Submitted:
24 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Tissues
2.2. Gene Expression by Real-Time Quantitative PCR Analysis (qRT-PCR)
2.3. Western Blotting
2.4. Statistical Analysis
3. Results
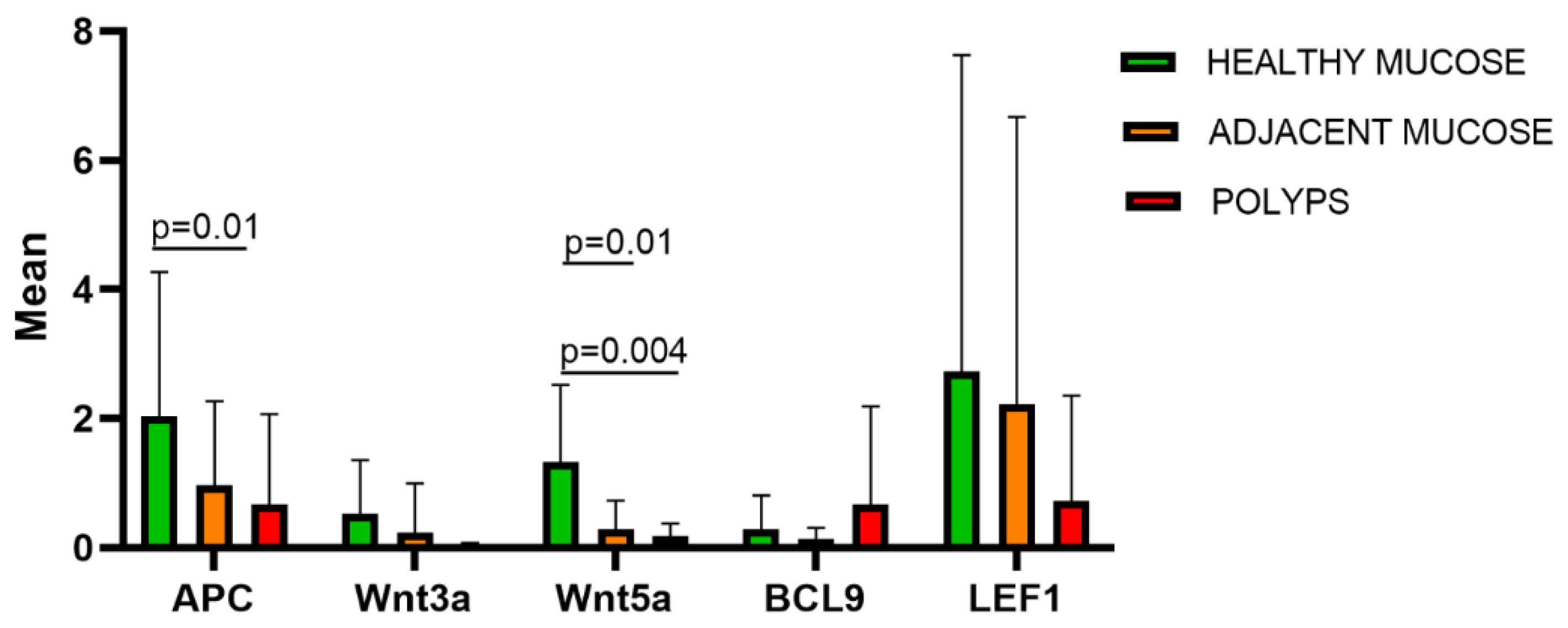
