Submitted:
24 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
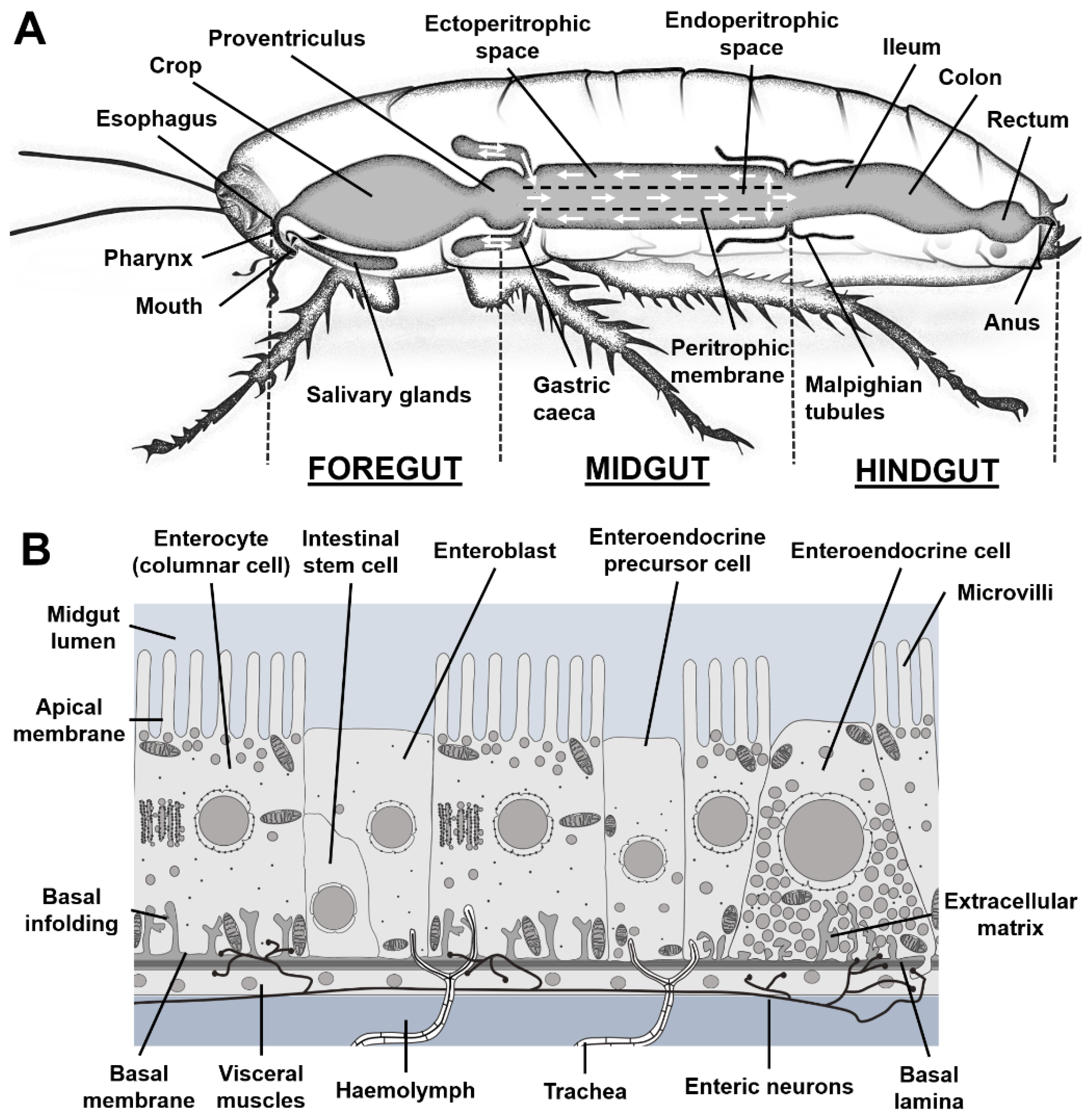
1. The Insect Digestive Tract
1.1. Structure of the Digestive Tract
1.2. Cell Types and Their Functions
2. Essential and Non-Essential Dietary Lipids
2.1. Dietary Lipids
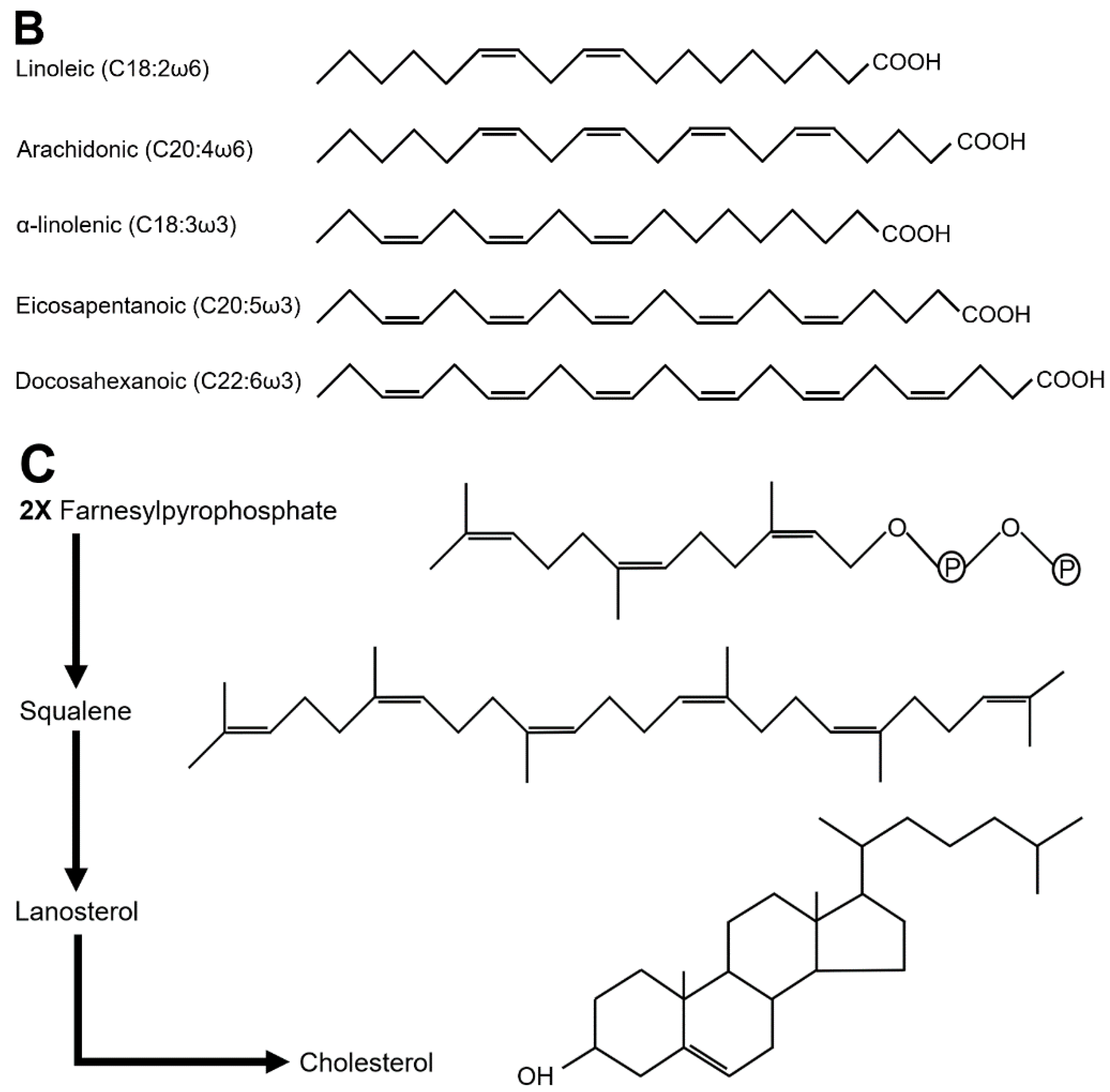
2.2. Essential Fatty Acids
2.3. Sterol Requirement
2.4. Carotenoids and Fat-Soluble Vitamins
2.5. Microbiota and Dietary Lipids
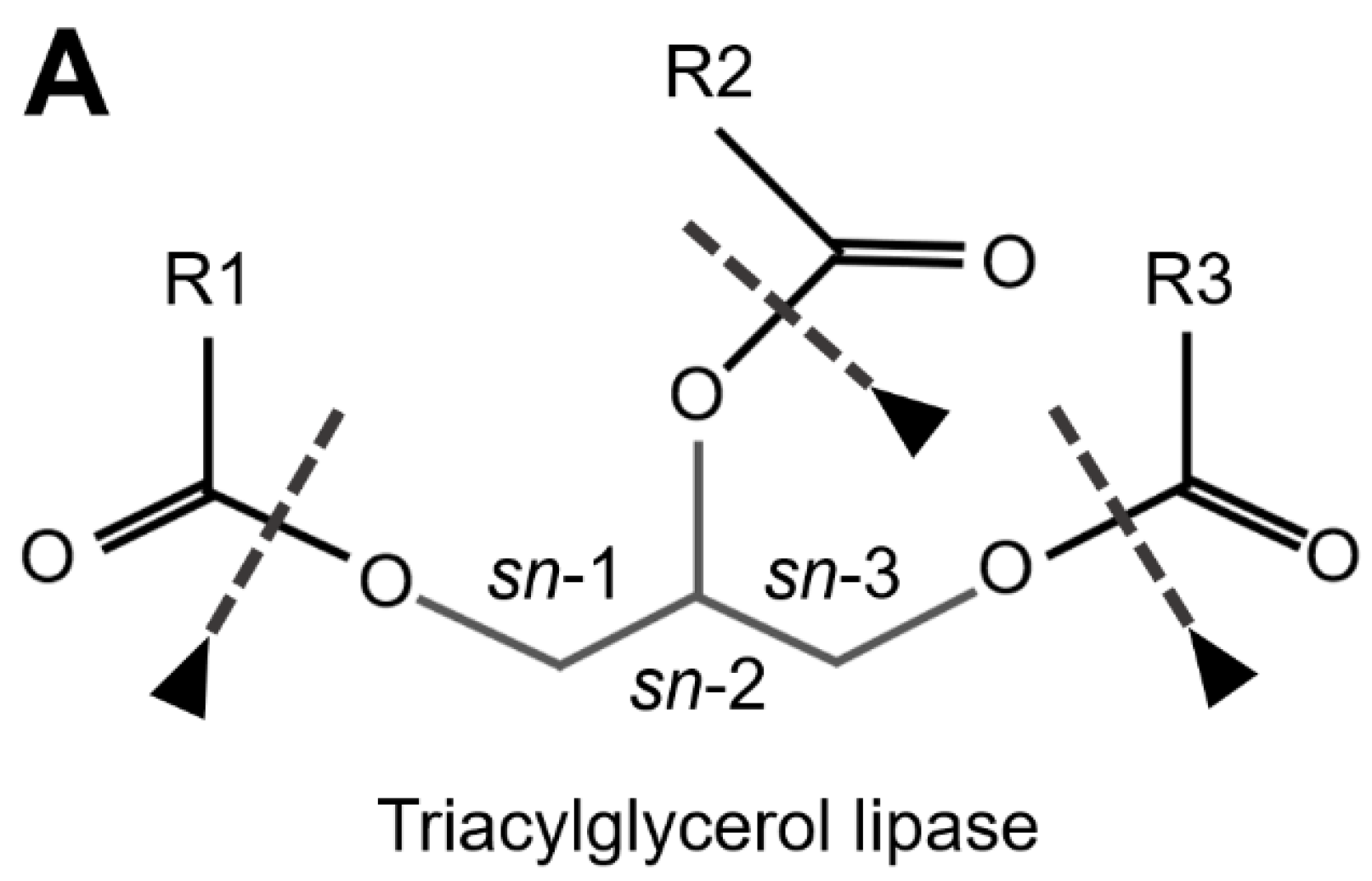
3. Lipid Hydrolysis in the Gut Lumen
3.1. Lipase Characterization
3.2. Insect Digestive Lipases
3.3. Lipase Activity and Expression
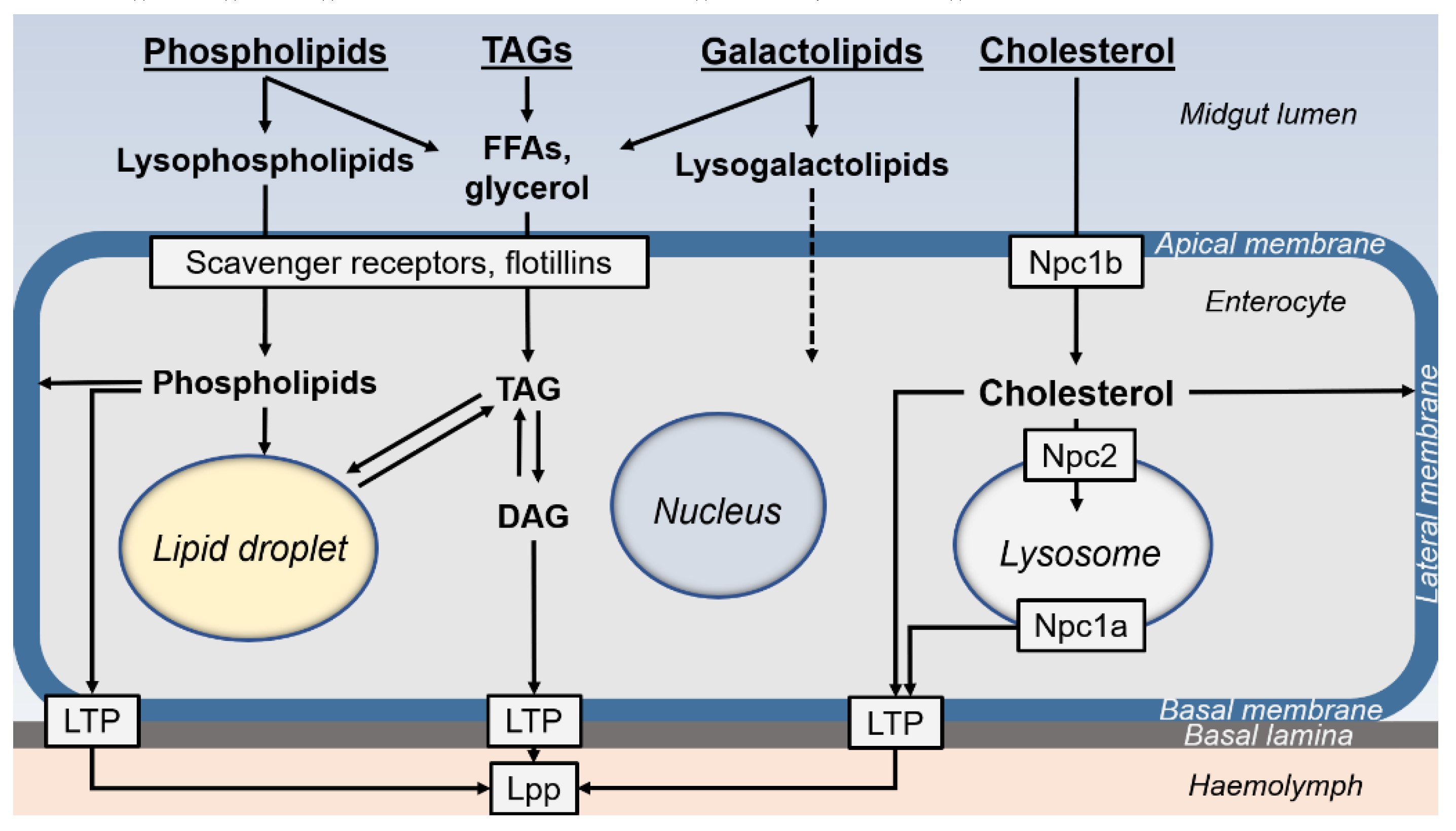
4. Lipid Uptake into Enterocytes
4.1. Lipid Emulsifiers
4.2. Lipid Transporters
5. Enterocyte Lipid Metabolism
5.1. Lipid Usage to Sustain Enterocyte Activity
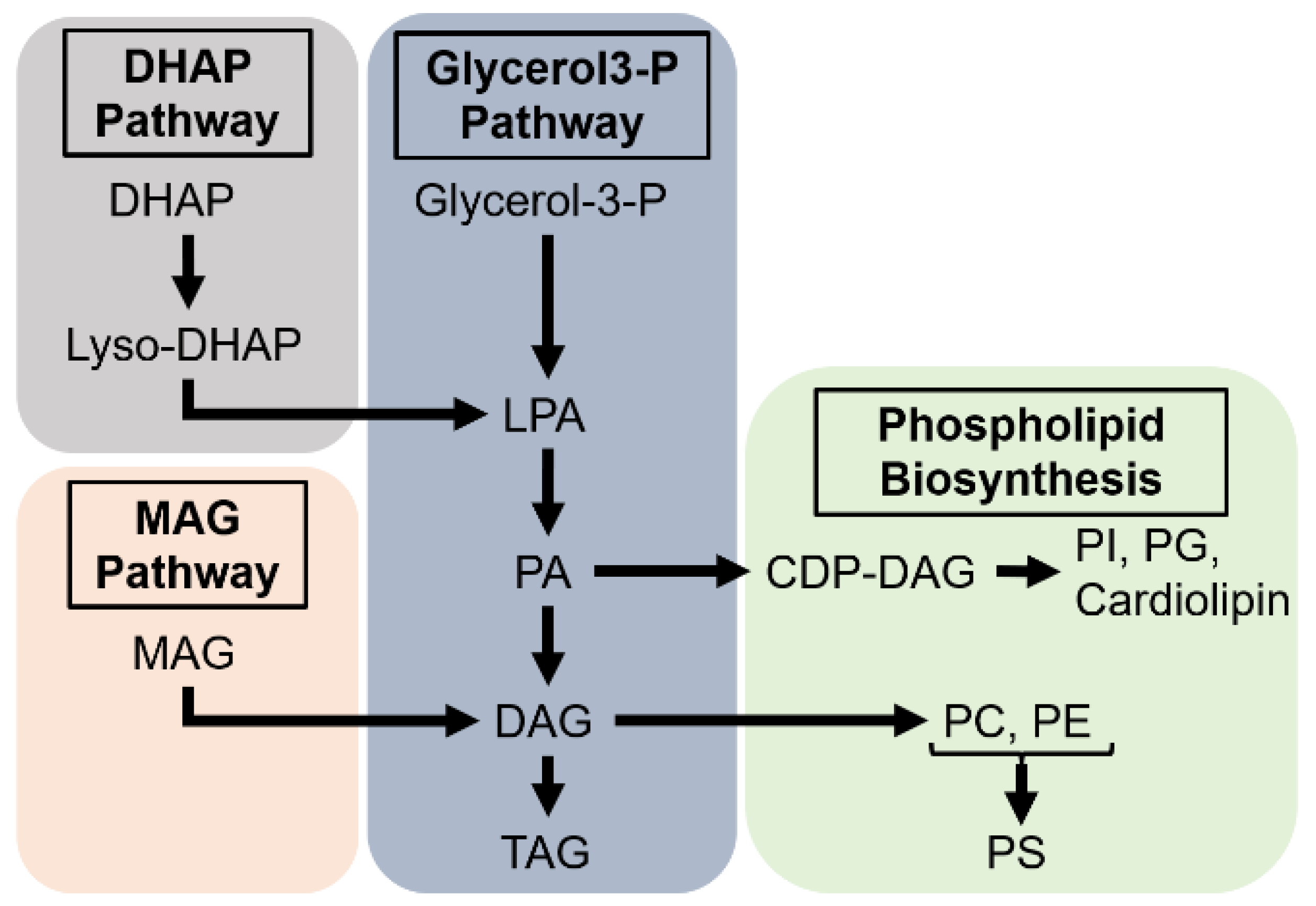
5.2. Lipid Metabolic Pathways in Enterocytes
5.3. Lipid Efflux to Haemolymph
6. The Dynamics of Lipid Digestion in Insects
6.1. An Attempt to Schematize the Lipid Digestive Process
6.2. Midgut Regionalization of Lipid Digestion
7. Conclusions
References
- Agarwal HC, Nair AM (1976) Studies on the cholesterol ester hydrolase of trogoderma (coleoptera). Biochem J. 157:111-116. [CrossRef]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlison JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science. 276:945-949.
- Albuquerque-Cunha JM, Gonzalez MS, Garcia ES, Mello CB, Azambuja P, Almeida JC, de Souza W, Nogueira NF (2009) Cytochemical characterization of microvillar and perimicrovillar membranes in the posterior midgut epithelium of rhodnius prolixus. Arthropod Struct Dev. 38:31-44. [CrossRef]
- Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA (2001) Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem Mol Biol. 31:7-17.
