Submitted:
24 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Narrative Review
Bioinformatics analyses
Results and Discussion
Association of Creatine Supplementation and renal injury in Humans
| Study | Object of study | Creatine intake protocol | Observations | |
|---|---|---|---|---|
| [16] | Five young, healthy males | 20 g of creatine monohydrate per day for 5 consecutive days | There was no detrimental effect on renal function; low sample size. | |
| [17] | Eight young men and one women | 1 to 80 g·day−1 for 10 months to 5 years | There was no detrimental effect on renal function; low sample size; long-term supplementation (years). | |
| [18] | Man, 25 years old, with a history of renal injury 8 years ago. | L: 15 g·day−1 for 1 week M: 2 g·day−1 for 7 weeks |
Previous renal injuries; low dosage in the maintenance phase (similar to amounts obtained dietetically and endogenously). | |
| [33] | 19 years old Man, soccer athlete. | 10 g·day−1 for 3 months | Renal insufficiency induced by creatine supplementation; serum creatinine was the sole marker of renal injury. | |
| [34] | Man, 20 years old. | 20 g·day−1 for 4 weeks | Severe interstitial nephritis four weeks after taking high dose creatine; insufficient past data on the patient. | |
| [35] | Male, 18 years old, renal insufficiency secondary to mitochondrial encephalopathy. | L: 20 g·day−1 for 12 days M: 5 g·day−1 for 28 months |
Previous renal injuries; creatinine levels were determined using a less accurate method; neuroprotective effect observed. | |
| [36] | Man, 22 years old, athlete | 200 g·day−1 continuously | Concomitant use of anabolic steroid; creatine overdose (200 g/day); renal injury markers were not reported. | |
| [37] | Man, 24 years old, athlete | 15 g·day−1 for 6 months | Acute renal failure; in addition to (low dose) creatine, other supplements were consumed for the purpose of bodybuilding. | |
| [38] | Man, 18 years old | L: 20 g·day−1 for 5 days M: 1 g·day−1 for 6 weeks |
Patient had acute renal failure while taking creatine; the study lacked a description of the participant's diet, whether he used anabolic steroids and a discussion of the possible contamination of the supplement | |
| [39] | Eighteen sedentary males performing resistance training | 10 g·day−1 for 3 months | Increased performance and body weight; no changes in metabolic and urine markers, or hepatic and renal function | |
| [40] | Man, 20 years old, single kidney | L: 20 g·day−1 for 5 days M: 5 g·day−1 for 30 days |
There were no changes in renal function; there was a slight improvement in glomerular filtration rate. | |
| [41] | 35 male individuals, 18 and 42 years, with a minimum of two consecutive months of training with resistance exercises | L: 20 g·day−1 for 7 days M: 0.03 g·kg-1day−1 for 7 weeks |
There were no changes in hepatic and renal function; relative small sample size and absence of long-term effects of creatine supplementation | |
| [42] | 25 men and women, > 45 years, with type 2 diabetes, physically inactive for at least 1 year, and with BMI ≥ 30 kg/m2 | 5 g·day−1 for 12 weeks | Creatine supplementation does not impair kidney function; study with short duration without long-term follow-up | |
| [43] | 18 healthy males | L: 0,3 g·kg-1day−1 for 7 days | Creatine supplementation did not alter metabolic and urine markers, hepatic, and renal function vs control group; short duration of supplementation | |
Association of creatine supplementation and renal injury in experimental models
| Study | Object of study | Creatine intake protocol | Conclusions | |
|---|---|---|---|---|
| [45] | 24 male Wistar rats; three dosages of creatine for 2 weeks | 0.5 g·kg-1day−1 1 g·kg-1day−1 2 g·kg-1day−1 |
Creatine supplementation did not result in renal and/or hepatic toxicity; short experimental period | |
| [47] | 23 male and 24 female Han:SPRD-cy rats (cystic kidney disease) | L: 2 g·kg-1day−1 for 1 week M: 0,4 g·kg-1day−1 for 35 days |
Creatine supplementation exacerbated pre-existing polycystic kidney disease; creatine was combined with glutamine | |
| [48] | 43 male Wistar rats; 23 with moderate renal failure | Creatine monohydrate (2% w/w) was added to this diet in the creatine-supplemented groups for 4 weeks | Creatine supplementation does not impair kidney function in animals with pre-existing renal failure or in control animals | |
| [49] | 60 male Sprague–Dawley rats; 40 with cisplatin-induced nephrotoxicity | 300 mg·kg-1day−1 for 30 days | Creatine administration was considered a promising adjuvant protective drug for reducing nephrotoxic effect of cisplatin | |
| [50] | 36 male Wistar rats | 2 g·kg-1day−1 for 10 weeks | The use of creatine alone induced an important and significant reduction of both renal plasma flow and glomerular filtration rate | |
| [51] | 72 male Wistar rats; swimming training | Short-term: 5 g·kg-1day−1 for 1 week Long-term: 1 g·kg-1day−1 for 4-8 weeks |
Long-term creatine supplementation impacted kidney and liver structure and function of sedentary but not of exercised rats | |
| [52] | 35 male young Wistar rats; swimming training | L: 5 g·kg-1day−1 for 1 week M: 1 g·kg-1day−1 for 40 days |
Increased hepatic and renal (urea and creatinine) biomarkers levels were observed in the groups supplemented with creatine | |
| [53] | 12 old Wistar rats | 0.3 mg/kg for 8 weeks. |
The supplemented group showed no significant organ damage, such as reductions in glomerular size or hepatic degeneration; however; low sample size | |
| [54] | 32 male Wistar rats, 16 with streptozotocin-induced type 1 DM | L: 13% (g·kg-1 of feed) for 5 days M: 2% (g·kg-1 of feed) for 35 days |
Creatine supplementation as adjuvant therapy for DM should be carefully evaluated |
Is there a therapeutic potential for creatine supplementation?
