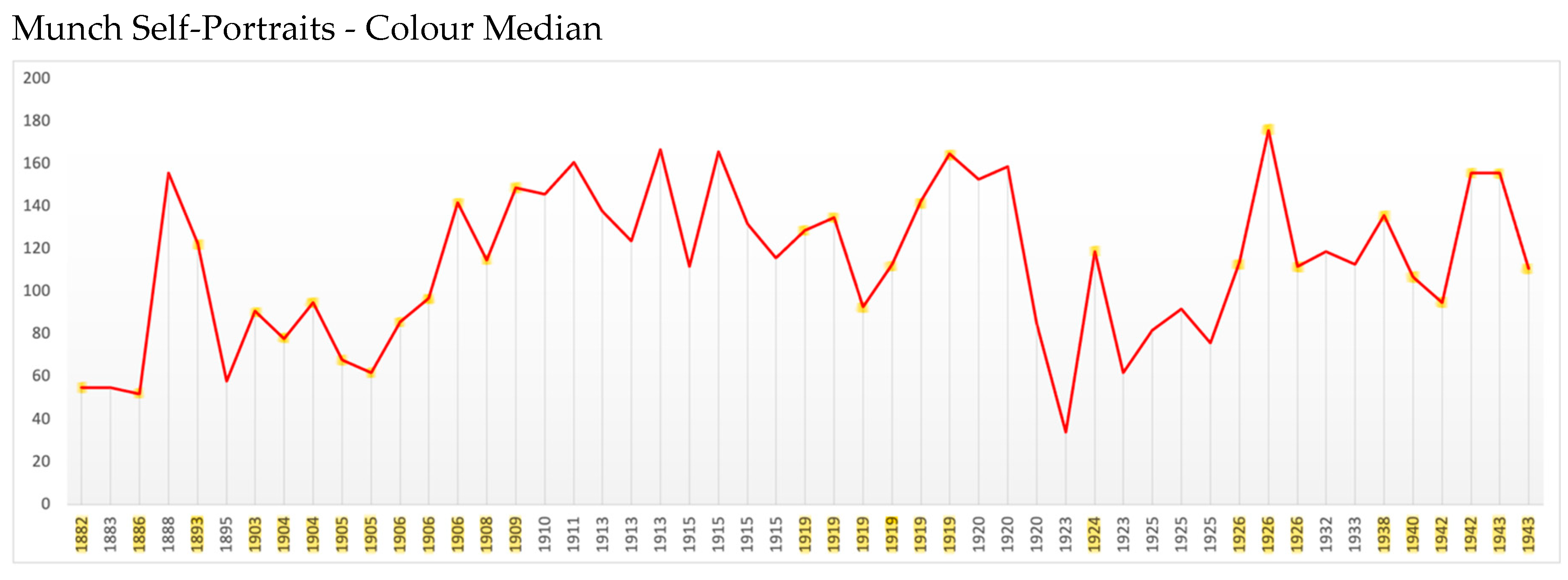
Colour
Fractals
Material
Methods
Contrast
Colour
Fractals
Results
Contrast
Colour
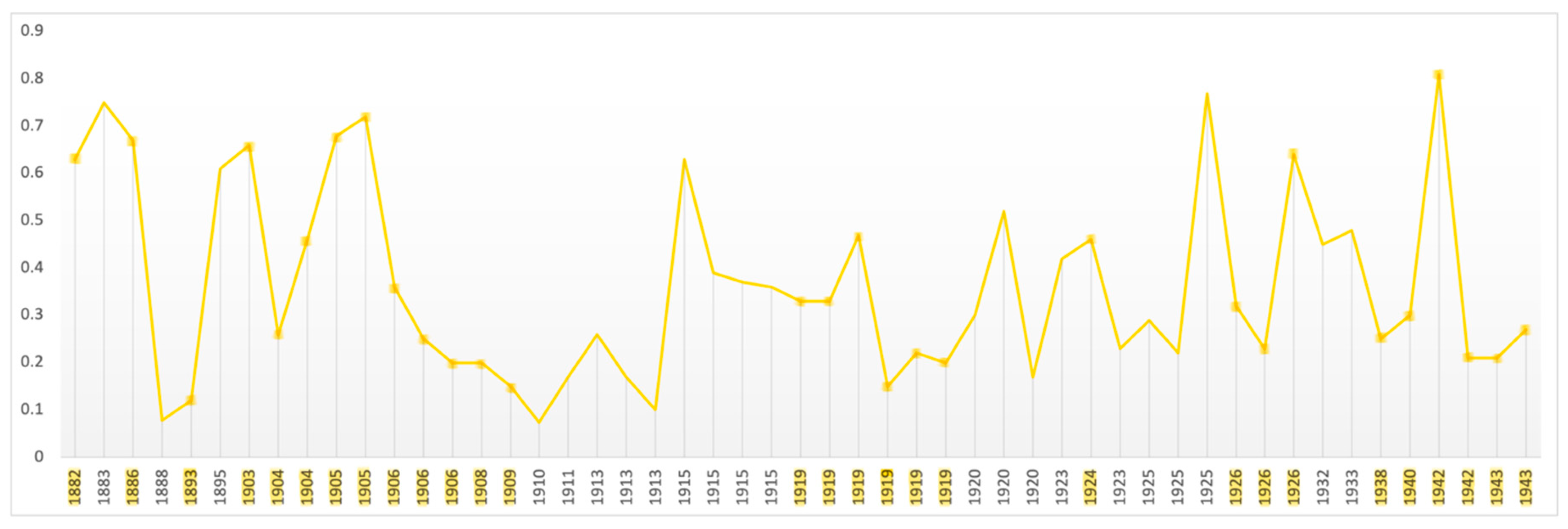
Fractals
Productivity
Discussion
Conclusions
Limitations
Acknowledgments
References
- Abely, P. De signe du mriroir dans les psychoses et plus spécialement dans la demence précoce. Ann Medicopsychol 1930, 28–36. [Google Scholar]
- Acar, S.; Chen, X.; Cayirdag, N. Schizophrenia and creativity: A meta-analytic review. Schizophr. Res. 2018, 195, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Acar, S.; Sen, S. A multilevel meta-analysis of the relationship between creativity and schizotypy. Psychol. Aesthetics, Creativity, Arts 2013, 7, 214–228. [Google Scholar] [CrossRef]
- Achim, A.M.; Maziade, M.; Raymond, E.; Olivier, D.; Merette, C.; Roy, M.-A. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr. Bull. 2009, 37, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.M.; Sutliff, S.; Samson, C.; Montreuil, T.C.; Lecomte, T. Attribution bias and social anxiety in schizophrenia. Schizophr. Res. Cogn. 2016, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Adámek, P.; Langová, V.; Horáček, J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Stephan, K.E.; Brown, H.R.; Frith, C.D.; Friston, K.J.; Adams, R.A.; Stephan, K.E.; Brown, H.R.; Frith, C.D.; Friston, K.J.; et al. The computational anatomy of psychosis. Front. Psychiatry 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.A.; Nasrallah, H.A. Multiple retinal anomalies in schizophrenia. Schizophr. Res. 2018, 195, 3–12. [Google Scholar] [CrossRef]
- Aderka, I.M.; Hofmann, S.G.; Nickerson, A.; Hermesh, H.; Gilboa-Schechtman, E.; Marom, S. Functional impairment in social anxiety disorder. J. Anxiety Disord. 2012, 26, 393–400. [Google Scholar] [CrossRef]
- Akinola, M.; Mendes, W.B. The dark side of creativity: biological vulnerability and negative emotions lead to greater artistic creativity. Pers. Soc. Psychol. Bull. 2008, 34, 1677–1686. [Google Scholar] [CrossRef]
- Alizadeh, M.; Delborde, Y.; Ahmadpanah, M.; Seifrabiee, M.A.; Jahangard, L.; Bazzazi, N.; Brand, S. Non-linear associations between retinal nerve fibre layer (RNFL) and positive and negative symptoms among men with acute and chronic schizophrenia spectrum disorder. J. Psychiatr. Res. 2021, 141, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Alzueta, E.; Melcón, M.; Jensen, O.; Capilla, A. The 'Narcissus Effect': Top-down alpha-beta band modulation of face-related brain areas during self-face processing. NeuroImage 2020, 213, 116754. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Edition, Text Revision (DSM-5-TR); American Psychiatric Publishing: Arlington (VA), 2022. [Google Scholar]
- American Psychiatric Association. Anxiety Disorders. In Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Text Revision; American Psychiatric Association, 2022; pp. 215–231. [Google Scholar]
- Andreasen, N.C.; Flaum, M. Schizophrenia: The characteristic symptoms. Schizophr. Bull. 1991, 17, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Higier, R.G.; Jimenez, A.M.; Hultman, C.M.; Borg, J.; Roman, C.; Kizling, I.; Larsson, H.; Cannon, T.D.; Sachsse, U.; VON DER Heyde, S.; et al. Creativity and mental illness: prevalence rates in writers and their first-degree relatives. Am. J. Psychiatry 1987, 144, 1288–1292. [Google Scholar] [CrossRef]
- Antonsen, S.; Mok, P.L.H.; Webb, R.T.; Mortensen, P.B.; McGrath, J.J.; Agerbo, E.; Brandt, J.; Geels, C.; Christensen, J.H.; Pedersen, C.B. Exposure to air pollution during childhood and risk of developing schizophrenia: a national cohort study. Lancet Planet. Heal. 2020, 4, e64–e73. [Google Scholar] [CrossRef] [PubMed]
- Appaji, A.; Nagendra, B.; Chako, D.M.; Padmanabha, A.; Hiremath, C.V.; Jacob, A.; Varambally, S.; Kesavan, M.; Venkatasubramanian, G.; Rao, S.V.; et al. Retinal vascular abnormalities in schizophrenia and bipolar disorder: A window to the brain. Bipolar Disord. 2019, 21, 634–641. [Google Scholar] [CrossRef] [PubMed]
- År Til År, F. Edvard Munch. A Year by Year Record of Edvard Munch’s Life; Aschehoug & Co., 1961. [Google Scholar]
- Ashok, A.H.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Azeem, H. The art of Edvard Munch: a window onto a mind. BJPsych Adv. 2015, 21, 51–53. [Google Scholar] [CrossRef]
- Baird, B.; Smallwood, J.; Mrazek, M.D.; Kam, J.W.Y.; Franklin, M.S.; Schooler, J.W. Inspired by distraction: Mind wandering facilitates creative incubation. Psychol. Sci. 2012, 23, 1117–1122. [Google Scholar] [CrossRef]
- Balaram, K.; Marwaha, R. Agoraphobia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island (FL), 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554387/.
- Balconi, M.; Fronda, G. Intra-Brain Connectivity vs. Inter-Brain Connectivity in Gestures Reproduction: What Relationship? Brain Sci. 2021, 11, 577. [Google Scholar] [CrossRef]
- Bannai, D.; Lizano, P.; Kasetty, M.; Lutz, O.; Zeng, V.; Sarvode, S.; Kim, L.A.; Hill, S.; Tamminga, C.; Clementz, B.; et al. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: A preliminary study. Psychiatry Res. Neuroimaging 2020, 299, 111061–111061. [Google Scholar] [CrossRef] [PubMed]
- Barrigón, M.L.; Diaz, F.J.; Gurpegui, M.; Ferrin, M.; Salcedo, M.D.; Moreno-Granados, J.; Cervilla, J.A.; Ruiz-Veguilla, M. Childhood trauma as a risk factor for psychosis: A sib-pair study. J. Psychiatr. Res. 2015, 70, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gitlin, M. Natural Course of Bipolar Disorder and Implications for Treatment. In The Essential Guide to Lithium Treatment; Springer: Cham, 2016. [Google Scholar] [CrossRef]
- Bernardin, F.; Schwitzer, T.; Angioi-Duprez, K.; Giersch, A.; Jansen, C.; Schwan, R.; Laprevote, V. Retinal ganglion cells dysfunctions in schizophrenia patients with or without visual hallucinations. Schizophr. Res. 2020, 219, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bernardin, F.; Schwitzer, T.; Angioi-Duprez, K.; Giersch, A.; Ligier, F.; Bourion-Bedes, S.; Jansen, C.; Schwan, R.; Laprevote, V. Retinal ganglion cell dysfunction is correlated with disturbed visual cognition in schizophrenia patients with visual hallucinations. Psychiatry Res. 2021, 298, 113780. [Google Scholar] [CrossRef] [PubMed]
- Bernardin, F.; Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Ligier, F.; Bourion-Bedes, S.; Jansen, C.; Giersch, A.; Laprevote, V. Altered central vision and amacrine cells dysfunction as marker of hypodopaminergic activity in treated patients with schizophrenia. Schizophr. Res. 2022, 239, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M. Alcohol and Depression Addiction. 2011, 106, 906–914. [Google Scholar] [PubMed]
- Bodis-Wollner, I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson's disease patients. Trends Neurosci. 1990, 13, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Bögels, S.M.; Mansell, W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin. Psychol. Rev. 2004, 24, 827–856. [Google Scholar] [CrossRef] [PubMed]
- Bonini, L.; Rotunno, C.; Arcuri, E.; Gallese, V. Mirror neurons 30 years later: implications and applications. Trends Cogn. Sci. 2022, 26, 767–781. [Google Scholar] [CrossRef]
- Boot, N.; Baas, M.; van Gaal, S.; Cools, R.; De Dreu, C.K. Creative cognition and dopaminergic modulation of fronto-striatal networks: Integrative review and research agenda. Neurosci. Biobehav. Rev. 2017, 78, 13–23. [Google Scholar] [CrossRef]
- Bortolon, C.; Capdevielle, D.; Altman, R.; Macgregor, A.; Attal, J.; Raffard, S. Mirror self-face perception in individuals with schizophrenia: Feelings of strangeness associated with one's own image. Psychiatry Res. 2017, 253, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.M. Munch and Agoraphobia: His Art and His Illness RACAR: revue d’art canadienne / Canadian Art Review. 1988, 15, 23–50. Available online: https://www.jstor.org/stable/42630379.
