Submitted:
24 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
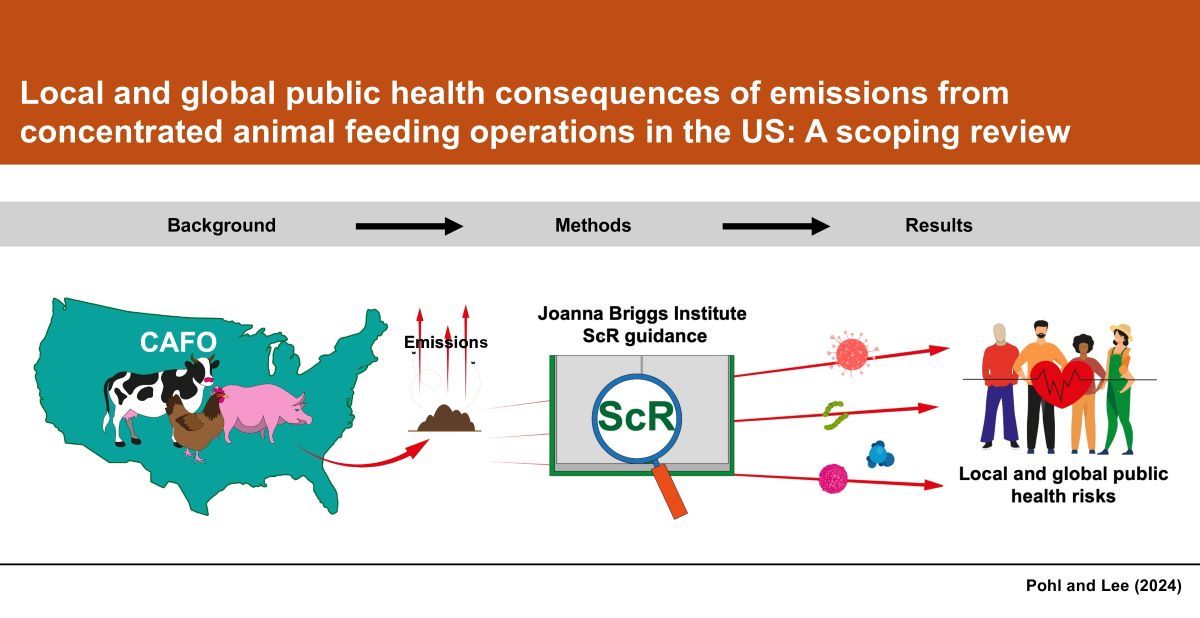
1. Introduction
1.1. Background
2. Materials and Methods
2.1. Inclusion Criteria Using the PCC Framework
2.1.1. Population
2.1.2. Concept
2.1.3. Context
2.2. Types of Sources
2.3. Exclusion Criteria
2.4. Search Strategy
2.5. Evidence Selection
2.6. Data Extraction
2.7. Synthesis of Results
3. Results
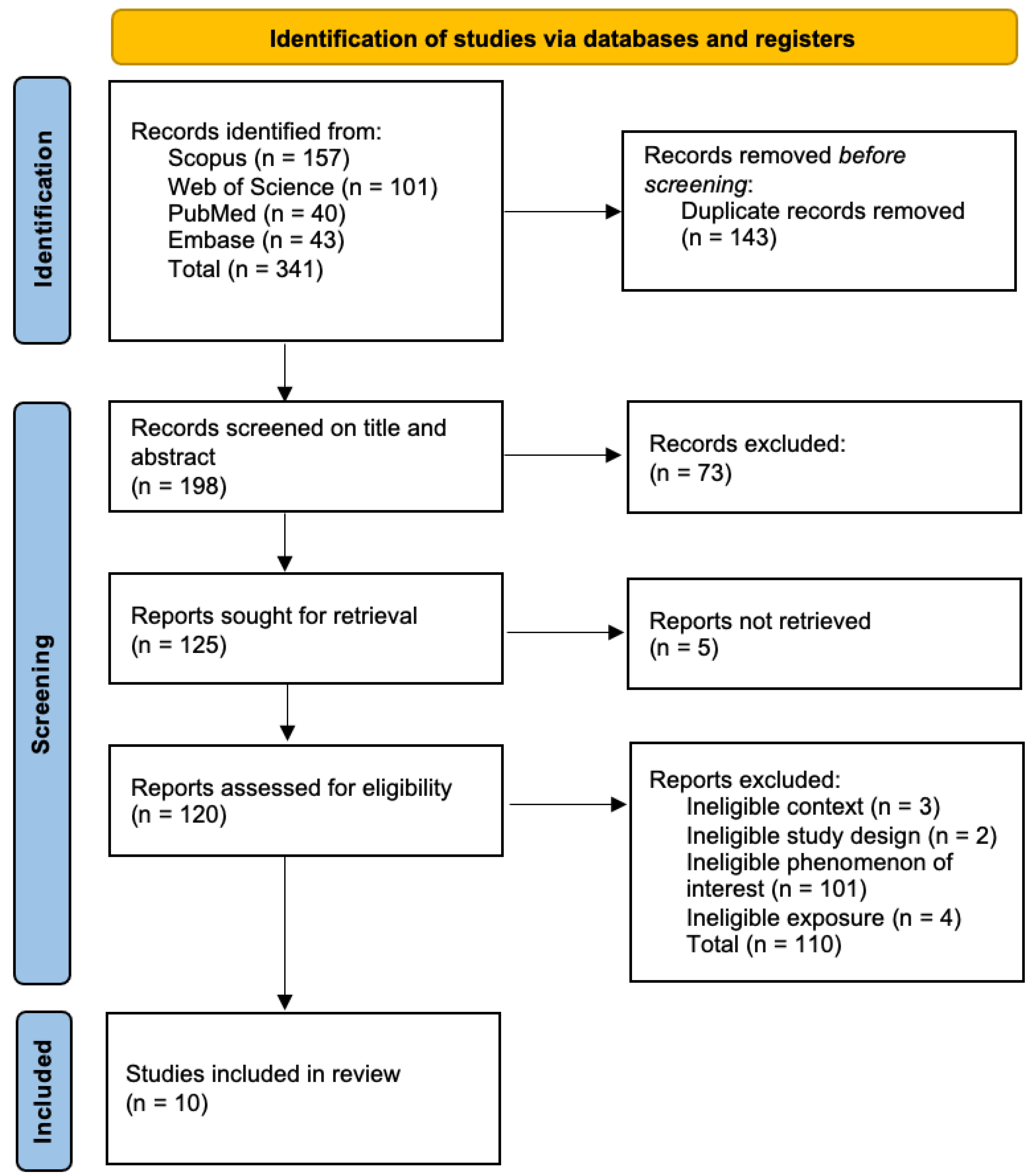
3.1. Study Identification
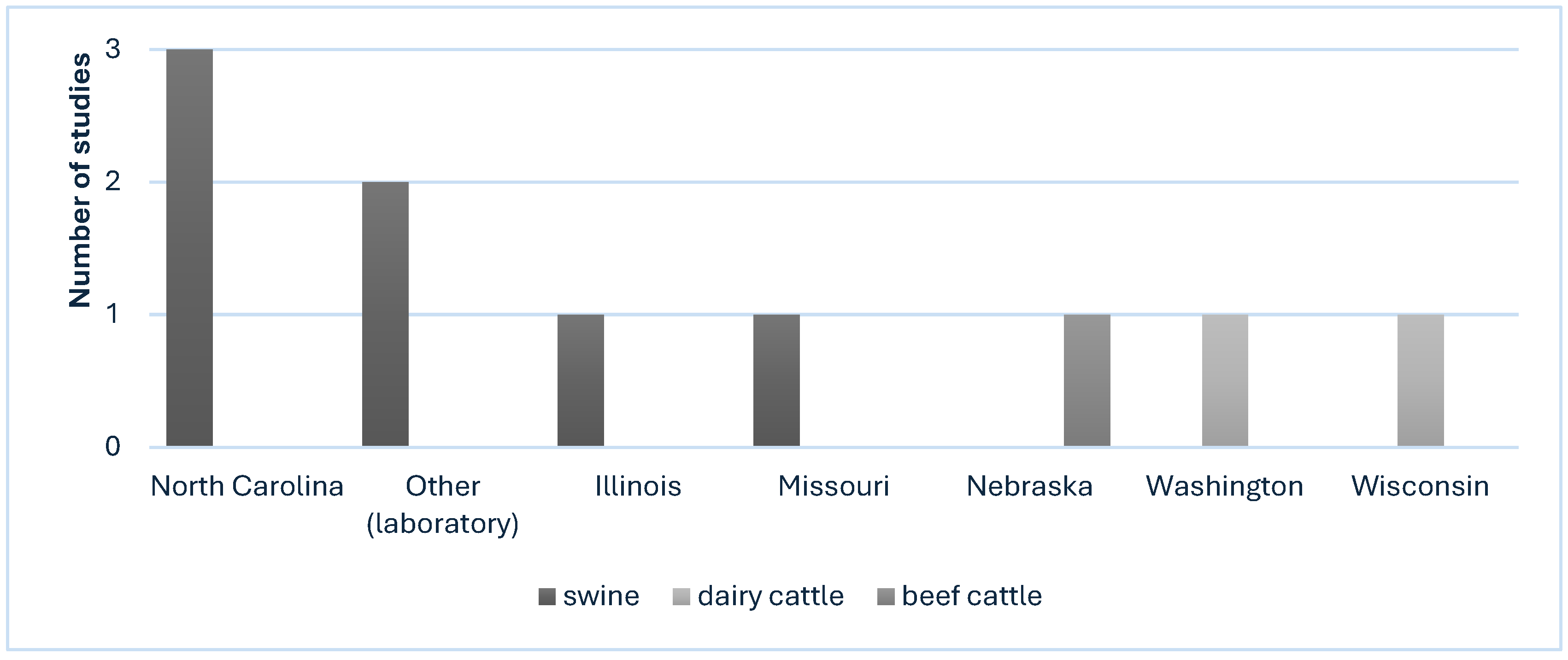
3.2. Characteristics of Included Studies
3.3. Key Findings of Included Studies
3.3.1. Occupational Health
3.3.2. Community Health Studies
3.3.4. Global Health
4. Discussion
4.1. Evidence Gaps and Suggestions for Future Research
4.2. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Search Strategy
Appendix B
Appendix C

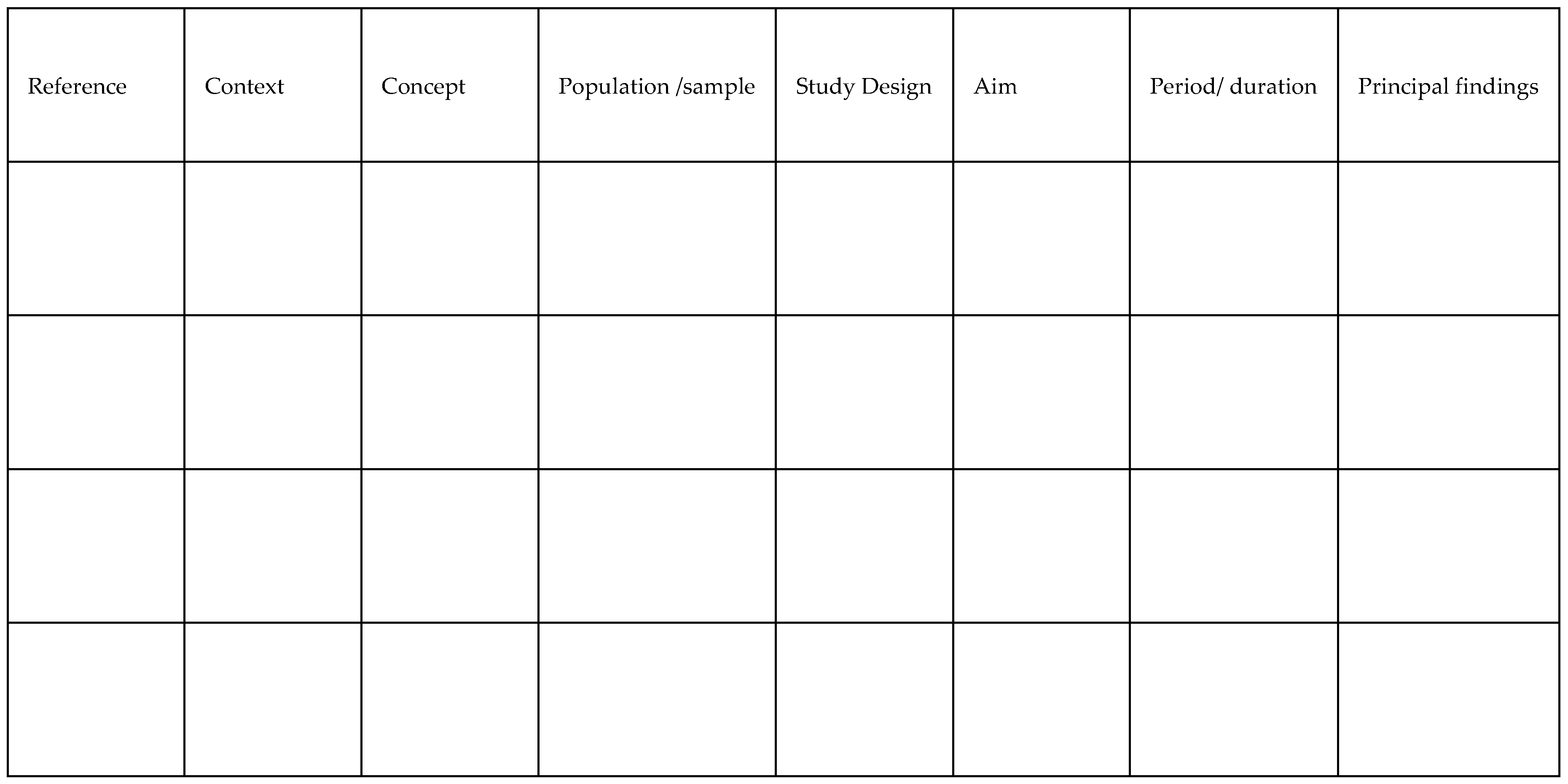
| Reference | Context | Concept | Population/ sample |
Study Design | Aim | Period/ duration | Principal findings |
| Beresin et al., 2017 | Illinois, swine, community level | MRSA | Inpatient hospitalizations for SSTI (n=215,218) | Observational | To examine and understand MRSA's swine-to-community linkages | 2008-2011 | Statistically significant link between ZIP code-level swine exposure and C.A. and H.A. MRSA for inpatient admissions suggests indirect swine production may elevate MRSA risk. |
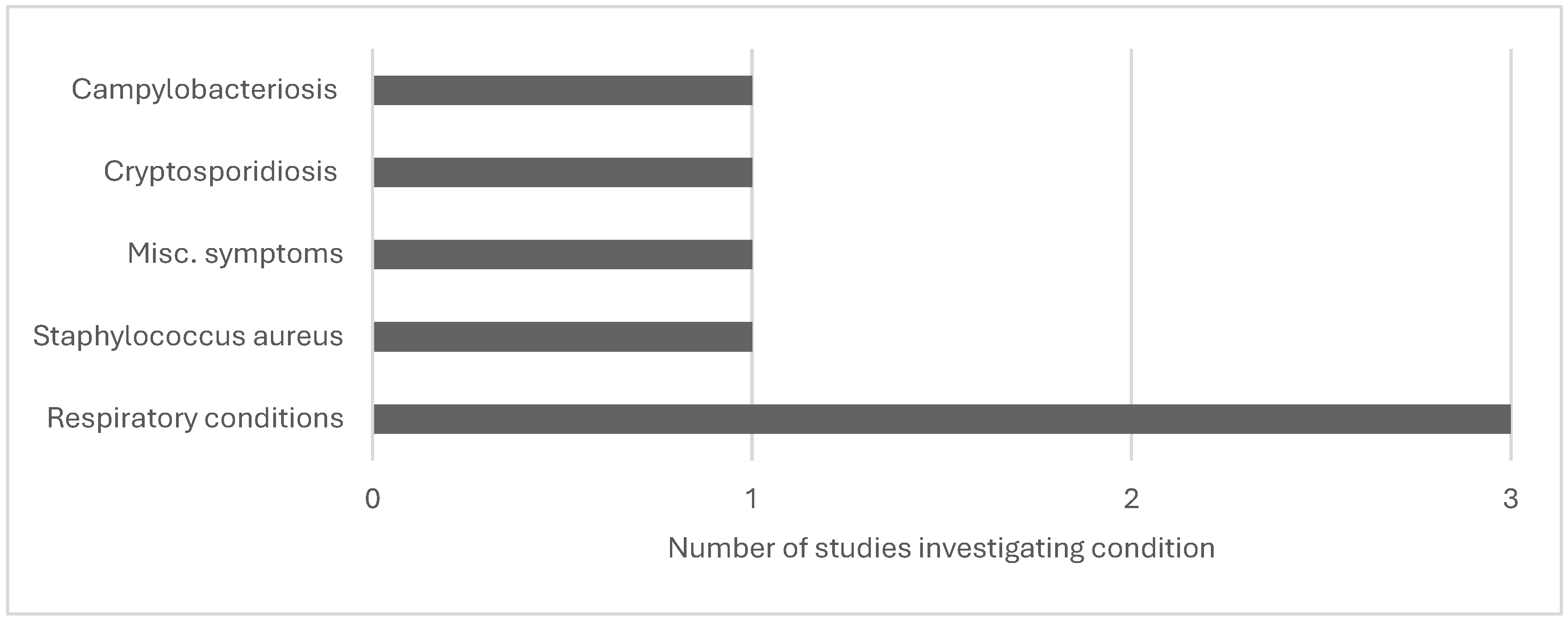
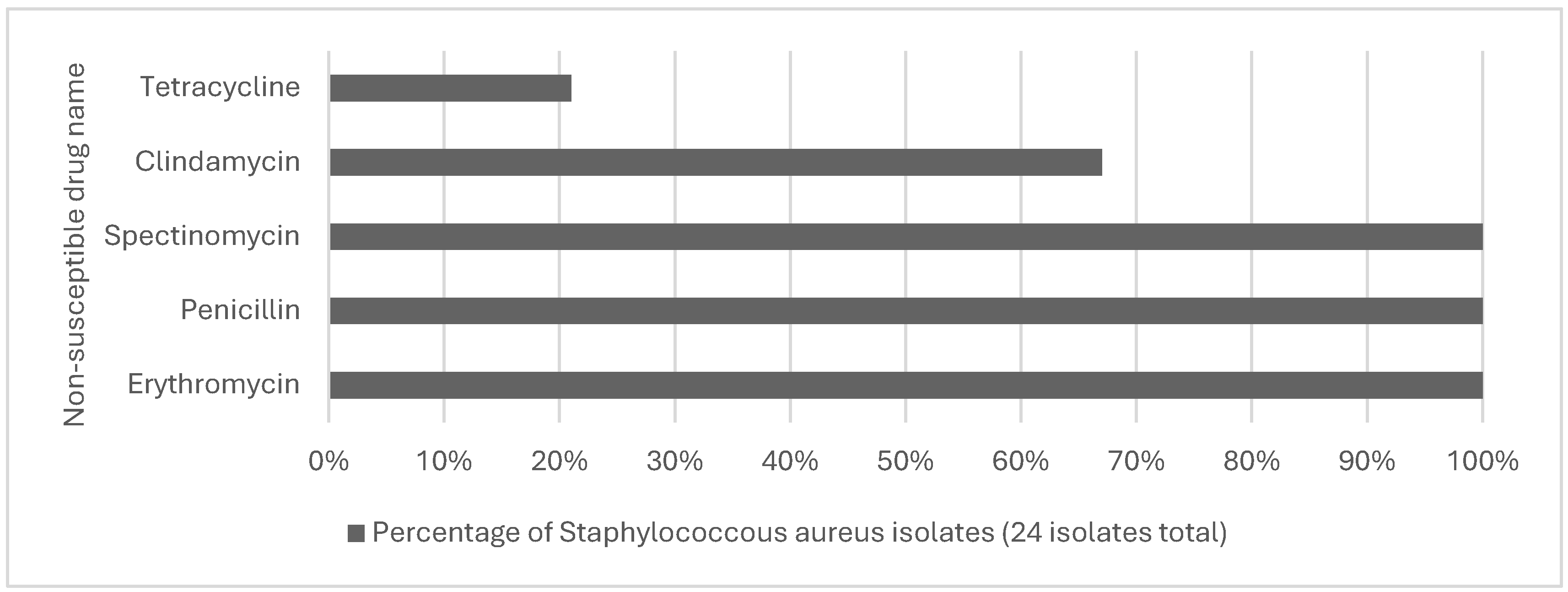
| Davis et al., 2018 | North Carolina, swine, occupational level | Staphylococcus Aureus | Worker surrogates (n=6) | Observational | To Identify worker MRSA exposure pathways | 120 to 150 minutes/2–2.5 hours | Breathing filters indicated presence of S. Aureus (7 and 9 CFU/m3, respectively, and S. Aureus isolates were MDRSA (spa type t337). At the grassland facility, by contrast, none of the worker samplers had a positive S. aureus test. |