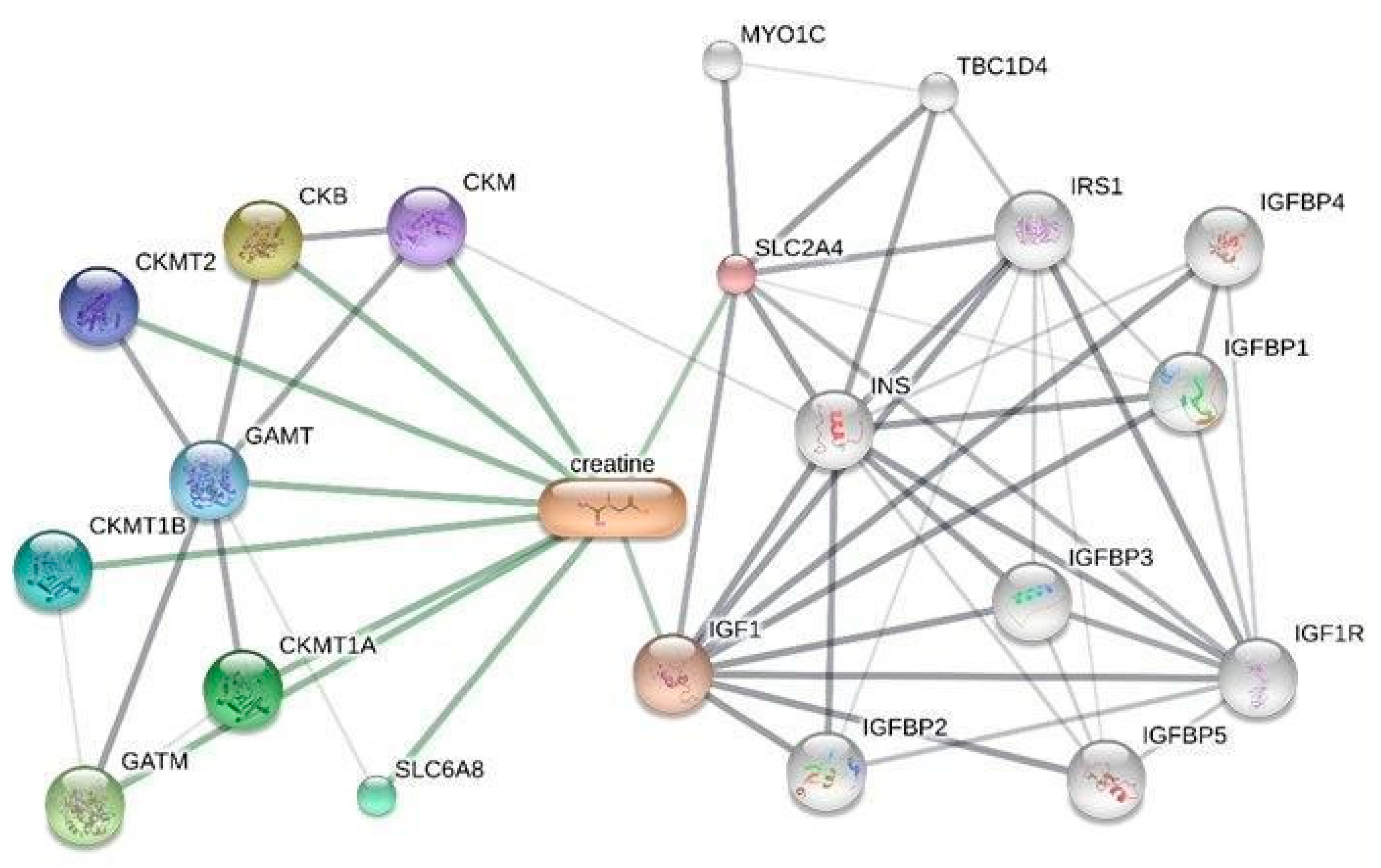
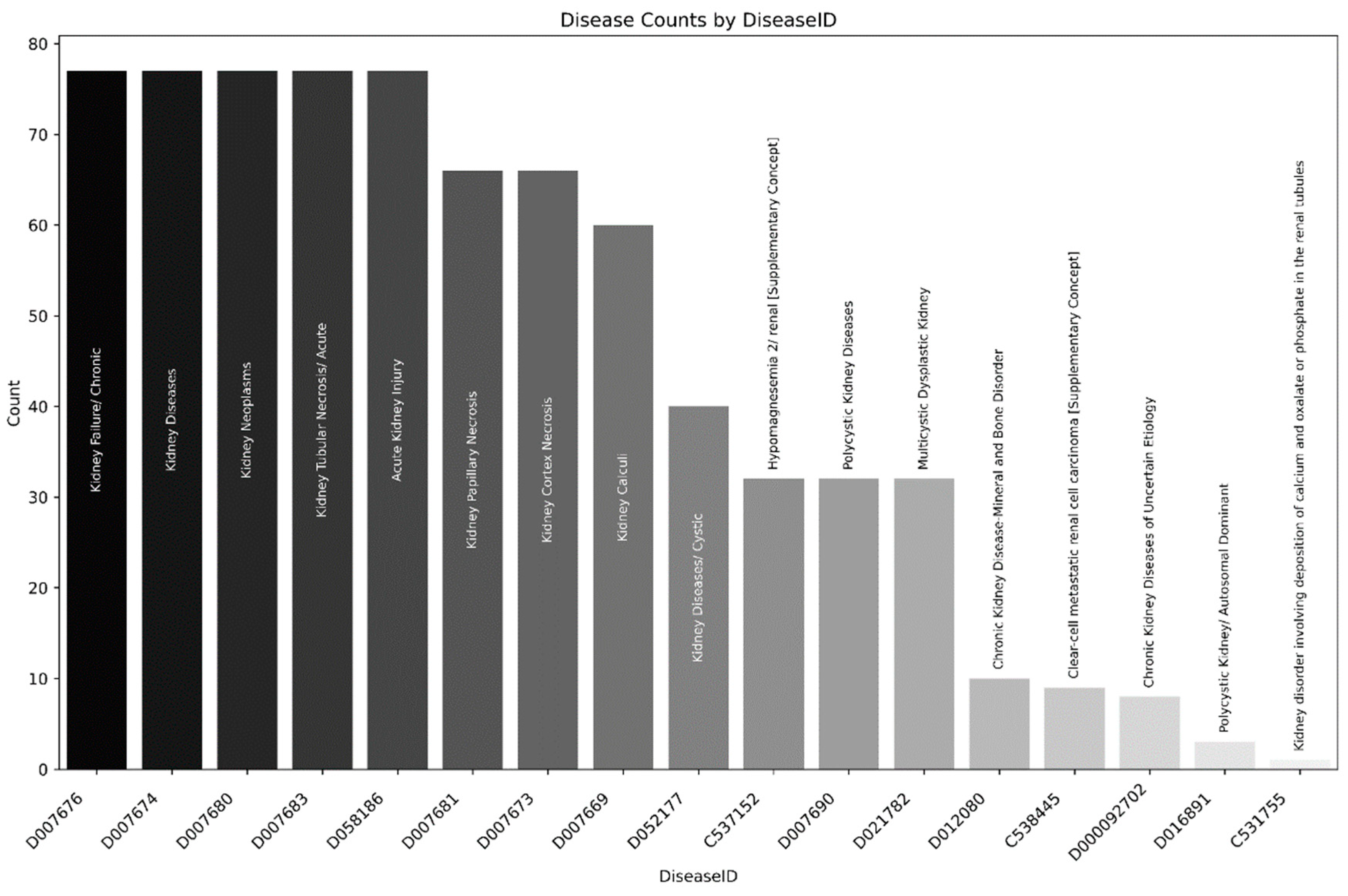
Comparative Toxicogenomics Database
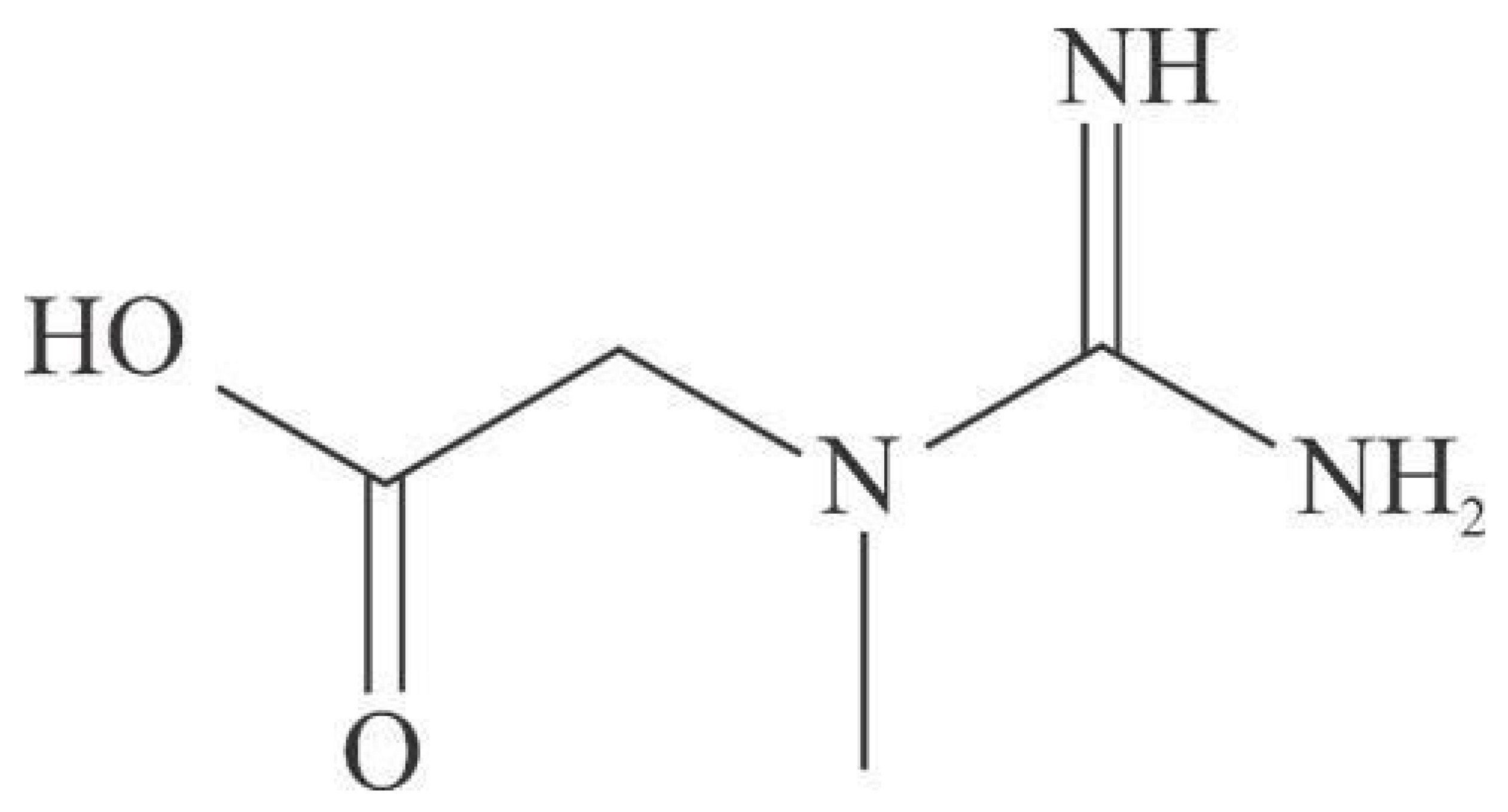
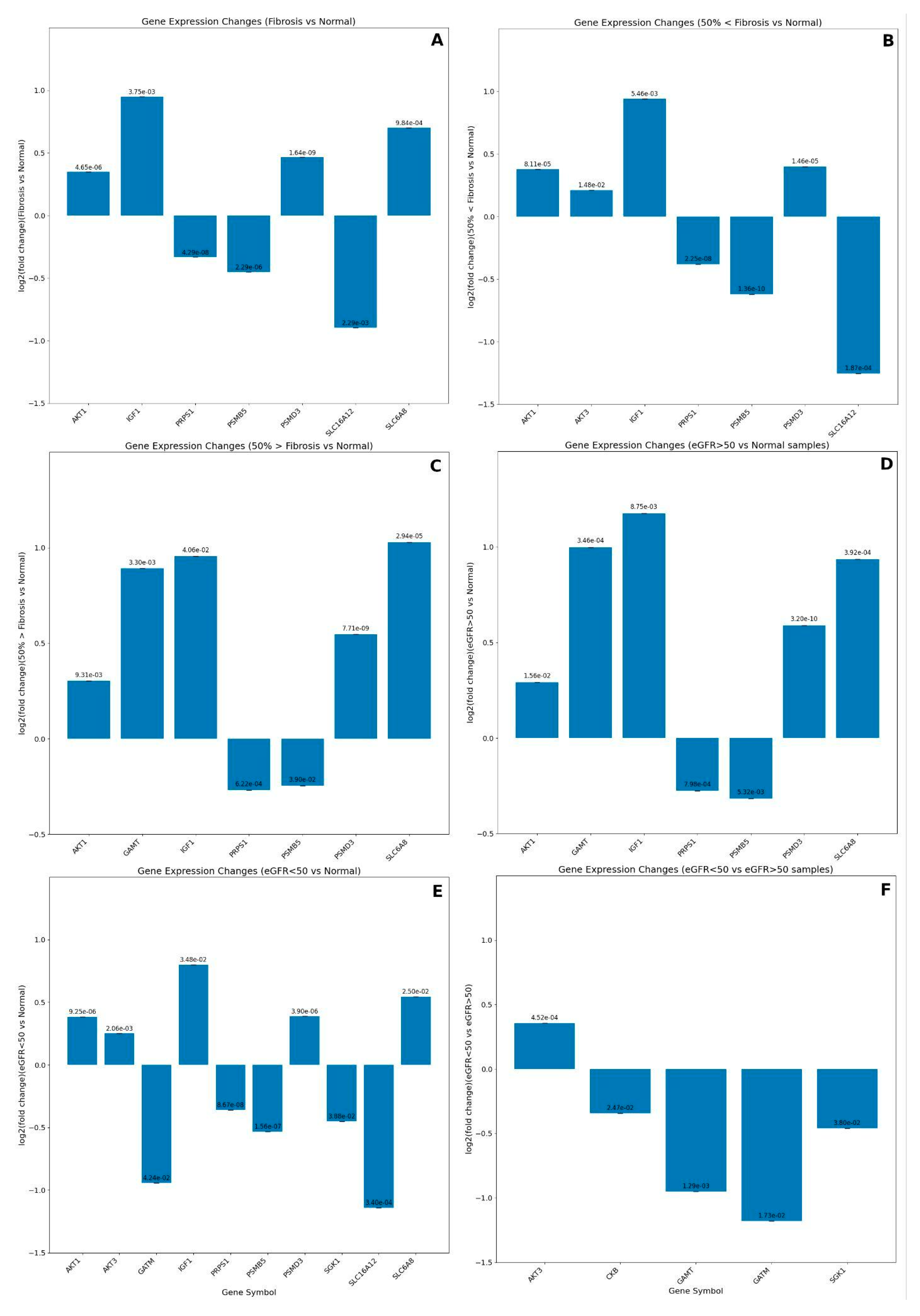
Gene Expression Analysis and Enrichment of Creatine-Related Genes
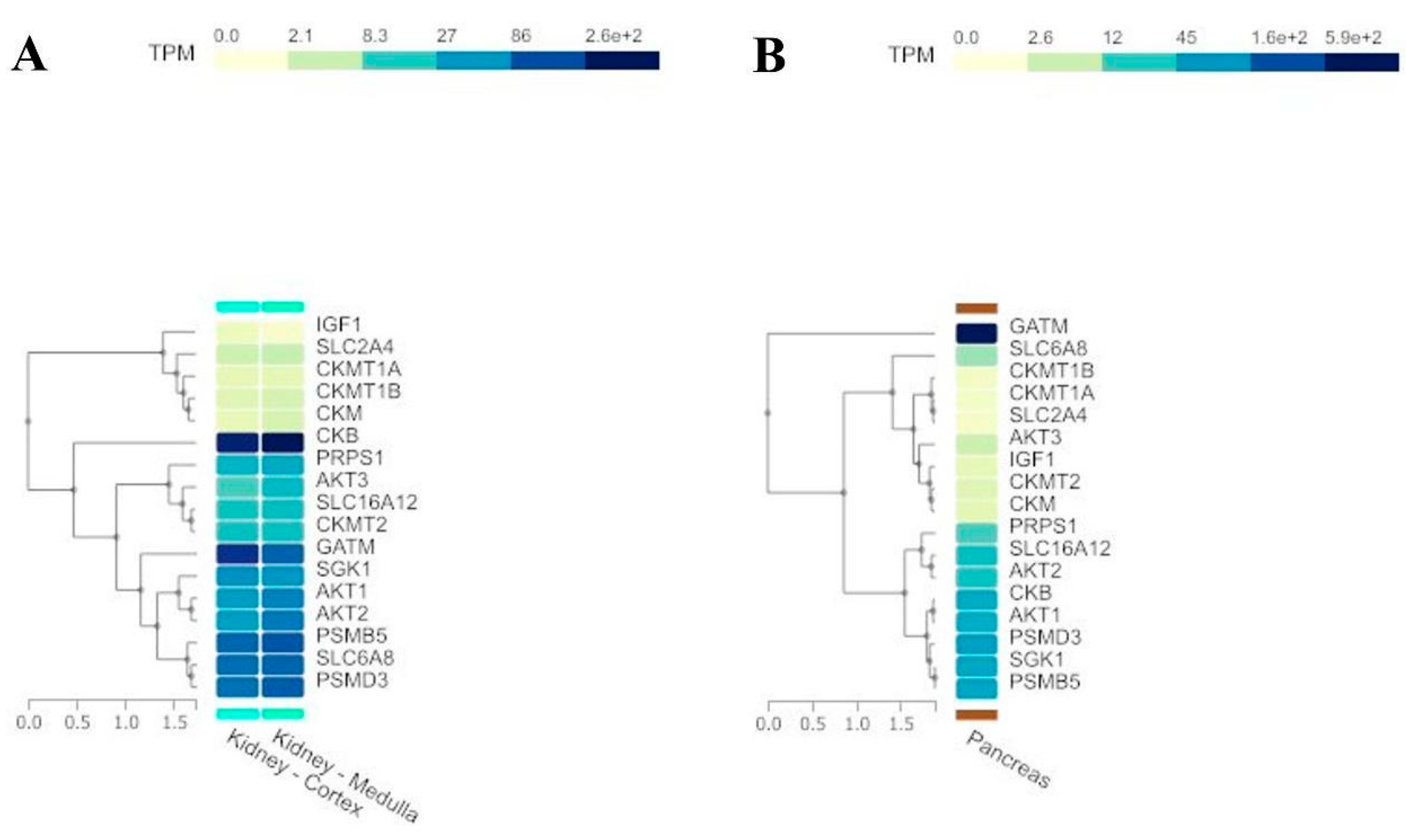
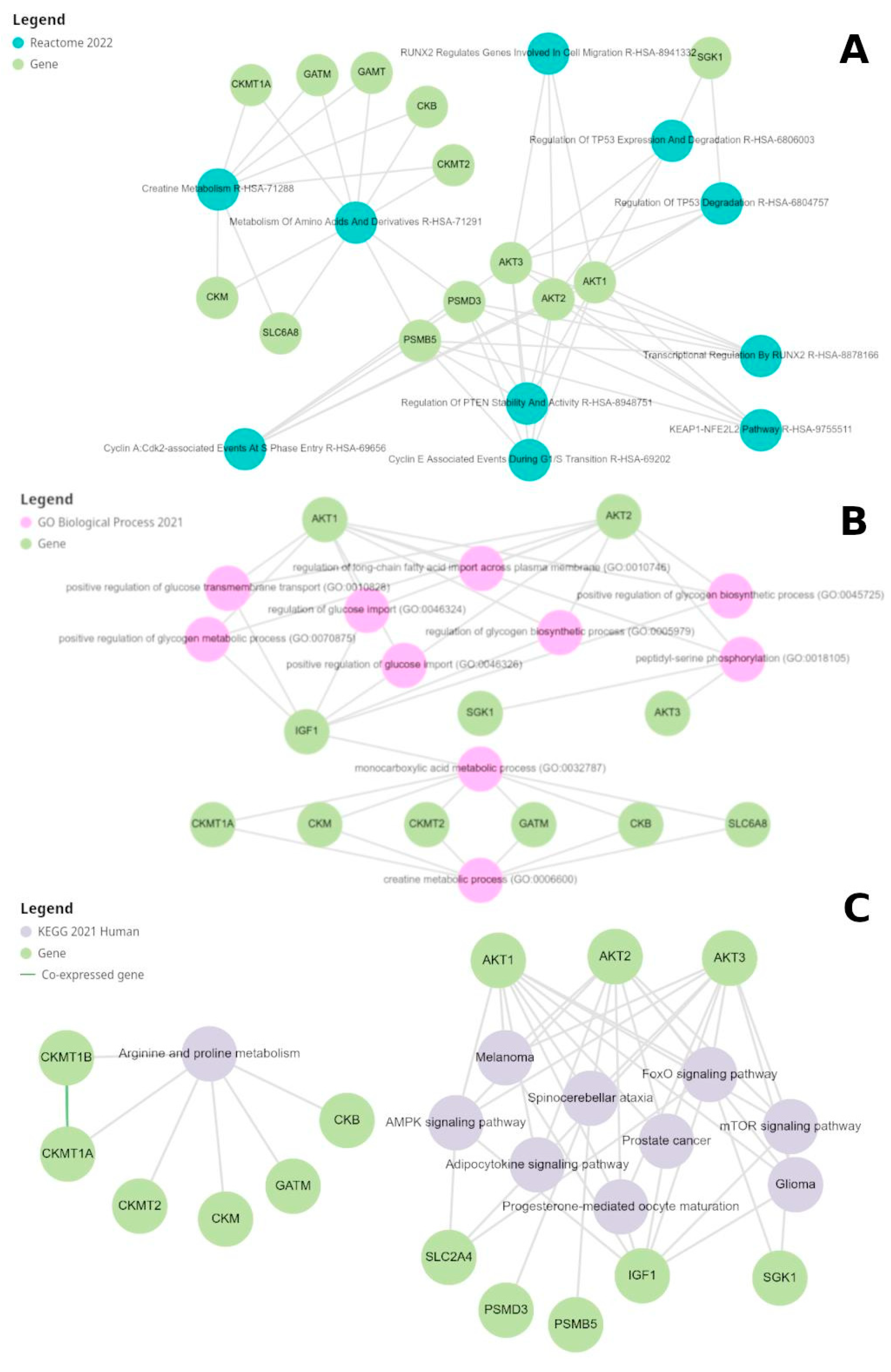
Pathway Analysis and Regulation by Kinases and Phosphatases
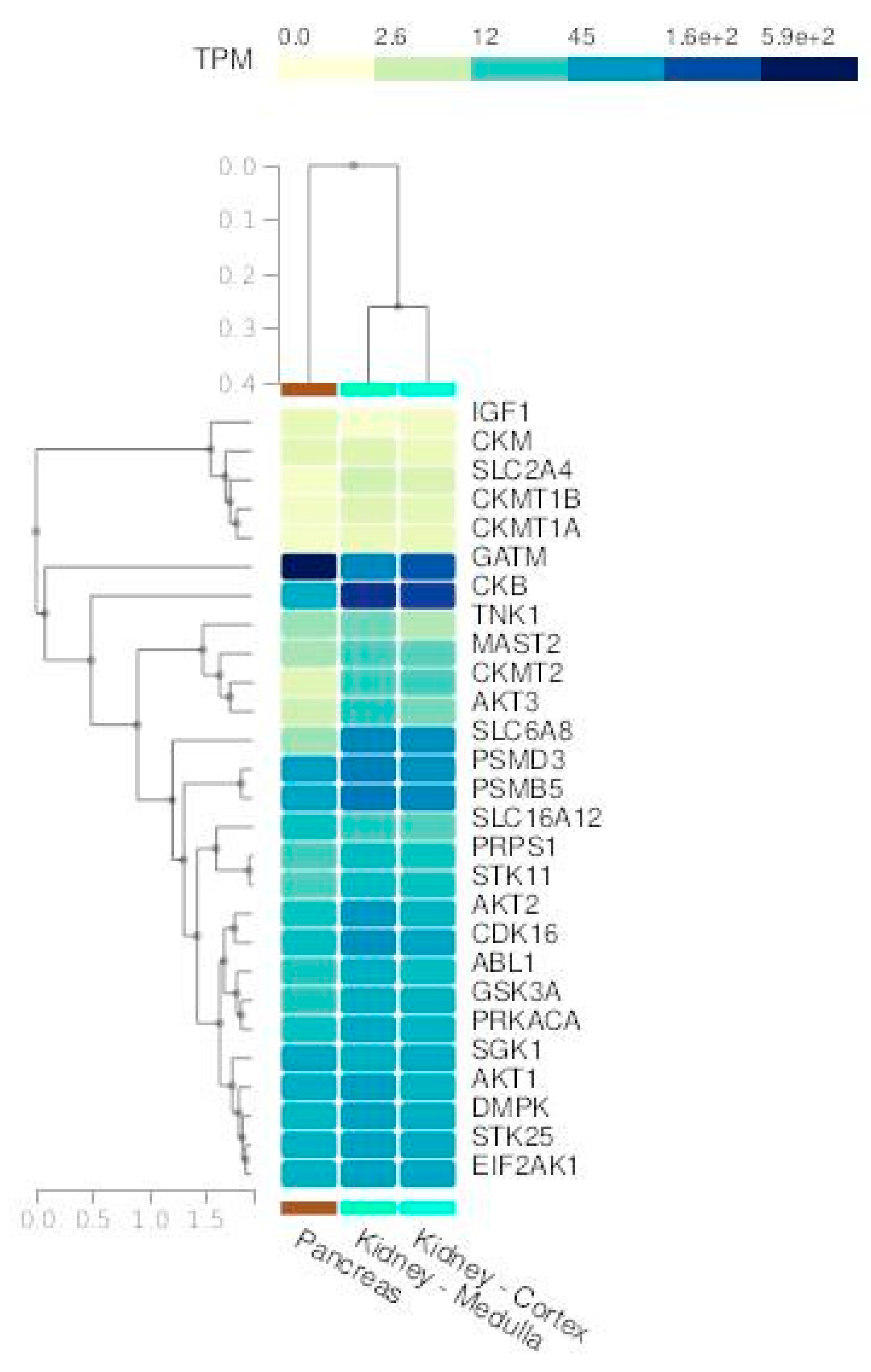
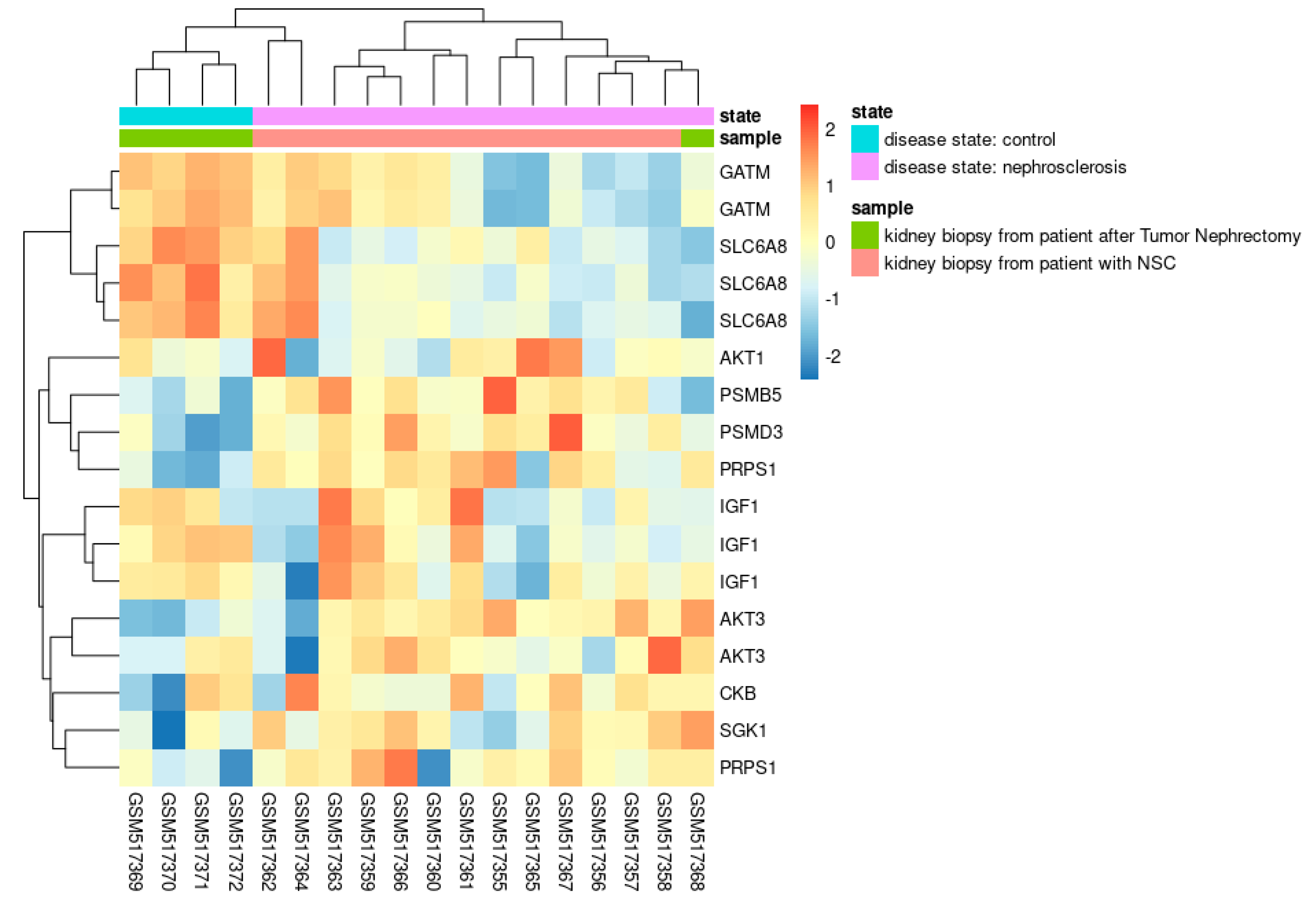
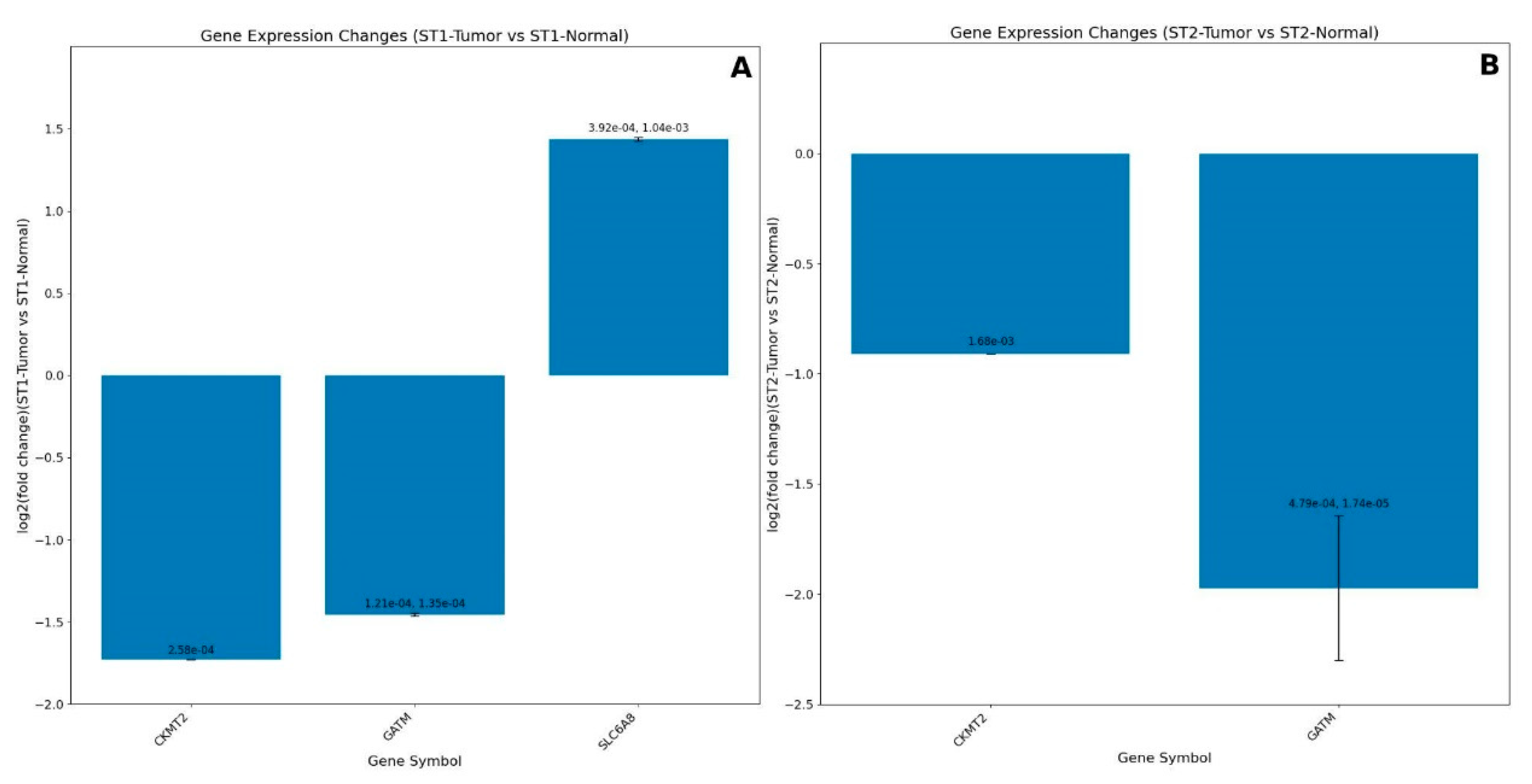
GEO datasets analyses
Future Directions
Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Gastin, P.B. Energy System Interaction and Relative Contribution During Maximal Exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Chanutin, A.B.; Guy, P. The fate of creatine when administered to man. J Biol. Chem. 1926, 67, 29–37. [Google Scholar] [CrossRef]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000, 30, 155–170. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Hall, M.; Trojian, T.H. Creatine supplementation. Curr. Sports Med. Rep. 2013, 12, 240–244. [Google Scholar] [CrossRef]
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Cordingley, D.M.; Cornish, S.M.; Gualano, B.; Roschel, H.; Ostojic, S.M.; Rawson, E.S.; Roy, B.D.; Prokopidis, K.; Giannos, P.; et al. Effects of Creatine Supplementation on Brain Function and Health. Nutrients 2022, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Triantafyllidis, K.K.; Kechagias, K.S.; Forbes, S.C.; Candow, D.G. Effects of creatine supplementation on memory in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2023, 81, 416–427. [Google Scholar] [CrossRef]
- Farquhar, W.B.; Zambraski, E.J. Effects of creatine use on the athlete's kidney. Curr. Sports Med. Rep. 2002, 1, 103–106. [Google Scholar] [CrossRef]
- Post, A.; Tsikas, D.; Bakker, S.J. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef]
- Post, A.; Groothof, D.; Kremer, D.; Knobbe, T.J.; Abma, W.; Koops, C.A.; Tsikas, D.; Wallimann, T.; Dullaart, R.P.; Franssen, C.F.; et al. Creatine homeostasis and the kidney: comparison between kidney transplant recipients and healthy controls. Amino Acids 2024, 56, 42. [Google Scholar] [CrossRef]
- Baker, S.A.; Gajera, C.R.; Wawro, A.M.; Corces, M.R.; Montine, T.J. GATM and GAMT synthesize creatine locally throughout the mammalian body and within oligodendrocytes of the brain. Brain Res. 2021, 1770, 147627. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Auquier, H.; Renaut, V.; Durussel, A.; Saugy, M.; Brisson, G.R. Effect of short-term creatine supplementation on renal responses in men. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 566–567. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med. Sci. Sports Exerc. 1999, 31, 1108–1110. [Google Scholar] [CrossRef]
