I. Introduction
A. Overview of Diastolic Dysfunction
Diastolic dysfunction, a hallmark of heart failure with preserved ejection fraction (HFpEF), manifests as impaired left ventricular relaxation and filling. Myofilament dysfunction, impacting cardiomyocyte contractility and myocardial stiffness, is pivotal in diastolic heart failure pathogenesis [
1]. Early pericyte loss initiates microvascular dysfunction, driving diastolic dysfunction progression [
2]. Additionally, cardiomyocytes contribute via altered calcium handling and sarcomeric protein isoform shifts [
3]. Coronary microvascular dysfunction exacerbates diastolic dysfunction, leading to left ventricular remodeling [
4]. These complexities underscore diastolic dysfunction’s multifactorial nature, involving cellular and microvascular components. Understanding these mechanisms is crucial for developing targeted HFpEF therapies.
B. Introduction to Galectin-3
Galectin-3, a member of the β-galactoside-binding lectin family, plays a pivotal role in cardiovascular pathology. Its relevance in HFpEF as a potential biomarker for diagnosing and prognosticating cardiac dysfunction is well-established [
5]. Moreover, galectin-3 influences fibrotic, inflammatory, and remodeling pathways across various cardiac conditions, extending beyond HFpEF [
6]. Investigations into galectin-3’s mechanisms offer novel therapeutic avenues, including inhibitors, for cardiovascular diseases [
7]. Clinical assessments affirm its diagnostic utility and prognostic significance in chronic heart failure [
8]. Understanding galectin-3’s multifaceted involvement in cardiovascular diseases is crucial for unlocking its therapeutic and diagnostic potential in managing conditions like diastolic dysfunction.
C. Rationale for Exploring Galectin-3 in Diastolic Dysfunction
The investigation of galectin-3 in diastolic dysfunction stems from its intricate involvement in cardiovascular pathology and its potential prognostic value. Galectin-3 levels are significantly associated with adverse outcomes across various cardiovascular disorders, notably HFpEF [
9]. Emerging evidence links galectin-3 with systemic proinflammatory-profibrotic responses in conditions such as aortic stenosis and diabetes, suggesting its role in myocardial remodeling and prognosis [
10]. Additionally, galectin-3 serves as an early indicator of diastolic dysfunction in pediatric hemodialysis patients, highlighting its potential in early detection [
11]. Given the limited treatment options for diastolic dysfunction and galectin-3’s importance in cardiovascular diseases, investigating its role offers promise for enhancing risk assessment and guiding therapeutic strategies.
II. Molecular Mechanisms of Galectin-3
A. Structure and Function of Galectin-3
Understanding the structural and functional intricacies of galectin-3 is paramount for deciphering its myriad roles in physiological and pathological processes, particularly in diastolic dysfunction. Galectin-3 comprises a carbohydrate recognition domain linked to a collagen-like N-terminal domain, facilitating interactions with β-galactosides on glycoproteins and glycolipids, thereby modulating diverse cellular processes [
12]. Functionally, galectin-3 participates in clathrin-independent endocytosis by associating with dynein, a microtubule motor protein, thereby facilitating intracellular trafficking crucial for cellular homeostasis [
13]. Moreover, galectin-3’s involvement in fibrosis and inflammation underscores its significance in cardiovascular conditions such as cirrhotic cardiomyopathy, contributing to disease pathogenesis and progression [
14]. Notably, targeting galectin-3 emerges as a potential therapeutic strategy, with neutralizing antibodies showing promise in mitigating fibrotic processes, as evidenced in systemic sclerosis treatment [
15].
B. Cellular Signaling Pathways
Galectin-3 intricately modulates cellular signaling pathways implicated in cardiovascular pathophysiology, with ramifications extending to diastolic dysfunction (
Figure 1). In vascular calcification, galectin-3 promotes calcification of vascular smooth muscle cells through the AMP-activated protein kinase/thioredoxin-interacting protein pathway, exacerbating arterial stiffness and atherosclerosis progression, crucial features linked to diastolic dysfunction [
16]. Furthermore, galectin-3 enhances atrial fibrosis via CD98 signaling, facilitating atrial fibrillation development, a prevalent arrhythmia in diastolic dysfunction patients [
17]. Additionally, galectin-3 mediates cardiac remodeling by interfering with glucose and lipid metabolism pathways, hindering Akt activation, and worsening cardiac dysfunction, which may further impair diastolic function [
18]. Galectin-3’s involvement in promoting calcification of human aortic valve interstitial cells through the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappa B) signaling pathway and inducing atrial fibrosis via the transforming growth factor beta1 (TGF beta1)/ suppressor of mother against decapentaplegic (Smad) pathway further highlights its role in valvular pathologies and atrial remodeling, impacting diastolic filling [
19,
20].
III. Role of Galectin-3 in Diastolic Dysfunction
A. Experimental Evidence from Animal Models
Experimental inquiries into the role of galectin-3 in diastolic dysfunction using animal models have yielded crucial insights. Investigations employing pressure-overloaded heart models revealed that stretch-induced sarcoplasmic reticulum calcium leak serves as a causal factor for atrial fibrillation, with galectin-3 inhibition exhibiting potential in mitigating doxorubicin-induced cardiac dysfunction [
26,
27]. Moreover, studies employing isolated subendocardial damage models unveiled alterations in myocardial microstructure and function, implicating galectin-3 in the pathophysiology of cardiac remodeling and dysfunction [
28]. Notably, galectin-3 has been linked to cardiac fibrosis and inflammation, thereby contributing to the progression of diastolic dysfunction. The CT-1 (cardiotrophin-1)-Gal-3 (galectin-3) axis emerged as a pivotal player in cardiac fibrosis, shedding light on galectin-3’s role in promoting adverse myocardial remodeling and functional impairment [
29]. Furthermore, evidence from cellular and animal models, coupled with clinical indices, suggests a correlation between galectin-3 levels and the severity of cardiac diastolic dysfunction [
30]. Remarkably, in mice with Pkd1(polycystic Kidney disease 1) deficiency—a model associated with cardiac dysfunction—galectin-3 knockout demonstrated a rescue effect on the phenotype, underscoring its involvement in the pathogenesis of cardiac abnormalities [
31]. Collectively, these findings underscore the multifaceted role of galectin-3 in the pathophysiology of diastolic dysfunction, encapsulating calcium handling abnormalities, myocardial microstructural alterations, fibrosis, and inflammation. Animal models serve as indispensable tools for elucidating these mechanisms, offering valuable insights into potential therapeutic strategies targeting galectin-3 to mitigate diastolic dysfunction and its associated complications.
B. Clinical Studies in Human Subjects
Clinical investigations provide robust evidence elucidating the involvement of galectin-3 in diastolic dysfunction across diverse patient cohorts (
Table 1). In individuals at risk of heart failure, elevated markers of type I collagen synthesis, including galectin-3, were correlated with impaired cardiac mechanics, suggesting a potential link between fibrosis and diastolic dysfunction [
32]. Similarly, in elderly patients, biomarkers of cardiac injury, stress, and fibrosis, including galectin-3, exhibited correlations with altered cardiac mechanics, implying a mechanistic role in the development of diastolic dysfunction [
33]. Anemia, a prevalent comorbidity in HFpEF, has been associated with diastolic dysfunction. Clinical studies have demonstrated associations between galectin-3 levels and the severity of anemia in HFpEF patients, suggesting its potential as a biomarker for identifying diastolic dysfunction in this population [
34]. Moreover, in individuals with ST-segment elevation myocardial infarction, elevated galectin-3 levels were predictive of post-infarction heart failure development, emphasizing its role in adverse cardiac remodeling and the progression of diastolic dysfunction [
35]. Longitudinal investigations have provided insights into the evolution of diastolic dysfunction following myocardial infarction. Patients with anterior Q-wave myocardial infarction demonstrated progressive diastolic dysfunction over time, with galectin-3 levels predicting left ventricular remodeling and adverse outcomes post-infarction [
36,
37]. Furthermore, in hemodialysis patients, galectin-3 emerged as a biomarker of diastolic dysfunction, reflecting its clinical utility in assessing cardiac function in high-risk populations [
38]. Overall, these clinical studies underscore the significance of galectin-3 in the pathogenesis and prognostication of diastolic dysfunction, highlighting its potential as a therapeutic target and diagnostic tool in cardiovascular disease management.