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Bowlby, J. Separation Anxiety. International Journal of Psychoanalysis 1960, 41, 89–113. [Google Scholar] [PubMed]
- Brandies, R.; Yehuda, S. The possible role of retinal dopaminergic system in visual performance. Neurosci. Biobehav. Rev. 2008, 32, 611–656. [Google Scholar] [CrossRef] [PubMed]
- Brasil, A.; Castro, A.J.O.; Martins, I.C.V.S.; Lacerda, E.M.C.B.; Souza, G.S.; Herculano, A.M.; Rosa, A.A.M.; Rodrigues, A.R.; Silveira, L.C.L. Colour Vision Impairment in Young Alcohol Consumers. PLOS ONE 2015, 10, e0140169. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, A.K.; Jankowski, Z.P.; Ekumaratilake, J.; et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [CrossRef]
- Brown, A.S.; Schaefer, C.A.; Wyatt, R.J.; Goetz, R.; Begg, M.D.; Gorman, J.M.; Susser, E.S. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: A prospective birth cohort study. Schizophr. Bull. 2000, 26, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S. Exposure to prenatal infection and risk of schizophrenia. Front. Psychiatry 2011, 2, 63. [Google Scholar] [CrossRef]
- Brown, A.S.; Derkits, E.J.; Kendler, K.S.; Lee, Y.H.; Cherkerzian, S.; Seidman, L.J.; Papandonatos, G.D.; Savitz, D.A.; Tsuang, M.T.; Goldstein, J.M.; et al. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Brown, G.; Rafaeli, E. Components of Self-Complexity as Buffers for Depressed Mood. J. Cogn. Psychother. 2007, 21, 310–333. [Google Scholar] [CrossRef]
- Bruteig, M.; Falck, U. K. Edvard Munch Works on Paper; Munch Museum, Yale University Press, 2013. [Google Scholar]
- Bruzzo, A. The chaotic nature of Self. Dialegesthai. Rivista telematica di filosofia 2007, 9. Available online: https://mondodomani.org/dialegesthai/>.
- Bucci, S.; Startup, M.; Wynn, P.; Heathcote, A.; Baker, A.; Lewin, T.J. Referential delusions of communication and reality discrimination deficits in psychosis. Br. J. Clin. Psychol. 2008, 47, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Bunney, W.E.; Hetrick, W.P.; Bunney, B.G.; Patterson, J.V.; Jin, Y.; Potkin, S.G.; Sandman, C.A. Structured Interview for Assessing Perceptual Anomalies (SIAPA). Schizophr. Bull. 1999, 25, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Burch, G.S.J.; Pavelis, C.; Hemsley, D.R.; Corr, P.J. Schizotypy and creativity in visual artists. Br. J. Psychol. 2006, 97, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.D.; Silverstein, S.M.; Dakin, S.C. Visual Perception and Its Impairment in Schizophrenia Biol Psychiatry. 2008, 64, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.D.; Abeles, I.Y.; Weiskopf, N.G.; Tambini, A.; Jalbrzikowski, M.; Legatt, M.E.; Zemon, V.; Loughead, J.; Gur, R.C.; Javitt, D.C. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr. Bull. 2009, 35, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.D.; Zemon, V.; Schechter, I.; Saperstein, A.M.; Hoptman, M.J.; Lim, K.O.; Revheim, N.; Silipo, G.; Javitt, D.C. Early-Stage Visual Processing and Cortical Amplification Deficits in Schizophrenia. ARCH GEN PSYCHIATRY/VOL 2005, 62. [Google Scholar] [CrossRef]
- Butler, P.D.; Javitt, D.C. Early-stage visual processing deficits in schizophrenia. Curr. Opin. Psychiatry 2005, 18, 151–157. [Google Scholar] [CrossRef]
- Byron, K.; Khazanchi, S. A meta-analytic investigation of the relationship of state and trait anxiety to performance on figural and verbal creative tasks. Pers. Soc. Psychol. Bull. 2011, 37, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Cadenhead, K.S.; Dobkins, K.; McGovern, J.; Shafer, K. Schizophrenia spectrum participants have reduced visual contrast sensitivity to chromatic (red/green) and luminance (light/dark) stimuli: new insights into information processing, visual channel function, and antipsychotic effects. Front. Psychol. 2013, 4, 535. [Google Scholar] [CrossRef] [PubMed]
- Cancel, A.; Dallel, S.; Zine, A.; El-Hage, W.; Fakra, E. Understanding the link between childhood trauma and schizophrenia: A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2019, 107, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D.; Kaprio, J.; Lönnqvist, J.; Huttunen, M.; Koskenvuo, M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch. Gen. Psychiatry 1998, 55, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.B.; Ferrucci, R.; Bortolomasi, M.; Giacopuzzi, M.; Priori, A.; Zago, S. Visual perception during mirror gazing at one's own face in schizophrenia. Schizophr. Res. 2012, 140, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Cardno, A.G.; Owen, M.J. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr. Bull. 2014, 40, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Cardno, A.G.; Marshall, E.J.; Coid, B.; Macdonald, A.M.; Ribchester, T.R.; Davies, N.J.; Venturi, P.; Jones, L.A.; Lewis, S.W.; Sham, P.C.; et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch. Gen. Psychiatry 1999, 56, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.H.; Randall, C.L. Self-medication in social phobia: A review of the alcohol literature. Addictive Behaviors 2003, 28, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.H.; Peterson, J.B.; Higgins, D.M. Decreased latent inhibition is associated with increased creative achievement in high-functioning individuals. J. Pers. Soc. Psychol. 2003, 85, 499–506. [Google Scholar] [CrossRef]
- Carson, S.H. Creativity and Psychopathology: A Shared Vulnerability Model. Can. J. Psychiatry 2011, 56, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Casares-López, M.; Castro-Torres, J.J.; Martino, F.; Ortiz-Peregrina, S.; Ortiz, C.; Anera, R.G. Contrast sensitivity and retinal straylight after alcohol consumption: effects on driving performance. Sci. Rep. 2020, 10, 13599. [Google Scholar] [CrossRef]
- Castillo-Carniglia, A.; Keyes, K.M.; Hasin, D.S.; Cerdá, M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry 2019, 6, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.d.O.; Rodrigues, A.R.; Côrtes, M.I.T.; Silveira, L.C.d.L. Impairment of color spatial vision in chronic alcoholism measured by psychophysical methods. Psychol. Neurosci. 2009, 2, 179–187. [Google Scholar] [CrossRef]
- Castro, J.J.; Pozo, A.M.; Rubiño, M.; Anera, R.G.; del Barco, L.J. Retinal-image quality and night-vision performance after alcohol consumption. J. Ophthalmol. 2014, 2014, 704823. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Kalenderoglu, A.; Karadag, A.S.; Egilmez, O.B.; Han-Almis, B.; Şimşek, A. Decreases in ganglion cell layer and inner plexiform layer volumes correlate better with disease severity in schizophrenia patients than retinal nerve fiber layer thickness: Findings from spectral optic coherence tomography. Eur. Psychiatry 2016, 32, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cernuschi, C. Sex and Psyche, Nature and Nurture. In The Personal and the Political: Edvard Munch and German Expressionism; Howe, J., Ed.; Edvard Munch, Psyche, Symbol and Expressionism. Boston College, 2001. [Google Scholar]
- Chakrabarti, S.; Singh, N. Psychotic symptoms in bipolar disorder and their impact on the illness: A systematic review. World J. Psychiatry 2022, 12, 1204–1232. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.; Krystal, J.H.; Self, D.W. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol. Psychiatry 2001, 50, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J. The Early Symptoms of Schizophrenia. Br. J. Psychiatry 1966, 112, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.W.; Loriemi, P.; Wensing, T.; Eickhoff, S.B.; Nickl-Jockschat, T. Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum. Brain Mapp. 2018, 39, 2917–2928. [Google Scholar] [CrossRef]
- Chatfield, C.; Xing, H. The Analysis of Time Series: An Introduction with R; Chapman and Hall/CRC: New York, 2019. [Google Scholar]
- Chen, Y.; Nakayama, K.; Levy, D.; Matthysse, S.; Holzman, P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr. Res. 2003, 61, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Levy, D.L.; Sheremata, S.; Nakayama, K.; Matthysse, S.; Holzman, P.S. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am. J. Psychiatry 2003, 160, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bidwell, L.; Holzman, P.S. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr. Res. 2005, 74, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bidwell, L.C.; Norton, D. Trait vs. State Markers for Schizophrenia: Identification and Characterization Through Visual Processes. Curr. Psychiatry Rev. 2006, 2, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Norton, D.; McBain, R. Can persons with schizophrenia appreciate visual art? Schizophr. Res. 2008, 105, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S. Dysfunction of magnocellular/dorsal processing stream in schizophrenia. Curr. Psychiatry Res. Rev. 2019, 15, 26–36. [Google Scholar] [CrossRef]
- Chilvers, I. Oxford Dictionary of Art & Artists, 4th ed.; Oxford University Press, 2009. [Google Scholar]
- Cho, W.; Shin, W.-S.; An, I.; Bang, M.; Cho, D.-Y.; Lee, S.-H. Biological Aspects of Aggression and Violence in Schizophrenia. Clin. Psychopharmacol. Neurosci. 2019, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Z.; Lennox, B. Maternal Immune Activation and Schizophrenia-Evidence for an Immune Priming Disorder. Front. Psychiatry 2021, 12, 585742. [Google Scholar] [CrossRef]
- Chouinard, V.-A.; Shinn, A.K.; Valeri, L.; Chouinard, P.A.; Gardner, M.E.; Asan, A.E.; Cohen, B.M.; Öngür, D. Visual hallucinations associated with multimodal hallucinations, suicide attempts and morbidity of illness in psychotic disorders. Schizophr. Res. 2019, 208, 196–201. [Google Scholar] [CrossRef]
- Clarke, M.C.; Tanskanen, A.; Huttunen, M.O.; Cannon, M. Sudden death of father or sibling in early childhood increases risk for psychotic disorder. Schizophr. Res. 2013, 143, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Coid, J.W.; Ullrich, S.; Kallis, C.; Keers, R.; Barker, D.; Cowden, F.; Stamps, R. The relationship between delusions and violence: Findings from the East London first episode psychosis study. JAMA Psychiatry 2013, 70, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.C. Religious psychopathology: The prevalence of religious content of delusions and hallucinations in mental disorder. Int. J. Soc. Psychiatry 2015, 61, 404–425. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Creupelandt, C.; D'Hondt, F.; Maurage, P. Towards a Dynamic Exploration of Vision, Cognition and Emotion in Alcohol-Use Disorders. Curr. Neuropharmacol. 2019, 17, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.; Guimaraes, A.; Wozniak, J.; Anjum, A.; Schulz, S.; White, T. Trajectories of social withdrawal and cognitive decline in the schizophrenia prodrome. Clin. Schizophr. Relat. Psychoses 2011, 4, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cunnigham Dax, E. The Cunnigham Dax Collection: Selected Works of Psychiatric Art; Melbourne University Press: Melbourne, 1998. [Google Scholar]
- Cutting, J.; Dunne, F. Subjective experience of schizophrenia. Schizophr. Bull. 1989, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Cutting, J.; Dunne, F. The Nature of the Abnormal Perceptual Experiences at the Onset of Schizophrenia. Psychopathology 1986, 19, 347–352. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, D.N.; de Andrade, M.J.O.; Cavalcanti-Gaudino, M.K.; Nogueira, R.M.T.B.L.; dos Santos, N.A. Effects of chronic alcoholism in the sensitivity to luminance contrast in vertical sinusoidal gratings. Psicol. E Crit. 2016, 29, 729. [Google Scholar] [CrossRef]
- Dakin, S.; Carlin, P.; Hemsley, D. Weak suppression of visual context in chronic schizophrenia. Curr. Biol. 2005, 15, R822–R824. [Google Scholar] [CrossRef]
- David, C.N.; Greenstein, D.; Clasen, L.; Gochman, P.; Miller, R.; Tossell, J.W.; Mattai, A.A.; Gogtay, N.; Rapoport, J.L. Childhood onset schizophrenia: High rate of visual hallucinations. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Welham, J.; Chant, D.; Torrey, E.F.; McGrath, J. A Systematic Review and Meta-analysis of Northern Hemisphere Season of Birth Studies in Schizophrenia. Schizophr. Bull. 2003, 29, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Daviet, R.; Aydogan, G.; Jagannathan, K.; Spilka, N.; Koellinger, P.D.; Kranzler, H.R.; Nave, G.; Wetherill, R.R. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat. Commun. 2022, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 2002, 27, 155–170. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, G.S.; Pereira, S.M.; Dos Santos, D.N.; Marinho, J.M.; Rodrigues, L.C.; Barreto, M.L. Common mental disorders associated with tuberculosis: A matched case-control study. PLOS ONE 2014, 9, e99551. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Saha, S.; Lim, C.C.W.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.H.; Bromet, E.J.; Bruffaerts, R.; Caldas-De-Almeida, J.M.; et al. The associations between psychotic experiences and substance use and substance use disorders: findings from the World Health Organization World Mental Health surveys. Addiction 2018, 113, 924–934. [Google Scholar] [CrossRef] [PubMed]
- de la Calleja, E.; Cervantes, F.; de la Calleja, J. Order-fractal transitions in abstract paintings. Ann. Phys. 2016, 371, 313–322. [Google Scholar] [CrossRef]
- Demmin, D.L.; Davis, Q.; Roché, M.; Silverstein, S.M. Electroretinographic anomalies in schizophrenia. J. Abnorm. Psychol. 2018, 127, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Demmin, D.L.; Netser, R.; Roché, M.W.; Thompson, J.L.; Silverstein, S.M. People with current major depression resemble healthy controls on flash Electroretinogram indices associated with impairment in people with stabilized schizophrenia. Schizophr. Res. 2020, 219, 69–76. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, K.; Cheng, D.; Zhang, J.; Chen, H.; Chen, B.; Li, Y.; Wang, W.; Kong, Y.; Wen, G. Ventral and dorsal visual pathways exhibit abnormalities of static and dynamic connectivities, respectively, in patients with schizophrenia. Schizophr. Res. 2019, 206, 103–110. [Google Scholar] [CrossRef]