- Pritchard, N.; Kalra, P. Renal dysfunction accompanying oral creatine supplements. Lancet 1998, 351, 1252–1253. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting Protein–Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): update 2023. Nucleic Acids Res. 2022, 51, D1257–D1262. [Google Scholar]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Evangelista, J.E.; Xie, Z.; Marino, G.B.; Nguyen, N.; Clarke, D.J.B.; Ma’ayan, A. Enrichr-KG: bridging enrichment analysis across multiple libraries. Nucleic Acids Res. 2023, 51, W168–W179. [Google Scholar] [CrossRef]
- Lachmann, A.; Torre, D.; Keenan, A.B.; Jagodnik, K.M.; Lee, H.J.; Wang, L.; Silverstein, M.C.; Ma’ayan, A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Johnson, J.L.; Yaron, T.M.; Huntsman, E.M.; Kerelsky, A.; Song, J.; Regev, A.; Lin, T.-Y.; Liberatore, K.; Cizin, D.M.; Cohen, B.M.; et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature 2023, 613, 759–766. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Davis, S.; Meltzer, P.S. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions using R and Bioconductor, R. Gentleman, V. Carey, S. Dudoit, R. Irizarry, W. Huber (eds.), Springer, New York, 2005, 397-420.
- Love, M.I.; Huber, W.; Anders, S. “Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. ” Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. https://ggplot2.tidyverse.org. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Centers for disease control and prevention (CDC). Hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers--North Carolina, Wisconsin, and Michigan, November-December 1997. MMWR. Morb. Mortal. Wkly. Rep. 1998, 47, 105–108. [Google Scholar]
- Kuehl, K.M.; Goldberg, L.M.; Elliot, D.M. Renal insufficiency after creatine supplementation in a college football athlete. Med. Sci. Sports Exerc. 1998, 30, 235. [Google Scholar] [CrossRef]
- Koshy, K.M.; Griswold, E.; E Schneeberger, E. Interstitial Nephritis in a Patient Taking Creatine. New Engl. J. Med. 1999, 340, 814–815. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of Oral Creatine Supplementation in a Patient with MELAS Phenotype and Associated Nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Révai, T.; Sápi, Z.; Benedek, S.; Kovács, A.; Kaszás, I.; Virányi, M.; Winkler, G. Severe nephrotic syndrome in a young man taking anabolic steroid and creatine long term. Orvosi hetilap. 2003, 144, 2425–2427. [Google Scholar] [PubMed]
- Thorsteinsdottir, B.; Grande, J.P.; Garovic, V.D. Acute Renal Failure in a Young Weight Lifter Taking Multiple Food Supplements, Including Creatine Monohydrate. J. Ren. Nutr. 2006, 16, 341–345. [Google Scholar] [CrossRef]
- Taner, B.; Aysim, O.; Abdulkadir, U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2011, 4, 23–24. [Google Scholar] [CrossRef]
- Gualano, B.; Ugrinowitsch, C.; Novaes, R.B.; Artioli, G.G.; Shimizu, M.H.; Seguro, A.C.; Harris, R.C.; Lancha, A.H., Jr. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur. J. Appl. Physiol. 2008, 103, 33–40. [Google Scholar] [CrossRef]
- Gualano, B.; Ferreira, D.C.; Sapienza, M.T.; Seguro, A.C.; Lancha, A.H., Jr. Effect of Short-term High-Dose Creatine Supplementation on Measured GFR in a Young Man With a Single Kidney. Am. J. Kidney Dis. 2010, 55, e7–e9. [Google Scholar] [CrossRef]
