Submitted:
24 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
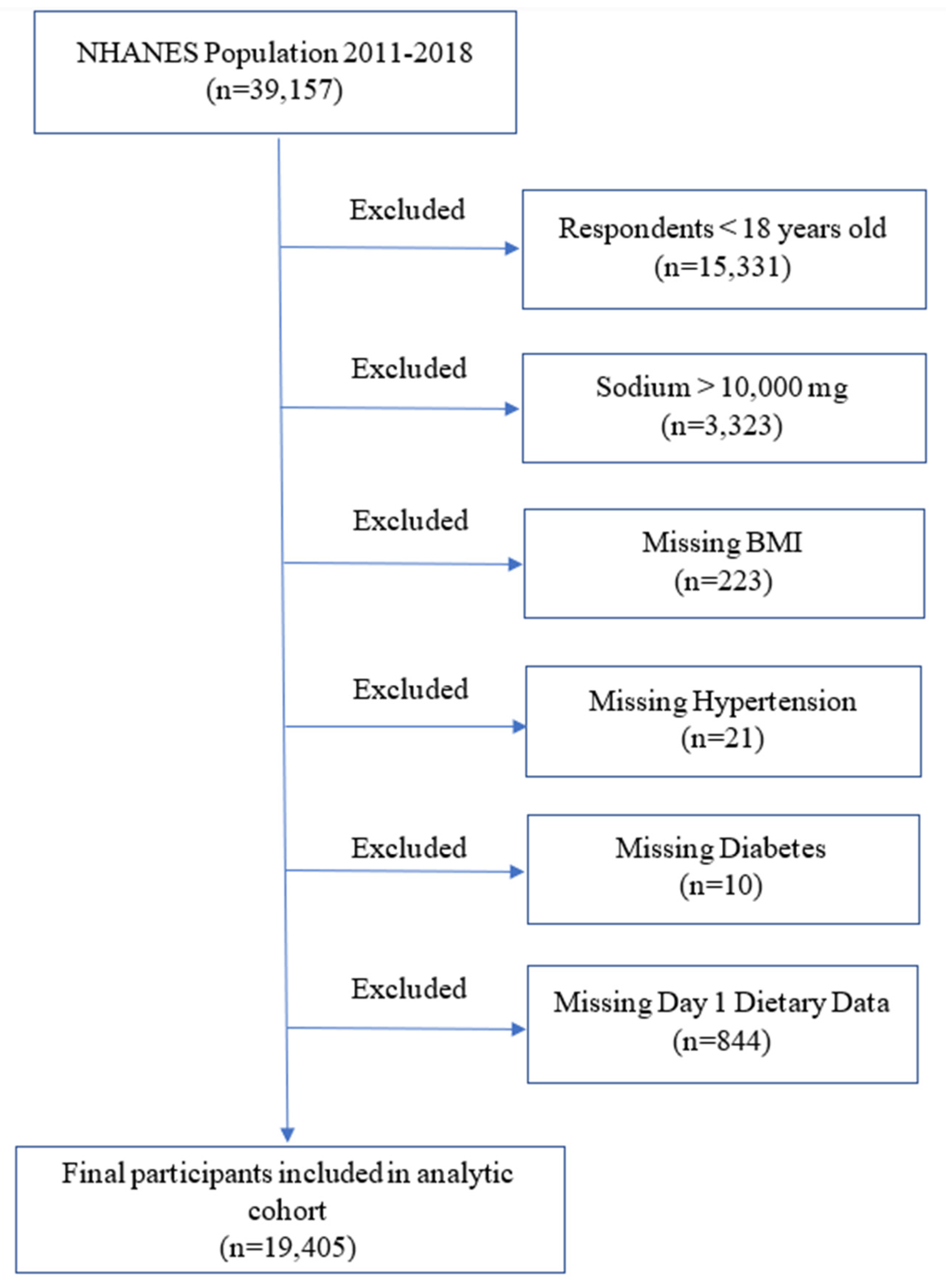
2.1. Study Population
2.2. Primary Predictors and Outcomes
2.3. Covariates
2.4. Data Analysis and Statistical Methods
2.5. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Dietary Sodium and Potassium Intakes and Prevalent Kidney Stone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63(5):1817-1823. [CrossRef]
- Saigal CS, Joyce G, Timilsina AR, Urologic Diseases in America P. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68(4):1808-1814. [CrossRef]
- Finkielstein, V.A.; Goldfarb, D.S. Strategies for preventing calcium oxalate stones. Can. Med Assoc. J. 2006, 174, 1407–1409. [CrossRef]
- Sakhaee, K.; Harvey, J.A.; Padalino, P.K.; Whitson, P.; Pak, C.Y. The Potential Role of Salt Abuse on the Risk for Kidney Stone Formation. J. Urol. 1993, 150, 310–312. [CrossRef]
- Taylor, E.N.; Curhan, G.C. Demographic, Dietary, and Urinary Factors and 24-h Urinary Calcium Excretion. Clin. J. Am. Soc. Nephrol. 2009, 4, 1980–1987. [CrossRef]
- Sorensen, M.D.; Kahn, A.J.; Reiner, A.P.; Tseng, T.Y.; Shikany, J.M.; Wallace, R.B.; Chi, T.; Wactawski-Wende, J.; Jackson, R.D.; O’Sullivan, M.J.; et al. Impact of Nutritional Factors on Incident Kidney Stone Formation: A Report From the WHI OS. J. Urol. 2012, 187, 1645–1650. [CrossRef]
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004;164(8):885-891. [CrossRef]
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232. [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A Prospective Study of Dietary Calcium and Other Nutrients and the Risk of Symptomatic Kidney Stones. N. Engl. J. Med. 1993, 328, 833–838. [CrossRef]