C. Galectin-3 as a Biomarker for Diastolic Dysfunction
Galectin-3 has emerged as a promising biomarker for evaluating diastolic dysfunction across diverse clinical contexts. Investigations in various patient populations consistently indicate associations between elevated galectin-3 levels and indicators of diastolic dysfunction. In individuals with chronic kidney disease (CKD), galectin-3 levels were correlated with echocardiography parameters indicative of diastolic dysfunction, suggesting its utility as a biomarker for cardiac fibrosis and dysfunction in this population [
39]. Similarly, in children with end-stage renal disease undergoing regular hemodialysis, galectin-3 was identified as an early marker of diastolic dysfunction, underscoring its potential role in early detection and monitoring of cardiac involvement in pediatric CKD [
11]. Furthermore, in patients with aortic stenosis and concomitant diabetes, galectin-3 levels were associated with systemic proinflammatory and profibrotic responses, myocardial remodeling, and adverse clinical outcomes, emphasizing its involvement in the pathogenesis of diastolic dysfunction in this high-risk population [
10]. Additionally, in patients with non-ischemic dilated cardiomyopathy, galectin-3 levels were correlated with circulating collagen turnover biomarkers and late gadolinium enhancement on cardiac magnetic resonance imaging, indicating its potential as a biomarker for cardiac fibrosis and adverse remodeling in this cohort [
40]. Moreover, meta-analyses have highlighted the clinical implications of plasma galectin-3 levels in HFpEF, showing consistent associations with diastolic dysfunction severity and adverse outcomes [
9]. Additionally, in patients with chronic Chagas cardiomyopathy, galectin-3 levels were correlated with cardiovascular biomarkers and diastolic dysfunction parameters, suggesting its potential as a prognostic marker in this specific cardiomyopathy [
41]. Overall, these findings underscore the value of galectin-3 as a biomarker for assessing diastolic dysfunction across different patient populations, providing insights into its pathophysiological role and clinical implications in cardiovascular disease management.
IV. Pathophysiological Insights
A. Inflammation and Fibrosis
Galectin-3 emerges as a pivotal mediator in the pathogenesis of cardiac inflammation and fibrosis, significantly impacting the development and progression of diastolic dysfunction. A comprehensive understanding of its multifaceted role in cardiac pathology has been elucidated through recent investigations. Seropian et al. (2023) delineated the involvement of galectin-3 in cardiac dysfunction and toxicity [
42], particularly in doxorubicin-treated murine models, where augmentation of galectin-3 correlated with heightened oxidative stress and fibrotic progression, thus exacerbating myocardial injury. Moreover, Wang et al. (2023) showcased the therapeutic potential of targeting galectin-3 post-infarction [
43], demonstrating its efficacy in attenuating progressive fibrosis by modulating inflammatory profibrotic pathways, thereby preserving diastolic function following myocardial infarction. Furthermore, Hu et al. (2023) unraveled a galectin-3-centered paracrine network orchestrating cardiac inflammation and fibrosis in response to beta-adrenergic insult [
44], underscoring its pivotal role in cardiac remodeling. Genetic studies have further underscored the significance of galectin-3, as reported by Fontana Estevez et al. (2022), wherein genetic deletion exacerbated age-related myocardial hypertrophy and fibrosis [
45], suggesting a protective role against pathological remodeling. Conversely, Vlachou et al. (2022) demonstrated the detrimental impact of galectin-3 interference in promoting cardiac dysfunction and comorbidities in a genetic heart failure model [
46], indicating a disruption in tissue repair mechanisms and functional recovery. These collective findings underscore the indispensable role of galectin-3 in mediating cardiac inflammation and fibrosis, thereby contributing to the pathogenesis of diastolic dysfunction and adverse cardiac remodeling. Targeting galectin-3 signaling pathways holds significant promise as a therapeutic strategy for managing diastolic dysfunction and enhancing clinical outcomes in heart failure.
B. Cardiac Remodeling
Cardiac remodeling, a multifaceted process encompassing structural and functional alterations in response to pathological stimuli, encompasses diastolic dysfunction, a hallmark feature of HFpEF. Galectin-3 emerges as a pivotal player in cardiac remodeling, exerting profound effects on the progression of diastolic dysfunction and HFpEF. Experimental evidence highlights the detrimental impact of galectin-3 on cardiac structure and function. Seropian et al. (2023) delineated its role in acute cardiac dysfunction and toxicity, primarily through mechanisms involving heightened oxidative stress and fibrosis [
42]. This pro-fibrotic action of galectin-3 exacerbates myocardial stiffness and impairs diastolic relaxation, thereby promoting diastolic dysfunction. Moreover, inhibition of galectin-3 holds promise in attenuating cardiac fibrosis and remodeling. Wang et al. (2023) demonstrated the efficacy of post-infarction galectin-3 inhibition in mitigating progressive fibrosis by modulating inflammatory profibrotic cascades [
43], offering potential therapeutic avenues for preserving diastolic function in various cardiac pathologies. Genetic studies further elucidate the significance of galectin-3 in cardiac remodeling. Fontana Estevez et al. (2022) demonstrated that genetic deletion exacerbates age-related myocardial hypertrophy and fibrosis [
47], highlighting its protective role against pathological cardiac remodeling. In a heart failure model, Sonkawade et al. (2021) explored the therapeutic potential of small endogenous peptides in mitigating myocardial remodeling induced by galectin-3 overexpression [
48], underscoring the promise of galectin-3 targeting for modulating pathological cardiac remodeling and improving diastolic function. Collectively, these studies underscore the critical role of galectin-3 in mediating cardiac fibrosis and diastolic dysfunction, highlighting its potential as a therapeutic target for managing HFpEF and other cardiac conditions characterized by impaired diastolic function.
C. Endothelial Dysfunction
Endothelial dysfunction, characterized by impaired vascular homeostasis and endothelial barrier integrity, contributes significantly to the pathogenesis of diastolic dysfunction and cardiovascular diseases. Galectin-3 emerges as a central mediator in endothelial dysfunction, exacerbating vascular inflammation and oxidative stress. Recent investigations have provided insights into the role of galectin-3 in mediating endothelial dysfunction across various cardiovascular conditions. Wang et al. (2024) elucidated its involvement in inflammation and fibrosis in arteriogenic erectile dysfunction, highlighting its pro-inflammatory role via the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)/NF-kappaB pathway [
49]. Additionally, Pang et al. (2023) identified Yes-associated protein (YAP)-galectin-3 signaling as a mediator of endothelial dysfunction in angiotensin II-induced hypertension, emphasizing the detrimental effects of galectin-3 on vascular homeostasis and endothelial function [
50]. Exploring the prognostic implications, Tsigkou et al. (2023) investigated the role of galectin-3 and endothelial function in heart failure [
51], suggesting a potential link between galectin-3-mediated endothelial dysfunction and adverse cardiovascular outcomes. Mechanistically, galectin-3 exacerbates endothelial injury by inducing inflammation and oxidative stress, as demonstrated by Chen et al. (2019) [
52], highlighting its role in promoting vascular inflammation and dysfunction. Overall, galectin-3-mediated endothelial dysfunction contributes significantly to the pathogenesis of diastolic dysfunction and cardiovascular diseases, underscoring its potential as a therapeutic target for mitigating vascular inflammation and improving endothelial function.
V. Diagnostic and Therapeutic Implications
A. Potential Diagnostic Utility of Galectin-3
Galectin-3 emerges as a promising diagnostic biomarker for diastolic dysfunction, reflecting its involvement in diverse underlying pathophysiological processes. Consistent findings link elevated galectin-3 levels to heightened cardiovascular event risk and heart failure incidence, underscoring its diagnostic potential [
53,
54,
55,
56]. Furthermore, galectin-3 levels correlate with disease severity and prognosis in heart failure and atrial fibrillation, offering valuable insights for risk stratification and treatment decision-making [
54,
56,
57]. Integrating galectin-3 measurements into clinical practice may refine diagnostic accuracy and facilitate tailored management strategies for cardiovascular diseases. Galectin-3 assessment holds promise for early detection and risk stratification in diastolic dysfunction patients, furnishing crucial prognostic information. Incorporating galectin-3 testing into diagnostic protocols for HFpEF could refine risk assessment and guide therapeutic interventions.
B. Therapeutic Targeting of Galectin-3
Therapeutic intervention targeting galectin-3 emerges as a promising avenue for managing diastolic dysfunction and its associated cardiovascular complications. Ongoing efforts focus on the development of drugs aimed at modulating galectin-3 activity to counter its pathological effects [
58]. Encouragingly, preclinical studies demonstrate the efficacy of galectin-3 inhibition in mitigating inflammation and fibrosis, hinting at its therapeutic potential [
59]. Furthermore, the prospect of targeting galectin-3 presents a novel therapeutic strategy for cardiovascular diseases by mitigating cardiac fibrosis, hypertrophy, and inflammation [
53,
60]. These insights underscore the therapeutic promise of galectin-3 inhibition in addressing the underlying mechanisms of cardiovascular pathologies, thus enhancing clinical outcomes. Clinical trials are actively investigating the safety and efficacy of galectin-3 inhibitors in heart failure patients, particularly those with preserved ejection fraction. The targeted modulation of galectin-3 signaling pathways represents a promising therapeutic approach for diastolic dysfunction, offering a complementary strategy to existing treatments.
C. Future Directions and Research Opportunities
Future research avenues in galectin-3 and diastolic dysfunction encompass elucidating molecular mechanisms, validating diagnostic and prognostic utility, developing novel therapeutics, and exploring galectin-3 as a biomarker for monitoring disease progression and treatment response. Prospective clinical studies are needed to standardize diagnostic algorithms and risk stratification models. Collaborative efforts across disciplines will expedite the translation of research findings into clinical practice, ultimately improving patient outcomes and advancing cardiovascular care.
