Submitted:
24 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Clinical Presentation of BMs from EOC
1.2. The Key role of Multimodal Treatment Plan for BMs from EOC
1.1. Biomarkers of CNS Spread and Potential Therapeutic Targets
2. Case Presentation
2.1. Case One

2.2. Case Two
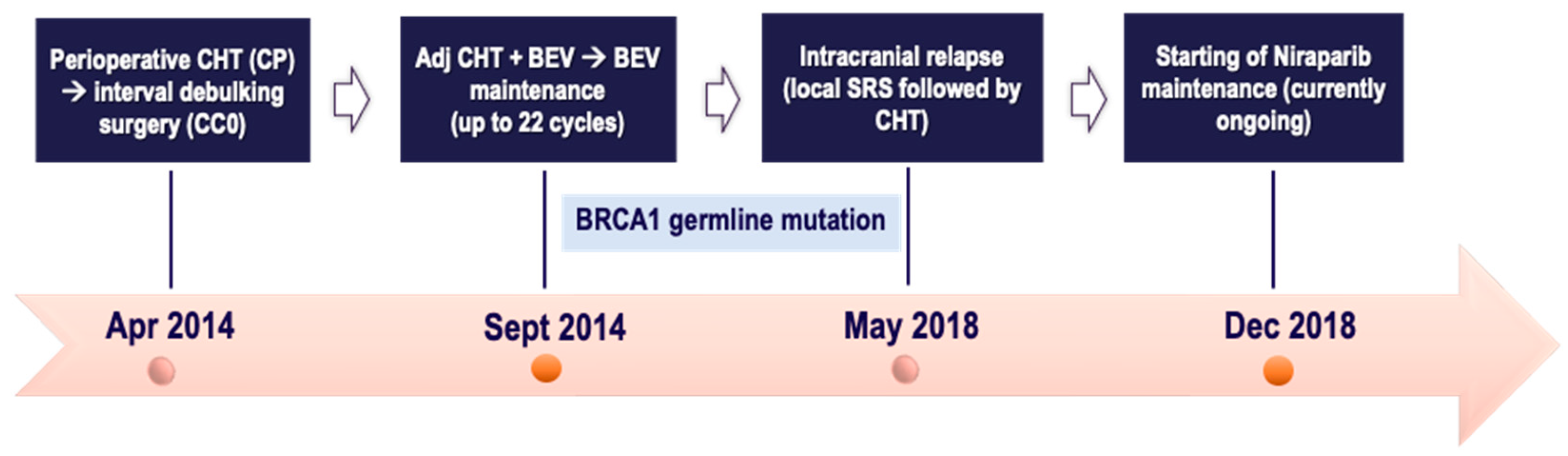
2.3. Case Three
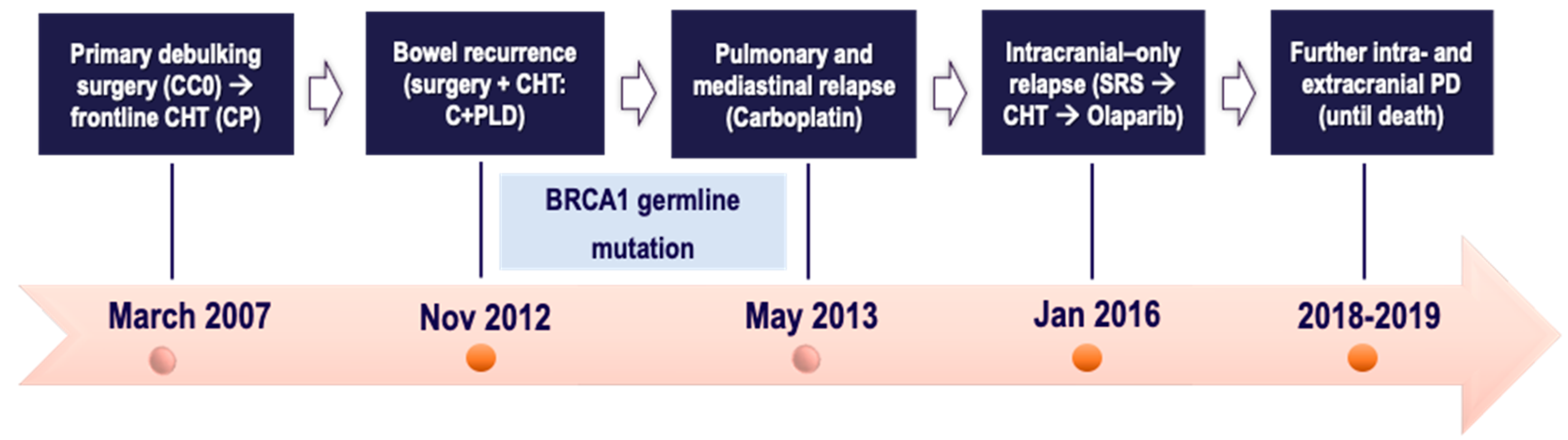
2.4. Case Four
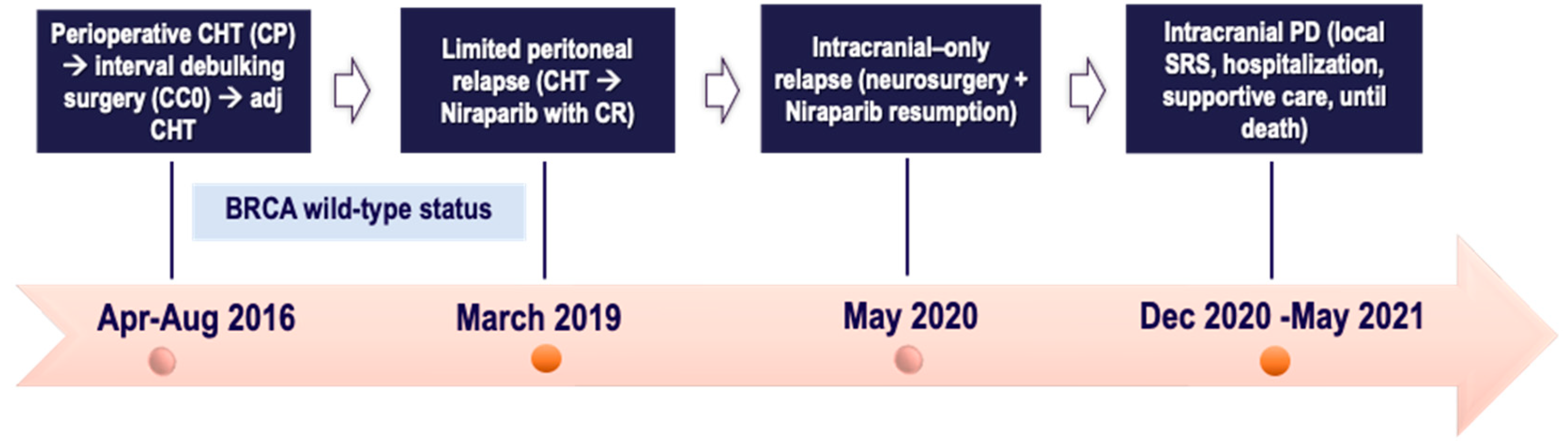
2.5. Case Five
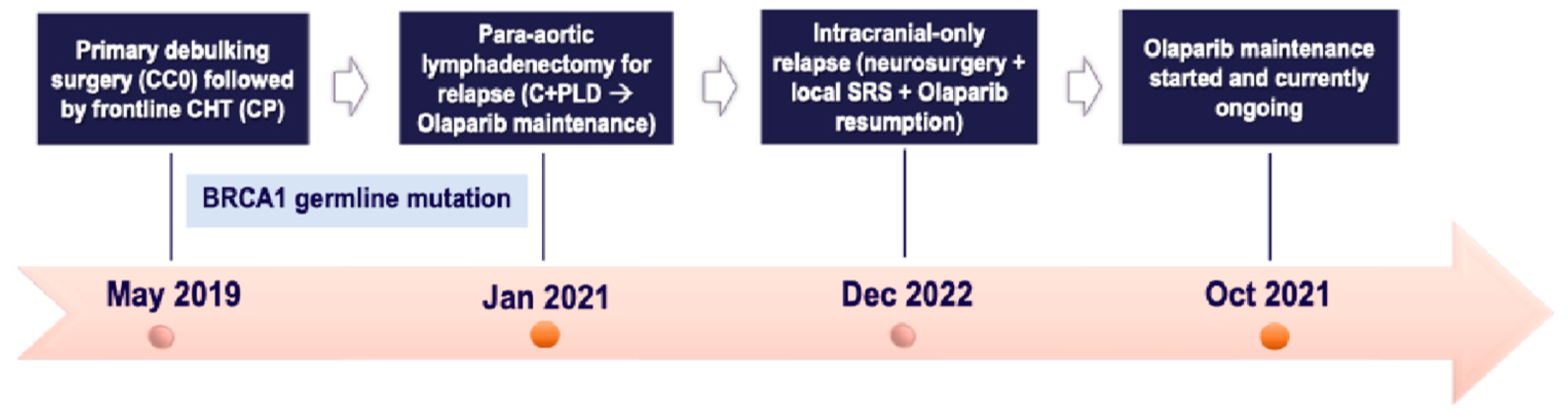
2.6. Case Six
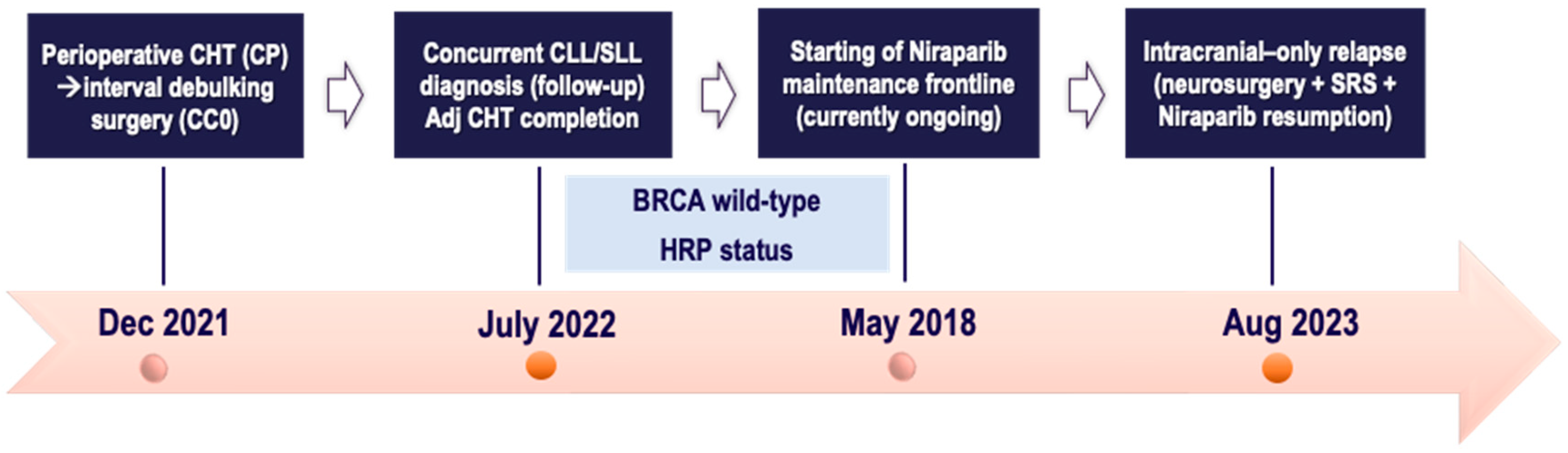
3. Discussion and Literature Review
3.1. What’s the Rationale Behind the PARPi Use in BMs from EOC?
3.2. What are the Main Highlights for Niraparib Maintenance in BMs from EOC?
3.3. What Evidence Supports Olaparib Maintenance in Patients with Intracranial Relapse?
3.4. What is the Rationale for Continuing PARPi Beyond Intracranial Progression in EOC?
4. Concluding Remarks and Future Challenges
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Access Statement
Conflicts of Interest declaration
References
- Jiri Hatina, Maximilian Boesch, Sieghart Sopper, Michaela Kripnerova, Dominik Wolf, Daniel Reimer, Christian Marth, and Alain G. Zeimet. (2021). Ovarian cancer stem cell heterogeneity. In A. Birbrair (Ed.), Stem Cells Heterogeneity in Cancer (pp. 201–216).
- Ledermann, J. A., Matias-Guiu, X., Amant, F., Concin, N., Davidson, B., Fotopoulou, C., González-Martin, A., Gourley, C., Leary, A., Lorusso, D., Banerjee, S., Chiva, L., Cibula, D., Colombo, N., Croce, S., Eriksson, A. G., Falandry, C., Fischerova, D., Harter, P., … Fagotti, A. (2024). ESGO–ESMO–ESP consensus conference recommendations on ovarian cancer: pathology and molecular biology and early, advanced and recurrent disease. Annals of Oncology. [CrossRef]
- National Comprehensive Cancer Network, Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN Practice Guidelines, Version 1, 2023.