- Lemann J, Jr., Pleuss JA, Gray RW, Hoffmann RG. Potassium administration reduces and potassium deprivation increases urinary calcium excretion in healthy adults [corrected]. Kidney Int. 1991;39(5):973-983. [CrossRef]
- Curhan, G.C.; Speizer, F.E.; Spiegelman, D.; Stampfer, M.J. Comparison of Dietary Calcium with Supplemental Calcium and Other Nutrients as Factors Affecting the Risk for Kidney Stones in Women. Ann. Intern. Med. 1997, 126, 497–504. [CrossRef]
- Ferraro PM, Mandel EI, Curhan GC, Gambaro G, Taylor EN. Dietary Protein and Potassium, Diet-Dependent Net Acid Load, and Risk of Incident Kidney Stones. Clin J Am Soc Nephrol. 2016;11(10):1834-1844. [CrossRef]
- US Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans. US Department of Health and Human Services: Washington, DC, 2015, p. 144.
- Weaver CM, Stone MS, Lobene AJ, Cladis DP, Hodges JK. What Is the Evidence Base for a Potassium Requirement? Nutr Today. 2018;53(5):184-195. [CrossRef]
- Medicine Io. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. National Academies Press. 2005.
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: implications for public health. Int. J. Epidemiol. 2009, 38, 791-813. [CrossRef]
- Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297(6644):319-328.
- Walser, M. Calcium clearance as a function of sodium clearance in the dog. Am. J. Physiol. Content 1961, 200, 1099–1104. [CrossRef]
- Muldowney, F.P.; Freaney, R.; Moloney, M.F. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982, 22, 292–296. [CrossRef]
- Kleeman, C.R.; Bohannan, J.; Bernstein, D.; Ling, S.; Maxwell, M.H. Effect of Variations in Sodium Intake on Calcium Excretion in Normal Humans.. Exp. Biol. Med. 1964, 115, 29–32. [CrossRef]
- Shortt, C.; Madden, A.; Flynn, A.; A Morrissey, P. Influence of dietary sodium intake on urinary calcium excretion in selected Irish individuals. Eur J Clin Nutr. 1988, 42, 595–603.
