Vascular rings have an estimated prevalence of 1 in 10,000 live births [1]. These rare congenital anomalies of the aortic arch encircle and compress the trachea and/or esophagus, presenting as nonspecific symptoms that vary in severity depending on the affected structure and the degree of compression [1,3]. Early surgical intervention is crucial in symptomatic vascular rings [1]. Surgical repair focuses on relieving tracheal and/or esophageal compression by dividing the vascular ring, thereby providing symptomatic relief and preventing serious complications such as sudden death or residual tracheobronchial damage [1,3]. However, determining symptoms in infants and pediatric patients can be challenging due to symptom overlap with common conditions in these age groups.
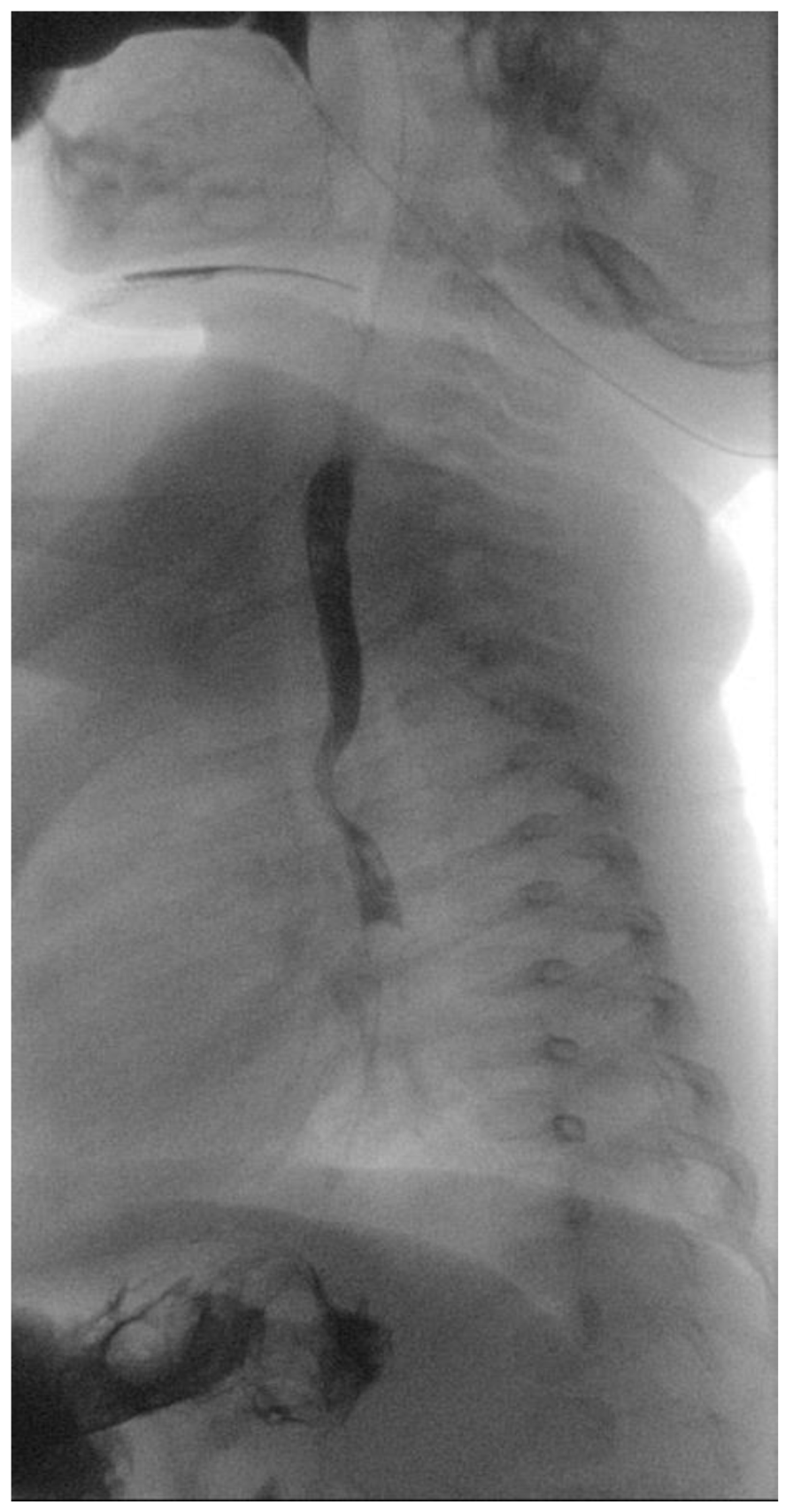
A 5-month-old female patient was being evaluated for failure to thrive secondary to dysphagia while on a liquid diet, requiring feeding aids (nasogastric tube). Past medical history accounts for in utero umbilical hemorrhage at 34 weeks’ gestation and intrauterine growth restriction. A barium swallow test was performed that revealed a posterior indentation of the proximal thoracic esophagus (
Figure 1).