- Atella GC, Gondim C, Masuda H (1995) Loading of lipophorin particles with phospholipids at the midgut of rhodnius prolixus. Arch Insect Biochem Physiol. 30:337-350. [CrossRef]
- Azevedo DO, Neves CA, Mallet JR, Goncalves TC, Zanuncio JC, Serrao JE (2009) Notes on midgut ultrastructure of cimex hemipterus (hemiptera: Cimicidae). J Med Entomol. 46:435-441. [CrossRef]
- Barroso IG, Cardoso C, Ferreira C, Terra WR (2021) Transcriptomic and proteomic analysis of the underlying mechanisms of digestion of triacylglycerols and phosphatides and absorption and fate of fatty acids along the midgut of musca domestica. Comp Biochem Physiol Part D Genomics Proteomics. 39:100826. [CrossRef]
- Bateta R, Wang J, Wu Y, Weiss BL, Warren WC, Murilla GA, Aksoy S, Mireji PO (2017) Tsetse fly (glossina pallidipes) midgut responses to trypanosoma brucei challenge. Parasit Vectors. 10:614. [CrossRef]
- Beenakkers AM, Van der Horst DJ, Van Marrewijk WJ (1985) Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 24:19-67. [CrossRef]
- Biagio FP, Tamaki FK, Terra WR, Ribeiro AF (2009) Digestive morphophysiology of gryllodes sigillatus (orthoptera: Gryllidae). J Insect Physiol. 55:1125-1133. [CrossRef]
- Billingsley PF (1990) Blood digestion in the mosquito, anopheles stephensi liston (diptera: Culicidae): Partial characterization and post-feeding activity of midgut aminopeptidases. Arch Insect Biochem Physiol. 15:149-163. [CrossRef]
- Blitzer EJ, Vyazunova I, Lan Q (2005) Functional analysis of aescp-2 using gene expression knockdown in the yellow fever mosquito, aedes aegypti. Insect Mol Biol. 14:301-307. [CrossRef]
- Blomquist G, Borgeson CE, Vundla M (1991) Polyunsaturated fatty acids and ecosanoids in insects. Insect Biochem. 21:99-106.
- Bollade D, Paris R, Moulins M (1970) Origine et mode d'action de la lipase intestinale chez les blattes. J Insect Physiol. 16:45-53.
- Bolognesi R, Terra WR, Ferreira C (2008) Peritrophic membrane role in enhancing digestive efficiency. Theoretical and experimental models. J Insect Physiol. 54:1413-1422. [CrossRef]
- Bonelli M, Bruno D, Caccia S, Sgambetterra G, Cappellozza S, Jucker C, Tettamanti G, Casartelli M (2019) Structural and functional characterization of hermetia illucens larval midgut. Front Physiol. 10:204. [CrossRef]
- Bownes M (1992) Why is there sequence similarity between insect yolk proteins and vertebrate lipases? J Lipid Res. 33:777-790.
- Breznak JA (1982) Intestinal microbiota of termites and other xylophagous insects. Annu Rev Microbiol. 36:323-343. [CrossRef]
- Brune A (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 12:168-180. [CrossRef]
- Brune A, Dietrich C (2015) The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Annu Rev Microbiol. 69:145-166. [CrossRef]
- Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, Lemaitre B (2013) Morphological and molecular characterization of adult midgut compartmentalization in drosophila. Cell Rep. 3:1725-1738. [CrossRef]
- Caccia S, Casartelli M, Tettamanti G (2019) The amazing complexity of insect midgut cells: Types, peculiarities, and functions. Cell Tissue Res. 377:505-525. [CrossRef]
- Canavoso LE, Frede S, Rubiolo ER (2004) Metabolic pathways for dietary lipids in the midgut of hematophagous panstrongylus megistus (hemiptera: Reduviidae). Insect Biochem Mol Biol. 34:845-854. [CrossRef]
- Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr. 21:23-46.
- Canavoso LE, Wells MA (2000) Metabolic pathways for diacylglycerol biosynthesis and release in the midgut of larval manduca sexta. Insect Biochem Mol Biol. 30:1173-1180. [CrossRef]
- Canavoso LE, Wells MA (2001) Role of lipid transfer particle in delivery of diacylglycerol from midgut to lipophorin in larval manduca sexta. Insect Biochem Mol Biol. 31:783-790. [CrossRef]
- Canton PE, Bonning BC (2020) Extraoral digestion: Outsourcing the role of the hemipteran midgut. Curr Opin Insect Sci. 41:86-91. [CrossRef]
- Carvalho M, Sampaio JL, Palm W, Brankatschk M, Eaton S, Shevchenko A (2012) Effects of diet and development on the drosophila lipidome. Mol Syst Biol. 8:600. [CrossRef]
- Carvalho M, Schwudke D, Sampaio JL, Palm W, Riezman I, Dey G, Gupta GD, Mayor S, Riezman H, Shevchenko A et al. (2010) Survival strategies of a sterol auxotroph. Development. 137:3675-3685.
- Chapman RF, Simpson SJ, Douglas AE. (2013). The insects. Structure and function.
- Chintapalli VR, Wang J, Dow JA (2007) Using flyatlas to identify better drosophila melanogaster models of human disease. Nat Genet. 39:715-720.
- Chng WA, Sleiman MSB, Schupfer F, Lemaitre B (2014) Transforming growth factor beta/activin signaling functions as a sugar-sensing feedback loop to regulate digestive enzyme expression. Cell Rep. 9:336-348. [CrossRef]
- Chow SL, Hollander D (1979) A dual, concentration-dependent absorption mechanism of linoleic acid by rat jejunum in vitro. J Lipid Res. 20:349-356.
- Christeller JT, Amara S, Carriere F (2011) Galactolipase, phospholipase and triacylglycerol lipase activities in the midgut of six species of lepidopteran larvae feeding on different lipid diets. J Insect Physiol. 57:1232-1239. [CrossRef]
- Christeller JT, Poulton J, Markwick NM, Simpson RM (2010) The effect of diet on the expression of lipase genes in the midgut of the lightbrown apple moth (epiphyas postvittana walker; tortricidae). Insect Mol Biol. 19:9-25. [CrossRef]
- Chu BS, Rich GT, Ridout MJ, Faulks RM, Wickham MS, Wilde PJ (2009) Modulating pancreatic lipase activity with galactolipids: Effects of emulsion interfacial composition. Langmuir. 25:9352-9360. [CrossRef]
- Cifarelli V, Abumrad NA (2018) Intestinal cd36 and other key proteins of lipid utilization: Role in absorption and gut homeostasis. Compr Physiol. 8:493-507. [CrossRef]
- Cioffi M (1984) Ultrastructure of arthropod transporting epithelia. Amer Zool. 24:139-156.
- Ciufo LF, Murray PA, Thompson A, Rigden DJ, Rees HH (2011) Characterisation of a desmosterol reductase involved in phytosterol dealkylation in the silkworm, bombyx mori. PLoS One. 6:e21316. [CrossRef]
- Clark AJ, Block K (1959) The absence of sterol synthesis in insects. J Biol Chem. 234:2578-2582.
- Clayton RB (1964) The utilization of sterols by insects. J Lipid Res. 5:3-19.
- Cleveland LR (1923) Symbiosis between termites and their intestinal protozoa. Proc Natl Acad Sci U S A. 9:424-428. [CrossRef]
- Cobbs C, Heath J, Stireman JO, 3rd, Abbot P (2013) Carotenoids in unexpected places: Gall midges, lateral gene transfer, and carotenoid biosynthesis in animals. Mol Phylogenet Evol. 68:221-228. [CrossRef]
- Coleman RA, Mashek DG (2011) Mammalian triacylglycerol metabolism: Synthesis, lipolysis, and signaling. Chem Rev. 111:6359-6386. [CrossRef]
- Collatz K-G, Mommsen T (1974) Die struktur der emulgierenden substanzen verschicdener invertebraten. J comp Physiol. 94:339-352.
- Consuegra J, Grenier T, Akherraz H, Rahioui I, Gervais H, da Silva P, Leulier F (2020a) Metabolic cooperation among commensal bacteria supports drosophila juvenile growth under nutritional stress. iScience. 23:101232. [CrossRef]
- Consuegra J, Grenier T, Baa-Puyoulet P, Rahioui I, Akherraz H, Gervais H, Parisot N, da Silva P, Charles H, Calevro F et al. (2020b) Drosophila-associated bacteria differentially shape the nutritional requirements of their host during juvenile growth. PLoS Biol. 18:e3000681. [CrossRef]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA (2003) A defective response to hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 33:508-513. [CrossRef]
- Coutinho-Abreu IV, Serafim TD, Meneses C, Kamhawi S, Oliveira F, Valenzuela JG (2020) Leishmania infection induces a limited differential gene expression in the sand fly midgut. BMC Genomics. 21:608. [CrossRef]
- Cruden DL, Markovetz AJ (1987) Microbial ecology of the cockroach gut. Annu Rev Microbiol. 41:617-643. [CrossRef]
- da Silva G, Costa Ramos LF, Dos Santos Seckler H, Mendonca Gomes F, Reis Cortines J, Ramos I, Dinis Anobom C, de Alcantara Machado E, Perpetua de Oliveira DM (2019) Biochemical characterization of digestive membrane-associated alkaline phosphatase from the velvet bean caterpillar anticarsia gemmatalis. Arch Insect Biochem Physiol. 102:e21591. [CrossRef]
- Dadd RH (1961) The nutritional requirements of locusts. J Ins Physiol. 6:126-145.