- Scotto, G., Borella, F., Turinetto, M., Tuninetti, V., Valsecchi, A. A., Giannone, G., Cosma, S., Benedetto, C., & Valabrega, G. (2021). Biomarkers of central nervous system involvement from epithelial ovarian cancer. Cells (Basel, Switzerland), 10(12), 3408. [CrossRef]
- Alizzi, Z., Roxburgh, P., Cartwright, D., McLaren, A., Park, S., Jones, R., Greening, S., Hudson, E., Green, C., Gray, S., Khalique, S., Karteris, E., & Hall, M. (2023). Description of a retrospective cohort of epithelial ovarian cancer patients with brain metastases: Evaluation of the role of PARP inhibitors in this setting. Journal of Clinical Medicine, 12(7), 2497. [CrossRef]
- Kolomainen, D. F., Larkin, J. M. G., Badran, M., A’Hern, R. P., King, D. M., Fisher, C., Bridges, J. E., Blake, P. R., Barton, D. P. J., Shepherd, J. H., Kaye, S. B., & Gore, M. E. (2002). Epithelial ovarian cancer metastasizing to the brain: a late manifestation of the disease with an increasing incidence. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 20(4), 982–986. [CrossRef]
- Marchetti, C., Ferrandina, G., Cormio, G., Gambino, A., Cecere, S., Lorusso, D., De Giorgi, U., Bogliolo, S., Fagotti, A., Mammoliti, S., Narducci, F., Bergamini, A., Scollo, P., Biglia, N., Breda, E., Tamberi, S., Marinaccio, M., Angioli, R., Salerno, L., … Panici, P. B. (2016). Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecologic Oncology, 143(3), 532–538. [CrossRef]
- Madariaga, A. (2020). Manage wisely: poly(ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. Int J of Gynecol Cancer, 30, 903–915. [CrossRef]
- Nasu, K., Satoh, T., Nishio, S., Nagai, Y., Ito, K., Otsuki, T., Hongo, A., Hirashima, Y., Ogura, T., & Shimada, M. (2013). Clinicopathologic features of brain metastases from gynecologic malignancies: a retrospective study of 139 cases (KCOG-G1001s trial). Gynecologic Oncology, 128(2), 198–203. [CrossRef]