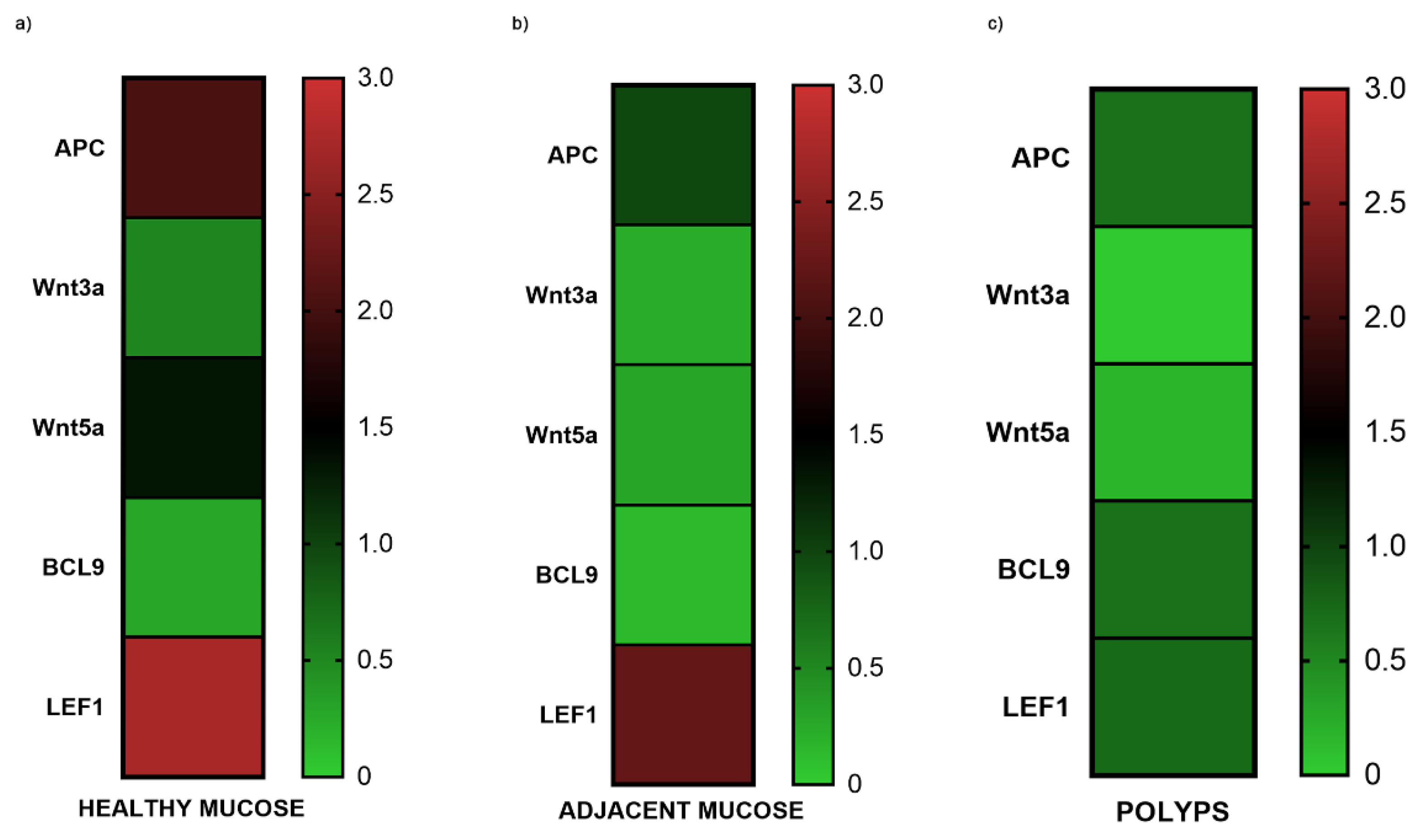
3.1. Gene Expression of APC, Wnt3A, Wnt5A, BCL9 and LEF1 in FAP and Tubular-Villous Adenomas
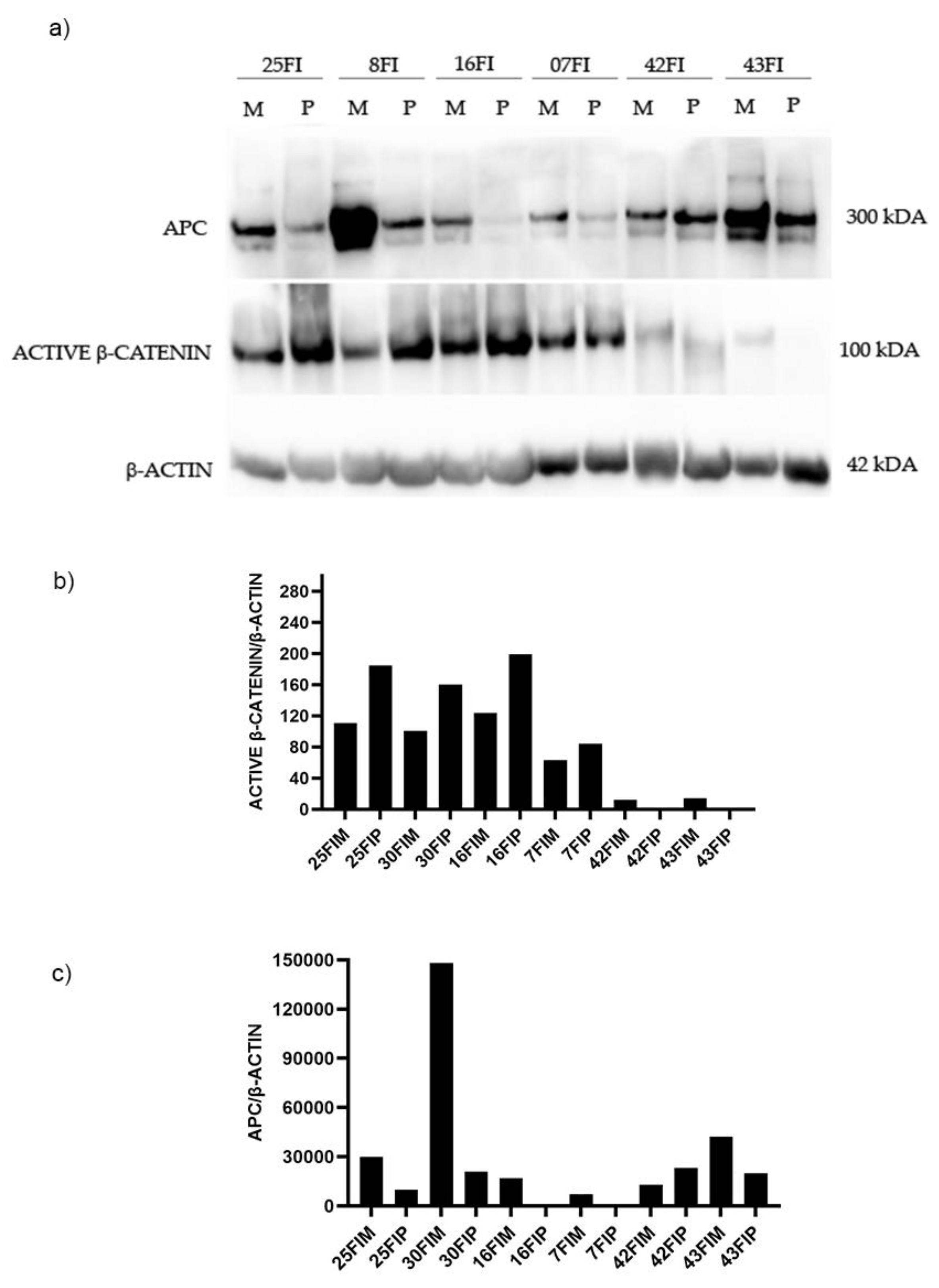
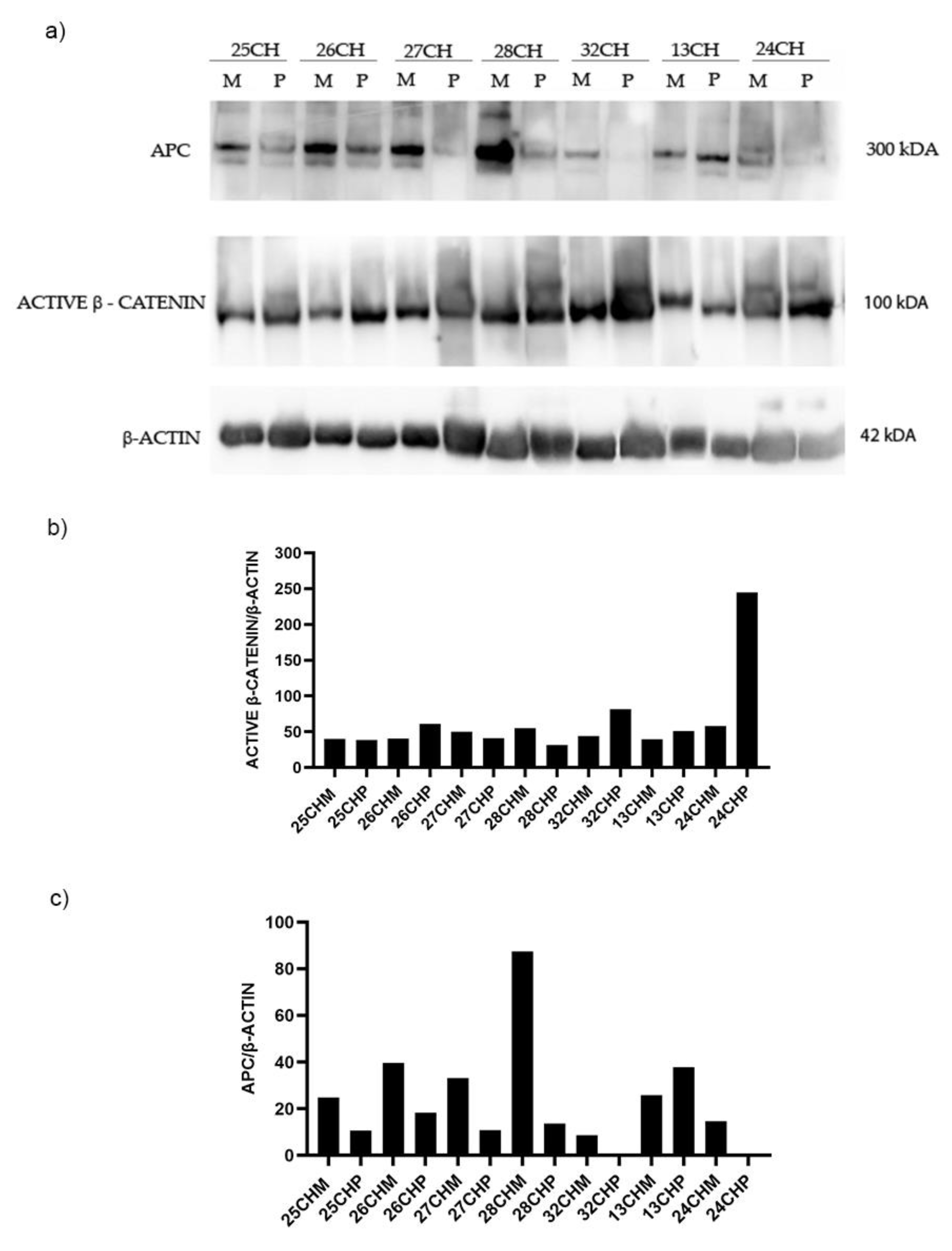
3.2.1. Protein Expression Assay by Western Blotting of APC and β-Catenin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol. 2016 Feb 14;22(6):1925-34. [CrossRef] [PubMed]
- Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA. 2018 May 15;319(19):2021-2031.
- Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986 Aug 15;38(2):173-6. [CrossRef] [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759-67. [CrossRef] [PubMed]
- Yang B, Mao L, Li Y, Li Q, Li X, Zhang Y, Zhai Z. β-catenin, leucine-rich repeat-containing G protein-coupled receptor 5 and GATA-binding factor 6 are associated with the normal mucosa-adenoma-adenocarcinoma sequence of colorectal tumorigenesis. Oncol Lett. 2018;15:2287–2295.
- Kim N. H., Jung Y. S., Jeong W. S., et al. Miss rate of colorectal neoplastic polyps and risk factors for missed polyps in consecutive colonoscopies. Intestinal Research. 2017;15(3):411–418. [CrossRef]
- International Agency for Research on Cancer. Colorectal cancer screening. In IARC Handbooks of Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2019; Volume 17, p. 300.
- Lieberman D. A., Rex D. K., Winawer S. J., et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. [CrossRef]
- Aceto GM, Catalano T, Curia MC. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. Biomed Res Int. 2020 Mar 16;2020:1726309. [CrossRef] [PubMed]
- Dornblaser D, Young S, Shaukat A. Colon polyps: updates in classification and management. Curr Opin Gastroenterol. 2024 Jan 1;40(1):14-20. Epub 2023 Nov 1. [CrossRef] [PubMed]
- Galuppini F, Fassan M, Mastracci L, Gafà R, Lo Mele M, Lazzi S, Remo A, Parente P, D'Amuri A, Mescoli C, Tatangelo F, Lanza G. The histomorphological and molecular landscape of colorectal adenomas and serrated lesions. Pathologica. 2021 Jun;113(3):218-229. [CrossRef] [PubMed]
- Dubé C, Yakubu M, McCurdy BR, et al.. Risk of advanced adenoma, colorectal cancer, and colorectal cancer cortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol 2017;112:1790-1801. [CrossRef]
- Lucci-Cordisco E., Risio M., Venesio T., Genuardi M. The growing complexity of the intestinal polyposis syndromes. American Journal of Medical Genetics. Part A. 2013;161(11):2777–2787. [CrossRef]
- Corley D. A., Jensen C. D., Marks A. R., et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clinical Gastroenterology and Hepatology. 2013;11(2):172–180. [CrossRef]
- Pommergaard H. C., Burcharth J., Rosenberg J., Raskov H. The association between location, age and advanced colorectal adenoma characteristics: a propensity-matched analysis. Scandinavian Journal of Gastroenterology. 2017;52(1):1–4. [CrossRef]
- Klein J. L., Okcu M., Preisegger K. H., Hammer H. F. Distribution, size and shape of colorectal adenomas as determined by a colonoscopist with a high lesion detection rate: influence of age, sex and colonoscopy indication. United European Gastroenterology Journal. 2016;4(3):438–448.
- IJspeert J. E., van Doorn S. C., van der Brug Y. M., et al. The proximal serrated polyp detection rate is an easy-to-measure proxy for the detection rate of clinically relevant serrated polyps. Gastrointestinal Endoscopy. 2015;82(5):870–877. [CrossRef]
- Kim K. H., Kim K. O., Jung Y., et al. Clinical and endoscopic characteristics of sessile serrated adenomas/polyps with dysplasia/adenocarcinoma in a Korean population: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter study. Scientific Reports. 2019;9(1):p. 3946. [CrossRef]
- Kahi C. J., Hewett D. G., Norton D. L., Eckert G. J., Rex D. K. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clinical Gastroenterology and Hepatology. 2011;9(1):42–46. [CrossRef]
- Leggett B., Whitehall V. Role of serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–2100. [CrossRef]
- Lochhead P, Chan AT, Giovannucci E, Fuchs CS, Wu K, Nishihara R, O'Brien M and Ogino S: Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 109:1205–1214. 2014.
- Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, Lai J and Guzman MA: Familial adenomatous polyposis syndrome: An update and review of extraintestinal manifestations. Arch Pathol Lab Med. 143:1382–1398. 2019.