- Carvalho, A.P.P.F.; Molina, G.E.; Fontana, K.E. Creatine supplementation associated with resistance training does not alter renal and hepatic functions. Rev. Bras. Med. Esporte. 2011, 17, 1–5. [Google Scholar]
- Gualano, B.; de Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; da Silva, M.E.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756. [Google Scholar] [CrossRef]
- Almeida, D.; Colombini, A.; Machado, M. Creatine supplementation improves performance, but is it safe? Double-blind placebo-controlled study. J. Sports Med. Phys. Fit. 2020, 60, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.K.; Carpentier, A.; Poortmans, J.R. Studies on the safety of creatine supplementation. Amino Acids 2011, 40, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Baracho, N.C.; Castro, L.P.; Borges, N.C.; Laira, P.B. Study of renal and hepatic toxicity in rats supplemented with creatine. Acta Cir. Bras. 2015, 30, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Karimzadeh, I.; Ezzatzadegan-Jahromi, S.; Sagheb, M.M. Potential Adverse Effects of Creatine Supplement on the Kidney in Athletes and Bodybuilders. Iran. J. Kidney Dis. 2018, 12, 253–260. [Google Scholar] [PubMed]
- Edmunds, J.W.; Jayapalan, S.; DiMarco, N.M.; Saboorian, H.; Aukema, H.M. Creatine Supplementation Increases Renal Disease Progression in Han:SPRD-cy Rats. Am. J. Kidney Dis. 2001, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Taes, Y.E.C.; Delanghe, J.R.; Wuyts, B.; van de Voorde, J.; Lameire, N.H. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol. Dial. Transplant. 2003, 18, 258–264. [Google Scholar] [CrossRef]
- Genc, G.; Okuyucu, A.; Meydan, B.C.; Yavuz, O.; Nisbet, O.; Hokelek, M.; Bedir, A.; Ozkaya, O. Effect of free creatine therapy on cisplatin-induced renal damage. Ren. Fail. 2014, 36, 1108–1113. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Bergamaschi, C.D.T.; Lazaretti-Castro, M.; Heilberg, I.P. Effects of Creatine Supplementation on Body Composition and Renal Function in Rats. Med. Sci. Sports Exerc. 2005, 37, 1525–1529. [Google Scholar] [CrossRef]
- A Souza, R.; Miranda, H.; Xavier, M.; A Lazo-Osorio, R.; A Gouvea, H.; Cogo, J.C.; Vieira, R.P.; Ribeiro, W. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J Sports Sci Med 2009, 8, 672–681. [Google Scholar]
- Souza, W.M.; Heck, T.G.; Wronski, E.C.; Ulbrich, A.Z.; Boff, E. Effects of creatine supplementation on biomarkers of hepatic and renal function in young trained rats. Toxicol. Mech. Methods 2013, 23, 697–701. [Google Scholar] [CrossRef]
- Fernandes, V.A.R.; Delforno, M.C.; Banov, G.C.; Shmayev, M.; Leandro, J.V.A.; Teixeira, K.F.G.; Iatecola, A.; Cardozo, M.F.I.; Caldeira, E.J.; da Cunha, M.R. Renal, hepatic and muscle effects of creatine supplementation in an older adults experimental model. Clin. Nutr. ESPEN 2022, 48, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.G.; Medeiros, M.A.; de Lemos, L.I.C.; Pedrosa, L.d.F.C.; Santos, P.P.d.A.; Abreu, B.J.; Lima, J.P.M.S. Effects of Creatine Supplementation on Histopathological and Biochemical Parameters in the Kidney and Pancreas of Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 431. [Google Scholar] [CrossRef]