- Cabitza, E., Pirola, M., Baldessari, C., Bernardelli, G., Zunarelli, E., Pipitone, S., Vitale, M. G., Nasso, C., Molinaro, E., Oltrecolli, M., D’Agostino, E., Mandato, V. D., Palicelli, A., Dominici, M., & Sabbatini, R. (2023). Cerebellar metastasis of ovarian cancer: a case report. Journal of Medical Case Reports, 17(1), 553. [CrossRef]
- Pakneshan, S., Safarpour, D., Tavassoli, F., & Jabbari, B. (2014). Brain metastasis from ovarian cancer: a systematic review. Journal of Neuro-Oncology, 119(1), 1–6. [CrossRef]
- Kim M. et al: Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm Res; 35(9): 177. [CrossRef]
- Limon, D., Shachar, E., Wolf, I., Adar, L., Peleg Hasson, S., Ferro, L., & Safra, T. (2022). Brain metastases in patients with ovarian cancer. Acta Oncologica (Stockholm, Sweden), 61(6), 757–763. [CrossRef]
- Gallego, A. (2021). Long-term response to olaparib in BRCA 1-related ovarian cancer with brain metastases. Int J Gynecol Cancer, 31, 1292–1296. [CrossRef]
- Kasherman, L., Madariaga, A., Rouzbahman, M., Murphy, K., Shultz, D., Stockley, T., & Oza, A. M. (2021). Across barriers: poly ADP-ribose polymerase inhibitors beyond progression in high grade serous ovarian cancer with brain metastases. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 31(1), 139–143. [CrossRef]
- Stasenko, M., Cybulska, P., Feit, N., Makker, V., Konner, J., O’Cearbhaill, R. E., Alektiar, K. M., Beal, K., Gardner, G. J., Long Roche, K. C., Sonoda, Y., Chi, D. S., Zivanovic, O., Leitao, M. M., Jr, Cadoo, K. A., & Tew, W. P. (2019). Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecologic Oncology, 154(1), 144–149. [CrossRef]
- Kwon, J.-W., Yoon, J. H., Lim, M. C., Joo, J., Yoo, H., Shin, S.-H., Park, S. Y., Lee, S. H., Kim, Y.-J., Kim, J.-Y., & Gwak, H.-S. (2018). Treatment results and prognostic factors of brain metastases from ovarian cancer: A single institutional experience of 56 patients. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 28(8), 1631–1638. [CrossRef]