VI. Conclusions
A. Summary of Key Findings
In this comprehensive review, we have meticulously examined the intricate interplay between galectin-3 and diastolic dysfunction, illuminating its multifaceted role in the pathophysiology of HFpEF. Our analysis reveals that galectin-3 functions both as a biomarker and mediator, orchestrating inflammation, fibrosis, and cardiac remodeling processes that drive the development and progression of diastolic dysfunction (
Figure 3). Through its modulation of cellular signaling pathways and interactions with extracellular matrix components, galectin-3 profoundly impacts myocardial stiffness, vascular function, and endothelial integrity. Clinical investigations underscore its diagnostic and prognostic value in identifying high-risk patients and guiding therapeutic strategies. Moving forward, further research is imperative to dissect the precise mechanisms underpinning galectin-3-mediated pathophysiology and to explore its therapeutic potential in ameliorating diastolic dysfunction and enhancing patient outcomes in HFpEF.
B. Clinical Relevance and Implications
Galectin-3 holds significant clinical relevance in diastolic dysfunction (
Table 2), serving as a promising diagnostic biomarker and therapeutic target for HFpEF. By elucidating its pivotal role in inflammation, fibrosis, and cardiac remodeling, our review underscores galectin-3’s utility as an indicator of disease severity and prognosis in diastolic dysfunction patients (
Table 3). Moreover, therapeutic interventions targeting galectin-3 offer a novel avenue for attenuating myocardial stiffness, improving vascular function, and preserving endothelial integrity, thereby mitigating disease progression and reducing HFpEF-related morbidity and mortality. Recognizing galectin-3 as a clinically relevant marker and therapeutic avenue underscores the importance of personalized management approaches tailored to the underlying pathophysiology of diastolic dysfunction, ultimately enhancing patient care and outcomes in HFpEF.
Author Contributions
Wen-Rui Hao and Chun-Han Cheng wrote the initial paper, Ju-Chi Liu, Huan-Yuan Chen and Jin-Jer Chen designed and drew the figures and table, Tzu-Hurng Cheng revised the paper.
Corresponding author: Tzu-Hurng Cheng, PhD, Professor, Department of Biochemistry, School of Medicine, College of Medicine, China Medical University, Taichung City 404333, Taiwan (R.O.C.) thcheng@mail.cmu.edu.tw.
References
- Aboonabi, A.; McCauley, M. D. Myofilament dysfunction in diastolic heart failure. Heart Fail Rev 2024, 29, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S. J.; Grootaert, M. O. J.; Cuijpers, I.; Carai, P.; Geuens, N.; Herwig, M.; Baatsen, P.; Hamdani, N.; Luttun, A.; Heymans, S.; Jones, E. A. V. Pericyte loss initiates microvascular dysfunction in the development of diastolic dysfunction. Eur Heart J Open 2024, 4, oead129. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J. V.; Raaijmakers, A. J. A.; Weeks, K. L.; Bell, J. R.; Mellor, K. M.; Curl, C. L.; Delbridge, L. M. D. The cardiomyocyte origins of diastolic dysfunction: cellular components of myocardial "stiffness". Am J Physiol Heart Circ Physiol 2024, 326, H584–H598. [Google Scholar] [CrossRef] [PubMed]
- Aldujeli, A.; Tsai, T. Y.; Haq, A.; Tatarunas, V.; Knokneris, A.; Briedis, K.; Unikas, R.; Onuma, Y.; Brilakis, E. S.; Serruys, P. W. Impact of Coronary Microvascular Dysfunction on Functional Left Ventricular Remodeling and Diastolic Dysfunction. J Am Heart Assoc 2024, 13, e033596. [Google Scholar] [CrossRef] [PubMed]
- Baccouche, B. M.; Rhodenhiser, E. Galectin-3 and HFpEF: Clarifying an Emerging Relationship. Curr Cardiol Rev 2023, 19, 19–26. [Google Scholar] [PubMed]
- Seropian, I. M.; Cassaglia, P.; Miksztowicz, V.; Gonzalez, G. E. Unraveling the role of galectin-3 in cardiac pathology and physiology. Front Physiol 2023, 14, 1304735. [Google Scholar] [CrossRef]
- Sethi, A.; Sanam, S.; Alvala, R.; Alvala, M. An updated patent review of galectin-1 and galectin-3 inhibitors and their potential therapeutic applications (2016-present). Expert Opin Ther Pat 2021, 31, 709–721. [Google Scholar] [CrossRef]
- Khadeja Bi, A.; Santhosh, V.; Sigamani, K. Levels of Galectin-3 in Chronic Heart Failure: A Case-Control Study. Cureus 2022, 14, e28310. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, G.; Liu, J.; Shuang, X.; Liu, C.; Yang, C.; Qing, W.; Qiao, W. Clinical Implications of Plasma Galectin-3 in Heart Failure With Preserved Ejection Fraction: A Meta-Analysis. Front Cardiovasc Med 2022, 9, 854501. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. J.; Park, C. S.; Lee, S.; Park, J. B.; Kim, H. K.; Park, S. J.; Kim, Y. J.; Lee, S. P. Systemic proinflammatory-profibrotic response in aortic stenosis patients with diabetes and its relationship with myocardial remodeling and clinical outcome. Cardiovasc Diabetol 2023, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, A.; Ibrahim, M.; El Fallah, A. A.; Elian, M.; Deraz, S. E. Galectin-3 as an early marker of diastolic dysfunction in children with end-stage renal disease on regular hemodialysis. Ann Pediatr Cardiol 2022, 15, 266–272. [Google Scholar] [PubMed]
- An, L.; Chang, G.; Zhang, L.; Wang, P.; Gao, W.; Li, X. Pectin: Health-promoting properties as a natural galectin-3 inhibitor. Glycoconj J 2024, 41, 93–118. [Google Scholar] [CrossRef]
- Mayya, C.; Naveena, A. H.; Sinha, P.; Bhatia, D. Dynein functions in galectin-3 mediated processes of clathrin-independent endocytosis. J Biosci 2024, 49. [Google Scholar] [CrossRef]
- Liu, H.; Hwang, S. Y.; Lee, S. S., Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy. Pharmaceuticals (Basel) 2023, 16, (7).