- Gualano, B.; Roschel, H.; Lancha, A.H., Jr.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: the widespread application of creatine supplementation. Amino Acids. 2012, 43, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef]
- Deminice, R.; Cella, P.S.; Padilha, C.S.; Borges, F.H.; da Silva, L.E.C.M.; Campos-Ferraz, P.L.; Jordao, A.A.; Robinson, J.L.; Bertolo, R.F.; Cecchini, R.; et al. Creatine supplementation prevents hyperhomocysteinemia, oxidative stress and cancer-induced cachexia progression in Walker-256 tumor-bearing rats. Amino Acids 2016, 48, 2015–2024. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C.; Fairman, C.M.; Gualano, B.; Roschel, H. Creatine supplementation for older adults: Focus on sarcopenia, osteoporosis, frailty and Cachexia. Bone 2022, 162, 116467. [Google Scholar] [CrossRef]
- Op 't Eijnde, B.; Ursø, B.; Richter, E.A.; Greenhaff, P.L.; Hespel, P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes 2001, 50, 18–23. [Google Scholar] [CrossRef]
- Young, J.C.; E Young, R. The effect of creatine supplementation on glucose uptake in rat skeletal muscle. Life Sci. 2002, 71, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Kineman, R. D.; Rio-Moreno, M.; del Sarmento-Cabral, A. 40 YEARS of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef]
- Bach, L.A.; Hale, L.J. Insulin-like Growth Factors and Kidney Disease. Am. J. Kidney Dis. 2015, 65, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mohebi, R.; Liu, Y.; Hansen, M.K.; Yavin, Y.; Sattar, N.; Pollock, C.A.; Butler, J.; Jardine, M.; Masson, S.; Heerspink, H.J.L.; et al. Insulin growth factor axis and cardio-renal risk in diabetic kidney disease: an analysis from the CREDENCE trial. Cardiovasc. Diabetol. 2023, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Tropak, M.B.; Tkachyova, I.; Gu, R.; Lee, A.; Schulze, A. Evidence of an intracellular creatine-sensing mechanism that modulates creatine biosynthesis via AGAT expression in human HAP1 cells. Sci. Rep. 2023, 13, 22392. [Google Scholar] [CrossRef]
- Kalucka, J.; Missiaen, R.; Georgiadou, M.; Schoors, S.; Lange, C.; De Bock, K.; Dewerchin, M.; Carmeliet, P. Metabolic control of the cell cycle. Cell Cycle 2015, 14, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-B. Creatine kinase in cell cycle regulation and cancer. Amino Acids 2016, 48, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Neusser, M.A.; Lindenmeyer, M.T.; Moll, A.G.; Segerer, S.; Edenhofer, I.; Sen, K.; Stiehl, D.P.; Kretzler, M.; Gröne, H.-J.; Schlöndorff, D.; et al. Human Nephrosclerosis Triggers a Hypoxia-Related Glomerulopathy. Am. J. Pathol. 2010, 176, 594–607. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Sun, Y.; Jin, T.; Zhu, P.; Wan, X.; Hou, Y.; Tu, G. SLC6A8-mediated intracellular creatine accumulation enhances hypoxic breast cancer cell survival via ameliorating oxidative stress. J. Exp. Clin. Cancer Res. 2021, 40, 168. [Google Scholar] [CrossRef]