- Diaconescu, A.O.; Hauke, D.J.; Borgwardt, S. Models of persecutory delusions: a mechanistic insight into the early stages of psychosis. Mol. Psychiatry 2019, 24, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Dietrich, D.E.; Dillo, W.; Emrich, H.M. Impaired top-down processes in schizophrenia: A DCM study of ERPs. NeuroImage 2010, 52, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Schroeder, J.; Yolken, R.H. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatr. Serv. 2013, 64, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Di leva, A. The Fractal Geometry of the Brain; Springer-Verlag New York Inc., 2016; ISBN 9781493981489. [Google Scholar] [CrossRef]
- Djamgoz, M.; Hankins, M.; Hirano, J.; Archer, S. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vis. Res. 1997, 37, 3509–3529. [Google Scholar] [CrossRef] [PubMed]
- Do, E.K.; Maes, H.H. Genotype × Environment Interaction in Smoking Behaviors: A Systematic Review. Nicotine Tob. Res. 2017, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Döges, J. The Famed Painting The Scream Holds a Hidden Message. Sci. Am. 2021, 32, 6. [Google Scholar] [CrossRef]
- Drake, R.E.; Osher, F.C.; Noordsy, D.L.; Hurlbut, S.C.; Teague, G.B.; Beaudett, M.S. Diagnosis of Alcohol Use Disorders in Schizophrenia. Schizophr. Bull. 1990, 16, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Tian, C.; Fu, C.; He, J.; Dai, J.; Shao, X.; Zhu, G. Analysis of color vision and cognitive function in first-episode schizophrenia before and after antipsychotic treatment. J. Psychiatr. Res. 2022, 152, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Aynsworth, C.; Cheetham, R.; McCarthy-Jones, S.; Collerton, D. Prevalence and characteristics of multi-modal hallucinations in people with psychosis who experience visual hallucinations. Psychiatry Res. 2018, 269, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, O.N.; Pucci, F.G.; Wong, K.Y.; Berson, D.M. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J. Comp. Neurol. 2009, 517, 226–244. [Google Scholar] [CrossRef]
- Duth, P.S.; Deepa, M.M. Color detection in RGB-modeled images using MAT LAB. Int. J. Eng. Technol. 2018, 7, 29–33. [Google Scholar] [CrossRef]
- Eggum, A. Edvard Munch Paintings, Sketches and Studies; Thames & Hudson: London, 1984. [Google Scholar]
- Elling, C.; Forstner, A.J.; Seib-Pfeifer, L.-E.; Mücke, M.; Stahl, J.; Geiser, F.; Schumacher, J.; Conrad, R. Social anxiety disorder with comorbid major depression – why fearful attachment style is relevant. J. Psychiatr. Res. 2022, 147, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Eng, W.; Heimberg, R.G.; Hart, T.A.; Schneier, F.R.; Liebowitz, M.R. Attachment in individuals with social anxiety disorder: The relationship among adult attachment styles, social anxiety, and depression. Emotion 2001, 1, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Enoch, M. Genetic and environmental influences on the development of alcoholism: Resilience vs. risk. Ann. New York Acad. Sci. 2006, 1094, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, M.A.; Zavala, J.M. Genetics of bipolar disorder. Dialog- Clin. Neurosci. 2008, 10, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, U.; Joober, R.; DE Guzman, R.; O’driscoll, G.A. Schizotypy, attention deficit hyperactivity disorder, and dopamine genes. Psychiatry Clin. Neurosci. 2006, 60, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, H.J. Creativity as a Product of lntelligence and Personality. In International Handbook of Personality and Intelligence; Saklofske, D.H., Zeidner, M., Eds.; Plenum Press, 1995. [Google Scholar]
- Faravelli, C.; Sauro, C.L.; Godini, L.; Lelli, L.; Benni, L.; Pietrini, F.; Lazzeretti, L.; Talamba, G.A.; Fioravanti, G.; Ricca, V. Childhood stressful events, HPA axis and anxiety disorders. World J. Psychiatry 2012, 2, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, K.A.; Keane, B.P.; Roché, M.W.; Silverstein, S.M. Contour integration impairment in schizophrenia and first episode psychosis: State or trait?. Schizophr. Res. 2014, 159, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Fein, G.; Torres, J.; Price, L.J.; Di Sclafani, V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol. Clin. Exp. Res. 2006, 30, 1538–1544. [Google Scholar] [CrossRef]
- Fernandes, T.M.d.P.; de Almeida, N.L.; dos Santos, N.A. Effects of smoking and smoking abstinence on spatial vision in chronic heavy smokers. Sci. Rep. 2017, 7, 1690. [Google Scholar] [CrossRef]
- Fernandes, T.M.P.; Andrade, S.M.; de Andrade, M.J.O.; Nogueira, R.M.T.B.L.; Santos, N.A. Colour discrimination thresholds in type 1 Bipolar Disorder: a pilot study. Sci. Rep. 2017, 7, 16405. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.d.P.; de Almeida, N.L.; dos Santos, N.A. Effects of smoking and smoking abstinence on spatial vision in chronic heavy smokers. Sci. Rep. 2017, 7, 1690. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.P.; de Andrade, M.J.O.; Santana, J.B.; Nogueira, R.M.T.B.L.; dos Santos, N.A. Tobacco Use Decreases Visual Sensitivity in Schizophrenia. Front. Psychol. 2018, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.P.; Silverstein, S.M.; de Almeida, N.L.; dos Santos, N.A. Psychophysical evaluation of contrast sensitivity using Gabor patches in tobacco addiction. J. Clin. Neurosci. 2018, 57, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.P.; Silverstein, S.M.; Butler, P.D.; Kéri, S.; Santos, L.G.; Nogueira, R.L.; Santos, N.A. Color vision impairments in schizophrenia and the role of antipsychotic medication type. Schizophr. Res. 2019, 204, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.P.; Silverstein, S.M.; Almeida, N.L.; Santos, N.A. Visual impairments in tobacco use disorder. Psychiatry Res. 2019, 271, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.; Wilkins, A.J. Uncomfortable images in art and nature. Perception 2008, 37, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Venskus, A.; Fotia, F.; Cooke, J.; Romei, V. Higher proneness to multisensory illusions is driven by reduced temporal sensitivity in people with high schizotypal traits. Conscious. Cogn. 2018, 65, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Fett, A.-K.J.; Hanssen, E.; Eemers, M.; Peters, E.; Shergill, S.S. Social isolation and psychosis: an investigation of social interactions and paranoia in daily life. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 119–127. [Google Scholar] [CrossRef]
- Foisy, M.-L.; Kornreich, C.; Petiau, C.; Parez, A.; Hanak, C.; Verbanck, P.; Pelc, I.; Philippot, P. Impaired emotional facial expression recognition in alcoholics: Are these deficits specific to emotional cues? Psychiatry Res. 2007, 150, 33–41. [Google Scholar] [CrossRef]
- Forsythe, A.; Williams, T.; Reilly, R.G. What paint can tell us: A fractal analysis of neurological changes in seven artists. Neuropsychology 2017, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Forteller, V. Edvard Munch Som Vi Kjente Ham: Vennene. 22ip il Dreyers Forlag. 1946. [Google Scholar]
- Fortgang, R.G.; Hoff, R.A.; Potenza, M.N. Problem and Pathological Gambling in Schizophrenia: Exploring Links with Substance Use and Impulsivity. J. Gambl. Stud. 2018, 34, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Doniger, G.M.; Javitt, D.C. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. NeuroReport 2001, 12, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Freud, S. Further Remarks on the Neuro-Psychoses of Defence. The Standard Edition of the Complete Psychological Works of Sigmund Freud 1896, 3, 157–185. [Google Scholar]
- Freud, S. The Interpretation of Dreams. The Standard Edition of the Complete Psychological Works of Sigmund Freud 1900, 4, ix-627. [Google Scholar]
- Frigg, R.; Howard, C. Fact and Fiction in the Neuropsychology of Art. In The Aesthetic Mind: Philosophy and Psychology; Schellekens, E., Goldie, P, Eds.; Oxford University Press, Scholarship on Line, 2011. [Google Scholar] [CrossRef]
- A Frye, M.; Salloum, I.M. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord. 2006, 8, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Pries, L.-K.; van Os, J.; Erzin, G.; Delespaul, P.; Kenis, G.; Luykx, J.J.; Lin, B.D.; Richards, A.L.; Akdede, B.; et al. Examining facial emotion recognition as an intermediate phenotype for psychosis: Findings from the EUGEI study. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2022, 113, 110440. [Google Scholar] [CrossRef] [PubMed]
- Gagné, A.-M.; Hébert, M.; Maziade, M. Revisiting visual dysfunctions in schizophrenia from the retina to the cortical cells: A manifestation of defective neurodevelopment. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2015, 62, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Galdino, M.K.C.; Mendes, L.C.; Vieira, J.G.; Simas, M.L.d.B.; dos Santos, N.A. Visual perception of radial sine-wave grating after the alcohol consumption. Psicol. USP 2011, 22, 99–115. [Google Scholar] [CrossRef]
- Gatzke-Kopp, L.M.; Beauchaine, T.P. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum. Dev. 2007, 38, 255–269. [Google Scholar] [CrossRef]
- Goghari, V.M.; Harrow, M.; Grossman, L.S.; Rosen, C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol. Med. 2013, 43, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Goghari, V.M.; Harrow, M. Twenty year multi-follow-up of different types of hallucinations in schizophrenia, schizoaffective disorder, bipolar disorder, and depression. Schizophr. Res. 2016, 176, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Götz, K.O.; Götz, K. Personality characteristics of professional artists. Percept. Mot. Ski. 1979, 49, 327–334. [Google Scholar] [CrossRef]
- Götz, K.O.; Götz, K. Personality Characteristics of Successful Artists. 1979, 49, 919–924. [Google Scholar] [CrossRef]
- Gozdzik-Zelazny, A.; Borecki, L.; Pokorski, M. Depressive symptoms in schizophrenic patients. Eur. J. Med Res. 2011, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Pavanati, M.; Cardelli, R.; Ferri, S.; Peron, L. HIV-risk behaviour and knowledge about HIV/AIDS among patients with schizophrenia. Psychol. Med. 1999, 29, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Grillo, L. A Possible Link between Anxiety and Schizophrenia and a Possible Role of Anhedonia. Schizophr. Res. Treat. 2018, 2018, 5917475. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, C.; Norgren, E.; Eriksson, L. A systematic review of the neural correlates of multisensory integration in schizophrenia. Schizophr. Res. Cogn. 2021, 27, 100219. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.C.; Durukan, A.H.; Mumcuoglu, T.; Sobacı, G.; Bayraktar, M.Z. Acute effects of cigarette smoking on pattern electroretinogram. Doc. Ophthalmol. 2006, 113, 115–121. [Google Scholar] [CrossRef]
- Gutiérrez-García, A.; Calvo, M.G. Social anxiety and trustworthiness judgments of dynamic facial expressions of emotion. J. Behav. Ther. Exp. Psychiatry 2016, 52, 119–127. [Google Scholar] [CrossRef]
- Gutiérrez-García, A.; Calvo, M.G. Social anxiety and threat-related interpretation of dynamic facial expressions: Sensitivity and response bias. Pers. Individ. Differ. 2017, 107, 10–16. [Google Scholar] [CrossRef]
- Hagerhall, C.M.; Purcell, T.; Taylor, R. Fractal dimension of landscape silhouette outlines as a predictor of landscape preference. J. Environ. Psychol. 2004, 24, 247–255. [Google Scholar] [CrossRef]
- Hagerhall, C.M.; Laike, T.; Taylor, R.P.; Küller, M.; Küller, R.; Martin, T.P. Investigations of human EEG response to viewing fractal patterns. Perception 2008, 37, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Gründer, G.; Hirth, N.; Noori, H.R.; Spanagel, R.; Sommer, W.H. Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci. Biobehav. Rev. 2019, 106, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Spencer, E.; Davidson, C.; Hutchinson, C.V. Selectively reduced contrast sensitivity in high schizotypy. Exp. Brain Res. 2020, 238, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C. Anxiety (Angst). Arch. Gen. Psychiatry 2004, 61, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C. Spring. Arch. Gen. Psychiatry 2007, 64, 996–997. [Google Scholar] [CrossRef]
- Haug, B.A.; Kolle, R.U.; Trenkwalder, C.; Oertel, W.H.; Paulus, W. Predominant affection of the blue cone pathway in Parkinson's disease. Brain 1995, 118, 771–778. [Google Scholar] [CrossRef]
- Heckers, S.; Konradi, C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr. Res. 2014, 167, 4–11. [Google Scholar] [CrossRef]
- Hedden, W.L.; Dowling, J.E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc. R. Soc. London. Ser. B. Biol. Sci. 1978, 201, 27–55. [Google Scholar] [CrossRef]
- Heerwagen, J. H.; Orians, G. H. Humans, habitats, and aesthetics. In The biophilia hypothesis; Kellert, S. R., Wilson, E. O., Eds.; Island Press: Covelo, CA, 1993; pp. 138–172. ISBN 9781559631471. [Google Scholar]
- Heffner, J.L.; Strawn, J.R.; DelBello, M.P.; Strakowski, S.M.; Anthenelli, R.M. The co-occurrence of cigarette smoking and bipolar disorder: phenomenology and treatment considerations. Bipolar Disord. 2011, 13, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Heller, R. Munch, His Life and Work; University of Chicago Press, 1984; ISBN 100226326438. [Google Scholar]
- Hodgins, S. Aggressive Behavior Among Persons With Schizophrenia and Those Who Are Developing Schizophrenia: Attempting to Understand the Limited Evidence on Causality. Schizophr. Bull. 2017, 43, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Asnaani, M.A.; Hinton, D.E. Cultural aspects in social anxiety and social anxiety disorder. Depression Anxiety 2010, 27, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Holland, J. G. (2005) (trans.) The Private Journals of Edvard Munch; We Are Flames Which Pour Out Of The Earth. University of Wisconsin Press. ISBN: 0-299-19810-3.
- Holt, N. J. (2015) Schizotypy: A creative advantage? In: Schizotypy: New Dimensions (eds) Editors: Mason, O. and Claridge, G.) Routledge. ISBN 9780815356981.