- Tao, Mengyu, Cheng, J., & Wu, X. (2020). Niraparib as maintenance therapy in germline ATM-mutated and somatic BRCA2-mutated ovarian cancer with brain metastases: A case report and literature review. OncoTargets and Therapy, 13, 12979–12986. [CrossRef]
- Matsuo, K., Eno, M. L., Ahn, E. H., Shahzad, M. M. K., Im, D. D., Rosenshein, N. B., & Sood, A. K. (2011). Multidrug resistance gene (MDR-1) and risk of brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer. American Journal of Clinical Oncology, 34(5), 488–493. [CrossRef]
- Le Page, C., Amuzu, S., Rahimi, K., Gotlieb, W., Ragoussis, J., & Tonin, P. N. (2021). Lessons learned from understanding chemotherapy resistance in epithelial tubo-ovarian carcinoma from BRCA1and BRCA2 mutation carriers. Seminars in Cancer Biology, 77, 110–126. [CrossRef]
- Vergote, I., González-Martín, A., Ray-Coquard, I., Harter, P., Colombo, N., Pujol, P., Lorusso, D., Mirza, M. R., Brasiuniene, B., Madry, R., Brenton, J. D., Ausems, M. G. E. M., Büttner, R., Lambrechts, D., & European experts’ consensus group. (2022). European experts’ consensus: BRCA/homologous recombination deficiency testing in first-line ovarian cancer. Annals of Oncology, 33(3), 276–287. [CrossRef]
- Jernigan, A. M., Mahdi, H., & Rose, P. G. (2015). Epithelial ovarian cancer metastatic to the central nervous system and a family history concerning hereditary breast and ovarian cancer--A potential relationship. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 25(7), 1232–1238. [CrossRef]
- Ratner, Elena, Bala, M., Louie-Gao, M., Aydin, E., Hazard, S., & Brastianos, P. K. (2019). Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecologic Oncology, 153(3), 568–573. [CrossRef]
- Balendran, S., Liebmann-Reindl, S., Berghoff, A. S., Reischer, T., Popitsch, N., Geier, C. B., Kenner, L., Birner, P., Streubel, B., & Preusser, M. (2017). Next-Generation Sequencing-based genomic profiling of brain metastases of primary ovarian cancer identifies a high number of BRCA-mutations. Journal of Neuro-Oncology, 133(3), 469–476. [CrossRef]