- Rashidi, A.; Billingham, L.K.; Zolp, A.; Chia, T.-Y.; Silvers, C.; Katz, J.L.; Park, C.H.; Delay, S.; Boland, L.; Geng, Y.; et al. Myeloid cell-derived creatine in the hypoxic niche promotes glioblastoma growth. Cell Metab. 2024, 36, 62–77.e8. [Google Scholar] [CrossRef] [PubMed]
- Dalga, D.; Verissimo, T.; Seigneux, S. de Gluconeogenesis in the Kidney: In Health and in Chronic Kidney Disease. Clin. Kidney J. 2023, 16, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Flechner, S.M.; Kurian, S.M.; Head, S.R.; Sharp, S.M.; Whisenant, T.C.; Zhang, J.; Chismar, J.D.; Horvath, S.; Mondala, T.; Gilmartin, T.; et al. Kidney Transplant Rejection and Tissue Injury by Gene Profiling of Biopsies and Peripheral Blood Lymphocytes. Am. J. Transplant. 2004, 4, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Zou, H.; Kreinest, P.A.; Childs, A.C.; Belmonte, L.S.; LeGrand, S.N.; Wu, K.J.; Luxon, B.A.; Sinha, M.; Parker, A.S.; et al. Secreted Frizzled-Related Protein 1 Loss Contributes to Tumor Phenotype of Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 4740–4749. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.W.; Marlow, L.A.; von Roemeling, C.A.; Cooper, S.J.; Kreinest, P.; Wu, K.; Luxon, B.A.; Sinha, M.; Anastasiadis, P.Z.; Copland, J.A. Pathway Signature and Cellular Differentiation in Clear Cell Renal Cell Carcinoma. PLOS ONE 2010, 5, e10696. [Google Scholar] [CrossRef]
- Rashidi, A.; Billingham, L.K.; Zolp, A.; Chia, T.-Y.; Silvers, C.; Katz, J.L.; Park, C.H.; Delay, S.; Boland, L.; Geng, Y.; et al. Myeloid cell-derived creatine in the hypoxic niche promotes glioblastoma growth. Cell Metab. 2024, 36, 62–77. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Hu, G.; Ren, L.; Qiu, C.; Li, S.; Zhou, X.; Chen, S.; Chen, R. Reprogramming alternative macrophage polarization by GATM-mediated endogenous creatine synthesis: A potential target for HDM-induced asthma treatment. Front. Immunol. 2022, 13, 937331. [Google Scholar] [CrossRef]
- Reichold, M.; Klootwijk, E.D.; Reinders, J.; Otto, E.A.; Milani, M.; Broeker, C.; Laing, C.; Wiesner, J.; Devi, S.; Zhou, W.; et al. Glycine Amidinotransferase (GATM), Renal Fanconi Syndrome, and Kidney Failure. J. Am. Soc. Nephrol. 2018, 29, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Šalamon, Š.; Bevc, S.; Ekart, R.; Hojs, R.; Potočnik, U. Polymorphism in the GATM Locus Associated with Dialysis-Independent Chronic Kidney Disease but Not Dialysis-Dependent Kidney Failure. Genes 2021, 12, 834. [Google Scholar] [CrossRef]
- Mohebi, R.; Liu, Y.; Hansen, M.K.; Yavin, Y.; Sattar, N.; Pollock, C.A.; Butler, J.; Jardine, M.; Masson, S.; Heerspink, H.J.L.; et al. Insulin growth factor axis and cardio-renal risk in diabetic kidney disease: an analysis from the CREDENCE trial. Cardiovasc. Diabetol. 2023, 22, 176. [Google Scholar] [CrossRef]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The betaine/GABA transporter and betaine: roles in brain, kidney, and liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Dittmann, K.; Wallaschofski, H.; Rettig, R.; Stracke, S.; Endlich, K.; Völzke, H.; Nauck, M.; Friedrich, N. Association between serum insulin-like growth factor I or IGF-binding protein 3 and estimated glomerular filtration rate: results of a population-based sample. BMC Nephrol. 2012, 13, 169. [Google Scholar] [CrossRef]