| Knoell et al., 2019 | Lab, swine, occupational level | Airway inflammation | Veterans in Iowa and Nebraska with 2 years of agriculture experience (n=123); C57BL mice (n=6) | Experimental | To determine whether zinc affects HDE-induced airway inflammation. | 3 weeks | Veterans with COPD who did not consume sufficient zinc had a worse pulmonary function. Inhaling hog dust extract triggered an increase in neutrophil and total cell influx, mediator hyperresponsiveness, and intensification of tissue disease. Effects were more pronounced in mice that were zinc deficient. |
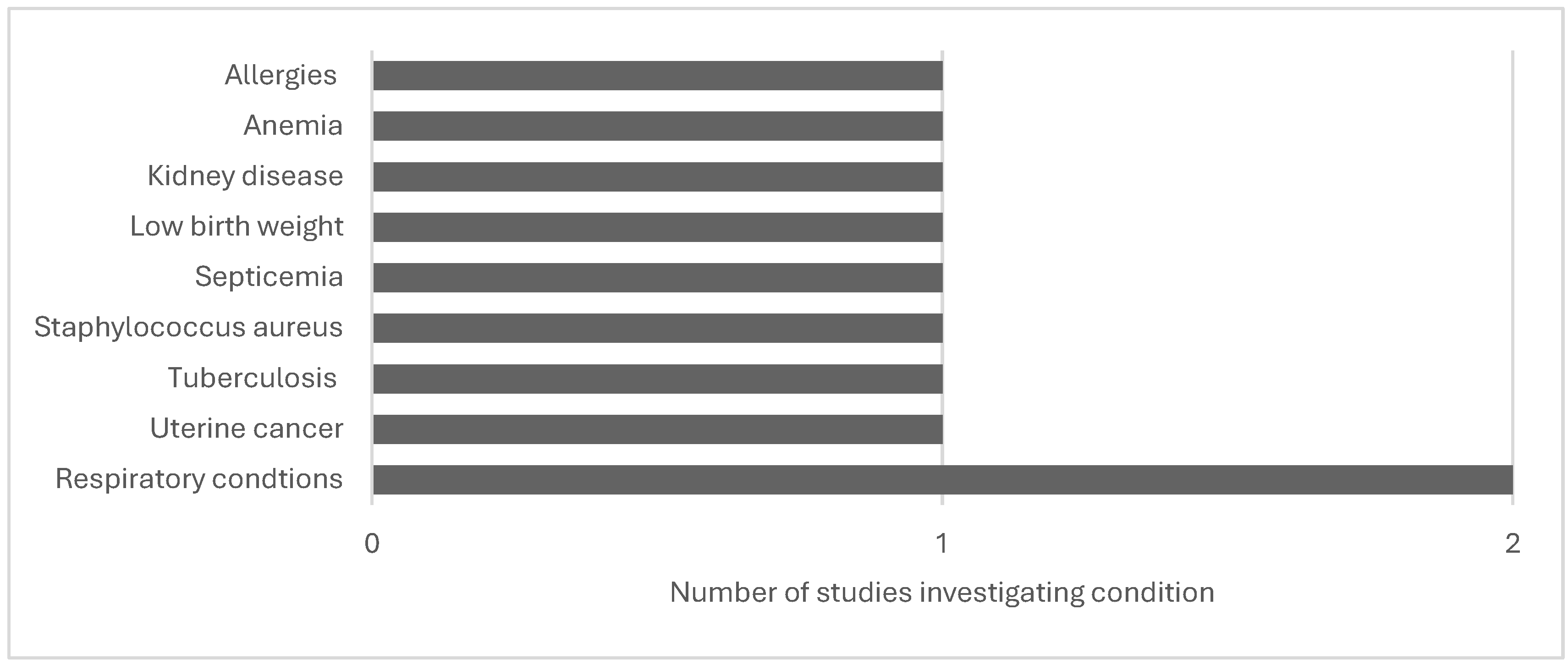
| Kravchenko et al., 2018 | North Carolina, swine, community level | Outcomes for kidney disease, anemia, tuberculosis, septicemia, low birth weight | Residents with emergency department or hospital admissions; From 221 zip codes with hog CAFO(s): (n≈2,260,000) |
Observational | To determine whether poor health outcomes are associated to swine CAFOs beyond demographic, socioeconomic, behavioral, or medical treatment access inequities | 2007-2013 | All disease-specific mortality rates in regions with > 215 swine/km2, were higher than in N.C. and the U.S. Communities near swine CAFOs had increased rates of all-cause, multi-morbidity, and neonatal mortality. The infant death rate was as high as 495/per 100,000 for infants less than one year. 56 zip codes (n=400,000 inhabitants) with the top quartile of density at >215swine/km rank #4 in the U. S. for all-cause death. #1 in the country for mortality from renal illness, #2 for septicemia, and #3 for T.B. as an underlying cause and secondary cause. |
