1. Introduction
Q fever is endemic zoonosis with global distribution, caused by intracellular bacterium
Coxiella burnetii and with great human and animal health significance [
1,
2,
3]. The last few years, the highest numbers of confirmed cases of Q fever in Europe were reported in Spain, France, Germany, Romania, Hungary and Bulgaria [
4,
5,
6,
7,
8,
9]. The primary source of infection to humans are domestic ruminants, such as goats, cattle, sheep, as well as pets, in which the infection is usually asymptomatic, but it could be also associated with infertility, abortions and premature birth [
10,
11,
12,
13]. The clinical manifestations of Q fever in human varies from non-specific, self-limiting disease to an acute form (fever of unknown origin, atypical pneumonia, granulomatous hepatitis) [
14,
15,
16] or chronic presentation (endocarditis, encephalopathy, etc.) [
17,
18]. Laboratory diagnosis of acute cases is based on: (i) isolation and cultivation, which requires BSL3 facilities for tissue cultures [
19]; (ii) serological tests, such as indirect immunofluorescence (IFA), recognized as the gold standard and enzyme-linked immunosorbent assay (ELISA) [
20,
21]; (iii) antigen detection assay such as immunohistochemical staining (IHC) [
22]; and (iv) molecular detection of
C. burnetii DNA by different PCR techniques for early diagnosis of acute phase [
23,
24]. The anti-
C. burnetii antibodies response is detectable after 2-3 weeks following infection. The level of anti-
C. burnetii IgM phase II increases rapidly after infection and are detectable for several months as a marker of recent infection. The presence of specific IgG phase II provides evidence of a recent
C. burnetii infection or a past exposure and can remain detectable for years [
25,
26].
Due to the fact that
C. burnetii is environmentally stable and can be transmitted from animal hosts to humans mainly via inhalation of contaminated aerosols or dust particles [
27,
28], the most vulnerable group are people who are in the higher occupational risk of exposure and daily contact with farm animals and pets [
29,
30,
31]. Veterinary medicine students remain at high risk of being infected with
C. burnetii during sixth-year veterinary curriculum, since this period from their training cover animal handling and treatment a wide variety of species [
32]. According to the data of scientific literature, few serological studies have reported on the seroprevalence for
C. burnetii among veterinary medicine students, [
33,
34,
35]. Only few studies have assessed zoonotic risks for veterinary medicine students [
36,
37,
38].
Q fever is considered endemic in Bulgaria, however, due to the nonspecific and pleomorphic clinical presentation of the C. burnetii infection it often remains neglected and underdiagnosed. Thus, little is known about seroprevalence of Q fever among various professional groups, occupationally exposed to infection with C. burnetii, including veterinary medicine students, because of their close contact with infected livestock and pets.
Therefore, the aim of the present study was to assess the prevalence of C. burnetii infection among veterinary medicine students at two Veterinary Medicine Faculties (Sofia and Stara Zagora), located in western and central part of the country. To the best of our knowledge, this is the first evidence of C. burnetii infection in veterinary medicine students from Bulgaria.
2. Materials and Methods
2.1. Target Population and Sample Collection
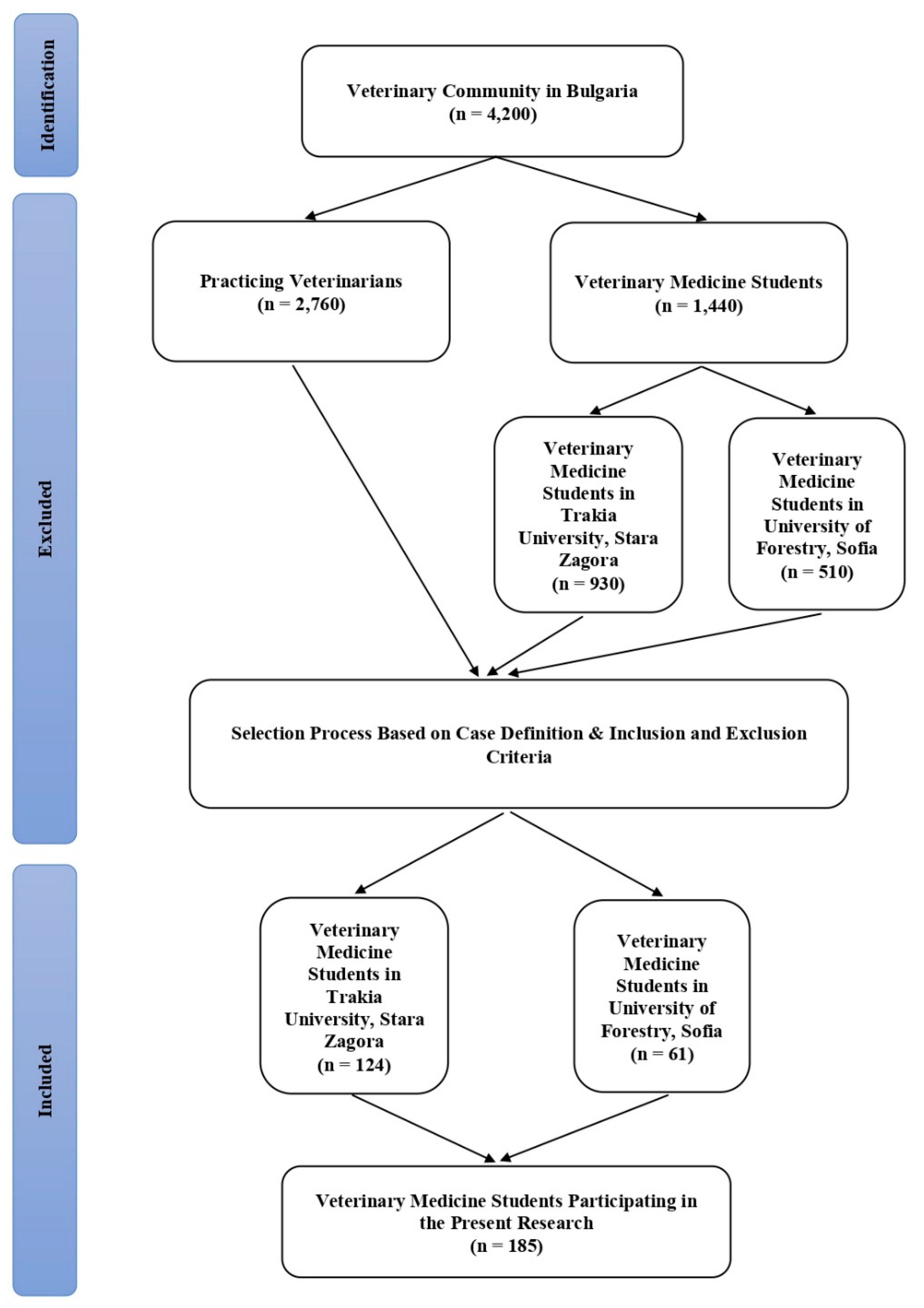