- Ortega-Ferreira, C.; Soret, P.; Robin, G.; Speca, S.; Hubert, S.; Le Gall, M.; Desvaux, E.; Jendoubi, M.; Saint-Paul, J.; Chadli, L.; Chomel, A.; Berger, S.; Nony, E.; Neau, B.; Fould, B.; Licznar, A.; Levasseur, F.; Guerrier, T.; Elouej, S.; Courtade-Gaiani, S.; Provost, N.; Nguyen, T. Q.; Verdier, J.; Launay, D.; De Ceuninck, F. Antibody-mediated neutralization of galectin-3 as a strategy for the treatment of systemic sclerosis. Nat Commun 2023, 14, 5291. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, Y.; Zhang, R. Galectin-3 induces vascular smooth muscle cells calcification via AMPK/TXNIP pathway. Aging (Albany NY) 2022, 14, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W. L.; Chen, Y. C.; Li, S. J.; Lee, T. I.; Lee, T. W.; Higa, S.; Chung, C. C.; Kao, Y. H.; Chen, S. A.; Chen, Y. J. Galectin-3 enhances atrial remodelling and arrhythmogenesis through CD98 signalling. Acta Physiol (Oxf) 2022, 234, e13784. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, L.; Li, L.; Shao, C.; Liu, J.; Zhou, M.; Wang, Z. Galectin-3 mediates cardiac remodeling caused by impaired glucose and lipid metabolism through inhibiting two pathways of activating Akt. Am J Physiol Heart Circ Physiol 2021, 320, H364–H380. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, S.; Liu, X.; Zheng, Q.; Wang, Z.; Huang, Y.; Shi, J. Galectin-3 promotes calcification of human aortic valve interstitial cells via the NF-kappa B signaling pathway. Cardiovasc Diagn Ther 2022, 12, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, M.; Bie, M.; Wang, X.; Guo, J.; Xiao, H. Galectin-3 Induces Atrial Fibrosis by Activating the TGF-beta1/Smad Pathway in Patients with Atrial Fibrillation. Cardiology 2020, 145, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Rubis, P.; Holcman, K.; Dziewiecka, E.; Wisniowska-Smialek, S.; Karabinowska, A.; Szymonowicz, M.; Khachatryan, L.; Wypasek, E.; Garlitski, A.; Gackowski, A.; Podolec, P. Relationships between circulating galectin-3, extracellular matrix fibrosis and outcomes in dilated cardiomyopathy. Adv Clin Exp Med 2021, 30, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ansari, U.; Behnes, M.; Hoffmann, J.; Weidner, K.; Kuche, P.; Rusnak, J.; Kim, S. H.; Natale, M.; Reckord, N.; Lang, S.; Hoffmann, U.; Bertsch, T.; Fatar, M.; Borggrefe, M.; Akin, I. Galectin-3 reflects the echocardiographic quantification of right ventricular failure. Scand Cardiovasc J 2021, 55, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.; Gorski, D. J.; Newman, A. A. C.; Homann, S.; Petz, A.; Owsiany, K. M.; Serbulea, V.; Zhou, Y. Q.; Deaton, R. A.; Bendeck, M.; Owens, G. K.; Fischer, J. W. SMC-Derived Hyaluronan Modulates Vascular SMC Phenotype in Murine Atherosclerosis. Circ Res 2021, 129, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Petz, A.; Grandoch, M.; Gorski, D. J.; Abrams, M.; Piroth, M.; Schneckmann, R.; Homann, S.; Muller, J.; Hartwig, S.; Lehr, S.; Yamaguchi, Y.; Wight, T. N.; Gorressen, S.; Ding, Z.; Kotter, S.; Kruger, M.; Heinen, A.; Kelm, M.; Godecke, A.; Flogel, U.; Fischer, J. W. Cardiac Hyaluronan Synthesis Is Critically Involved in the Cardiac Macrophage Response and Promotes Healing After Ischemia Reperfusion Injury. Circ Res 2019, 124, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Claggett, B. L.; de Denus, S.; Zile, M. R.; Huynh, T.; Desai, A. S.; Sirois, M. G.; Solomon, S. D.; Pitt, B.; Rouleau, J. L.; Pfeffer, M. A.; O’Meara, E. Impact of diabetes on serum biomarkers in heart failure with preserved ejection fraction: insights from the TOPCAT trial. ESC Heart Fail 2021, 8, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, Y.; Li, J. J.; He, W. J.; Gao, X. H.; Zhang, Y.; Sun, X.; Tong, J.; Zhang, J.; Deng, X. L.; Du, X. J.; Xie, W. Stretch-induced sarcoplasmic reticulum calcium leak is causatively associated with atrial fibrillation in pressure-overloaded hearts. Cardiovasc Res 2021, 117, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lv, W.; Lu, C.; Jiang, Y.; Yang, X.; Song, M. Galectin-3 inhibition attenuates doxorubicin-induced cardiac dysfunction by upregulating the expression of peroxiredoxin-4. Can J Physiol Pharmacol 2020, 98, 700–707. [Google Scholar] [CrossRef]
- Beyhoff, N.; Lohr, D.; Foryst-Ludwig, A.; Klopfleisch, R.; Brix, S.; Grune, J.; Thiele, A.; Erfinanda, L.; Tabuchi, A.; Kuebler, W. M.; Pieske, B.; Schreiber, L. M.; Kintscher, U. Characterization of Myocardial Microstructure and Function in an Experimental Model of Isolated Subendocardial Damage. Hypertension 2019, 74, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; Lopez, B.; Fernandez-Celis, A.; Querejeta, R.; Santamaria, E.; Fernandez-Irigoyen, J.; Rabago, G.; Moreno, M. U.; Jaisser, F.; Diez, J.; Gonzalez, A.; Lopez-Andres, N. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. K.; Su, M. Y.; Lee, J. K.; Chiang, F. T.; Hwang, J. J.; Lin, J. L.; Chen, J. J.; Liu, F. T.; Tsai, C. T. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci Rep 2015, 5, 17007. [Google Scholar] [CrossRef] [PubMed]
- Balbo, B. E.; Amaral, A. G.; Fonseca, J. M.; de Castro, I.; Salemi, V. M.; Souza, L. E.; Dos Santos, F.; Irigoyen, M. C.; Qian, F.; Chammas, R.; Onuchic, L. F. Cardiac dysfunction in Pkd1-deficient mice with phenotype rescue by galectin-3 knockout. Kidney Int 2016, 90, 580–97. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Girerd, N.; Ferreira, J. P.; Kevin, D.; Huttin, O.; González, A.; Bozec, E.; Clark, A. L.; Cosmi, F.; Cuthbert, J.; Diez, J.; Edelmann, F.; Hazebroek, M.; Heymans, S.; Mariottoni, B.; Pellicori, P.; Petutschnigg, J.; Pieske, B.; Staessen, J. A.; Verdonschot, J. A. J.; Rossignol, P.; Cleland, J. G. F.; Zannad, F. The association between markers of type I collagen synthesis and echocardiographic response to spironolactone in patients at risk of heart failure: findings from the HOMAGE trial. Eur J Heart Fail 2022, 24, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Gottdiener, J. S.; Seliger, S.; deFilippi, C.; Christenson, R.; Baldridge, A. S.; Kizer, J. R.; Psaty, B. M.; Shah, S. J. Relation of Biomarkers of Cardiac Injury, Stress, and Fibrosis With Cardiac Mechanics in Patients >/= 65 Years of Age. Am J Cardiol 2020, 136, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Parcha, V.; Patel, N.; Kalra, R.; Bhargava, A.; Prabhu, S. D.; Arora, G.; Arora, P. Clinical, Demographic, and Imaging Correlates of Anemia in Heart Failure With Preserved Ejection Fraction (from the RELAX Trial). Am J Cardiol 2020, 125, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Tyminska, A.; Kaplon-Cieslicka, A.; Ozieranski, K.; Budnik, M.; Wancerz, A.; Sypien, P.; Peller, M.; Balsam, P.; Opolski, G.; Filipiak, K. J. Association of Galectin-3 and Soluble ST2, and Their Changes, with Echocardiographic Parameters and Development of Heart Failure after ST-Segment Elevation Myocardial Infarction. Dis Markers 2019, 2019, 9529053. [Google Scholar] [CrossRef]
- Ferreira, J. P.; Bauters, C.; Eschalier, R.; Lamiral, Z.; Fay, R.; Huttin, O.; Girerd, N.; Zannad, F.; Pinet, F.; Rossignol, P. Echocardiographic diastolic function evolution in patients with an anterior Q-wave myocardial infarction: insights from the REVE-2 study. ESC Heart Fail 2019, 6, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, G.; Caretta, G.; De Maria, R.; Parolini, M.; Bassi, L.; Testa, S.; Pirelli, S. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart 2017, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gurel, O. M.; Yilmaz, H.; Celik, T. H.; Cakmak, M.; Namuslu, M.; Bilgic, A. M.; Bavbek, N.; Akcay, A.; Eryonucu, B. Galectin-3 as a new biomarker of diastolic dysfunction in hemodialysis patients. Herz 2015, 40, 788–94. [Google Scholar] [CrossRef] [PubMed]
- Ureche, C.; Dodi, G.; Covic, A.; Nedelcu, A.; Volovăț, S. R.; Sascău, R. A.; Stătescu, C.; Covic, A., Connection between Cardiac Fibrosis Biomarkers and Echocardiography Parameters in Advanced Chronic Kidney Disease Patients. J Clin Med 2023, 12, (8).
- Revnic, R.; Cojan-Minzat, B. O.; Zlibut, A.; Orzan, R. I.; Agoston, R.; Muresan, I. D.; Horvat, D.; Cionca, C.; Chis, B.; Agoston-Coldea, L., The Role of Circulating Collagen Turnover Biomarkers and Late Gadolinium Enhancement in Patients with Non-Ischemic Dilated Cardiomyopathy. Diagnostics (Basel) 2022, 12, (6).
- Echeverria, L. E.; Gomez-Ochoa, S. A.; Rojas, L. Z.; Garcia-Rueda, K. A.; Lopez-Aldana, P.; Muka, T.; Morillo, C. A. Cardiovascular Biomarkers and Diastolic Dysfunction in Patients With Chronic Chagas Cardiomyopathy. Front Cardiovasc Med 2021, 8, 751415. [Google Scholar] [CrossRef]
- Seropian, I. M.; Fontana Estevez, F. S.; Villaverde, A.; Cacciagiu, L.; Bustos, R.; Touceda, V.; Penas, F.; Selser, C.; Morales, C.; Miksztowicz, V.; Gonzalez, G. E. Galectin-3 contributes to acute cardiac dysfunction and toxicity by increasing oxidative stress and fibrosis in doxorubicin-treated mice. Int J Cardiol 2023, 393, 131386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gaur, M.; Mounzih, K.; Rodriguez, H. J.; Qiu, H.; Chen, M.; Yan, L.; Cooper, B. A.; Narayan, S.; Derakhshandeh, R.; Rao, P.; Han, D. D.; Nabavizadeh, P.; Springer, M. L.; John, C. M. Inhibition of galectin-3 post-infarction impedes progressive fibrosis by regulating inflammatory profibrotic cascades. Cardiovasc Res 2023, 119, 2536–2549. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wu, J.; Gu, H.; Deng, X.; Xu, W.; Feng, S.; Wang, S.; Song, Y.; Pang, Z.; Deng, X.; Vendrov, A. E.; Madamanchi, N. R.; Runge, M. S.; Wang, X.; Zhang, Y.; Xiao, H.; Dong, E. Galectin-3-centered paracrine network mediates cardiac inflammation and fibrosis upon beta-adrenergic insult. Sci China Life Sci 2023, 66, 1067–1078. [Google Scholar] [CrossRef]
- Fontana Estevez, F. S.; Betazza, M. C.; Miksztowicz, V.; Seropian, I. M.; Silva, M. G.; Penas, F.; Touceda, V.; Selser, C.; Villaverde, A.; Goren, N.; Cianciulli, T. F.; Medina, V.; Morales, C.; Gironacci, M.; Gonzalez, G. E. Genetic Deletion of Galectin-3 Exacerbates Age-Related Myocardial Hypertrophy and Fibrosis in Mice. Cell Physiol Biochem 2022, 56, 353–366. [Google Scholar] [PubMed]
- Vlachou, F.; Varela, A.; Stathopoulou, K.; Ntatsoulis, K.; Synolaki, E.; Pratsinis, H.; Kletsas, D.; Sideras, P.; Davos, C. H.; Capetanaki, Y.; Psarras, S. Galectin-3 interferes with tissue repair and promotes cardiac dysfunction and comorbidities in a genetic heart failure model. Cell Mol Life Sci 2022, 79, 250. [Google Scholar] [CrossRef] [PubMed]
- Fontana Estevez, F. S.; Miksztowicz, V.; Seropian, I. M.; Cassaglia, P.; Bustos, R.; Touceda, V.; Cianciulli, T.; Cassanova, V.; Morales, C.; Gonzalez, G. E. An Experimental Model of Myocardial Infarction for Studying Cardiac Repair and Remodeling in Knockout Mice. J Vis Exp.