- Holt, D.J.; Weiss, A.P.; Rauch, S.L.; Wright, C.I.; Zalesak, M.; Goff, D.C.; Ditman, T.; Welsh, R.C.; Heckers, S. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol. Psychiatry 2005, 57, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Kunkel, L.; Weiss, A.P.; Goff, D.C.; Wright, C.I.; Shin, L.M.; Rauch, S.L.; Hootnick, J.; Heckers, S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr. Res. 2006, 82, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Holzman, P.S.; Proctor, L.R.; Levy, D.L.; Yasillo, N.J.; Meltzer, H.Y.; Hurt, S.W. Eye-Tracking Dysfunctions in Schizophrenic Patients and Their Relatives. Arch. Gen. Psychiatry 1974, 31, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.E.; Schroeder, M.; Ross, T.J.; Buchholz, B.; Salmeron, B.J.; Wonodi, I.; Thaker, G.K.; Stein, E.A. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr. Bull. 2009, 37, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Hoptman, M.J. Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectrums 2015, 20, 280–286. [Google Scholar] [CrossRef]
- Horney, K. Self-analysis; W. W. Norton: New York, 1942. [Google Scholar]
- Howe, J. (ed) (2001) Edvard Munch: Psyche, Symbolism and Expression. Boston: McMullen Museum of Art. ISBN-1892850-02-8.
- Howes, O.D.; McCutcheon, R.; Owen, M.J.; Murray, R.M. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef]
- Huang, H.; Dong, M.; Zhang, L.; Zhong, B.-L.; Ng, C.H.; Ungvari, G.S.; Yuan, Z.; Meng, X.; Xiang, Y.-T. Psychopathology and extrapyramidal side effects in smoking and non-smoking patients with schizophrenia: Systematic review and meta-analysis of comparative studies. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2019, 92, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Murray, R.; Asher, L.; Leonardi-Bee, J. The Effects of Tobacco Smoking, and Prenatal Tobacco Smoke Exposure, on Risk of Schizophrenia: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2020, 22, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.; MacCabe, J. Paradoxes in creativity and psychiatric conditions. In The Paradoxical Brain; Pascual-Leone, A., Ramachandran, V., Cole, J., Della Sala, S., Manly, T., Mayes, A., et al., Eds.; Cambridge University Press: Cambridge, 2011; pp. 289–300. [Google Scholar] [CrossRef]
- Hwang, C.K.; Chaurasia, S.S.; Jackson, C.R.; Chan, G.C.-K.; Storm, D.R.; Iuvone, P.M. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J. Neurosci. 2013, 33, 14989–14997. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Ruan, G.-X.; Aseem, F.; Abey, J.; Gamble, K.; Stanwood, G.; Palmiter, R.D.; Iuvone, P.M.; McMahon, D.G. Retinal dopamine mediates multiple dimensions of light-adapted vision. J. Neurosci. 2012, 32, 9359–9368. [Google Scholar] [CrossRef] [PubMed]
- Janet, P. The major symptoms of hysteria; Macmillan Publishing, 1907. [Google Scholar]
- James, W. The principles of psychology; Holt: New York, 1890. [Google Scholar]
- Jamison, K.R. Mood disorders and patterns of creativity in British writers and artists. Psychiatry 1989, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, K. (1913/1997) Allgemeine Psychopathologie. Berlin: Springer; English translation of the 7th edition: General Psychopathology. Baltimore: Johns Hopkins University Press.
- Javitt, D.C. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull. 2009, 35, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Meeting overview: Sensory perception and schizophrenia, Lausanne, Switzerland June 31–July 1, 2014. Schizophr. Res. Cogn. 2015, 2, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Freedman, R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophreni. Am. J. Psychiatry 2015, 172, 17–31. [Google Scholar] [CrossRef]
- Jerotic, S.; Ristic, I.; Pejovic, S.; Mihaljevic, M.; Pavlovic, Z.; Britvic, D.; Ignjatovic, Z.; Silverstein, S.M.; Maric, N.P. Retinal structural abnormalities in young adults with psychosis spectrum disorders. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2019, 98, 109825. [Google Scholar] [CrossRef]
- Jiang, N.; Lee, Y.O.; Ling, P.M. Association between tobacco and alcohol use among young adult bar patrons: a cross-sectional study in three cities. BMC Public Heal. 2014, 14, 500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Cowell, R.M.; Nakazawa, K. Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front. Behav. Neurosci. 2013, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Murray, G.; Fredrickson, B.; Youngstrom, E.A.; Hinshaw, S.; Bass, J.M.; Deckersbach, T.; Schooler, J.; Salloum, I. Creativity and bipolar disorder: touched by fire or burning with questions? Clin. Psychol. Rev. 2012, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Juda, A. The relationship between highest mental capacity and psychic abnormalities. Am. J. Psychiatry 1949, 106, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, D.; Cavar, I.; Sesar, A.; Sesar, I.; Vukojevic, J.; Curkovic, M. New Insights into Schizophrenia: a Look at the Eye and Related Structures. Psychiatr. Danub. 2020, 32, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kalenderoglu, A.; Çelik, M.; Sevgi-Karadag, A.; Egilmez, O.B. Optic coherence tomography shows inflammation and degeneration in major depressive disorder patients correlated with disease severity. J. Affect. Disord. 2016, 204, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.L. Genetic association of giftedness and creativity with schizophrenia. Hereditas 2009, 66, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, N.; Mortimer, A. Causes, diagnosis and treatment of visceral hallucinations. Progress in Neurology and Psychiatry January/February. 2013. [Google Scholar]
- Kean, C. Silencing the Self: Schizophrenia as a Self-disturbance. Schizophr. Bull. 2009, 35, 1034–1036. [Google Scholar] [CrossRef]
- Kelemen, O.; Kiss, I.; Benedek, G.; Kéri, S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2013, 47, 13–19. [Google Scholar] [CrossRef]
- Kelly, C.; McCreadie, R. Cigarette smoking and schizophrenia. Adv. Psychiatr. Treat. 2000, 6, 327–331. [Google Scholar] [CrossRef]
- Kendall, K.M.; Van Assche, E.; Andlauer, T.F.M.; Choi, K.W.; Luykx, J.J.; Schulte, E.C.; Lu, Y. The genetic basis of major depression. Psychol. Med. 2021, 51, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Kéri, S.; Antal, A.; Szekeres, G.; Benedek, G.; Janka, Z. Spatiotemporal visual processing in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences 2002, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kéri, S.; Benedek, G. Visual contrast sensitivity alterations in inferred magnocellular pathways and anomalous perceptual experiences in people at high-risk for psychosis. Vis. Neurosci. 2007, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Keromnes, G.; Chokron, S.; Celume, M.-P.; Berthoz, A.; Botbol, M.; Canitano, R.; Du Boisgueheneuc, F.; Jaafari, N.; Lavenne-Collot, N.; Martin, B.; et al. Exploring Self-Consciousness From Self- and Other-Image Recognition in the Mirror: Concepts and Evaluation. Front. Psychol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Norton, D.; McBain, R.; Ongur, D.; Chen, Y. Deficient biological motion perception in schizophrenia: results from a motion noise paradigm. Front. Psychol. 2013, 4, 46070. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Fábián, A.; Benedek, G.; Kéri, S. When doors of perception open: Visual contrast sensitivity in never-medicated, first-episode schizophrenia. J. Abnorm. Psychol. 2010, 119, 586–593. [Google Scholar] [CrossRef]
- Klosterkötter, J.; Hellmich, M.; Steinmeyer, E.M.; Schultze-Lutter, F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry 2001, 58, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Koethe, D.; Kranaster, L.; Hoyer, C.; Gross, S.; Neatby, M.A.; Schultze-Lutter, F.; Ruhrmann, S.; Klosterkötter, J.; Hellmich, M.; Leweke, F.M. Binocular depth inversion as a paradigm of reduced visual information processing in prodromal state, antipsychotic-naïve and treated schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kogata, T.; Iidaka, T. A review of impaired visual processing and the daily visual world in patients with schizophrenia. Nagoya journal of medical science 2018, 80, 317–328. [Google Scholar] [CrossRef]
- Kogata, T.; Iidaka, T. Lateralization of Color Discrimination Performance and Lexical Effects in Patients With Chronic Schizophrenia. Front. Hum. Neurosci. 2021, 15, 702086. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.G.; Turner, T.H.; Bilker, W.B.; Brensinger, C.M.; Siegel, S.J.; Kanes, S.J.; Gur, R.E.; Gur, R.C. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003, 160, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.G.; Walker, J.B.; Martin, E.A.; Healey, K.M.; Moberg, P.J. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010, 36, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Korver-Nieberg, N.; Berry, K.; Meijer, C.J.; de Haan, L. Adult attachment and psychotic phenomenology in clinical and non-clinical samples: A systematic review. Psychol. Psychother. Theory, Res. Pr. 2014, 87, 127–154. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Shetty, A. Frequency of psychiatric comorbid symptoms in bipolar disorder patients in remission. Ind. Psychiatry J. 2022, 31, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kozáková, E.; Bakštein, E.; Havlíček, O.; Bečev, O.; Knytl, P.; Zaytseva, Y.; Španiel, F. Disrupted Sense of Agency as a State Marker of First-Episode Schizophrenia: A Large-Scale Follow-Up Study. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kraepelin. E. (1896/1971) Dementia Praecox and Paraphrenia. (8th ed.) (trans Barclay, R.M.) Huntington, NY: Krieger Publishing.
- Kretschmer, E. (1970) Physique and Character: An Investigation of the Nature of Constitution and of the Theory of Temperament. (Trans. Miller. E.) New York: Cooper Square Publishers, Inc. ISBN 9781138875401.
- Krynicki, C.R.; Upthegrove, R.; Deakin, J.F.W.; Barnes, T.R.E. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr. Scand. 2018, 137, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; D’souza, D.C.; Gallinat, J.; Driesen, N.; Abi-Dargham, A.; Petrakis, I.; Heinz, A.; Pearlson, G. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotox. Res. 2006, 10, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V., & Ettinger, U. (2010) Latent inhibition in schizophrenia and schizotypy: a review of the empirical literature Chap. 17. In Latent Inhibition Cognition, Neuroscience and Applications to Schizophrenia (R. and Weiner, I. eds) Cambridge University Press. ISBN: 9780521517331.
- Kuo, S.-C.; Chen, Y.-T.; Li, S.-Y.; Lee, Y.-T.; Yang, A.C.; Chen, T.-L.; Liu, C.-J.; Chen, T.-J.; Su, I.-J.; Fung, C.-P. Incidence and outcome of newly-diagnosed tuberculosis in schizophrenics: a 12-year, nationwide, retrospective longitudinal study. BMC Infect. Dis. 2013, 13, 351–351. [Google Scholar] [CrossRef] [PubMed]
- Kyaga, S.; Lichtenstein, P.; Boman, M.; Hultman, C.; Långström, N.; Landén, M. Creativity and mental disorder: Family study of 300 000 people with severe mental disorder. Br. J. Psychiatry 2011, 199, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kyaga, S.; Landén, M.; Boman, M.; Hultman, C.M.; Långström, N.; Lichtenstein, P. Mental illness, suicide and creativity: 40-Year prospective total population study. J. Psychiatr. Res. 2013, 47, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Laskemoen, J.F.; Simonsen, C.; Büchmann, C.; Barrett, E.A.; Bjella, T.; Lagerberg, T.V.; Vedal, T.J.; Andreassen, O.A.; Melle, I.; Aas, M. Sleep disturbances in schizophrenia spectrum and bipolar disorders – a transdiagnostic perspective. Compr. Psychiatry 2019, 91, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Agerbo, E.; Pedersen, C.B. Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J. Clin. Psychiatry 2009, 70, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Lavin, R.; Bucci, S.; Varese, F.; Berry, K. The relationship between insecure attachment and paranoia in psychosis: A systematic literature review. Br. J. Clin. Psychol. 2020, 59, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Ross, T.J.; A Stein, E. Cognitive Mechanisms of Nicotine on Visual Attention. Neuron 2002, 36, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Cherkerzian, S.; Seidman, L.J.; Papandonatos, G.D.; Savitz, D.A.; Tsuang, M.T.; Goldstein, J.M.; Buka, S.L. Maternal Bacterial Infection During Pregnancy and Offspring Risk of Psychotic Disorders: Variation by Severity of Infection and Offspring Sex. Am. J. Psychiatry 2020, 177, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Mexal, S.; Freedman, R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J. Dual Diagn. 2007, 3, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Lew, B.J.; I Wiesman, A.; Rezich, M.T.; Wilson, T.W. Altered neural dynamics in occipital cortices serving visual-spatial processing in heavy alcohol users. J. Psychopharmacol. 2020, 34, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, L.; Tao, Y. Diagnosis and treatment of congenital tuberculosis: a systematic review of 92 cases. Orphanet J. Rare Dis. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; An, F.-R.; Zhang, L.; Ungvari, G.S.; Jackson, T.; Yuan, Z.; Xiang, Y.-T. Prevalence of comorbid depression in schizophrenia: A meta-analysis of observational studies. J. Affect. Disord. 2020, 273, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Olsen, J.; Yuan, W.; Cnattingus, S.; Vestergaard, M.; Obel, C.; Gissler, M.; Li, J. Early Life Bereavement and Schizophrenia: A Nationwide Cohort Study in Denmark and Sweden. Medicine 2016, 95, e2434–e2434. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chen, S.; Zhao, L.; Miao, D. Categorization of emotional faces in schizophrenia patients: An ERP study. Neurosci. Lett. 2019, 713, 134493. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Yip, B.H.; Björk, C.; Pawitan, Y.; Cannon, T.D.; Sullivan, P.F.; Hultman, C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009, 373, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Hoek, H.W.; Deen, M.L.; Blom, J.D.; Bruggeman, R.; Cahn, W.; de Haan, L.; Kahn, R.S.; Meijer, C.J.; Myin-Germeys, I.; et al. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr. Res. 2016, 176, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Linville, P.W. Self-complexity as a cognitive buffer against stress-related illness and depression. Journal of personality and social psychology, 1987; 52, 663–676. [Google Scholar]
- Lisman, J.E.; Coyle, J.T.; Green, R.W.; Javitt, D.C.; Benes, F.M.; Heckers, S.; Grace, A.A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008, 31, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lizano, P.; Lutz, O.; Ling, G.; Lee, A.M.; Eum, S.; Bishop, J.R.; Kelly, S.; Pasternak, O.; Clementz, B.; Pearlson, G.; et al. Association of Choroid Plexus Enlargement With Cognitive, Inflammatory, and Structural Phenotypes Across the Psychosis Spectrum. Am. J. Psychiatry 2019, 176, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Lizano, P.; Bannai, D.; Lutz, O.; A Kim, L.; Miller, J.; Keshavan, M. A Meta-analysis of Retinal Cytoarchitectural Abnormalities in Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2020, 46, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Loewy, R.L.; Corey, S.; Amirfathi, F.; Dabit, S.; Fulford, D.; Pearson, R.; Hua, J.P.; Schlosser, D.; Stuart, B.K.; Mathalon, D.H.; et al. Childhood trauma and clinical high risk for psychosis. Schizophr. Res. 2019, 205, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lubow, A. (2006) Edvard Munch: Beyond the Scream. Smithsonian Magazine; March.