In the present study, blood samples were taken from a total of 185 veterinary medicine students, in the 5th year of study during the academic year 2022–2023 from the two Veterinary Faculties at the University of Forestry in Sofia and Trakia University in Stara Zagora, Bulgaria, respectively. Participants in the current research were selected based on case definition as well as the inclusion and exclusion criteria (
Figure 1).
The students, included in the study have to meet the following criteria: (a) age > 18 years; (b) persons, studying in a specialty “Veterinary Medicine” in the last but one year of study; (c) participants, in the end of their curriculum, which are in close contact with farm animal and pets during practical training; (d) obtained written informed consent from each student to participate in a scientific survey; (e) female participants, who did not report a possible pregnancy at the moment of the survey. The exclusion criteria for the current research include: (a) individuals younger than 18 years; (b) persons from other university specialties (students of specialties, such as mathematics, engineering, finance and economics, law, human sciences, etc; (c) individuals with contraindications for venepuncture; (d) persons who refused to give written informed consent; (e) participant with Q fever vaccination history; (f) persons with a severe clinical form of an acute or chronic disease.
The samples from students were taken during routine diagnostic tests following standard procedure. After obtaining a written consent and completing a questionnaire, students were invited to donate a blood sample of 10 ml by venepuncture for testing. Blood samples were delivered to the National Reference Laboratory (NRL) “Rickettsia and cell cultures”, Department of Virology, National Centre of Infectious and Parasitic Diseases, Sofia (Bulgaria). Sera were separated by centrifugation at 1500 rpm for 10 min, transferred into sterile vacuum tubes and stored at −20°C until processed. Whole blood samples (200µL), collected in EDTA tubes were used for DNA extraction.
2.2. Case Definition
The presence of anti-C. burnetii phase II IgG antibody positivity was assumed to indicate a possibly acute or a history of past C. burnetii infection, i.e. previous encounter with this pathogen. Acute phase of C. burnetii infection was defined when one of the following criteria were present of: (a) a 4-fold increase of the IgG phase II antibodies titer to C. burnetii between paired serum samples as evidence by ELISA; (b) positive ELISA result for IgM antibodies against phase II antigens of C. burnetii; (c) detection of the presence of C. burnetii DNA in EDTA blood specimens via amplification of a specific target by PCR assay, < 21 days of onset of disease; (d) a negative blood PCR result but a positive ELISA result for IgM and IgG against phase II antigens of C. burnetii.
2.3. Serological Tests
The serum samples were examined for the presence of anti-
C. burnetii phase II IgM and IgG antibodies using a commercial ELISA kit (EUROIMMUN, Lübeck, Germany) according to the manufacture`s recommendations. The absorbance values were determined at 450 nm (reference wavelength of 620–690 nm) using an Epoch ELISA reader (BioTek Instruments, Inc., VT, USA), on the Gen5TM data analysis software (BioTek Instruments, Inc., VT, USA). Optical density (OD), cut-off values and controls were checked. Results were evaluated semiquantitatively by calculating a ratio (R) of the extinction value of the patient sample over the extinction value of the calibrator. The results were categorised as follow: R ≥ 1.1 was taken positive and indicating a possibly acute or evidence for past infection; R< 0.8 considered as negative and R ≥ 0.8 to < 1.1 indicated as suspicious (equivocal) [
39].