- Dadd RH, Kleinjan JE (1979) Essential fatty acid for the mosquito culex pipiens: Arachidonic acid. J Insect Physiol. 25:495-502. [CrossRef]
- de Oliveira Souza A, Couto-Lima CA, Catalao CHR, Santos-Junior NN, Dos Santos JF, da Rocha MJA, Alberici LC (2019) Neuroprotective action of eicosapentaenoic (epa) and docosahexaenoic (dha) acids on paraquat intoxication in drosophila melanogaster. Neurotoxicology. 70:154-160. [CrossRef]
- de Sousa ME, Wanderley-Teixeira V, Teixeira AA, de Siqueira HA, Santos FA, Alves LC (2009) Ultrastructure of the alabama argillacea (hubner) (lepidoptera: Noctuidae) midgut. Micron. 40:743-749. [CrossRef]
- Derewenda ZS (1994) Structure and function of lipases. Adv Protein Chem. 45:1-52. [CrossRef]
- Dewett D, Lam-Kamath K, Poupault C, Khurana H, Rister J (2021) Mechanisms of vitamin a metabolism and deficiency in the mammalian and fly visual system. Dev Biol. 476:68-78. [CrossRef]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990) Plant strategies of manipulating predatorprey interactions through allelochemicals: Prospects for application in pest control. J Chem Ecol. 16:3091-3118. [CrossRef]
- Dragh MA, Xu Z, Al-Allak ZS, Hong L (2017) Vitamin k2 prevents lymphoma in drosophila. Sci Rep. 7:17047. [CrossRef]
- Dubreuil RR (2004) Copper cells and stomach acid secretion in the drosophila midgut. Int J Biochem Cell Biol. 36:745-752. [CrossRef]
- Dutta D, Dobson AJ, Houtz PL, Glasser C, Revah J, Korzelius J, Patel PH, Edgar BA, Buchon N (2015) Regional cell-specific transcriptome mapping reveals regulatory complexity in the adult drosophila midgut. Cell Rep. 12:346-358. [CrossRef]
- Eguchi M (1995) Alkaline phosphatase isozymes in insects and comparison with mammalian enzyme. Comp Biochem Physiol B Biochem Mol Biol. 111:151-162. [CrossRef]
- Engel P, Moran NA (2013) The gut microbiota of insects - diversity in structure and function. FEMS Microbiol Rev. 37:699-735. [CrossRef]
- Entringer PF, Majerowicz D, Gondim KC (2021) The fate of dietary cholesterol in the kissing bug rhodnius prolixus. Front Physiol. 12:654565. [CrossRef]
- Erkosar B, Leulier F (2014) Transient adult microbiota, gut homeostasis and longevity: Novel insights from the drosophila model. FEBS Lett. 588:4250-4257. [CrossRef]
- Faber V, Komnick H (1989) Peroxisomes of the midgut epithelium, malpighian tubules and fat body of larvae of the dragonfly, aeshna cyanea. Tissue Cell. 21:917-924. [CrossRef]
- Ferreira C, Capella AN, Sitnik R, Terra WR (1994) Properties of the digestive enzymes and the permeability of the peritrophic membrane of spodoptera frugiperda (lepidoptera) larvae. Comp Biochem Physiol. 107A:631-640.
- Fialho MCQ, Terra WR, Moreira NR, Zanuncio JC, Serrao JE (2013) Ultrastructure and immunolocalization of digestive enzymes in the midgut of podisus nigrispinus (heteroptera: Pentatomidae). Arthropod Struct Dev. 42:277-285. [CrossRef]
- Fluegel ML, Parker TJ, Pallanck LJ (2006) Mutations of a drosophila npc1 gene confer sterol and ecdysone metabolic defects. Genetics. 172:185-196. [CrossRef]
- Flybase (2003) The flybase database of the drosophila genome projects and community literature. Nucleic Acids Res. 31:172-175. [CrossRef]
- Ford PS, Van Heusden MC (1994) Triglyceride-rich lipophorin in aedes aegypti (diptera: Culicidae). J Med Entomol. 31:435-441. [CrossRef]
- Fraenkel G, Blewett M (1946) Linoleic acid, vitamin e and other fat-soluble substances in the nutrition of certain insects, ephestia kuehniella, e. Elutella, e. Cautella and plodia interpunctella (lep.). J Exp Biol. 22:172-190. [CrossRef]
- Fruttero LL, Rubiolo ER, Canavoso LE (2009) Biochemical and cellular characterization of lipophorin-midgut interaction in the hematophagous panstrongylus megistus (hemiptera: Reduviidae). Insect Biochem Mol Biol. 39:322-331. [CrossRef]
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T et al. (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 469:543-547. [CrossRef]
- Fuzita FJ, Pimenta DC, Palmisano G, Terra WR, Ferreira C (2019) Detergent-resistant domains in spodoptera frugiperda midgut microvillar membranes and their relation to microapocrine secretion. Comp Biochem Physiol B Biochem Mol Biol. 235:8-18. [CrossRef]
- Galbiati F, Volonte D, Goltz JS, Steele Z, Sen J, Jurcsak J, Stein D, Stevens L, Lisanti MP (1998) Identification, sequence and developmental expression of invertebrate flotillins from drosophila melanogaster. Gene. 210:229-237. [CrossRef]
- Gandotra S, Bhuyan PM, Gogoi DK, Kumar A, Subramanian S (2016) Screening of nutritionally important gut bacteria from the lepidopteran insects through qualitative enzyme assays. Proc Natl Acad Sci, India. 88:329-337. [CrossRef]
- Garrido D, Rubin T, Poidevin M, Maroni B, Le Rouzic A, Parvy JP, Montagne J (2015) Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 11:e1004995. [CrossRef]
- Gazara RK, Cardoso C, Bellieny-Rabelo D, Ferreira C, Terra WR, Venancio TM (2017) De novo transcriptome sequencing and comparative analysis of midgut tissues of four non-model insects pertaining to hemiptera, coleoptera, diptera and lepidoptera. Gene. 627:85-93. [CrossRef]
- Ge L, Qi W, Wang LJ, Miao HH, Qu YX, Li BL, Song BL (2011) Flotillins play an essential role in niemann-pick c1-like 1-mediated cholesterol uptake. Proc Natl Acad Sci U S A. 108:551-556. [CrossRef]
- Gondim KC, Wells MA (2000) Characterization of lipophorin binding to the midgut of larval manduca sexta. Insect Biochem Mol Biol. 30:405-413. [CrossRef]
- Gong J, Hou Y, Zha XF, Lu C, Zhu Y, Xia QY (2006) Molecular cloning and characterization of bombyx mori sterol carrier protein x/sterol carrier protein 2 (scpx/scp2) gene. DNA Seq. 17:326-333. [CrossRef]
- Goudriaan JR, Dahlmans VE, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ (2002) Intestinal lipid absorption is not affected in cd36 deficient mice. Mol Cell Biochem. 239:199-202.
- Grenier T, Leulier F (2020) How commensal microbes shape the physiology of drosophila melanogaster. Curr Opin Insect Sci. 41:92-99. [CrossRef]
- Grillo LA, Majerowicz D, Gondim KC (2007) Lipid metabolism in rhodnius prolixus (hemiptera: Reduviidae): Role of a midgut triacylglycerol-lipase. Insect Biochem Mol Biol. 37:579-588. [CrossRef]
- Grillo LA, Pontes EG, Gondim KC (2003) Lipophorin interaction with the midgut of rhodnius prolixus: Characterization and changes in binding capacity. Insect Biochem Mol Biol. 33:429-438. [CrossRef]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP (2005) Brummer lipase is an evolutionary conserved fat storage regulator in drosophila. Cell Metab. 1:323-330. [CrossRef]
- Guedes BAM, Zanuncio JC, Ramalho FS, Serrao JE (2007) Midgut morphology and enzymes of the obligate zoophytophagous stinkbug brontocoris tabidus (signoret, 1963) (heteroptera: Pentatomidae). Pan-Pac Entomol. 83:66-74.
- Guo XR, Zheng SC, Liu L, Feng QL (2009) The sterol carrier protein 2/3-oxoacyl-coa thiolase (scpx) is involved in cholesterol uptake in the midgut of spodoptera litura: Gene cloning, expression, localization and functional analyses. BMC Mol Biol. 10:102. [CrossRef]
- Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol. 55:593-608. [CrossRef]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore manduca sexta (lepidoptera, sphingidae) and its natural host nicotiana attenuata. Iii. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 125:711-717. [CrossRef]
- Harrison JF (2001) Insect acid-base physiology. Annu Rev Entomol. 46:221-250. [CrossRef]
- Harrison JF, Kaiser A, VandenBrooks JM (2010) Atmospheric oxygen level and the evolution of insect body size. Proc Biol Sci. 277:1937-1946. [CrossRef]
- Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 54:285-302. [CrossRef]
- Hobson RP (1935) On a fat-soluble growth factor required by blow-fly larvae: Identity of the growth factor with cholesterol. Biochem J. 29:2023-2026. [CrossRef]
- Hoffman AGD, Downer RGH (1976) The crop as an organ of glyceride absorption in the american cockroach, periplaneta americana l. Can J Zool. 54:1165-1171.
- Hoffman AGD, Downer RGH (1979) End product specificity of triacylglycerol lipases from intestine, fat body, muscle and haemolymph of the american cockroach, periplaneta americana l. Lipids. 14:893-899.
- Holmquist M (2000) Alpha/beta-hydrolase fold enzymes: Structures, functions and mechanisms. Curr Protein Pept Sci. 1:209-235. [CrossRef]
- Holtof M, Lenaerts C, Cullen D, Vanden Broeck J (2019) Extracellular nutrient digestion and absorption in the insect gut. Cell Tissue Res. 377:397-414. [CrossRef]
- Horne I, Haritos VS, Oakeshott JG (2009) Comparative and functional genomics of lipases in holometabolous insects. Insect Biochem Mol Biol. 39:547-567. [CrossRef]
- House HL (1966) Effects of vitamins e and a on growth and development, and the necessity of vitamin e for reproduction in the parasitoid agria affinis (fallen) (diptera, sarcophagidae). J Insect Physiol. 12:409-417. [CrossRef]
- Hu X, Chen L, Xiang X, Yang R, Yu S, Wu X (2012) Proteomic analysis of peritrophic membrane (pm) from the midgut of fifth-instar larvae, bombyx mori. Mol Biol Rep. 39:3427-3434. [CrossRef]
- Huang X, Ma J, Qin X, Tu X, Cao G, Wang G, Nong X, Zhang Z (2017) Biology, physiology and gene expression of grasshopper oedaleus asiaticus exposed to diet stress from plant secondary compounds. Sci Rep. 7:8655. [CrossRef]
- Huang X, Suyama K, Buchanan J, Zhu AJ, Scott MP (2005) A drosophila model of the niemann-pick type c lysosome storage disease: Dnpc1a is required for molting and sterol homeostasis. Development. 132:5115-5124. [CrossRef]
- Huang X, Warren JT, Buchanan J, Gilbert LI, Scott MP (2007) Drosophila niemann-pick type c-2 genes control sterol homeostasis and steroid biosynthesis: A model of human neurodegenerative disease. Development. 134:3733-3742.