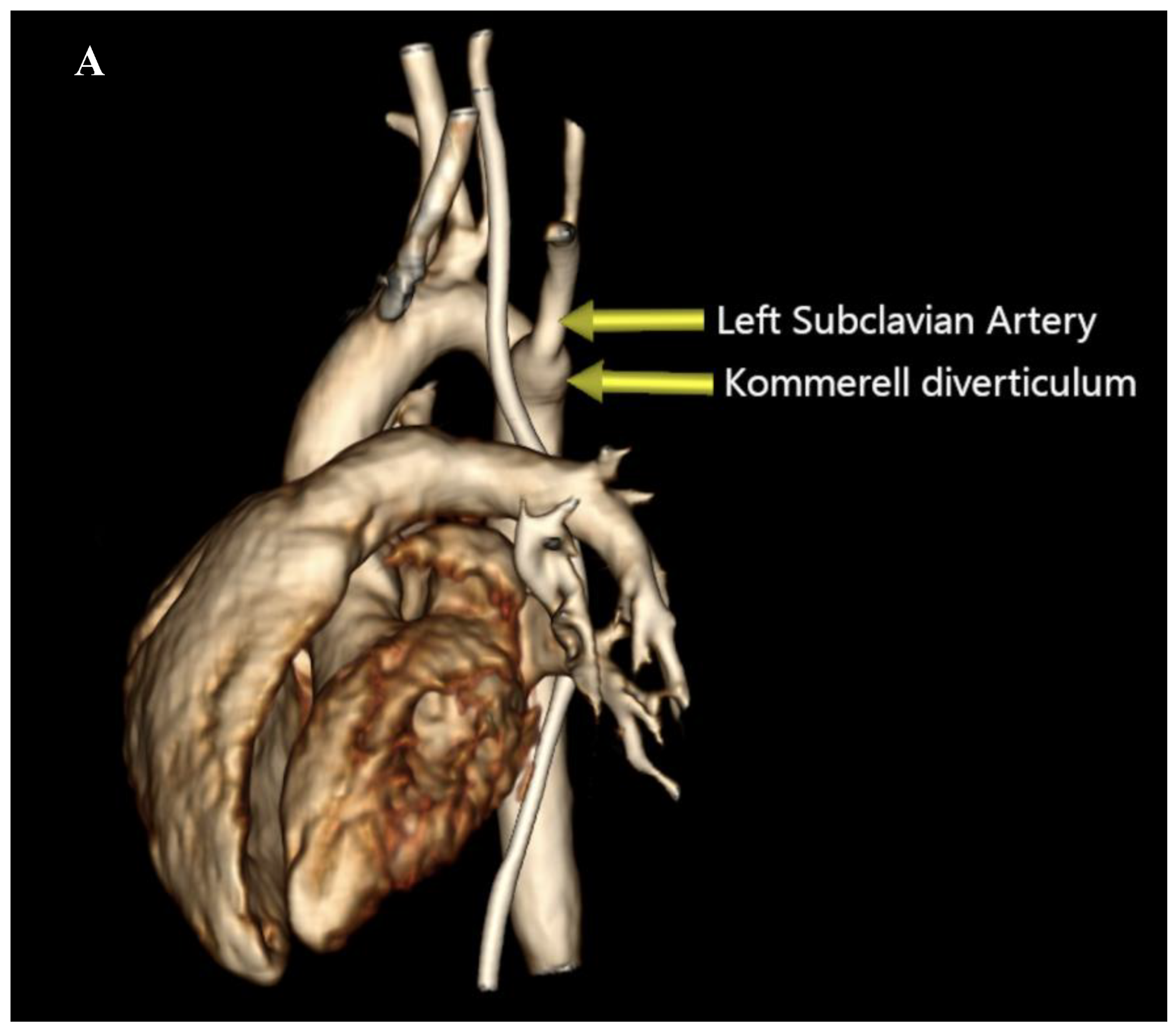
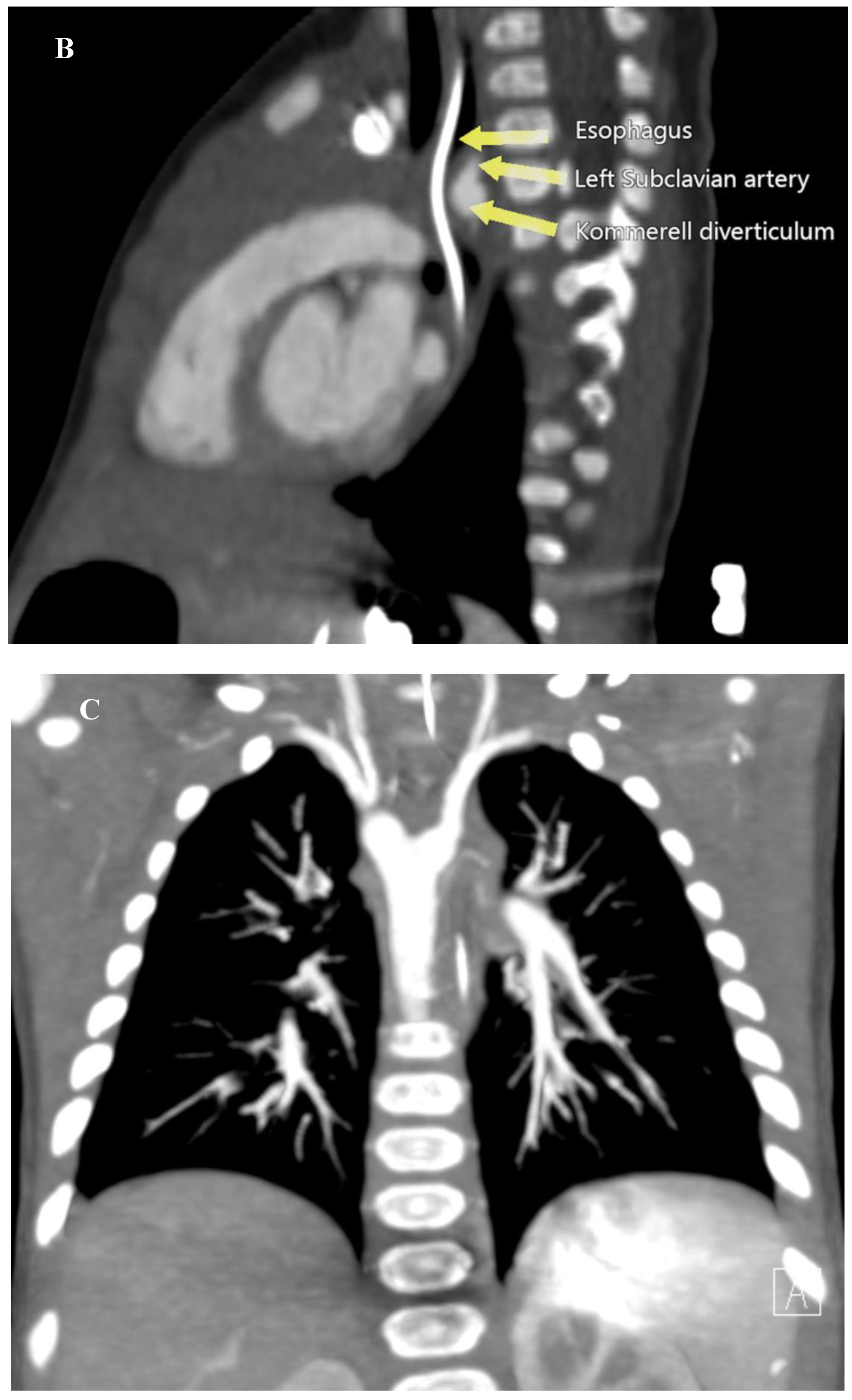
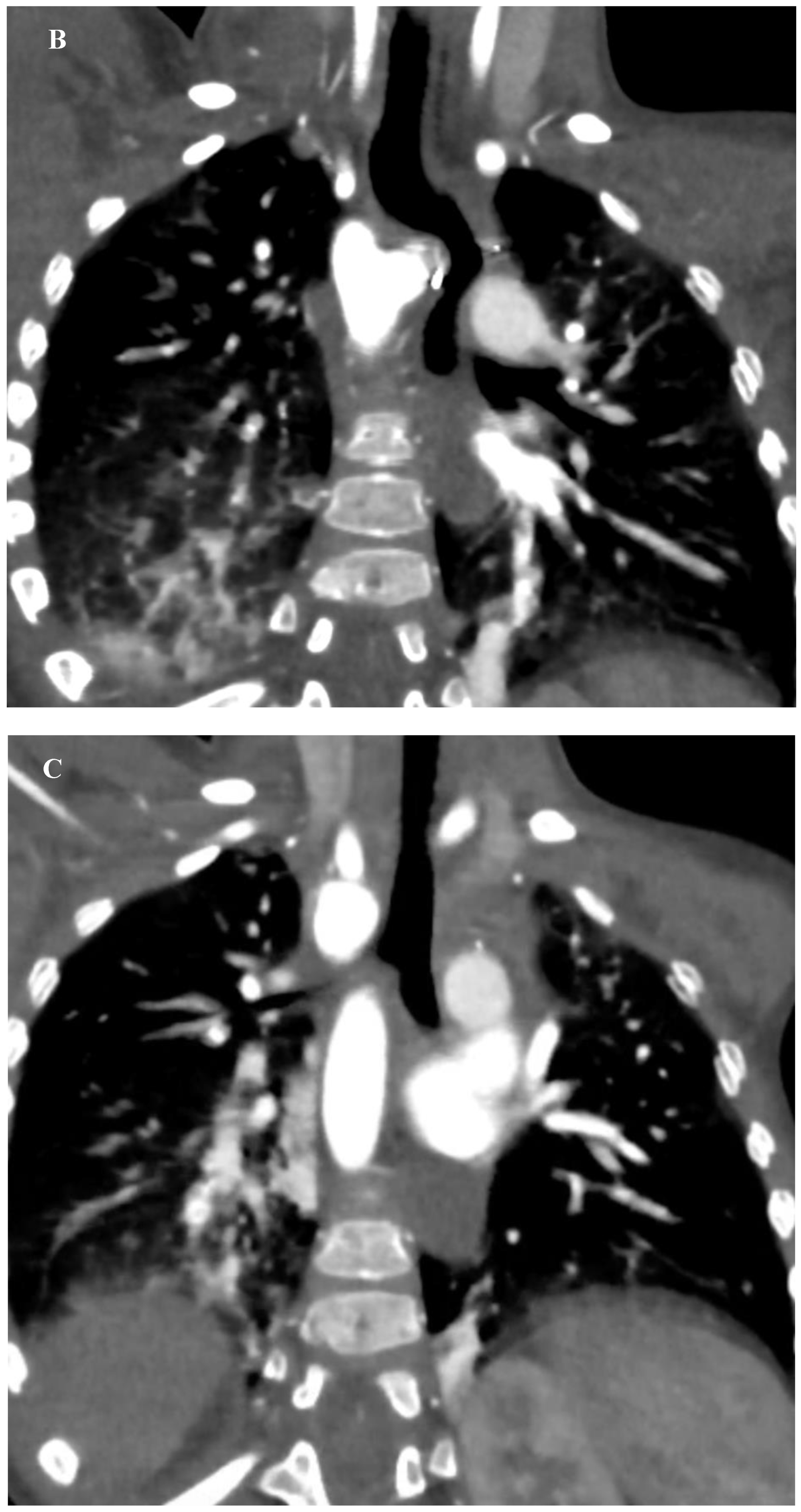
This finding prompted a Computed Tomography Angiography (CTA) which revealed a vascular ring formed by a Right Aortic Arch (RAA), an aberrant Left Subclavian Artery (LSA) originating from an aneurysmal dilation at its base, the so-called Kommerell’s diverticulum (KD), and a Left Ligamentum Arteriosum (LLA) (
Figure 2) (
Supplementary material 1).