| Kravchenko et al., 2020 | North Carolina, swine, community level | Uterine cancer | Uterine cancer mortality and hospital admissions in 56 southeastern NC zip codes with (>215hogs/km2); (n=1,393,824 person-years) | Observational | To determine how demographic, socioeconomic, behavioral, and medical care access affect uterine cancer mortality and hospitalization. | 2007-2013 | Higher odds of uterine cancer mortality in White females living in southeastern N.C. in zip codes with swine CAFOS than in White women from areas without swine CAFOS. |
| Loftus et al., 2020 | Washington, dairy cattle, community level | Asthma | Children 6-16 with asthma living in Yakima Valley (n=58) | Observational | To determine whether air pollution may be associated with health impacts in children with asthma. | 2010-2013 | Weakened lung function in children in the days after increased exposure to air pollution. No observed correlation with asthma symptoms. |
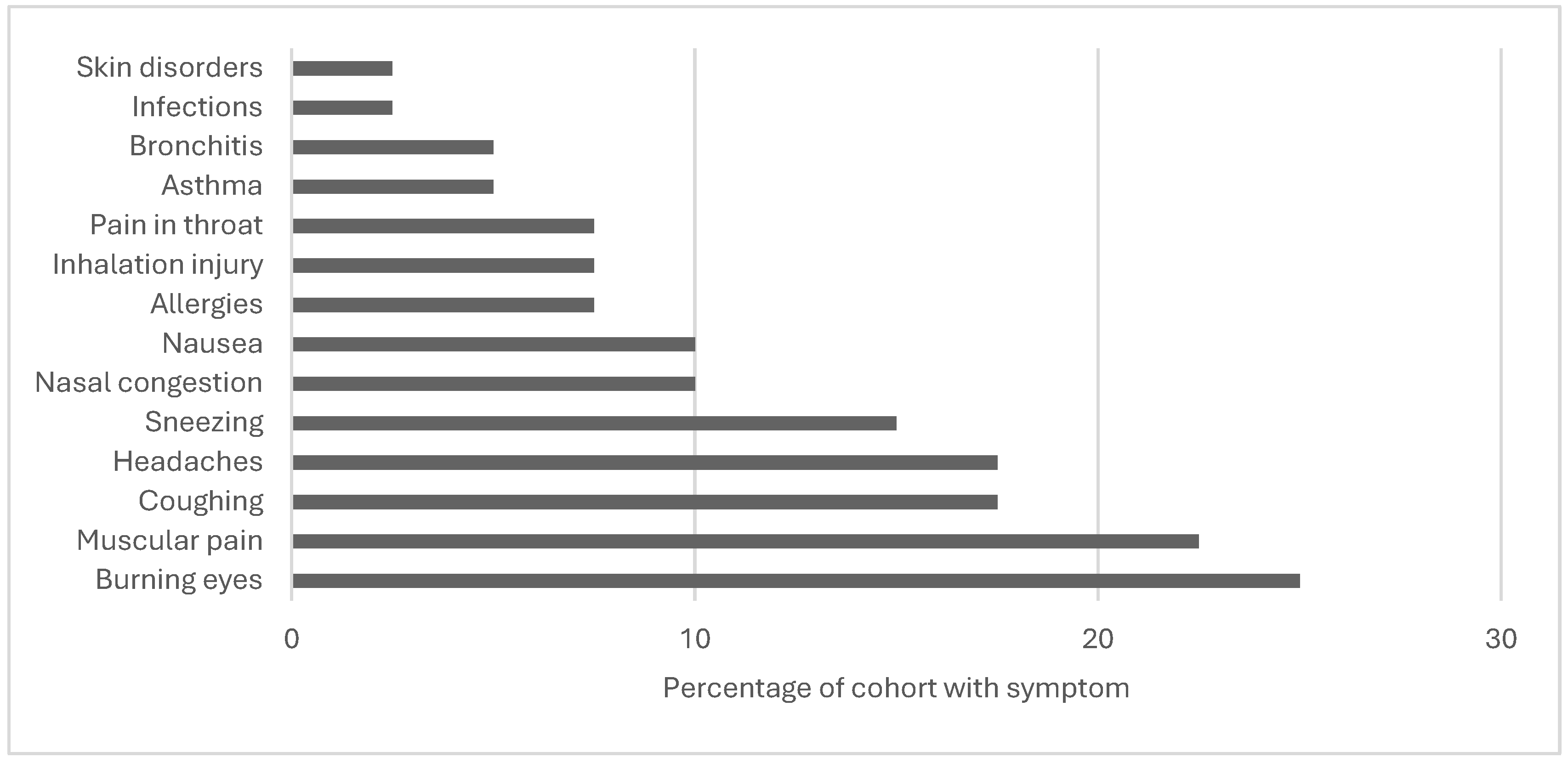
| Ramos et al., 2018 | Missouri, swine, occupational level | Occupational health concerns | Latino immigrant CAFO workers, ages 18+, in Audrain, Linn, and Sullivan counties (n=40) |
Qualitative | Examine self-reported workplace injuries and health concerns | June, July, August 2015 | Size of the facilities varied from 2,800 swine to 80,000 swine. 42.5 percent of employees reported poor or fair health; 28.2 percent experienced occupational health issues such as burning eyes, muscular discomfort, headache, coughing, nausea, nasal congestion, and sneezing. Twelve could not afford to visit a doctor in the previous 12 months, forgoing necessary medical visits. Coughing reports rose as job duration increased (r =.80 and p.01). Being a current smoker was shown to be substantially linked with reporting allergies (r =.57 and p.05). |