- Ian De Veau EJ, Schultz JC (1992) Reassessment of interaction between gut detergents and tannins in lepidoptera and significance for gypsy moth larvae. J Chem Ecol. 18:1437-1453. [CrossRef]
- Issaq HJ, Chan KC, Blonder J, Ye X, Veenstra TD (2009) Separation, detection and quantitation of peptides by liquid chromatography and capillary electrochromatography. J Chromatogr A. 1216:1825-1837. [CrossRef]
- Jing TZ, Qi FH, Wang ZY (2020) Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome. 8:38. [CrossRef]
- Jing X, Behmer ST (2020) Insect sterol nutrition: Physiological mechanisms, ecology, and applications. Annu Rev Entomol. 65:251-271. [CrossRef]
- Jing X, Vogel H, Grebenok RJ, Zhu-Salzman K, Behmer ST (2012) Dietary sterols/steroids and the generalist caterpillar helicoverpa zea: Physiology, biochemistry and midgut gene expression. Insect Biochem Mol Biol. 42:835-845. [CrossRef]
- Jouni ZE, Zamora J, Wells MA (2002) Absorption and tissue distribution of cholesterol in manduca sexta. Arch Insect Biochem Physiol. 49:167-175. [CrossRef]
- Jugder BE, Kamareddine L, Watnick PI (2021) Microbiota-derived acetate activates intestinal innate immunity via the tip60 histone acetyltransferase complex. Immunity. 54:1683-1697 e1683. [CrossRef]
- Jupatanakul N, Sim S, Dimopoulos G (2014) Aedes aegypti ml and niemann-pick type c family members are agonists of dengue virus infection. Dev Comp Immunol. 43:1-9. [CrossRef]
- Jurzitza G (1974) [furnishing of sterol by the yeast-like endosymbionts of lasioderma serricorne f. (coleoptera, anobiidae) and its ecological importance for the host]. Oecologia. 16:163-172. [CrossRef]
- Kamareddine L, Robins WP, Berkey CD, Mekalanos JJ, Watnick PI (2018) The drosophila immune deficiency pathway modulates enteroendocrine function and host metabolism. Cell Metab. 28:449-462 e445. [CrossRef]
- Kesnerova L, Mars RAT, Ellegaard KM, Troilo M, Sauer U, Engel P (2017) Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 15:e2003467. [CrossRef]
- Kiefer C, Sumser E, Wernet MF, Von Lintig J (2002) A class b scavenger receptor mediates the cellular uptake of carotenoids in drosophila. Proc Natl Acad Sci U S A. 99:10581-10586. [CrossRef]
- Kim MS, Lan Q (2010) Sterol carrier protein-x gene and effects of sterol carrier protein-2 inhibitors on lipid uptake in manduca sexta. BMC Physiol. 10:9. [CrossRef]
- Kim Y, Stanley D (2021) Eicosanoid signaling in insect immunology: New genes and unresolved issues. Genes (Basel). 12. [CrossRef]
- Kirfel G, Komnick H (1999) Differential absorption and esterification of dietary long-chain fatty acids by larvae of the dragonfly, aeshna cyanea. Arch Insect Biochem Physiol. 40:183-193. [CrossRef]
- Ko CW, Qu J, Black DD, Tso P (2020) Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 17:169-183. [CrossRef]
- Komnick H, Bauerfeind R (1991) Intestinal absorption of defined lipids by the larval dragonfly aeshna cyanea (insecta, odonata): Wax ester and fatty alcohols. J Insect Physiol. 37:179-191.
- Kong HG, Kim HH, Chung JH, Jun J, Lee S, Kim HM, Jeon S, Park SG, Bhak J, Ryu CM (2019) The galleria mellonella hologenome supports microbiota-independent metabolism of long-chain hydrocarbon beeswax. Cell Rep. 26:2451-2464 e2455. [CrossRef]
- Kuhnlein RP (2012) Thematic review series: Lipid droplet synthesis and metabolism: From yeast to man. Lipid droplet-based storage fat metabolism in drosophila. J Lipid Res. 53:1430-1436.
- Kuhns EH, Seidl-Adams I, Tumlinson JH (2012) A lepidopteran aminoacylase (l-acy-1) in heliothis virescens (lepidoptera: Noctuidae) gut lumen hydrolyzes fatty acid-amino acid conjugates, elicitors of plant defense. Insect Biochem Mol Biol. 42:32-40. [CrossRef]
- Kunding AH, Christensen SM, Danielsen EM, Hansen GH (2010) Domains of increased thickness in microvillar membranes of the small intestinal enterocyte. Mol Membr Biol. 27:170-177. [CrossRef]
- Kvietys PR, Specian RD, Grisham MB, Tso P (1991) Jejunal mucosal injury and restitution: Role of hydrolytic products of food digestion. Am J Physiol. 261:G384-391. [CrossRef]
- Kwong WK, Medina LA, Koch H, Sing KW, Soh EJY, Ascher JS, Jaffe R, Moran NA (2017) Dynamic microbiome evolution in social bees. Sci Adv. 3:e1600513. [CrossRef]
- LaJeunesse DR, Johnson B, Presnell JS, Catignas KK, Zapotoczny G (2010) Peristalsis in the junction region of the drosophila larval midgut is modulated by dh31 expressing enteroendocrine cells. BMC Physiol. 10:14. [CrossRef]
- Lan Q, Massey RJ (2004) Subcellular localization of the mosquito sterol carrier protein-2 and sterol carrier protein-x. J Lipid Res. 45:1468-1474. [CrossRef]
- Lan Q, Wessely V (2004) Expression of a sterol carrier protein-x gene in the yellow fever mosquito, aedes aegypti. Insect Mol Biol. 13:519-529. [CrossRef]
- Lee WJ, Hase K (2014) Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 10:416-424. [CrossRef]
- Lehane MJ (1977) Transcellular absorption of lipids in the midgut of the stablefly, stomoxys calcitrans. J Insect Physiol. 23:945-954. [CrossRef]
- Lehane MJ (1997) Peritrophic matrix structure and function. Annu Rev Entomol. 42:525-550. [CrossRef]
- Li S, Jing X (2020) Fates of dietary sterols in the insect alimentary canal. Curr Opin Insect Sci. 41:106-111. [CrossRef]
- Lin X, Wen X, Wei Z, Guo K, Shi F, Huang T, Wang W, Zheng J (2021) Vitamin k2 protects against abeta42-induced neurotoxicity by activating autophagy and improving mitochondrial function in drosophila. Neuroreport. 32:431-437. [CrossRef]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr., Ory DS, Schaffer JE (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 100:3077-3082. [CrossRef]
- Male KB, Storey KB (1981) Enzyme activities and isozyme composition of triglyceride, diglyceride and monoglyceride lipases in periplaneta americana, locusta migratoria and polia adjuncta. Insect Biochem. 11:423-427.
- Marianes A, Spradling AC (2013) Physiological and stem cell compartmentalization within the drosophila midgut. Elife. 2:e00886. [CrossRef]
- Markvoort AJ, Marrink SJ (2011) Lipid acrobatics in the membrane fusion arena. Curr Top Membr. 68:259-294. [CrossRef]
- Matic D, Vlahovic M, Ilijin L, Grcic A, Filipovic A, Todorovic D, Peric-Mataruga V (2021) Implications of long-term exposure of a lymantria dispar l. Population to pollution for the response of larval midgut proteases and acid phosphatases to chronic cadmium treatment. Comp Biochem Physiol C Toxicol Pharmacol. 250:109172. [CrossRef]
- Mattila J, Havula E, Suominen E, Teesalu M, Surakka I, Hynynen R, Kilpinen H, Vaananen J, Hovatta I, Kakela R et al. (2015) Mondo-mlx mediates organismal sugar sensing through the gli-similar transcription factor sugarbabe. Cell Rep. 13:350-364.
- McFarlane JE (1976) Vitamin k: Growth factor for the house cricket (orthoptera: Gryllidae). Can Entomol. 108:391-394.
- McFarlane JE, Alli I (1985) Volatile fatty acids of frass of certain omnivorous insects. J Chem Ecol. 11:59-63. [CrossRef]
- Mei Y, Jing D, Tang S, Chen X, Chen H, Duanmu H, Cong Y, Chen M, Ye X, Zhou H et al. (2022) Insectbase 2.0: A comprehensive gene resource for insects. Nucleic Acids Res. 50:D1040-D1045. [CrossRef]
- Meikle JES, McFarlane JE (1965) The role of lipid in the nutrition of the house cricket, acheta domesticus l. Can J Zool. 43:87-98.
- Merritt RW, Dadd RH, Walker ED (1992) Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 37:349-376. [CrossRef]
- Miao Z, Cao X, Jiang H (2020) Digestion-related proteins in the tobacco hornworm, manduca sexta. Insect Biochem Mol Biol. 126:103457. [CrossRef]
- Miguel-Aliaga I, Jasper H, Lemaitre B (2018) Anatomy and physiology of the digestive tract of drosophila melanogaster. Genetics. 210:357-396. [CrossRef]
- Miled N, Canaan S, Dupuis L, Roussel A, Riviere M, Carriere F, de Caro A, Cambillau C, Verger R (2000) Digestive lipases: From three-dimensional structure to physiology. Biochimie. 82:973-986. [CrossRef]
- Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J (2006) Cellular uptake of fatty acids driven by the er-localized acyl-coa synthetase fatp4. J Cell Sci. 119:4678-4688. [CrossRef]
- Moadeli T, Mainali B, Ponton F, Taylor PW (2020) Effects of fatty acids and vitamin e in larval diets on development and performance of queensland fruit fly. J Insect Physiol. 125:104058. [CrossRef]
- Montigny C, Lyons J, Champeil P, Nissen P, Lenoir G (2016) On the molecular mechanism of flippase- and scramblase-mediated phospholipid transport. Biochim Biophys Acta. 1861:767-783. [CrossRef]
- Moran NA, Jarvik T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 328:624-627. [CrossRef]
- Nassir F, Wilson B, Han X, Gross RW, Abumrad NA (2007) Cd36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 282:19493-19501. [CrossRef]
- Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P (2006) Cd36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 131:1197-1207. [CrossRef]
- Neira Oviedo M, Vanekeris L, Corena-McLeod MD, Linser PJ (2008) A microarray-based analysis of transcriptional compartmentalization in the alimentary canal of anopheles gambiae (diptera: Culicidae) larvae. Insect Mol Biol. 17:61-72. [CrossRef]
- Niot I, Poirier H, Tran TT, Besnard P (2009) Intestinal absorption of long-chain fatty acids: Evidence and uncertainties. Prog Lipid Res. 48:101-115. [CrossRef]
- Nor Aliza AR, Othman M, Sabri M, Stanley DW (2018) A midgut digestive phospholipase a2 in larval mosquitoes, aedes albopictus and culex quinquefasciatus. Enzyme Res. 2018:9703413. [CrossRef]
- Nor Aliza AR, Stanley D (1998) A digestive phospholipase a2 in larval mosquitoes, aedes aegypti. Insect Biochem Mol Biol. 28:561-569.