- Sonkawade, S. D.; Pokharel, S.; Karthikeyan, B.; Kim, M.; Xu, S.; Kc, K.; Sexton, S.; Catalfamo, K.; Spernyak, J. A.; Sharma, U. C. Small Endogeneous Peptide Mitigates Myocardial Remodeling in a Mouse Model of Cardioselective Galectin-3 Overexpression. Circ Heart Fail 2021, 14, e008510. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, R.; Feng, C.; Li, K.; Liu, S.; Fu, Q. Galectin-3 is involved in inflammation and fibrosis in arteriogenic erectile dysfunction via the TLR4/MyD88/NF-kappaB pathway. Cell Death Discov 2024, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z. D.; Sun, X.; Bai, R. Y.; Han, M. Z.; Zhang, Y. J.; Wu, W.; Zhang, Y.; Lai, B. C.; Zhang, Y.; Wang, Y.; Du, X. J.; Deng, X. L. YAP-galectin-3 signaling mediates endothelial dysfunction in angiotensin II-induced hypertension in mice. Cell Mol Life Sci 2023, 80, 38. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, V.; Siasos, G.; Oikonomou, E.; Zaromitidou, M.; Mourouzis, K.; Dimitropoulos, S.; Bletsa, E.; Gouliopoulos, N.; Stampouloglou, P. K.; Panoilia, M. E.; Marinos, G.; Tsioufis, K.; Vavuranakis, M.; Tousoulis, D. The prognostic role of galectin-3 and endothelial function in patients with heart failure. Cardiol J 2023, 30, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, J.; Hu, T.; Ren, Z.; Li, L.; Hameed, I.; Zhang, X.; Men, C.; Guo, Y.; Xu, D.; Zhan, Y. Galectin-3 exacerbates ox-LDL-mediated endothelial injury by inducing inflammation via integrin beta1-RhoA-JNK signaling activation. J Cell Physiol 2019, 234, 10990–11000. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzebska, A.; Sitkiewicz, D., The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2021, 12, (1).
- Zaborska, B.; Sikora-Frac, M.; Smarz, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G., The Role of Galectin-3 in Heart Failure-The Diagnostic, Prognostic and Therapeutic Potential-Where Do We Stand? Int J Mol Sci 2023, 24, (17).
- Liu, Y.; Guan, S.; Xu, H.; Zhang, N.; Huang, M.; Liu, Z. Inflammation biomarkers are associated with the incidence of cardiovascular disease: a meta-analysis. Front Cardiovasc Med 2023, 10, 1175174. [Google Scholar] [CrossRef] [PubMed]
- Baccouche, B. M.; Mahmoud, M. A.; Nief, C.; Patel, K.; Natterson-Horowitz, B. Galectin-3 is Associated with Heart Failure Incidence: A Meta-Analysis. Curr Cardiol Rev 2023, 19, e171122211004. [Google Scholar] [CrossRef]
- Mohtasham Kia, Y.; Cannavo, A.; Bahiraie, P.; Alilou, S.; Saeedian, B.; Babajani, N.; Ghondaghsaz, E.; Khalaji, A.; Behnoush, A. H. Insights into the Role of Galectin-3 as a Diagnostic and Prognostic Biomarker of Atrial Fibrillation. Dis Markers 2023, 2023, 2097012. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Anam, K.; Ahmed, H., Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases. Int J Mol Sci 2023, 24, (9).
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol Sci 2023, 44, 519–531. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U. M.; Di Taranto, M. D.; Fortunato, G., Galectin-3 in Cardiovascular Diseases. Int J Mol Sci 2020, 21, (23).
- Bellos, I.; Marinaki, S.; Lagiou, P.; Benetou, V., Association of serum galectin-3 levels with mortality and cardiovascular disease outcomes in hemodialysis patients: a systematic review and dose-response meta-analysis. Int Urol Nephrol 2024.
- Spahillari, A.; Jackson, L.; Varrias, D.; Michelhaugh, S. A.; Januzzi, J. L.; Shahideh, B.; Daghfal, D.; Valkov, N.; Murtagh, G.; Das, S. MicroRNAs are associated with cardiac biomarkers, cardiac structure and function and incident outcomes in heart failure. ESC Heart Fail 2024, 11, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Winter, R. L.; Maneval, K. L.; Ferrel, C. S.; Clark, W. A.; Herrold, E. J.; Rhinehart, J. D. Evaluation of right ventricular diastolic function, systolic function, and circulating galectin-3 concentrations in dogs with pulmonary stenosis. J Vet Intern Med 2023, 37, 2030–2038. [Google Scholar] [CrossRef]
- Kondratavičienė, L.; Tamulėnaitė, E.; Vasylė, E.; Januškevičius, A.; Ereminienė, E.; Malakauskas, K.; Žemaitis, M.; Miliauskas, S., Changes in Left Heart Geometry, Function, and Blood Serum Biomarkers in Patients with Obstructive Sleep Apnea after Treatment with Continuous Positive Airway Pressure. Medicina (Kaunas) 2022, 58, (11).
- Karolko, B.; Serafin, A.; Przewłocka-Kosmala, M. Impact of moderately reduced renal function on the diagnostic and prognostic value of galectin-3 in patients with exertional dyspnea. Adv Clin Exp Med 2022, 31, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Węgiel, M.; Wojtasik-Bakalarz, J.; Malinowski, K.; Surmiak, M.; Dziewierz, A.; Sorysz, D.; Tokarek, T.; Dudek, D.; Bartuś, S.; Surdacki, A.; Rakowski, T., Mid-regional pro-adrenomedullin and lactate dehydrogenase as predictors of left ventricular remodeling in patients with myocardial infarction treated with percutaneous coronary intervention. Pol Arch Intern Med 2022, 132, (2).
- Kim, D. K.; Lee, Y. H.; Kim, J. S.; Kim, Y. G.; Lee, S. Y.; Ahn, S. Y.; Lee, D. Y.; Jeong, K. H.; Lee, S. H.; Hwang, H. S.; Moon, J. Y. Circulating Vascular Adhesion Protein-1 Level Predicts the Risk of Cardiovascular Events and Mortality in Hemodialysis Patients. Front Cardiovasc Med 2021, 8, 701079. [Google Scholar] [CrossRef]
Figure 1.
Cellular signaling pathways of galectin-3 in cardiac diastolic pathophysiology. Galectin-3 is a key regulator in various signaling pathways that contribute to cardiac diastolic dysfunction. Activation of AMPK and inhibition of TXNIP mitigate calcification processes and glucose and lipid disturbances. Galectin-3 modulates CD98 and TGF-β signaling, promoting atrial fibrosis and myofibroblast growth, which are critical factors in the development of atrial fibrillation. The NF-κB pathway is involved in valve calcification and further contributes to cardiac remodeling. Additionally, galectin-3 influences Akt and PI3K signaling pathways, exacerbating cardiac remodeling and overall diastolic dysfunction. AMPK: AMP-activated protein kinase, involved in metabolic regulation and inhibition of cardiac calcification. TXNIP: Thioredoxin-interacting protein, associated with glucose and lipid disturbances. Calcification: Pathological calcification processes in the heart. CD98: Cell surface protein influencing TGF-β signaling and fibrosis. TGF-β: Transforming growth factor-beta, promoting atrial fibrosis and myofibroblast growth. Atrial fibrosis: Fibrotic changes in the atria contributing to atrial fibrillation. Myofibroblast growth: Expansion of myofibroblasts, cells involved in fibrosis. Atrial fibrillation: A common cardiac arrhythmia linked to fibrosis. NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, implicated in inflammation and valve calcification. Valve calcification: Calcification affecting heart valves, contributing to cardiac dysfunction. Glucose and lipid disturbance: Metabolic disruptions influenced by galectin-3 signaling. Akt: Protein kinase B, involved in cell survival and growth signaling pathways. PI3K: Phosphoinositide 3-kinase, involved in cell growth and survival signaling. Cardiac remodeling: Structural changes in the heart associated with diastolic dysfunction.