| Schultz et al., 2019 | Wisconsin, dairy cattle, community level | Respiratory and allergy | 2008-2016 Survey of the Health of Wisconsin respondent, rural resident, aged 18+ (n = 5338) |
Mixed methods | To evaluate respiratory and allergy health of residents near dairy CAFOs | 2008-2016 | Compared to persons who lived 5 miles from a CAFO, those who lived 1.5 miles away had a higher incidence of self-reported nasal allergies, lung allergies, asthma, asthma medication, uncontrolled asthma, and having had an asthma attack in the previous 12 months. |
| Su et al., 2017 | Nebraska, beef cattle, occupational level | Campylobacteriosis, cryptosporidiosis | Confirmed/probable campylobacteriosis or cryptosporidiosis cases; 2005–2015 aged 14+ (n=3,352) | Observational | To estimate animal-related occupational livestock exposures | 2005-2015 | Exposures in occupational settings occurred in 16.6% of 3,352 campylobacteriosis patients and 8.7% of 1,070 cryptosporidiosis cases. Farming and ranching were the most prevalent (68.2 percent and 78.5 percent, respectively), followed by slaughtering and processing (16.3 percent and 5.4 percent, respectively). Cattle/beef was the most prevalent animal occupational exposure, with exposure to feedlots (concentrated animal feeding operations) recorded in 29.9 percent of campylobacteriosis and 7.9 percent of cryptosporidiosis cases. |