The patient was then placed on a gastrostomy tube and followed up until 14 months of age, showing persistent dysphagia and weighing 8.2 kg. Hence, the decision was made to perform a surgical repair of the vascular ring. Prior to the surgical incision, an endoscopic and EndoFLIP
TM evaluation were conducted, demonstrating an esophageal narrowing at the level of the vascular ring and an abnormal increase in esophageal impedance, respectively. Subsequently, a limited left posterior thoracotomy was performed, allowing the visualization of a left-sided esophagus with a RAA originating from behind, and an aberrant LSA exiting from a KD. The LLA, observed compressing the esophagus, was divided. The KD was obliterated with two purse-string sutures, and the aberrant LSA was dissected and divided from the aorta, then re-implanted into the left carotid artery (LCA) via an end-to-side anastomosis. Following closure of the chest, another endoscopic and EndoFLIP
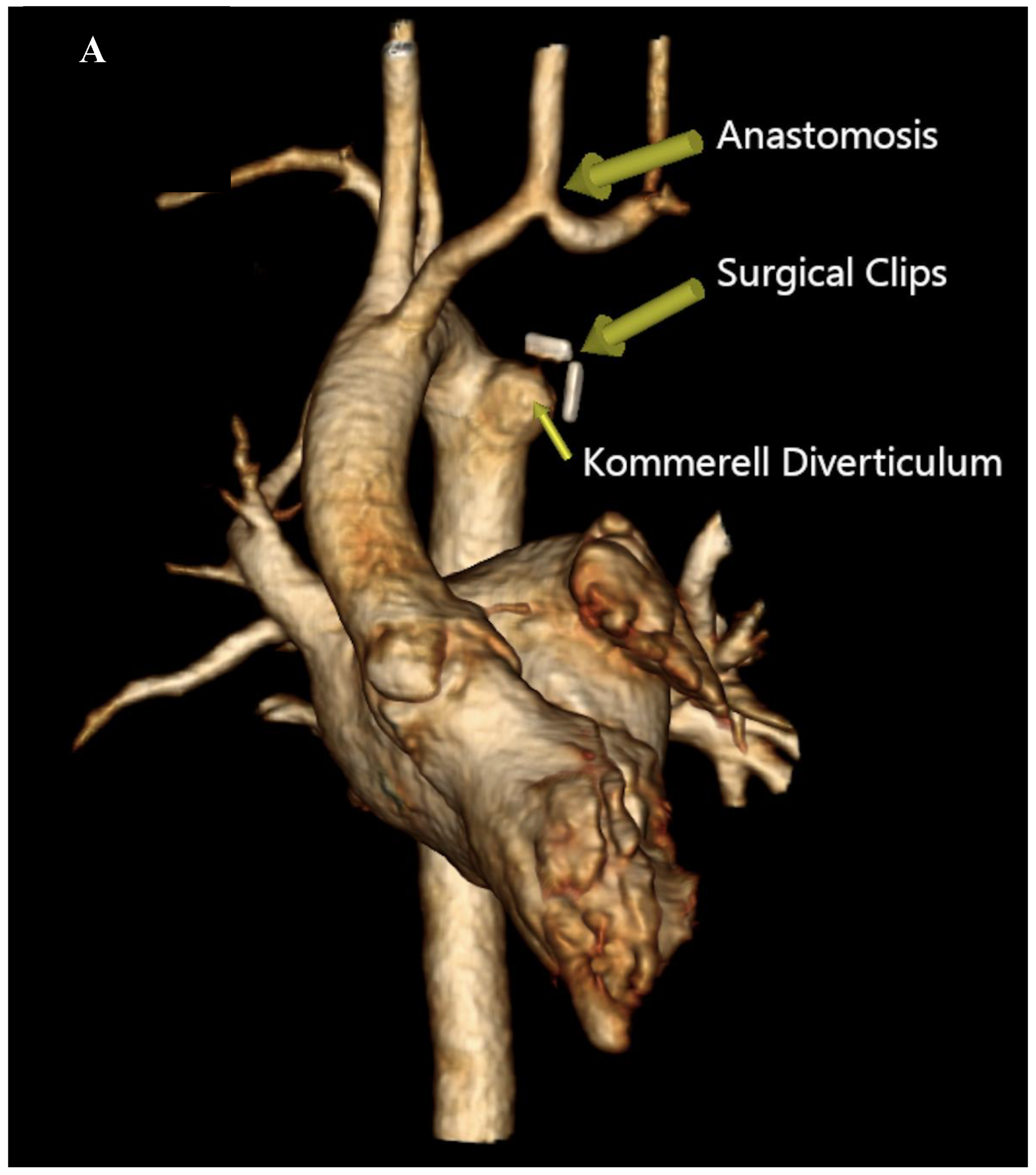
TM evaluation were performed which evidenced an improvement in esophageal narrowing and impedance, respectively. The patient’s recovery was uneventful, and she was discharged on postoperative day 4. During the follow-up visits, she was asymptomatic and meeting her growth milestones. A recent CTA performed 2 years post- procedure revealed a patent re-implanted LSA into the LCA and no tracheal deformities (
Figure 3) (
Supplementary material 2, 3, and 4).