2.4. PCR Assay
Whole blood samples were tested for the presence/absence of
C. burnetii DNA using end-point PCR by specific primers. Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer's instruction. DNA was eluted with forty microliters of elution buffer and stored at 4°C until use. The primers were designed to amplify a 687-bp fragment of the transposon-like repetitive region of the bacterial genome (
IS1111) of
C. burnetii, using Trans-1 and Trans-2 primers with the following sequence: Trans-1 (5’-TAT GTA TCC ACC GTA GCCAGT C-3’) and Tans-2 (5’-CCC AAC AACACC TCC TTATTC-3’) (Metabion, Germany) [
40]. The Trans-PCR was carried out as described [
41]. The resulting amplicons were electrophoresed on 1.5% agarose gel and visualized under UV light [
42].
2.5. Statistical Analysis
Data analysis was performed with the help of SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) and Excel 2016 (Microsoft, Redmond, WA, USA). Data were entered and arranged in MS Excel. The risk exposure of C. burnetii among different veterinary student groups, categorized by sex and location, was calculated. Statistical analysis was performed to compare the relative risks (RR) as proportion of exposed over unexposed students. A Chi-squared test was utilized for this comparison, and a P-value < 0.05 was considered statistically significant. The final results obtained from the statistical analysis were confirmed by all scientists involved in this study.
2.6. Ethical Considerations
All veterinary medicine students signed a written informed consent to participate in scientific research. Participants gave written informed consent for the invasive procedures conducted on them (venepuncture). Every student had access to their laboratory result. Scientists participating in this research conducted their activities according to the ethical principles of Declaration of Helsinki (adopted in June 1964, last revision in October 2013). The current survey was approved by the Local Ethics Committee of National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria (NCIPD-02/07 February 2023), who confirmed that the research was in full accordance with all ethical principles and practices. The authors have not used artificial intelligence (AI) to create this manuscript.
3. Results
3.1. Characterization of Study Population
A two-step approach was used to discriminate acute from past C. burnetii infection. At first, all serum samples were screened by a commercial ELISA kit for the presence of anti-C. burnetii phase II IgM and IgG antibodies. Then the positive, were analysed by end-point PCR assay, targeting IS1111 transposable element, which was routinely used marker in the epidemiological investigation of C. burnetii infections.
3.2. Serology Analysis
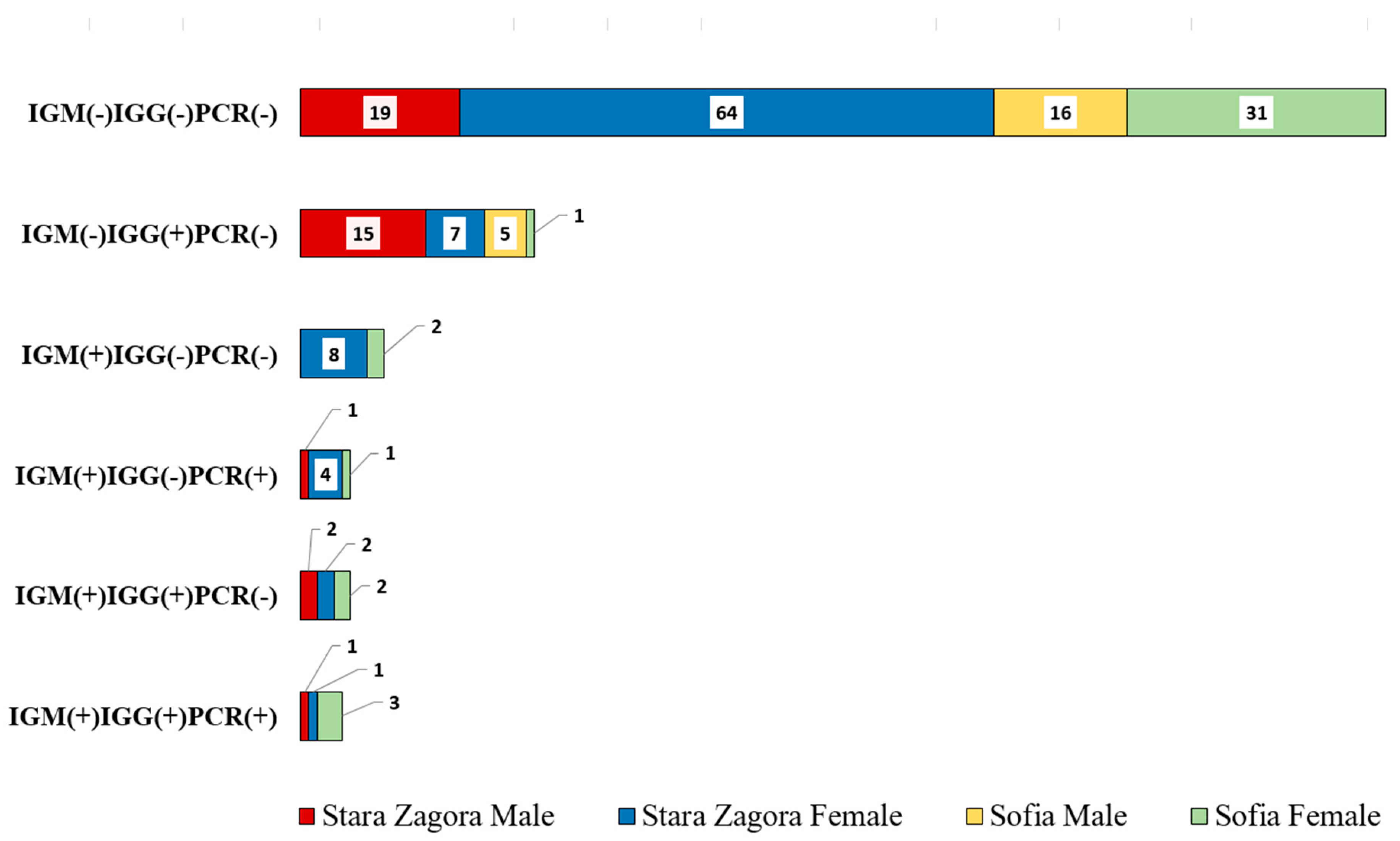
The result in
Figure 2 showed the distribution of persons with various immune responses and PCR results across four groups: Stara Zagora Male, Stara Zagora Female, Sofia Male, and Sofia Female. The categories include combinations of IgM, IgG, and PCR results, indicating different stages and types of exposure or infection with
C. burnetii. Out of all 185 serum samples studied, 55 (29.7%) were positive for at least one marker anti-