- Noriega FG (2014) Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int Sch Res Notices. 2014:967361. [CrossRef]
- Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S (2012) Lipoproteins in drosophila melanogaster--assembly, function, and influence on tissue lipid composition. PLoS Genet. 8:e1002828. [CrossRef]
- Parvy JP, Napal L, Rubin T, Poidevin M, Perrin L, Wicker-Thomas C, Montagne J (2012) Drosophila melanogaster acetyl-coa-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet. 8:e1002925. [CrossRef]
- Peller CR, Bacon EM, Bucheger JA, Blumenthal EM (2009) Defective gut function in drop-dead mutant drosophila. J Insect Physiol. 55:834-839. [CrossRef]
- Pennington JE, Wells MA (2002) Triacylglycerol-rich lipophorins are found in the dipteran infraorder culicomorpha, not just in mosquitoes. J Insect Sci. 2:15. [CrossRef]
- Perez-Salas U, Garg S, Gerelli Y, Porcar L (2021) Deciphering lipid transfer between and within membranes with time-resolved small-angle neutron scattering. Curr Top Membr. 88:359-412. [CrossRef]
- Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) Signalp 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 8:785-786. [CrossRef]
- Plateau F (1876). Les phenomenes de la digestion chez la blatte americaine (periplaneta americana). In: Hayez F, editor.: Imprimeur de l’Académie Royale de Belgique.
- Rana RL, Hoback WW, Rahim NAR, Bedick J, Stanley DW (1997) Pre-oral digestion: A phospholipase a2 associated with oral secretions in adult burying beetles, nicrophorus marginatus. Comp Biochem Physiol. 118:375-380.
- Rana RL, Stanley DW (1999) In vitro secretion of digestive phospholipase a(2) by midguts isolated from tobacco hornworms, manduca sexta. Arch Insect Biochem Physiol. 42:179-187. [CrossRef]
- Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P (2006) Scavenger receptor class b type i (sr-bi) is involved in vitamin e transport across the enterocyte. J Biol Chem. 281:4739-4745. [CrossRef]
- Reiher W, Shirras C, Kahnt J, Baumeister S, Isaac RE, Wegener C (2011) Peptidomics and peptide hormone processing in the drosophila midgut. J Proteome Res. 10:1881-1892. [CrossRef]
- Renne MF, Hariri H (2021) Lipid droplet-organelle contact sites as hubs for fatty acid metabolism, trafficking, and metabolic channeling. Front Cell Dev Biol. 9:726261. [CrossRef]
- Rewitz KF, Rybczynski R, Warren JT, Gilbert LI (2006) The halloween genes code for cytochrome p450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 34:1256-1260.
- Ribeiro JM, Genta FA, Sorgine MH, Logullo R, Mesquita RD, Paiva-Silva GO, Majerowicz D, Medeiros M, Koerich L, Terra WR et al. (2014) An insight into the transcriptome of the digestive tract of the bloodsucking bug, rhodnius prolixus. PLoS Negl Trop Dis. 8:e2594. [CrossRef]
- Rietveld A, Neutz S, Simons K, Eaton S (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with drosophila raft lipid microdomains. J Biol Chem. 274:12049-12054. [CrossRef]
- Rodriguez-Vazquez M, Vaquero D, Parra-Peralbo E, Mejia-Morales JE, Culi J (2015) Drosophila lipophorin receptors recruit the lipoprotein ltp to the plasma membrane to mediate lipid uptake. PLoS Genet. 11:e1005356. [CrossRef]
- Rost-Roszkowska MM, Pilka M, Szymska R, Klag J (2007) Ultrastructural studies of midgut epithelium formation in lepisma saccharina l. (insecta, zygentoma). J Morphol. 268:224-231. [CrossRef]
- Rudin W, Hecker H (1979) Functional morphology of the midgut of aedes aegypti l. (insecta, diptera) during blood digestion. Cell Tissue Res. 200:193-203. [CrossRef]
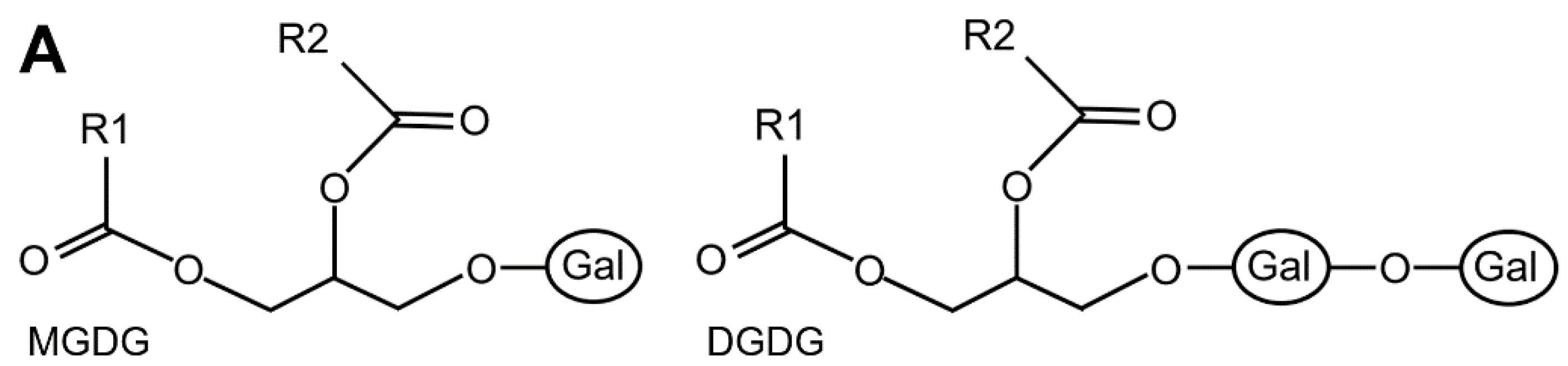
- Sahaka M, Amara S, Wattanakul J, Gedi MA, Aldai N, Parsiegla G, Lecomte J, Christeller JT, Gray D, Gontero B et al. (2020) The digestion of galactolipids and its ubiquitous function in nature for the uptake of the essential alpha-linolenic acid. Food Funct. 11:6710-6744. [CrossRef]
- Sajjadian SM, Vatanparast M, Stanley D, Kim Y (2019) Secretion of secretory phospholipase a2 into spodoptera exigua larval midgut lumen and its role in lipid digestion. Insect Mol Biol. 28:773-784. [CrossRef]
- Santos HP, Rost-Roszkowska M, Vilimova J, Serrao JE (2017) Ultrastructure of the midgut in heteroptera (hemiptera) with different feeding habits. Protoplasma. 254:1743-1753. [CrossRef]
- Schmidt K, Engel P (2021) Mechanisms underlying gut microbiota-host interactions in insects. J Exp Biol. 224. [CrossRef]
- Shen LR, Lai CQ, Feng X, Parnell LD, Wan JB, Wang JD, Li D, Ordovas JM, Kang JX (2010) Drosophila lacks c20 and c22 pufas. J Lipid Res. 51:2985-2992. [CrossRef]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 334:670-674. [CrossRef]
- Shukla SP, Plata C, Reichelt M, Steiger S, Heckel DG, Kaltenpoth M, Vilcinskas A, Vogel H (2018) Microbiome-assisted carrion preservation aids larval development in a burying beetle. Proc Natl Acad Sci U S A. 115:11274-11279. [CrossRef]
- Siddiqi S, Sheth A, Patel F, Barnes M, Mansbach CM, 2nd (2013) Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim Biophys Acta. 1831:1311-1321. [CrossRef]
- Sieber MH, Thummel CS (2009) The dhr96 nuclear receptor controls triacylglycerol homeostasis in drosophila. Cell Metab. 10:481-490.
- Sieber MH, Thummel CS (2012) Coordination of triacylglycerol and cholesterol homeostasis by dhr96 and the drosophila lipa homolog magro. Cell Metab. 15:122-127. [CrossRef]
- Silva W, Cardoso C, Ribeiro AF, Terra WR, Ferreira C (2013) Midgut proteins released by microapocrine secretion in spodoptera frugiperda. J Insect Physiol. 59:70-80. [CrossRef]
- Smith AF, Tsuchida K, Hanneman E, Suzuki TC, Wells MA (1992) Isolation, characterization, and cdna sequence of two fatty acid-binding proteins from the midgut of manduca sexta larvae. J Biol Chem. 267:380-384.
- Somerville HJ, Pockett HV (1976) Phospholipase activity in gut juice of lepidopterous larvae. Insect Biochem. 6:351-353.
- Song W, Veenstra JA, Perrimon N (2014) Control of lipid metabolism by tachykinin in drosophila. Cell Rep. 9:40-47. [CrossRef]
- Spates GE, Bull DL, Chen AC (1990) Hydrolysis of sphingomyelin and phosphatidylcholine by midgut homogenates of the stable fly. Arch Insect Biochem Physiol. 14:1-12. [CrossRef]
- Spiteller D, Boland W (2003) N-(17-acyloxy-acyl)-glutamines: Novel surfactants from oral secretions of lepidopteran larvae. J Org Chem. 68:8743-8749. [CrossRef]
- Spiteller D, Dettner K, Boland W (2000) Gut bacteria may be involved in interactions between plants, herbivores and their predators: Microbial biosynthesis of n-acylglutamine surfactants as elicitors of plant volatiles. Biol Chem. 381:755-762.
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA et al. (1999) Identification of the major intestinal fatty acid transport protein. Mol Cell. 4:299-308. [CrossRef]
- Stanley-Samuelson DW (1994) Prostaglandins and related eicosanoids in insects. Adv Insect Physiol. 24:115-212.
- Stanley D (2006) The non-venom insect phospholipases a2. Biochim Biophys Acta. 1761:1383-1390. [CrossRef]
- Stanley DW, Sarath G, Rana RL (1998) A digestive phospholipase a(2) in midguts of tobacco hornworms, manduca sexta l. J Insect Physiol. 44:297-303. [CrossRef]
- Stark AH, Crawford MA, Reifen R (2008) Update on alpha-linolenic acid. Nutr Rev. 66:326-332. [CrossRef]
- Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F (2011) Lactobacillus plantarum promotes drosophila systemic growth by modulating hormonal signals through tor-dependent nutrient sensing. Cell Metab. 14:403-414. [CrossRef]
- Storelli G, Strigini M, Grenier T, Bozonnet L, Schwarzer M, Daniel C, Matos R, Leulier F (2018) Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont lactobacillus plantarum. Cell Metab. 27:362-377 e368. [CrossRef]
- Svoboda JA (1999) Variability of metabolism and function of sterols in insects. Crit Rev Biochem Mol Biol. 34:49-57. [CrossRef]
- Tamaki FK, Pimentel AC, Dias AB, Cardoso C, Ribeiro AF, Ferreira C, Terra WR (2014) Physiology of digestion and the molecular characterization of the major digestive enzymes from periplaneta americana. J Insect Physiol. 70:22-35. [CrossRef]
- Teixeira A, Fialho Mdo C, Zanuncio JC, Ramalho Fde S, Serrao JE (2013) Degeneration and cell regeneration in the midgut of podisus nigrispinus (heteroptera: Pentatomidae) during post-embryonic development. Arthropod Struct Dev. 42:237-246. [CrossRef]
- Terra WR (2001) The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol. 47:47-61. [CrossRef]
- Terra WR, Costa RH, Ferreira C (2006) Plasma membranes from insect midgut cells. An Acad Bras Cienc. 78:255-269. [CrossRef]
- Terra WR, Ferreira C (2020) Evolutionary trends of digestion and absorption in the major insect orders. Arthropod Struct Dev. 56:100931. [CrossRef]
- Terra WR, Ferreira C, Garcia ES (1988) Origin, distribution, properties and functiosn of the major rhodnius prolixus midgut hydrolases. Insect Biochem. 18:423-434.