We describe the case of a 14-month-old female patient with a vascular ring who underwent successful surgical repair. Intraoperative demonstration of esophageal compression relief was achieved using endoscopic and EndoFLIP™ technology. Transposition of the LSA into the LCA was performed, and patency of the re-implanted artery, without evident tracheal deformities or esophageal narrowing, was confirmed by the two-year postoperative CTA.
As vascular rings can compress the trachea and/or esophagus, they manifest as various symptoms, ranging from respiratory distress in newborns to swallowing difficulties in older children [2]. Evidencing symptoms in small infants can pose a significant diagnostic challenge due to the overlap with common manifestations observed in this age group, such as stridor, wheezing, recurrent respiratory infections, vomiting, and dysphagia [1–3,5]. Early surgical repair of symptomatic vascular rings is crucial, aiming to relieve the compression while reducing the associated potential complications [1]. The two primary factors causing compression in this type of vascular ring (i.e., RAA with aberrant LSA and LLA) are the space-occupying effect of the KD and the sling-like effect of the aberrant LSA [6]. Therefore, to relieve the compression, the arterial ligament should be divided, the KD should be obliterated, and the LSA should be transferred to the LCA [6]. EndoFLIPTM technology, employed for assessing esophageal cross-sectional area via high-resolution impedance planimetry during volume-controlled distension, has been utilized in the pediatric population for diagnosing and managing esophageal disorders, and it can serve for the intraoperative assessment of the esophageal compression release during vascular ring repair [7]. In our patient, endoscopy and EndoFLIPTM technology provided an objective intraoperative measurement of the esophageal narrowing and impedance improvement. A limited left posterior thoracotomy was the chosen surgical approach considering the absence of additional intracardiac abnormalities, allowing the visualization of the vascular ring’s anatomy. During the surgical procedure the LLA was divided, the KD was obliterated, and despite the patient's weight of 8.2 kg, the LSA was successfully transferred to the LCA. Re-implantation of the LSA in this type of vascular ring is feasible even for infants, having a good patency as confirmed by the follow-up CTA at mid-term. This case underscores the significance of managing symptomatic vascular rings via early surgical repair and introduces an alternative method for objective intraoperative assessment of esophageal compression relief in the vascular ring population, representing a safer intervention compared to the higher risks associated with the potential complications resulting from tracheoesophageal compression.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Video S1: Preoperative Computed Tomography Angiography, 3D reconstruction. Video S2: Postoperative Computed Tomography Angiography, 3D reconstruction. Video S3: Postoperative Computed Tomography Angiography, coronal view. Video S4: Postoperative Computed Tomography Angiography, axial view.
Author Contributions
Conceptualization, V.B-H. and M.A.R.B.; writing—original draft preparation, M.A.R.B.; writing—review and editing, M.A.R.B., K.D.K., A.T.A., G.P., and V.B-H.; supervision, V.B-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained withing the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yoshimura N, Fukahara K, Yamashita A, et al. Congenital vascular ring. Surg Today. 2020 Oct;50(10):1151-1158.
- Worhunsky DJ, Levy BE, Stephens EH, et al. Vascular rings. Semin Pediatr Surg. 2021 Dec;30(6):151128.
- Schmidt AMS., Larsen SH, Hjortdal VE. Vascular ring: Early and long-term mortality and morbidity after surgical repair. J Pediatr Surg. 2018 Oct;53(10):1976-1979. [CrossRef]
- Backer CL, Mavroudis C. Congenital heart surgery nomenclature and database project: vascular rings, tracheal stenosis, pectus excavatum. Ann Thorac Surg. 2000:69:S308-S318. [CrossRef]
- Biermann D, Holst T, Hüners I, et al. Right aortic arch forming a true vascular ring: a clinical review. Eur J Cardiothorac Surg. 2021 Nov 2;60(5):1014-1021. [CrossRef]
- Backer CL, Hillman N, Mavroudis C, et al. Resection of Kommerell's diverticulum and left subclavian artery transfer for recurrent symptoms after vascular ring division. Eur J Cardiothorac Surg. 2002 Jul;22(1):64-9. [CrossRef]
- Ng K, Mogul D, Hollier J, et al. Utility of functional lumen imaging probe in esophageal measurements and dilations: a single pediatric center experience. urg Endosc. 2020 Mar;34(3):1294-1299. Epub 2019 Jun 11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).