- Nordin, B.E.C.; Need, A.G.; A Morris, H.; Horowitz, M. The Nature and Significance of the Relationship between Urinary Sodium and Urinary Calcium in Women. J. Nutr. 1993, 123, 1615–1622. [CrossRef]
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3-10. [CrossRef]
- Eisner, B.H.; Eisenberg, M.L.; Stoller, M.L. Impact of Urine Sodium on Urine Risk Factors for Calcium Oxalate Nephrolithiasis. J. Urol. 2009, 182, 2330–2333. [CrossRef]
- Wang, M.-X.; Cuevas, C.A.; Su, X.-T.; Wu, P.; Gao, Z.-X.; Lin, D.-H.; McCormick, J.A.; Yang, C.-L.; Wang, W.-H.; Ellison, D.H. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018, 93, 893–902. [CrossRef]
- Sebastian, A.; Hernandez, R.E.; Portale, A.A.; Colman, J.; Tatsuno, J.; Morris, R.C. Dietary potassium influences kidney maintenance of serum phosphorus concentration. Kidney Int. 1990, 37, 1341–1349. [CrossRef]
- Osorio, A.V.; Alon, U.S. The Relationship Between Urinary Calcium, Sodium, and Potassium Excretion and the Role of Potassium in Treating Idiopathic Hypercalciuria. 1997, 100, 675–681. [CrossRef]
- Osis, G.; Webster, K.L.; Harris, A.N.; Lee, H.-W.; Chen, C.; Fang, L.; Romero, M.F.; Khattri, R.B.; Merritt, M.E.; Verlander, J.W.; et al. Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A. Am. J. Physiol. Physiol. 2019, 317, F489–F501. [CrossRef]
- Domrongkitchaiporn, S.; Stitchantrakul, W.; Kochakarn, W. Causes of Hypocitraturia in Recurrent Calcium Stone Formers: Focusing on Urinary Potassium Excretion. Am. J. Kidney Dis. 2006, 48, 546–554. [CrossRef]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009, 11, 134–44.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290-2298. [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT study findings. Public health and medical care implications.. Hypertension 1989, 14, 570–577. [CrossRef]
- Cook NR, Obarzanek E, Cutler JA, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169(1):32-40. [CrossRef]
- Mattes, R.D.; Donnelly, D. Relative contributions of dietary sodium sources. J. Am. Coll. Nutr. 1991, 10, 383–393. [CrossRef]
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M.; et al. Medical Management of Kidney Stones: AUA Guideline. J. Urol. 2014, 192, 316–324. [CrossRef]
| Stone Former | Non-Stone Former | P Value | ||
|---|---|---|---|---|
| Total number | 1895 | 17510 | ||
| Age (years) | 54 (± 0.4) | 47 (± 0.3) | <0.001 | |
| Sex (male %) | 985 (52) | 8230 (47) | 0.01 | |
| Race (Non-Hispanic White %) | 1421 (75) | 11382 (65) | <0.001 | |
| BMI (mg/m2) | 30.9 (± 0.2) | 29.2 (± 0.1) | <0.001 | |
| History of hypertension (%) | 910 (48) | 5603 (32) | <0.001 | |
| History of diabetes (%) | 360 (19) | 1576 (9) | <0.001 | |
| History of dyslipidemia (%) | 872 (46) | 5778 (33) | <0.001 | |
| Cardiovascular disease (%) | 284 (15) | 1401 (8) | <0.001 | |
| Thiazide use (%) | 208 (11) | 1226 (7) | <0.001 | |
| Smoking (%) | Everyday/some day | 379 (20) | 3152 (18) | <0.001 |
| Past smoker | 569 (30) | 4202 (24) | ||
| Not at all | 948 (50) | 10156 (58) | ||
| Alcohol (%) | Heavy | 38 (2) | 1051 (6) | 0.001 |
| Light | 360 (19) | 3852 (22) | ||
| None | 1477 (78) | 12607 (72) | ||
| DSI | Mean (mg) | 3438 (± 56) | 3532 (± 17) | 0.1 |