Figure 1.
Cellular signaling pathways of galectin-3 in cardiac diastolic pathophysiology. Galectin-3 is a key regulator in various signaling pathways that contribute to cardiac diastolic dysfunction. Activation of AMPK and inhibition of TXNIP mitigate calcification processes and glucose and lipid disturbances. Galectin-3 modulates CD98 and TGF-β signaling, promoting atrial fibrosis and myofibroblast growth, which are critical factors in the development of atrial fibrillation. The NF-κB pathway is involved in valve calcification and further contributes to cardiac remodeling. Additionally, galectin-3 influences Akt and PI3K signaling pathways, exacerbating cardiac remodeling and overall diastolic dysfunction. AMPK: AMP-activated protein kinase, involved in metabolic regulation and inhibition of cardiac calcification. TXNIP: Thioredoxin-interacting protein, associated with glucose and lipid disturbances. Calcification: Pathological calcification processes in the heart. CD98: Cell surface protein influencing TGF-β signaling and fibrosis. TGF-β: Transforming growth factor-beta, promoting atrial fibrosis and myofibroblast growth. Atrial fibrosis: Fibrotic changes in the atria contributing to atrial fibrillation. Myofibroblast growth: Expansion of myofibroblasts, cells involved in fibrosis. Atrial fibrillation: A common cardiac arrhythmia linked to fibrosis. NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, implicated in inflammation and valve calcification. Valve calcification: Calcification affecting heart valves, contributing to cardiac dysfunction. Glucose and lipid disturbance: Metabolic disruptions influenced by galectin-3 signaling. Akt: Protein kinase B, involved in cell survival and growth signaling pathways. PI3K: Phosphoinositide 3-kinase, involved in cell growth and survival signaling. Cardiac remodeling: Structural changes in the heart associated with diastolic dysfunction.
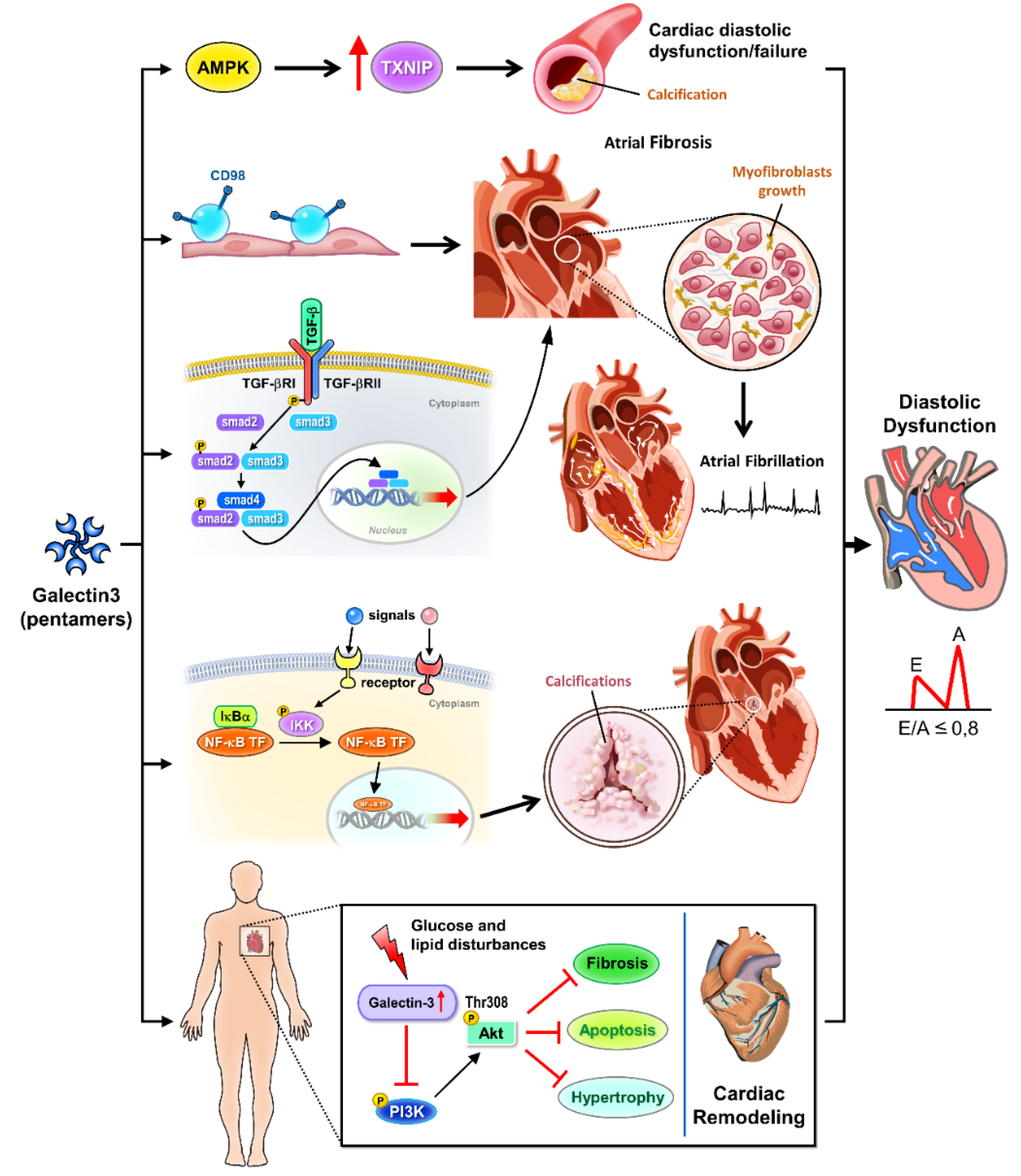
Figure 2.
The pathophysiology of galectin-3 interacted with extracellular matrix components in cardiac diastolic dysfunction. Galectin-3 interacts with various extracellular matrix (ECM) components, playing a pivotal role in the pathophysiology of cardiac diastolic dysfunction. This figure illustrates the mechanisms by which galectin-3 influences cardiac structure and function through its interactions with ECM components. Through these interactions, galectin-3 contributes to the progression of diastolic dysfunction by promoting ECM deposition, vascular remodeling, and alterations in vascular smooth muscle cell phenotype, leading to increased myocardial stiffness and impaired cardiac function. Hyaluronic acid: An ECM glycosaminoglycan that interacts with galectin-3, contributing to tissue stiffness and fibrosis. Vascular smooth muscle cell phenotype: Galectin-3 affects the phenotype of vascular smooth muscle cells, promoting a pro-fibrotic and pro-inflammatory state. Vascular remodeling: Structural changes in blood vessels induced by galectin-3, leading to increased stiffness and altered function. Diastolic dysfunction: Impaired relaxation and filling of the heart during diastole, exacerbated by galectin-3-mediated ECM alterations and vascular remodeling.
Figure 2.
The pathophysiology of galectin-3 interacted with extracellular matrix components in cardiac diastolic dysfunction. Galectin-3 interacts with various extracellular matrix (ECM) components, playing a pivotal role in the pathophysiology of cardiac diastolic dysfunction. This figure illustrates the mechanisms by which galectin-3 influences cardiac structure and function through its interactions with ECM components. Through these interactions, galectin-3 contributes to the progression of diastolic dysfunction by promoting ECM deposition, vascular remodeling, and alterations in vascular smooth muscle cell phenotype, leading to increased myocardial stiffness and impaired cardiac function. Hyaluronic acid: An ECM glycosaminoglycan that interacts with galectin-3, contributing to tissue stiffness and fibrosis. Vascular smooth muscle cell phenotype: Galectin-3 affects the phenotype of vascular smooth muscle cells, promoting a pro-fibrotic and pro-inflammatory state. Vascular remodeling: Structural changes in blood vessels induced by galectin-3, leading to increased stiffness and altered function. Diastolic dysfunction: Impaired relaxation and filling of the heart during diastole, exacerbated by galectin-3-mediated ECM alterations and vascular remodeling.