- Tinker KA, Ottesen EA (2016) The core gut microbiome of the american cockroach, periplaneta americana, is stable and resilient to dietary shifts. Appl Environ Microbiol. 82:6603-6610. [CrossRef]
- Toprak U, Hegedus D, Dogan C, Guney G (2020) A journey into the world of insect lipid metabolism. Arch Insect Biochem Physiol. 104:e21682. [CrossRef]
- Treherne JE (1958) The digestion and absorption of tripalmitin in the cockroach, periplanata americana l. J Exp Biol. 35:862-870.
- Trigatti BL, Anderson RG, Gerber GE (1999) Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 255:34-39. [CrossRef]
- Tsuchida K, Wells MA (1988) Digestion, absoprtion, transport and storage of fat during the last larval stadium of manduca sexta. Changes in the role of lipophorin in the delivery of dietary lipid to the fat body. Insect Biochem. 18:263-268.
- Turunen S (1975) Absorption and transport of dietary lipid in pieris brassicae. J Insect Physiol. 21:1521-1529.
- Turunen S (1988a) Digestion and absorption of glycerophospholipid in pieris brassicae. Comp Biochem Physiol. 89A:19-24.
- Turunen S (1988b) Uptake of dietary lipids: A novel pathway in pieris brassicae. Insect Biochem. 18:499-505.
- Turunen S (1990) Absorption of choline, myo-inositol, and oleic acid in th emidgut of pieris brassicae: Sectional differentiation and uptake into the haemolymph. J Insect Physiol. 36:737-741.
- Turunen S (1993) Metabolic pathways in the midgut epithelium of pieris brassicae during carbohydrate and lipid assimilation. Insect Biochem Molec Biol. 23:681-689.
- Turunen S, Chippendale GM (1977) Lipid absorption and transport: Sectional analysis of the larval midgut of the corn borer, diatraea grandiosella. Insect Biochem. 7:203-208.
- Turunen S, Chippendale GM (1989) Relationship between dietary lipids, midgut lipids, and lipid absorption in eight species of lepidoptera reared on artificial and natural diets J Insect Physiol. 35:627-633.
- Turunen S, Kastari T (1979) Digestion and absorption of lecithin in larvae of the cabbage butterfly, pieris brassicae. Comp Biochem Physiol. 62A:933-937.
- Uscian JM, Miller JS, Sarah G, Stanley-Samuelson DW (1995) A digestive phospholipase a2 in the tiger beetle cicindella circumpicta. J Insect Physiol. 41:135-141.
- Valzania L, Coon KL, Vogel KJ, Brown MR, Strand MR (2018) Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito aedes aegypti. Proc Natl Acad Sci U S A. 115:457-465. [CrossRef]
- Vance JE (2015) Phospholipid synthesis and transport in mammalian cells. Traffic. 16:1-18. [CrossRef]
- Vasconcelos MA, Orsolin PC, Oliveira VC, Lima P, Naves MPC, de Morais CR, Nicolau-Junior N, Bonetti AM, Spano MA (2020) Modulating effect of vitamin d3 on the mutagenicity and carcinogenicity of doxorubicin in drosophila melanogaster and in silico studies. Food Chem Toxicol. 143:111549. [CrossRef]
- Vatanparast M, Ahmed S, Herrero S, Kim Y (2018) A non-venomous spla2 of a lepidopteran insect: Its physiological functions in development and immunity. Dev Comp Immunol. 89:83-92. [CrossRef]
- Veenstra JA (2009) Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 336:309-323. [CrossRef]
- Veenstra JA, Agricola HJ, Sellami A (2008) Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334:499-516. [CrossRef]
- Verger R, Mieras MC, de Haas GH (1973) Action of phospholipase a at interfaces. J Biol Chem. 248:4023-4034.
- Visotto LE, Oliveira MG, Guedes RN, Ribon AO, Good-God PI (2009) Contribution of gut bacteria to digestion and development of the velvetbean caterpillar, anticarsia gemmatalis. J Insect Physiol. 55:185-191. [CrossRef]
- Vogel H, Shukla SP, Engl T, Weiss B, Fischer R, Steiger S, Heckel DG, Kaltenpoth M, Vilcinskas A (2017a) The digestive and defensive basis of carcass utilization by the burying beetle and its microbiota. Nat Commun. 8:15186. [CrossRef]
- Vogel KJ, Valzania L, Coon KL, Brown MR, Strand MR (2017b) Transcriptome sequencing reveals large-scale changes in axenic aedes aegypti larvae. PLoS Negl Trop Dis. 11:e0005273. [CrossRef]
- Voght SP, Fluegel ML, Andrews LA, Pallanck LJ (2007) Drosophila npc1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab. 5:195-205. [CrossRef]
- Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP (1999) Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem. 274:12702-12709. [CrossRef]
- Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B, Meganathan R et al. (2012) Vitamin k2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 336:1306-1310. [CrossRef]
- Wada-Katsumata A, Zurek L, Nalyanya G, Roelofs WL, Zhang A, Schal C (2015) Gut bacteria mediate aggregation in the german cockroach. Proc Natl Acad Sci U S A. 112:15678-15683. [CrossRef]
- Wegener C, Veenstra JA (2015) Chemical identity, function and regulation of enteroendocrine peptides in insects. Curr Opin Insect Sci. 11:8-13. [CrossRef]
- Weidlich S, Hoffmann KH, Woodring J (2015) Secretion of lipases in the digestive tract of the cricket gryllus bimaculatus. Arch Insect Biochem Physiol. 90:209-217. [CrossRef]
- Weiher B, Komnick H (1997) Digestion of phosphatidylcholines, absorption, and esterification of lipolytic products by aeshna cyanea larvae as studied in vivo and in vitro. Arch Insect Biochem Physiol. 36:273-293. [CrossRef]
- Weintraub H, Tietz A (1973) Triglyceride digestion and absorption in the locust, locusta migratoria. Biochim Biophys Acta. 306:31-41. [CrossRef]
- Weintraub H, Tietz A (1978) Lipid absorption by isolated intestinal preparations. Insect Biochem. 8:267-274.
- Wicker-Thomas C, Garrido D, Bontonou G, Napal L, Mazuras N, Denis B, Rubin T, Parvy JP, Montagne J (2015) Flexible origin of hydrocarbon/pheromone precursors in drosophila melanogaster. J Lipid Res. 56:2094-2101. [CrossRef]
- Woodring J, Lorenz MW (2007) Feeding, nutrient flow, and functional gut morphology in the cricket gryllus bimaculatus. J Morphol. 268:815-825. [CrossRef]
- Yamanaka N, Rewitz KF, O'Connor MB (2013) Ecdysone control of developmental transitions: Lessons from drosophila research. Annu Rev Entomol. 58:497-516.