- https://www.smithsonianmag.com/arts-culture/edvard-munch-beyond-the-scream-111810150/.
- Ludwig, A.M. Creative achievement and psychopathology: comparison among professions. Am. J. Psychother. 1992, 46, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Hasson-Ohayon, I.; Wiesepape, C.; Huling, K.; Musselman, A.; Lysaker, J.T. Social Dysfunction in Psychosis Is More Than a Matter of Misperception: Advances From the Study of Metacognition. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- MacCabe, J.H.; Sariaslan, A.; Almqvist, C.; Lichtenstein, P.; Larsson, H.; Kyaga, S. Artistic creativity and risk for schizophrenia, bipolar disorder and unipolar depression: a Swedish population-based case–control study and sib-pair analysis. Br. J. Psychiatry 2018, 212, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, D.W. The nature and nurture of creative talent. Am. Psychol. 1962, 17, 484–495. [Google Scholar] [CrossRef]
- Malaspina, D.; Owen, M.J.; Heckers, S.; Tandon, R.; Bustillo, J.; Schultz, S.; Barch, D.M.; Gaebel, W.; Gur, R.E.; Tsuang, M.; et al. Schizoaffective Disorder in the DSM-5. Schizophr. Res. 2013, 150, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.G.; Morgan, V.A.; Mitchell, P.B.; Berk, M.; Young, A.; Castle, D.J. A comparison of schizophrenia, schizoaffective disorder, and bipolar disorder: Results from the Second Australian national psychosis survey. J. Affect. Disord. 2015, 172, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Manjeese, W.; Mvubu, N.E.; Steyn, A.J.C.; Mpofana, T. Mycobacterium tuberculosis causes a leaky blood-brain barrier and neuroinflammation in the prefrontal cortex and cerebellum regions of infected mice offspring. Int. J. Dev. Neurosci. 2021, 81, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Manzella, F.; E Maloney, S.; Taylor, G.T. Smoking in schizophrenic patients: A critique of the self-medication hypothesis. World J. Psychiatry 2015, 5, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Margolis, A.E.; Lee, S.H.; Liu, R.; Goolsby, L.; Champagne, F.; Herbstman, J.; Beebe, B. Associations between prenatal exposure to second hand smoke and infant self-regulation in a New York city longitudinal prospective birth cohort. Environ. Res. 2023, 227, 115652. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.C.V.d.S.; Souza, G.d.S.; Brasil, A.; Herculano, A.M.; Lacerda, E.M.d.C.B.; Rodrigues, A.R.; Rosa, A.A.M.; Ventura, D.F.; Castro, A.J.d.O.; Silveira, L.C.d.L. Psychophysical Evaluation of Visual Functions of Ex-Alcoholic Subjects After Prolonged Abstinence. Front. Neurosci. 2019, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Maurage, P.; Philippot, P.; Verbanck, P.; Noel, X.; Kornreich, C.; Hanak, C.; Campanella, S. Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddball paradigm. Clin. Neurophysiol. 2007, 118, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Maurage, P.; Campanella, S.; Philippot, P.; de Timary, P.; Constant, E.; Gauthier, S.; Miccichè, M.-L.; Kornreich, C.; Hanak, C.; Noel, X.; et al. Alcoholism leads to early perceptive alterations, independently of comorbid depressed state: An ERP study. Neurophysiol. Clin. 2008, 38, 83–97. [Google Scholar] [CrossRef]
- Maziade, M.; Bureau, A.; Jomphe, V.; Gagné, A. Retinal function and preclinical risk traits in children and adolescents at genetic risk of schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2022, 112. [Google Scholar] [CrossRef] [PubMed]
- Maziade, M.; Silverstein, S.M. The place of the retina in psychiatry: Uniting neurobiological and neurodevelopmental research with clinical research in psychiatric disorders. Schizophr. Res. 2020, 219, 1–4. [Google Scholar] [CrossRef] [PubMed]
- McCarthy-Jones, S.; Smailes, D.; Corvin, A.; Gill, M.; Morris, D.W.; Dinan, T.G.; Murphy, K.C.; O′Neill, F.A.; Waddington, J.L.; et al. Occurrence and co-occurrence of hallucinations by modality in schizophrenia-spectrum disorders, Australian Schizophrenia Research Bank, Donohoe, G., Dudley, R. Psychiatry Res. 2017, 252, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.M. Munch and Agoraphobia: His Art and His Illness. RACAR : Revue d’art canadienne / Canadian Art Review. 1988, 15, 23–50. [Google Scholar] [CrossRef]
- McElroy, S.L. Diagnosing and treating comorbid (complicated) bipolar disorder. 2004, 65, 35–44. [Google Scholar] [PubMed]
- McGilchrist, I. (2009/2019). The Master and his Emissary. The Divided Brain and the Making of the Western World. Yale University Press. ISBN-10 0300188374.
- McGilchrist, I. (2021) The Matter with Things. Our Brains Our Delusions and the Unmaking of the World. Vol. 1 Perspectiva. ISBN-10 1914568060.
- McTeague, L.M.; Laplante, M.-C.; Bulls, H.W.; Shumen, J.R.; Lang, P.J.; Keil, A. Face Perception in Social Anxiety: Visuocortical Dynamics Reveal Propensities for Hypervigilance or Avoidance. Biol. Psychiatry 2018, 83, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Deckert, J. Genetics of Anxiety Disorders. Curr. Psychiatry Rep. 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Merritt, K.; Egerton, A.; Kempton, M.J.; Taylor, M.J.; McGuire, P.K. Nature of Glutamate Alterations in Schizophrenia. JAMA Psychiatry 2016, 73, 665–74. [Google Scholar] [CrossRef] [PubMed]
- Michail, M.; Birchwood, M. Social anxiety in first-episode psychosis: The role of childhood trauma and adult attachment. J. Affect. Disord. 2014, 163, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Fone, K.; Steckler, T.; Horan, W.P. Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014, 24, 645–692. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Messias, E.; Miettunen, J.; Alaräisänen, A.; Järvelin, M.-R.; Koponen, H.; Räsänen, P.; Isohanni, M.; Kirkpatrick, B. Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophr. Bull. 2011, 37, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.A.; Gupta, T.; Keane, B.P.; Silverstein, S.M. Visual context processing dysfunctions in youth at high risk for psychosis: Resistance to the Ebbinghaus illusion and its symptom and social and role functioning correlates. J. Abnorm. Psychol. 2015, 124, 953–960. [Google Scholar] [CrossRef] [PubMed]
- C, M.M.; C, E.M.; D, M.M. Edvard Munch: enfermedad y genialidad en el gran artista noruego [Edvard Munch: disease and genius of the great Norwegian artist]. Rev. Medica De Chile 2013, 141, 774–779. [Google Scholar] [CrossRef]
- Misra, S.; Gelaye, B.; Koenen, K.C.; Williams, D.R.; Borba, C.P.; Quattrone, D.; Di Forti, M.; La Cascia, C.; La Barbera, D.; Tarricone, I.; et al. Early Parental Death and Risk of Psychosis in Offspring: A Six-Country Case-Control Study. J. Clin. Med. 2019, 8, 1081. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Ristic, M.; Dimitrijevic, B.; Pesic, M.H. Facial Emotion Recognition and Persecutory Ideation in Paranoid Schizophrenia. Psychol. Rep. 2020, 123, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Mize, R.R., Marc, R.E., Sillito, A.M. (1992) Gaba in the Retinal and Central Visual System. Elsevier Science Publishers. ISBN: 0-444-81446-9.
- Moberg, P. J.; Agrin, R.; Gur, R. E.; Gur, R. C.; Turetsky, B. I.; Doty, R. L. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999, 21, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.M.; Docherty, N.M. Schizophrenia and the sense of self. Schizophr. Bull. 2013, 40, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Monistrol-Mula, A.; Felez-Nobrega, M.; Oh, H.; Haro, J.M.; Koyanagi, A. Association between tuberculosis and psychotic experiences: Mediating factors and implications for patient care in low- and middle-income countries. J. Glob. Heal. 2024, 14, 04005. [Google Scholar] [CrossRef] [PubMed]
- Mosolov, S.N.; Yaltonskaya, P.A. Primary and Secondary Negative Symptoms in Schizophrenia. Front. Psychiatry 2022, 12, 766692. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Ohström, K.-R.P.; Lindenberger, U. Interactive brains, social minds: Neural and physiological mechanisms of interpersonal action coordination. Neurosci. Biobehav. Rev. 2021, 128, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Müller-Westermann (2005) Munch by Himself. Royal Academy of Arts. ISBN 1-903973-64-3.
- Murphy, F.; Nasa, A.; Cullinane, D.; Raajakesary, K.; Gazzaz, A.; Sooknarine, V.; Haines, M.; Roman, E.; Kelly, L.; O'Neill, A.; et al. Childhood Trauma, the HPA Axis and Psychiatric Illnesses: A Targeted Literature Synthesis. Front. Psychiatry 2022, 13, 748372. [Google Scholar] [CrossRef] [PubMed]
- Muros, N.I.; García, A.S.; Forner, C.; López-Arcas, P.; Lahera, G.; Rodriguez-Jimenez, R.; Nieto, K.N.; Latorre, J.M.; Fernández-Caballero, A.; Fernández-Sotos, P. Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars. J. Clin. Med. 2021, 10, 1904. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Zsiros, V.; Jiang, Z.; Nakao, K.; Kolata, S.; Zhang, S.; Belforte, J.E. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012, 62, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426–107426. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.R.; Mishra, J. An improved method to estimate the fractal dimension of colour images. Perspect. Sci. 2016, 8, 412–416. [Google Scholar] [CrossRef]
- Nelson, B.; Rawlings, D. Relating schizotypy and personality to the phenomenology of creativity. Schizophr. Bull. 2010, 36, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Nettle, D.; Clegg, H. Schizotypy, creativity and mating success in humans. Proc. R. Soc. B: Biol. Sci. 2006, 273, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Núñez, D.; Rauch, J.; Herwig, K.; Rupp, A.; Andermann, M.; Weisbrod, M.; Resch, F.; Oelkers-Ax, R. Evidence for a magnocellular disadvantage in early-onset schizophrenic patients: A source analysis of the N80 visual-evoked component. Schizophr. Res. 2013, 144, 16–23. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, B.F.; Swearer, J.M.; Smith, L.T.; Nestor, P.G.; E Shenton, M.; McCarley, R.W. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry 1996, 153, 687–692. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, B.F.; Bismark, A.; Hetrick, W.P.; Bodkins, M.; Vohs, J.L.; Shekhar, A. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2006, 86, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Onstad, S.; Skre, I.; Torgersen, S.; Kringlen, E. Twin concordance for DSM-III-R schizophrenia. Acta Psychiatr. Scand. 1991, 83, 395–401. [Google Scholar] [CrossRef] [PubMed]
- O’toole, M.; Hougaard, E.; Mennin, D. Social anxiety and emotion knowledge: A meta-analysis. J. Anxiety Disord. 2013, 27, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M. Impulsivity in schizophrenia: A comprehensive update. Aggress. Violent Behav. 2013, 18, 247–254. [Google Scholar] [CrossRef]
- Pallanti, S.; Quercioli, L.; Hollander, E. Social anxiety in outpatients with schizophrenia: a relevant cause of disability. Am. J. Psychiatry 2004, 161, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.V.; Marraccini, M.E.; Weyandt, L.L.; Wilder-Smith, O.; McGee, H.A.; Liu, S.; Goodwin, M.S. Interpersonal Autonomic Physiology: A Systematic Review of the Literature. Pers. Soc. Psychol. Rev. 2017, 21, 99–141. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Zhou, A.B. Altered Auditory Self-recognition in People with Schizophrenia. Span. J. Psychol. 2020, 23, e52. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, Y.; Xiang, Y.; Yu, J. Retinal nerve fiber layer thickness changes in Schizophrenia: A meta-analysis of case–control studies. Psychiatry Res. 2018, 270, 786–791. [Google Scholar] [CrossRef]
- Parker, G. The suprasensory world of bipolar II disorder. Am. J. Psychiatry 2014, 171, 614–615. [Google Scholar] [CrossRef]
- Parker, G.; Paterson, A.; Romano, M.; Graham, R. Altered Sensory Phenomena Experienced in Bipolar Disorder. Am. J. Psychiatry 2017, 174, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Parnas, J.; Henriksen, M.G. Disordered Self in the Schizophrenia Spectrum. Harv. Rev. Psychiatry 2014, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Kotlicka-Antczak, M.; Łojek, E.; Ruszpel, A.; Pawełczyk, T. Schizophrenia patients have higher-order language and extralinguistic impairments. Schizophr. Res. 2018, 192, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Peeling, J.L, Muzio, M.R. (2023) Conversion Disorder. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551567/.