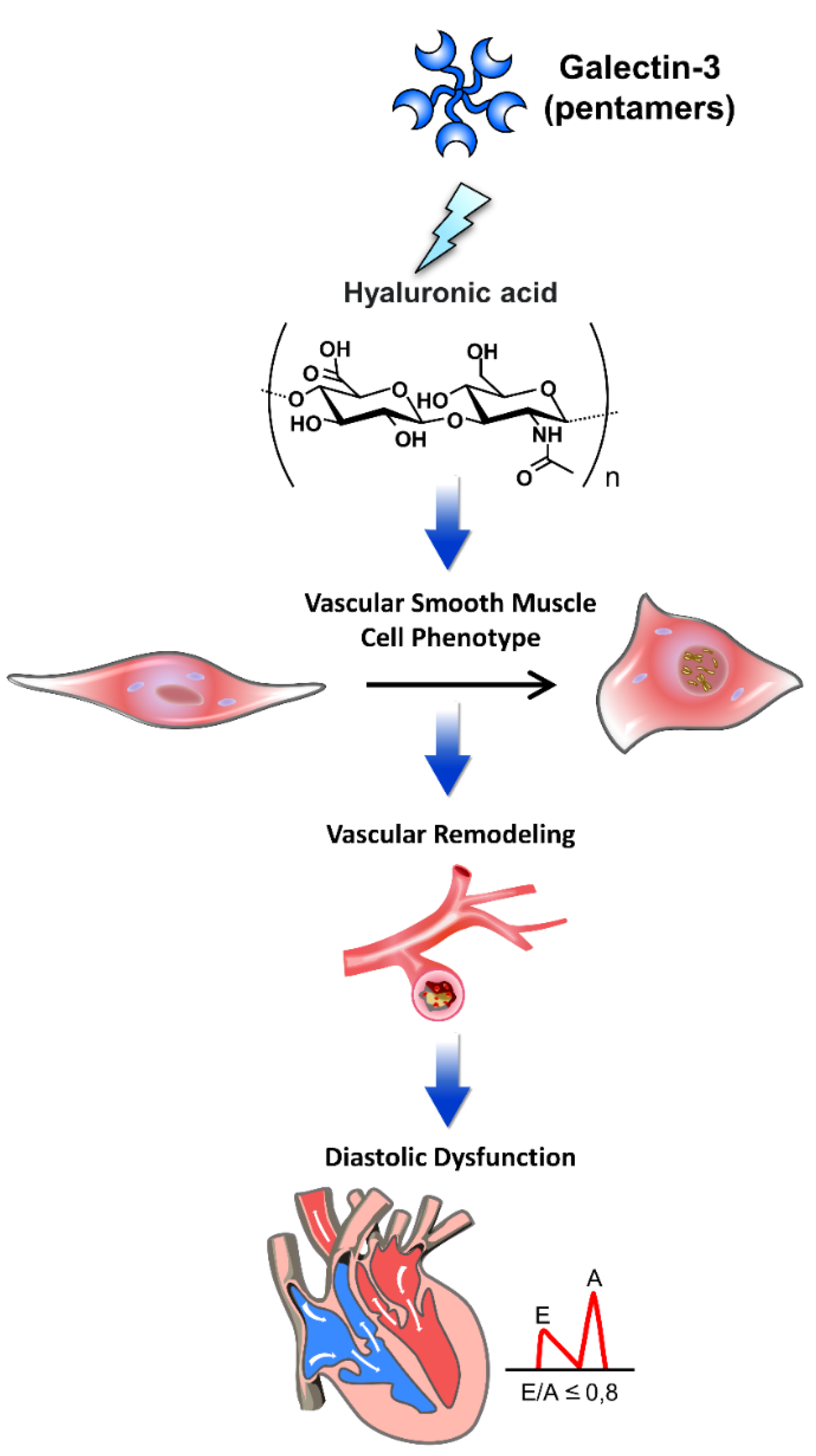
Figure 3.
The role of galectin-3 in inflammation, fibrosis, and heart remodeling leading to cardiac diastolic dysfunction. Galectin-3 (Gal-3), secreted by macrophages, plays a crucial role in the progression of cardiac diastolic dysfunction through its involvement in inflammation, fibrosis, and heart remodeling. This figure illustrates the pathways and mechanisms by which Gal-3 contributes to these pathological processes, ultimately leading to heart failure. Through these mechanisms, galectin-3 significantly contributes to the pathophysiology of cardiac diastolic dysfunction, highlighting its potential as a therapeutic target and diagnostic marker. Macrophage-secreted Gal-3: Galectin-3 released by macrophages acts as a key mediator in the inflammatory response. Biomarker: Galectin-3 serves as a biomarker for inflammation and fibrosis in cardiac tissues. Mediators: Various inflammatory mediators are regulated by Gal-3, contributing to cardiac inflammation. Inflammation: Gal-3 promotes inflammation, which exacerbates cardiac tissue damage and remodeling. Cardiac remodeling: Structural changes in the heart induced by Gal-3, including hypertrophy and fibrosis, impair cardiac function. Fibrosis: Gal-3 stimulates fibroblast activation and collagen deposition, leading to increased stiffness and fibrosis of the cardiac tissue. Heart failure: The combined effects of inflammation, fibrosis, and remodeling contribute to the development of heart failure. Diastolic dysfunction: Impaired relaxation and filling of the heart during diastole, driven by Gal-3-mediated pathological changes.
Figure 3.
The role of galectin-3 in inflammation, fibrosis, and heart remodeling leading to cardiac diastolic dysfunction. Galectin-3 (Gal-3), secreted by macrophages, plays a crucial role in the progression of cardiac diastolic dysfunction through its involvement in inflammation, fibrosis, and heart remodeling. This figure illustrates the pathways and mechanisms by which Gal-3 contributes to these pathological processes, ultimately leading to heart failure. Through these mechanisms, galectin-3 significantly contributes to the pathophysiology of cardiac diastolic dysfunction, highlighting its potential as a therapeutic target and diagnostic marker. Macrophage-secreted Gal-3: Galectin-3 released by macrophages acts as a key mediator in the inflammatory response. Biomarker: Galectin-3 serves as a biomarker for inflammation and fibrosis in cardiac tissues. Mediators: Various inflammatory mediators are regulated by Gal-3, contributing to cardiac inflammation. Inflammation: Gal-3 promotes inflammation, which exacerbates cardiac tissue damage and remodeling. Cardiac remodeling: Structural changes in the heart induced by Gal-3, including hypertrophy and fibrosis, impair cardiac function. Fibrosis: Gal-3 stimulates fibroblast activation and collagen deposition, leading to increased stiffness and fibrosis of the cardiac tissue. Heart failure: The combined effects of inflammation, fibrosis, and remodeling contribute to the development of heart failure. Diastolic dysfunction: Impaired relaxation and filling of the heart during diastole, driven by Gal-3-mediated pathological changes.
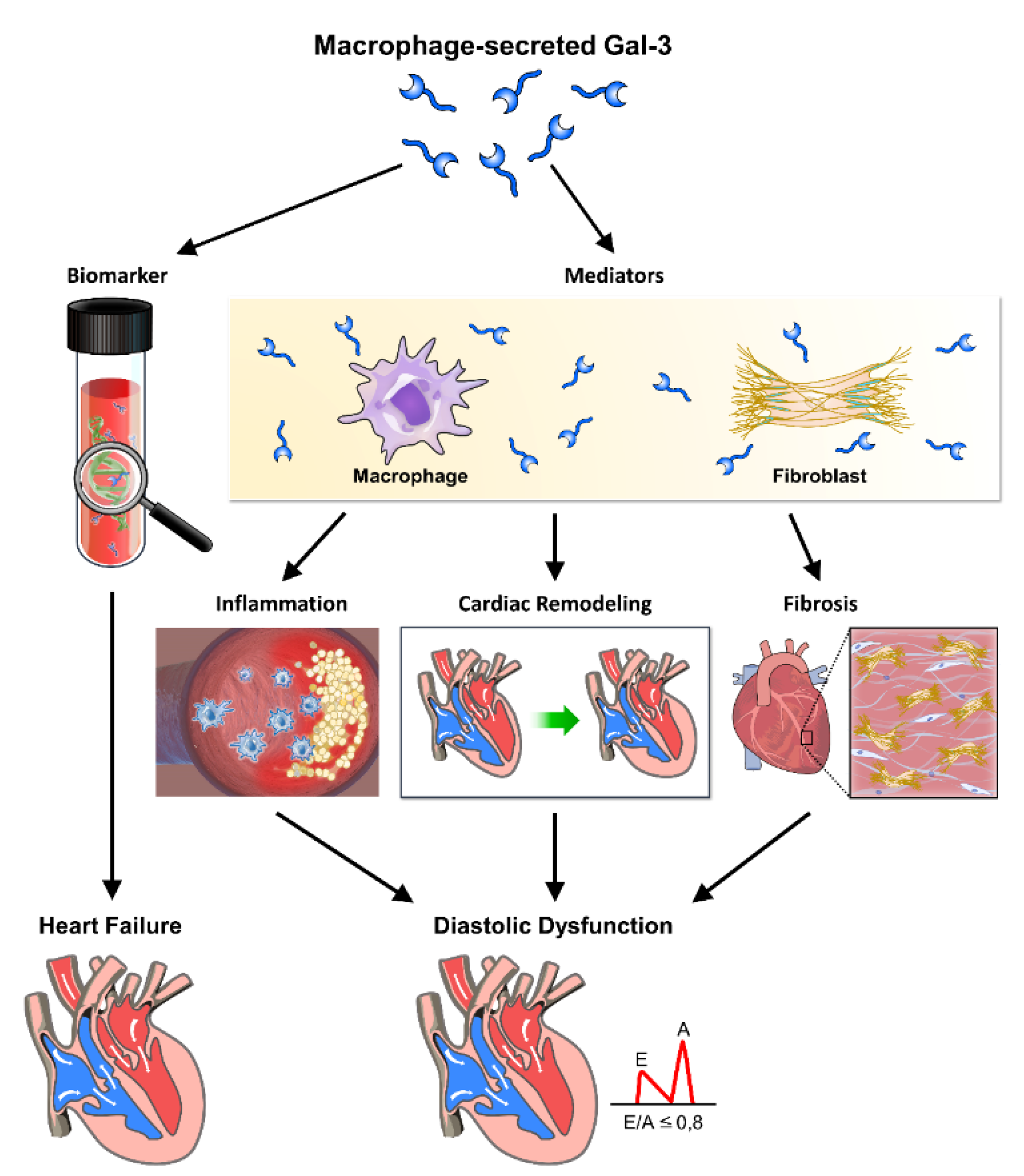
Table 1.