C. burnetii antibody for phase II or presence of
C. burnetii DNA (Chi-squared (1) = 27.88; P < 0.001). Veterinary students from Stara Zagora showed significantly more frequent seropositive status of Q fever, than those who studied in capital city of Sofia, (42 out of 125 (33.6%) vs 14 out of 61 (23%), Chi-squared (1) = 2.22; P < 0.05). By sex, 24 out of 59 (40.7%) of male samples were positive, whereas 31 out of 126 (24.6%) of female samples were positive (Chi square (1) = 4.00, P < 0.05).
Among all tested veterinary students, who provided a serum samples, 28 (14.6%) were seropositive for anti-C. burnetii phase II IgM antibody and therefore had evidence for recent exposition. Of those, 4 (14.8%) were male and 23 (85.2%) were female (Chi square (1) = 3.98, P < 0.05). Eight of 61 serum samples (13.1%) from veterinary students in Sofia were positive to phase II IgM antibodies. Seroprevalence among students in Stara Zagora was 15.3% (19/126) (Chi square (1) = 0.14, P = 0.71).
Presence of anti-C. burnetii IgG antibody phase II was detected in 40 out of 185 (21.6%) examined serum sample. The seroprevalence for phase II antibodies to IgG reached in our group of subjects were 14.8% and 25%, respectively for University of Forestry and Trakia University, however this observation does not reach statistical significance (Chi square (1) = 2.44, P > 0.1). It should be noted that the frequency of positive serum titer for Q fever (phase II) was greater in man than women, Chi square (1) = 6.84, P < 0.01). Moreover, incidences of seropositive for IgG male subjects were almost as twice as much in males (39%) then in females (13.5%) (Chi square (1) = 6.84, P < 0.01). Of the 10 patients with suspected acute Q fever infection (presence of both class phase II anti-C. burnetii IgM/IgG(+) antibodies), the proportion of women was higher than that of man.
3.3. Molecular Analysis
In the current study, EDTA blood samples were further screened by end-point PCR assay for detection of C. burnetii DNA. End-point PCR assay detected as positive at 687bp by Trans-1 and Trans-2 primers 11 of 185 (5.9%) blood samples. In all, 7.1% of the females and 3.4% of the males tested positive.
3.4. Relative Risk Assessment Q Fever Exposure
The relative risk (RR) of Q fever exposure among veterinary students was assessed by comparing the prevalence of anti-
C. burnetii antibodies between different groups, specifically focusing on sex and University affiliation (
Table 1). For clarity all cases showed at least one positive test marker (IgG, IgM or PCR) were pooled together and designated as “exposed” and were compared to frequency of negative cases (“unexposed“). The relative risk of Q fever exposure for students in Stara Zagora was 33.1% and 23% in Sofia, respectively. The relative risk (RR) of Q fever exposure for male students was 40.7%, whereas for female students, the RR was 24.6%. Location-by-sex RR analysis showed that the relative risk of Q fever exposure for male students in Stara Zagora is 50%, followed by female students in Stara Zagora (25.6%). Male veterinary students in Sofia had an RR of 23.8%, and female students in Sofia had an RR of 22.5%.