- Peschard, V.; Philippot, P. Overestimation of threat from neutral faces and voices in social anxiety. J. Behav. Ther. Exp. Psychiatry 2017, 57, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Vlamings, P.H.J.M.; Goffaux, V.; Kemner, C. Is the early modulation of brain activity by fearful facial expressions primarily mediated by coarse low spatial frequency information? J. Vis. 2009, 9, 12–12. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, O.T.; Harris, J.P. Perceptual changes in schizophrenia: a questionnaire survey. Psychol. Med. 1985, 15, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Pincus, D.; Cadsky, O.; Berardi, V.; Asuncion, C.M.; Wann, K. Fractal Self-Structure and Psychological Resilience. Nonlinear dynamics, psychology, and life sciences 2019, 23, 57–78. [Google Scholar] [PubMed]
- Post, F. Creativity and psychopathology: a study of 291world-famous men. Br. J. Psychiatry 1994, 165, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Postmes, L.; Sno, H.; Goedhart, S.; van der Stel, J.; Heering, H.; de Haan, L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr. Res. 2014, 152, 41–50. [Google Scholar] [CrossRef] [PubMed]
- A Power, R.; Steinberg, S.; Bjornsdottir, G.; A Rietveld, C.; Abdellaoui, A.; Nivard, M.M.; Johannesson, M.; E Galesloot, T.; Hottenga, J.J.; Willemsen, G.; et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat. Neurosci. 2015, 18, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.R.; Kelley, M.; Corlett, P.R. Hallucinations as Top-Down Effects on Perception. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2016, 1, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Prideaux S. (2005) Edvard Munch: Behind the Scream. New Haven, Conn: Yale University Press. ISBN 978-0-300-25000.
- Price, M.J.; Feldman, R.G.; Adelberg, D.; Kayne, H. Abnormalities in color vision and contrast sensitivity in Parkinson's disease. Neurology 1992, 42, 887–887. [Google Scholar] [CrossRef] [PubMed]
- Pries, L.-K.; Guloksuz, S.; Have, M.T.; de Graaf, R.; van Dorsselaer, S.; Gunther, N.; Rauschenberg, C.; Reininghaus, U.; Radhakrishnan, R.; Bak, M.; et al. Evidence That Environmental and Familial Risks for Psychosis Additively Impact a Multidimensional Subthreshold Psychosis Syndrome. Schizophr. Bull. 2018, 44, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Purty, A.; Nestadt, G.; Samuels, J. Genetics of obsessive-compulsive disorder. Indian J. Psychiatry 2019, 61, 37–S42. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.; MacCabe, J.H. The relationship between nicotine and psychosis. Ther. Adv. Psychopharmacol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Radua, J.; Ramella-Cravaro, V.; Ioannidis, J.P.; Reichenberg, A.; Phiphopthatsanee, N.; Amir, T.; Thoo, H.Y.; Oliver, D.; Davies, C.; Morgan, C.; et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 2018, 17, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.; Arvind, S.; Dutt, B.P.; Mamta, B.; Bhavneesh, S.; Kavita, M.; Navneet, K.; Shrutika, G.; Priyanka, B.; Arun, K.; et al. The Role of Religiosity and Guilt in Symptomatology and Outcome of Obsessive Compulsive Disorder. Psychopharmacology bulletin 2021, 51, 38–49. [Google Scholar]
- Redies, C.; Hänisch, J.; Blickhan, M.; Denzler, J. Artists portray human faces with the Fourier statistics of complex natural scenes. Network: Comput. Neural Syst. 2007, 18, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Redies, C.; Hasenstein, J.; Denzler, J. Fractal-like image statistics in visual art: similarity to natural scenes. Spat. Vis. 2007, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Read, J.; Bentall, R.P. Negative childhood experiences and mental health: theoretical, clinical and primary prevention implications. Br. J. Psychiatry 2012, 200, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Reeve, S.; Nickless, A.; Sheaves, B.; Freeman, D. Insomnia, negative affect, and psychotic experiences: Modelling pathways over time in a clinical observational study. Psychiatry Res. 2018, 269, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Farmer, M.E.; Rae, D.S.; Locke, B.Z.; Keith, S.J.; Judd, L.L.; Goodwin, F.K. Comorbidity of Mental Disorders With Alcohol and Other Drug Abuse. JAMA 1990, 264, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Revheim, N.; Corcoran, C.M.; Dias, E.; Hellmann, E.; Martinez, A.; Butler, P.D.; Lehrfeld, J.M.; DiCostanzo, J.; Albert, J.; Javitt, D.C. Reading Deficits in Schizophrenia and Individuals at High Clinical Risk: Relationship to Sensory Function, Course of Illness, and Psychosocial Outcome. Am. J. Psychiatry 2014, 171, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Kinney, D.K.; Lunde, I.; Benet, M.; Merzel, A.P. Creativity in manic-depressives, cyclothymes, their normal relatives, and control subjects. J. Abnorm. Psychol. 1988, 97, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J. (1991) A life of Picasso Vol. 1 1881-1906. Jonathan Cape. ISBN-100394531922.
- Richter, J.; Hölz, L.; Hesse, K.; Wildgruber, D.; Klingberg, S. Measurement of negative and depressive symptoms: Discriminatory relevance of affect and expression. Eur. Psychiatry 2019, 55, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ritsner, M. The attribution of somatization in schizophrenia patients: a naturalistic follow-up study. J. Clin. Psychiatry 2003, 64, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Bergen, S.E. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front. Genet. 2021, 12, 686666. [Google Scholar] [CrossRef] [PubMed]
- Roquelaure, Y.; LE Gargasson, J.F.; Kupper, S.; Girre, C.; Hispard, E.; Dally, S. Alcohol consumption and visual contrast sensitivity. Alcohol Alcohol. 1995, 30, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Repossi, M.; Florio, V.; Demartini, B.; Conca, A.; Gambini, O.; Maravita, A. Sense of body ownership and body agency in schizophrenia. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, A. Bipolar Illness, Creativity, and Treatment. Psychiatr. Q. 2001, 72, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, A. (2015) Creativity and Mental Illness II: The Scream. Psychology Today, 24 March. https://www.psychologytoday.com/gb/blog/creative-explorations/201503/creativity-and-mental-illness-iithe-scream.
- Roy, M.-A.; Demers, M.-F.; Achim, A.M. Social anxiety disorder in schizophrenia: a neglected, yet potentially important comorbidity. J. Psychiatry Neurosci. 2018, 43, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Roy, M.; Berman, J.; Gonzalez, B. Blue cone electroretinogram amplitudes are related to dopamine function in cocaine-dependent patients. Psychiatry Res. 2003, 117, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Han, J.; Coughlin, J.M.; Hill, S.K.; Bishop, J.R.; Tamminga, C.A.; Clementz, B.A.; Pearlson, G.D.; Keshavan, M.S.; Gershon, E.S.; et al. Real-time facial emotion recognition deficits across the psychosis spectrum: A B-SNIP Study. Schizophr. Res. 2022, 243, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Runco, M.A.; Noble, E.P.; Reiter-Palmon, R.; Acar, S.; Ritchie, T.; Yurkovich, J.M. The genetic basis of creativity and ideational fluency. Creativity Res. J. 2011, 23, 376–380. [Google Scholar] [CrossRef]
- Ruocco, A.C.; Reilly, J.L.; Rubin, L.H.; Daros, A.R.; Gershon, E.S.; Tamminga, C.A.; Pearlson, G.D.; Hill, S.K.; Keshavan, M.S.; Gur, R.C.; et al. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr. Res. 2014, 158, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.N.; A Proudlock, F.; Siram, V.; Suraweera, C.; Hutchinson, C.; Nelson, C.P.; Al-Uzri, M.; Gottlob, I. Retinal Layer Abnormalities as Biomarkers of Schizophrenia. Schizophr. Bull. 2018, 44, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Samsom, J.N.; Wong, A.H.C. Schizophrenia and Depression Co-Morbidity: What We have Learned from Animal Models. Front. Psychiatry 2015, 6, 13–13. [Google Scholar] [CrossRef] [PubMed]
- Sandsten, K.E.; Nordgaard, J.; Kjaer, T.W.; Gallese, V.; Ardizzi, M.; Ferroni, F.; Petersen, J.; Parnas, J. Altered self-recognition in patients with schizophrenia. Schizophr. Res. 2020, 218, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R.; Kay, S.R.; Merriam, A.E. Atrophy of the cerebellar vermis: relevance to the symptoms of schizophrenia. Int. J. Neurosci. 1991, 57, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Saramba, M.I.; Zhao, D. A Perspective of the Diagnosis and Management of Congenital Tuberculosis. J. Pathog. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schallmo, M.-P.; Sponheim, S.R.; Olman, C.A. Reduced Contextual Effects on Visual Contrast Perception in Schizophrenia and Bipolar Affective Disorder. Psychol. Med. 2015, 45, 3527–3537. [Google Scholar] [CrossRef] [PubMed]
- Schechter, I.; Butler, P.D.; Jalbrzikowski, M.; Pasternak, R.; Saperstein, A.M.; Javitt, D.C. A new dimension of sensory dysfunction: stereopsis deficits in schizophrenia. Biol. Psychiatry 2006, 60, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Schneier, F.R.; Johnson, J.; Hornig, C.D.; Liebowitz, M.R.; Weissman, M.M. Social phobia: comorbidity and morbidity in an epidemiological sample. Arch Gen Psychiatry, 1992; 55, 322–331. [Google Scholar]
- Schönfeldt-Lecuona, C.; Kregel, T.; Schmidt, A.; Kassubek, J.; Dreyhaupt, J.; Freudenmann, R.W.; Connemann, B.J.; Gahr, M.; Pinkhardt, E.H. Retinal single-layer analysis with optical coherence tomography (OCT) in schizophrenia spectrum disorder. Schizophr. Res. 2020, 219, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt-Lecuona, C.; Kregel, T.; Schmidt, A.; Pinkhardt, E.H.; Lauda, F.; Kassubek, J.; Connemann, B.J.; Freudenmann, R.W.; Gahr, M. From Imaging the Brain to Imaging the Retina: Optical Coherence Tomography (OCT) in Schizophrenia. Schizophr. Bull. 2016, 42, sbv073–14. [Google Scholar] [CrossRef] [PubMed]
- Schubert, E.W.; Henriksson, K.M.; McNeil, T.F. A prospective study of offspring of women with psychosis: visual dysfunction in early childhood predicts schizophrenia-spectrum disorders in adulthood. Acta Psychiatr. Scand. 2005, 112, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Schuler, A.-L.; Tik, M.; Sladky, R.; Luft, C.D.B.; Hoffmann, A.; Woletz, M.; Zioga, I.; Bhattacharya, J.; Windischberger, C. Modulations in resting state networks of subcortical structures linked to creativity. NeuroImage 2019, 195, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; McNeill, Y.; Cavanagh, J.; Cannon, M.; Murray, R. Exposure to obstetric complications and subsequent development of bipolar disorder. Br. J. Psychiatry 2006, 189, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pedraza, I.; Romero-Ferreiro, V.; Read, J.C.A.; Diã©Guez-Risco, T.; Bagney, A.; Caballero-Gonzã¡Lez, M.; Rodrã guez-Torresano, J.; Rodriguez-Jimenez, R. Reduced visual surround suppression in schizophrenia shown by measuring contrast detection thresholds. Front. Psychol. 2014, 5, 1431–1431. [Google Scholar] [CrossRef] [PubMed]
- Shantna, K.; Chaudhury, S.; Verma, A.; Singh, A. Comorbid psychiatric disorders in substance dependence patients: A control study. Ind. Psychiatry J. 2009, 18, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sorrell, J.H.; Peralta, N.R. A Protracted Case of Psychosis, Motor Abnormalities, and Agitation. Prim. Care Companion J. Clin. Psychiatry 2007, 9, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Sheinbaum, T.; Bedoya, E.; Ros-Morente, A.; Kwapil, T.R.; Barrantes-Vidal, N. Association between attachment prototypes and schizotypy dimensions in two independent non-clinical samples of Spanish and American young adults. Psychiatry Res. 2013, 210, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Shoham, N.; Eskinazi, M.; Hayes, J.F.; Lewis, G.; Theodorsson, M.; Cooper, C. Associations between psychosis and visual acuity impairment: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2021, 144, 6–27. [Google Scholar] [CrossRef]
- Shuwairi, S.M.; Cronin-Golomb, A.; McCarley, R.W.; O'Donnell, B.F. Color discrimination in schizophrenia. Schizophr. Res. 2002, 55, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Souto, J.J.; Fernandes, T.P.; Bonifacio, T.A.; Almeida, N.L.; Gomes, G.H.; Felisberti, F.M.; Santos, N.A. Impairments of facial detection in tobacco use disorder: baseline data and impact of smoking duration. Rev. Bras. de Psiquiatr. 2021, 43, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Keane, B.P. Perceptual Organization Impairment in Schizophrenia and Associated Brain Mechanisms: Review of Research from 2005 to 2010. Schizophr. Bull. 2011, 37, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Rosen, R. Schizophrenia and the eye. Schizophr. Res. Cogn. 2015, 2, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S. M. (2016). Visual perception disturbances in schizophrenia: A unified model. In M. Li & W. D. Spaulding (Eds.), The neuropsychopathology of schizophrenia: Molecules, brain systems, motivation, and cognition (pp. 77–132). Springer International Publishing AG.