| Wyatt et al., 2017 | Lab, swine, occupational level- Nebraska | Inflammatory lung disease | Human bronchial epithelial cells (BEAS-2B); C57BL mice (n=6) | Mixed methods | To determine whether alcohol inhibits HDE-induced TNF, ICAM-1, and neutrophil adhesion by inhibiting TNF converting enzyme (TACE) activity. | 8 weeks | Mice which were fed alcohol exhibited reduced airway epithelium ICAM-1 expression which was triggered by hog dust extract. Lung inflammation induced by hog dust inhalation associated with increases in TNF, IL-6, IL-8, ICAM-1, and neutrophil adhesion. |
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. The Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Nestle, M. A Call for Food System Change. The Lancet 2020, 395, 1685–1686. [Google Scholar] [CrossRef]
- Machovina, B.; Feeley, K.J.; Ripple, W.J. Biodiversity Conservation: The Key Is Reducing Meat Consumption. Science of the Total Environment 2015, 536, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Jolley, C. Modern Farming Techniques Are Essential to Provide Food to a Fast-Growing World Population. In Factory Farming; Miller, D.A., Ed.; Greenhaven Press: Farmington Hills, MI, 2010; ISBN 9780737749106. [Google Scholar]
- Fetrow, J. Hormone Use in Dairy Cows Increases Milk Production and Is Safe. In Food; Grover, J., Ed.; Greenhaven Press: Farmington Hills, MI, 2008; ISBN 9780737737936. [Google Scholar]
- USDA American Agricultural Exports Shattered Records in 2021 Available online:. Available online: https://www.usda.gov/media/press-releases/2022/02/08/american-agricultural-exports-shattered-records-2021 (accessed on 26 July 2022).
- Roser, M.; Ritchie, H. Meat Consumption vs. GDP per Capita 1990-2017 Available online:. Available online: https://ourworldindata.org/meat-production (accessed on 4 August 2022).