- O’Malley, D. M., Krivak, T. C., Kabil, N., Munley, J., & Moore, K. N. (2023). PARP inhibitors in ovarian cancer: A review. Targeted Oncology, 18(4), 471–503. [CrossRef]
- Gray, S., Khor, X. Y., & Yiannakis, D. (2019). Niraparib as maintenance therapy in a patient with ovarian cancer and brain metastases. BMJ Case Reports, 12(8), e230738. [CrossRef]
- Sun K, Mikule K, Wang Z, et al. A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget 2018;9:37080–96. [CrossRef]
- Mikule, K., & Wilcoxen, K. (2015). Abstract B168: The PARP inhibitor, niraparib, crosses the blood brain barrier in rodents and is efficacious in a BRCA2-mutant intracranial tumor model. Molecular Cancer Therapeutics, 14(12_Supplement_2), B168–B168. [CrossRef]
- Ning, J., & Wakimoto, H. (2020). Therapeutic application of PARP inhibitors in neuro-oncology. Trends in Cancer, 6(2), 147–159. [CrossRef]
- Wang, X., Ellenbogen, Y., Mojica, C., Matos, G. D. R., Alsajjan, R., Ramos, R., Climans, S. A., Voisin, M., Patil, V., Baker, S., Chan, Y.-L., Pimentel Muniz, T., Gao, A., Wang, B. X., Zadeh, G., Mason, W. P., & Chen, E. X. (2024). A phase II trial of olaparib and durvalumab in patients with recurrent IDH-mutated gliomas. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 42(16_suppl), 2013–2013. [CrossRef]
- Sanai, N., Umemura, Y., Margaryan, T., Molloy, J., Zhang, H., Knight, W., Harmon, J., Hong, A., Wanebo, J., Braun, K., Kennedy, W. R., Garcia, M. A., Barani, I. J., Yoo, W., Tien, A.-C., Tovmasyan, A., & Mehta, S. (2024). Niraparib efficacy in patients with newly-diagnosed glioblastoma: Clinical readout of a phase 0/2 “trigger” trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 42(16_suppl), 2002–2002. [CrossRef]
- Zhang, Z., Xu, M., Sakandar, A., Du, X., He, H., He, W., Li, D., & Wen, Q. (2022). Successful treatment of a patient with brain metastasis from ovarian cancer with BRCA wild type using niraparib: A case report and review of the literature. Frontiers in Oncology, 12, 873198. [CrossRef]