- Silverstein, S.M.; Kovács, I.; Corry, R.; Valone, C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr. Res. 2000, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Lai, A. The Phenomenology and Neurobiology of Visual Distortions and Hallucinations in Schizophrenia: An Update. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Lai, A.; Green, K.M.; Crosta, C.; I Fradkin, S.; Ramchandran, R.S. Retinal Microvasculature in Schizophrenia. Eye Brain 2021, 13, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Silvia, P.J.; Kimbrel, N.A. A dimensional analysis of creativity and mental illness: Do anxiety and depression symptoms predict creative cognition, creative accomplishments, and creative self-concepts? Psychol. Aesthetics, Creativity, Arts 2010, 4, 2–10. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Thompson, J.L. A vision science perspective on schizophrenia. Schizophr. Res. Cogn. 2015, 2, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, D.; Chang, K.; Strong, C.; Ketter, T. Creativity in familial bipolar disorder. J. Psychiatr. Res. 2005, 39, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Simonton, D.K. Creativity in the Later Years: Optimistic Prospects for Achievement. Gerontol. 1990, 30, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, V.Y.; Skryabina, A.A.; Torrado, M.V.; Gritchina, E.A. Edvard Munch: the collision of art and mental disorder. Ment. Heal. Relig. Cult. 2020, 23, 570–578. [Google Scholar] [CrossRef]
- Slaghuis, W.A. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol. 1998, 107, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Pantelis, C.; McGrath, J.; Tangas, C.; Copolov, D. Ocular Abnormalities in Chronic Schizophrenia: Clinical Implications. Aust. New Zealand J. Psychiatry 1997, 31, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Soares-Weiser, K.; Maayan, N.; Bergman, H.; Davenport, C.; Kirkham, A.J.; Grabowski, S.; E Adams, C. First rank symptoms for schizophrenia. Cochrane Database Syst. Rev. 2015, 2015, CD010653. [Google Scholar] [CrossRef]
- Solesvik, M.; Joa, I.; Larsen, T.K.; Langeveld, J.; Johannessen, J.O.; Bjørnestad, J.; Anda, L.G.; Gisselgård, J.; Hegelstad, W.T.V.; Brønnick, K. Visual Hallucinations in First-Episode Psychosis: Association with Childhood Trauma. PLOS ONE 2016, 11, e0153458–e0153458. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Ganesh, R.; Mahapatra, A.; Verma, R.; Chadda, R.K. Somatic symptoms in schizophrenia: Association with socio-demographic and clinical characteristics, disability and quality of life. Indian J. Psychiatry 2023, 65, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sossen, B.; Richards, A.S.; Heinsohn, T.; Frascella, B.; Balzarini, F.; Oradini-Alacreu, A.; Odone, A.; Rogozinska, E.; Häcker, B.; Cobelens, F.; et al. The natural history of untreated pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Respir. Med. 2023, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Spehar, B.; Clifford, C.W.; Newell, B.R.; Taylor, R.P. Universal aesthetic of fractals. Comput. Graph. 2003, 27, 813–820. [Google Scholar] [CrossRef]
- Clair, D.S.; Lang, B. Schizophrenia: a classic battle ground of nature versus nurture debate. Sci. Bull. 2021, 66, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectrums 2018, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Stang, R. (1977). Edvard Munch: The Man and His Art (trans. Culverwell, G.). Abbeville Press. ISBN: 0-89659-025-9.
- Stankewicz HA, Salen P. (2018) Alcohol related psychosis. Treasure Island, FL: StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK459134/.
- Stanton, K.J.; Denietolis, B.; Goodwin, B.J.; Dvir, Y. Childhood Trauma and Psychosis. Child Adolesc. Psychiatr. Clin. North Am. 2020, 29, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B. An epidemiologic perspective on social anxiety disorder. . 2006, 3–8. [Google Scholar]
- Stein, M.B.; Stein, D.J. Social anxiety disorder. Lancet 2008, 371, 1115–1125. [Google Scholar] [CrossRef]
- Stenersen, R. (1944/1969) Edvard Munch – Close-Up of a Genius. Stockholm: Sem & Stenersen A/S. ISBN 82-7046-057-5.
- Stern, D.N. The Interpersonal World of the Infant. Basic Books. ISBN-10185575200X. 1985. [Google Scholar]
- Sterzer, P.; Adams, R.A.; Fletcher, P.; Frith, C.; Lawrie, S.M.; Muckli, L.; Petrovic, P.; Uhlhaas, P.; Voss, M.; Corlett, P.R. The Predictive Coding Account of Psychosis. Biol. Psychiatry 2018, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Stuve, T.A.; Friedman, L.; Jesberger, J.A.; Gilmore, G.C.; Strauss, M.E.; Meltzer, H.Y. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol. Med. 1997, 27, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a Complex Trait. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Swanton, T. The dopamine, glutamate, and GABA hypotheses of schizophrenia: Glutamate may be the key. ANU Undergraduate Research Journal, 2020; 10, 88–96. [Google Scholar]
- Swee, M.B.; Hudson, C.C.; Heimberg, R.G. Examining the relationship between shame and social anxiety disorder: A systematic review. Clin. Psychol. Rev. 2021, 90, 102088. [Google Scholar] [CrossRef] [PubMed]
- Tadin, D.; Kim, J.; Doop, M.L.; Gibson, C.; Lappin, J.S.; Blake, R.; Park, S. Weakened Center-Surround Interactions in Visual Motion Processing in Schizophrenia. J. Neurosci. 2006, 26, 11403–11412. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, M.; Bodis-Wollner, I.; Kovanecz, I.; Stanzione, P. Spatial frequency tuning of the monkey pattern erg depends on d2 receptor-linked action of dopamine. Vis. Res. 1994, 34, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia,“Just the Facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophrenia Res. 2009, 110, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R., Newell, B., Spehar, B. (2005) Fractals: A Resonance between Art and Nature Mathematics and Culture II, ISBN : 978-3-540-21368-0. [CrossRef]
- Taylor, R. Reduction of Physiological Stress Using Fractal Art and Architecture. Leonardo 2006, 39, 245–251. [Google Scholar] [CrossRef]
- Taylor, R.P.; Sprott, J.C. Biophilic fractals and the visual journey of organic screen-savers. . 2008, 12, 117–29. [Google Scholar] [PubMed]
- Taylor, R.P.; Spehar, B.; Van Donkelaar, P.; Hagerhall, C.M. Perceptual and Physiological Responses to Jackson Pollock's Fractals. Front. Hum. Neurosci. 2011, 5, 10034. [Google Scholar] [CrossRef] [PubMed]
- Ryymin, T. “Tuberculosis-threatened Children”: The Rise and Fall of a Medical Concept in Norway, c.1900–1960. Med Hist. 2008, 52, 347–364. [Google Scholar] [CrossRef] [PubMed]
- ter Borg, M.; Trenité, D.K.-N. The cultural context of diagnosis: The case of Vincent van Gogh. Epilepsy Behav. 2012, 25, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Thor, S.; Hemmingsson, T.; Danielsson, A.-K.; Landberg, J. Fathers’ alcohol consumption and risk of substance-related disorders in offspring. Drug Alcohol Depend. 2022, 233, 109354. [Google Scholar] [CrossRef] [PubMed]
- Tibber, M.S.; Anderson, E.J.; Bobin, T.; Antonova, E.; Seabright, A.; Wright, B.; Carlin, P.; Shergill, S.S.; Dakin, S.C. Visual Surround Suppression in Schizophrenia. Front. Psychol. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Tibbo, P.; Swainson, J.; Chue, P.; LeMelledo, J.-M. Prevalence and relationship to delusions and hallucinations of anxiety disorders in schizophrenia. Depression Anxiety 2003, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ting, J. H.; Zainal, N.; Kanagasundram, S.; Mohamed, S. Conversion Symptoms In Schizophrenia: A Case Report. Malaysian Journal Of Psychiatry 2010, 18, 18. [Google Scholar]
- Tomar, R.; Gupta, A.; Prasad, T.; Bhalla, P.; Murthy, G. Congenital Tuberculosis. Med J. Armed Forces India 2008, 64, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Tøjner, P. E. (2001) Munch in His Own Words. Prestel, Munich. ISBN: 3-7913-2494-2.
- Trotta, A.; Kang, J.; Stahl, D.; Yiend, J. Interpretation Bias in Paranoia: A Systematic Review and Meta-Analysis. Clin. Psychol. Sci. 2021, 9, 3–23. [Google Scholar] [CrossRef]
- True, W.R.; Xian, H.; Scherrer, J.F.; Madden, P.A.F.; Bucholz, K.K.; Heath, A.C.; Eisen, S.A.; Lyons, M.J.; Goldberg, J.; Tsuang, M. Common Genetic Vulnerability for Nicotine and Alcohol Dependence in Men. Arch. Gen. Psychiatry 1999, 56, 655–661. [Google Scholar] [CrossRef]
- Tsitsipa, E.; Fountoulakis, K.N. The neurocognitive functioning in bipolar disorder: a systematic review of data. Ann. Gen. Psychiatry 2015, 14, 1–29. [Google Scholar] [CrossRef]
- Tsoi, D.T.; Lee, K.-H.; Khokhar, W.A.; Mir, N.U.; Swalli, J.S.; Gee, K.A.; Pluck, G.; Woodruff, P.W. Is facial emotion recognition impairment in schizophrenia identical for different emotions? A signal detection analysis. Schizophr. Res. 2008, 99, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, B.I.; Kohler, C.G.; Indersmitten, T.; Bhati, M.T.; Charbonnier, D.; Gur, R.C. Facial emotion recognition in schizophrenia:When and why does it go awry? Schizophr. Res. 2007, 94, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Fagerholm, E.D.; Vignando, M.; Dafflon, J.; Da Costa, P.F.; Dazzan, P.; Leech, R. A GABA Interneuron Deficit Model of the Art of Vincent van Gogh. Front. Psychiatry 2020, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Leech, R.; Expert, P.; Lord, L.-D.; Vernon, A.C. The brain's code and its canonical computational motifs. From sensory cortex to the default mode network: A multi-scale model of brain function in health and disease. Neurosci. Biobehav. Rev. 2015, 55, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Mota, N.; Bolton, J.; Sareen, J. Self-medication with alcohol or drugs for mood and anxiety disorders: A narrative review of the epidemiological literature. Depression Anxiety 2018, 35, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Silverstein, S.M. Perceptual Organization in Schizophrenia Spectrum Disorders: Empirical Research and Theoretical Implications. Psychol. Bull. 2005, 131, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Birchwood, M.; Ross, K.; Brunett, K.; McCollum, R.; Jones, L. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr. Scand. 2010, 122, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ustvedt, O. (2020) Edvard Munch: An Inner Life. London: Thames & Hudson. ISBN 978-0-500-29576-2.
- Vardaxi, C.C.; Gonda, X.; Fountoulakis, K.N. Life events in schizoaffective disorder: A systematic review. J. Affect. Disord. 2018, 227, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Valle, R. Schizophrenia in ICD-11: Comparison of ICD-10 and DSM-5. Span. J. Psychiatry Ment. Heal. 2020, 13, 95–104. [Google Scholar] [CrossRef]
- van der Weiden, A.; Prikken, M.; van Haren, N.E. Self–other integration and distinction in schizophrenia: A theoretical analysis and a review of the evidence. Neurosci. Biobehav. Rev. 2015, 57, 220–237. [Google Scholar] [CrossRef] [PubMed]
- van Ommen, M.M.; van Beilen, M.; Cornelissen, F.W.; Smid, H.G.O.M.; Knegtering, H.; Aleman, A.; van Laar, T. ; GROUP Investigators The prevalence of visual hallucinations in non-affective psychosis, and the role of perception and attention. Psychol. Med. 2016, 46, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Vardaxi, C.C.; Gonda, X.; Fountoulakis, K.N. Life events in schizoaffective disorder: A systematic review. J. Affect. Disord. 2018, 227, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Chaos, Ruiz-Esteban, R.; Mestre De Juan, M.J. Fractals, and Our Concept of Disease. Perspectives in Biology and Medicine 2010, 53, 584–595. [Google Scholar]
- Varghese, S.B.; Reid, J.C.; Hartmann, E.E.; Keyser, K.T. The Effects of Nicotine on the Human Electroretinogram. Investig. Opthalmology Vis. Sci. 2011, 52, 9445–9451. [Google Scholar] [CrossRef] [PubMed]
- Varsamis, J.; Adamson, J.D. Somatic Symptoms in Schizophrenia. Can. Psychiatr. Assoc. J. 1976, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, T.; Fisher, H.L.; Mason, O.; Johnson, S. Childhood trauma and schizotypy: a systematic literature review. Psychol. Med. 2015, 45, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Velthorst, E.; Fett, A.-K.J.; Reichenberg, A.; Perlman, G.; van Os, J.; Bromet, E.J.; Kotov, R. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 2017, 174, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, P.; Joorman, J.; Khan, R. Why We Sing the Blues: The Relation Between Self-Reflective Rumination, Mood, and Creativity. Emotion 2005, 5, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Viengkham, C.; Spehar, B. Preference for Fractal-Scaling Properties Across Synthetic Noise Images and Artworks. Front. Psychol. 2018, 9, 1439. [Google Scholar] [CrossRef] [PubMed]
- Viertiö, S.; Laitinen, A.; Perälä, J.; Saarni, S.I.; Koskinen, S.; Lönnqvist, J.; Suvisaari, J. Visual impairment in persons with psychotic disorder. Chest 2007, 42, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Vila-Badia, R.; Butjosa, A.; Del Cacho, N.; Serra-Arumí, C.; Esteban-Sanjusto, M.; Ochoa, S.; Usall, J. Types, prevalence and gender differences of childhood trauma in first-episode psychosis. What is the evidence that childhood trauma is related to symptoms and functional outcomes in first episode psychosis? A systematic review. Schizophr. Res. 2021, 228, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Vitay J. & Hamker F.H. (2007) On the Role of Dopamine in Cognitive Vision. In: Paletta L., Rome E. (eds) Attention in Cognitive Systems. Theories and Systems from an Interdisciplinary Viewpoint. LNAI 4840, pp. 352–366 WAPCV. Lecture Notes in Computer Science, vol 4840. Springer, Berlin, Heidelberg.
- Vollmer-Larsen, A.; Handest, P.; Parnas, J. Reliability of Measuring Anomalous Experience: The Bonn Scale for the Assessment of Basic Symptoms. Psychopathology 2007, 40, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Voskuil, P. Vincent van Gogh and his illness. A reflection on a posthumous diagnostic exercise. Epilepsy Behav. 2020, 111, 107258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Z.; Pan, Z.; Zhao, K.; Zhao, Q.; Zhou, D.; Shen, H.-W.; Wu, X. Impaired Binocular Depth Perception in First-Episode Drug-Naive Patients With Schizophrenia. Front. Psychol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Warick, L., & Warick, E., (2014) Edvard Munch A Study of Loss, Grief and Creativity 22 Nov. https://www.msu.edu/course/ha/446/panter.htm.
- Waters, F.; Collerton, D.; Ffytche, D.H.; Jardri, R.; Pins, D.; Dudley, R.; Blom, J.D.; Mosimann, U.P.; Eperjesi, F.; Ford, S.; et al. Visual Hallucinations in the Psychosis Spectrum and Comparative Information From Neurodegenerative Disorders and Eye Disease. Schizophr. Bull. 2014, 40, S233–S245. [Google Scholar] [CrossRef] [PubMed]
- Waters, F.; Woodward, T.; Allen, P.; Aleman, A.; Sommer, I. Self-recognition Deficits in Schizophrenia Patients With Auditory Hallucinations: A Meta-analysis of the Literature. Schizophr. Bull. 2010, 38, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.G.; Kucala, T.; Tilleskjor, C.; Jacobs, L. Schizophrenic Birth Seasonality in Relation to the Incidence of Infectious Diseases and Temperature Extremes. Arch. Gen. Psychiatry 1984, 41, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Weber-Stadlbauer, U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl. Psychiatry 2017, 7, e1113–e1113. [Google Scholar] [CrossRef] [PubMed]
- Weir, David (2023) Bohemians: A Very Short Introduction. Oxford University Press. ISBN: 9780197538296.