4. Discussion
Q fever is among the most common zoonotic diseases and even information for some countries is limited, available data showed a high prevalence of infection, particularly amongst the people, professionally involved in animal husbandry. The results of our study are in agreement with similar data reported by other authors from Europe. In 2000, Valencia et al. reported 11.02%
C. burnetii seroprevalence in veterinary students from Zaragoza, Spain [
38]. Turkish authors found 7.0% and 8.0%
C. burnetii antibodies among 83 veterinarians from two distinct geographic regions [
44]. German scientists detected 38.0% positive sera for
C. burnetii phase II IgG antibodies among 424 veterinarians [
45]. In 2012, de Rooij et al. reported on 18.7% IgG antibodies against
C. burnetii in Dutch veterinary medicine students [
36]. One year later, another Dutch scientific team found 65.1% antibodies against
C. burnetii among 189 livestock veterinarians [
46]. Similar data are reported also by other authors [
47,
48,
49]. In short, our results and data from other authors from Europe show a moderate and high level of
C. burnetii seropositivity among risk groups (persons in contact with animals; predominantly veterinarians).
To assess the importance of
C. burnetii infection among risk groups (veterinarians, hunters, etc.), it is recommended to make a comparison with seropositivity in the general population. Tiscione et al. reported lower levels of
C. burnetii antibodies among an urban group with no potential animal contact (6.1%) compared to the higher levels, found in a rural group with higher potential exposure to animals (49.1%) [
50]. A few years later, Spanish authors found 3.0% positive serum samples for Q fever in healthy population from Lanzarote, Canary Islands [
51]. Irish scientists detected 12.8% positive results for
C. burnetii among 2,394 randomly selected subjects (humans) from the northern part of the country [
52]. By testing plasma samples obtained from individuals living in Southern Netherlands, Brandwagt et al [
53] reported significant dynamics in Q fever seroprevalence levels, ranging from 62.5%, as detected in 1983 to 14.4% in 1987, and decreasing to 1% in 2008 and 2.3% in 2010, respectively. Similar data are reported also by other authors [
54,
55,
56,
57]. All this clearly showed that there is a lower seropositivity for
C. burnetii infection in the general population compared to the risk groups. This confirms the role of
C. burnetii as an occupational disease pathogen and highlights the need for more detailed epidemiological investigations of infection amongst them, especially.
In our survey, high prevalence rates of antibodies against C. burnetii (30.8%) among veterinary medicine students, were found. The potential reasons for this fact can be many and of a different nature. As a first, Q fever is endemic to Bulgaria, and high environmental contamination can be expected due to the wide prevalence of C. burnetii infection found amongst ruminant herds/flocks in the country (Simeonov K, unpublished data). In addition, C. burnetii infection is easily acquired by inhaling of contaminated aerosol, which facilitates the transmission to people and the spread of the bacterium. The lack of protection skills in veterinary medicine students also increases the risk of infection when working with animals.
The elevated seroprevalence of anti-C. burnetii IgM (+) phase II antibodies among students in Trakia University suggests a direct correlation with their frequent contact with ruminants. The practical courses that involve hands-on livestock handling and farming activities contribute to increased exposure to C. burnetii. This cohort of students exhibited the highest seropositivity for anti-C. burnetii IgG (+) phase II antibodies. The curriculum at Trakia University includes a number of practical lessons involving farm animals, which lead to increased exposure to C. burnetii and a higher risk of infection. In contrast, practical training at the University of Forestry is focused on the pet animals, which represent a lower epidemiological risk. This may explain to some extent the smaller number of students from this University, who had contact with the infection.
Our study revealed a higher seropositivity rate among male students as compared to the female ones, with 32.8% of males testing positive for anti-
C. burnetii phase II IgG antibodies versus 16.0% of females. This sex disparity is in agreement with our previous observations [
9] and suggests that male students might have more intense or prolonged exposure to risk factors during their practical training.
The detection of C. burnetii DNA in some blood samples implies that certain infections were in the incubation period or early acute phase. These findings are critical for early diagnosis and prompt treatment to prevent the progression of Q fever.
The study emphasizes the need for improved safety protocols and infection control measures in veterinary training programs. Educating students about the risks and implementing strategies to reduce exposure to C. burnetii are essential steps in safeguarding their health.
The present study has some limitations that need to be addressed. First, the research involved a small number of participants. Second, we present results from the serological and PCR assays but do not have data on the associated risk factors. Third, study participants did not have clinical signs and symptoms of Q fever, i.e. all students were clinically healthy and laboratory and imaging studies were not performed. Despite these limitations, this research has its merits. To the best of our knowledge, this is the first study that presents serological evidence of C. burnetii infection among veterinary medicine students from Bulgaria. In addition, we performed PCR analysis of the studied serum samples. These are important contributions to the knowledge of this infection in our country and the region of Southeastern Europe (Balkan peninsula).
5. Conclusions
In the present study, we presented data on the prevalence of C. burnetii infection among a risk group (veterinary medicine students) from Bulgaria. We found high prevalence rates of this pathogen (30.8%) among study participants. This shows that veterinarians in our country are exposed to an increased risk of this infection, which can occur as an acute disease or a chronic infection. All this requires the national health authorities to take adequate measures for the control and surveillance of Q fever among risk groups. In this direction, an important step is to increase the awareness of the veterinary community in our country regarding the measures to prevent Q fever.