- Taherian M, Lotfollahzadeh S, Daneshpajouhnejad P, Arora K. Tubular Adenoma. 2023 Jun 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed]
- Tischoff I, Tannapfel A. Präkanzerosen im Kolon [Precancerous colorectal tumors]. Internist (Berl). 2013 Jun;54(6):691-8. German. [CrossRef] [PubMed]
- Zhang L, Shay JW. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J Natl Cancer Inst. 2017 Aug 1;109(8):djw332. [CrossRef] [PubMed]
- Schatoff EM, Leach BI, Dow LE. Wnt Signaling and Colorectal Cancer. Curr Colorectal Cancer Rep. 2017 Apr;13(2):101-110. Epub 2017 Feb 28. [CrossRef] [PubMed]
- Hankey W, Frankel WL, Groden J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev. 2018 Mar;37(1):159-172. [CrossRef] [PubMed]
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001 Oct;1(1):55-67. [CrossRef] [PubMed]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787-90. [CrossRef] [PubMed]
- Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018 Jun 8;145(11):dev146589. [CrossRef] [PubMed]
- Han, W., He, L., Cao, B., Zhao, X., Zhang, K., Li, Y., … Wang, H. (2017). Differential expression of LEF1/TCFs family members in colonic carcinogenesis. Molecular Carcinogenesis, 56(11), 2372–2381. [CrossRef]
- F.H. Brembeck, Maria Wiese, Nathalie Zatula, Tamara Grigoryan, Yiyang Dai, Johannes Fritzmann, Walter Birchmeier, BCL9-2 Promotes Early Stages of Intestinal Tumor Progression,Gastroenterology,Volume 141, Issue 4,2011,Pages 1359-1370.e3,ISSN 0016-5085. [CrossRef]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004 Sep 15;18(18):2225-30. Epub 2004 Sep 1. [CrossRef] [PubMed]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Züllig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002 Apr 5;109(1):47-60. [CrossRef] [PubMed]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002 May;4(5):367-73. [CrossRef] [PubMed]
- Wu M, Dong H, Xu C, Sun M, Gao H, Bu F, Chen J. The Wnt-dependent and Wnt-independent functions of BCL9 in development, tumorigenesis, and immunity: Implications in therapeutic opportunities. Genes Dis. 2023 Apr 11;11(2):701-710. [CrossRef] [PubMed]
- Tufail, M., Wu, C. Wnt3a is a promising target in colorectal cancer. Med Oncol 40, 86 (2023). [CrossRef]
- Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T, Anchang B, Demir K, Boehm C, Leible S, Ball CR, Glimm H, Spang R, Boutros M. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun. 2013;4:2610. [CrossRef] [PubMed]
- Qi L, Sun B, Liu Z, Cheng R, Li Y, Zhao X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J Exp Clin Cancer Res. 2014 Dec 11;33(1):107. [CrossRef] [PubMed]
- Ferrer-Mayorga G, Niell N, Cantero R, González-Sancho JM, Del Peso L, Muñoz A, Larriba MJ. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci Rep. 2019 May 30;9(1):8085. [CrossRef] [PubMed]
- Torres M. A., Yang-Snyder J. A., Purcell S. M., DeMarais A. A., McGrew L. L., Moon R. T. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. Journal of Cell Biology . 1996;133(5):1123–1137. [CrossRef]
- Bauer M., Bénard J., Gaasterland T., Willert K., Cappellen D. WNT5A encodes two isoforms with distinct functions in cancers. PLoS One . 2013;8(11). [CrossRef]
- Asem M., Buechler S., Wates R., Miller D., Stack M. Wnt5a signaling in cancer. Cancers . 2016;8(9):p. 79. [CrossRef]
- Muhammad Tufail, Changxin Wu,WNT5A: a double-edged sword in colorectal cancer progression,Mutation Research/Reviews in Mutation Research,Volume 792,2023,108465,ISSN 1383-5742. [CrossRef]
- Ficari F, Cama A, Valanzano R, Curia MC, Palmirotta R, Aceto G, Esposito DL, Crognale S, Lombardi A, Messerini L, Mariani-Costantini R, Tonelli F, Battista P. APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis. Br J Cancer. 2000 Jan;82(2):348-53. [CrossRef] [PubMed]
- Catalano T, D'Amico E, Moscatello C, Di Marcantonio MC, Ferrone A, Bologna G, Selvaggi F, Lanuti P, Cotellese R, Curia MC, Lattanzio R, Aceto GM. Oxidative Distress Induces Wnt/β-Catenin Pathway Modulation in Colorectal Cancer Cells: Perspectives on APC Retained Functions. Cancers (Basel). 2021 Nov 30;13(23):6045. [CrossRef] [PubMed]
- Silviera ML, Smith BP, Powell J, Sapienza C. Epigenetic differences in normal colon mucosa of cancer patients suggest altered dietary metabolic pathways. Cancer Prev Res (Phila). 2012 Mar;5(3):374-84. Epub 2012 Feb 1. [CrossRef] [PubMed]
- Zhan, T., Rindtorff, N. & Boutros, M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017). [CrossRef]
- Hankey, W., Frankel, W.L. & Groden, J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev 37, 159–172 (2018). [CrossRef]
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007 Oct 1;120(Pt 19):3327-35. [CrossRef] [PubMed]
- Xu D., Yuan L., Liu X., Li M., Zhang F., Gu X., Zhang D., Yang Y., Cui B., Tong J., Zhou J., Yu Z. EphB6 overexpression and Apc mutation together promote colorectal cancer. Oncotarget. 2016; 7: 31111-31121. Retrieved from https://www.oncotarget.com/article/9080/text/.