- Yoshinaga N, Aboshi T, Abe H, Nishida R, Alborn HT, Tumlinson JH, Mori N (2008) Active role of fatty acid amino acid conjugates in nitrogen metabolism in spodoptera litura larvae. Proc Natl Acad Sci U S A. 105:18058-18063. [CrossRef]
- Yun HK, Jouni ZE, Wells MA (2002) Characterization of cholesterol transport from midgut to fat body in manduca sexta larvae. Insect Biochem Mol Biol. 32:1151-1158. [CrossRef]
- Zeng X, Hou SX (2015) Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult drosophila posterior midgut. Development. 142:644-653. [CrossRef]
- Zhang T, Yuan D, Xie J, Lei Y, Li J, Fang G, Tian L, Liu J, Cui Y, Zhang M et al. (2019) Evolution of the cholesterol biosynthesis pathway in animals. Mol Biol Evol. [CrossRef]
- Zhao X, Karpac J (2020) The drosophila midgut and the systemic coordination of lipid-dependent energy homeostasis. Curr Opin Insect Sci. 41:100-105. [CrossRef]
- Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA (2017) Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci U S A. 114:4775-4780. [CrossRef]
- Zheng JC, Sun SL, Yue XR, Liu TX, Jing X (2018) Phylogeny and evolution of the cholesterol transporter npc1 in insects. J Insect Physiol. 107:157-166. [CrossRef]
- Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ (2002) Nutrient control of gene expression in drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 21:6162-6173. [CrossRef]
- Zwick RK, Ohlstein B, Klein OD (2019) Intestinal renewal across the animal kingdom: Comparing stem cell activity in mouse and drosophila. Am J Physiol Gastrointest Liver Physiol. 316:G313-G322. [CrossRef]
| Neutral | Acid | Lipase3 | GDSL | HSL | ATGL | Total | |
| Aedes aegypti | 42 | 23 | 2 | 4 | 1 | 0 | 72 |
| Anopheles gambiae | 23 | 13 | 2 | 5 | 1 | 1 | 45 |
| Apis mellifera | 14 | 5 | 1 | 5 | 1 | 1 | 27 |
| Bombyx mori | 25 | 17 | 1 | 1 | 1 | 1 | 46 |
| Cimex lectularius | 14 | 12 | 2 | 1 | 1 | 2 | 32 |
| Drosophila melanogaster | 37 | 25 | 1 | 2 | 1 | 2 | 68 |
| Locusta migratoria | 22 | 14 | 2 | 3 | 1 | 1 | 43 |
| Manduca sexta | 45 | 28 | 1 | 1 | 1 | 1 | 77 |
| Musca domestica | 54 | 39 | 1 | 6 | 1 | 1 | 102 |
| Rhodnius prolixus | 18 | 6 | 3 | 2 | 1 | 0 | 30 |
| Spodoptera frugiperda | 57 | 22 | 1 | 2 | 1 | 0 | 83 |
| Tenebrio molitor | 28 | 25 | 2 | 1 | 1 | 1 | 58 |
| Activity | Species | Reference |
| TAG to sn-2-MAG |
Periplaneta americana; Blabera craniifer Pieris brassicae Periplaneta americana |
(Bollade et al. 1970) (Turunen 1975) (Hoffman and Downer 1979) |
| TAG to FFAs+glycerol |
Locusta migratoria Periplaneta americana; Locusta migratoria Manduca sexta Panstrongylus megistus |
(Weintraub and Tietz 1973) (Male and Storey 1981) (Tsuchida and Wells 1988) (Canavoso et al. 2004) |
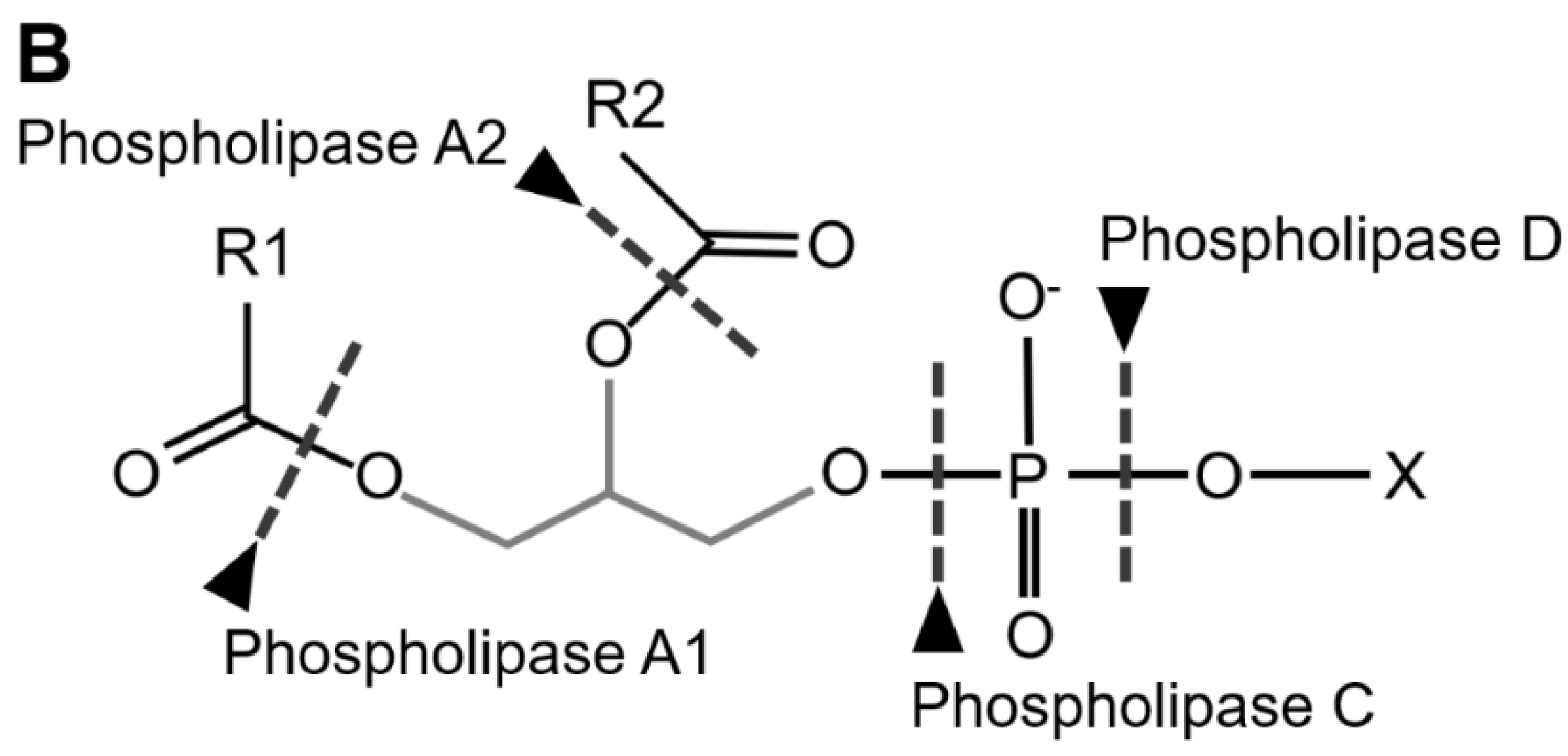
| Phospholipase A1 |
Drosophila melanogaster Lepidoptera species Musca domestica |
Table 3 (Canavoso et al. 2001) (Barroso et al. 2021) |
| Phospholipase A2 |
Cicindella circumpicta Manduca sexta Aedes aegypti Spodoptera exigua Musca domestica |
(Uscian et al. 1995) (Stanley et al. 1998) (Nor Aliza and Stanley 1998) (Sajjadian et al. 2019) (Barroso et al. 2021) |
| Phospholipase B | Drosophila melanogaster | Table 3 |
| Phospholipase C |
Stomoxys calcitrans Aeshna cyanea Musca domestica |
(Spates et al. 1990) (Weiher and Komnick 1997) (Barroso et al. 2021) |
| Phospholipase D | Pieris brassicae | (Turunen 1988a) |
| Galactolipase | Epiphyas postvittana; Helicoverpa armigera | (Christeller et al. 2011) |
| Sterol dealkilation | Bombyx mori | (Ciufo et al. 2011) |
| Sterol esterase | Drosophila melanogaster | (Sieber and Thummel 2012) |
| Wax hydrolysis |
Aeshna cyanea Galleria mellonella |
(Komnick and Bauerfeind 1991) (Kong et al. 2019) |
| Lipid synthesis |
Locusta migratoria Pieris brassicae |
(Weintraub and Tietz 1978) (Turunen 1988b) |
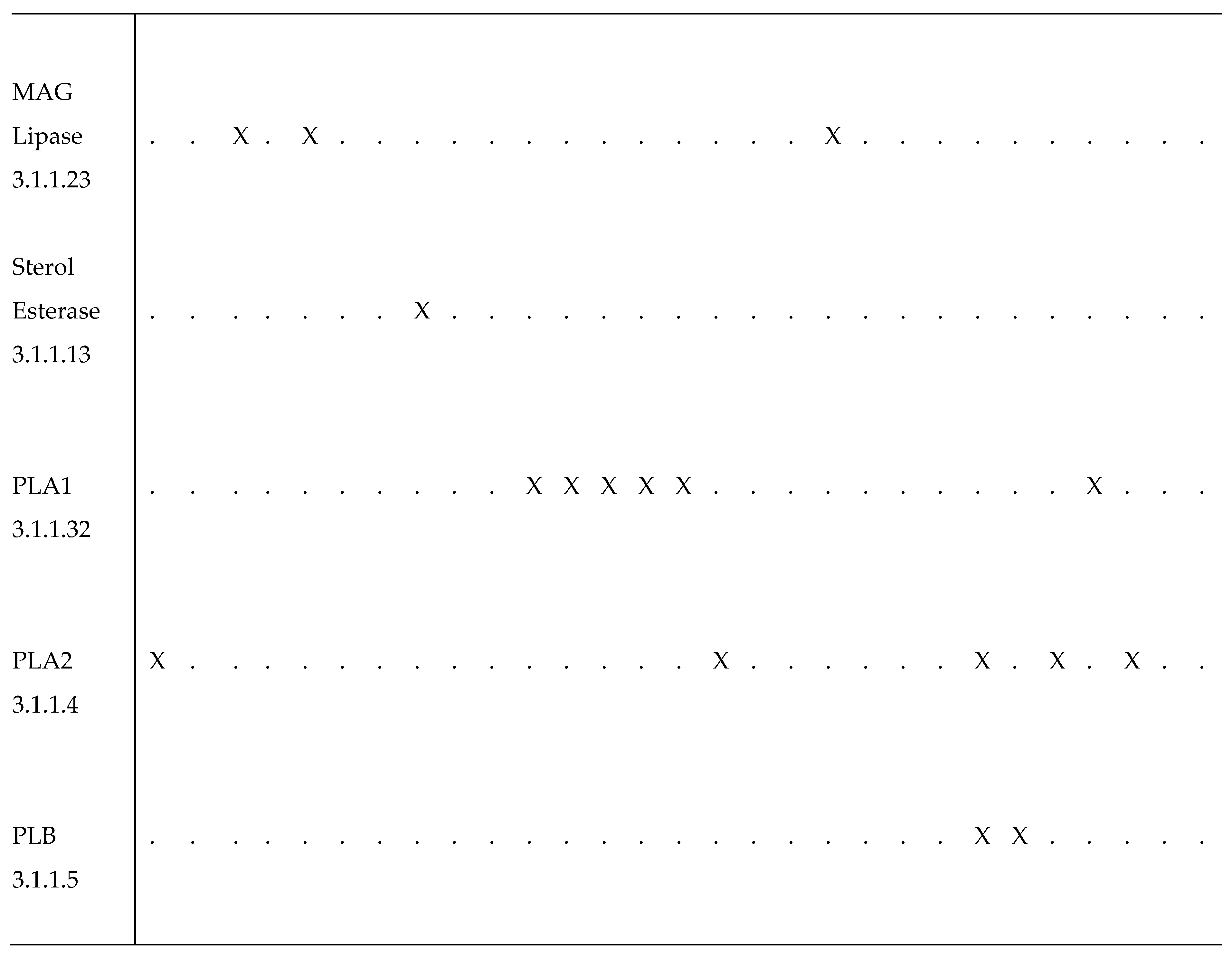
| Scavenger receptor | FABP | FATP | Npc1a | Npc1b | Npc2 | Flo | |
| Aedes aegypti | 3 | 1 | 2 | 0 | 1 | 4 | 2 |
| Anopheles gambiae | 4 | 2 | 2 | 1 | 1 | 4 | 3 |
| Apis mellifera | 1 | 2 | 3 | 1 | 1 | 2 | 2 |
| Bombyx mori | 1 | 5 | 5 | 1 | 1 | 1 | 3 |
| Cimex lectularius | 3 | 2 | 4 | 1 | 1 | 3 | 2 |
| Drosophila melanogaster | 3 | 1 | 3 | 1 | 1 | 8 | 2 |
| Locusta migratoria | 3 | 7 | 4 | 1 | 1 | 1 | 2 |
| Manduca sexta | 1 | 18 | 5 | 1 | 1 | 2 | 2 |
| Musca domestica | 4 | 2 | 2 | 1 | 1 | 9 | 3 |
| Rhodnius prolixus | 2 | 1 | 7 | 1 | 1 | 1 | 2 |
| Spodoptera frugiperda | 2 | 6 | 4 | 2 | 1 | 1 | 2 |
| Tenebrio molitor | 3 | 3 | 2 | 2 | 1 | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