Author Contributions
Conceptualization, P.G.K., I.T. and M.B.; methodology, P.G.K. and M.B.; formal analysis, P.G.K. and M.B.; investigation, P.G.K., I.T., R.P., B.V., M.B. and P.E.F.; resources, I.T., R.P., B.B.M. and B.C.; data curation, P.G.K., V.D., Y.H., K.T., S.K., K.S. and M.B.; statistical analysis, Y.H.; writing – original draft preparation, P.G.K. and M.B.; writing – review and editing, P.G.K., I.T., M.B., S.P. and P.E.F.; visualization, P.G.K., Y.H. and M.B.; supervision, P.G.K., I.T., M.B. and P.E.F.; project administration, P.G.K. and I.T.; funding acquisition, I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bulgarian National Science Fund under Grant number KP-06-N33/3, from 13 December 2019, entitled: “Molecular-genetic identification and creation of an archive genomic bank of the circulating human and animal C. burnetii genotypes and determination of their role as particularly dangerous infectious agents causing epidemiological outbreaks on the territory of Bulgaria”. The Article Publishing Charge (APC) was funded by the Bulgarian Ministry of Education and Science in the frames of the Bulgarian National Recovery and Resilience Plan, Component “Innovative Bulgaria”, Project No. BG-RRP-2.004- 0006-C02 “Development of research and innovation at Trakia University in service of health and sustainable well-being”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee of National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria (NCIPD-02 / 07 February 2023). The authors have not used artificial intelligence (AI) to create this manuscript.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Acknowledgments
Part of the work on this article was supported by the Bulgarian National Science Fund under Grant number KP-06-N33/3, from 13 December 2019. We are grateful to all veterinary medicine students who participated in this study. Furthermore, we thank our families for providing us with the time and support required to complete this survey in a timely manner.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Q.; Jamil, T.; Saqib, M.; Iqbal, M.; Neubauer, H. Q fever – A neglected zoonosis. Microorganisms 2022, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Zendoia, I.I.; Alonso, E.; Beraza, X.; Bidaurrazaga, J.; Ocabo, B.; Arrazola, I.; Cevidanes, A.; Barandika, J.F.; Garcia-Perez, A.L. A Q fever outbreak among visitors to a natural cave, Bizkaia, Spain, December 2020 to October 2021. Euro Surveill. 2023, 28, 2200824. [Google Scholar] [CrossRef] [PubMed]
- Melenotte, C.; Protopopescu, C.; Million, M.; Edouard, S.; Carrieri, M.P.; Eldin, C.; Angelakis, E.; Djossou, F.; Bardin, N.; Fournier, P.E.; et al. Clinical features and complications of Coxiella burnetii infections from the French national reference center for Q Fever. JAMA Netw. Open. 2018, 1, e181580. [Google Scholar] [CrossRef] [PubMed]
- Winter, F.; Campe, A. Q fever expertise among human and veterinary health professionals in Germany – A stakeholder analysis of knowledge gaps. PLoS One 2022, 17, e0264629. [Google Scholar] [CrossRef] [PubMed]
- Negru, A.R.; Popescu, C.; Radulescu, M.; Lobodan, A.; Lapadat, I.; Gliga, S.; Dulama, R.; Cristea, D.; Arama, V. Coxiella burnetii endocarditis – A real threat in the context of Q fever re-emergence. BMC Infect. Dis. 2014, 14 (Suppl. S7), P32. [Google Scholar] [CrossRef]
- Sulyok, K.M.; Kreizinger, Z.; Hornstra, H.M.; Pearson, T.; Szigeti, A.; Dan, A.; Balla, E.; Keim, P.S.; Gyuranecz, M. Genotyping of Coxiella burnetii from domestic ruminants and human in Hungary: Indication of various genotypes. BMC Vet. Res. 2014, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Genova-Kalou, P.; Vladimirova, N.; Stoitsova, S.; Krumova, S.; Kurchatova, A.; Kantardjiev, T. Q fever in Bulgaria: Laboratory and epidemiological findings on human cases and outbreaks, 2011 to 2017. Euro Surveill. 2019, 24, 1900119. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Seegers, H.; Taurel, A.F.; Joly, A.; Beaudeau, F. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Vet. Microbiol. 2011, 149, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A. Q fever, state of art: Epidemiology, diagnosis and prophylaxis. Small Ruminant Res. 2006, 62, 121–124. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; Zaher, H.M. Parturient cat as a potential reservoir for Coxiella burnetii: A hidden threat to pet owners. Vector Borne Zoonotic Dis. 2021, 21, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S. Coxiella burnetii associated reproductive disorders in domestic animals – A critical review. Acta Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.R.; Barralet, J.H.; Bell, A.M. Q fever. Lancet 2006, 367, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J. Coxiella burnetii (Q fever) pneumonia. Clin. Infect. Dis. 1995, 21 (Suppl 3), S253–S264. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Fournier, P.E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 2001, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Bernit, E.; Pouget, J.; Janbon, F.; Dutronc, H.; Martinez, P.; Brouqui, P.; Raoult, D. Neurological involvement in acute Q fever: A report of 29 cases and review of the literature. Arch. Intern. Med. 2002, 162, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Mertens, K.; Cutler, S.J.; Santos, A.S. Critical aspects for detection of Coxiella burnetii. Vector Borne Zoonotic Dis. 2017, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Meekelenkamp, J.C.; Schneeberger, P.M.; Wever, P.C.; Leenders, A.C. Comparison of ELISA and indirect immunofluorescent antibody assay detecting Coxiella burnetii IgM phase II for the diagnosis of acute Q fever. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.; Gerlach, C.; Neubauer, H.; Henning, K. Q fever – An update. Curr. Clin. Microbiol. Rep. 2017, 4, 61–70. [Google Scholar] [CrossRef]
- Lepidi, H.; Houpikian, P.; Liang, Z.; Raoult, D. Cardiac valves in patients with Q fever endocarditis: Microbiological, molecular, and histologic studies. J. Infect. Dis. 2003, 187, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Rawool, D.B.; Vinod, V.K.; Malik, S.V.S.; Barbuddhe, S.B. Current approaches for the detection of Coxiella burnetii infection in humans and animals. J. Microbiol. Methods 2020, 179, 106087. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever – United States, 2013: Recommendations from CDC and the Q fever working group. MMWR Recomm. Rep. 2013, 62, 1–30, Erratum in: MMWR Recomm. Rep. 2013, 62, 730. [Google Scholar] [PubMed]
- Dupuis, G.; Peter, O.; Peacock, M.; Burgdorfer, W.; Haller, E. Immunoglobulin responses in acute Q fever. J. Clin. Microbiol. 1985, 22, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Marmion, B.P.; Storm, P.A.; Lockhart, M.; Turra, M.; Graves, S. Long-term persistence after acute Q fever of non-infective Coxiella burnetii cell components, including antigens. QJM 2010, 103, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Marrie, T.; Mege, J. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- McQuiston, J.H.; Childs, J.E. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2002, 2, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Groten, T.; Kuenzer, K.; Moog, U.; Hermann, B.; Maier, K.; Boden, K. Who is at risk of occupational Q fever: New insights from a multi-profession cross-sectional study. BMJ Open 2020, 10, e030088. [Google Scholar] [CrossRef] [PubMed]
- Whitney, E.A.; Massung, R.F.; Candee, A.J.; Ailes, E.C.; Myers, L.M.; Patterson, N.E.; Berkelman, R.L. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin. Infect. Dis. 2009, 48, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Palkhade, R.; Mishra, S.; Barbuddhe, S. Occupation-related biological health hazards and infection control practices among Indian veterinarians. Vet. Med. Int. 2022, 2022, 2503399. [Google Scholar] [CrossRef] [PubMed]
- Conan, A.; Gallagher, C.A.; Erskine, N.; Howland, M.; Smith-Anthony, M.; Marchi, S.; Magouras, I.; Muller, A.; Becker, A.A.M.J. Is there a higher risk of exposure to Coxiella burnetii for pre-clinical veterinary students? One Health 2023, 16, 100485. [Google Scholar] [CrossRef] [PubMed]
- Riemann, H.P.; Brant, P.C.; Franti, C.E.; Reis, R.; Buchanan, A.M.; Stormont, C.; Behymer, D.E. Antibodies to Toxoplasma gondii and Coxiella burnetii among students and other personnel in veterinary colleges in California and Brazil. Am. J. Epidemiol. 1974, 100, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Dorko, E.; Kalinova, Z.; Pilipcinec, E. Seroprevalence of Coxiella burnetii antibodies among students of the Faculty of Medicine in Kosice (Slovakia). Folia Microbiol. (Praha) 2008, 53, 563–568. [Google Scholar] [CrossRef] [PubMed]
- de Lange, M.M.A.; van der Hoek, W.; Schneeberger, P.M.; Swart, A.; Heederik, D.J.J.; Schimmer, B.; Wouters, I.M. High Coxiella burnetii seroconversion rate in veterinary students, the Netherlands, 2006-2010. Emerg. Infect. Dis. 2020, 26, 3086–3088. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.M.; Schimmer, B.; Versteeg, B.; Schneeberger, P.; Berends, B.R.; Heederik, D.; van der Hoek, W.; Wouters, I.M. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS One 2012, 7, e32108. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Qorbani, A.; Sharifi, H.; Golchin, M. Prevalence and risk factor of Q fever among veterinary students in Iran. Trop. Biomed. 2015, 32, 704–709. [Google Scholar] [PubMed]