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996 Aug 15;382(6592):638-42. [CrossRef] [PubMed]
- Xiao, L.; Zhang, C.; Li, X.; Jia, C.; Chen, L.; Yuan, Y.; Gao, Q.; Lu, Z.; Feng, Y.; Zhao, R.; et al. LEF1 Enhances the Progression of Colonic Adenocarcinoma via Remodeling the Cell Motility Associated Structures. Int. J. Mol. Sci. 2021, 22, 10870. [Google Scholar] [CrossRef] [PubMed]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. [CrossRef]
- Gao X., Mi Y., Ma Y., Jin W. LEF1 regulates glioblastoma cell proliferation, migration, invasion, and cancer stem-like cell self-renewal. Tumour Biol. 2014;35:11505–11511. [CrossRef]
- Hao Y.H., Lafita-Navarro M.C., Zacharias L., Borenstein-Auerbach N., Kim M., Barnes S., Kim J., Shay J., DeBerardinis R.J., Conacci-Sorrell M. Induction of LEF1 by MYC activates the WNT pathway and maintains cell proliferation. Cell Commun. Signal. 2019;17:129. [CrossRef]
- Xie Q, Tang T, Pang J, Xu J, Yang X, Wang L, Huang Y, Huang Z, Liu G, Tong D, Zhang Y, Wang L, Zhang D, Lan W, Liu Q, Jiang J. LSD1 Promotes Bladder Cancer Progression by Upregulating LEF1 and Enhancing EMT. Front Oncol. 2020 Jul 28;10:1234. [CrossRef] [PubMed]
- Yuan M., Zhang X., Zhang J., Wang K., Zhang Y., Shang W., Zhang Y., Cui J., Shi X., Na H., et al. DC-SIGN-LEF1/TCF1-miR-185 feedback loop promotes colorectal cancer invasion and metastasis. Cell Death Differ. 2020;27:379–395. [CrossRef]
- Kim G.H., Fang X.Q., Lim W.J., Park J., Kang T.B., Kim J.H., Lim J.H. Cinobufagin Suppresses Melanoma Cell Growth by Inhibiting LEF1. Int. J. Mol. Sci. 2020;21:6706. [CrossRef]
- Blazquez R., Rietkötter E., Wenske B., Wlochowitz D., Sparrer D., Vollmer E., Müller G., Seegerer J., Sun X., Dettmer K., et al. LEF1 supports metastatic brain colonization by regulating glutathione metabolism and increasing ROS resistance in breast cancer. Int. J. Cancer. 2020;146:3170–3183. [CrossRef]
- Heino S, Fang S, Lähde M, Högström J, Nassiri S, Campbell A, Flanagan D, Raven A, Hodder M, Nasreddin N, Xue HH, Delorenzi M, Leedham S, Petrova TV, Sansom O, Alitalo K. Lef1 restricts ectopic crypt formation and tumor cell growth in intestinal adenomas. Sci Adv. 2021 Nov 19;7(47):eabj0512. Epub 2021 Nov 17. [CrossRef] [PubMed]
- Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol Cell. 2006 Oct 20;24(2):293-300. [CrossRef] [PubMed]
- Habib SJ, Acebrón SP. Wnt signalling in cell division: from mechanisms to tissue engineering. Trends Cell Biol. 2022 Dec;32(12):1035-1048. Epub 2022 Jun 15. [CrossRef] [PubMed]
- Chen J, Rajasekaran M, Xia H, Kong SN, Deivasigamani A, Sekar K, Gao H, Swa HL, Gunaratne J, Ooi LL, Xie T, Hong W, Hui KM. CDK1-mediated BCL9 phosphorylation inhibits clathrin to promote mitotic Wnt signalling. EMBO J. 2018 Oct 15;37(20):e99395.Epub 2018 Sep 14. [CrossRef] [PubMed]
- Suzuki K, Okuno Y, Kawashima N, Muramatsu H, Okuno T, Wang X, Kataoka S, Sekiya Y, Hamada M, Murakami N, Kojima D, Narita K, Narita A, Sakaguchi H, Sakaguchi K, Yoshida N, Nishio N, Hama A, Takahashi Y, Kudo K, Kato K, Kojima S. MEF2D-BCL9 Fusion Gene Is Associated With High-Risk Acute B-Cell Precursor Lymphoblastic Leukemia in Adolescents. J Clin Oncol. 2016 Oct 1;34(28):3451-9. Epub 2016 Aug 9. [CrossRef] [PubMed]
- Feng M, Wu Z, Zhou Y, Wei Z, Tian E, Mei S, Zhu Y, Liu C, He F, Li H, Xie C, Jin J, Dong J, Yang D, Yu K, Qian J, Lambrechts D, Wang MW, Zhu D. BCL9 regulates CD226 and CD96 checkpoints in CD8+ T cells to improve PD-1 response in cancer. Signal Transduct Target Ther. 2021 Aug 20;6(1):313. [CrossRef] [PubMed]
- Kaur N, Chettiar S, Rathod S, Rath P, Muzumdar D, Shaikh ML, Shiras A. Wnt3a mediated activation of Wnt/β-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci. 2013 May;54:44-57. Epub 2013 Jan 19. [CrossRef] [PubMed]
- Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013 Jul 4;8(7):e67811. [CrossRef] [PubMed]
- Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004 Dec 15;64(24):8860-6. [CrossRef] [PubMed]
- Fox SA, Richards AK, Kusumah I, Perumal V, Bolitho EM, Mutsaers SE, Dharmarajan AM. Expression profile and function of Wnt signaling mechanisms in malignant mesothelioma cells. Biochem Biophys Res Commun. 2013 Oct 11;440(1):82-7. Epub 2013 Sep 13. [CrossRef] [PubMed]
- Qiang YW, Shaughnessy JD Jr, Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008 Jul 15;112(2):374-82. Epub 2008 Mar 14. [CrossRef] [PubMed]
- Nygren MK, Døsen G, Hystad ME, Stubberud H, Funderud S, Rian E. Wnt3A activates canonical Wnt signalling in acute lymphoblastic leukaemia (ALL) cells and inhibits the proliferation of B-ALL cell lines. Br J Haematol. 2007 Feb;136(3):400-13. Epub 2006 Dec 8. [CrossRef] [PubMed]
- Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK, Kim HN. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer. 2014 Feb 24;14:125. [CrossRef] [PubMed]
- Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao & Weiwei Tang (2021) WNT5a in Colorectal Cancer: Research Progress and Challenges, Cancer Management and Research,, 2483-2498. [CrossRef]
- Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. 1999 Oct;81(3):496-502. [CrossRef] [PubMed]
- Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008 Jan 1;14(1):55-61. [CrossRef] [PubMed]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005 Apr 14;434(7035):843-50. [CrossRef] [PubMed]
- Abdelmaksoud-Dammak R, Miladi-Abdennadher I, Saadallah-Kallel A, Khabir A, Sellami-Boudawara T, Frikha M, Daoud J, Mokdad-Gargouri R. Downregulation of WIF-1 and Wnt5a in patients with colorectal carcinoma: clinical significance. Tumour Biol. 2014 Aug;35(8):7975-82. Epub 2014 May 16. [CrossRef] [PubMed]
- Hibi K, Mizukami H, Goto T, Kitamura Y, Sakata M, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y. WNT5A gene is aberrantly methylated from the early stages of colorectal cancers. Hepatogastroenterology. 2009 Jul-Aug;56(93):1007-9. [PubMed]
- Cao YC, Yang F, Liu XH, Xin X, Wang CC, Geng M. [Expression of Wnt5a, APC, β-catenin and their clinical significance in human colorectal adenocarcinoma]. Zhonghua Zhong Liu Za Zhi. 2012 Sep;34(9):674-8. Chinese. [CrossRef] [PubMed]
- Rawson JB, Mrkonjic M, Daftary D, Dicks E, Buchanan DD, Younghusband HB, Parfrey PS, Young JP, Pollett A, Green RC, Gallinger S, McLaughlin JR, Knight JA, Bapat B. Promoter methylation of Wnt5a is associated with microsatellite instability and BRAF V600E mutation in two large populations of colorectal cancer patients. Br J Cancer. 2011 Jun 7;104(12):1906-12. Epub 2011 May 17. [CrossRef] [PubMed]
- Li Q, Chen H. Silencing of Wnt5a during colon cancer metastasis involves histone modifications. Epigenetics. 2012 Jun 1;7(6):551-8. Epub 2012 Jun 1. [CrossRef] [PubMed]
- Lai C, Robinson J, Clark S, Stamp G, Poulsom R, Silver A. Elevation of WNT5A expression in polyp formation in Lkb1+/- mice and Peutz-Jeghers syndrome. J Pathol. 2011 Apr;223(5):584-92. Epub 2011 Feb 21. [CrossRef] [PubMed]
- Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis. 2013 Nov;34(11):2629-38. Epub 2013 Jun 12. [CrossRef] [PubMed]
- Dong X, Liao W, Zhang L, Tu X, Hu J, Chen T, Dai X, Xiong Y, Liang W, Ding C, Liu R, Dai J, Wang O, Lu L, Lu X. RSPO2 suppresses colorectal cancer metastasis by counteracting the Wnt5a/Fzd7-driven noncanonical Wnt pathway. Cancer Lett. 2017 Aug 28;402:153-165. Epub 2017 Jun 6. [CrossRef] [PubMed]
- Chen Z, Tang C, Zhu Y, Xie M, He D, Pan Q, Zhang P, Hua D, Wang T, Jin L, Qi X, Zhu Y, Yao X, Jin J, Ma X. TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer. Clin Sci (Lond). 2017 Feb 1;131(3):227-237. Epub 2016 Nov 28. [CrossRef] [PubMed]
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021 Oct;21(10):653-667. [CrossRef]