- Wermes, R.; Lincoln, T.M.; Helbig-Lang, S. Anxious and alert? Hypervigilance in social anxiety disorder. Psychiatry Res. 2018, 269, 740–745. [Google Scholar] [CrossRef] [PubMed]
- West, B.J. Where Medicine Went Wrong - Rediscovering the Path to Complexity; World Scientific Publishing, 2006; ISBN 9789810213770. [Google Scholar]
- Whitfield, C.L.; Dube, S.R.; Felitti, V.J.; Anda, R.F. Adverse childhood experiences and hallucinations. Child Abus. Negl. 2005, 29, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; Gulati, G.; Geddes, J.R.; Fazel, S. Association of Schizophrenia Spectrum Disorders and Violence Perpetration in Adults and Adolescents From 15 Countries. JAMA Psychiatry 2022, 79, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Woll, G. (2001) Edvard Munch, The Complete Graphic Works. Abrams, New York. ISBN-100810908743.
- Woll, G. (2008) Edvard Munch Complete Paintings, Catalogue Raissone. London: Thames & Hudson. ISBN 13: 9780500093450.
- Wylie, H.W., Jr. Edvard Munch. American Imago , Winter 1980, 37, 413–443. [Google Scholar]
- Wylie, M.L.; Wylie, H.W., Jr. The Creative Relationship of Internal and External Determinants in the Life of an Artist. Annual of Psychoanalysis 1989, 17, 73–128. [Google Scholar]
- Yakovenko, I.; Clark, C.M.; Hodgins, D.C.; Goghari, V.M. A qualitative analysis of the effects of a comorbid disordered gambling diagnosis with schizophrenia. Schizophr. Res. 2016, 171, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Maddock, R.J.; Rokem, A.; Silver, M.A.; Minzenberg, M.J.; Ragland, J.D.; Carter, C.S. GABA Concentration Is Reduced in Visual Cortex in Schizophrenia and Correlates with Orientation-Specific Surround Suppression. J. Neurosci. 2010, 30, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Maddock, R.J.; Cui, E.D.; Minzenberg, M.J.; Niendam, T.A.; Lesh, T.; Solomon, M.; Ragland, J.D.; Carter, C. Reduced in vivo visual cortex GABA in schizophrenia, a replication in a recent onset sample. Schizophr. Res. 2020, 215, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Young, B.G. A phenomenological comparison of LSD and schizophrenic states. Br J Psychiatry 1974, 124, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-Y.; Hur, J.-W.; Jung, W.H.; Jang, J.H.; Youn, T.; Kang, D.-H.; Park, S.; Kwon, J.S. Dysfunctional role of parietal lobe during self-face recognition in schizophrenia. Schizophr. Res. 2014, 152, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lu, Z.; Li, Y.; Cowart, L.A.; Lopes-Virella, M.F.; Huang, Y. Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLoS One 2016, 11, e0193343. [Google Scholar] [CrossRef] [PubMed]
- Zemon, V.; Herrera, S.; Gordon, J.; Revheim, N.; Silipo, G.; Butler, P.D. Contrast sensitivity deficits in schizophrenia: A psychophysical investigation. Eur. J. Neurosci. 2021, 53, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.; Zhang, J. Association of COMT and COMT-DRD2 interaction with creative potential. Front. Hum. Neurosci. 2014, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; King, A.; McNamara, P.; Pokorny, J.; Cao, D. Differential effects of alcohol on contrast processing mediated by the magnocellular and parvocellular pathways. J. Vis. 2012, 12, 16–16. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Fang, T.; Chen, C.; Chen, M.; Sun, Y.; Ma, X.; Li, R.; Tian, H.; Ping, J. Brain imaging features in schizophrenia with co-occurring auditory verbal hallucinations and depressive symptoms—Implication for novel therapeutic strategies to alleviate the reciprocal deterioration. Brain Behav. 2020, 11, e01991. [Google Scholar] [CrossRef]
- Żochowska, A.; Nowicka, M.M.; Wójcik, M.J.; Nowicka, A. Self-face and emotional faces—are they alike? Soc. Cogn. Affect. Neurosci. 2021, 16, 593–607. [Google Scholar] [CrossRef]
| 1 | The Scream is among the most famous paintings in the world ((https://edition.cnn.com/style/article/most-famous-paintings/index.html). On the reverse Munch wrote: “Can only have been painted by a madman” (Döges, 2021). |
| 2 | For images of his work in chronological order:https://en.wikipedia.org/wiki/List_of_paintings_by_Edvard_Munch. |
| 3 | “My fear of life is necessary to me, as is my illness. Without anxiety and illness, I am a ship without a rudder. My art is grounded in reflections over being different from others. My sufferings are part of my self and my art. They are indistinguishable from me, and their destruction would destroy my art. I want to keep those sufferings.” (Prideaux, 2005, 251; Tøjner, 2001, hereafter respectively ‘Prideaux’ and ‘Tøjner’). |
| 4 | Munch left several thousand pages of journals, correspondence, poetry, and fiction. His exhibitions are well documented, there are numerous contemporary reviews of his art and a significant number of people he knew wrote memoires of him (for translated materials see: https://www.emunch.no/english.xhtml; for memoires see: Stang; Är Til Är, 1961 and Stenersen, 1944 (hereafter, ‘Stenersen’). |
| 5 | Although a consequence of spinal tuberculosis, it was a source of Munch’s belief in his hereditary madness (Prideaux; Tøjner). |
| 6 | This may be compared to Picasso’s Blue Period precipitated by death of a close friend (Richardson 1991). |
| 7 | For a detailed explanation of colour extraction using MatLab (see: Duth & Deepa, 2018). |
| 8 | Paintings completed in periods of crisis in his life ‘Critical Years’ are highlighted. |
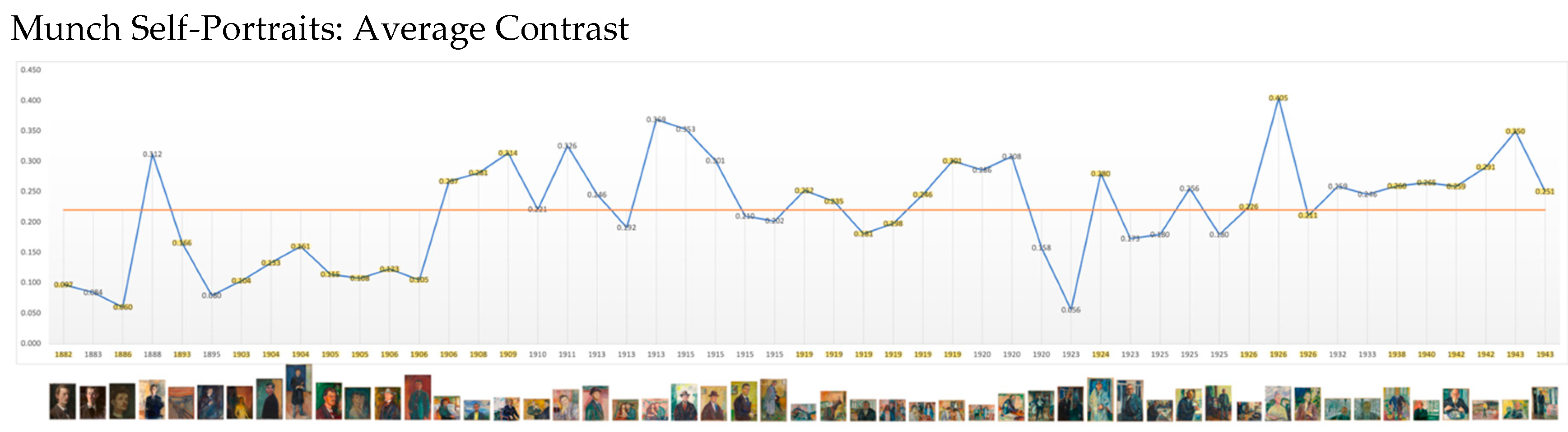
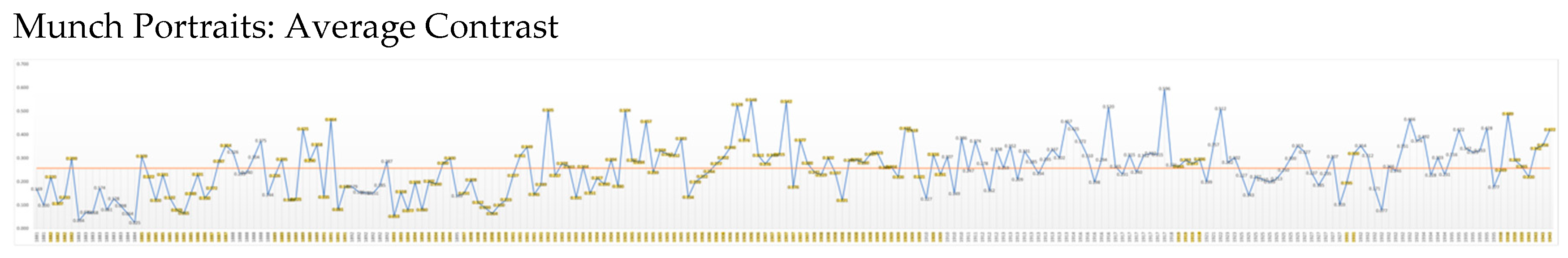

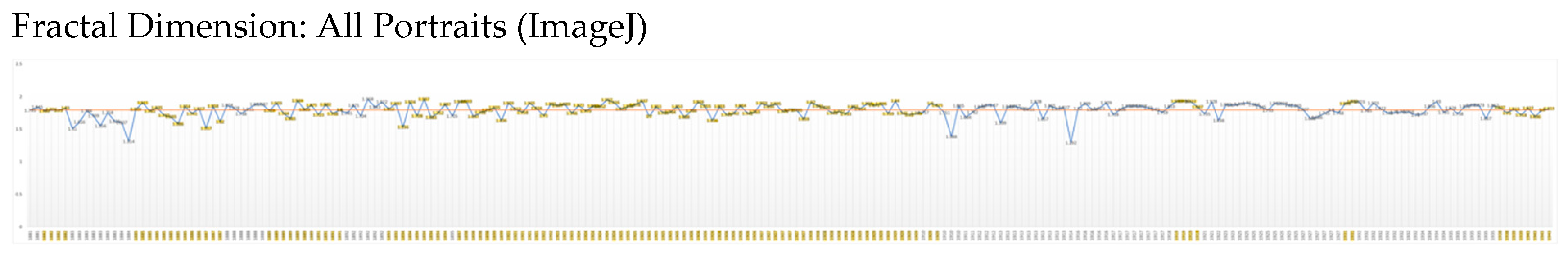
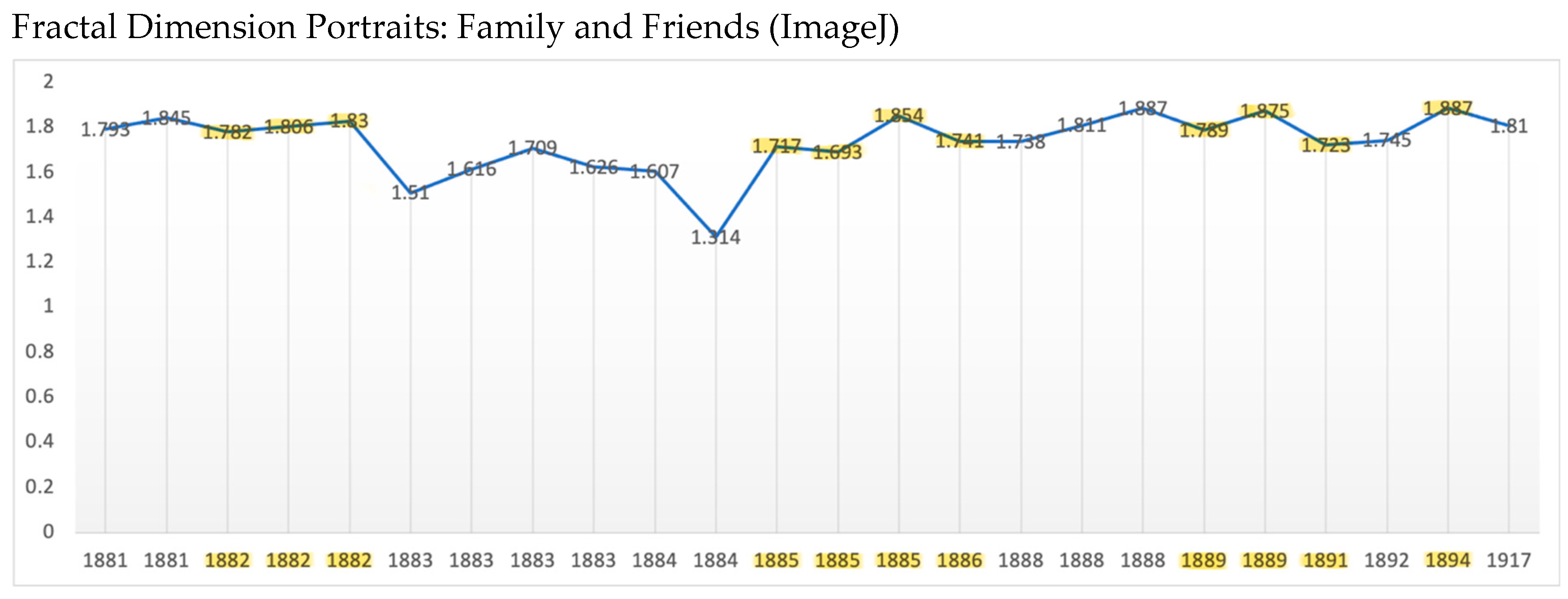

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