- van der Veen, Y.; Post, A.; Kremer, D.; Koops, C.A.; Marsman, E.; Appeldoorn, T.Y.J.; Touw, D.J.; Westerhuis, R.; Heiner-Fokkema, M.R.; Franssen, C.F.M.; et al. Chronic Dialysis Patients Are Depleted of Creatine: Review and Rationale for Intradialytic Creatine Supplementation. Nutrients 2021, 13, 2709. [Google Scholar] [CrossRef] [PubMed]
- Pasala, S.; Carmody, J.B. How to use… serum creatinine, cystatin C and GFR. Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Ferreira, J.C.; de Siqueira-Filho, M.A.; Carvalho, C.R.; Lancha, A.H., Jr.; Gualano, B. Creatine-induced glucose uptake in type 2 diabetes: a role for AMPK-α? Amino Acids. 2012, 43, 1803–1807. [Google Scholar] [CrossRef] [PubMed]

| Clinical conditions | Effect of creatine supplementation |
|---|---|
| GAMT deficiency; AGAT deficiency; Chronic Heart Failure, Myotonic dystrophy type II, Huntington’s disease, Dystrophinopathies, Sarcopenia | Most likely beneficial |
| Cancer, Mitochondrial cytopathies, Type II diabetes, Depression, Osteoarthritis, Osteoporosis, post-traumatic stress disorder, Alzheimer’s disease, Dyslipidemia, Traumatic brain injury, Charcot Marie-Tooth disease, Fibromyalgia, Myositis, Drug addiction, Parkinson’s disease | Possibly beneficial |
| HIV infection, Myotonic dystrophy type I, Creatine transporter deficiency, Chronic obstructive pulmonary disease, Facioscapulohumeral dystrophy, Amyotrophic lateral sclerosis, Schizophrenia | Unlikely beneficial |
| Gene Symbol (Homo sapiens, txid:9606) | Short description |
|---|---|
| SLC2A4 | Insulin-regulated facilitative glucose transporter GLUT4. |
| IGF1 | Insulin-like growth factor 1, a hormone involved in growth and development. |
| GATM | Glycine amidinotransferase, key in creatine biosynthesis. |
| GATM | Guanidinoacetate N-Methyltransferase, converts GAA in creatine. |
| SLC6A8 | Creatine transporter involved in transporting creatine into cells. |
| AKT1 | AKT serine-threonine protein kinase 1, involved in signaling pathways regulating cell growth and survival. |
| AKT2 | AKT serine-threonine protein kinase 2, involved in metabolism and insulin signaling. |
| AKT3 | AKT serine-threonine protein kinase 3, involved in brain development and function. |
| PSMB5 | A component of the 20S core proteasome complex involved in protein degradation. |
| PSMD3 | A non-ATPase subunit of the 26S proteasome involved in protein degradation. |
| SGK1 | Serum/glucocorticoid regulated kinase 1, involved in cellular stress response. |
| PRPS1 | Phosphoribosyl pyrophosphate synthetase 1, involved in purine metabolism and nucleotide biosynthesis. |
| CKB | Creatine kinase B, which plays a role in energy homeostasis by transferring phosphate between ATP and various phosphogens. |
| CKM | Creatine kinase M, involved in energy homeostasis and is a marker for myocardial infarction. |
| CKMT1A | Ubiquitous mitochondrial creatine kinase, involved in energy transduction and impaired in various diseases. |