- Brozek, W.; Falkenberg, C. Industrial Animal Farming and Zoonotic Risk: Covid-19 as a Gateway to Sustainable Change? A Scoping Study. Sustainability (Switzerland) 2021, 13, 1–30. [Google Scholar] [CrossRef]
- Delgado, C.L. Rising Consumption of Meat and Milk in Developing Countries Has Created a New Food Revolution. Journal of Nutrition 2003, 133, 3907–3910. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.K. Livestock Production: Recent Trends, Future Prospects. Philosophical Transactions of the Royal Society B: Biological Sciences 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.M.R.; Ogneva-Himmelberger, Y. Distribution of Industrial Farms in the United States and Socioeconomic, Health, and Environmental Characteristics of Counties. Geography Journal 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Leibler, J.H.; Otte, J.; Roland-Holst, D.; Pfeiffer, D.U.; Soares Magalhaes, R.; Rushton, J.; Graham, J.P.; Silbergeld, E.K. Industrial Food Animal Production and Global Health Risks: Exploring the Ecosystems and Economics of Avian Influenza. Ecohealth 2009, 6, 58–70. [Google Scholar] [CrossRef] [PubMed]
- USEPA National Pollution Discharge Elimination System Permit Regulation and Effluent Limitations Guidelines and Standards for Concentrated Animal Feeding Operations (CAFOs) Available online:. Available online: https://www.federalregister.gov/documents/2003/02/12/03-3074/national-pollutant-discharge-elimination-system-permit-regulation-and-effluent-limitation-guidelines (accessed on 17 April 2022).
- USDA Animal Feeding Operations Nutrient Management Plan Available online:. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/plantsanimals/livestock/afo/ (accessed on 14 July 2022).
- Gurian-Sherman, D. CAFOs Uncovered: The Untold Costs of Confined Animal Feeding Operations. Union of Concerned Scientists 2008, 1–86. [Google Scholar]
- Lappé, F.M.; Collins, J.; Rosset, P.; Esparz, L. World Hunger 12 Myths; 2nd, *!!! REPLACE !!!* (Eds.) ; Institute for Food and Development Policy: Oakland, Calif., 2014; ISBN 9781315071121.
- Gunderson, R.; Stuart, D.; Petersen, B. Factory Farming:; Miller, D., Ed.; Current controversies; Greenhaven Press, 2017; ISBN 9780737749090.
- Rossi, J.; Garner, S.A. Industrial Farm Animal Production: A Comprehensive Moral Critique. J Agric Environ Ethics 2014, 27, 479–522. [Google Scholar] [CrossRef]
- Donham, K.J.; Wing, S.; Osterberg, D.; Flora, J.L.; Hodne, C.; Thu, K.M.; Thorne, P.S. Community Health and Socioeconomic Issues Surrounding Concentrated Animal Feeding Operations. Environ Health Perspect 2007, 115, 317–320. [Google Scholar] [CrossRef] [PubMed]
- E: GAO Concentrated Animal Feeding Operations, 2011.
- 2: and Water Watch Factory Farm Nation, 2020.
- 2013.
- Hribar, C. 2010.
- Sierra Club Why Are CAFOs Bad? Available online:. Available online: https://www.sierraclub.org/michigan/why-are-cafos-bad#basics (accessed on 23 February 2022).
- Hooser, S.B.; van Alstine, W.; Kiupel, M.; Sojka, J. Acute Pit Gas (Hydrogen Sulfide) Poisoning in Confinement Cattle. J Vet Diagn Invest 2000, 12, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Mallin, M.A.; Cahoon, L.B. Industrialized Animal Production - A Major Source of Nutrient and Microbial Pollution to Aquatic Ecosystems. Popul Environ 2003, 24, 369–385. [Google Scholar] [CrossRef]
- Todd, R.W.; Cole, N.A.; Clark, R.N.; Flesch, T.K.; Harper, L.A.; Baek, B.H. Ammonia Emissions from a Beef Cattle Feedyard on the Southern High Plains. Atmos Environ 2008, 42, 6797–6805. [Google Scholar] [CrossRef]
- Greger, M.; Koneswaran, G. The Public Health Impacts of Concentrated Animal Feeding Operations on Local Communities. Fam Community Health 2010, 33, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M. From Hogs to HABs: Impacts of Industrial Farming in the US on Nitrogen and Phosphorus and Greenhouse Gas Pollution; Springer International Publishing, 2020; Vol. 150; ISBN 0123456789.
- Donham, K.J. The Concentration of Swine Production. Effects on Swine Health, Productivity, Human Health, and the Environment. Vet Clin North Am Food Anim Pract 2000, 16, 559–597. [Google Scholar] [CrossRef]
- Gerald, C.L.; Pender, R.J.; Watson, C.Y.; Adler, K.B.; Waterman, J.T. Oxidant Stress In Airway Epithelial Cells Following Swine Confinement Facility Dust Exposure. American Thoracic Society International Conference Meetings Abstracts American Thoracic Society International Conference Meetings Abstracts 2010, A4695–A4695. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.; Peters, T.; Donham, K.; Taylor, C.; Altmaier, R.; Kelly, K. Assessment of Swine Worker Exposures to Dust and Endotoxin during Hog Load-out and Power Washing. Annals of Occupational Hygiene 2012, 56, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J. The Concentration of Swine Production: Effects on Swine Health, Productivity, Human Health, and the Environment. Veterinary Clinics of North America: Food Animal Practice 2000, 16, 559–597. [Google Scholar] [CrossRef]
- Von Essen, S.; Romberger, D. The Respiratory Inflammatory Response to the Swine Confinement Building Environment: The Adaptation to Respiratory Exposures in the Chronically Exposed Worker. J Agric Saf Health 2003, 9, 185–196. [Google Scholar] [CrossRef]
- Monson, R.; Gustafson, K. Acute Toxic Exposure to Gases from Liquid Manure. Journal of Occupational Medicine 1982, 24, 142–145. [Google Scholar]
- Donham, K.J.; Rubino, M.; Thedell, T.D.; Kammermeyer, J. Potential Health Hazards to Agricultural Workers in Swine Confinement Buildings. Journal of Occupational Medicine 1977, 19, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Merchant, J.A.; Naleway, A.L.; Svendsen, E.R.; Kelly, K.M.; Burmeister, L.F.; Stromquist, A.M.; Taylor, C.D.; Thorne, P.S.; Reynolds, S.J.; Sanderson, W.T.; et al. Asthma and Farm Exposures in a Cohort of Rural Iowa Children. Environ Health Perspect 2005, 113, 350–356. [Google Scholar] [CrossRef]
- Donham, K.J.; Lee, J.A.; Thu, K.; Reynolds, S.J. Assessment of Air Quality at Neighbor Residences in the Vicinity of Swine Production Facilities. J Agromedicine 2006, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sneeringer, S. Anational, Longitudinal Study of the Effects of Concentrated Hog Production on Ambient Air Pollution. Am J Agric Econ 2010, 92, 821–835. [Google Scholar] [CrossRef]
- Tajik, M.; Muhammad, N.; Lowman, A.; Thu, K.; Wing, S.; Grant, G.R. Impact of Odor from Industrial Hog Operations on Daily Living Activities. New Solutions 2008, 18, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Pavilonis, B.T.; O’Shaughnessy, P.T.; Altmaier, R.; Metwali, N.; Thorne, P.S. Passive Monitors to Measure Hydrogen Sulfide near Concentrated Animal Feeding Operations. Environmental Sciences: Processes and Impacts 2013, 15, 1271–1278. [Google Scholar] [CrossRef]
- Wing, S.; Wolf, S. Intensive Livestock Operations, Health, and Quality of Life among Eastern North Carolina Residents. Environ Health Perspect 2000, 108, 233–238. [Google Scholar] [CrossRef]