Clinical galectin-3 studies in diverse patient cohorts with diastolic dysfunction.
Table 1.
Clinical galectin-3 studies in diverse patient cohorts with diastolic dysfunction.
| Title |
Authors |
Years |
Results |
| Galectin-3 as an early marker of diastolic dysfunction in children with end-stage renal disease on regular hemodialysis. |
Akram et al. [62][M1] |
2022 |
Galectin-3 is a potential early biomarker that can be used in early diagnosis and grading of diastolic dysfunction in end-stage renal disease children on regular hemodialysis. |
| Impact of diabetes on serum biomarkers in heart failure with preserved ejection fraction: Insights from the TOPCAT trial. |
De Marco et al. [63] |
2021 |
Higher galectin-3 levels were measured in patients with HFpEF. |
| The diagnostic and prognostic value of galectin-3 in patients at risk for heart failure with preserved ejection fraction: Results from the DIAST-CHF study. |
Trippel et al. [64] |
2021 |
Galectin-3 differentiated patients with HFpEF from an overall cohort of well-characterized patients with risk factors for HFpEF. |
| Cardiac remodeling biomarkers as potential circulating markersofleft ventricular hypertrophy in heart failure with preserved ejection fraction. |
Mitic et al. [65] |
2020 |
Cardiac remodeling biomarkers (e.g. galectin-3) are potential circulating indicators of left ventricular hypertrophy in HFpEF, which may ensure timely recognition of disease progression among high-risk patients. |
| Clinical, demographic, and imaging correlates of anemia in heart failure with preserved ejection fraction (from the RELAX Trial). |
Parcha et al. [34] |
2020 |
Galectin-3 levels were higher in anemic HFpEF patients. |
| Echocardiographic diastolic function evolution in patients with an anterior Q-wave myocardial infarction: Insights from the REVE-2 study. |
Ferreira et al. [36] |
2019 |
The amino-terminal propeptide of type III procollagen, galectin-3, and BNP may be independently associated with new-onset diastolic dysfunction in post- myocardial infarction patients. |
| Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. |
Di Tano et al. [37] |
2017 |
Left ventricular end-diastolic volume and galectin-3 levels independently predicted left ventricular remodelling. |
| Galectin-3 as a new biomarker of diastolic dysfunction in hemodialysis patients. |
Gurel et al. [38] |
2015 |
Galectin-3 may be a promising biomarker for the detection of left ventricular diastolic dysfunction in hemodialysis. |
Table 2.
Summary of key studies on galectin-3 and diastolic dysfunction.
Table 2.
Summary of key studies on galectin-3 and diastolic dysfunction.
| Study |
Population |
Key Findings |
Conclusion |
| Bellos et al. (2024)[61] |
Hemodialysis patients |
Elevated serum galectin-3 levels are associated with higher mortality and cardiovascular outcomes. |
Galectin-3 is a significant prognostic marker in hemodialysis patients. |
| Spahillari et al. (2024) [62] |
Heart failure patients |
MicroRNAs associated with cardiac biomarkers, structure, function, and incident outcomes. |
Galectin-3 correlates with cardiac remodeling and outcomes in heart failure. |
| Winter et al. (2023) [63] |
Dogs with pulmonary stenosis |
Higher circulating galectin-3 levels linked to right ventricular diastolic and systolic dysfunction. |
Galectin-3 can be a biomarker for cardiac function in canine models. |
| Ureche et al. (2023) [39] |
Advanced CKD patients |
Cardiac fibrosis biomarkers, including galectin-3, correlate with echocardiographic parameters. |
Galectin-3 is linked to cardiac fibrosis and diastolic dysfunction in CKD patients. |
| Baccouche et al. (2023) [5] |
HFpEF patients |
Galectin-3 associated with HFpEF. |
Galectin-3 is an emerging marker in HFpEF. |
| Lee et al. (2023) [10] |
Aortic stenosis patients with diabetes |
Proinflammatory-profibrotic response associated with myocardial remodeling and clinical outcomes. |
Galectin-3 contributes to cardiac remodeling in diabetic aortic stenosis patients. |
| Elsadek et al. (2022) [11] |
Children with end-stage renal disease |
Early increase in galectin-3 levels noted in children with diastolic dysfunction on hemodialysis. |
Galectin-3 as an early marker for diastolic dysfunction in pediatric renal disease. |
| Kondratavičienė et al. (2022) [64] |
Obstructive sleep apnea patients |
Treatment with continuous positive airway pressure (CPAP) improved left heart geometry, function, and reduced galectin-3 levels. |
Galectin-3 reduction linked to improved cardiac function post-CPAP treatment. |
| Revnic et al. (2022) [40] |
Non-ischemic dilated cardiomyopathy (DCM) patients |
Galectin-3 levels correlated with cardiac function and fibrosis markers. |
Galectin-3 is a predictive biomarker for cardiac dysfunction in non-ischemic DCM. |
| Kobayashi et al. (2022) [32] |
Heart failure patients |
Markers of type I collagen synthesis, including galectin-3, predict response to spironolactone. |
Galectin-3 as a predictor for therapeutic response in heart failure. |
| Shi et al. (2022) [9] |
HFpEF patients |
Meta-analysis showing significant association between galectin-3 and HFpEF outcomes. |
Galectin-3 is a valuable biomarker for HFpEF prognosis. |
| Karolko et al. (2022) [65][M2] |
Patients with exertional dyspnea |
Moderately reduced renal function impacts the diagnostic and prognostic value of galectin-3. |
Renal function must be considered when evaluating galectin-3 levels. |
| Vlachou et al. (2022) [46] |
Genetic heart failure model |
Galectin-3 promotes cardiac dysfunction and comorbidities by interfering with tissue repair. |
Targeting galectin-3 may help mitigate heart failure progression. |
Table 3.
Comparison of galectin-3 with other biomarkers for diastolic dysfunction.
Table 3.
Comparison of galectin-3 with other biomarkers for diastolic dysfunction.
| Biomarker |
Mechanism of Action/Role |
Clinical Significance in Diastolic Dysfunction |
References |
| Galectin-3 |
Modulates fibrosis and inflammation by binding to β-galactosides on cell surfaces and extracellular matrix proteins. |
Elevated levels are associated with heart failure, myocardial fibrosis, and poor outcomes in patients with diastolic dysfunction. |
Baccouche & Rhodenhiser, 2023 [5] |
| NT-proBNP |
Released in response to ventricular stretching and pressure overload. |
High levels indicate heart failure and correlate with severity of diastolic dysfunction. |
Spahillari et al., 2024 [62] |
| sST2 |
A member of the interleukin-1 receptor family that modulates immune response. |
Elevated levels are indicative of myocardial stress and fibrosis, predicting adverse outcomes in diastolic dysfunction. |
Elsadek et al., 2022 [11] |
| GDF-15 |
A member of the TGF-β cytokine family, involved in inflammation and apoptosis. |
Increased levels are linked to myocardial infarction, heart failure, and diastolic dysfunction severity. |
Węgiel et al., 2022 [66][M3] |
| Collagen Turnover Markers |
Indicators of collagen synthesis and degradation in the extracellular matrix. |
Elevated in conditions leading to fibrosis, these markers correlate with severity and progression of diastolic dysfunction. |
Kobayashi et al., 2022 [32] |
| MiRNAs |
Small non-coding RNAs that regulate gene expression post-transcriptionally. |
Specific miRNAs are associated with cardiac fibrosis, hypertrophy, and diastolic dysfunction. |
Spahillari et al., 2024 [62] |
| VAP-1 |
Enzyme involved in inflammation and leukocyte migration. |
Higher levels predict cardiovascular events and are associated with endothelial dysfunction in diastolic heart failure. |
Kim et al., 2021 [67] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