- Jridi I, Canté-Barrett K, Pike-Overzet K, Staal FJT. Inflammation and Wnt Signaling: Target for Immunomodulatory Therapy? Front Cell Dev Biol. 2021 Feb 4;8:615131. [CrossRef]
- Cui G. TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front Oncol. 2019 Oct 4;9:1002. [CrossRef]
- Liu JL, Yang M, Bai JG, Liu Z, Wang XS. "Cold" colorectal cancer faces a bottleneck in immunotherapy. World J Gastrointest Oncol. 2023 Feb 15;15(2):240-250. [CrossRef]
| Patients with FAP polyps | ||||||
|---|---|---|---|---|---|---|
| Case | age | sex | Phenotype | Site and size of polyps | Dysplasia (L or H) |
n.of polyps |
| 5FI | 25 | F | Adenomatous | Diffuse or “carpet”, <1cm | HGD | 1060 |
| 6FI a,e | 58 | M | Adenomatous | Diffuse | HGD | 25 |
| 7FI a,e | 28 | F | Adenomatous | Diffuse | HGD | 375 |
| 8FI b,e | 18 | F | Adenomatous (Tubular-villous) |
Diffuse | HGD | 415 |
| 9FI c,e | 15 | F | Adenomatous | Diffuse | LGD | 375 |
| 16FI | n.a | F | Adenomatous | Diffuse | LGD | n.a |
| 25FI | n.a. | M | Adenomatous | Diffuse | LGD | n.a. |
| 26FI | 46 | F | Adenomatous | Diffuse | LGD | 835 |
| 31FI | M | Adenomatous | Diffuse | |||
| 33FI | 49 | F | Adenomatous | Diffuse | LGD | 97 |
| 35FI c,e | 31 | M | Adenomatous | Diffuse | LGD | 550 |
| 36FI | 42 | F | Adenomatous | Diffuse | LGD | 250 |
| 39FI | 42 | M | Adenomatous | Diffuse | LGD | 430 |
| 40FI a,d,e | 61 | F | Adenomatous | Diffuse | HGD | 730 |
| 41FI | 49 | M | Adenomatous | Diffuse | LGD | 1025 |
| 42FI | 42 | F | Adenomatous and amartomatous | Diffuse | LGD | 210 |
| 43FI | 36 | M | Adenomatous | Diffuse | LGD? | n.a |
| Patients with sporadic polyps | ||||||
| case | age | sex | Phenotype | Site and size of polyps |
Dysplasia (L or H) |
Morphology |
| 1CH | 50 | M | Hyperplastic | Sigma, 6mm | LGD | Spl |
| 2CH | 67 | M | Tubular | Sigma, 10mm | LGD | Spl |
| 3CH | 49 | M | Hyperplastic | Sigma, 4mm | LGD | Spl |
| 9CH | 47 | M | Tubular-villous | Retto, 15mm | LGD | Ppl |
| 11CH | 57 | F | Hyperplastic-adenomatous | Descending, 4mm |
LGD | Spl |
| 13CH | 83 | F | Tubular | Descending, 15mm | LGD | Spl |
| 15CH | 37 | F | Villous | Sigma, 50mm |
HGD | Ppl |
| 16CH | 60 | M | Tubular | Cecum, 15mm |
LGD | Ppl |
| 17CH | 66 | M | Tubular-villous | Sigma, 15mm | LGD | Ppl |
| 18CH | 64 | M | Tubular-villous | Descending, 8mm | LGD | Spl |
| 21CH | 78 | M | Tubular-villous | Sigma, 10mm |
LGD | Spl |
| 22CH | 67 | M | Tubular-villous | Rectum, 10mm |
LGD | Spl |
| 23CH | 68 | F | Tubular-villous | Sigma, 10mm |
LGD | Ppl |
| 24CH | 59 | M | Tubular-villous | Ascending | LGD | Ppl |
| 25CH | 77 | M | Tubular-villous | Descending | HGD | Ppl |
| 26CH | 69 | M | Tubular | Splenic flexure, 10mm | LGD | Spl |
| 27CH | 61 | F | Tubular-villous | Sigma, 15mm | LGD | Ppl |
| 28CH | 77 | M | Tubular-villous | Hepatic flexure, 5mm | LGD | Spl |
| 29CH | 47 | M | Hyperplastic-adenomatous | Descending, 20mm | Not atypical | Ppl |
| 30CH | 53 | M | Hyperplastic-adenomatous | Retto-sigma, 7mm |
Not atypical | Spl |
| 31CH | 76 | M | Tubular | Ascending, 5mm | LGD | Spl |
| 32CH | 51 | M | Tubular-villous | Ascending, 45mm | LGD | Spl |
| 33CH | 68 | F | Tubular-villous | Colon, 40mm |
LGD | LST-G |
| 34CH | 67 | M | Tubular | Colon sx, 7mm |
LGD | Ppl |
| Wnt3a | BCL9 | |||||
|---|---|---|---|---|---|---|
| Group |
Spearman’s rho (2-tailed) |
P-value |
Spearman’s rho (2-tailed) |
P-value | ||
| Polyps and adjacent mucosa | n. 42 | |||||
| Wnt3a | 0,336376 | 0,0294 | ||||
| BCL9 | 0,336376 | 0,0294 | ||||
| Wnt5a | LEF1 | |||||
| Group |
Spearman’s rho (2-tailed) |
P-value |
Spearman’s rho (2-tailed) |
P-value | ||
| Normal colonic mucosa | n. 10 | |||||
| Wnt5a | 0,69697 | 0,025097 | ||||
| LEF1 | 0,69697 | 0,025097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