| <2300mg/d (%) | 493 (26) | 4202 (24) | 0.2 | |
| DPI | Mean (mg) | 2572 (± 43) | 2665 (± 17) | 0.03 |
| ≥3500 mg/d (%) | 360 (19) | 3677 (21) | 0.2 | |
| DSI/DPI | 1.5 (± 0.03) | 1.4 (± 0.01) | 0.2 | |
| DSI (mg/day) | P Value | |||||
|---|---|---|---|---|---|---|
| 0 - 2226 | 2227 - 3149 | 3150 - 4321 | >4321 | |||
| Age (years) | 50 (± 0.4) | 49 (± 0.4) | 47 (± 0.4) | 43 (± 0.4) | <0.0001 | |
| Sex (male %) | 1538 (30) | 1999 (39) | 2566 (50) | 3634 (71) | <0.0001 | |
| Race (Non-Hispanic White %) | 3230 (63) | 3434 (67) | 3438 (67) | 3327 (65) | 0.0003 | |
| BMI (kg/m2) | 28.8 (± 0.2) | 29.1 (± 0.2) | 29.2 (± 0.2) | 29.8 (± 0.2) | <0.0001 | |
| History of hypertension (%) | 1846 (36) | 1691 (33) | 1642 (32) | 1638 (32) | 0.0003 | |
| History of diabetes (%) | 564 (11) | 564 (11) | 462 (9) | 461 (9) | 0.1 | |
| History of dyslipidemia (%) | 1794 (35) | 1794 (35) | 1642 (32) | 1587 (31) | 0.01 | |
| Cardiovascular disease (%) | 564 (11) | 513 (10) | 411 (8) | 307 (6) | <0.0001 | |
| Thiazide use (%) | 410 (8) | 410 (8) | 359 (7) | 358 (7) | 0.3 | |
| Smoking (%) | Active | 1025 (20) | 820 (16) | 872 (17) | 972 (19) | <0.0001 |
| Past smoker | 1128 (22) | 1230 (24) | 1334 (26) | 1280 (25) | ||
| Not at all | 2974 (58) | 3024 (59) | 2925 (57) | 2866 (56) | ||
| Alcohol (%) | Heavy | 3999 (78) | 3844 (75) | 3695 (72) | 3634 (71) | <0.0001 |
| Light | 974 (19) | 1128 (22) | 1180 (23) | 1126 (22) | ||
| None | 154 (3) | 154 (3) | 257 (5) | 205 (4) | ||
| DPI (mg/day) | P Value | |||||
|---|---|---|---|---|---|---|
| 0-1698 | 1699-2374 | 2375-3209 | >3209 | |||
| Age (years) | 45 (± 0.5) | 47 (± 0.5) | 48 (± 0.4) | 48 (± 0.4) | 0.0003 | |
| Sex (male %) | 1693 (33) | 2049 (40) | 2462 (48) | 3481 (68) | <0.0001 | |
| Race (Non-Hispanic White %) | 3027 (59) | 3278 (64) | 3540 (69) | 3532 (69) | <0.0001 | |
| BMI (kg/m2) | 29.8 (± 0.2) | 29.2 (± 0.2) | 29.3 (± 0.2) | 28.7 (± 0.2) | 0.0001 | |
| History of hypertension (%) | 1744 (34) | 1741 (34) | 1693 (33) | 1638 (32) | 0.6 | |
| History of diabetes (%) | 616 (12) | 512 (10) | 513 (10) | 461 (9) | 0.0006 | |
| History of dyslipidemia (%) | 1539 (30) | 1741 (34) | 1744 (34) | 1792 (35) | 0.0002 | |
| Cardiovascular disease (%) | 564 (11) | 461 (9) | 410 (8) | 410 (8) | 0.0002 | |
| Thiazide use (%) | 359 (7) | 410 (8) | 410 (8) | 358 (7) | 0.4 | |
| Smoking (%) | Active | 1180 (23) | 973 (19) | 821 (16) | 870 (17) | <0.0001 |
| Past smoker | 975 (19) | 1127 (22) | 1334 (26) | 1485 (29) | ||
| Not at all | 2975 (58) | 3022 (59) | 2975 (58) | 3020 (59) | ||
| Alcohol (%) | Heavy | 4258 (83) | 3893 (76) | 3642 (71) | 3532 (69) | <0.0001 |
| Light | 770 (15) | 1076 (21) | 1283 (25) | 1229 (24) | ||
| None | 103 (2) | 154 (3) | 205 (4) | 870 (17) | ||
| DSI/DPI | P Value | |||||
|---|---|---|---|---|---|---|
| <1.01 | 1.01-1.35 | 1.35-1.77 | >1.77 | |||
| Age (years) | 52 (± 0.4) | 49 (± 0.4) | 46 (± 0.4) | 41 (± 0.4) | <0.0001 | |
| Sex (male %) | 2181 (43) | 2477 (48) | 2553 (50) | 2686 (52) | <0.0001 | |
| Race (Non-Hispanic White %) | 3500 (69) | 3457 (67) | 3369 (66) | 3100 (60) | <0.0001 | |
| BMI (kg/m2) | 28.1 (± 0.2) | 28.9 (± 0.2) | 29.8 (± 0.2) | 30.2 (± 0.2) | <0.0001 | |