- Valencia, M.C.; Rodriguez, C.O.; Punet, O.G.; de Blas Giral, I. Q fever seroprevalence and associated risk factors among students from the Veterinary School of Zaragoza, Spain. Eur. J. Epidemiol. 2000, 16, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Bautista, J.F.; Tarrino, M.; Gonzalez, A.; Olivares Duran, M.J.; Cobo, F.; Reguera, J.A.; Rodriguez-Granger, J.; Sampedro, A. Comparison of an enzyme linked-immunosorbent assay and a chemiluminescent immunoassay with an immunofluorescence assay for detection of phase II IgM and IgG antibodies to Coxiella burnetii. Microorganisms 2024, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Hoover, T.A.; Vodkin, M.H.; Williams, J.C. A Coxiella burnetii repeated DNA element resembling a bacterial insertion sequence. J. Bacteriol. 1992, 174, 5540–5548. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Laroucau, K.; Rodolakis, A. The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Vet. Microbiol. 2000, 72, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Naderi, H.R.; Salehnia, N.; Abiri, Z. Detection of Coxiella burnetii in acute undifferentiated febrile illnesses (AUFIs) in Iran. Trop. Doct. 2016, 46, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Lobato, L.; Bethony, J.M.; Pereira, F.B.; Grahek, S.L.; Diemert, D.; Gazzinelli, M.F. Impact of gender on the decision to participate in a clinical trial: A cross-sectional study. BMC Public Health 2014, 14, 1156. [Google Scholar] [CrossRef] [PubMed]
- Ergonul, O.; Zeller, H.; Kilic, S.; Kutlu, S.; Kutlu, M.; Cavusoglu, S.; Esen, B.; Dokuzoguz, B. Zoonotic infections among veterinarians in Turkey: Crimean-Congo hemorrhagic fever and beyond. Int. J. Infect. Dis. 2006, 10, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.; Brockmann, S.O.; Kleinkauf, N.; Klinc, C.; Wagner-Wiening, C.; Stark, K.; Jansen, A. High seroprevalence of Coxiella burnetii antibodies in veterinarians associated with cattle obstetrics, Bavaria, 2009. Vector Borne Zoonotic Dis. 2012, 12, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Schimmer, B.; Schneeberger, P.M.; Swart, W.A.; van der Hoek, W.; Vellema, P. Seroepidemiological survey for Coxiella burnetii antibodies and associated risk factors in Dutch livestock veterinarians. PLoS One 2013, 8, e54021. [Google Scholar] [CrossRef] [PubMed]
- Fenga, C.; Gangemi, S.; De Luca, A.; Calimeri, S.; Lo Giudice, D.; Pugliese, M.; Licitra, F.; Alibrandi, A.; Costa, C. Seroprevalence and occupational risk survey for Coxiella burnetii among exposed workers in Sicily, Southern Italy. Int. J. Occup. Med. Environ. Health 2015, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.G.; Vesco, G.; Villari, S.; Galluzzo, P.; Gargano, V.; Matranga, D.; De Marchis, P.; Picciotto, D. Analysis of seroprevalence against Coxiella burnetii in a sample of farm workers in Western Sicily. Ann. Agric. Environ. Med. 2016, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzo, F.; Martinelle, L.; Leonard, P.; Renaville, B.; Renaville, R.; Thys, C.; Smeets, F.; Czaplicki, G.; Van Esbroeck, M.; Saegerman, C. Q fever serological survey and associated risk factors in veterinarians, Southern Belgium, 2013. Transbound. Emerg. Dis. 2017, 64, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Tiscione, E.; Ademollo, B.; Donato, R.; Roller, S.; Signorini, L.F. Prevalence of antibodies against Coxiella burnetii in 2 geographical zones of Tuscany [Article in Italian]. Ann. Ig. 1989, 1, 1133–1143. [Google Scholar] [PubMed]
- Pascual Velasco, F.; Otero Ferrio, I.; Borobio Enciso, M.V. Prevalence of antibodies against Coxiella burnetii in a healthy population in Lanzarote (Canary Islands) [Article in Spanish]. An. Med. Interna. 1991, 8, 233–234. [Google Scholar] [PubMed]
- McCaughey, C.; McKenna, J.; McKenna, C.; Coyle, P.V.; O'Neill, H.J.; Wyatt, D.E.; Smyth, B.; Murray, L.J. Human seroprevalence to Coxiella burnetii (Q fever) in Northern Ireland. Zoonoses Public Health 2008, 55, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Brandwagt, D.A.; Herremans, T.; Schneeberger, P.M.; Hackert, V.H.; Hoebe, C.J.; Paget, J.; van Der Hoek, W. Waning population immunity prior to a large Q fever epidemic in the South of the Netherlands. Epidemiol. Infect. 2016, 144, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Lopez, J.; Acosta-Jamett, G.; Edouard, S.; Parola, P.; Abarca, K. Absence of convincing evidence of Coxiella burnetii infection in Chile: A cross-sectional serosurvey among healthy adults in four different regions. BMC Infect. Dis. 2016, 16, 541. [Google Scholar] [CrossRef] [PubMed]
- Neare, K.; Janson, M.; Hutt, P.; Lassen, B.; Viltrop, A. Coxiella burnetii antibody prevalence and risk factors of infection in the human population of Estonia. Microorganisms 2019, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.; Naze, F.; Camuset, G.; Larrieu, S.; Pascalis, H.; Guernier, V.; Naty, N.; Bertolotti, A.; Manaquin, R.; Mboussou, Y.; et al. Seroprevalence of Coxiella burnetii (Q fever) exposure in humans on Reunion Island. Open Forum Infect. Dis. 2019, 6, ofz227. [Google Scholar] [CrossRef] [PubMed]
- Kersh, G.J.; Fitzpatrick, K.; Pletnikoff, K.; Brubaker, M.; Bruce, M.; Parkinson, A. Prevalence of serum antibodies to Coxiella burnetii in Alaska native persons from the Pribilof Islands. Zoonoses Public Health 2020, 67, 89–92. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).