- Mirabelli, M.C.; Wing, S.; Marshall, S.W.; Wilcosky, T.C. Asthma Symptoms among Adolescents Who Attend Public Schools That Are Located near Confined Swine Feeding Operations. Pediatrics 2006, 118, e66–e75. [Google Scholar] [CrossRef]
- Mirabelli, M.C.; Wing, S.; Marshall, S.W.; Wilcosky, T.C. Race, Poverty, and Potential Exposure of Middle-School Students to Air Emissions from Confined Swine Feeding Operations. Environ Health Perspect 2006, 114, 591–596. [Google Scholar] [CrossRef]
- Sigurdarson, S.T.; Kline, J.N. School Proximity to Concentrated Animal Feeding Operations and Prevalence of Asthma in Students. Chest 2006, 129, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Sneeringer, S. Does Animal Feeding Operation Pollution Hurt Public Health? A National Longitudinal Study of Health Externalities Identified by Geographic Shifts in Livestock Production. Am J Agric Econ 2009, 91, 124–137. [Google Scholar] [CrossRef]
- Thu, K.; Donham, K.; Ziegenhorn, R.; Reynolds, S.; Thorne, P.S.; Subramanian, P.; Whitten, P.; Stookesberry, J. A Control Study of the Physical and Mental Health of Residents Living Near a Large-Scale Swine Operation. J Agric Saf Health 1997, 3, 13–26. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Sattely Miller, E.A.; Suggs, M.S.; Graham, B.G. The Effect of Environmental Odors Emanating from Commercial Swine Operations on the Mood of Nearby Residents. Brain Res Bull 1995, 37, 369–375. [Google Scholar] [CrossRef]
- Aneja, V.P.; Schlesinger, W.H.; Erisman, J.W. Effects of Agriculture upon the Air Quality and Climate: Research, Policy, and Regulations. Environ Sci Technol 2009, 43, 4234–4240. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow 2006.
- Leibler, J.H.; Dalton, K.; Pekosz, A.; Gray, G.C.; Silbergeld, E.K. Epizootics in Industrial Livestock Production: Preventable Gaps in Biosecurity and Biocontainment. Zoonoses Public Health 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Grossi, G.; Goglio, P.; Vitali, A.; Williams, A.G. Livestock and Climate Change: Impact of Livestock on Climate and Mitigation Strategies. Animal Frontiers 2019, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. W: Change 2014 Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects, 2014.
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Valle, S. Del; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet Sci 2019, 6. [Google Scholar] [CrossRef]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between Environmental Issues and Zoonotic Diseases: With Reference to COVID-19 Pandemic. Environmental Sustainability 2021, 4, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Price, L.B.; Roess, A.; Graham, J.P.; Baqar, S.; Vailes, R.; Sheikh, K.A.; Silbergeld, E. Neurologic Symptoms and Neuropathologic Antibodies in Poultry Workers Exposed to Campylobacter Jejuni. J Occup Environ Med 2007, 49, 748–755. [Google Scholar] [CrossRef]
- Okamatsu, M.; Saito, T.; Yamamoto, Y.; Mase, M.; Tsuduku, S.; Nakamura, K.; Tsukamoto, K.; Yamaguchi, S. Low Pathogenicity H5N2 Avian Influenza Outbreak in Japan during the 2005-2006. Vet Microbiol 2007, 124, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, E.R.; Johnson, K.R.; Karpati, A.; Rubin, C.S.; Kolpin, D.W.; Meyer, M.T.; Esteban, J.E.; Currier, R.W.; Smith, K.; Thu, K.M.; et al. Antimicrobial Residues in Animal Waste and Water Resources Proximal to Large-Scale Swine and Poultry Feeding Operations. Science of the Total Environment 2002, 299, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.-F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes Following Land Application of Manure Waste. J Environ Qual 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ben, W.; Yang, M.; Zhang, Y.; Qiang, Z. Dissemination of Veterinary Antibiotics and Corresponding Resistance Genes from a Concentrated Swine Feedlot along the Waste Treatment Paths. Environ Int 2016, 92–93, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Rollin, B.E. Antibiotic Use and the Demise of Husbandry. Journal of Ethics 2018, 22, 45–57. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Aminov, R.I.; Krapac, I.J.; Garrigues-Jeanjean, N.; Mackie, R.I. Occurrence and Diversity of Tetracycline Resistance Genes in Lagoons and Groundwater Underlying Two Swine Production Facilities. Appl Environ Microbiol 2001, 67, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.-F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes Following Land Application of Manure Waste. J Environ Qual 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- He, L.Y.; Ying, G.G.; Liu, Y.S.; Su, H.C.; Chen, J.; Liu, S.S.; Zhao, J.L. Discharge of Swine Wastes Risks Water Quality and Food Safety: Antibiotics and Antibiotic Resistance Genes from Swine Sources to the Receiving Environments. Environ Int 2016, 92–93, 210–219. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic Resistance Genes from Livestock Waste: Occurrence, Dissemination, and Treatment. NPJ Clean Water 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Feingold, B.J.; Silbergeld, E.K.; Curriero, F.C.; van Cleef, B.A.G.L.; Heck, M.E.O.C.; Kluytmans, J.A.J.W. Livestock Density as Risk Factor for Livestock-Associated Methicillin-Resistant Staphylococcus Aureus, the Netherlands. Emerg Infect Dis 2012, 18, 1841–1849. [Google Scholar] [CrossRef]
- Larsen, J.; Petersen, A.; Sørum, M.; Stegger, M.; van Alphen, L.; Valentiner-Branth, P.; Knudsen, L.K.; Larsen, L.S.; Feingold, B.; Price, L.B.; et al. Meticillin-Resistant Staphylococcus Aureus CC398 Is an Increasing Cause of Disease in People with No Livestock Contact in Denmark, 1999 to 2011. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef]
- Carrel, M.; Schweizer, M.L.; Sarrazin, M.V.; Smith, T.C.; Perencevich, E.N. Residential Proximity to Large Numbers of Swine in Feeding Operations Is Associated with Increased Risk of Methicillin-Resistant Staphylococcus Aureus Colonization at Time of Hospital Admission in Rural Iowa Veterans. Infect Control Hosp Epidemiol 2014, 35, 190–192. [Google Scholar] [CrossRef]
- Schinasi, L.; Wing, S.; Augustino, K.L.; Ramsey, K.M.; Nobles, D.L.; Richardson, D.B.; Price, L.B.; Aziz, M.; Macdonald, P.D.M.; Stewart, J.R. A Case Control Study of Environmental and Occupational Exposures Associated with Methicillin Resistant Staphylococcus Aureus Nasal Carriage in Patients Admitted to a Rural Tertiary Care Hospital in a High Density Swine Region. Environ Health 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Hoover, J.N. Can’t You Smell That Smell? Clean Air Act Fixes for Factory Farm Air Pollution. Stanford Journal of Animal Law & Policy 2013, 6. [Google Scholar]
- Stella, C.; Conner, H.; van Saun, A.; Russ, A.; Heinzen, T.; Newell, B. Petition to Rescind the Air Consent Agreement and Enforce Clean Air Laws Against Animal Feeding Operations. 2021.