- Wang, Q., Zhang, F., Gao, H., & Xu, Y. (2021). Successful treatment of a patient with brain metastases from endometrial cancer using Niraparib: a case report. Annals of Palliative Medicine, 10(1), 818–827. [CrossRef]
- Bangham, M., Goldstein, R., Walton, H., & Ledermann, J. A. (2016). Olaparib treatment for BRCA-mutant ovarian cancer with leptomeningeal disease. Gynecologic Oncology Reports, 18, 22–24. [CrossRef]
- Sakamoto, I., Hirotsu, Y., Nakagomi, H., Ikegami, A., Teramoto, K., & Omata, M. (2019). Durable response by olaparib for a Japanese patient with primary peritoneal cancer with multiple brain metastases: A case report: Durable response by olaparib. The Journal of Obstetrics and Gynaecology Research, 45(3), 743–747. [CrossRef]
- Mohler, A., Salinas, B., Nazur, K., Odia, Y., & Lambrou, N. (2021). THER-02. PARP inhibitor tolerability and impact on progression-free survival in patients with high-grade, ovarian carcinoma with brain metastasis: A case-series. Neuro-Oncology Advances, 3(Supplement_3), iii12–iii13. [CrossRef]
- Magdalena Sliwinska, Malgorzata Cieslak-Stec, Izabela Laprus, Rafal Tarnawski. 482 Brain metastases in patients with advanced BRCA-associated ovarian cancer receiving systemic treatment and adjuvant maintenance Olaparib therapy according to the criteria of study 19 clinical trial–single-centre experience (2023). [CrossRef]
- Favier, L., Truc, G., Boidot, R., & Bengrine-Lefevre, L. (2020). Long-term response to Olaparib in carcinomatous meningitis of a BRCA2 mutated ovarian cancer: A case report. Molecular and Clinical Oncology, 13(1), 73–75. [CrossRef]
- Yano, H., Nagao, S., & Yamaguchi, S. (2020). Leptomeningeal metastases arising from gynecological cancers. International Journal of Clinical Oncology, 25(2), 391–395. [CrossRef]