| History of hypertension (%) | 1724 (34) | 1754 (34) | 1736 (34) | 1601 (31) | 0.05 | |
| History of diabetes (%) | 507 (10) | 568 (11) | 511 (10) | 517 (10) | 0.6 | |
| History of dyslipidemia (%) | 1877 (37) | 1858 (36) | 1685 (33) | 1395 (27) | <0.0001 | |
| Cardiovascular disease (%) | 558 (11) | 464 (9) | 408 (8) | 362 (7) | 0.001 | |
| Thiazide use (%) | 406 (8) | 413 (8) | 408 (8) | 362 (7) | 0.6 | |
| Smoking (%) | Active | 862 (17) | 826 (16) | 919 (18) | 1137 (22) | <0.0001 |
| Past smoker | 1319 (26) | 1393 (27) | 1225 (24) | 1033 (20) | ||
| Not at all | 2891 (57) | 2941 (57) | 2961 (58) | 2996 (58) | ||
| Alcohol (%) | Heavy | 3601 (71) | 3767 (73) | 3727 (73) | 4081 (79) | <0.0001 |
| Light | 1217 (24) | 1135 (22) | 1123 (22) | 930 (18) | ||
| None | 254 (5) | 258 (5) | 204 (4) | 207 (4) | ||
| OR (95% CI) | P Value | ||
|---|---|---|---|
| DSI | |||
| Continuous variable | 0.99 (0.99-1.00) | 0.2 | |
| Categorial variable | Quartile 4 vs. 1 | 0.84 (0.68-1.04) | 0.1 |
| Quartile 3 vs. 1 | 1.05 (0.85-1.30) | 0.6 | |
| Quartile 2 vs. 1 | 0.95 (0.79-1.10) | 0.6 | |
| ≤2300 mg vs. >2300 mg | 1.10 (0.93-1.20) | 0.3 | |
| DPI | |||
| Continuous variable | 0.99 (0.99-0.99) | 0.02 | |
| Categorial variable | Quartile 4 vs. 1 | 0.75 (0.60-0.94) | 0.01 |
| Quartile 3 vs. 1 | 0.82 (0.67-1.01) | 0.06 | |
| Quartile 2 vs. 1 | 0.82 (0.68-0.97) | 0.02 | |
| >3500 mg vs. ≤ 3500 mg | 0.87 (0.72-1.04) | 0.1 | |
| DSI/DPI | |||
| Continuous variable | 1.10 (1.01-1.20) | 0.03 | |
| Categorial variable | Quartile 4 vs. 1 | 1.30 (1.10-1.70) | 0.008 |
| Quartile 3 vs. 1 | 1.20 (0.99-1.40) | 0.06 | |
| Quartile 2 vs. 1 | 1.20 (0.91-1.50) | 0.2 | |
| >0.6 vs. ≤ 0.6 | 1.20 (0.86-1.67) | 0.3 | |
| DSI | DPI | DSI/DPI | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P Value | |
| Age (years) | 1.02 (1.01-1.02) | <0.001 | 1.02 (1.01-1.02) | <0.001 | 1.02 (1.01-1.02) | <0.001 |
| Sex (Male) | 1.20 (1.04-1.50) | 0.02 | 1.30 (1.10-1.50) | 0.005 | 1.2 (1.01-1.40) | 0.03 |
| Race (White) | 2.30 (1.90-2.70) | <0.001 | 2.30 (2.00-2.70) | <0.001 | 2.3 (1.97-2.70) | <0.001 |
| BMI (>30 kg/m2) | 1.70 (1.40-1.96) | <0.001 | 1.60 (1.40-1.90 | <0.001 | 1.6 (1.40-1.90) | <0.001 |
| History of hypertension | 1.30 (1.04-1.50) | 0.02 | 1.20 (1.03-1.50) | 0.02 | 1.2 (1.03-1.50) | 0.02 |
| History of diabetes | 1.50 (1.20-1.80) | <0.001 | 1.50 (1.20-1.80) | <0.001 | 1.5 (1.20-1.80) | <0.001 |
| History of dyslipidemia | 1.20 (1.01-1.30 | 0.03 | 1.20 (1.01-1.30 | 0.03 | 1.2 (1.03-1.50) | 0.03 |
| Cardiovascular disease | 1.20 (0.92-1.50) | 0.2 | 1.1 (0.91-1.50) | 0.3 | 1.2 (0.92-1.50) | 0.2 |
| Thiazide use | 1.10 (0.86-1.30) | 0.6 | 1.1 (0.85-1.30) | 0.6 | 1.05 (0.85-1.30) | 0.6 |
| Smoking (active) | 1.30 (1.10-1.50) | 0.01 | 1.3 (1.04-1.50) | 0.02 | 1.3 (1.10-1.50) | 0.01 |
| Alcohol (heavy) | 0.49 (0.32-0.76) | 0.002 | 0.51 (0.33-0.78) | 0.002 | 0.5 (0.33-0.76) | 0.002 |
| DSI | 0.99 (0.99-1.00) | 0.2 | N/A | N/A | N/A | N/A |
| DPI | N/A | N/A | 0.99 (0.99-0.99) | 0.02 | N/A | N/A |
| DSI/DPI | N/A | N/A | N/A | N/A | 1.1 (1.01-1.20) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