| CKMT1B | Identical protein to CKMT1A and is also involved in energy transduction in tissues with large energy demands. |
| CKMT2 | Sarcomeric mitochondrial creatine kinase, involved in energy transduction in muscle tissues. |
| SLC16A12 | Transporter likely involved in monocarboxylic acid transport and associated with juvenile cataracts and renal glucosuria. |
| Kinase Gene Symbol | Short description (Source: GeneCards) |
|---|---|
| CAMKK2 | Calcium/calmodulin-dependent protein kinase kinase 2, involved in various signaling pathways. |
| MAP2K2 | Mitogen-activated protein kinase kinase 2, a key component in the MAPK/ERK pathway. |
| VRK1 | Serine/threonine-protein kinase VRK1, involved in cell cycle and nuclear envelope formation. |
| PDK4 | Pyruvate dehydrogenase kinase isozyme 4, regulating glucose and fatty acid metabolism. |
| PLK4 | Polo-like kinase 4, a regulator of centriole duplication in cell cycle progression. |
| PDK1 | Pyruvate dehydrogenase kinase 1, regulating glucose metabolism by phosphorylating pyruvate dehydrogenase. |
| DMPK | Myotonic dystrophy protein kinase, involved in muscle, heart, and brain function. |
| AAK1 | AP2 associated kinase 1, involved in clathrin-mediated endocytosis. |
| TBK1 | TANK-binding kinase 1, central in innate immune response and inflammation. |
| GAK | Cyclin G-associated kinase, involved in cell cycle regulation and clathrin-mediated endocytosis |
| NEK7 | Serine/threonine-protein kinase involved in mitotic cell cycle progression and cytokinesis. |
| PBK | Serine/threonine-protein kinase involved in mitotic cell cycle and DNA damage response. |
| BMP2K | Kinase implicated in bone morphogenic protein signaling and osteoblast differentiation. |
| MASTL | Kinase that regulates mitosis entry and maintenance which is involved in cell cycle checkpoint recovery. |
| VRK2 | Kinase involved in cell cycle regulation and neuronal apoptosis. |
| STK25 | Kinase that regulates stress response and cell migration. |
| CDK16 | Kinase involved in vesicle-mediated transport and exocytosis. |
| MAST2 | Kinase that regulates spermatid differentiation and interleukin-12 production. |
| PRKACA | Catalytic subunit of protein kinase A, involved in various cellular processes including metabolism and memory. |
| ABL1 | Tyrosine-protein kinase involved in cell differentiation, division, adhesion, and stress response. |
| STK11 | Serine/threonine kinase 11, also known as liver kinase B1 (LKB1), which regulates cell polarity and functions as a tumor suppressor. |
| TNK1 | Tyrosine kinase non-receptor 1, involved in negative regulation of cell growth and acting as a tumor suppressor. |
| EIF2AK1 | Eukaryotic translation initiation factor 2-alpha kinase 1, which plays a role in the cellular response to stress by phosphorylating the alpha subunit of the eukaryotic translation initiation factor 2 (eIF2). |
| GSK3A | Glycogen synthase kinase 3 alpha, involved in energy metabolism, neuronal cell development, and body pattern formation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