- Jang, A. I., Bernstock, J. D., Segar, D. J., Distasio, M., Matulonis, U., & Bi, W. L. (2021). Case report: Frontoparietal metastasis from a primary Fallopian tube carcinoma. Frontiers in Surgery, 8. [CrossRef]
- Cerda, V. R., Lu, D., Scott, M., Kim, K. H., Rimel, B. J., & Kamrava, M. (2022). Evaluation of patterns of progression on poly (ADP-ribose) polymerase inhibitor (PARPi) maintenance in ovarian cancer: a cross-sectional study. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society, 32(2), 153–158. [CrossRef]
- Matulonis, U., Herrstedt, J., Oza, A., Mahner, S., Redondo, A., Berton, D., Berek, J., Lund, B., Marmé, F., González-Martín, A., Tinker, A., Ledermann, J., Benigno, B., Lindahl, G., Colombo, N., Li, Y., Gupta, D., Monk, B., & Mirza, M. (2021). Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecologic Oncology, 162, S24–S25. [CrossRef]
|
Case ID |
Age |
BRCA mutation status |
CNS site(s) |
PARPi agent and duration |
PARPi setting at time of BMs |
Extra- cranial site(s) |
Local therapies for BMs |
CNS BOR and survival time with BMs |
| 1 | 53 | BRCA1 PV |
Single (cerebellar) BM |
Niraparib 18 m (ongoing) |
PSR | Para-aortic lymph node |
Surgery RT (SRS) |
CR 32 m (alive) |
| 2 | 52 | BRCA1 PV |
Single (right parietal) BM | Niraparib 64 m (ongoing) |
PSR | No | Surgery RT (SRS) |
CR 72 m (alive) |
| 3 | 47 | BRCA1 PV |
Multiple (parietal, occipital) BMs | Olaparib 21 m |
PSR | Lung; media-stinum |
RT (SRS) | PR 46 m (dead) |
| 4 | 65 | BRCA1-2 wild-type | Single (cerebellar) BM | Niraparib 18 m |
PSR (beyond CNS oligo-recurrence) |
No |
Surgery RT (SRS) |
PR 12 m (dead) |
| 5 | 52 | BRCA1 PV |
Single (single left parieto-occipital) BM | Olaparib 30 m (ongoing) |
PSR (beyond CNS oligo-recurrence) |
No | Surgery RT (SRS) |
CR 18 m (alive) |
| 6 | 73 | BRCA1-2 wild-type | Single (cerebellar) BM | Niraparib 18 m (ongoing) |
Frontline (beyond CNS oligo-recurrence) |
No | Surgery RT (SRS) |
CR 10 m (alive) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