- O’Connor, A.M.; Auvermann, B.; Bickett-Weddle, D.; Kirkhorn, S.; Sargeant, J.M.; Ramirez, A.; von Essen, S.G. The Association between Proximity to Animal Feeding Operations and Community Health: A Systematic Review. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Auvermann, B.W.; Dzikamunhenga, R.S.; Glanville, J.M.; Higgins, J.P.T.; Kirychuk, S.P.; Sargeant, J.M.; Totton, S.C.; Wood, H.; von Essen, S.G. Updated Systematic Review: Associations between Proximity to Animal Feeding Operations and Health of Individuals in Nearby Communities. Syst Rev 2017, 6. [Google Scholar] [CrossRef]
- Douglas, P.; Robertson, S.; Gay, R.; Hansell, A.L.; Gant, T.W. A Systematic Review of the Public Health Risks of Bioaerosols from Intensive Farming. Int J Hyg Environ Health 2018, 221, 134–173. [Google Scholar] [CrossRef]
- JBI JBI Manual for Evidence Synthesis; Aromataris, E. , Munn, Z., Eds.; Joanna Briggs Institute: Adelaide, 2020; ISBN 9780648848806. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Munn, Z.; Aromataris, E.; Tufanaru, C.; Stern, C.; Porritt, K.; Farrow, J.; Lockwood, C.; Stephenson, M.; Moola, S.; Lizarondo, L.; et al. The Development of Software to Support Multiple Systematic Review Types: The Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). Int J Evid Based Healthc 2019, 17, 36–43. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. The BMJ 2021, 372. [Google Scholar] [CrossRef]
- Ramos, A.K.; Fuentes, A.; Carvajal-Suarez, M. Self-Reported Occupational Injuries and Perceived Occupational Health Problems among Latino Immigrant Swine Confinement Workers in Missouri. J Environ Public Health 2018, 2018. [Google Scholar] [CrossRef]
- Davis, M.F.; Pisanic, N.; Rhodes, S.M.; Brown, A.; Keller, H.; Nadimpalli, M.; Christ, A.; Ludwig, S.; Ordak, C.; Spicer, K.; et al. Occurrence of Staphylococcus Aureus in Swine and Swine Workplace Environments on Industrial and Antibiotic-Free Hog Operations in North Carolina, USA: A One Health Pilot Study. Environ Res 2018, 163, 88–96. [Google Scholar] [CrossRef]
- Wyatt, T.A.; Canady, K.; Heires, A.J.; Poole, J.A.; Bailey, K.L.; Nordgren, T.M.; Romberger, D.J. Alcohol Inhibits Organic Dust-Induced ICAM-1 Expression on Bronchial Epithelial Cells. Safety 2017, 3. [Google Scholar] [CrossRef]
- Knoell, D.L.; Smith, D.A.; Sapkota, M.; Heires, A.J.; Hanson, C.K.; Smith, L.M.; Poole, J.A.; Wyatt, T.A.; Romberger, D.J. Insufficient Zinc Intake Enhances Lung Inflammation in Response to Agricultural Organic Dust Exposure. Journal of Nutritional Biochemistry 2019, 70, 56–64. [Google Scholar] [CrossRef]
- Su, C.; Stover, D.T.; Buss, B.F.; Carlson, A. V.; Luckhaupt, S.E. Occupational Animal Exposure Among Persons with Campylobacteriosis and Cryptosporidiosis — Nebraska, 2005–2015. MMWR Morb Mortal Wkly Rep 2017, 66, 955–958. [Google Scholar] [CrossRef]
- Beresin, G.A.; Wright, J.M.; Rice, G.E.; Jagai, J.S. Swine Exposure and Methicillin-Resistant Staphylococcus Aureus Infection among Hospitalized Patients with Skin and Soft Tissue Infections in Illinois: A ZIP Code-Level Analysis. Environ Res 2017, 159, 46–60. [Google Scholar] [CrossRef]
- Kravchenko, J.; Rhew, S.H.; Akushevich, I.; Agarwal, P.; Lyerly, H.K. Mortality and Health Outcomes in North Carolina Communities Located in Close Proximity to Hog Concentrated Animal Feeding Operations. N C Med J 2018, 79, 278–288. [Google Scholar] [CrossRef]
- Kravchenko, J.; Akushevich, I.; Rhew, S.H.; Agarwal, P.; Lyerly, H.K. Uterine Cancer Mortality in White and African American Females in Southeastern North Carolina. J Environ Public Health 2020, 2020, 9–13. [Google Scholar] [CrossRef]
- Loftus, C.; Afsharinejad, Z.; Sampson, P.; Vedal, S.; Torres, E.; Arias, G.; Tchong-French, M.; Karr, C. Estimated Time-Varying Exposures to Air Emissions from Animal Feeding Operations and Childhood Asthma. Int J Hyg Environ Health 2020, 223, 187–198. [Google Scholar] [CrossRef]
- Schultz, A.A.; Peppard, P.; Gangnon, R.E.; Malecki, K.M.C. Residential Proximity to Concentrated Animal Feeding Operations and Allergic and Respiratory Disease. Environ Int 2019, 130, 104911. [Google Scholar] [CrossRef] [PubMed]
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine Disrupting Chemicals and Endometrial Cancer: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Int J Environ Res Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Afsharinejad, Z.; Sampson, P.; Vedal, S.; Torres, E.; Arias, G.; Tchong-French, M.; Karr, C.; Sciences, O.H.; Farm, V.; et al. Estimated Time-Varying Exposures to Air Emissions from Animal Feeding Operations and Childhood Asthma. Int J Hyg Environ Health 2021, 223, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.D.; Johansson, R.C.; Peters, M. The Manure Hits the Land: Economic and Environmental Implications When Land Application of Nutrients Is Constrained. Am J Agric Econ 2004, 86, 688–700. [Google Scholar] [CrossRef]
- Ribaudo, M.O.; Gollehon, N.R.; Agapoff, J. Land Application of Manure by Animal Feeding Operations: Is More Land Needed? J Soil Water Conserv 2003, 58, 30–38. [Google Scholar]
- Costanza, J.K.; Marcinko, S.E.; Goewert, A.E.; Mitchell, C.E. Potential Geographic Distribution of Atmospheric Nitrogen Deposition from Intensive Livestock Production in North Carolina, USA. Science of the Total Environment 2008, 398, 76–86. [Google Scholar] [CrossRef]
- Schlesinger, W.H. On the Fate of Anthropogenic Nitrogen. Proc Natl Acad Sci U S A 2009, 106, 203–208. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikulewicz, M. Bioaccumulation. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123864543. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




